Submitted:
22 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Description of study site

2.2. Methods of Sampling and Laboratory Studies
3. Results
3.1. Physical and chemical properties of waters
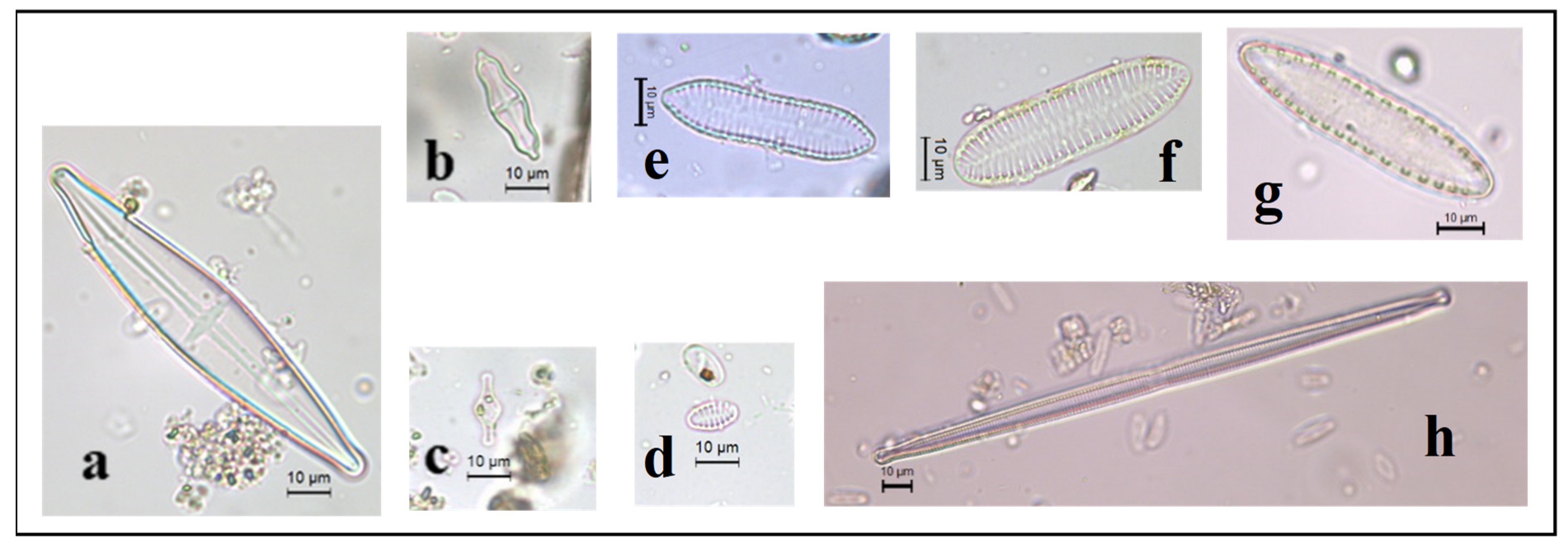
3.2. Floristic composition