Introduction
The study start-up phase at the site level represents a significant administrative and logistical undertaking. The initiation of a new clinical trial typically involves completion of a feasibility questionnaire, budget and contract negotiation, Institutional Review Board (IRB) preparation and submission, regulatory essential documents collection, establishment of study logistics in collaboration with ancillary services within the institution and development a patient recruitment strategy [1]. Successful navigation of this phase in any stage of the clinical trial life cycle is essential to the timely and successful start of a research. However, there are few common milestone measurements and the duration of the various start-up phase stages varies throughout sites, making it challenging to determine how well a site is performing in comparison to others. Our experience suggests that complicated study protocols and procedures, such as the need for Institutional Biosafety Committee (IBC) approval and the inclusion of interventional procedures, may lengthen the time needed to complete the study start-up process [2]. An increase in the number of ancillary services—such as those offered by different departments or external services housed within the same institution—as well as the requirement for pharmacy services, imaging core facilities, pathology laboratory facilities, etc.
Under the NIH Guidelines, an institutional committee called the IBC Institutional Biosafety was established to examine studies employing synthetic nucleic acid and recombinant DNA. The IBC also oversees and is in charge of the organization’s biosafety program (hospital, university, private practice), and it reviews all research involving biohazardous materials (i.e., biological specimens collected during the study, such as blood, urine, tissue samples, etc.). The IBC performs these duties in accordance with guidelines established by organizations, as well as federal, state, and municipal entities. Furthermore, the duration of a study’s start-up is often influenced by external factors like the usage of a local rather than centralized IRB, project manager expertise, and the utilization of Contract Research Organizations (CROs) [3].
In order to determine what factors are most crucial in determining the time from a sponsor’s or its representatives’ initial contact with a potential clinical site to the enrollment of the first patient, the current investigation set out to identify every factor that could contribute to either a delay or an acceleration of the start-up phase of a clinical study. We characterized the role that each component of a study has in relation to the time it takes to attain milestones during the initiation process using the data that was available at our site from prior research start-ups. It is challenging to adopt general metrics for study start-up performance and apply them to all investigations because each study has distinct characteristics. On the other hand, stratification according to study type and attributes can help determine more realistic timeframes for subsequent research with similar features. Most significantly, patients experience a delay in receiving therapy and lose out on opportunities due to research start-up delays [4]. All clinical trials must be conducted well, but Phase III trials are usually the biggest and most intricate before a medicine is approved. The total cost of a Phase III, randomized trial can range from $11.5 million to $52.9 million, contingent on the study’s complexity and therapeutic area. Despite the fact that delays in clinical trials are well-documented, this study provides clinical trial managers with a comprehensive start-up checklist as a helpful tool for increasing trial efficiency.
Literature Review
Six major factors were found in the literature review to be responsible for regulatory delays: inconsistent regulations, late submissions, extra requirements after regulatory approval, local ethics committee/IRB use, infrequent ethics committee/IRB meetings, and regulatory backlogs/clock-stops. Study start-up timeframes are significantly impacted by different rules and variations in start-up processes between nations. When numerous countries are involved, the complexity and level of cooperation required for regulatory submission packages increases significantly since the start-up team must closely monitor the timing and needs of each country.
Furthermore, some nations include hard-to-get documentation in their regulatory submissions, such as completed site contracts or insurance policies. This makes it significantly slower to submit the regulatory package and, as a result, delays clearance. A site contract may require a great deal of time to negotiate and sign. Some nations have extra regulatory requirements that must be started after receiving regulatory approval from the appropriate authority. Only then can the country acquire full approval and the sites open for enrollment. For instance, an import license can be necessary before trial medication can be imported, and it can take weeks for the study to begin because the import license application cannot be made until the country has given its consent [5].
Whether a site can use a central Institutional Review Board (IRB)/Ethics Committee (EC) or a local IRB/EC determines the length of the regulatory delays at the site level. Research indicates that the time it takes to get IRB permission is significantly shorter when a centralized IRB oversees numerous sites instead of a local IRB managing each site. Retrospective research revealed that central IRBs were linked to significantly faster cycle times, with protocol reviews taking an average of 7 days as opposed to 35 days for local IRBs. The frequency of IRB/EC meetings affects startup schedules as well. Schedules for meetings might differ significantly between locations and take place every week, every month, every quarter, or even just twice a year.
