Introduction
Cancer mortality is the leading cause of death before age of 70 years in 57 countries including the US, Canada, most European countries, China, and Australia.1 It is estimated that 10 million people died of cancer worldwide in 2020.1 Therefore, there is an urgent medical need to identify modifiable risk factors to develop preventative strategies to reduce cancer mortality in the general population.
Diabetes is frequently found to be associated with high risks of cancer incidence2-4 and cancer mortality.3,5,6 Postprandial plasma glucose (PPG) has long been recognized to play an important role in diabetes-associated complications.7-11 However, it is unknown whether PPG is associated with cancer mortality in the general population.
Only one study12 has investigated the association between PPG and cancer mortality. That study investigated PPG at 2 h after breakfast in 1,582 Japanese patients with type 2 diabetes and found that PPG was positively associated with cancer mortality after a median follow-up of 19.4 years.12 That study has some limitations. First, measuring glucose at 2 h after a meal may not be ideal, as variation in diet could change PPG by more than 20 mg/dL,13 and variation in blood collection time (2 ± 0.5 h in practice12) could introduce bias as PPG could be time-sensitive around 2 h.13 Second, the use of antidiabetic medications could produce bias, as these medications affect PPG.
It has been shown
13 that plasma glucose returned to baseline four hours after a meal regardless of mealtime (breakfast, lunch and dinner) and meal type (normal or high carbohydrate,
eFigure 1 in the Supplement). In addition, a population-based study showed that PPG reached a relatively stable state only from 4 h after a meal in 34,907 US adults.
14 Therefore, the current study categorized PPG as 0-3.9 h (PPG
0-3.9h) and 4-7.9 h (PPG
4-7.9h).
This study aimed to investigate whether PPG (PPG0-3.9h and PPG4-7.9h) was associated with cancer mortality using a representative cohort of US adults who attended the third National Health and Nutrition Examination Survey (NHANES III) from 1988 to 1994. As antidiabetic medications affect PPG levels, this study excluded participants with prescribed diabetes medications. Therefore, participants of this study represented US general adult population excluding those with prescribed diabetes medications. Among the study participants, 626 had a hemoglobin A1c (HbA1c) of ≥6.5% and 413 had a prior diagnosis of cancer.
Methods
Study Participants
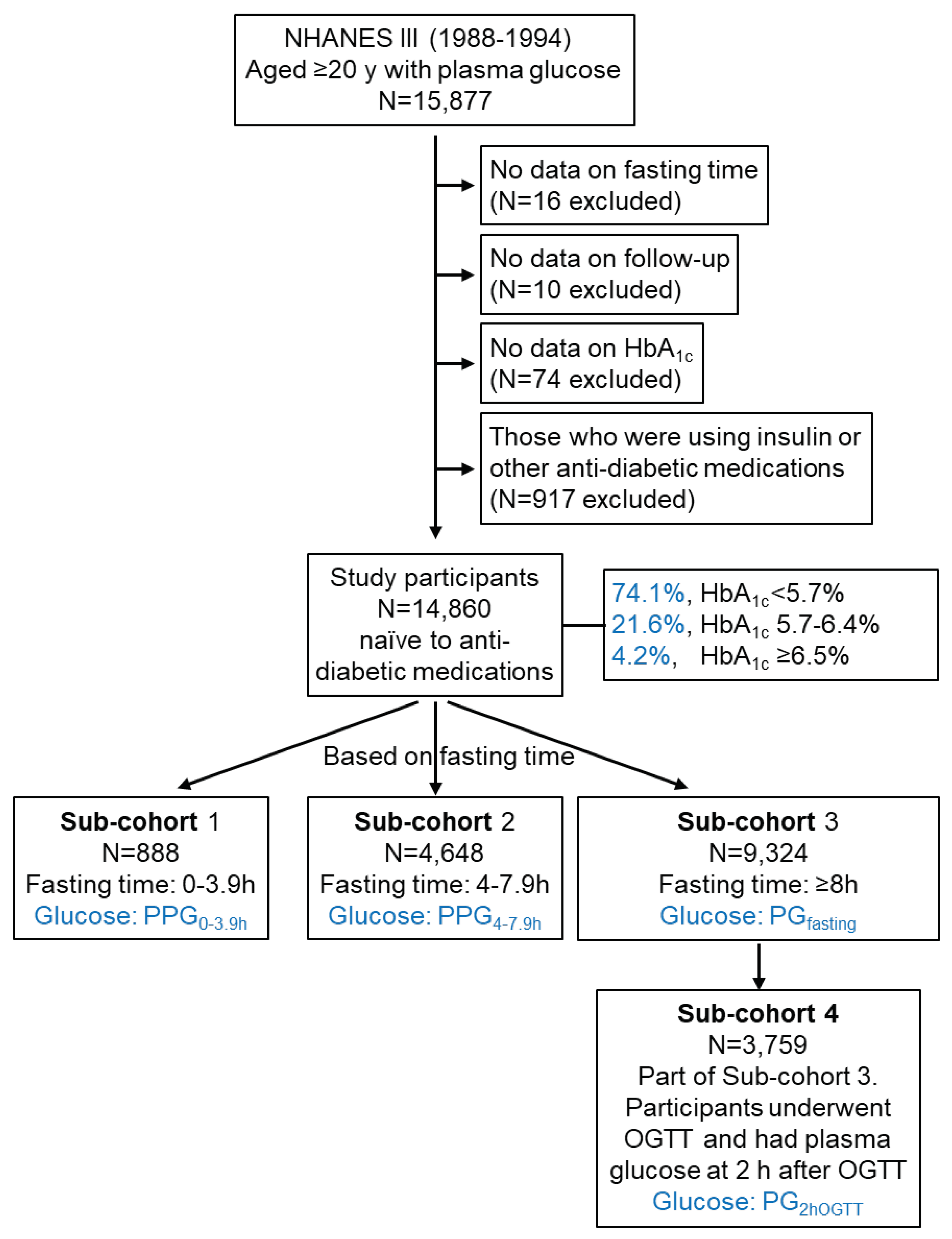
A total of 15,877 adults aged ≥ 20 years who attended the NHANES III had recorded plasma glucose data. Those who did not have a fasting time (N=16), follow-up time (N=10), or
HbA1c (N=74) were excluded. In addition, those who were prescribed insulin or other diabetes medications (N=917) were excluded, as these drugs affect plasma glucose levels. Therefore, the remaining 14,860 participants were included in this cohort study, among which 4.2% had an HbA
1c of ≥ 6.5% (
Figure 1). They comprised 888 participants whose plasma glucose was measured from blood taken with a fasting time of 0-3.9 h (Sub-cohort 1), 4,648 with a fasting time of 4-7.9 h (Sub-cohort 2), and 9,324 with a fasting time of ≥8 h (Sub-cohort 3). The plasma glucose in these sub-cohorts was termed PPG
0-3.9h, PPG
4-7.9h, and PG
fasting, respectively (
Figure 1). Among Sub-cohort 3 (fasting), 3,759 participants had plasma glucose at 2 h after an oral glucose tolerance test (OGTT) with 75 g glucose, which plasma glucose was termed PG
2hOGTT in this study. NHANES III was approved by the National Center for Health Statistics Institutional Review Board.
