1. Introduction
With the aging global population, the prevalence of total hip replacements has surged, exceeding 330,000 procedures annually in the United States alone [
1]. Contemporary total hip arthroplasty (THA) implants predominantly utilize titanium alloys due to their favourable properties, including exceptional corrosion resistance, a modulus of elasticity conducive to osteointegration, and low weight [
2]. While titanium alloys are generally associated with fewer adverse local tissue reactions (ALTR) compared to cobalt-chromium (CoCr) alloys, the phenomenon of trunnionosis has emerged, linking it to pain, ALTR, aseptic loosening, and fractures of the femoral prosthesis neck [
3,
4,
5].
Despite case reports demonstrating pseudo-tumours and ALTR without the presence of CoCr implants, investigations into the effects of titanium alloy debris on periprosthetic tissues have primarily focused on primary THA cases involving bimodular stems [
6,
7]. Bimodular stems, distinguished by their design, feature an exchangeable neck connected to the femoral stem via an additional Morse taper. This unique construction results in the release of titanium alloy debris not only from the proximal trunnion between the head and neck but also from the distal trunnion between the neck and stem, intensifying the dissemination of metal degradation products into surrounding tissues [
8]. Consequently, bimodular stem THA systems release a higher volume of degradation products than standard single-modular stem THA systems [
8]. These degradation products pose a potential threat to soft tissues and bone, and their association with the onset of osteosarcoma has been suggested [
9].
However, even in bimodular stem THA systems, the release of titanium alloy debris is typically gradual, resulting in low blood concentrations of trace elements in patients with well-functioning implants [
10,
11]. Conversely, suboptimal THA performance may lead to increased titanium alloy debris release, provoking a heightened tissue and immune response [
12]. This study aims to present a unique case of primary THA malfunction characterized by an unprecedented, extensive release of titanium alloy debris, a phenomenon not previously documented in the literature.
2. Materials and Methods
A 35-year-old male, actively engaged in farming, began experiencing progressive hip pain in 2006 following a bull kick. Subsequently, he underwent osteosynthesis for a femoral neck fracture using a Dynamic Hip Screw (DHS, Synthes GmbH, Oberdorf, Switzerland). Aseptic necrosis of the femoral head ensued, leading to the removal of the osteosynthetic material eight months later. During the same surgical session, the patient underwent THA, where a 52-mm porous Ti6Al4V alloy press-fit acetabular cup (Trilogy AB, Zimmer, Warsaw, IN, USA) with an aluminum oxide ceramic shell insert (Biolox Forte, CeramTec, Plochlingen, Germany – distributed by Zimmer) was combined with a #18 Versys femoral stem (Zimmer). The femoral stem featured a Ti6Al4V alloy collarless fiber metal taper with a standard neck offset and was coupled with a 28-mm alumina ceramic femoral head (Biolox Forte, CeramTec – distributed by Zimmer) with a 0 mm neck length via 12/14 mm Morse taper. The patient's postoperative course was uneventful, with discharge instructions to use crutches for six weeks, followed by unrestricted weight-bearing.
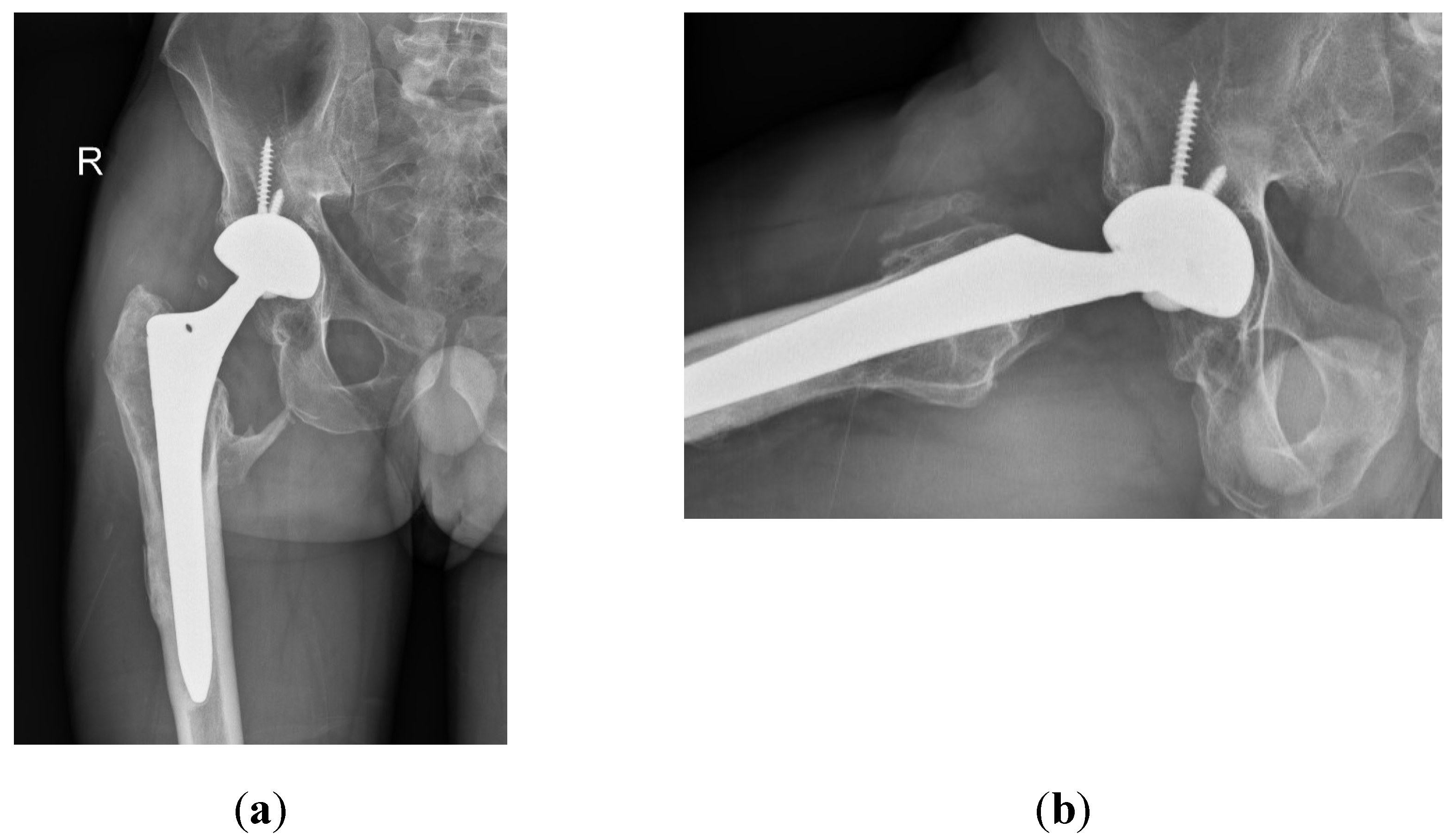
Despite the absence of pain, the patient reported unusual sounds in the hip during weight-bearing, linked to the ceramic-on-ceramic (CoC) bearing. Standard radiographs of the right hip were deemed normal, as depicted in the first available postoperative X-ray (
Figure 1). Therefore, no further evaluation or specific treatment was advised for the patient.