Finally, longer regulatory assessment periods may result from backlogs and clock-stops at the national or site level. For example, prior to changing their regulatory review methods in 2019, China had a high regulatory review backlog of almost 52,000 applications. Trial managers working with sites in China would have to prepare for longer regulatory review times during this time as well as the chance that their sites could become part of an international study after sites in other countries had started recruiting participants [6].
Site Contracts and Budgets
The process of negotiating site contracts (clinical trial agreements) and investigator grants (the study funding for an investigative site) is clearly one of the additional factors contributing to the start-up delay. It may require many months to arrange and carry out contract and financial agreements between sponsors and clinical sites. Contract executions lasted an average of 7.9 months for US locations (range 2.5–17.2 months) and 8.7 months for sites outside the US (range 2.5–24.9 months) in a global study that was carried out at 57 centers in 16 countries. Longer legal reviews, insufficient budget templates, inexperienced staff, and constrained negotiation boundaries are some of the factors that contribute to extended contract and budget cycle durations. Cycle times may be significantly shortened if a sponsor has previously worked with the site by utilizing already established contract and budget parameters. Clinical research organizations (CROs) frequently handle startup tasks for sponsor businesses, including negotiating clinical trial agreements (CTAs). Significant sponsor oversight is necessary for CRO-managed discussions, and neglecting to do so could lead to weeks of delay and harm the study site’s relationship. It is crucial to give sites a sponsor contact so that problems can be escalated during negotiations if needed. Sites may demand prepayments once contracts are finalized before they formally launch the trial and begin enrolling. Since it takes time for the payer to set up the site in their systems and generate the payments, pre-payments may also cause delays [2,6,7,9].
Insurance
Clinical trial managers might undervalue the complexity and importance of obtaining liability insurance when starting a clinical experiment. Given that obtaining proof of insurance is sometimes a requirement for the filing and approval of regulatory documents, it could significantly increase the expense of the study and cause a delay in the start-up process. Similar to other facets of international clinical trials, indemnity insurance is governed by national laws in each nation. A variety of policies are used in multinational trials to reduce risk to the sponsor in the event that a participant is hurt and receives financial recompense. Typically, the sponsor firm maintains an annual global master liability policy that is sufficient to cover a number of nations, including the United States, Canada, and New Zealand [10]. Certain nations mandate local insurance policies that are provided by locally authorized insurance providers. Unless the study goes longer than the first term covered, local policies usually span the duration of the investigation and have differing criteria. Policies may need to be updated in the event that the number of sites or expected participants to be screened or randomized in that nation varies, depending on the information required on the insurance certificate. It is crucial to get this properly because making changes to an insurance policy might take weeks and impede the nation’s startup process.
Clinical Supplies
Another area where the start of international clinical trials may be seriously hampered is clinical supplies. It might be particularly difficult to prepare and distribute clinical goods to remote areas of the globe because every nation has different regulations and linguistic needs. Furthermore, the mandatory data items on medicine labels vary by country and need to be translated into the local languages. Smith-Gick et al. listed 19 data pieces (drug name, storage conditions, and the phrase “clinical trial use”) that, depending on the nation, might need to be on the label. Packing and labeling take roughly 30 weeks from the time standard booklet labels are prepared and authorized until the kits are distributed to the sites. Including the utilization of If electronic labels, or eLabels, are used, this time can be cut down to 16 weeks. The clinical supply manager in charge of international research must keep up to date on delivery timetables and local import and export regulations. Lamberti and associates examined logistics information from seventy-three clinical trials, covering all stages and a variety of therapeutic domains. They found that while actual shipping timeframes varied widely depending on geography and supply strategy (e.g., distribution via central, local, or regional depots), the average time to transport clinical goods to clinical sites was 3-4 days.
When conducting clinical trials involving medications (such biologics) that must be refrigerated or frozen, a robust cold chain approach is necessary. Maintaining the cold chain gets considerably more challenging when multiple countries are involved and may necessitate remote locations with limited courier access. To ensure that local drug depots are adequately stocked and to lessen drug supply worries, stakeholders must be knowledgeable about import/export laws, shipping schedules, and allow for adequate product overage when predicting drug supply demands. Finally, stakeholders need to thoroughly examine the site logistics in order to understand the movement of healthcare goods from supplier to pharmacy to patient and expedite temperature excursions. In many trials, the administration of the investigational agent is combined with the acquisition and administration of comparator drugs and co-therapies as part of the research. These additional drugs are hard to locate and track down, add a lot of expense to trials, and sometimes result in longer research cycles and delays. The primary cause of the delay is obtaining the required paperwork, which consists of stability data to support decisions on temperature excursions and certificates of analysis to support regulatory submissions. Comparable products may need to be repackaged or relabeled once they are obtained, depending on county-specific regulations [14].
