1. Introduction
Since 4 May 2023, Coronavirus Disease 2019 (COVID-19) is an established and ongoing health issue which no longer constitutes a public health emergency of international concern. However, according to the World Health Organization (WHO), the next pandemic is a matter of ‘when’ not ‘if’, and therefore we need to treasure past experiences to be better prepared for the future, with the awareness of having new technological platforms at our disposal, such as that based on nucleoside-modified mRNA (modRNA) [
1].
It was 11 March 2020 when the WHO declared COVID-19 a pandemic; since then, there have been more than 750 million confirmed cases of COVID-19, including nearly 7 million deaths [
2]. It represents the most dramatic pandemic of the new millennium, the viral infection with the highest media coverage in history, and the human disease that has put a strain on health, social and political systems, globally. Although it had been expected for years by insiders after the past outbreak of the Severe Acute Respiratory Syndrome (SARS), the world was not ready to face COVID-19; however, thanks to an extraordinary research work, specific vaccines have been discovered in record time, and made available on a large scale in early 2021 [
1]. To date, more than 13 billion doses have been administered worldwide, of which almost 150 million in Italy [
2,
3].
Originally identified in the Chinese city of Wuhan, Italy was the first Western country to be hit hard by COVID-19, and to experience lockdown restrictions under a democratic framework, numbering 58.85 million people [
4]. Like all respiratory virosis, COVID-19 is influenced by environmental temperature and solar ultraviolet radiation; in fact, the virulence of the SARS Coronavirus 2 (SARS-CoV-2), its etiological agent, has been found maximum below 10°C and 40 kJ/m
2, respectively [
5,
6]. In Italy, these meteorological conditions are typical of the autumn-winter periods; therefore, we have focused our real-world nationwide study on these seasons.
Just over four years after that fateful 11 March 2020, let’s take stock of the COVID-19 pandemic in Italy during the autumn-winter of 2020, before mass vaccination but when the emergency machinery was fully operative in terms of tracing and swabs, comparing this period with the autumn-winter of 2021, i.e. after mass vaccination campaign; moreover, we here provide a retrospective analysis of the annual mortality by age groups and life stages in the years 2019 (pre-COVID-19), 2020 (before mass vaccination) and 2021 (after mass vaccination) among the whole Italian population, in order to evaluate the death burden attributable to COVID-19.
2. Materials and Methods
2.1. Data Collection
During the state of national health emergency (expired on 31 March 2022), the Civil Defense Department daily released the aggregate data coming from the Higher Institute of Health, the Ministry of Health, the Italian Regions (Lombardia, Lazio, Campania, Veneto, Sicilia, Emilia-Romagna, Piemonte, Puglia, Toscana, Calabria, Sardegna, Liguria, Marche, Abruzzo, Friuli-Venezia Giulia, Umbria, Basilicata, Molise, and Valle d’Aosta), and the Independent Provinces (Trento and Bolzano) in order to inform the population about the pandemic situation in Italy. Among these data, on a daily basis, there were the number of contagions, performed swabs, hospitalizations in Intensive Care Units (ICU), non-ICU patients, and deaths.
By means of a team effort, we have collected all these data and elaborated the respective graphs, comparing the COVID-19 pandemic in Italy during the autumn-winter of 2020 (before mass vaccination) with the autumn-winter of 2021 (after mass vaccination).
2.2. Data Mining
In addition, we have consulted the database of the National Institute of Statistics [
7], and extracted the total number of annual deaths in the years 2019 (pre-COVID-19), 2020 (before mass vaccination) and 2021 (after mass vaccination), broken down into gender and age groups by years (<4; 5-9; 10-14; 15-19; 20-24; 25-29; 30-34; 35-39; 40-44; 45-49; 50-54; 55-59; 60-64; 65-69; 70-74; 75-79; 80-84; 85-89; 90-94; >95), in order to evaluate the mortality burden attributable to COVID-19.
3. Results
3.1. COVID-19 Deaths
In the autumn-winter of 2020 there were 68934 deaths due to COVID-19, of which 33092 in autumn (22 September 2020 ‒ 20 December 2020) and 35842 in winter (21 December 2020 ‒ 20 March 2021), while in the autumn-winter of 2021 there were 27365 deaths, of which 5359 in autumn (22 September 2021 ‒ 20 December 2021) and 22006 in winter (21 December 2021 ‒ 20 March 2022) (
Table 1).
