Submitted:
23 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
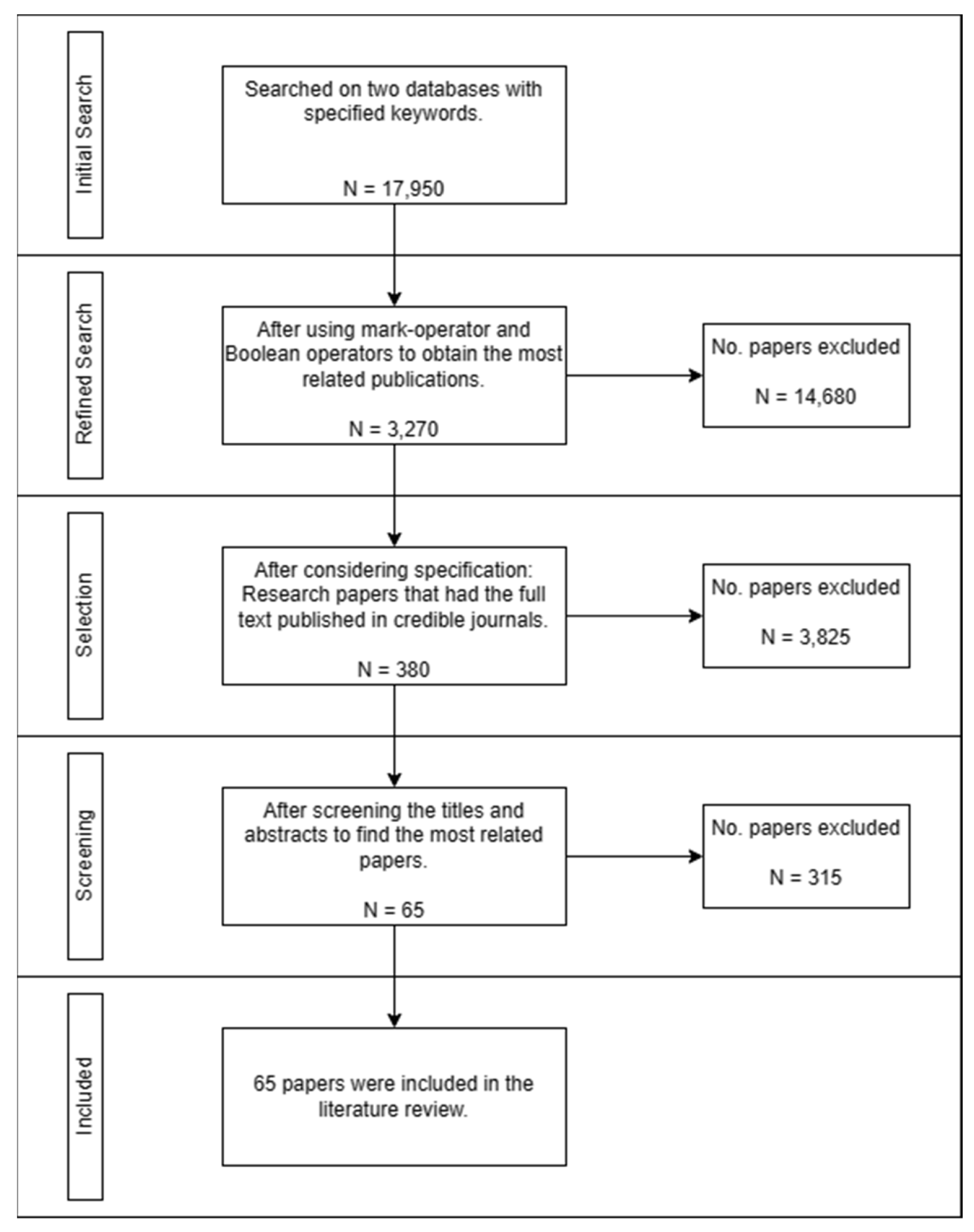
2.1. Literature Exploration
2.2. Study Selection and Screening
- A paper must have been published in a journal or conference booklet to be selected. Books, book series, chapters, and others were not considered.
- It must be a research paper, not a review, a meta-analysis, or a literature review.
- We considered the credibility and quality of the publisher. To do this, we cross-checked the publisher and journal with Scimago/Scopus and Clarivate or the Web of Science.
- The final consideration is that the published papers should include the full text. Our university has limited access to journal subscriptions, which limited us somewhat in selecting the papers.