Side Contracts and Budgets
The growing rivalry for skilled, established clinical locations is a major issue for the selection of sites. In general, selecting sites for complex trials is more difficult for sponsors and CROs. when those with interest select areas. They carefully consider key site requirements before selecting a venue to take part in a clinical investigation. Among the assessment elements are prior experience in research and the therapeutic field being studied, access to participants who meet eligibility standards, appropriate staff, facilities, equipment, training, and a willingness to participate enthusiastically. Sponsors and CROs typically reach out to prospective sites to see if they’re interested, and then they ask them to sign a confidentiality agreement (CDA). There is a possibility of delay as the parties discuss the terms of the contract. Once a CDA is in place, a thorough feasibility questionnaire is given to the site for completion. Feasibility questionnaires are frequently created hastily since sites must be chosen promptly in order to prepare and submit regulatory submissions and record as many crucial enrollment months as feasible. Owing to hurried start-up schedules, venues frequently have limited time to complete feasibility studies. Consequently, questionnaires may provide erroneous or partial information and potentially excessively optimistic enrollment projections [15]. Sponsors often use the enrollment projection of the site and discount the patient numbers they supply, although the real performance of the site is rarely reflected in the findings. Important documents, such as the complete procedure and budget, are frequently unavailable to sites as it becomes feasible. Following completion and return of the feasibility questionnaire by the sites, an evaluation of the data takes place, and a subset of the interested and qualified sites is chosen to proceed to a pre-study visit. It is important to evaluate the site infrastructure to make sure it can meet the study’s technological requirements and has internet connectivity. It might be necessary for sponsors to remove technological obstacles by offering Internet access or delivering equipment that complies with local laws. Cycle times can be shortened by choosing “repeat” sites—those that have collaborated with a sponsor or CRO on a prior trial. Compared to freshly picked sites, cycle times for repeat sites were 28% shorter. Nevertheless, many locations decide not to take part in a follow-up clinical research after having already participated in one. Workload balance, time and money constraints, intricate contracts and rules, a lack of infrastructure, poor training, and difficulties gathering data are some of the major obstacles faced by investigators [18].
The sponsors/CROs have a list of documents that must be in place before opening a site to enrollment. These documents include an FDA 1572 form or equivalent statement of investigator, a signed contract, a budget, an IRB/EC approval, medical licenses, CVs, and financial disclosure forms from the principal investigator and all sub investigators. The documentation needed prior to the initiation of a clinical investigation is described in Sect. 8.2 of ICH E6 (R2). Completing site start-up duties accurately and promptly is essential to prevent delays and further regulatory review cycles. According to ICH E6 (R2), Sect. 5.14.2, the sponsor is not allowed to provide a study drug to a clinical site until all necessary paperwork, such as an approval from the IRB/EC and regulatory bodies, is in order. A small mistake on a vital document, like an import license, insurance policy, or informed consent form, might have a significant impact because the website might not be able to accept participants until the mistake is fixed. cycle times are not regularly gathered by studies, sponsors, or CROs, and it is recommended that the industry track significant site start-up cycle intervals in order to evaluate the effectiveness of multisite trials. They consist of the following dates: (1) the clinical site received the final protocol; (2) the IRB made a decision; (3) the site received the contract (first draft or template); (4) the site signed the contract; (5) the site was activated (open to enrollment); and (6) the date the first patient provided consent. Clinical trial managers can determine opportunities for improvement and evaluate the efficacy of improvement activities by utilizing standard metrics [16].
Table 1.
Multivariate analysis of time to budget and contract approval, Institutional Review Board approval, and first patient enrollment as a function of Institutional Review Board type, project manager experience, and use of a Contract Research Organization.
Table 1.