15 All participants provided written informed consent.
Exposures
Exposure of the study was plasma glucose, including PPG0-3.9h, PPG4-7.9h, PGfasting, and PG2hOGTT. Plasma glucose was measured using the hexokinase-mediated reaction method with a high precision (interassay coefficient of variation, <2.5%).16
Outcomes
Data on mortality from all causes and cancer (C00-C97) were directly retrieved from NHANES-linked mortality files17 with underlying cause of death being coded according to the International Classification of Diseases, 9th Revision (ICD-9) or the International Classification of Diseases, 10th Revision (ICD–10).18,19 Follow-up time was the duration from the time when the participant was examined at the Mobile Examination Center until death, or until the end of follow-up (December 31, 2019), whichever occurred first.20,21
Confounders
Confounding covariates included age (continuous), sex (male or female), ethnicity (non-Hispanic white, non-Hispanic black, Mexican-American, or other), body mass index (continuous), education (<high school, high school, >high school, or unknown), poverty-income ratio (<130%, 130%-349%, ≥350%, or unknown),22 and survey periods (1988-1991 or 1991-1994). Lifestyle confounders included physical activity (inactive, insufficiently active, or active),17 alcohol consumption (never, <1 drink per week, 1-6 drinks per week, ≥7 drinks per week, or unknown),23 and smoking status (past smoker, current smoker, or other). Clinical confounders included systolic blood pressure (continuous), total cholesterol (continuous), high-density lipoprotein (HDL) cholesterol (continuous), HbA1c (continuous), and family history of diabetes (yes, no, or unknown). In addition, fasting time (continuous) was adjusted in the analyses.
Statistical Analyses
Baseline characteristics were presented as median (interquartile range) for not normally distributed continuous variables, mean (standard deviation) for normally distributed continuous variables, or number (percentage) for categorical variables. Differences in continuous variables between two groups were analyzed using Mann Whitney U test (not normally distributed) or Student’s t-test (normally distributed). Differences among categorical variables were analyzed using Pearson’s chi-square test.18
Out of 14,860 participants, a total of 335 (2.3%) had missing data including body mass index (N=38), systolic blood pressure (N=28), total cholesterol (190), or HDL cholesterol (N=271). The missing data were imputed via multiple imputation by chained equations, with 20 imputed data sets being created.19,24 Little’s test showed that the missing data were not missing completely at random (P<0.001). In all the regression analyses, body mass index, systolic blood pressure, total cholesterol, HDL cholesterol, and HbA1c were natural log transformed to improve data distribution.
Cox proportional hazards models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of plasma glucose (continuous) or HbA1c (continuous) for mortality.25 In further analyses, plasma glucose was treated as a dichotomous categorical variable using the top decile as the cutoff, as 8.5% of adults may have diabetes according to the World Health Organization,26 or using clinical cutoffs [200 mg/dL for high PG2hOGTT,27,28 and 126 (criterion after 1997)27,28 or 140 mg/dL (criterion before 1997)28 for high PGfasting]. Further analyses were conducted with stratification of a prior diagnosis of cancer.
The association of PPG4-7.9h with HbA1c was analyzed by linear regression and Pearman’s rho analysis.29 Receiver operating characteristic (ROC) curves30 were constructed and the area under the curve (AUC) was calculated to assess the discriminatory power of PPG4-7.9h for high (≥6.5%) or normal HbA1c (<5.7%).27 The optimal cutoff of PPG4-7.9h was determined by the Youden Index.31 The Kaplan-Meier analyses were used to generate and compare PPG4-7.9h (normal, borderline high, or high) and mortality curves with a log-rank test.
The difference between hourly PPG4-7.9h was analyzed using Kruskal Wallis one-way ANOVA. The difference among 3 lines (hourly PPG4-7.9h over time in those with normal, borderline high, or high PPG4-7.9h) was analyzed using multiple linear regression with PPG4-7.9h as the outcome variable, and time and PPG4-7.9h categories as the predictor variables.
Sensitivity analyses were conducted when the imputed data were not used, i.e., excluding those 335 (2.3%) participants with missing data from the analysis or when those with a follow-up time of <1 year (N=138) were excluded.
The null hypothesis was rejected for two-sided P values of <0.05. All analyses were performed using SPSS version 27.0 (IBM SPSS Statistics for Windows, Armonk, NY, IBM Corporation).
Results
General Characteristics
This cohort included 14,860 adult participants with a mean (standard deviation) age of 47 (19) years, among which 74.1% had an HbA
1c of <5.7% (normal), 21.6% had an HbA
1c of 5.7%- 6.4% (pre-diabetic), and 4.2% had an HbA
1c of ≥ 6.5% (diabetic;
eTable 1 in the Supplement and
Figure 1). Compared with those with lower plasma glucose, those with higher plasma glucose were older and had higher HbA
1c, body mass index, systolic blood pressure, and total cholesterol, as well as less education (
eTable 2-5 in the Supplement). Other characteristics of the cohort or sub-cohorts were described in
eTables 1-5 in the Supplement.
Association of Plasma Glucose with Mortality
This cohort was followed up for 332,313 person-years with a mean follow-up of 22.4 years. During the follow-up, 5,996 all-cause deaths were recorded, which included 1,388 cancer deaths (
eTable 6 in the Supplement).