2.3. Data Extraction
2.4. Literature Review Diagram Flow
2.5. Classification of the Paper
3. Results
3.1. Predictive Analytics of Heart Failure Prediction
3.2. Predictive Analytics for the Prediction of Readmission or Mortality
3.2.1. Readmission
3.2.2. Mortality
3.2.3. Both Readmission and Mortality
4. Discussion
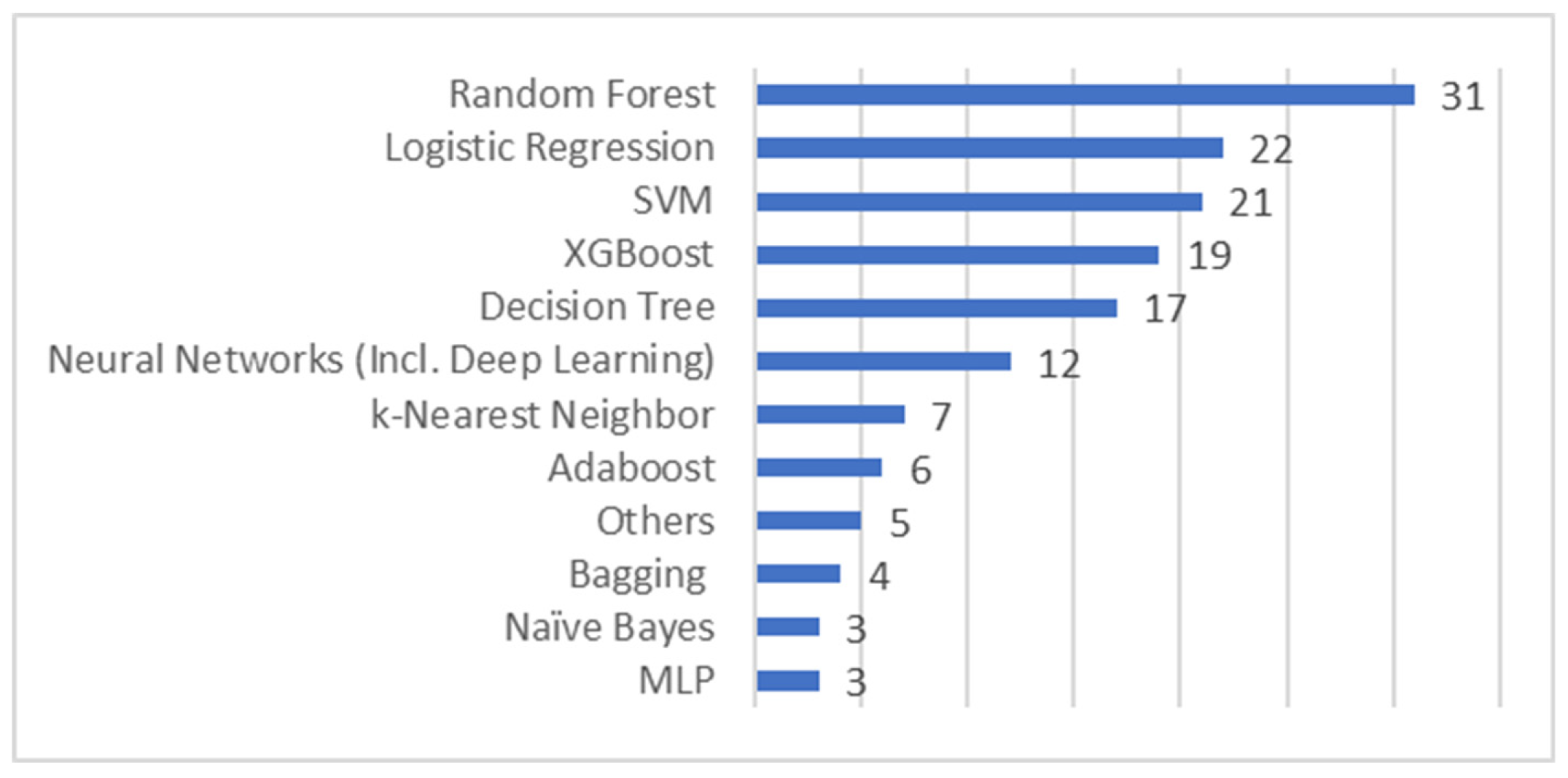
4.1. Machine Learning Algorithms Used for Building Predictive Models
4.2. Data Pre-Processing Implementation in Building Predictive Models
| Pre-processing Step | Article | Frequent method |
|---|---|---|