Multivariate analysis of time to budget and contract approval, Institutional Review Board approval, and first patient enrollment as a function of Institutional Review Board type, project manager experience, and use of a Contract Research Organization.
| |
Mean ratio |
95% confidence interval |
p-value |
| Budget/contract finalization |
|
|
|
| Local IRB |
1.02 |
0.86-1.18 |
0.90 |
| CRO |
0.85 |
0.71-0.99 |
0.33 |
| <48months project manager experience |
1.05 |
0.88-1.22 |
0.78 |
| IRB approval |
|
|
|
| Local IRB |
2.06 |
1.56-2.56 |
0.01 |
| CRO |
0.88 |
0.67-1.09 |
0.62 |
| <48months project manager experience |
0.89 |
0.67-1.11 |
0.65 |
| First Subject enrollment |
|
|
|
| Local IRB |
1.22 |
0.88-1.56 |
0.50 |
| CRO |
1.54 |
1.11-1.97 |
0.15 |
| <48months project manager experience |
1.45 |
1.04-1.86 |
0.24 |
Methodology
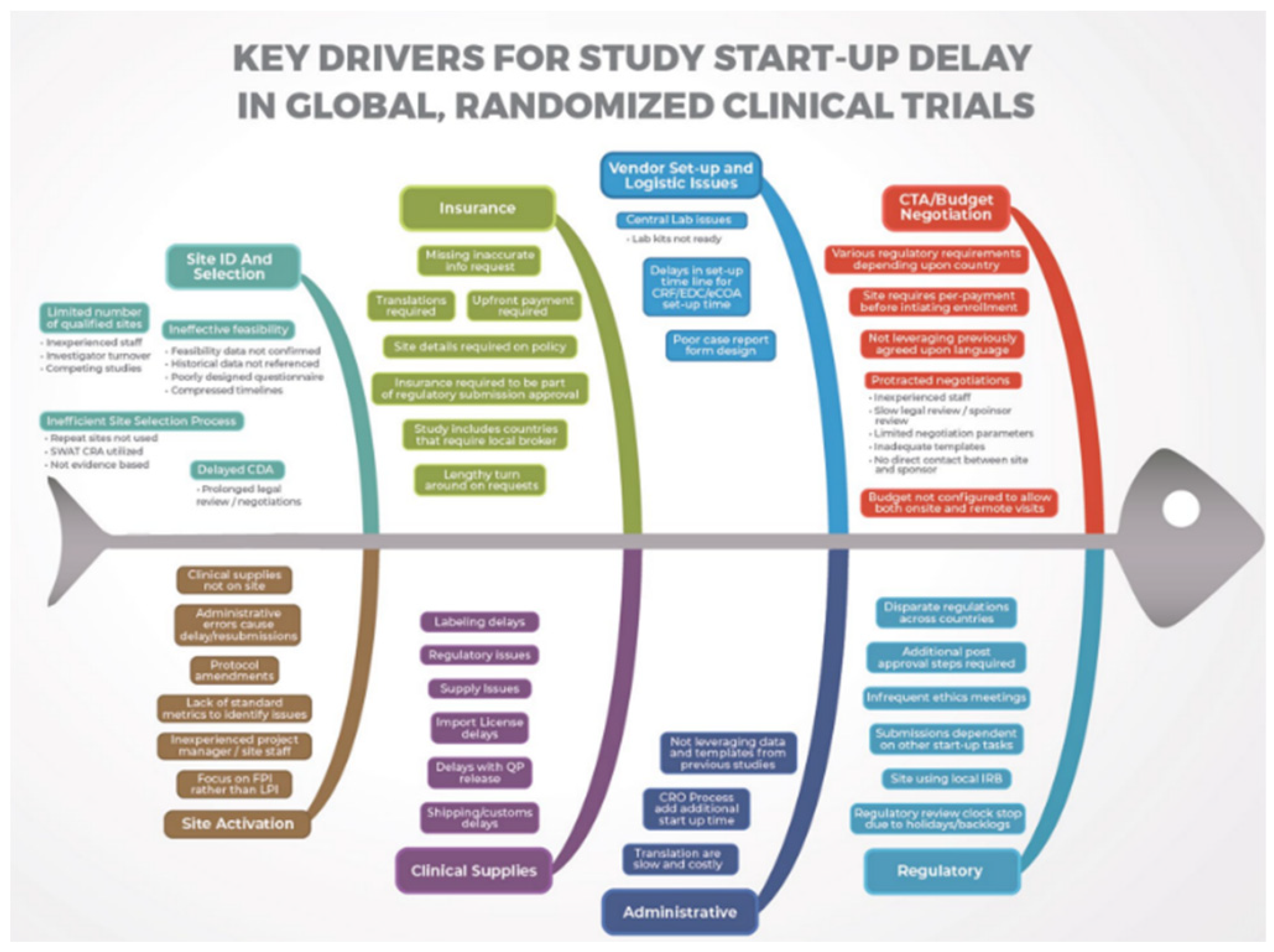
A comprehensive search of good clinical practice guidelines was carried out using search tactics modeled after the recommendations of the ADAPTE Collaboration (2007), guided by the research objectives. Three primary areas were the focus of the search: restricted internet search, clinical practice guidelines database, and bibliographic database. Additionally, a thorough search of the references listed in pertinent guidelines documents was done. Three parts make up this qualitative integrative analysis: (1) an overview of the body of research on the subject of clinical trial start-up; (2) a fishbone diagram designed to enumerate the primary causes of start-up delays in Phase III international clinical trials; and (3) a checklist for study start-up that clinical trial managers can utilize to prepare their trials. Regulatory permissions, site contracts and budgets, insurance, clinical supplies, site identification and selection, site activation, and inefficient processes/pitfalls were all deemed to be within the purview of this evaluation.
Results
The terms “study start-up and delays” and “clinical trial start-up and delays” were used to review the literature. A book, several industry white papers, and 19 peer-reviewed academic articles were included in the review. The research start-up delay was attributed to a number of well-documented factors, the main drivers of which were outlined in a fishbone diagram (
Figure 1). The main causes of start-up delays in randomized controlled trials have been shown to be regulatory permissions, site contracts and budgets, insurance, clinical supplies, activation of the site, inefficient processes, CROs, and translations. A brief discussion of the key findings in each of these areas will follow.
