Submitted:
19 January 2024
Posted:
23 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
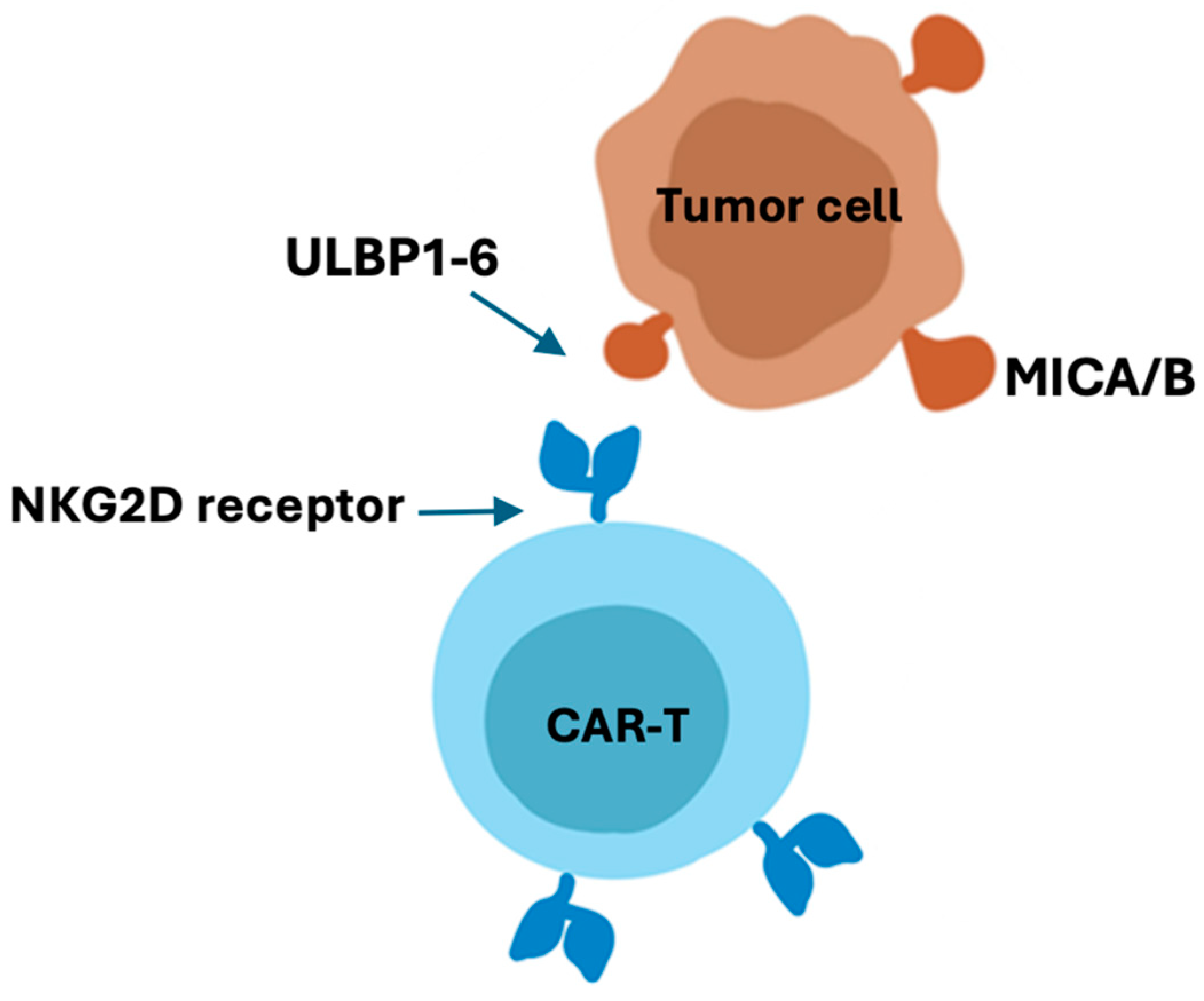
NKG2D CAR-T cell therapy against solid tumors
Advantages of NKG2D CAR-T cell therapy
- Enhanced Specificity: NKG2D CAR-T cells are engineered to express a chimeric antigen receptor containing the NKG2D receptor, which provides enhanced specificity in targeting cancer cells. The NKG2D receptor recognizes stress-induced ligands that are often overexpressed on the surface of cancer cells, thus allowing for precise targeting of tumor cells.