| Data Cleaning | [7], [13], [19], [20], [23], [26], [28], [32], [33], [34], [35], [36], [39], [40], [42], [41], [45], [46], [47], [54], [55], [60], [64], [65], [66], [68], [69] | Mean imputation, predictive mean matching, median imputation, random forest imputation, kNN imputation, XGBoost imputation, and missForest |
| Data Transformation | [13], [14], [22], [23], [24], [25], [26], [30], [31], [34], [36], [39], [41], [42], [43], [47], [54], [55], [58], [60], [62], [33], [63], [64], [65], [69] | Recursive feature elminiation, SelectKBest, Chi-Square, Pearson's correlation, KS-Test, T-Test |
| Data Reduction | [47], [50], [54] | PCA |
| Data Balancing | [13], [14], [21], [22], [23], [32], [33], [39], [43], [55], [62], [65], [66] | SMOTE, Under-sampling, Over-sampling, ADASYSN |
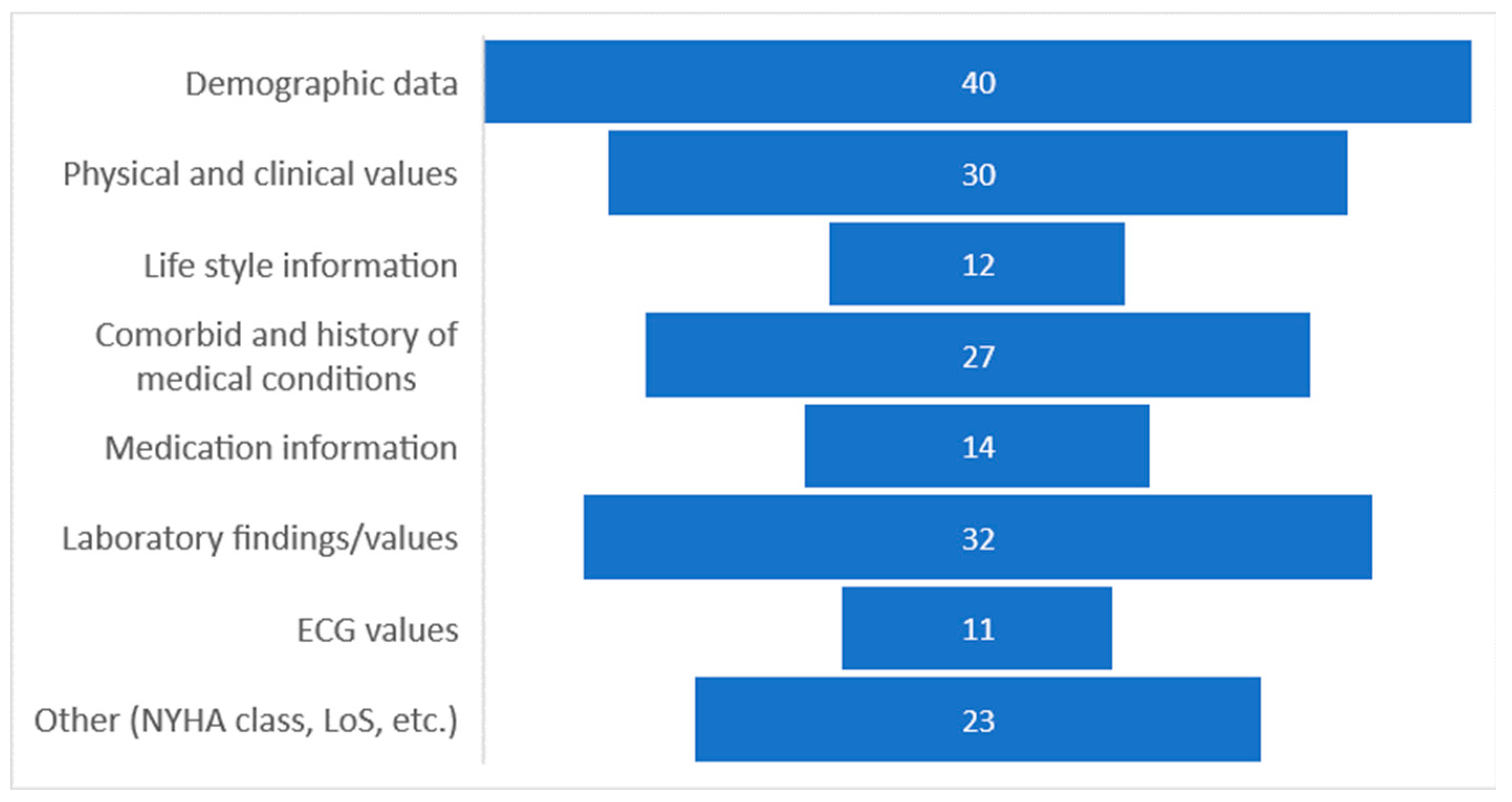
4.3. Data Specification Used in Building Predictive Models
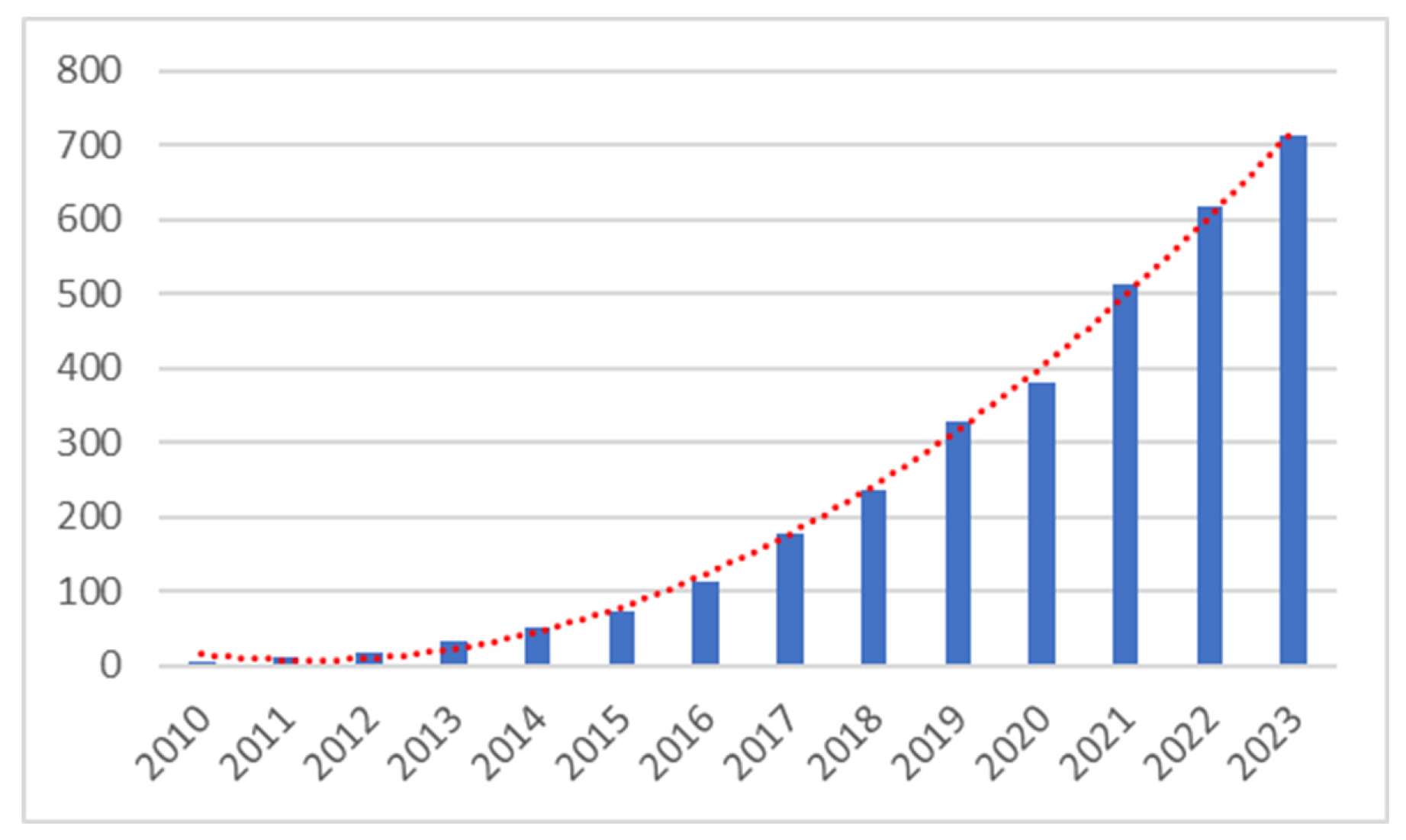
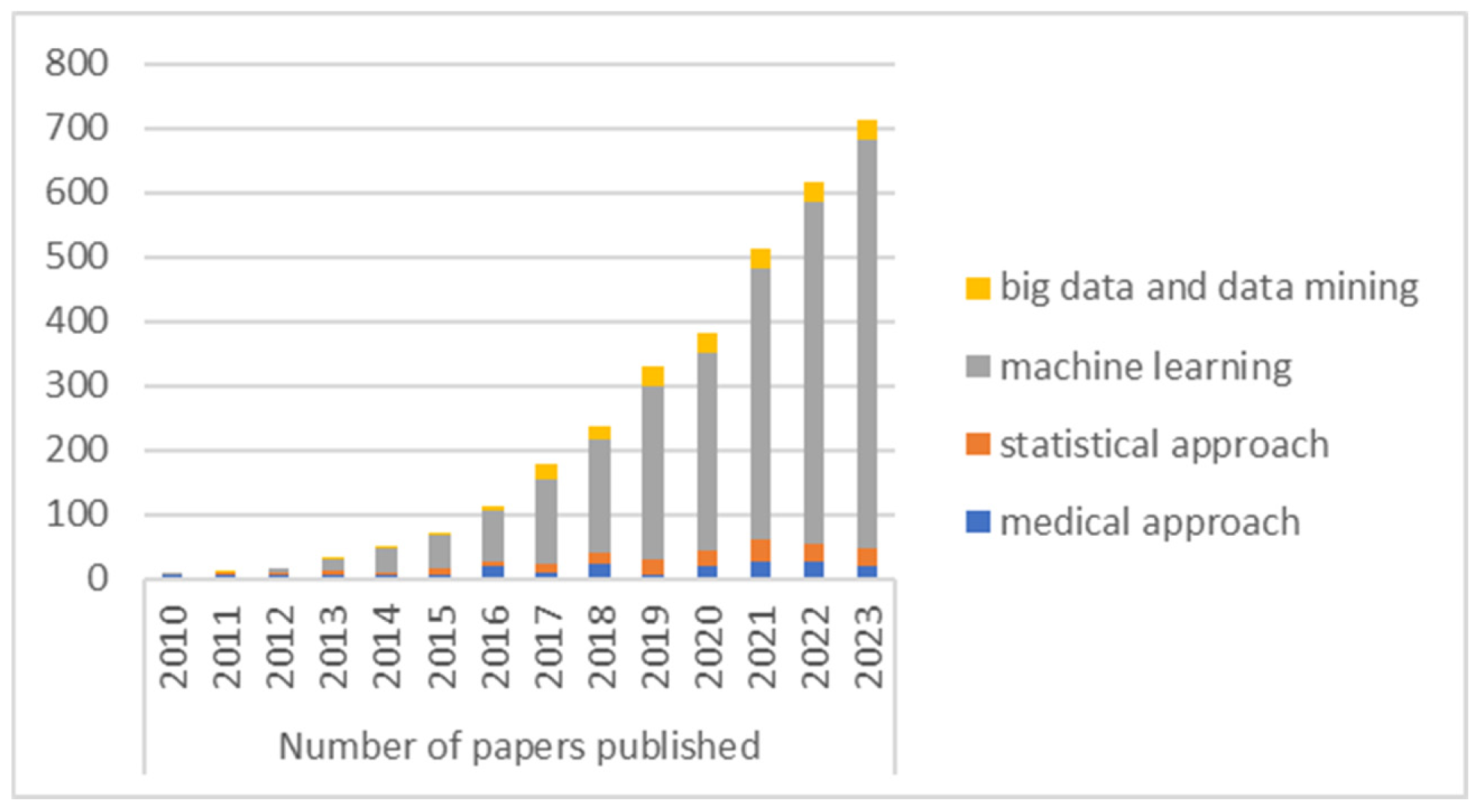
4.4. Publication by Year
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Association, A.H. What Is Heart Failure?. Available online: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure.