Table I.
Reasons for delays in clinical trials due to country-level approvals.
Table I.
Reasons for delays in clinical trials due to country-level approvals.
| Country |
Average Approval Time (in months) |
Reasons for Delays |
| United States |
6-8 |
Stringent FDA regulations and compliance requirements |
| |
|
Lengthy protocol reviews by regulatory bodies |
| |
|
Delays in Institutional Review Board (IRB) or Ethics Committee (IEC) approvals |
| United Kingdom |
2-6 |
National Health Service (NHS) and Health Research Authority (HRA) approvals |
| |
|
MHRA (Medicines and Healthcare products Regulatory Agency) regulations and reviews |
| European Union |
6-12 |
Varied regulations across member states leading to harmonization challenges |
| |
|
European Medicines Agency (EMA) approvals and dossier evaluations |
| Asia |
8-18 |
Diverse regulatory landscapes across countries |
| |
|
Local Institutional Review Board (IRB) or Ethics Committee (EC) approvals in each country |
| |
|
Language and documentation barriers |
Discussion:
The results of our analysis indicate that better efficiency is needed when initiating large-scale, multicenter randomized clinical studies. Due to the complexity of these projects, any delay in their implementation has a substantial financial impact and increases the amount of time it takes for therapies that could save lives to reach the market. In order to avoid delays caused by all of the identified drivers, the research start-up team should include local specialists who have a solid awareness of the legislation and requirements in each participating country. These professionals can assist in organizing a productive submission procedure and provide precise start-up schedule estimates. Precise step-by-step coordination can optimize startup when utilizing countries with larger startup periods.
By enabling decentralized clinical trials (DCTs), or trials where at least some of the activities are conducted at the patient’s house, telemedicine and other technologies can help improve the caliber and effectiveness of clinical studies. Faster trial participant recruitment, better trial participant retention, enhanced trial participant comfort and convenience, and easier trial access are among the benefits of DCTs. Starting up decentralized clinical trials, however, may need taking into account extra factors, such as creating new procedures and training manuals, acquiring equipment, and adhering to regulatory and legal criteria [17].