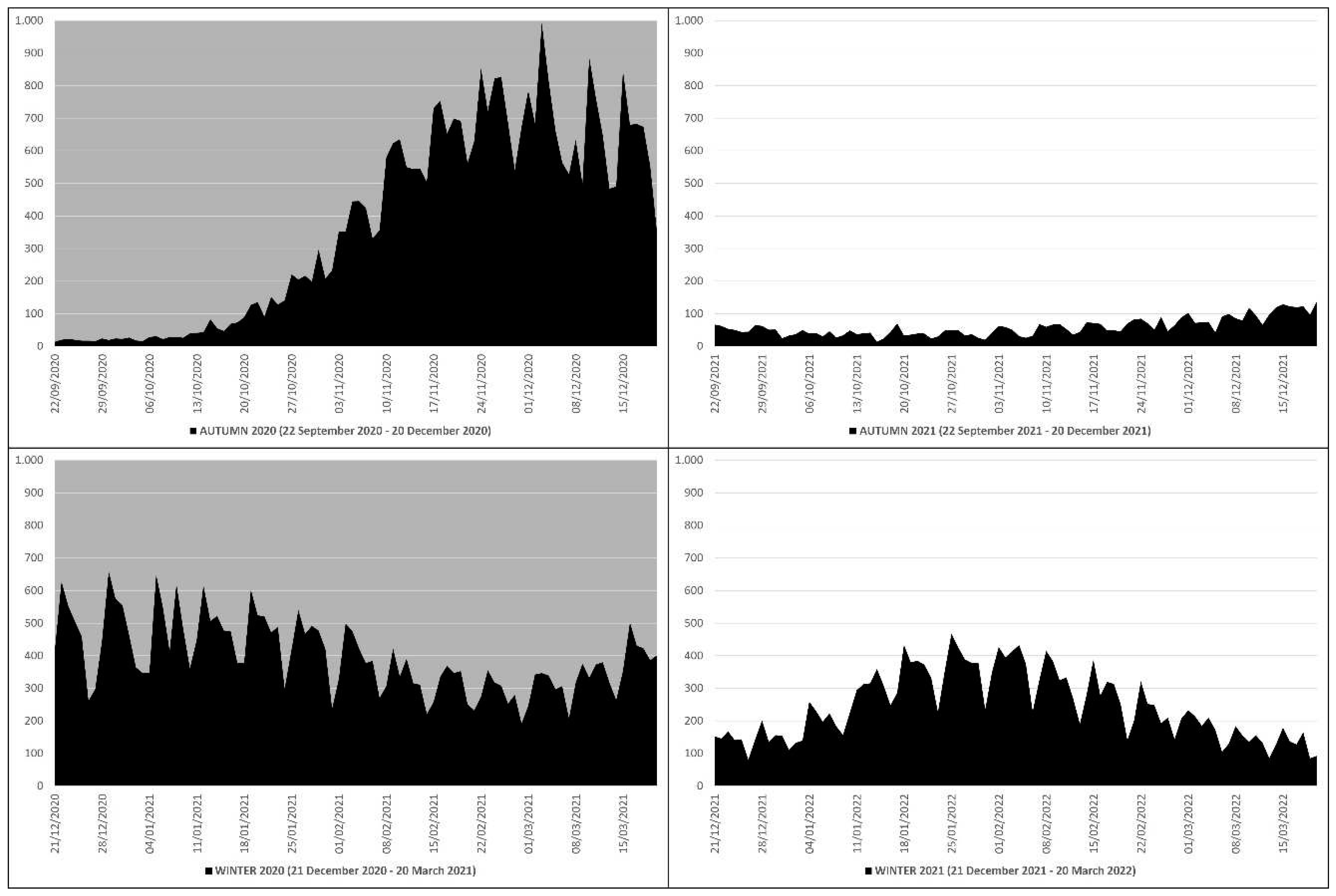
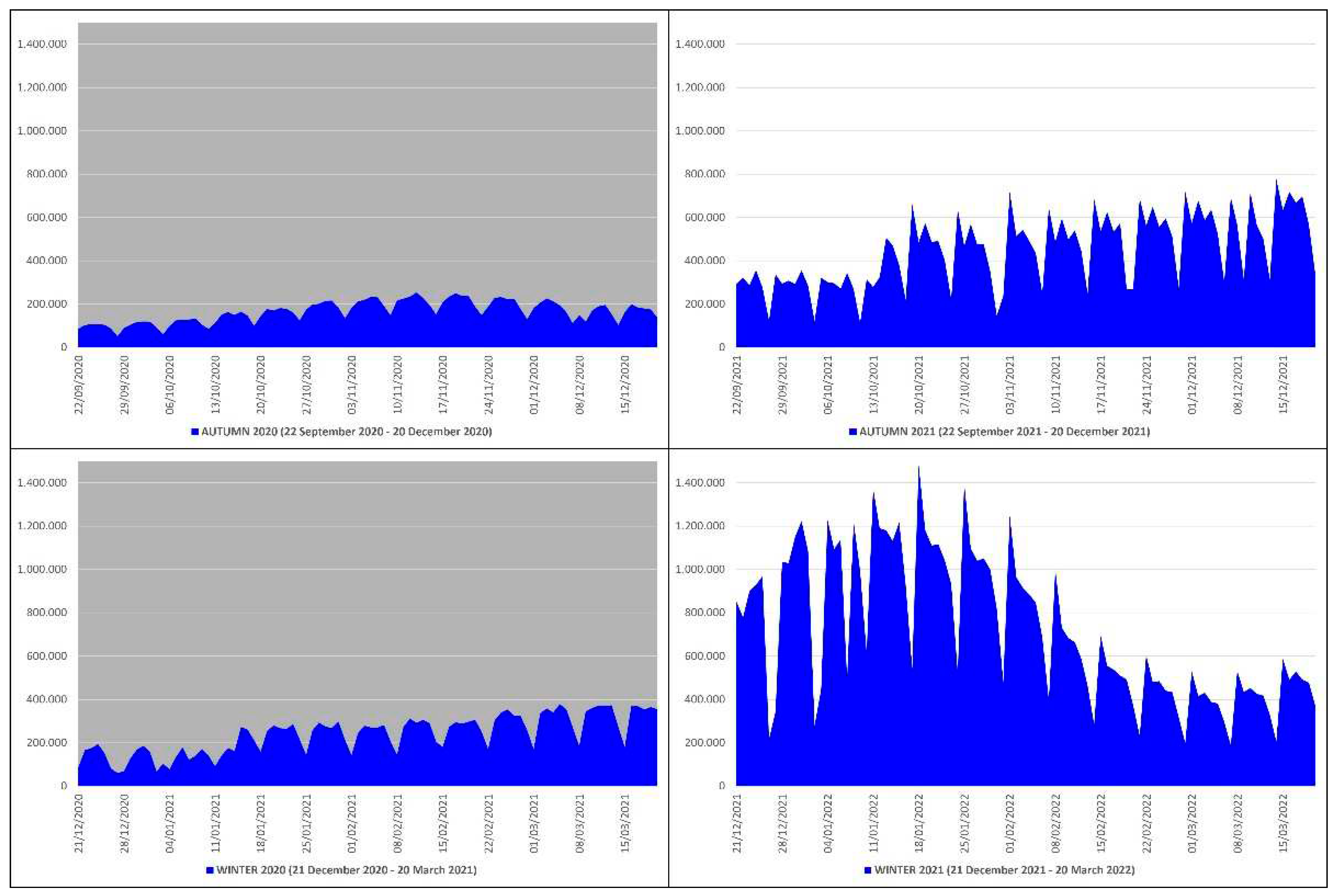
This means that in the autumn-winter of 2020 (before mass vaccination) there were 41569 more deaths than in the autumn-winter of 2021 (after mass vaccination), which equals ≈2.51 times more; in practice, from the autumn-winter of 2020 (before mass vaccination) to the autumn-winter of 2021 (after mass vaccination) there was a drop in deaths of ≈251%. The peak of deaths in the autumn-winter of 2020 (n. 993) occurred on 3 December 2020, while in the autumn-winter of 2021 (n. 468) on 25 January 2022 (
Figure 1).
3.2. COVID-19 ICU Hospitalizations
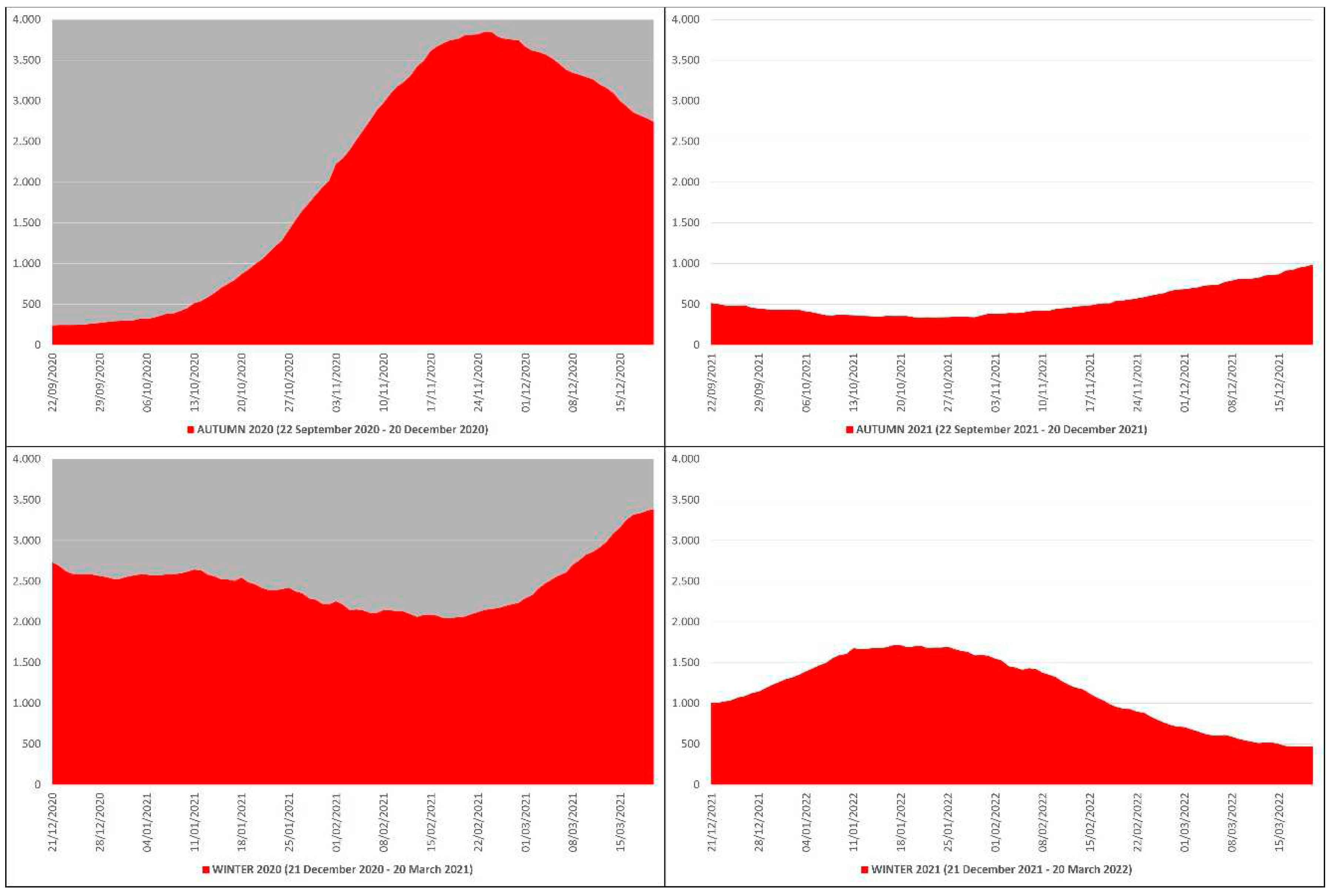
In the autumn-winter of 2020 the daily ICU hospitalizations due to COVID-19 averaged 2281, with an autumn average of 2091, a winter average of 2470 and a peak of 3848 ICU patients on 25 November 2020, while in the autumn-winter of 2021 the daily ICU hospitalizations averaged 843, with an autumn average of 522, a winter average of 1163 and a peak of 1717 ICU patients on 17 January 2022 (
Figure 2). This means that in the autumn-winter of 2020 (before mass vaccination) the daily ICU hospitalization rate was ≈224% higher than in the autumn-winter of 2021 (after mass vaccination); in practice, from the autumn-winter of 2020 (before mass vaccination) to the autumn-winter of 2021 (after mass vaccination) the rate fell ≈2.24 times.