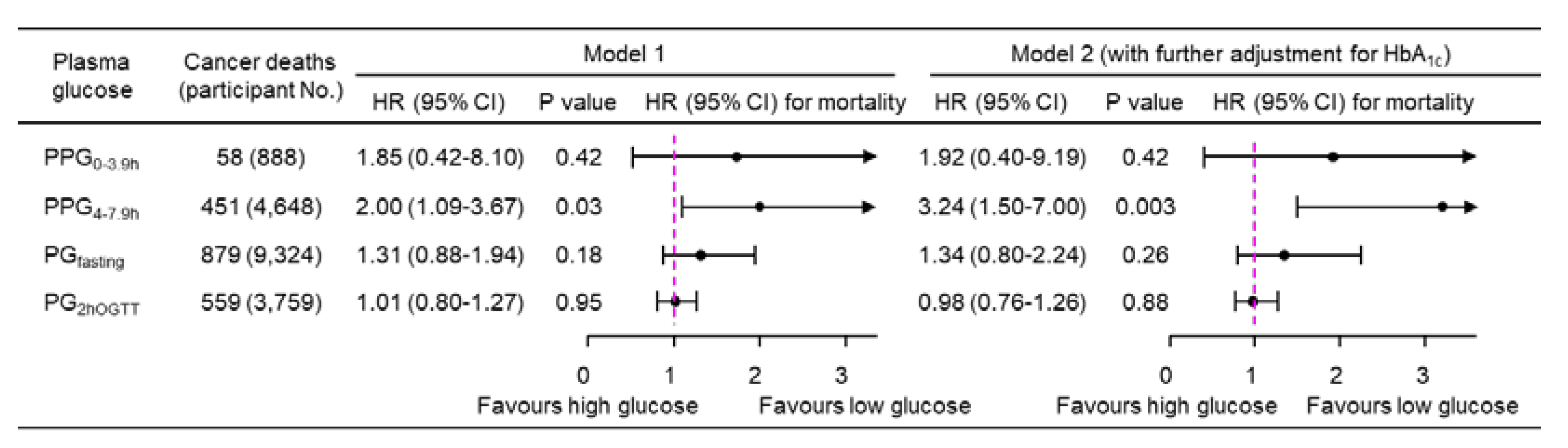
A 1-natural-log-unit increase in PPG
4-7.9h was associated with a higher multivariate-adjusted risk of cancer mortality (HR, 3.24; 95% CI, 1.50-7.00; P=0.003;
Figure 2), whereas PPG
0-3.9h, PG
fasting, and PG
2hOGTT were not significantly associated with cancer mortality (
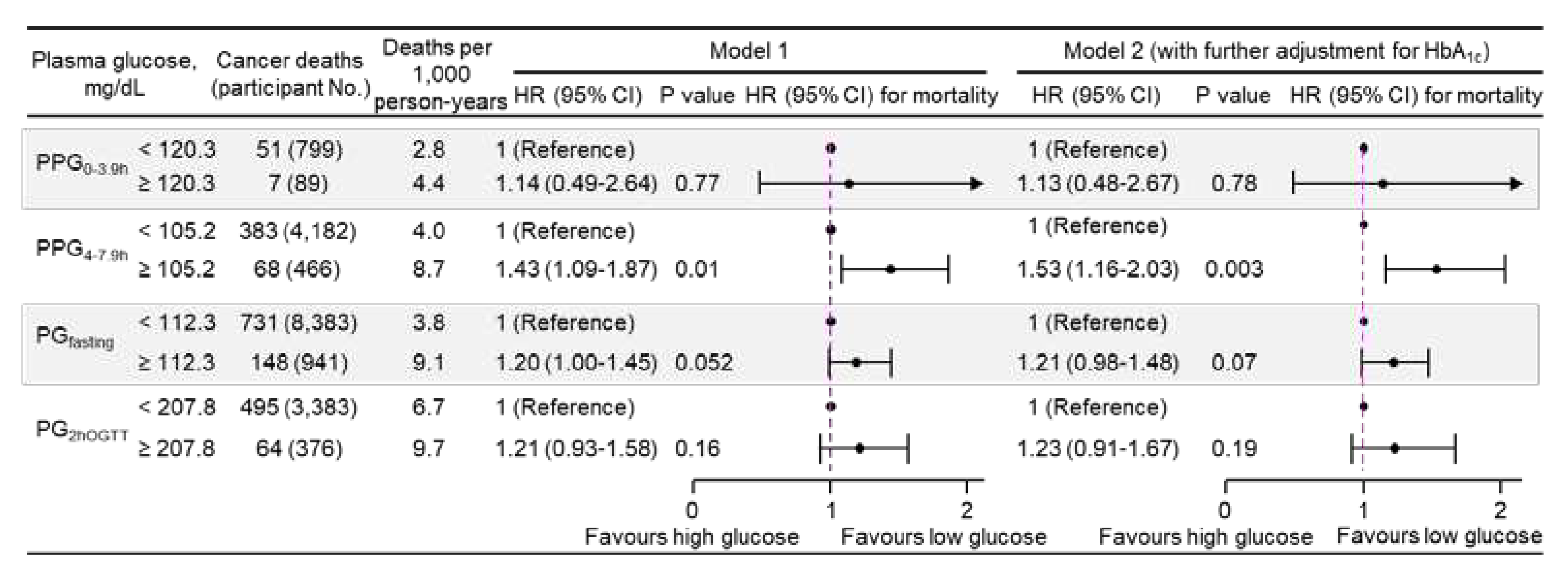
Figure 2). Similar results were obtained when plasma glucose was treated as a dichotomous variable using the top decile as the cutoff (
Figure 3), or when using clinical cutoffs for PG
fasting and PG
2hOGTT (
eFigure 2 in the Supplement). In addition, PPG
4-7.9h was positively associated with all-cause mortality (
eFigure 3 in the Supplement).
Sensitivity analyses showed that PPG
4-7.9h remained positively associated with cancer mortality and all-cause mortality when imputed data were not used,
i.e., by excluding those 335 participants with missing data (
eFigure 4 in the Supplement), or when those with a follow-up time of <1 year were excluded (
eFigure 5 in the Supplement). HbA
1c was not associated with cancer mortality in the whole cohort or any of the sub-cohorts (
eTable 7 in the Supplement).
Determination and Validation of Cutoffs of PPG4-7.9h
PPG
4-7.9h was positively associated with HbA
1c (
eFigure 6 in the Supplement). ROC curve analysis showed that the optimal cutoff of PPG
4-7.9h was 94 mg/dL for normal HbA
1c (<5.7%) and 101 mg/dL for high HbA
1c (≥6.5%) (Figures 4A-B). These results suggested the following classification of PPG
4-7.9h: <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high).