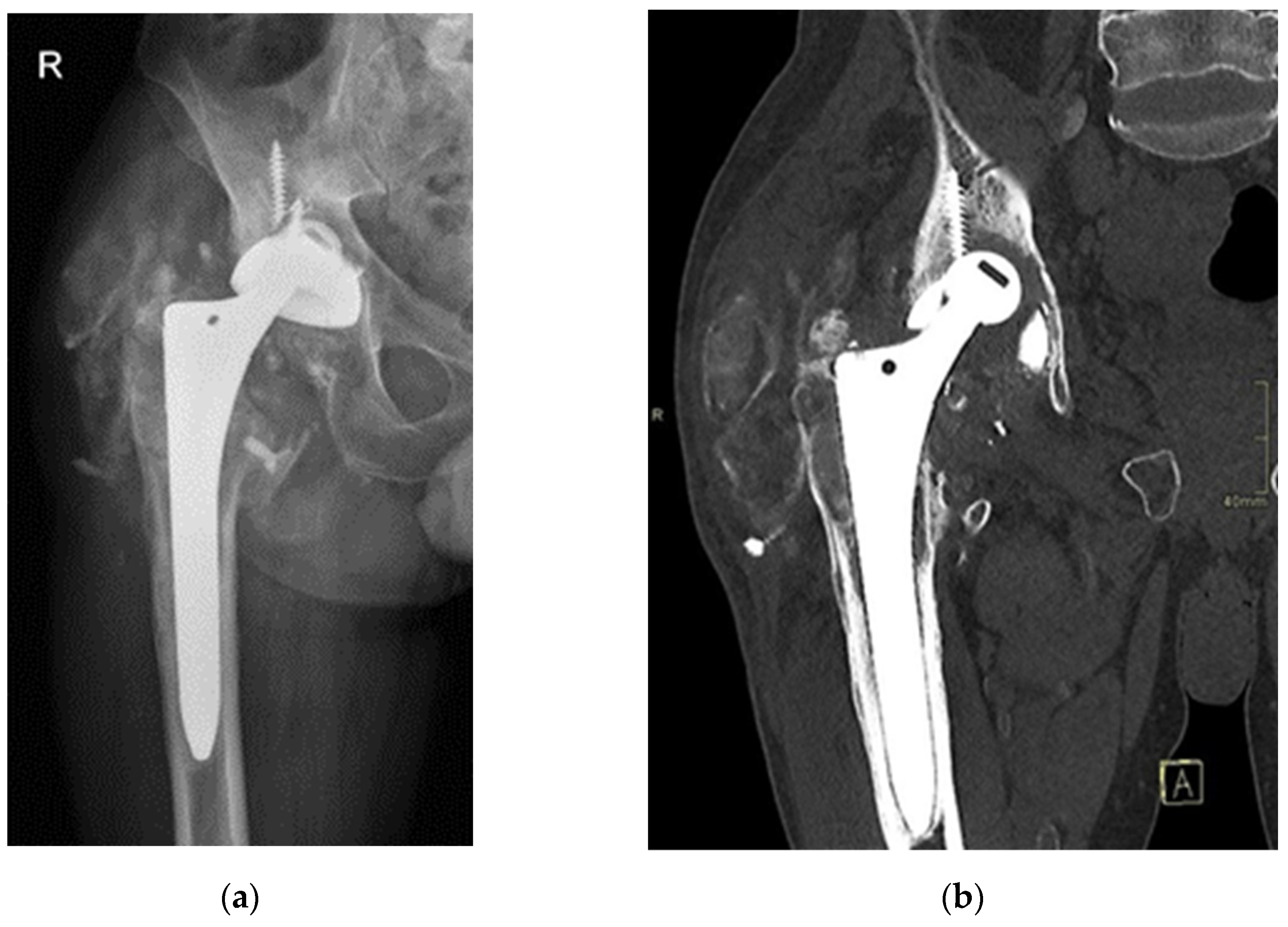
Seventeen years post-THA, the now 52-year-old patient presented to our outpatient clinics with groin pain and severely limited range of motion. The patient’s Harris Hip Score (HHS) was 48 points. Laboratory parameters, including white cell count, haemoglobin level, liver and kidney function, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) level, were within normal limits. Separate blood samples were taken and stored in special containers (BD Vacutainer® Trace Element, Becton Dickinson, Franklin Lakes, NJ, USA). Serum levels of titanium, aluminium, and vanadium were determined at the nationally accredited laboratory (Clinical Institute for Clinical Chemistry and Biochemistry, Ljubljana, Slovenia) for trace element analysis. X-rays and computer tomography (CT) evaluation revealed protrusion of the alumina ceramic head through the Ti6Al4V acetabular cup (
Figure 2), prompting scheduling for an early THA revision.