- Ponikowski, P.; Anker, S.D.; AlHabib, K.F.; Cowie, M.R.; Force, T.L.; Hu, S.; Jaarsma, T.; Krum, H.; Rastogi, V.; Rohde, L.E.; et al. Heart Failure: Preventing Disease and Death Worldwide. ESC Heart Failure 2014, 1, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N Engl J Med 2009, 360, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hao, Y.; Hwang, K.; Wang, L.; Wang, L. Disease Prediction by Machine Learning Over Big Data From Healthcare Communities. IEEE Access 2017, 5, 8869–8879. [Google Scholar] [CrossRef]
- Guidi, G.; Pettenati, M.C.; Melillo, P.; Iadanza, E. A Machine Learning System to Improve Heart Failure Patient Assistance. IEEE J. Biomed. Health Inform. 2014, 18, 1750–1756. [Google Scholar] [CrossRef]
- Yajuan Wang; Ng, K.; Byrd, R.J.; Jianying Hu; Ebadollahi, S.; Daar, Z.; deFilippi, C.; Steinhubl, S.R.; Stewart, W.F. Early Detection of Heart Failure with Varying Prediction Windows by Structured and Unstructured Data in Electronic Health Records. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); IEEE: Milan, August 2015; pp. 2530–2533. [CrossRef]
- Ng, K.; Steinhubl, S.R.; deFilippi, C.; Dey, S.; Stewart, W.F. Early Detection of Heart Failure Using Electronic Health Records: Practical Implications for Time Before Diagnosis, Data Diversity, Data Quantity, and Data Density. Circ: Cardiovascular Quality and Outcomes 2016, 9, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Rammal, H.F.; Z., A. Heart Failure Prediction Models Using Big Data Techniques. ijacsa 2018, 9. [CrossRef]
- Nagrecha, S.; Thomas, P.B.; Feldman, K.; Chawla, N.V. Predicting Chronic Heart Failure Using Diagnoses Graphs. In Machine Learning and Knowledge Extraction; Holzinger, A., Kieseberg, P., Tjoa, A.M., Weippl, E., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, 2017; Vol. 10410, pp. 295–312. [CrossRef]
- Krittayaphong, R.; Chichareon, P.; Komoltri, C.; Sairat, P.; Lip, G.Y.H. Predicting Heart Failure in Patients with Atrial Fibrillation:A Report from the Prospective COOL-AF Registry. Journal of clinical medicine 2023, 12, 4, 1265. [Google Scholar] [CrossRef]
- Austin, P.C.; Tu, J.V.; Ho, J.E.; Levy, D.; Lee, D.S. Using Methods from the Data-Mining and Machine-Learning Literature for Disease Classification and Prediction: A Case Study Examining Classification of Heart Failure Subtypes. Journal of Clinical Epidemiology 2013, 66, 398–407. [Google Scholar] [CrossRef]
- Blecker, S.; Sontag, D.; Horwitz, L.I.; Kuperman, G.; Park, H.; Reyentovich, A.; Katz, S.D. Early Identification of Patients With Acute Decompensated Heart Failure. Journal of Cardiac Failure 2018, 24, 357–362. [Google Scholar] [CrossRef]
- Plati, D.K.; Tripoliti, E.E.; Bechlioulis, A.; Rammos, A.; Dimou, I.; Lakkas, L.; Watson, C.; McDonald, K.; Ledwidge, M.; Pharithi, R.; et al. A Machine Learning Approach for Chronic Heart Failure Diagnosis. Diagnostics 2021, 11, 1863. [Google Scholar] [CrossRef]
- Wang, K.; Tian, J.; Zheng, C.; Yang, H.; Ren, J.; Li, C.; Han, Q.; Zhang, Y. Improving Risk Identification of Adverse Outcomes in Chronic Heart Failure Using SMOTE+ENN and Machine Learning. RMHP 2021, Volume 14, 2453–2463. [Google Scholar] [CrossRef]
- Quesada, J.A.; Lopez-Pineda, A.; Gil-Guillén, V.F.; Durazo-Arvizu, R.; Orozco-Beltrán, D.; López-Domenech, A.; Carratalá-Munuera, C. Machine Learning to Predict Cardiovascular Risk. Int J Clin Pract 2019, 73. [Google Scholar] [CrossRef]
- Kolukula, N.R.; Pothineni, P.N.; Chinta, V.M.K.; Boppana, V.G.; Kalapala, R.P.; Duvvi, S. Predictive Analytics of Heart Disease Presence with Feature Importance Based on Machine Learning Algorithms. IJEECS 2023, 32, 1070. [Google Scholar] [CrossRef]
- Sornsuwit, P.; Jundahuadong, P.; Pongsakornrungsilp, S. A New Efficiency Improvement of Ensemble Learning for Heart Failure Classification by Least Error Boosting. Emerg Sci J 2022, 7, 135–146. [Google Scholar] [CrossRef]
- Ahmed, S.; Shaikh, S.; Ikram, F.; Fayaz, M.; Alwageed, H.S.; Khan, F.; Jaskani, F.H. Prediction of Cardiovascular Disease on Self-Augmented Datasets of Heart Patients Using Multiple Machine Learning Models. Journal of Sensors 2022, 2022, 1–21. [Google Scholar] [CrossRef]
- Praveena Rachel Kamala, S.; Gayathri, S.; Pillai, N.M.; Anto Gracious, L.A.; Varun, C.M.; Siva Subramanian, R. Predictive Analytics for Heart Disease Detection: A Machine Learning Approach. In Proceedings of the 2023 4th International Conference on Electronics and Sustainable Communication Systems (ICESC); IEEE: Coimbatore, India, July 6 2023; pp. 1583–1589. [CrossRef]