Next, we investigated whether this PPG
4-7.9h classification would differentiate cancer mortality. Kaplan-Meier curve analysis supported such a classification because cumulative survival was lower in participants with PPG
4-7.9h in the higher categories (P<0.001,
Figure 4C). Cox regression analysis also validated such a classification: those with high PPG
4-7.9h had a 41% higher multivariate-adjusted risk of cancer mortality compared with those with normal PPG
4-7.9h (HR, 1.41; 95% CI, 1.10-1.82; P=0.008;
Figure 4D). In addition, all-cause mortality validated this classification: those with high PPG
4-7.9h had a 16% higher multivariate-adjusted risk of all-cause mortality compared with those with normal PPG
4-7.9h (HR, 1.16; 95% CI, 1.03-1.30; P=0.02;
eFigure 7 in the Supplement)
Association of PPG4-7.9h with Cancer Mortality in Those with or without a Prior Diagnosis of Cancer
A prior diagnosis of cancer may affect the association between PPG
4-7.9h and cancer mortality risk. Therefore, further analyses were conducted with stratification of a prior diagnosis of cancer. The results showed that, in participants without a prior diagnosis, those with high PPG
4-7.9h had a 45% higher multivariate-adjusted risk of cancer mortality compared with those with normal PPG
4-7.9h (HR, 1.45; 95% CI, 1.09-1.92; P=0.01;
eFigure 8 in the Supplement). However, such an association was not significant in those 413 participants with such a diagnosis (high vs normal PPG
4-7.9h: HR, 1.64; 95% CI, 0.88-3.07; P=0.12;
eFigure 8 in the Supplement).
Variation of PPG4-7.9h over the Duration from 4 to 7.9 h
Hourly PPG
4-7.9h over the duration from 4 to 7.9 h was comparable in the whole sub-cohort of participants who had PPG
4-7.9h data (
eFigure 9A in the Supplement). Similarly, hourly PPG
4-7.9h was comparable in each PPG
4-7.9h category (normal, borderline high, or high;
eFigure 9B in the Supplement).
Discussion
Using a general cohort of US adults, this study demonstrates that PPG4-7.9h is associated with high cancer mortality. PPG4-7.9h could be classified as <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high). Participants with high PPG4-7.9h had a 41% higher multivariate-adjusted risk of cancer mortality compared with those with normal PPG4-7.9h. In addition, the high-PPG4-7.9h-associated increase in cancer mortality remained in those without a prior diagnosis of cancer.
Different from PPG4-7.9h, other glucose parameters including PPG0-3.9h, PGfasing, and PG2hOGTT were not significantly associated with cancer mortality. The reason for this is unknown. One possible explanation could be due to difference in repeatability. It has been shown that plasma glucose returns to baseline from 4 h after a meal regardless of mealtime and meal type in 22 healthy participants.13 In addition, a population-based study showed that PPG reached a relatively stable state from 4 h after a meal in 34,907 US adults.14 The current study confirmed that hourly PPG4-7.9h was similar in adults from the general population who were taking meals of free choice. All these results suggest that plasma glucose returns to its baseline 4 h after a meal of free choice in the general population and PPG4-7.9h is reproducible. In contrast, PG0-3.9h could be time- and meal type-sensitive.13,14 Similarly, PGfasing is affected by “dawn phenomenon" (increases in the early morning).32 It has been shown that PGfasing and PG2hOGTT have poor reproducibility.33-35 For example, only 61% of adults were classified in the same category (normal, prediabetes, diabetes) using two PG2hOGTT readings (6 weeks apart), and this figure was 75% when PGfasting was used.33
In the current study, 4.2% of participants had high HbA1c (≥6.5%, diabetic). PPG4-7.9h could detect those with high HbA1c with an accuracy of 88%. An accuracy of 80% to 90% is considered excellent, and > 90% is outstanding.30,36 Therefore, PPG4-7.9h had an excellent discriminatory power for high HbA1c. Based on HbA1c cutoffs [<5.7% (normal), 5.7%-6.4% (borderline high), and ≥6.5% (high)], ROC curve analysis suggested that PPG4-7.9h could be classified as <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high).
This classification was validated by the high-PPG4-7.9h-associated increase in cancer mortality and all-cause mortality. In addition, the cutoff of 101 mg/dL for high PPG4-7.9h seems to be supported by literature reports. Peter et al37 reported that PPG at 4 h after breakfast, lunch, and dinner was 102 mg/dL in 18 men with type 2 diabetes with HbA1c<7.3%. In addition, PPG at 5 h after lunch was 104 mg/dL in 20 patients with type 2 diabetes with good glycemic control (HbAlc <7.0%).38 Therefore, the PPG4-7.9h cutoff of 101 mg/dL may represent the PPG4-7.9h level of those with mild type 2 diabetes.
PPG
4-7.9h was 27 mg/dL lower than PG
fasting in the above two studies
37,38 (
eTable 8 in the Supplement). This phenomenon that PG
fasting is higher than PPG
4-7.9h in those with type 2 diabetes is likely due to the "dawn phenomenon" (blood glucose increases in the early morning),
32 resulting from a transient increase in both glycogenolysis and gluconeogenesis.
32 Therefore, 101 mg/dL in PPG
4-7.9h may equate to 128 mg/dL in fasting plasma glucose in those with mild type 2 diabetes, which is just above the diabetes cutoff,
i.e., 126 mg/dL in fasting plasma glucose.
27
The high-PPG4-7.9h-associated increase in cancer mortality is consistent with the reports which found that diabetes is associated with high risks of cancer incidence2-4 and cancer mortality.3,5,6,39 The mechanism underlying this observation is not clear. One explanation is that high PPG4-7.9h may promote cancer formation and progression. One of the cancer characteristics is an increased glucose uptake and metabolism to support cancer growth (the Warburg effect),40 and therefore, an increase in PPG may confer a growth advantage, leading to cancer formation. This explanation was supported by the observation that the positive association between PPG4-7.9h and cancer mortality remained in those without a prior diagnosis of cancer. It is worthwhile to investigate whether lowering PPG4-7.9h prevents cancer incidence and cancer mortality in the general population in the future.
The current study revealed that PPG4-7.9h was not significantly associated with cancer mortality in patients with existing cancer (HR, 1.64; 95%CI, 0.88-3.07; P=0.12). The lack of statistical significance, however, could be due to the small sample size of this sub-cohort (N=413). Therefore, the association between PPG4-7.9h and cancer mortality in those with existing cancer needs to be investigated with a larger sample size in future studies.