- Broad-spectrum Antitumor Activity: The NKG2D receptor has the ability to bind to a diverse range of stress-induced ligands expressed on various types of cancer cells. As a result, NKG2D CAR-T therapy has the potential to target a broad spectrum of solid and hematologic malignancies.
- Resistance to Tumor Immune Evasion: Solid tumors often create an immunosuppressive microenvironment that impedes the function of traditional immune cells. NKG2D CAR-T cells circumvent these evasion tactics by recognizing stress-induced ligands, enhancing their ability to target cancer cells in the immunosuppressive tumor microenvironment.
Disadvantages of NKG2D CAR-T cell therapy
- Toxicity: Targeting stress-induced ligands expressed on many types of cells including some healthy tissues may lead to off-target effects or unintended toxicities. This could result in damage to normal cells expressing these ligands and potential adverse effects.
- Tumor Immune Evasion Mechanisms: Despite the specificity of NKG2D CAR-T therapy, cancer cells may still evolve immune evasion mechanisms to avoid NKG2D-mediated recognition, which could compromise the therapy's effectiveness over time.
- Tumor Microenvironment Complexity: Solid tumors exhibit a complex microenvironment characterized by immunosuppressive factors and cellular components that can hinder the function of immune cells, including CAR-T cells. Overcoming this hurdle to ensure effective CAR-T cell infiltration and function within the tumor remains a challenge.
- Tumor Escape Variants: While stress-induced ligands may be relatively stable, there is still the potential for tumor cells to adapt and evade NKG2D CAR-T cell recognition by downregulating or mutating the targeted antigens, posing a risk of treatment resistance.
- Cytokine Release Syndrome (CRS) and Neurotoxicity: As with other CAR-T therapies, NKG2D CAR-T cell therapy carries the risk of inducing immune-related adverse events such as CRS and neurotoxicity, which can be severe in some cases.
- Manufacturing Complexity: The engineering and production of NKG2D CAR-T cells can be technically challenging and time-consuming, limiting its widespread availability. Scalability and reproducibility of manufacturing processes are important considerations for broad clinical application.
- Cost and Accessibility: CAR-T cell therapies are resource-intensive and costly, potentially limiting patient access and healthcare system adoption. These therapies may also present logistical challenges due to the need for specialized facilities and expertise in cell processing and delivery.
- Regulatory and Ethical Considerations: The regulatory landscape for advanced cell therapies, including CAR-T, remains dynamic, and establishing frameworks for ensuring the safety and consistent quality of NKG2D CAR-T products is an ongoing concern.
Concluding remarks
Funding
Acknowledgments
References
- Sentman, C.L.; Meehan, K.R. NKG2D CARs as Cell Therapy for Cancer. Cancer J 2014, 20, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zhang, J.; Mao, L. Novel Cellular Immunotherapy Using NKG2D CAR-T for the Treatment of Cervical Cancer. Biomedicine & Pharmacotherapy 2020, 131, 110562. [Google Scholar] [CrossRef]
- Baumeister, S.H.; Murad, J.; Werner, L.; Daley, H.; Trebeden-Negre, H.; Gicobi, J.K.; Schmucker, A.; Reder, J.; Sentman, C.L.; Gilham, D.E.; et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients with AML/MDS and Multiple Myeloma. Cancer Immunol Res 2019, 7, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yang, D.; Dai, H.; Liu, X.; Jia, R.; Cui, X.; Li, W.; Cai, C.; Xu, J.; Zhao, X. Eradication of Hepatocellular Carcinoma by NKG2D-Based CAR-T Cells. Cancer Immunol Res 2019, 7, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Spear, P.; Barber, A.; Rynda-Apple, A.; Sentman, C.L. NKG2D CAR T-cell Therapy Inhibits the Growth of NKG2D Ligand Heterogeneous Tumors. Immunol Cell Biol 2013, 91, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gao, F.; Li, N.; Li, Q.; Zhou, Y.; Yang, T.; Cai, Z.; Du, P.; Chen, F.; Cai, J. Antitumor Activity of NKG2D CAR-T Cells against Human Colorectal Cancer Cells in Vitro and in Vivo. Am J Cancer Res 2019, 9, 945–958. [Google Scholar] [PubMed]
- Ebrahimiyan, H.; Tamimi, A.; Shokoohian, B.; Minaei, N.; Memarnejadian, A.; Hossein-Khannazer, N.; Hassan, M.; Vosough, M. Novel Insights in CAR-NK Cells beyond CAR-T Cell Technology; Promising Advantages. Int Immunopharmacol 2022, 106, 108587. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).