3.3. COVID-19 Non-ICU Hospitalizations
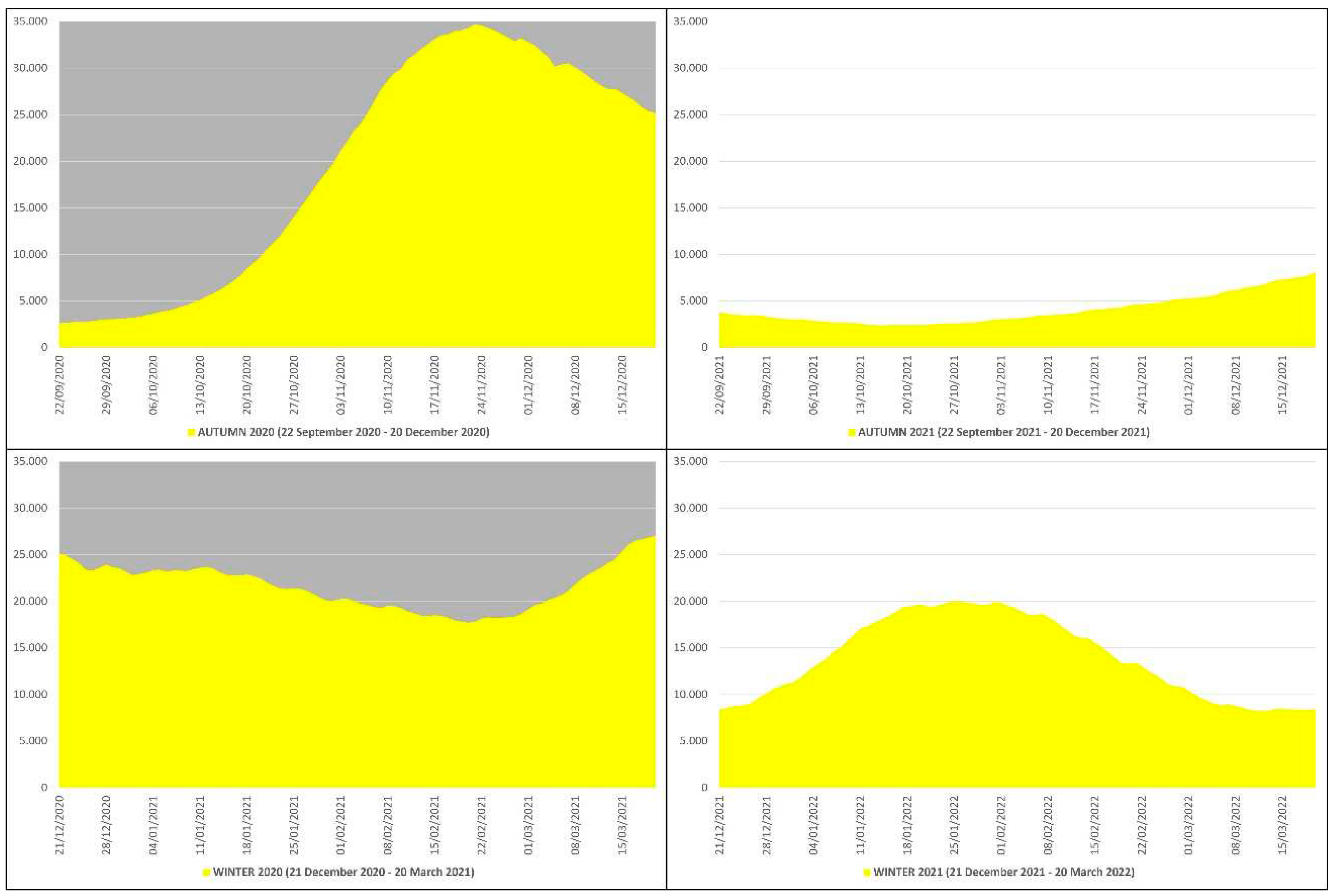
In the autumn-winter of 2020 the daily non-ICU hospitalizations due to COVID-19 averaged 20489, with an autumn average of 19357, a winter average of 21621 and a peak of 34697 non-ICU patients on 23 November 2020, while in the autumn-winter of 2021 the daily non-ICU hospitalizations averaged 8977, with an autumn average of 4024, a winter average of 13929 and a peak of 20027 non-ICU patients on 25 January 2022 (
Figure 3). This means that in the autumn-winter of 2020 (before mass vaccination) the daily non-ICU hospitalization rate was ≈228% higher than in the autumn-winter of 2021 (after mass vaccination); in practice, from the autumn-winter of 2020 (before mass vaccination) to the autumn-winter of 2021 (after mass vaccination) the rate fell ≈2.28 times.