- Alotaibi, F.S. Implementation of Machine Learning Model to Predict Heart Failure Disease. IJACSA 2019, 10. [Google Scholar] [CrossRef]
- Mamun, M.; Farjana, A.; Mamun, M.A.; Ahammed, M.S.; Rahman, M.M. Heart Failure Survival Prediction Using Machine Learning Algorithm: Am I Safe from Heart Failure? In Proceedings of the 2022 IEEE World AI IoT Congress (AIIoT); IEEE: Seattle, WA, USA, June 6 2022; pp. 194–200. [CrossRef]
- Nishat, M.M.; Faisal, F.; Ratul, I.J.; Al-Monsur, A.; Ar-Rafi, A.M.; Nasrullah, S.M.; Reza, T.; Khan, R.H. A Comprehensive Investigation of the Performances of Different Machine Learning Classifiers with SMOTE-ENN Oversampling Technique and Hyperparameter Optimization for Imbalanced Heart Failure Dataset. Scientific Programming, 2022, 1-17. [CrossRef]
- Senan, E.M.; Abunadi, I.; Jadhav, M.E.; Fati, S.M. Score and Correlation Coefficient-Based Feature Selection for Predicting Heart Failure Diagnosis by Using Machine Learning Algorithms. Computational and Mathematical Methods in Medicine 2021, 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Al-Yarimi, F.A.M.; Munassar, N.M.A.; Bamashmos, M.H.M.; Ali, M.Y.S. Feature Optimization by Discrete Weights for Heart Disease Prediction Using Supervised Learning. Soft Comput 2021, 25, 1821–1831. [Google Scholar] [CrossRef]
- Bharti, R.; Khamparia, A.; Shabaz, M.; Dhiman, G.; Pande, S.; Singh, P. Prediction of Heart Disease Using a Combination of Machine Learning and Deep Learning. Computational Intelligence and Neuroscience 2021, 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kanagarathinam, K.; Sankaran, D.; Manikandan, R. Machine Learning-Based Risk Prediction Model for Cardiovascular Disease Using a Hybrid Dataset. Data & Knowledge Engineering 2022, 140, 102042. [Google Scholar] [CrossRef]
- Venkatesh, R.; Balasubramanian, C.; Kaliappan, M. Development of Big Data Predictive Analytics Model for Disease Prediction Using Machine Learning Technique. J Med Syst 2019, 43, 272. [Google Scholar] [CrossRef] [PubMed]
- Alsubai, S.; Alqahtani, A.; Binbusayyis, A.; Sha, M.; Gumaei, A.; Wang, S. Heart Failure Detection Using Instance Quantum Circuit Approach and Traditional Predictive Analysis. Mathematics 2023, 11, 1467. [Google Scholar] [CrossRef]
- Botros, J.; Mourad-Chehade, F.; Laplanche, D. CNN and SVM-Based Models for the Detection of Heart Failure Using Electrocardiogram Signals. Sensors 2022, 22, 9190. [Google Scholar] [CrossRef] [PubMed]
- Alsinglawi, B.; Alnajjar, F.; Mubin, O.; Novoa, M.; Alorjani, M.; Karajeh, O.; Darwish, O. Predicting Length of Stay for Cardiovascular Hospitalizations in the Intensive Care Unit: Machine Learning Approach. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC); IEEE: Montreal, QC, Canada, July 2020; pp. 5442–5445. [CrossRef]
- Shameer, K.; Johnson, K.W.; Yahi, A.; Miotto, R.; Li, L.; Ricks, D.; Jebakaran, J.; Kovatch, P.; Sengupta, P.P.; Gelijns, S.; et al. PREDICTIVE MODELING OF HOSPITAL READMISSION RATES USING ELECTRONIC MEDICAL RECORD-WIDE MACHINE LEARNING: A CASE-STUDY USING MOUNT SINAI HEART FAILURE COHORT. In Proceedings of the Biocomputing 2017; WORLD SCIENTIFIC: Kohala Coast, Hawaii, USA, January 2017; pp. 276–287. [CrossRef]
- Bat-Erdene, B.-I.; Zheng, H.; Son, S.H.; Lee, J.Y. Deep Learning-Based Prediction of Heart Failure Rehospitalization during 6, 12, 24-Month Follow-Ups in Patients with Acute Myocardial Infarction. Health Informatics J 2022, 28, 146045822211015. [Google Scholar] [CrossRef]
- Rizinde, T.; Ngaruye, I.; Cahill, N.D. Comparing Machine Learning Classifiers for Predicting Hospital Readmission of Heart Failure Patients in Rwanda. JPM 2023, 13, 1393. [Google Scholar] [CrossRef] [PubMed]
- Landicho, J.A.; Esichaikul, V.; Sasil, R.M. Comparison of Predictive Models for Hospital Readmission of Heart Failure Patients with Cost-Sensitive Approach. International Journal of Healthcare Management 2021, 14, 1536–1541. [Google Scholar] [CrossRef]
- Sohrabi, B.; Vanani, I.R.; Gooyavar, A.; Naderi, N. Predicting the Readmission of Heart Failure Patients through Data Analytics. J. Info. Know. Mgmt. 2019, 18, 1950012. [Google Scholar] [CrossRef]
- AbdelRahman, S.E.; Zhang, M.; Bray, B.E.; Kawamoto, K. A Three-Step Approach for the Derivation and Validation of High-Performing Predictive Models Using an Operational Dataset: Congestive Heart Failure Readmission Case Study. BMC Med Inform Decis Mak 2014, 14, 41. [Google Scholar] [CrossRef]