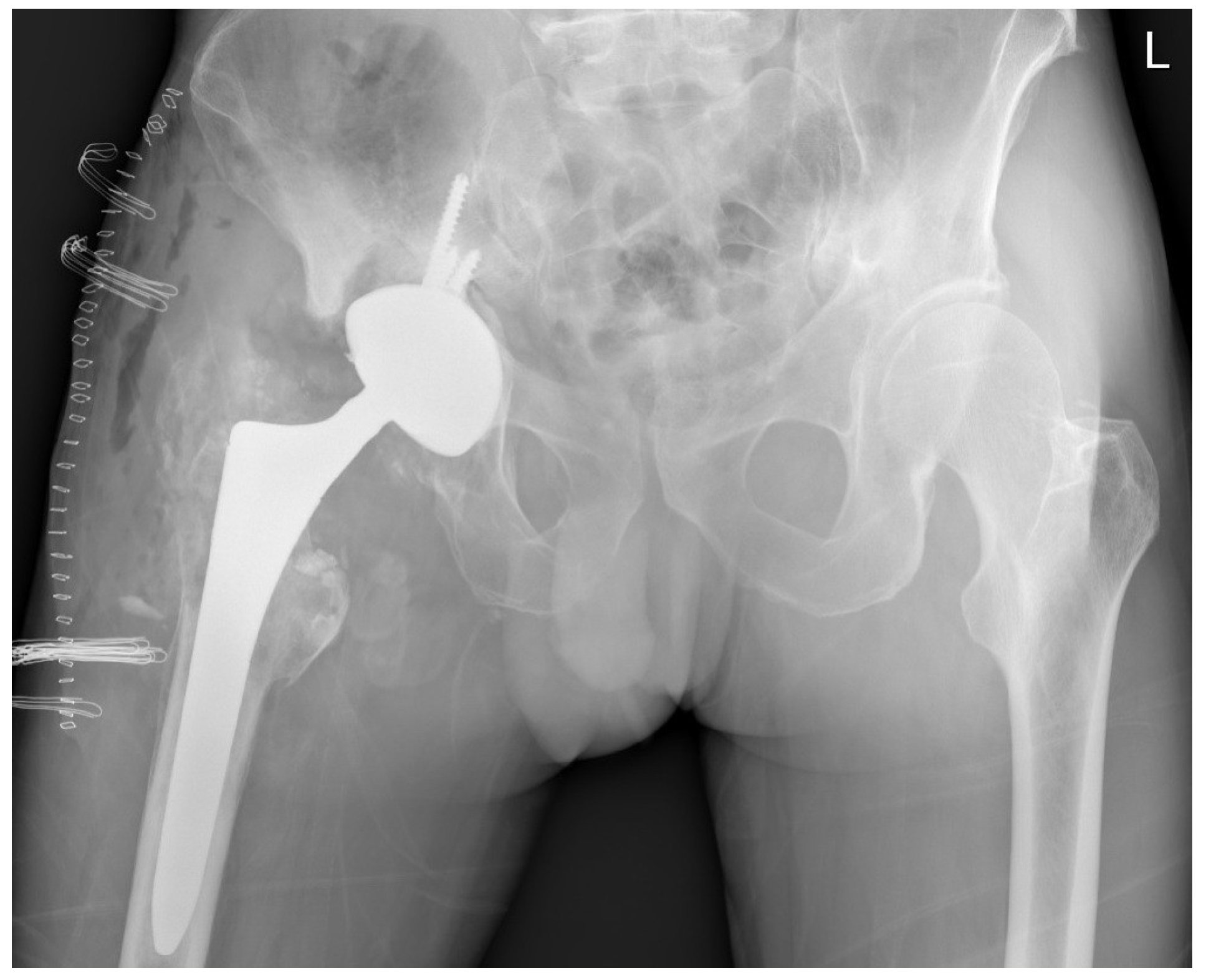
During the revision surgery, the absence of an acetabular liner was noted, and the worn-out ceramic head had directly contacted the acetabular cup, protruding through a central hole formed over time (
Figure 3). Pseudo-tumours in soft tissue and severe metallosis near the implant were excised, with tissue samples sent for histological analysis and cultures. Acetabular screws underwent sonication. The stable femoral stem with an intact Morse taper for femoral neck–head coupling necessitated only the exchange of the acetabular component and femoral head. A 58-mm Ti6Al4V press-fit acetabular cup (Delta-TT, Limacorporate, Udine, Italy) with an aluminum oxide matrix composite ceramic liner (Biolox Delta, CeramTec) was inserted and secured with three screws. A 36-mm aluminum oxide matrix composite ceramic revision femoral head with a Ti6Al4V sleeve (Biolox Delta Option, CeramTec) was attached to the original femoral stem, followed by repositioning (
Figure 4).
The soft tissues obtained during revision surgery were formalin-fixed, underwent routine tissue processing and was paraffin-embedded. Out of each paraffin-embedded tissue block, 3–5 µm thick paraffin sections were cut and placed on glass slide (Superfrost, Waldemar Knittel Glasbearbeitungs GmbH, Braunschweig, Germany). Hematoxylin and eosine (HE) staining were performed on automated Ventana HE 600 system (Ventana Medical Systems, Roche, Basel, Switzerland). Microphotographs of tissue slides were taken with Aperio ScanScope CS (Aperio, Leica Biosystems, Nußloch, Germany) pathology slide scanner with 20× scanning magnification, and exported with Aperio's free image viewer (ImageScope (version: 12.4.6.5003), Leica Biosystems).
Magnetic resonance imaging (MRI) of the patient’s hip was performed with 1,5 T MRI machine (Magnetom Sola, Siemens, Munich, Germany). Protocol parameters were as follows: STIR TSE sequence, slice thickness: 3 mm, body coil, flip angle 150, TR 3790, TE 35 ms.