3.4. COVID-19 Contagions
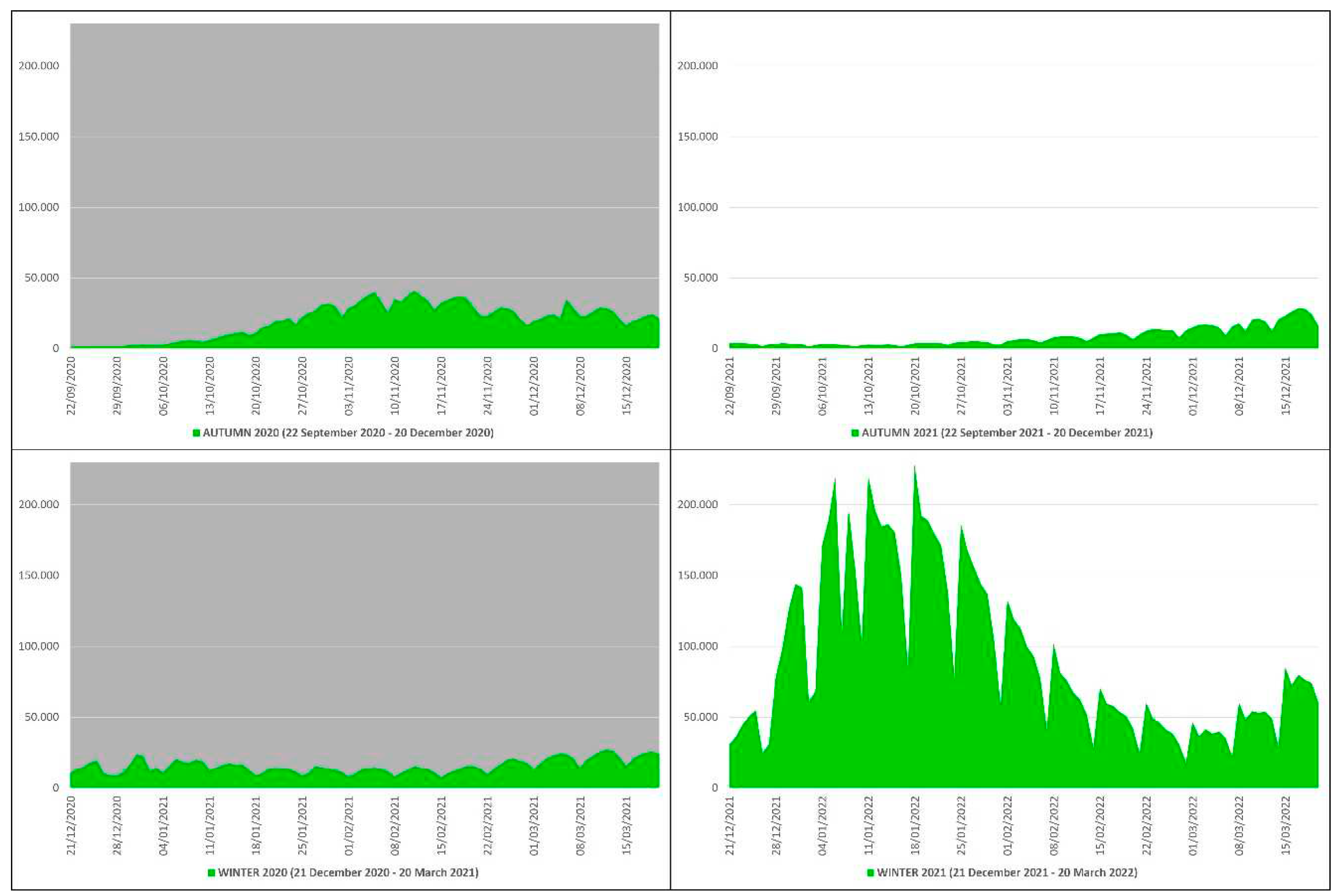
In the autumn-winter of 2020 the COVID-19 contagions were 3,179,094, of which 1,774,864 in autumn and 1,404,230 in winter, while in the autumn-winter of 2021 they were 9,068,535, of which 762,613 in autumn and 8,305,922 in winter (
Table 1). This means that from the autumn-winter of 2020 (before mass vaccination) to the autumn-winter of 2021 (after mass vaccination) the COVID-19 contagions increased by 5,889,441 subjects, an increase of ≈2.85 times equal to ≈285%. The peak of contagions in the autumn-winter of 2020 (n. 40902) occurred on 13 November 2020, while in the autumn-winter of 2021 (n. 228,179) on 18 January 2022 (
Figure 4).