and diversity of diatoms
3.3. Bioindicators
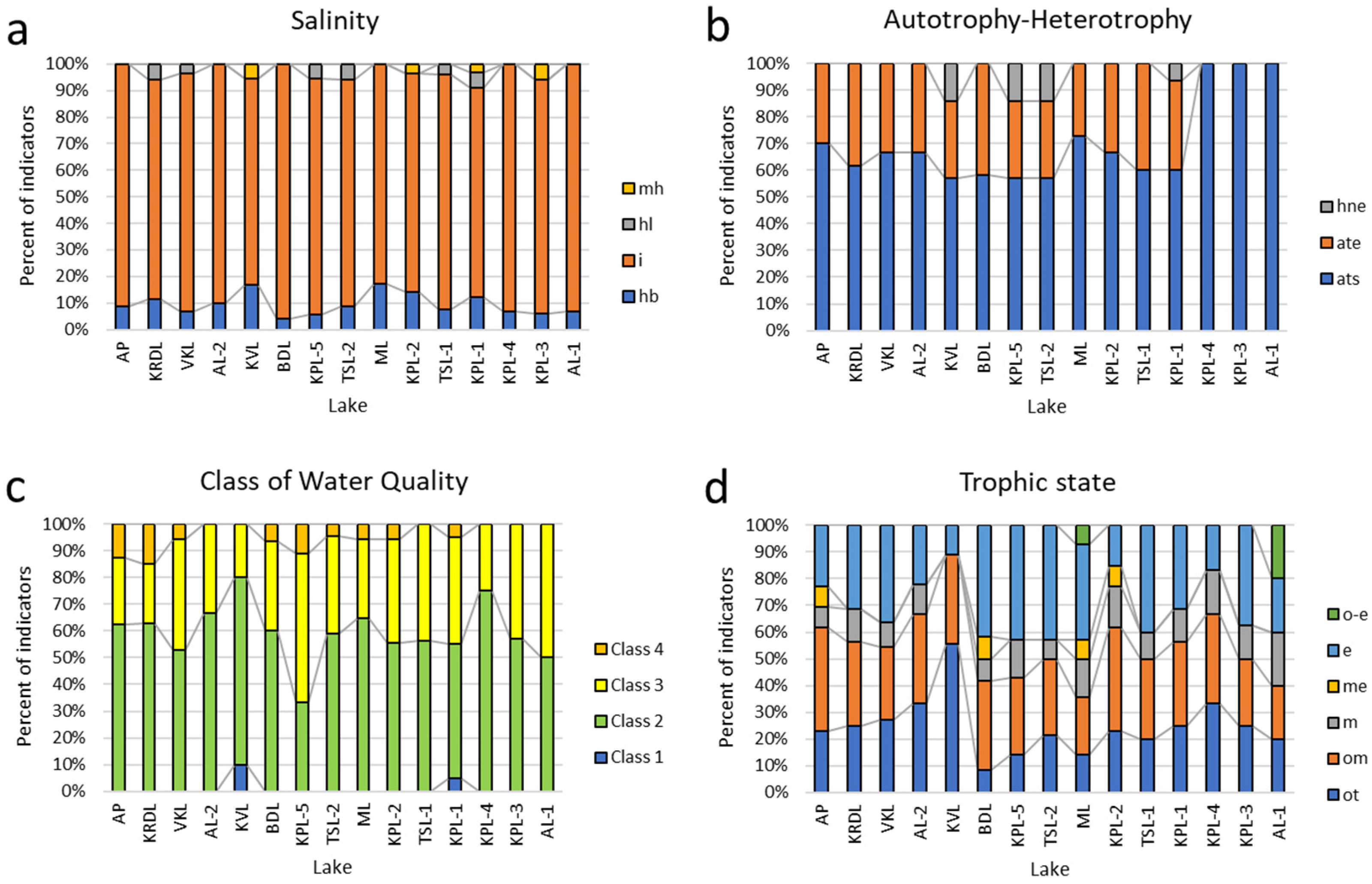