3. Results
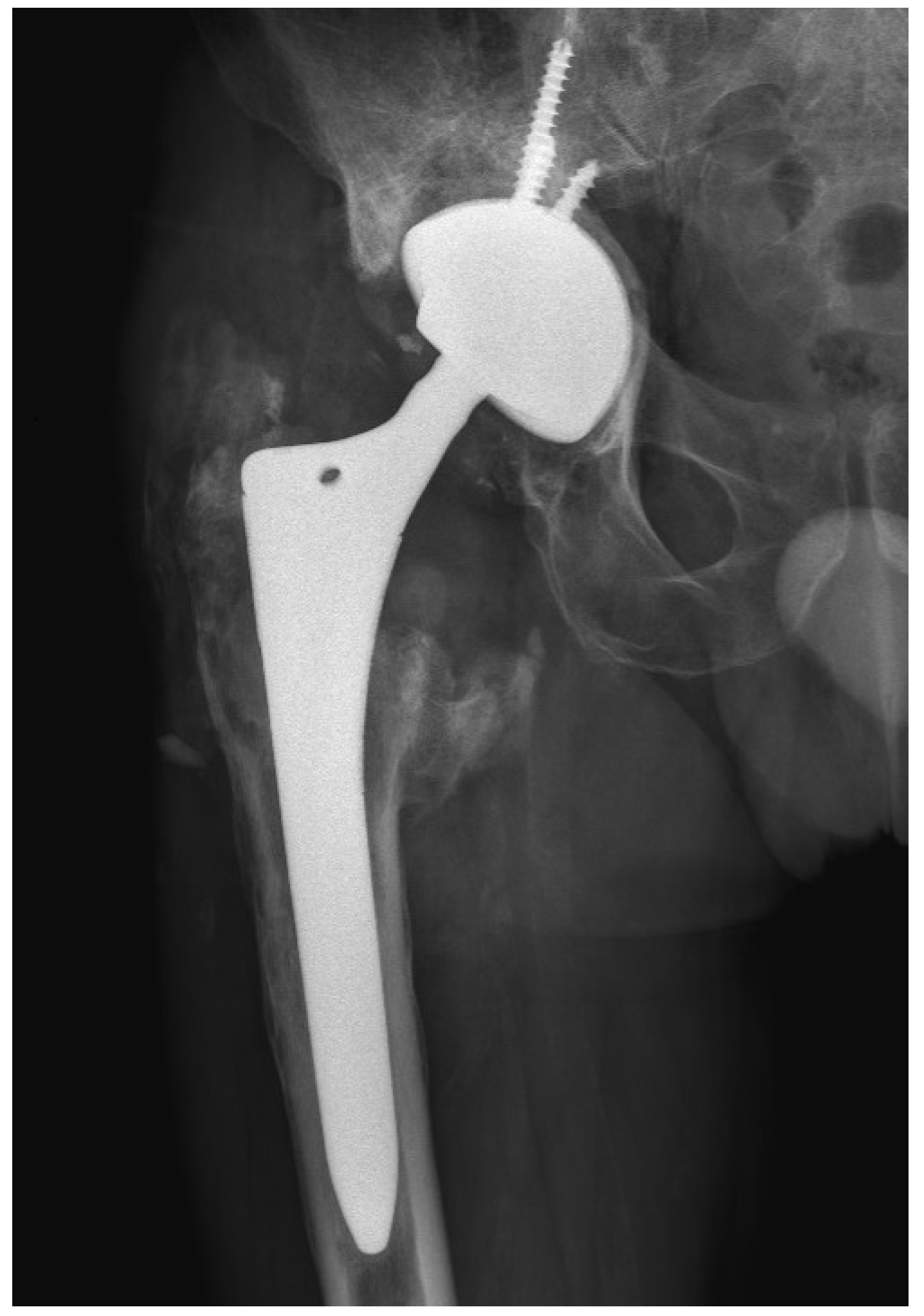
The wound healed uneventfully, and the patient was discharged on the ninth postoperative day. After a 10-day incubation period, all samples were found to be sterile. At the six-month follow-up after the revision THA, the patient reported favorable outcomes. The patient’s HHS was 97 points. Laboratory parameters, including white cell count, hemoglobin level, liver and kidney function, as well as ESR and CRP level, were within normal limits. The radiograph of the patient’s hip depicted stable THA construct without additional osteolysis (
Figure 5).
3.1. Serum trace element analysis
Serum trace element analysis was performed on the day before revision surgery, at 4 months follow up (titanium only), and at 6 months follow up. Trace element analysis indicated significantly elevated levels of serum titanium (norm <6.0 μg/L), aluminium (norm <10.0 μg/L), and vanadium (norm <0.14 μg/L) at all times. The serum levels of titanium peaked to 1237.6 μg/L at 4 months after revision surgery. At 6 months follow up, the serum levels of titanium and vanadium were steady declining. However, the aluminium levels remain elevated and were even inclining at 6 moths follow up regarding the basic level before revision surgery (
Table 1). The values for cobalt and chromium were in the normal range at all times (norm <0.65 μg/L for cobalt and <0.75 μg/L for chromium).