Our study found that HbA1c was not associated with cancer mortality. This is in agreement with previous reports using the US NHANES participants41,42 as well as Japanese12 and Swedish participants.43 The reason for the lack of association between HbA1c and cancer mortality is unclear, and poor reproducibility of HbA1c44 may be a contributor. It is worth noting, however, that some studies observed that HbA1c was positively associated with cancer mortality.45,46
Many guidelines have started to recommend non-fasting lipids (total, HDL & LDL cholesterol as well as triglyceride) as the standard for cardiovascular risk assessment.
47,48 The current study suggests that non-fasting glucose (PPG
4-7.9h) may be used for cancer mortality risk assessment. The PPG
4-7.9h test is low-cost and more convenient than a fasting glucose test or oral glucose tolerance test. In addition, most of the tests could be done on the same day as the clinical visit. Moreover, the stability of PPG
4-7.9h throughout 4 to 7.9 h makes the PPG
4-7.9h test more reliable and practical than measuring PPG at an exact time point,
e.g., 2 h, which could be time- and meal type-sensitive.
13,14 Therefore, this low-cost, convenient, reliable, and practical test of PPG
4-7.9h may be potentially used as a treatment target (
Figure 5), and whether lowering PPG
4-7.9h below 101 mg/dL reduces cancer mortality needs to be investigated in the future.
Strengths and Limitations
One strength of this study is its analysis of PPG after meals of free choice in a large number of US adults. Another strength is its prospective study design with a long follow-up (mean, 22 years). A third strength is that participants with anti-diabetic drugs were excluded to avoid confounding effects from these medications.49 In addition, this study adjusted for many confounding factors. This study also has several limitations. First, cancer type information was not available. Second, mortality outcomes were ascertained by linkage to the National Death Index (NDI) records with a probabilistic match,18,19 which may lead to misclassification. However, this matching method has been shown highly accurate (accuracy, 98.5%).50
Conclusions
High PPG4-7.9h is associated with a higher cancer mortality risk in US adults. Lowering PPG4-7.9h below 101 mg/dL may reduce cancer mortality.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization: Y.W.; Data curation: Y.W., Y. F.; data analysis: Y.W.; writing - original draft preparation, Y.W., Y. F.; writing - review and editing: Y.W., Y.F, A.J.R.H., J.G., E.L.G., A.C.
Conflict of Interest Disclosures
A.C. is a speaker and consultant for Astra Zeneca, Bayer, Berlin Chemie, Guidotti, Lilly, Novo Nordisk, Roche Diagnostic, Sanofi. J.G. is supported by grants from the National Health and Medical Research Council of Australia, Medical Research Futures Fund, Townsville Hospital and Health Services, The Heart Foundation of Australia and Diabetes Australia.
Funding/Support
This study was funded by a grant from the National Health and Medical Research Council of Australia (1062671).
Role of the Funder/Sponsor
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021;71(3):209-249. [CrossRef]
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16(4):1103-23. [CrossRef]
- Bjornsdottir HH, Rawshani A, Rawshani A, et al. A national observation study of cancer incidence and mortality risks in type 2 diabetes compared to the background population over time. Scientific Reports. 2020;10(1):17376. [CrossRef]
- Ballotari P, Vicentini M, Manicardi V, et al. Diabetes and risk of cancer incidence: results from a population-based cohort study in northern Italy. BMC Cancer. 2017;17(1):703. [CrossRef]
- Lam EKK, Batty GD, Huxley RR, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. 2011;22(3):730-738. https://doi.org/10.1093/annonc/mdq405. [CrossRef]
- Wang M, Sperrin M, Rutter MK, Renehan AG. Cancer is becoming the leading cause of death in diabetes. The Lancet. 2023;401(10391):1849. https://doi.org/10.1016/S0140-6736(23)00445-2. [CrossRef]
- Woo V, Shestakova MV, Ørskov C, Ceriello A. Targets and tactics: the relative importance of HbA, fasting and postprandial plasma glucose levels to glycaemic control in type 2 diabetes. Int J Clin Pract. 2008;62(12):1935-42. https://doi.org/10.1111/j.1742-1241.2008.01941.x. [CrossRef]
- Ceriello A, Colagiuri S, Gerich J, Tuomilehto J. Guideline for management of postmeal glucose. Nutr Metab Cardiovasc Dis. 2008;18(4):S17-33. https://doi.org/10.1016/j.numecd.2008.01.012. [CrossRef]
- Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003;163(11):1306-16. [CrossRef]
- Peter R, Okoseime OE, Rees A, Owens DR. Postprandial glucose - a potential therapeutic target to reduce cardiovascular mortality. Curr Vasc Pharmacol. 2009;7(1):68-74. [CrossRef]
- Association AD. Postprandial Blood Glucose. Diabetes Care. 2001;24(4):775-778. [CrossRef]
- Takao T, Takahashi K, Suka M, Suzuki N, Yanagisawa H. Association between postprandial hyperglycemia at clinic visits and all-cause and cancer mortality in patients with type 2 diabetes: A long-term historical cohort study in Japan. Diabetes Res Clin Pract. 2019;148:152-159. [CrossRef]
- Eichenlaub MM, Khovanova NA, Gannon MC, Nuttall FQ, Hattersley JG. A Glucose-Only Model to Extract Physiological Information from Postprandial Glucose Profiles in Subjects with Normal Glucose Tolerance. J Diabetes Sci Technol. 2022;16(6):1532-1540. [CrossRef]
- Wang Y, Fang Y. Late non-fasting plasma glucose predicts cardiovascular mortality independent of hemoglobin A1c. Sci Rep. 2022;12:7778. [CrossRef]
- Wang Y. Definition, prevalence, and risk factors of low sex hormone-binding globulin in US adults. J Clin Endocrinol Metab. 2021;106(10):e3946–e3956. [CrossRef]
- Gunter EW, Lewis BG, Koncikowski SM. Laboratory Procedures Used for the Third National Health and Nutrition Examination Survey (NHANES III), 1988-1994. https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/labman.pdf.