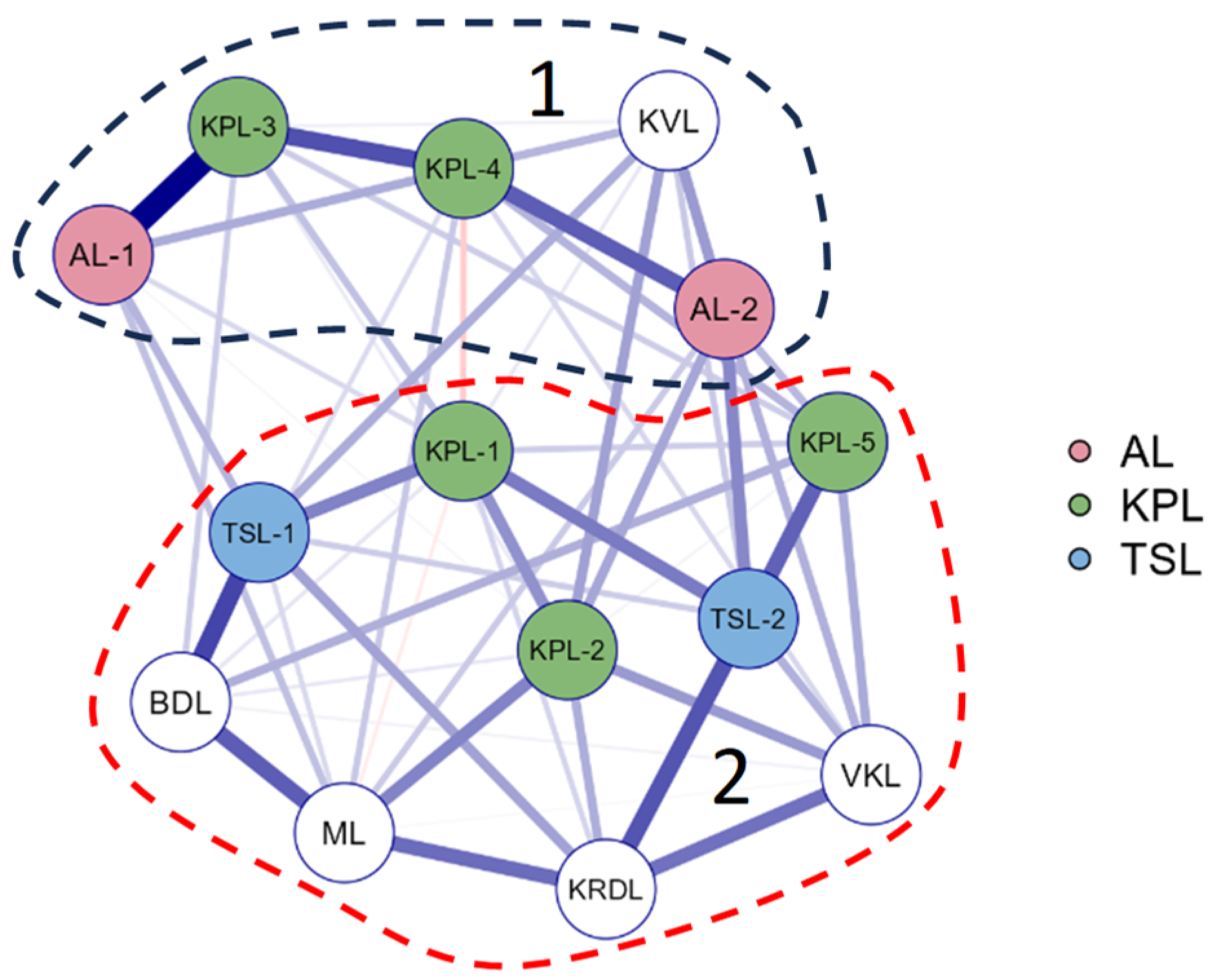
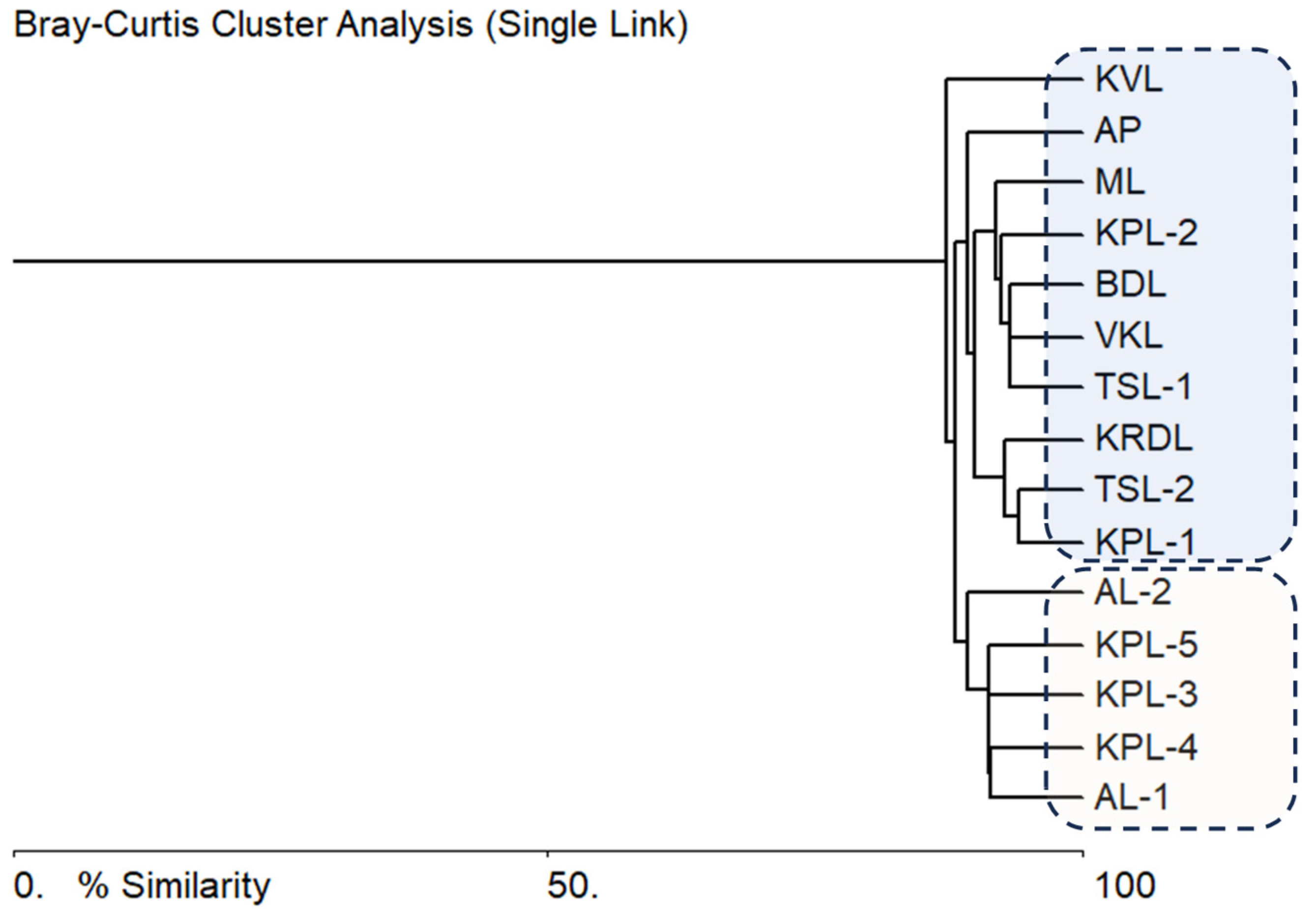