Direct patient delivery of therapeutic supplies, electronic informed consent (eConsent), home health visits, telemedicine visits, remote site monitoring, and digital data collecting technologies are DCT components that call for extra care. research teams should proactively map data flow, data collection, data storage, and research methods in order to negotiate some of these obstacles. They should also create thorough training programs for stakeholders. The most unexpected possible start-up delay was related to clinical trial insurance. This is not a topic that is highlighted much, but there is a lot of opportunity for delay because of different national regulations and the obligation to send information from the clinical operations team/CRO to an insurance agent, who then relays it to a local broker. The requirement for translations and original documents bearing signatures in some areas further complicates this. Even a minor mistake on an insurance policy might cause a significant delay in a regulatory application or the inability to activate a site after all other requirements have been met.
Clinical trial start-up is affected by the Benjamin Franklin adage, “an ounce of prevention is worth a pound of cure,” since it is far better to avoid start-up delays whenever possible than to deal with and address them as they arise. Even seemingly insignificant delays might accumulate over time and result in significant delays across multiple workflows. Checklist is meant to assist clinical trial managers in keeping track of study start-up operations and managing them as effectively as possible, even when industry practice changes to include technology and apply evidence-based enhancements.
Conclusion and recommendations
Based on the analysis of 19 studies and the input received from
the research retreat, a list of recommendations was created. The recommendations below will help to reduce clinical trial startup times.
Recommendation 1. A centralized email address needs to be created so that Sponsors and CROs have one area to send their regulatory documents. This recommendation will help alleviate emails getting buried or startups being delayed due to vacations or illnesses. Also, this gives the startup team an exact date of when documents are received on site without having to rely on shared drives or memory to retrieve these possible inaccurate dates.
Recommendation 2. The feasibility tool, which focuses on scientific merit, population, and budget, should continue to be utilized before a study is approved at the institution. For this recommendation, new changes that should be maintained include pulling three years of population history for a study. This would accurately depict if the institution has adequate patient population for the study.
Recommendation 3. A Feasibility Committee was formed during this project and should continue. It was created to properly vet potential clinical studies. This new committee was formed and implemented to take the feasibility review out of the weekly research meetings and put it into a more controlled and unbiased environment where the potential studies are closely analyzed.
Recommendation 4. The Coverage Analysis should be centralized and only done by the Administrative Coverage Analysis team. The Administrative Coverage Analysis team are experts in their field and have a wealth of experience with Center for Medicare and Medicaid Services coding and billing. Coverage Analysis’ are only done once per study and having the administrative team conduct these, will cut down on study startup times.
Recommendation 5. Allow the local institution to conduct their own budget negotiations. Currently, a contracting team in administration conducts these negotiations but rely heavily on local institution input. The local institution has a copy of the Sponsor’s budget and they know what is needed to start a study, financially, as well as the manpower needed to conduct the study in its entirety. Having the local institution control their own budget negotiations cuts down on the current back and forth with the administrative team acting as the liaison between the local institution and the CRO or Sponsor.
Recommendation 6. Standardize the study startup costs based on the complexity of the clinical trial. The internal budget is composed of study startup costs, labor, and laboratory tests and procedures. Study startup costs include the time to prepare IRB paperwork, obtain signatures, and train staff on the study. The study startup costs have traditionally caused the most disagreements in the budget negotiations. Every study, regardless of whether a master serviceagreement exists or not, have differing study startup costs. Standardizing them alleviates these disagreements. For smaller, less complex or risky clinical trials startups should be $10,000. Mid-size trials $15,000 and large and complex trials $20,000.
Recommendation 7. The institution needs to purchase an electronic system or software that can accurately track startup times. Currently, Smartsheets tracks cycle times but requires a staff member to manually input dates which can lead to errors. The research community has numerous platforms available that can help facilitate study startup and accurately track when cycle times begin by electronically timestamping them when they are received.
Study startup delays cost money and time for both the Sponsor of a clinical trial as well as the local institutions. Most importantly, patients miss the opportunity to participate in trials that could benefit their health and well-being. During the analysis, delays were discovered in different time metric cycles. Those delays were analyzed for ways in which the institution could work to reduce them. Recommendations were made based on the findings and actions were taken. Continued progress will help the local institution establish a more efficient study startup process.
References
- Schimanski, C. Streamline and improve study start up. Appl. Clin. Trials 2013, 22, 22–25. [Google Scholar]
- Treweek, S.; Bevan, S.; Bower, P.; Campbell, M.; Christie, J.; Clarke, M.; Collett, C.; Cotton, S.; Devane, D.; El Feky, A.; et al. Trial Forge Guidance 1: what is a Study Within A Trial (SWAT)? Trials 2018, 19, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Transcelerate BioPharma Inc. Beyond COVID-19: Modernizing Clinical Trial Conduct. Published July 2020.