3.2. Histological analysis
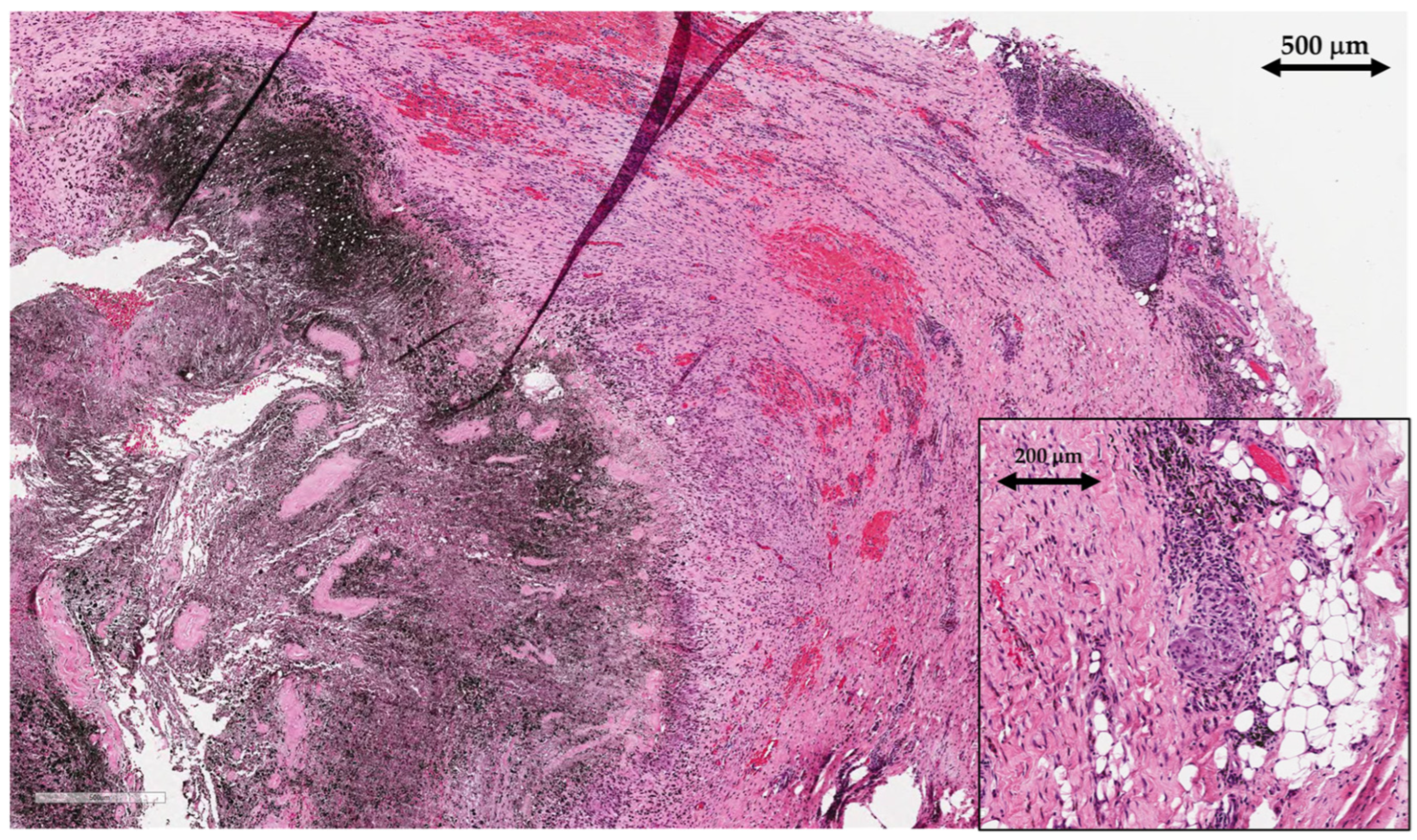
Tissue samples of periprosthetic soft tissue, that gave a macroscopic impression of pseudotumors were sent to the pathology lab for histological analysis. Tissue samples were of irregular shapes, soft, greyish brown to black color and up to 4 cm in size. Microscopic examination showed areas of necrotic tissue and fibrin depositions admixed with abundant metallic microparticles (
Figure 6). There was extensive infiltration with predominantly macrophages, containing metallic micro and macroparticles as well as areas of granulomatous inflammatory reaction with foreign-body giant cells. Lymphoplasmacytic infiltrate was present but there were no granulocytes, indicating the absence of acute inflammation and therefore excluding infection.
After joint replacement surgery, regenerated synovial tissue and the periprosthetic membrane are referred to using the term “synovial-like interface membrane” (SLIM). Histological findings of SLIM pathology are classified into groups that aid the recognition of underlying pathophysiological mechanisms leading to implant failure. Based on the International Expanded SLIM Consensus Classification, the patient had a wear-induced synovitis/SLIM, leading to adverse local tissue reaction to implant wear particles (type VI) with a predominantly macrophagic pattern with absent or minimal lymphocytic response [
13]. The patient did not meet criteria for periprosthetic joint infection (no neutrophils present in the tissue).
3.3. MR imaging
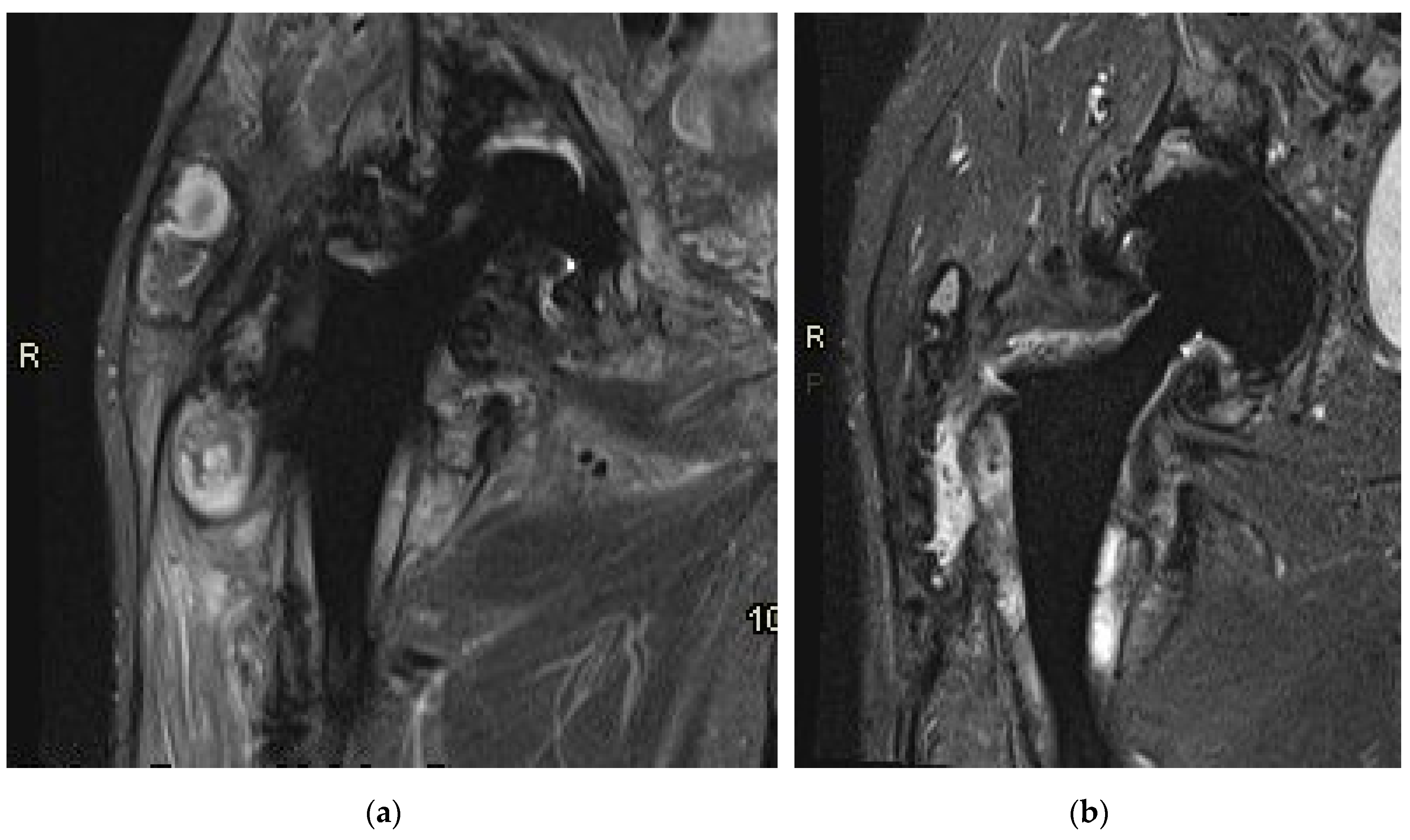
Prior to the revision surgery, MRI revealed substantial soft tissue alterations around the endo-prosthesis, particularly in the muscles proximal to the greater trochanter of the femur. Primarily, these changes manifested as solid chronic granulation with a smaller cystic component that was partially calcified. In both the described soft tissue changes and the periprosthetic osteolytic regions, minute hypointense inclusions of metal microparticles were observed (
Figure 7a). Six months post-revision surgery, the previously extensive granulation changes in the surrounding soft tissues were diminished. There was reduced soft tissue edema, and indications of local irritation had also decreased. The presence of slightly increased fluid around the endoprosthesis indicated residual seroma following the surgery (
Figure 7b).