- Wang Y, Fang Y, Magliano DJ, et al. Fasting triglycerides are positively associated with cardiovascular mortality risk in people with diabetes. Cardiovasc Res. 2023;119(3):826–834. [CrossRef]
- Wang Y, Fang Y, Witting PK, et al. Dietary fatty acids and mortality risk from heart disease in US adults: an analysis based on NHANES. Scientific Reports. 2023;13(1):1614. [CrossRef]
- Wang Y, Fang Y, Sobey CG, Drummond GR. Prior cancer diagnosis and mortality profile in US adults. Am J Med Sci. 2023;365(2):176-183. [CrossRef]
- Wang Y. Higher fasting triglyceride predicts higher risks of diabetes mortality in US adults. Lipids in Health and Disease. 2021;20(1):181. [CrossRef]
- Fang Y, Wang Y. Fasting status modifies the association between triglyceride and all-cause mortality: A cohort study. Health Sci Rep. 2022;5:e642. [CrossRef]
- Kim H, Hu EA, Rebholz CM. Ultra-processed food intake and mortality in the USA: results from the Third National Health and Nutrition Examination Survey (NHANES III, 1988-1994). Public Health Nutr. 2019;22(10):1777-1785. [CrossRef]
- El Saadany T, Richard A, Wanner M, Rohrmann S. Sex-specific effects of leisure-time physical activity on cause-specific mortality in NHANES III. Prev Med. 2017;101:53-59. [CrossRef]
- Kubo Y, Noguchi T, Hayashi T, Tomiyama N, Ochi A, Hayashi H. Eating alone and weight change in community-dwelling older adults during the coronavirus pandemic: A longitudinal study. Nutrition. 2022;102:111697. [CrossRef]
- Wang Y. Stage 1 hypertension and risk of cardiovascular disease mortality in United States adults with or without diabetes. J Hypertens. 2022;40:794–803. [CrossRef]
- World Health Organization. Key facts-Diabetes. Availabe at https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed on 18 October 2023.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(the Supplement):S15-S33. [CrossRef]
- Diagnosis TECot, Mellitus CoD. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183-1197. [CrossRef]
- Wang Y, Zhang W, Qian T, et al. Reduced renal function may explain the higher prevalence of hyperuricemia in older people. Scientific Reports. 2021;11(1):1302. [CrossRef]
- Wang Y, Fang Y. Postabsorptive homeostasis model assessment for insulin resistance is a reliable biomarker for cardiovascular disease mortality and all-cause mortality. Diabetes Epidemiology and Management. 2021;6:100045. [CrossRef]
- Perkins NJ, Schisterman EF. The inconsistency of "optimal" cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol. 2006;163(7):670-5. [CrossRef]
- O’Neal TB, Luther EE. Dawn Phenomenon. StatPearls. StatPearls Publishing; 2022.
- Tjaden AH, Edelstein SL, Arslanian S, et al. Reproducibility of Glycemic Measures Among Dysglycemic Youth and Adults in the RISE Study. J Clin Endocrinol Metab. 2023;108(10):e1125-e1133. [CrossRef]
- Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231-7. [CrossRef]
- Ko GT, Chan JC, Woo J, et al. The reproducibility and usefulness of the oral glucose tolerance test in screening for diabetes and other cardiovascular risk factors. Ann Clin Biochem. 1998;35 ( Pt 1):62-7. [CrossRef]
- Mandrekar JN. Receiver Operating Characteristic Curve in Diagnostic Test Assessment. Journal of Thoracic Oncology. 2010;5(9):1315-1316. [CrossRef]
- Peter R, Dunseath G, Luzio SD, Chudleigh R, Roy Choudhury S, Owens DR. Daytime variability of postprandial glucose tolerance and pancreatic B-cell function using 12-h profiles in persons with Type 2 diabetes. Diabet Med. 2010;27(3):266-73. [CrossRef]
- Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997;20(12):1822-6. [CrossRef]
- Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53(9):1867-76. [CrossRef]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science. 2009;324(5930):1029-1033. [CrossRef]
- Parekh N, Lin Y, Hayes RB, Albu JB, Lu-Yao GL. Longitudinal associations of blood markers of insulin and glucose metabolism and cancer mortality in the third National Health and Nutrition Examination Survey. Cancer Causes & Control. 2010;21(4):631-642. [CrossRef]
- Hsu CN, Chang CH, Lin YS, Lin JW, Caffrey JL. Association of serum C-peptide concentrations with cancer mortality risk in pre-diabetes or undiagnosed diabetes. PLoS One. 2013;8(2):e55625. [CrossRef]
- Otten J, Tavelin B, Söderberg S, Rolandsson O. Fasting C-peptide at type 2 diabetes diagnosis is an independent risk factor for total and cancer mortality. Diabetes Metab Res Rev. 2022;38(3):e3512. [CrossRef]
- Simon D, Senan C, Balkau B, Saint-Paul M, Thibult N, Eschwège E. Reproducibility of HbA1c in a healthy adult population: the Telecom Study. Diabetes Care. 1999;22(8):1361-1363. [CrossRef]
- Ramdass V, Caskey E, Sklarz T, et al. Association Between Obesity and Cancer Mortality: An Internal Medicine Outpatient Clinic Perspective. J Clin Med Res. 2021;13(7):377-386. [CrossRef]
- Nakanishi S, Yamada M, Hattori N, Suzuki G. Relationship between HbA(1)c and mortality in a Japanese population. Diabetologia. 2005;48(2):230-4. [CrossRef]
- Darras P, Mattman A, Francis GA. Nonfasting lipid testing: the new standard for cardiovascular risk assessment. CMAJ. 2018;190(45):E1317-E1318. [CrossRef]
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082-e1143. [CrossRef]
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33(7):1674-85. [CrossRef]
- Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114(13):1388-94. [CrossRef]
Figure 1.
Study participants. HbA1c, hemoglobin A1c; NHANES III, the third National Health and Nutrition Examination Survey; OGTT, oral glucose tolerance test; PG, plasma glucose; PPG, postprandial plasma glucose.
Figure 1.
Study participants. HbA1c, hemoglobin A1c; NHANES III, the third National Health and Nutrition Examination Survey; OGTT, oral glucose tolerance test; PG, plasma glucose; PPG, postprandial plasma glucose.
Figure 2.
Cancer mortality risk associated with a 1-natural-log-unit increase in plasma glucose in 14,860 participants. Model 1: adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, and fasting time. Model 2: adjusted for all the factors in Model 1 plus HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PG2hOGTT, plasma glucose measured from blood taken at 2 h after an oral glucose tolerance test; PGfasting, plasma glucose measured from blood taken in a fasting state (fasting time ≥8 h); PPG0-3.9h, postprandial plasma glucose measured from blood taken between 0 and 3.9 h; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h.
Figure 2.
Cancer mortality risk associated with a 1-natural-log-unit increase in plasma glucose in 14,860 participants. Model 1: adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, and fasting time. Model 2: adjusted for all the factors in Model 1 plus HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PG2hOGTT, plasma glucose measured from blood taken at 2 h after an oral glucose tolerance test; PGfasting, plasma glucose measured from blood taken in a fasting state (fasting time ≥8 h); PPG0-3.9h, postprandial plasma glucose measured from blood taken between 0 and 3.9 h; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h.