3.4. Statistical Analysis
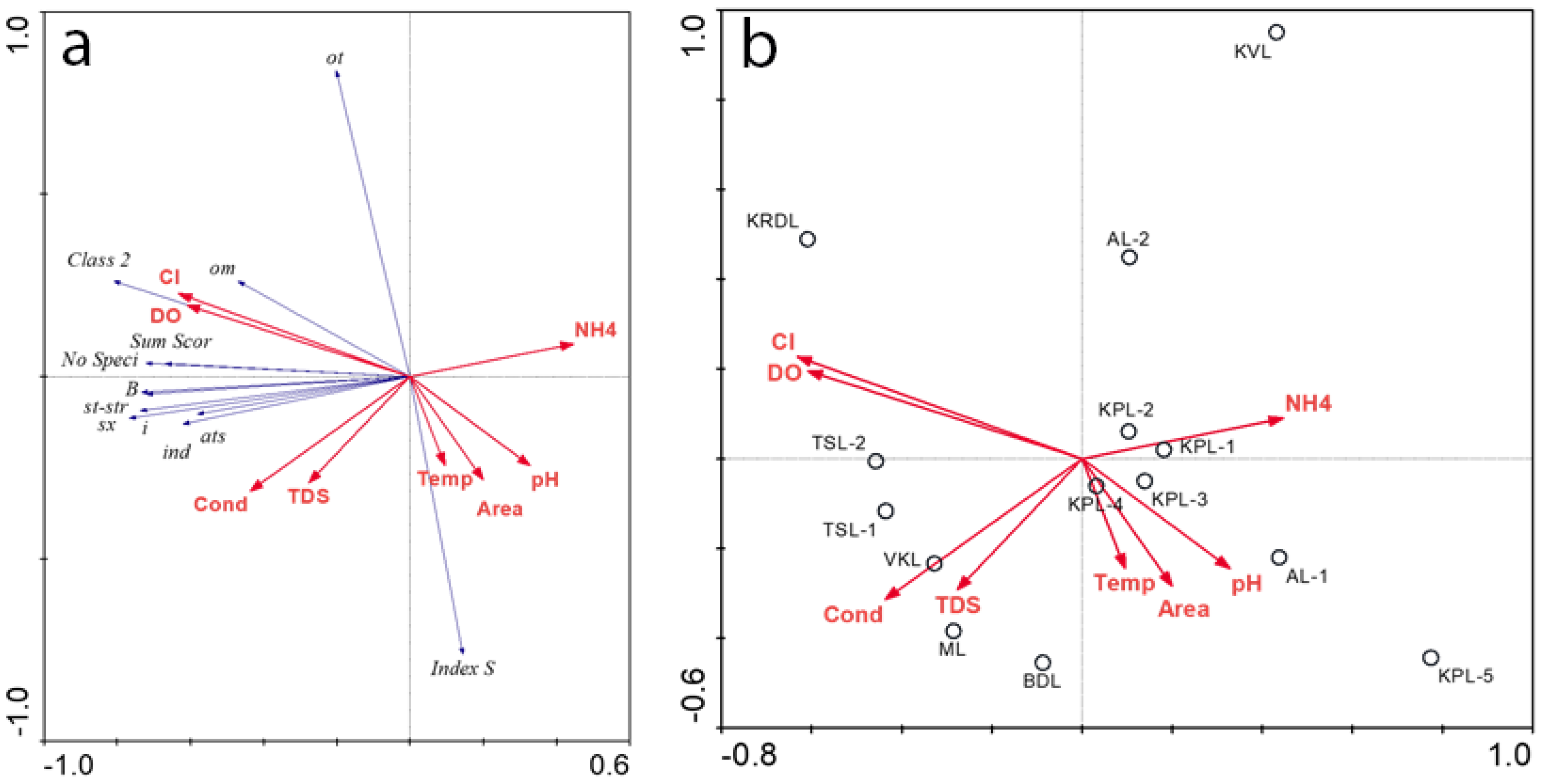
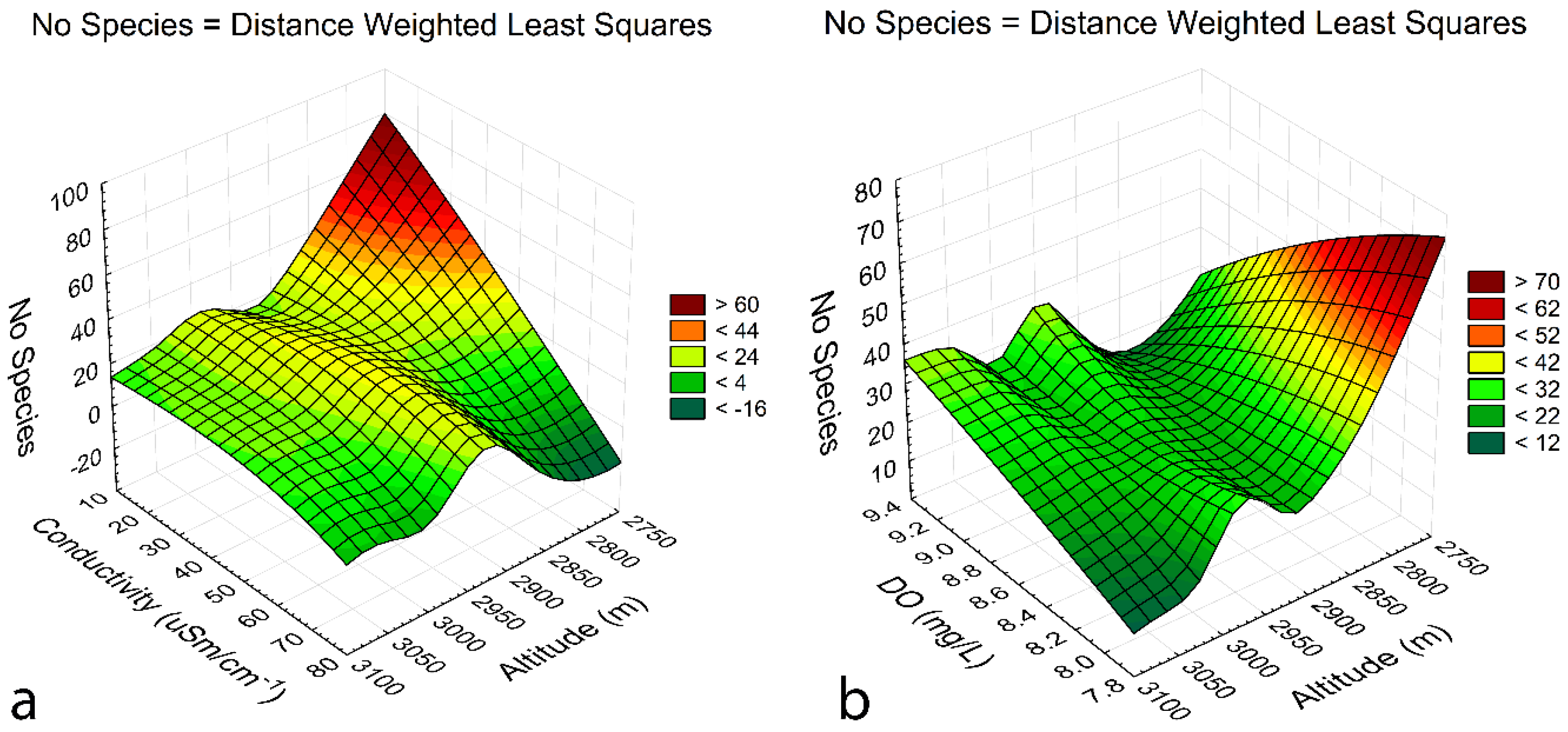