4. Discussion
To the best of our knowledge, this investigation presents the highest documented serum concentration of titanium in a patient with a failed orthopedic implant. Patients undergoing hip replacement with loosened titanium components commonly exhibit elevated serum titanium levels at the time of implant failure. Previous studies exploring this phenomenon were typically limited in scale, employed various implant designs, and utilized diverse analytical methods to assess the titanium content in the blood or serum of affected patients, contributing to the observed variability [
14,
15,
16]. Instances of fractured ceramic liners in CoC prostheses have also been demonstrated to result in heightened systemic titanium levels. Swiatkowska et al. documented a case within their institution where such an occurrence was associated with a blood titanium level of 21.5 μg/L just before the revision [
17]. The substantial increase was linked to mechanical abrasion between the femoral head and fragments of the fractured ceramic liner, as well as contact between the titanium femoral neck and the edge of the acetabular cup [
17]. In addition to the observed increase in serum titanium levels, Sakamoto et al. have conclusively demonstrated the capacity of titanium particles to give rise to symptomatic solid pseudo-tumors [
18].
Titanium is a key material in various medical devices, including joint implants, has been associated with wear and corrosion, releasing inflammatory byproducts into tissues and blood [
19]. Unlike cobalt, titanium accumulates in organs, posing a risk of tissue injury [
20]. Despite emerging safety concerns, titanium's impact remains understudied. The mechanisms, species (ions, particles), and cellular fate of titanium released from implants are unclear. Establishing baseline and toxic titanium levels in biological fluids is challenging due to limited reliable detection methods. While high-resolution instruments show promise for blood titanium measurement, accessibility remains restricted to specialized laboratories, hindering routine monitoring. Inconsistencies in titanium measurements can arise due to the absence of standardized protocols across laboratories, complicating inter-study comparisons. Recent findings by Koller and colleagues underscored significant variations in results when identical baseline pooled blood samples were analyzed by different laboratories [
21]. These discrepancies were attributed to differences in sample preparation, instrument types, and analytical approaches. Given these challenges, it is recommended to employ a single laboratory for titanium testing to ensure direct comparability, as values from different laboratories may not align accurately. In our investigation, we adhered to these guidelines and utilized a solitary laboratory accredited for titanium testing.
Based on Histological particle algorithm, proposed by Perino et al., microparticles in tissue samples were mostly likely conventional non-ferrous metallic particles due to implant-wear
[22]. Described micro and macro particles were greyish to intensively black, which is consistent with metal. Ceramic particles could also be present in blackish color if oxidized and can also be abundant but usually present only as microparticles except in cases of prosthesis fracture, where larger microparticles were described as well. Other implant wear-particles and endogenous particles (such as hemosiderin) differ from metal particles in shape, size, color, or are birefringent.
Several researchers have validated the occurrence of a deleterious pseudotumor post THA even in the absence of a metal-on-metal (MoM) bearing [
23,
24]. Notably, these instances involved the utilization of metal heads in conjunction with titanium-alloy femoral stems. The responsibility of cobalt and chromium in ALTR generation has been well established [
25,
26]. Bisseling et al. reported a case involving a patient who underwent revision THA with a double-mobility metal-on-polyethylene (MoP) bearing, two years after the primary procedure due to the presence of a soft tissue mass at the posterolateral aspect of the greater trochanter [
27]. During the initial THA, an uncemented titanium-niobium (Ti-6Al-7Nb) stem (Zweymuller Alloclassic; Zimmer Orthopaedics, Warsaw, IN, USA) was implanted, featuring a 12/14 mm trunnion combined with an XXL (+10.5 mm) 28-mm cobalt-chromium head with a 12/14 mm tapered bore (Biomet, Warsaw, IN, USA). Despite chromium levels below the detection threshold and unreported serum titanium levels, elevated cobalt serum levels (5.7 µg/L) were observed. The authors concluded that a robust patient-specific immunological response to a modest quantity of metal debris and corrosion by-products resulting from the mismatched head-neck junction prompted the early revision. Emphasizing the potential role of the head-neck junction in generating metal debris and corrosion by-products, which may contribute to ALTR, the authors cautioned against intermixing THA components from diverse manufacturers [
27]. It is noteworthy that in our case, components exclusively from the same manufacturer were employed, and none contained cobalt or chromium.