- Vedomske, M.A.; Brown, D.E.; Harrison, J.H. Random Forests on Ubiquitous Data for Heart Failure 30-Day Readmissions Prediction. In Proceedings of the 2013 12th International Conference on Machine Learning and Applications; IEEE: Miami, FL, December 2013; pp. 415–421. [CrossRef]
- Hilbert, J.P.; Zasadil, S.; Keyser, D.J.; Peele, P.B. Using Decision Trees to Manage Hospital Readmission Risk for Acute Myocardial Infarction, Heart Failure, and Pneumonia. Appl Health Econ Health Policy 2014, 12, 573–585. [Google Scholar] [CrossRef]
- Zolbanin, H.M.; Delen, D. Processing Electronic Medical Records to Improve Predictive Analytics Outcomes for Hospital Readmissions. Decision Support Systems 2018, 112, 98–110. [Google Scholar] [CrossRef]
- Golas, S.B.; Shibahara, T.; Agboola, S.; Otaki, H.; Sato, J.; Nakae, T.; Hisamitsu, T.; Kojima, G.; Felsted, J.; Kakarmath, S.; et al. A Machine Learning Model to Predict the Risk of 30-Day Readmissions in Patients with Heart Failure: A Retrospective Analysis of Electronic Medical Records Data. BMC Med Inform Decis Mak 2018, 18, 44. [Google Scholar] [CrossRef]
- Mortazavi, B.J.; Downing, N.S.; Bucholz, E.M.; Dharmarajan, K.; Manhapra, A.; Li, S.-X.; Negahban, S.N.; Krumholz, H.M. Analysis of Machine Learning Techniques for Heart Failure Readmissions. Circ: Cardiovascular Quality and Outcomes 2016, 9, 629–640. [Google Scholar] [CrossRef]
- Lorenzoni, G.; Sabato, S.S.; Lanera, C.; Bottigliengo, D.; Minto, C.; Ocagli, H.; De Paolis, P.; Gregori, D.; Iliceto, S.; Pisanò, F. Comparison of Machine Learning Techniques for Prediction of Hospitalization in Heart Failure Patients. JCM 2019, 8, 1298. [Google Scholar] [CrossRef]
- Sundararaman, A.; Valady Ramanathan, S.; Thati, R. Novel Approach to Predict Hospital Readmissions Using Feature Selection from Unstructured Data with Class Imbalance. Big Data Research 2018, 13, 65–75. [Google Scholar] [CrossRef]
- Liu, X.; Chen, Y.; Bae, J.; Li, H.; Johnston, J.; Sanger, T. Predicting Heart Failure Readmission from Clinical Notes Using Deep Learning. In Proceedings of the 2019 IEEE International Conference on Bioinformatics and Biomedicine (BIBM); IEEE: San Diego, CA, USA, November 2019; pp. 2642–2648. [CrossRef]
- Sharma, V.; Kulkarni, V.; McAlister, F.; Eurich, D.; Keshwani, S.; Simpson, S. H.; Voaklander, D.; Samanani, S. Predicting 30-Day Readmissions in Patients With Heart Failure Using Administrative Data: A Machine Learning Approach. Journal of Cardiac Failure 2022, 28, 5, 710–722. [Google Scholar] [CrossRef]
- Shams, I.; Ajorlou, S.; Yang, K. A Predictive Analytics Approach to Reducing 30-Day Avoidable Readmissions among Patients with Heart Failure, Acute Myocardial Infarction, Pneumonia, or COPD. Health Care Manag Sci 2015, 18, 19–34. [Google Scholar] [CrossRef]
- Ben-Assuli, O.; Heart, T.; Klempfner, R.; Padman, R. Human-Machine Collaboration for Feature Selection and Integration to Improve Congestive Heart Failure Risk Prediction. Decision Support Systems 2023, 172, 113982. [Google Scholar] [CrossRef]
- Jing, L.; Ulloa Cerna, A.E.; Good, C.W.; Sauers, N.M.; Schneider, G.; Hartzel, D.N.; Leader, J.B.; Kirchner, H.L.; Hu, Y.; Riviello, D.M.; et al. A Machine Learning Approach to Management of Heart Failure Populations. JACC: Heart Failure 2020, 8, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Kamio, T.; Ikegami, M.; Machida, Y.; Uemura, T.; Chino, N.; Iwagami, M. Machine Learning-Based Prognostic Modeling of Patients with Acute Heart Failure Receiving Furosemide in Intensive Care Units. DIGITAL HEALTH 2023, 9, 20552076231194933. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.D.; Voors, A.A.; Klein, L.; Macheret, F.; Braun, O.O.; Urey, M.A.; Zhu, W.; Sama, I.; Tadel, M.; Campagnari, C.; et al. Improving Risk Prediction in Heart Failure Using Machine Learning. European J of Heart Fail 2020, 22, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lagu, T.; Pekow, P.S.; Shieh, M.-S.; Stefan, M.; Pack, Q.R.; Kashef, M.A.; Atreya, A.R.; Valania, G.; Slawsky, M.T.; Lindenauer, P.K. Validation and Comparison of Seven Mortality Prediction Models for Hospitalized Patients With Acute Decompensated Heart Failure. Circ: Heart Failure 2016, 9, e002912. [Google Scholar] [CrossRef] [PubMed]
- Panahiazar, M.; Taslimitehrani, V.; Pereira, N.; Pathak, J. Using EHRs and Machine Learning for Heart Failure Survival Analysis. Studies in health technology 2015, 2016, 40–44. [Google Scholar] [CrossRef]