Figure 3.
Cancer mortality risk associated with categorical plasma glucose in 14,860 participants. Plasma glucose was dichotomous, using the top decile as the cutoff. Model 1: adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, and fasting time. Model 2: adjusted for all the factors in Model 1 plus HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PG2hOGTT, plasma glucose measured from blood taken at 2 h after an oral glucose tolerance test; PGfasting, plasma glucose measured from blood taken in a fasting state (fasting time ≥8 h); PPG0-3.9h, postprandial plasma glucose measured from blood taken between 0 and 3.9 h; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h.
Figure 3.
Cancer mortality risk associated with categorical plasma glucose in 14,860 participants. Plasma glucose was dichotomous, using the top decile as the cutoff. Model 1: adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, and fasting time. Model 2: adjusted for all the factors in Model 1 plus HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PG2hOGTT, plasma glucose measured from blood taken at 2 h after an oral glucose tolerance test; PGfasting, plasma glucose measured from blood taken in a fasting state (fasting time ≥8 h); PPG0-3.9h, postprandial plasma glucose measured from blood taken between 0 and 3.9 h; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h.
Figure 4.
Determination and validation of cutoff of PPG4-7.9h. A, ROC curve of PPG4-7.9h to classify normal HbA1c (<5.7%). The optimal cutoff was 94 mg/dL, with a sensitivity of 59.3%, specificity of 67.3%, and an area under the curve (AUC) of 0.674. B, ROC curve of PPG4-7.9h to classify high HbA1c (≥6.5%). The optimal cutoff was 101 mg/dL, with a sensitivity of 76.8%, specificity of 85.8%, and AUC of 0.881. Panels A-B suggested the following classification for PPG4-7.9h: <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high). C, Kaplan-Meier survival curves. D, Cancer mortality risk associated with PPG4-7.9h categories. The analysis was adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, fasting time, and HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h; ROC, receiver operating characteristic.
Figure 4.
Determination and validation of cutoff of PPG4-7.9h. A, ROC curve of PPG4-7.9h to classify normal HbA1c (<5.7%). The optimal cutoff was 94 mg/dL, with a sensitivity of 59.3%, specificity of 67.3%, and an area under the curve (AUC) of 0.674. B, ROC curve of PPG4-7.9h to classify high HbA1c (≥6.5%). The optimal cutoff was 101 mg/dL, with a sensitivity of 76.8%, specificity of 85.8%, and AUC of 0.881. Panels A-B suggested the following classification for PPG4-7.9h: <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high). C, Kaplan-Meier