3.5. COVID-19 Swab Tests
In the autumn-winter of 2020 the swabs performed to detect COVID-19 were 36,396,019, of which 14,878,410 in autumn and 21,517,609 in winter, while in the autumn-winter of 2021 they were 105,553,802, of which 40,618,200 in autumn and 64,935,602 in winter (
Table 1). This means that from the autumn-winter of 2020 (before mass vaccination) to the autumn-winter of 2021 (after mass vaccination) the swab tests increased by 69,157,783 units, an increase of ≈2.90 times equal to ≈290%. The peak of performed tests in the autumn-winter of 2020 (n. 378,463) occurred on 5 March 2021, while in the autumn-winter of 2021 (n. 1,481,349) on 18 January 2022 (
Figure 5).
3.6. Mortality Burden
By analyzing the mortality data in Italy, it has emerged that in 2019 (pre-COVID-19) 634,417 people died, of which 303,652 males and 330,764 females, while in 2020 (before mass vaccination) there were 740,317 deaths, 359,418 males and 380,899 females (
Table 2).
Therefore, from 2019 to 2020 there was an excess of mortality quantifiable in 105,900 more deaths attributable to COVID-19, as the only significant variable changed between the two years. This death burden represents the ≈14.3% of the total. If then apply the standardized age disaggregation groups recommended for data analysis by life stages [
8], 103,183 out of 105,900 deaths occurred in older adults (≥60 years), which is equivalent to ≈97.4%. In 2021 (after mass vaccination) there were 701,346 deaths, of which 340,210 males and 361,136 females (
Table 2). Comparing 2019 (pre-COVID-19) to 2021 (after mass vaccination) an excess of mortality is confirmed, quantifiable in 66929 more deaths, a value however lower than in 2020 thanks to mass vaccination; in fact, from 2020 to 2021 the only significant additional variable changed between the two pandemic years has been the mass vaccination campaign. Among adults (25-59 years) in 2019, 2020 and 2021 there were 42163, 45114 and 45531 deaths, respectively (
Table 2). As achievable, COVID-19 has caused a mortality excess of 2951 more deaths (2125 males and 826 females) from 2019 (pre-COVID-19) to 2020 (before mass vaccination) in this life stage, of which 2807 in over 50, i.e. ≈95.1%. Among young adults (20-24 years), older adolescents (15-19 years), young adolescents (10-14 years), older children (5-9 years), young children (1-4 years), post-neonatal infants (28-364 days), late neonates (7-27 days) and early neonates (0-6 days) in 2019, 2020 and 2021 there were 2962, 2728 and 2820 deaths, respectively (
Table 2). Therefore, in 2019 (pre-COVID-19) there were 238 and 142 more deaths than in the years 2020 and 2021, both impacted by COVID-19.
Surprisingly, the excess of mortality under the age of 60 continued to grow by 509 subjects (316 males and 193 females) from 2020 (before mass vaccination) to 2021, the unprecedented year of the mass vaccination campaign and the only significant additional variable changed between the two pandemic years. If we further restrict the field of analysis to subjects under the age of 40, it emerges that in 2019, 2020 and 2021 there were 7103, 6808 and 7165 deaths, respectively (
Table 2). Therefore, during 2020 (before mass vaccination) there were 295 fewer deaths (246 males and 49 females) than in 2019 (pre-COVID-19), equivalent to ≈4.2% less, while during 2021 (after mass vaccination) there were 357 more deaths (247 males and 110 females) than in 2020 (before mass vaccination) and 62 more deaths (1 male and 61 females) than in 2019 (pre-COVID-19), equivalent to ≈5.2% and ≈0.9% more.
4. Discussion
COVID-19 was first confirmed to have spread to Italy on 31 January 2020, when two Chinese tourists in Rome tested positive for the virus; one week later an Italian man, repatriated to Italy from the city of Wuhan, was hospitalized and ascertained as the third case. From that day Italy was overwhelmed by four waves of the pandemic [
9], and 2020 became the year with the highest number of deaths since 1945, when Italy was fighting in World War II on its soil. The four waves are timed as follows: first wave from February to May 2020; second wave from October to December 2020; third wave from January to May 2021; fourth wave from November 2021 to March 2022 [
9]. All the waves’ peaks coincide with the coldest seasons in Italy; in fact, SARS-CoV-2 virulence has been found maximum below 10°C and 40 kJ/m
2 [
6]. This behavior towards environmental temperature and solar ultraviolet radiation is quite similar to that of other viral respiratory infections, in primis influenza [
10]. Our study has therefore been focused on the coldest seasons of the year in Italy, i.e. the autumn-winter periods. The Italian government faced the aforementioned waves with gradually decreasing lockdown restrictions thanks to the progressive availability of masks, swabs, antivirals and above all, starting from the beginning of 2021, of new generation vaccines, such as adenovirus vector-based or modRNA vaccines [
1]. By virtue of this, health policies were adopted that strongly encouraged free mass vaccination, achieving a 90.25% coverage of the population over 12 with regards to the primary vaccination cycle [
11].
By comparing the trend of COVID-19 pandemic in Italy during the autumn-winter of 2020, which encompasses the second and third waves, to the autumn-winter of 2021, that includes the fourth wave, we have found that the contagions increased by ≈285%, however against a ≈290% increase in the swab tests. The absolute peak of contagions coincided with 18 January 2022, the very day on which the highest number of swabs was performed. The mean positivity rate therefore passed from ≈8.74% before mass vaccination to ≈8.59% after mass vaccination. All this means that, from one side, COVID-19 vaccines are not able to stop the contagions, but, from the other side, they have been able to reduce the mean positivity rate by ≈0.15% despite the progressive easing of lockdown, which remains the most effective health policy measure to block the contagions’ surge in an emergency phase when specific vaccines are not yet available, together with to wear mask indoors (preferably filtering face piece), to practice hand hygiene (with soap or hydroalcoholic solution), and to keep interpersonal social distancing (at least 1m).