Several researchers have advocated for the assessment of serum titanium levels in individuals with titanium-based orthopedic prostheses due to its potential as a diagnostic marker for implant performance [
28,
29]. This recommendation stems from the observation that implants, particularly those displaying looseness or signs of wear, including polyethylene wear-through, often release higher concentrations of titanium compared to well-functioning implants. Jacobs et al. demonstrated that elevated serum titanium levels could function as an indicator of patellar component failure or accelerated femoral component wear in total knee replacements with titanium alloy bearings [
30]. Notably, patients with failed patellar components exhibited titanium concentrations approximately 50 times higher than those with intact components, with no discernible differences in aluminum and vanadium levels. Despite the authors acknowledging the unknown toxicological implications of these findings, we, too, did not observe any adverse effects of potential titanium toxicity in our patient.
Titanium, characterized by poor solubility, tends to accumulate in organ tissues such as the liver and spleen, while a fraction is excreted in urine. Monitoring renal function may be crucial, but our patient showed no signs of impaired excretion processes. We attributed the skewed results to significant titanium release from the poorly functioning implant. A temporary rise in serum titanium concentration at the 4-month follow-up was likely due to additional titanium release from soft tissue pseudo-tumors, only partially accessible for excision. Despite titanium being considered less toxic than cobalt, our case highlights the potential for adverse tissue reactions in the absence of CoCr alloy components. Unfortunately, no titanium chelating agents, comparable to those recently developed for cobalt, are currently available [
31].
The etiology behind the catastrophic failure of the titanium-alloy acetabular cup remains elusive. Although the original manufacturer's sticker, containing all pertinent data of the implant, was located in the patient's medical records, no evidence of the acetabular liner was discovered during revision surgery. Additionally, despite employing various radiographic procedures (X-rays, CT, and MRI), we were unable to identify fragments or remnants of the ceramic liner. It is plausible that the entire acetabular liner was ground into tiny and fine-grain debris; however, such debris was not identified in histological samples obtained during revision surgery. Furthermore, the absence of larger pieces of alumina ceramic liner, which would likely have eluded the grinding process between the intact alumina ceramic femoral head and the dome of the acetabular cup, speaks against this hypothesis. Conversely, it would be highly unusual for an orthopedic surgeon not to insert the acetabular liner into the acetabular cup. In such a scenario, over the years, the harder ceramic femoral head would inevitably create a void in the softer titanium-alloy acetabular cup, particularly in a young and physically active patient.
5. Conclusions
This case serves as an informative example for examining the significant release of titanium-alloy debris from dysfunctional orthopaedic implants. Fortunately, no clinically relevant consequences on the patient's overall health were detected. Nevertheless, given the substantial exposure to elevated titanium levels, persisting even at the 6-month follow-up, the patient will undergo close monitoring for potential long-term consequences.
Author Contributions
Conceptualization, S.K.F.; methodology, S.K.F., Ž.L. and M.K.D., software, Ž.L.; validation, S.K.F., I.N., Ž.L. and M.K.D.; formal analysis, Ž.L. and M.K.D.; investigation, S.K.F. and I.N.,.; resources, S.K.F.; data curation, I.N.; writing—original draft preparation, S.K.F.; writing—review and editing, S.K.F., Ž.L. and M.K.D.; visualization, Ž.L. and M.K.D.; supervision, S.K.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of University Medical Centre Maribor (protocol code UKC-MB-KME-38/2, May 6th, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (privacy reason).
Acknowledgments
This study was performed at the University Medical Centre Maribor.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement--a materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.B.; Chughtai, M.; Elmallah, R.K.; Diedrich, A.; Le, S.; Thomas, M.; Mont, M.A. Trunnionosis in total hip arthroplasty: a review. Journal of orthopaedics and traumatology : official journal of the Italian Society of Orthopaedics and Traumatology 2016, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- van Doesburg, P.G.; van Langelaan, E.J.; Apachitei, I.; Bénard, M.R.; Verdegaal, S.H.M. Femoral prosthesis neck fracture following total hip arthroplasty - a systematic review. Arthroplasty (London, England) 2020, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Berstock, J.R.; Whitehouse, M.R.; Duncan, C.P. Trunnion corrosion: what surgeons need to know in 2018. The bone & joint journal 2018, 100-B(1 Supple A), 44–49. [Google Scholar] [CrossRef]
- Rowan, F.E.; Wach, A.; Wright, T.M.; Padgett, D.E. The onset of fretting at the head-stem connection in hip arthroplasty is affected by head material and trunnion design under simulated corrosion conditions. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 2018, 36, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Podlipec, R.; Punzón-Quijorna, E.; Pirker, L.; Kelemen, M.; Vavpetič, P.; Kavalar, R.; Hlawacek, G.; Štrancar, J.; Pelicon, P.; Fokter, S.K. Revealing Inflammatory Indications Induced by Titanium Alloy Wear Debris in Periprosthetic Tissue by Label-Free Correlative High-Resolution Ion, Electron and Optical Microspectroscopy. Materials (Basel, Switzerland) 2021, 14, 3048. [Google Scholar] [CrossRef] [PubMed]
- Fokter, S.K.; Noč, N.; Levašič, V.; Hanc, M.; Zajc, J. Dual-Modular Versus Single-Modular Stems for Primary Total Hip Arthroplasty: A Long-Term Survival Analysis. Medicina (Kaunas, Lithuania) 2023, 59, 290. [Google Scholar] [CrossRef] [PubMed]