survival curves. D, Cancer mortality risk associated with PPG4-7.9h categories. The analysis was adjusted for age, sex, ethnicity, body mass index, education, poverty-income ratio, survey period, physical activity, alcohol consumption, smoking status, systolic blood pressure, total cholesterol, HDL cholesterol, family history of diabetes, fasting time, and HbA1c. CI, confidence interval; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HR, hazard ratio; No., number; PPG4-7.9h, postprandial plasma glucose measured from blood taken between 4 and 7.9 h; ROC, receiver operating characteristic.
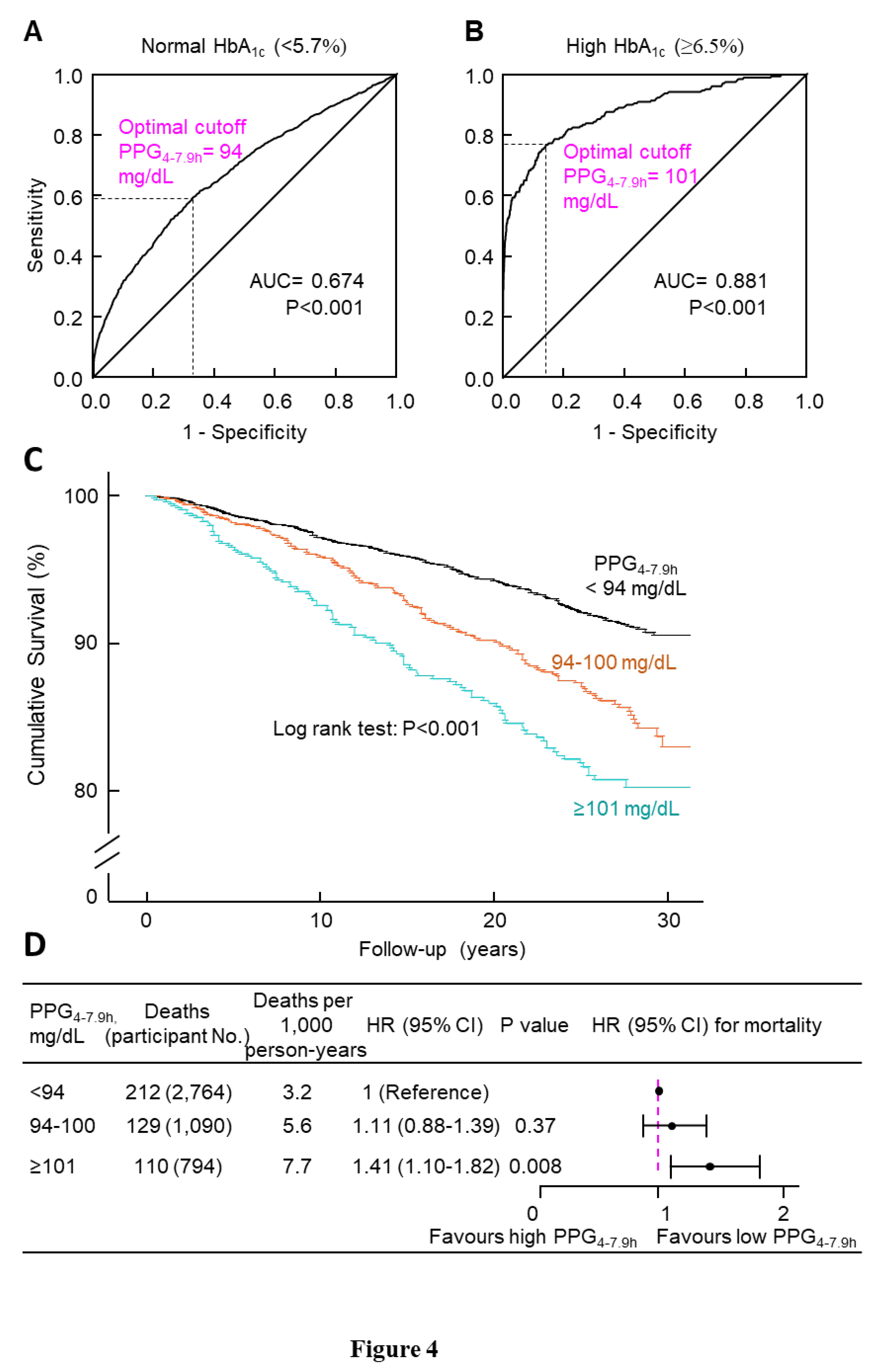
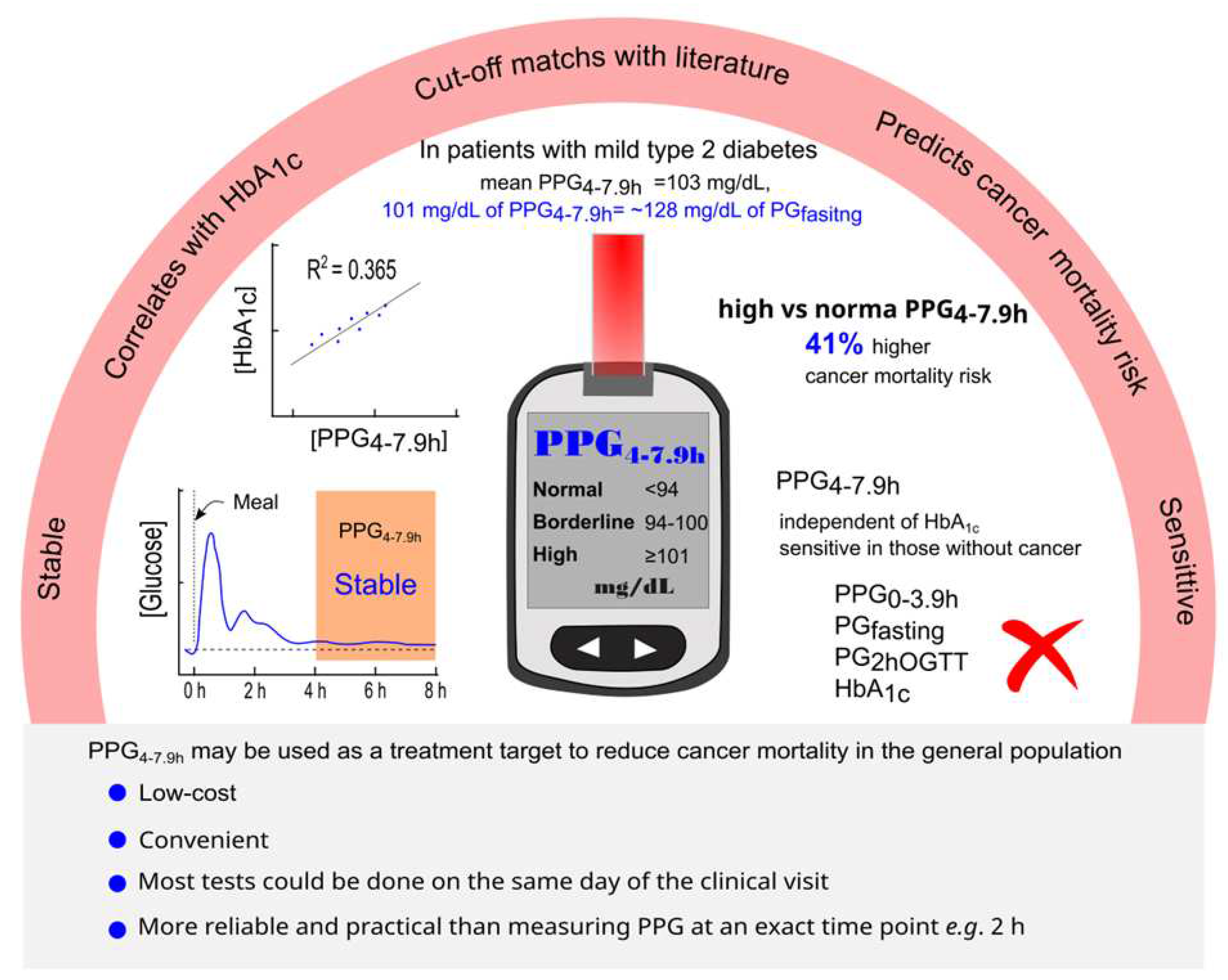
Figure 5.
Summary diagram. PPG4-7.9h is stable, correlates with HbA1c, and predicts mortality from all causes and cancer. The proposed classification of PPG4-7.9h is <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high). The PPG4-7.9h cutoff of 101 mg/dL represents the PPG4-7.9h level in those with mild type 2 diabetes and equates to 128 mg/dL in fasting plasma glucose. Those with high PPG4-7.9h have a 41% higher cancer mortality risk compared to those with normal PPG4-7.9h. The positive association of PPG4-7.9h with cancer mortality is independent of HbA1c and remains in those without a prior diagnosis of cancer. However, other glucose parameters and HbA1c are not associated with cancer mortality. The measurement of PPG4-7.9h is low-cost, convenient, reliable, and practical. PPG4-7.9h may be used as a treatment target to reduce cancer mortality in the general population. HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test; PG, plasma glucose; PPG, postprandial plasma glucose.
Figure 5.
Summary diagram. PPG4-7.9h is stable, correlates with HbA1c, and predicts mortality from all causes and cancer. The proposed classification of PPG4-7.9h is <94 (normal), 94-100 (borderline high), and ≥101 mg/dL (high). The PPG4-7.9h cutoff of 101 mg/dL represents the PPG4-7.9h level in those with mild type 2 diabetes and equates to 128 mg/dL in fasting plasma glucose. Those with high PPG4-7.9h have a 41% higher cancer mortality risk compared to those with normal PPG4-7.9h. The positive association of PPG4-7.9h with cancer mortality is independent of HbA1c and remains in those without a prior diagnosis of cancer. However, other glucose parameters and HbA1c are not associated with cancer mortality. The measurement of PPG4-7.9h is low-cost, convenient, reliable, and practical. PPG4-7.9h may be used as a treatment target to reduce cancer mortality in the general population. HbA1c, hemoglobin A1c; OGTT, oral glucose tolerance test; PG, plasma glucose; PPG, postprandial plasma glucose.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).