- Corneli, A.; Pierre, C.; Hinkley, T.; Lin, L.; Fordyce, C.B.; Hamre, G.; Roe, M.T. One and done: Reasons principal investigators conduct only one FDA-regulated drug trial. Contemp. Clin. Trials Commun. 2017, 6, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Briel, M.; Speich, B.; von Elm, E.; Gloy, V. Comparison of randomized controlled trials discontinued or revised for poor recruitment and completed trials with the same research question: a matched qualitative study. Trials 2019, 20, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crow, R.A.; Hart, K.A.; McDermott, M.P.; Tawil, R.; Martens, W.B.; Herr, B.E.; McColl, E.; Wilkinson, J.; Kirschner, J.; King, W.M.; et al. A checklist for clinical trials in rare disease: obstacles and anticipatory actions—lessons learned from the FOR-DMD trial. Trials 2018, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.D.; Bull, J.; McKee, K.J.; Mahon, E.; Harper, B.; Roberts, J.N. Clinical trials recruitment planning: A proposed framework from the Clinical Trials Transformation Initiative. Contemp. Clin. Trials 2018, 66, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Chingarande, G.R.; Moodley, K. Disparate compensation policies for research related injury in an era of multinational trials: a case study of Brazil, Russia, India, China and South Africa. BMC Med Ethic- 2018, 19, 1–12. [Google Scholar] [CrossRef]
- Tang, M.; Joensuu, H.; Simes, R.J.; Price, T.J.; Yip, S.; Hague, W.; Sjoquist, K.M.; Zalcberg, J. Challenges of international oncology trial collaboration—a call to action. Br. J. Cancer 2019, 121, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Smith-Gick, J, Barnes, N, Barone, R, Bedford, J, James, J, Reisner, S, et al. The near-term viability and benefts of elabels for patients, clinical sites, and sponsors. Ther Innov Regul Sci. 2018, 52, 537–545. [CrossRef]
- Pike, E.R. In need of remedy: US policy for compensating injured research participants. J. Med. Ethic 2013, 40, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Forney, L.; Brinton, D.L.; Simpson, K.N. Drivers of Start-Up Delays in Global Randomized Clinical Trials. Ther. Innov. Regul. Sci. 2020, 55, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Abu-Shaheen, A.; Al Badr, A.; Al Fayyad, I.; Al Qutub, A.; Faqeih, E.A.; Al-Tannir, M. Streamlining and cycle time reduction of the startup phase of clinical trials. Trials 2020, 21, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Atassi, N.; Yerramilli-Rao, P.; Szymonifka, J.; Yu, H.; Kearney, M.; Grasso, D.; Deng, J.; Levine-Weinberg, M.; Shapiro, J.; Lee, A.; et al. Analysis of start-up, retention, and adherence in ALS clinical trials. Neurology 2013, 81, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Krafcik, B.M.; Doros, G.; A Malikova, M. A single center analysis of factors influencing study start-up timeline in clinical trials. Futur. Sci. OA 2017, 3, FSO223. [Google Scholar] [CrossRef] [PubMed]
- Bieske, L.; Zinner, M.; Dahlhausen, F.; Truebel, H. Critical path activities in clinical trial setup and conduct: How to avoid bottlenecks and accelerate clinical trials. Drug Discov. Today 2023, 28, 103733. [Google Scholar] [CrossRef] [PubMed]
- Biberstein, A. Study Start-Up Times: One Site’s Analysis of the Process, the Problem, and the Plan to Shorten Times. 2018.
- Cernik, C.; Shergina, E.; Thompson, J.; Blackwell, K.; Stephens, K.; Kimminau, K.S.; Wick, J.; Mayo, M.S.; Gajewski, B.; He, J.; et al. Non-cancer clinical trials start-up metrics at an academic medical center: Implications for advancing research. Contemp. Clin. Trials Commun. 2021, 22, 100774. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Wilkinson, M.; Harper, B.; Morgan, C.; Getz, K. Assessing Study Start-up Practices, Performance, and Perceptions Among Sponsors and Contract Research Organizations. Ther. Innov. Regul. Sci. 2018, 52, 572–578. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).