- Almazroi, A.A. Survival Prediction among Heart Patients Using Machine Learning Techniques. MBE 2022, 19, 134–145. [Google Scholar] [CrossRef]
- Özbay Karakuş, M.; Er, O. A Comparative Study on Prediction of Survival Event of Heart Failure Patients Using Machine Learning Algorithms. Neural Comput & Applic 2022, 34, 13895–13908. [Google Scholar] [CrossRef]
- Zaman, S.M.M.; Qureshi, W.M.; Raihan, M.S.; Shams, A.B.; Sultana, S. Survival Prediction of Heart Failure Patients Using Stacked Ensemble Machine Learning Algorithm. In 2021 IEEE International Women in Engineering (WIE) Conference on Electrical and Computer Engineering. IEEE: Dhaka, Bangladesh, 19 July 2022; pp. 117-120. 19 July. [CrossRef]
- Newaz, A.; Ahmed, N.; Shahriyar Haq, F. Survival Prediction of Heart Failure Patients Using Machine Learning Techniques. Informatics in Medicine Unlocked 2021, 26, 100772. [Google Scholar] [CrossRef]
- Kedia, S.; Bhushan, M. Prediction of Mortality from Heart Failure Using Machine Learning. In Proceedings of the 2022 2nd International Conference on Emerging Frontiers in Electrical and Electronic Technologies (ICEFEET); IEEE: Patna, India, June 24 2022; pp. 1–6. [CrossRef]
- Chicco, D.; Jurman, G. Machine Learning Can Predict Survival of Patients with Heart Failure from Serum Creatinine and Ejection Fraction Alone. BMC Med Inform Decis Mak 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xin, H.; Zhang, J.; Fu, M.; Zhou, J.; Lian, Z. Prediction Model of In--hospital Mortality in Intensive Care Unit Patients with Heart Failure: Machine Learning-- Based, Retrospective Analysis of the MIMIC- III Database. BMJ open 2021, 11, 7. [Google Scholar] [CrossRef]
- Luo, C.; Zhu, Y.; Zhu, Z.; Li, R.; Chen, G.; Wang, Z. A Machine Learning-Based Risk Stratification Tool for in-Hospital Mortality of Intensive Care Unit Patients with Heart Failure. J Transl Med 2022, 20, 136. [Google Scholar] [CrossRef]
- Chen, Z.; Li, T.; Guo, S.; Zeng, D.; Wang, K. Machine Learning-Based in-Hospital Mortality Risk Prediction Tool for Intensive Care Unit Patients with Heart Failure. Frontiers in Cardiovascular Medicine 2023, 10, 1119699. [Google Scholar] [CrossRef]
- Awan, S.E.; Bennamoun, M.; Sohel, F.; Sanfilippo, F.M.; Dwivedi, G. Machine Learning-based Prediction of Heart Failure Readmission or Death: Implications of Choosing the Right Model and the Right Metrics. ESC Heart Failure 2019, 6, 428–435. [Google Scholar] [CrossRef]
- Awan, S.E.; Bennamoun, M.; Sohel, F.; Sanfilippo, F.M.; Chow, B.J.; Dwivedi, G. Feature Selection and Transformation by Machine Learning Reduce Variable Numbers and Improve Prediction for Heart Failure Readmission or Death. PLoS ONE 2019, 14, e0218760. [Google Scholar] [CrossRef] [PubMed]
- Sarijaloo, F.; Park, J.; Zhong, X.; Wokhlu, A. Predicting 90 DAY Acute Heart Failure Readmission and Death Using Machine LEARNING-SUPPORTED Decision Analysis. Clinical Cardiology 2021, 44, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Yan, J.; Han, G.; Du, Y.; Hu, X.; He, Z.; Han, Q.; Zhang, Y. Machine Learning Prognosis Model Based on Patient-Reported Outcomes for Chronic Heart Failure Patients after Discharge. Health Qual Life Outcomes 2023, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, X.; Wang, B.; Wang, S.; Du, X.; Tan, Q.; Hao, Z.; Liu, Y.; Yan, J.; Xia, Y. Machine Learning–Driven Models to Predict Prognostic Outcomes in Patients Hospitalized With Heart Failure Using Electronic Health Records: Retrospective Study. J Med Internet Res 2021, 23, e24996. [Google Scholar] [CrossRef]
- Eapen, Z.J.; Liang, L.; Fonarow, G.C.; Heidenreich, P.A.; Curtis, L.H.; Peterson, E.D.; Hernandez, A.F. Validated, Electronic Health Record Deployable Prediction Models for Assessing Patient Risk of 30-Day Rehospitalization and Mortality in Older Heart Failure Patients. JACC: Heart Failure 2013, 1, 245–251. [Google Scholar] [CrossRef]
- Zhao, H.; Li, P.; Zhong, G.; Xie, K.; Zhou, H.; Ning, Y.; Xu, D.; Zeng, Q. Machine Learning Models in Heart Failure with Mildly Reduced Ejection Fraction Patients. Front. Cardiovasc. Med. 2022, 9, 1042139. [Google Scholar] [CrossRef]
- Beecy, A.N.; Gummalla, M.; Sholle, E.; Xu, Z.; Zhang, Y.; Michalak, K.; Dolan, K.; Hussain, Y.; Lee, B.C.; Zhang, Y.; et al. Utilizing Electronic Health Data and Machine Learning for the Prediction of 30-Day Unplanned Readmission or All-Cause Mortality in Heart Failure. Cardiovascular Digital Health Journal 2020, 1, 71–79. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