- Kavalar, R.; Fokter, S.K.; Lamovec, J. Total hip arthroplasty-related osteogenic osteosarcoma: case report and review of the literature. European journal of medical research 2016, 21, 8. [Google Scholar] [CrossRef]
- Yao, J.J.; Lewallen, E.A.; Thaler, R.; Dudakovic, A.; Wermers, M.; Day, P.; Eckdahl, S.; Jannetto, P.; Bornhorst, J.A.; Larson, A.N.; Abdel, M.P.; Lewallen, D.G.; van Wijnen, A.J. Challenges in the Measurement and Interpretation of Serum Titanium Concentrations. Biological trace element research 2020, 196, 20–26. [Google Scholar] [CrossRef]
- Fokter, S.K.; Zajc, J.; Merc, M. Interchangeable neck failures of bi-modular femoral stems in primary total hip arthroplasty cannot be predicted from serum trace element analysis. International orthopaedics 2021, 45, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.M.; O'Byrne, T.J.; Brennan, P.C.; Jannetto, P.J.; Pavelko, K.D.; Lewallen, D.G.; Vassilaki, M.; Maradit Kremers, H. Cross-sectional association between systemic metal concentrations and immune markers in patients with total joint arthroplasty. Frontiers in immunology 2023, 14, 1130209. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Perino, G. Histological Diagnosis of Implant-Associated Pathologies. Springer: Berlin, Heidelberg, Germany 2017; pp. 1-44. [CrossRef]
- Jacobs, J. J.; Skipor, A. K.; Black, J. , Urban, R.m; Galante, J. O. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. The Journal of bone and joint surgery. American volume 1991, 73, 1475–1486. [Google Scholar] [PubMed]
- Omlor, G. W.; Kretzer, J. P.; Reinders, J.; Streit, M. R.; Bruckner, T.; Gotterbarm, T.; Aldinger, P. R.; Merle, C. In vivo serum titanium ion levels following modular neck total hip arthroplasty--10 year results in 67 patients. Acta biomaterialia 2013, 9, 6278–6282. [Google Scholar] [CrossRef] [PubMed]
- McAlister, I. P.; Abdel, M. P. Elevated Serum Titanium Level as a Marker for Failure in a Titanium Modular Fluted Tapered Stem. Orthopedics 2016, 39, e768–e770. [Google Scholar] [CrossRef] [PubMed]
- Swiatkowska, I.; Martin, N.; Hart, A. J. Blood titanium level as a biomarker of orthopaedic implant wear. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS) 2019, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Watanabe, H.; Higashi, H.; Kubosawa, H. Pseudotumor Caused by Titanium Particles From a Total Hip Prosthesis. Orthopedics, 2016, 39, e162–e165. [Google Scholar] [CrossRef] [PubMed]
- Agins, H. J.; Alcock, N. W.; Bansal, M.; Salvati, E. A.; Wilson, P.D., Jr; Pellicci, P. M.; Bullough, P. G. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. The Journal of Bone and Joint Surgery 1988, 70, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Moran, C. A.; Mullick, F. G.; Ishak, K. G.; Johnson, F. B.; Hummer, W. B. (1991). Identification of titanium in human tissues: probable role in pathologic processes. Human pathology 1991, 22, 450–454. [Google Scholar] [CrossRef]
- Koller, D.; Bramhall, P.; Devoy, J.; Goenaga-Infante, H.; Harrington, C. F.; Leese, E.; Morton, J.; Nuñez, S.; Rogers, J.; Sampson, B.; Powell, J. J. (2018). Analysis of soluble or titanium dioxide derived titanium levels in human whole blood: consensus from an inter-laboratory comparison. The Analyst 2018, 143, 5520–5529. [Google Scholar] [CrossRef]
- Perino, G.; Sunitsch, S.; Huber, M.; Ramirez, D.; Gallo, J.; Vaculova, J.; Natu, S.; Kretzer, J. P.; Müller, S.; Thomas, P.; Thomsen, M.; Krukemeyer, M.G.; Resch, H.; Hügle, T.; Waldstein, W.; Böettner, F.; Gehrke, T.; Sesselmann, S.; Rüther, W.; Xia, Z.; … Krenn, V. Diagnostic guidelines for the histological particle algorithm in the periprosthetic neo-synovial tissue. BMC clinical pathology 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed]
- Fokter, S. K.; Repse-Fokter, A.; Takac, I. Case report: femoral neuropathy secondary to total hip arthroplasty wear debris. Clinical orthopaedics and related research, 2009, 467, 3032–3035. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Tay, G. H.; Godbolt, D. B.; Crawford, R. W. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. The Journal of arthroplasty 2012, 27, 493.e13–493.e4.93E17. [Google Scholar] [CrossRef]
- Snyder, M. J.; Weber, M. A.; Kromka, J. J.; Sims, M. M.; Smith, C. N.; Daji, A. V.; Kumar, D.; Borrero, C. G.; Cordle, A. C.; DiGioia, A. M.; Hamlin, B. R.; Plakseychuk, A. Y.; Urish, K. L. ;Predictors of Adverse Local Tissue Reaction in a High-Risk Population. Arthroplasty today 2022, 13, 125–129. [Google Scholar] [CrossRef]
- Taunton M., J. How to Interpret Metal Ions in THA. The Journal of arthroplasty, 2020, 35(6S), S60–S62. [Google Scholar] [CrossRef]
- Bisseling, P.; Tan, T.; Lu, Z.; Campbell, P. A.; Susante, J. L. The absence of a metal-on-metal bearing does not preclude the formation of a destructive pseudotumor in the hip--a case report. Acta orthopaedica 2013, 84, 437–441. [Google Scholar] [CrossRef] [PubMed]
- von Schroeder, H. P.; Smith, D. C.; Gross, A. E.; Pilliar, R. M.; Kandel, R. A.; Chernecky, R.; Lugowski, S. J. Titanemia from total knee arthroplasty. A case resulting from a failed patellar component. The Journal of arthroplasty 1966, 11, 620–625. [Google Scholar] [CrossRef]
- Nam, D.; Keeney, J. A.; Nunley, R. M.; Johnson, S. R.; Clohisy, J. C.; Barrack, R. L. Metal Ion Concentrations in Young, Active Patients Following Total Hip Arthroplasty with the Use of Modern Bearing Couples. The Journal of arthroplasty 2015, 30, 2227–2232. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J. J.; Silverton, C.; Hallab, N. J.; Skipor, A. K.; Patterson, L.; Black, J.; Galante, J. O. Metal release and excretion from cementless titanium alloy total knee replacements. Clinical orthopaedics and related research 1999, 358, 173–180. [Google Scholar] [CrossRef]
- Ude, C. C.; Schmidt, S. J.; Laurencin, S.; Shah, S.; Esdaille, J.; Kan, H. M.; Holt, B. D.; Arnold, A. M.; Wolf, M. E.; Nair, L. S.; Sydlik, S. A.; Laurencin, C. T. Hyaluronic acid-British anti-Lewisite as a safer chelation therapy for the treatment of arthroplasty-related metallosis. Proceedings of the National Academy of Sciences of the United States of America 2023, 120, e2309156120. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).