Moreover, we have found that, from the autumn-winter of 2020 to the autumn-winter 2021, the mass vaccination campaign, without precedented in Italy as in the rest of the world, allowed a ≈251% abatement in COVID-19 deaths, and a reduction of ≈224% and ≈228% in daily ICU hospitalizations and daily non-ICU hospitalizations due to COVID-19, respectively. These results are impressive and definitively confirm the usefulness of anti-SARS-CoV-2 vaccination in reducing the number of deaths and hospitalizations on a large scale; this is reflected in a significant lowering of pressure on hospitals and, in particular, on ICU departments, thus safeguarding the health service in the public interest.
Going into the details of COVID-19 deaths, in 2020 there was a mortality excess of ≈14.3% quantifiable in 105,900 more deaths compared to 2019, the pre-COVID-19 year; 103,183 out of 105,900 deaths occurred in older adults, which is equivalent to ≈97.4%. Therefore, COVID-19 is confirmed as a potentially life-threating disease in older adults, who are the most at risk and vulnerable segment of population, given their immunosenescence. Immunosenescence is the gradual deterioration of the immune system, determined by natural age advancement, and represents a contributory factor to the increased frequency of mortality and morbidity among the elderly; both the host’s capacity to respond to infections and the development of long-term immune memory are affected by immunosenescence [
12]. Based on our research, from 2020 (before mass vaccination) to 2021 (after mass vaccination) the death burden in older adults is decreased by 39480 subjects, a gain in terms of life attributable to vaccine immunization, the only significant additional variable changed between the two pandemic years. Immunosenescence may also explain the mortality excess of 2807 more deaths from 2019 to 2020 found by us among adults over 50, the segment of population just below older adults. Another possible explanation lies in the concept of inflammaging, which represents a further risk factor for mortality and morbidity with advancing age [
13]. Unlike immunosenescence, inflammaging is a chronic low-grade inflammation in the absence of overt infection that occurs in aging, and may contribute to the clinical manifestations of other age-related pathologies; it is probably due to a loss of power of adaptive immunity and to a loss of control over systemic inflammation resulting in the release of pro-inflammatory cytokines, such as interleukin 6, the well-known molecule involved in those serious forms of COVID-19 characterized by «cytokine storm» [
13,
14,
15,
16].
Surprisingly, from our real-world study on a very large population, the picture has completely changed by varying the population segment taken into consideration. In fact, it is emerged that under the age of 40 in the years 2019, 2020 and 2021 there were 7103, 6808 and 7165 deaths, respectively. Therefore, during 2020 (before mass vaccination) there were 295 fewer deaths than in 2019 (pre-COVID-19), equivalent to ≈4.2% less, while during 2021 (after mass vaccination) there were 357 more deaths than in 2020 (before mass vaccination), equivalent to ≈5.2% more. COVID-19 is so confirmed as a potentially lethal illness in older adults, but not in young people; indeed, from our study results, the benefit-risk balance of COVID-19 vaccination steps down under the age of 40. Scientific proof of the possible complications, even fatal, in young people due to COVID-19 vaccination lies in the Vaccine-induced Immune Thrombotic Thrombocytopenia (VITT), a new blood clotting syndrome initially described in association with adenovirus vector-based COVID-19 vaccines [
17,
18,
19]. VITT is characterized by both arterial and venous thrombotic events also in unusual sites, such as cerebral venous sinus and splanchnic veins, accompanied by low platelets count (<150 ×10
3/mm
3), markedly elevated D-dimer (>4 times upper limit of normal) and positive Platelet Factor 4 (PF4) Heparin-Induced Thrombocytopenia (HIT) Enzyme-Linked Immuno-Sorbent Assay (ELISA), 4-42 days after vaccine administration; pulmonary embolism and disseminated intravascular coagulation may also occur [
20] Among the lab tests available today for the detection of pathological platelet-activating antibodies against PF4, that is to say VITT triggers, HIT ELISA is the one with the greatest validity and reliability, and with the most appropriate sensitivity, reaching around 95% [
20]. Although these adverse events are very rare, they exceeded what would be expected in the general population [
21].
5. Conclusions
In conclusion, supported by a real-world nationwide study on a population of 58.85 million people, just over four years after COVID-19 outbreak, we can now state that: I) COVID-19 is a potential life-threatening disease mainly in older adults, as they are more vulnerable because of inherent immunosenescence and inflammaging; II) vaccination is useful for reducing deaths and ICU/non-ICU hospitalizations due to COVID-19 in this segment of population, reducing at the same time the pressure on the hospitals in the public interest; III) COVID-19 vaccination shows a favorable benefit-risk balance in older adults, while this balance falls under the age of 40 from our study results; IV) subjects under the age of 40 should be therefore exempt from any mandatory vaccination campaign; V) an effective vaccination program against COVID-19 must be primarily aimed at people over 60 and at patients of any age with immune deficits, and secondly at people over 50; VI) in the event of a future coronavirus threat, lockdown remains the most effective measure of health policy to stop the contagions, when specific vaccines are not yet available, together with wearing mask indoors, practicing hand hygiene, and keeping interpersonal social distancing.
Author Contributions
Conceptualization, L.R.; methodology, L.R.; software, L.R., C.G. and G.B.; validation, S.C.; formal analysis, L.R., C.G. and G.B.; investigation, L.R., C.G. and G.B.; resources, C.G. and G.B.; data curation, L.R.; writing—original draft preparation, L.R.; writing—review and editing, L.R.; visualization, S.C.; supervision, L.R. and S.C.; project administration, L.R. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study; further requests for data should be addressed to the corresponding author.
Acknowledgments
The authors thank the Civil Defense Department of the Italian Republic, the Ministry of Health, the Higher Institute of Health, the National Institute of Statistics, the Independent Provinces (Bolzano and Trento), and all the Italian Regions (Abruzzo, Basilicata, Calabria, Campania, Emilia-Romagna, Friuli-Venezia Giulia, Lazio, Liguria, Lombardia, Marche, Molise, Piemonte, Puglia, Sardegna, Sicilia, Toscana, Umbria, Valle d’Aosta, and Veneto) for data sharing and press releases.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Roncati, L.; Corsi, L. Nucleoside-modified messenger RNA COVID-19 vaccine platform. J. Med. Virol. 2021, 93, 4054–4057. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 January 2024).
- Italian Government. Anti COVID-19 Vaccines Report. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 24 September 2023).
- Italian Institute of Statistics. Demographic Indicators—Year 2022. Available online: https://www.istat.it/it/files//2023/04/indicatori-anno-2022.pdf (accessed on 7 April 2023).
- Ma, Y.; Pei, S.; Shaman, J.; Dubrow, R.; Chen, K. Role of meteorological factors in the transmission of SARS-CoV-2 in the United States. Nat. Commun. 2021, 12, 3602. [Google Scholar] [CrossRef] [PubMed]
- Balboni, E.; Filippini, T.; Rothman, K.J.; Costanzini, S.; Bellino, S.; Pezzotti, P.; Brusaferro, S.; Ferrari, F.; Orsini, N.; Teggi, S.; et al. The influence of meteorological factors on COVID-19 spread in Italy during the first and second wave. Environ. Res. 2023, 228, 115796. [Google Scholar] [CrossRef] [PubMed]
- Italian Institute of Statistics. Deaths. Available online: http://dati.istat.it/Index.aspx?lang=en&SubSessionId=950b3a5b-2916-4ba0-975a-6d3cfc712ac5 (accessed on 28 August 2023).
- Diaz, T.; Strong, K.L.; Cao, B.; Guthold, R.; Moran, A.C.; Moller, A.B.; Requejo, J.; Sadana, R.; Thiyagarajan, J.A.; Adebayo, E.; et al. A call for standardised age-disaggregated health data. Lancet Healthy Longev. 2021, 2, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, M.; Melani, C. The COVID-19 pandemic spread on hospital admissions in the Bolzano Province (Italy): A descriptive study (February 2020-March 2022). Boll. Epidemiol. Naz. 2022, 3, 17–21. [Google Scholar] [CrossRef]
- Park, J.E.; Son, W.S.; Ryu, Y.; Choi, S.B.; Kwon, O.; Ahn, I. Effects of temperature, humidity, and diurnal temperature range on influenza incidence in a temperate region. Influenza Other Respir. Viruses 2020, 14, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Bartolacelli, G.; Galeazzi, C.; Caramaschi, S. Trends in the COVID-19 pandemic in Italy during the summers of 2020 (before mass vaccination), 2021 (after primary mass vaccination) and 2022 (after booster mass vaccination): A real-world nationwide study based on a population of 58.85 million people. Pathogens 2023, 12, 1376. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.S.; Bellantuono, I.; Lord, J.M.; Sapey, E.; Mannick, J.B.; Partridge, L.; Gordon, A.L.; Steves, C.J.; Witham, M.D. Tackling immunosenescence to improve COVID-19 outcomes and vaccine response in older adults. Lancet Healthy Longev. 2020, 1, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Kasler, H.; Verdin, E. How inflammaging diminishes adaptive immunity. Nat Aging 2021, 1, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Brábek, J.; Jakubek, M.; Vellieux, F.; Novotný, J.; Kolář, M.; Lacina, L.; Szabo, P.; Strnadová, K.; Rösel, D.; Dvořánková, B.; et al. Interleukin-6: molecule in the intersection of cancer, ageing and COVID-19. Int. J. Mol. Sci. 2020, 21, 7937. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Ligabue, G.; Fabbiani, L.; Malagoli, C.; Gallo, G.; Lusenti, B.; Nasillo, V.; Manenti, A.; Maiorana, A. Type 3 hypersensitivity in COVID-19 vasculitis. Clin. Immunol. 2020, 217, 108487. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Nasillo, V.; Lusenti, B.; Riva, G. Signals of Th2 immune response from COVID-19 patients requiring intensive care. Ann. Hematol. 2020, 99, 1419–1420. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Roncati, L.; Manenti, A.; Corsi, L. A three-case series of thrombotic deaths in patients over 50 with comorbidities temporally after modRNA COVID-19 vaccination. Pathogens 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. VAXZEVRIA/COVID-19 Vaccine AstraZeneca: Link between the Vaccine and the Occurrence of Thrombosis in Combination with Thrombocytopenia. Available online: https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-vaxzevria-previously-covid-19-vaccine-astrazeneca_en-0.pdf (accessed on 13 April 2021).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).