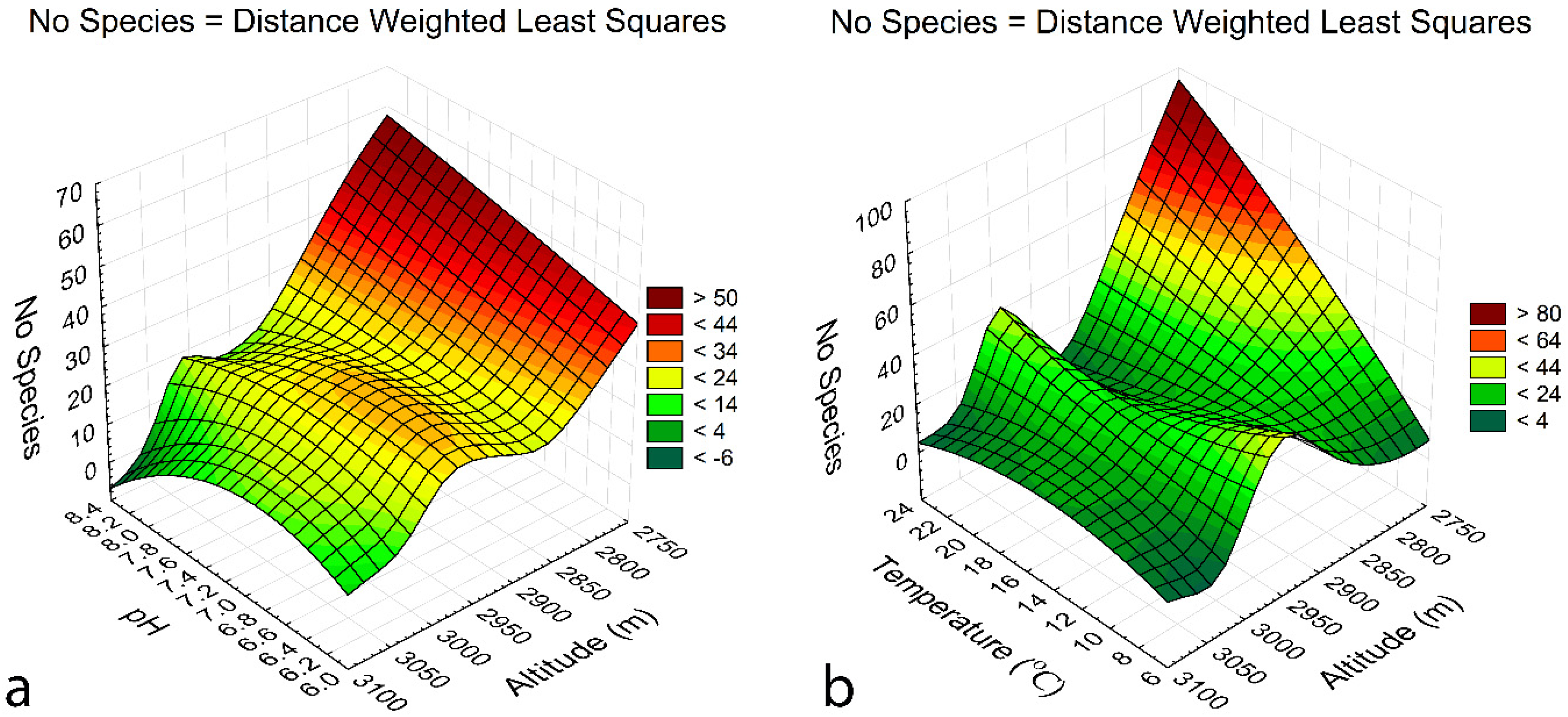
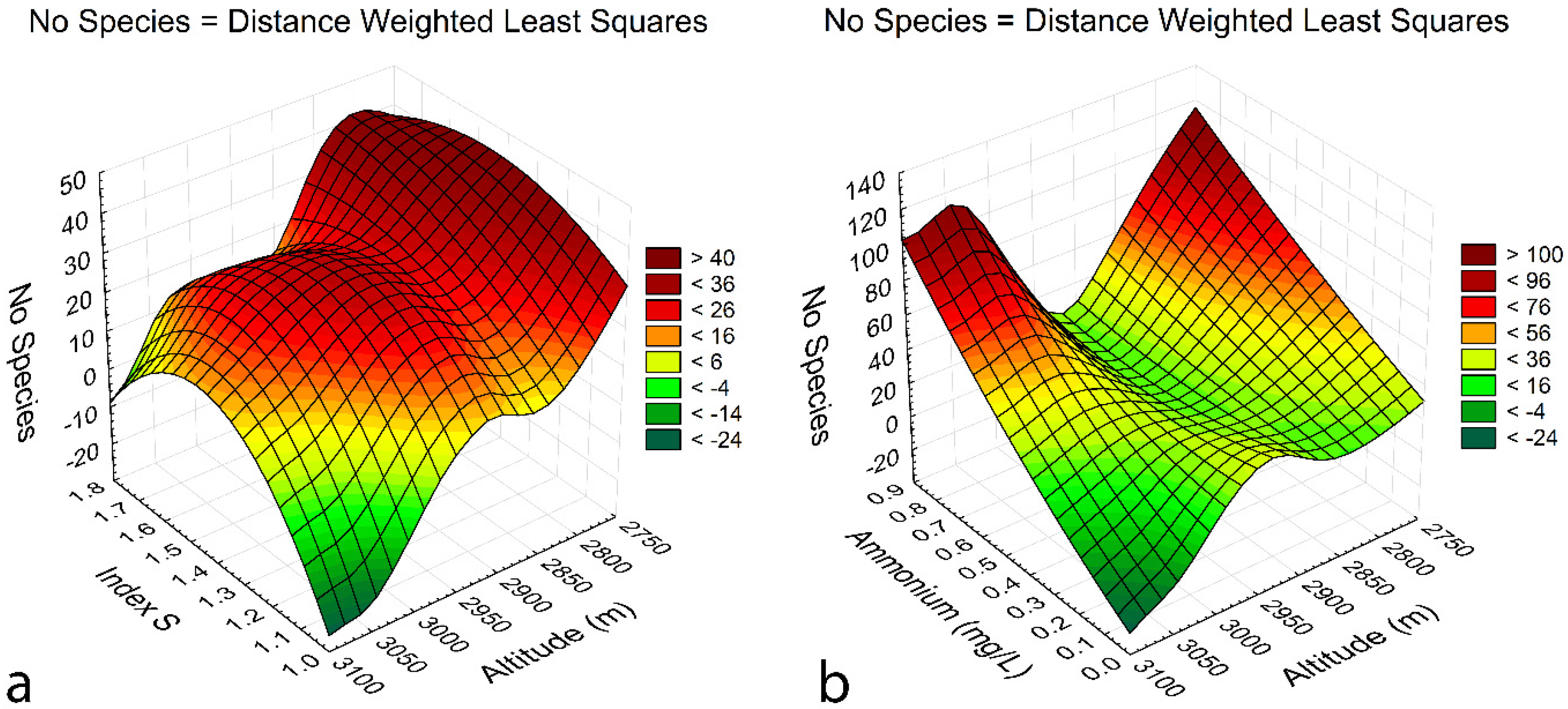
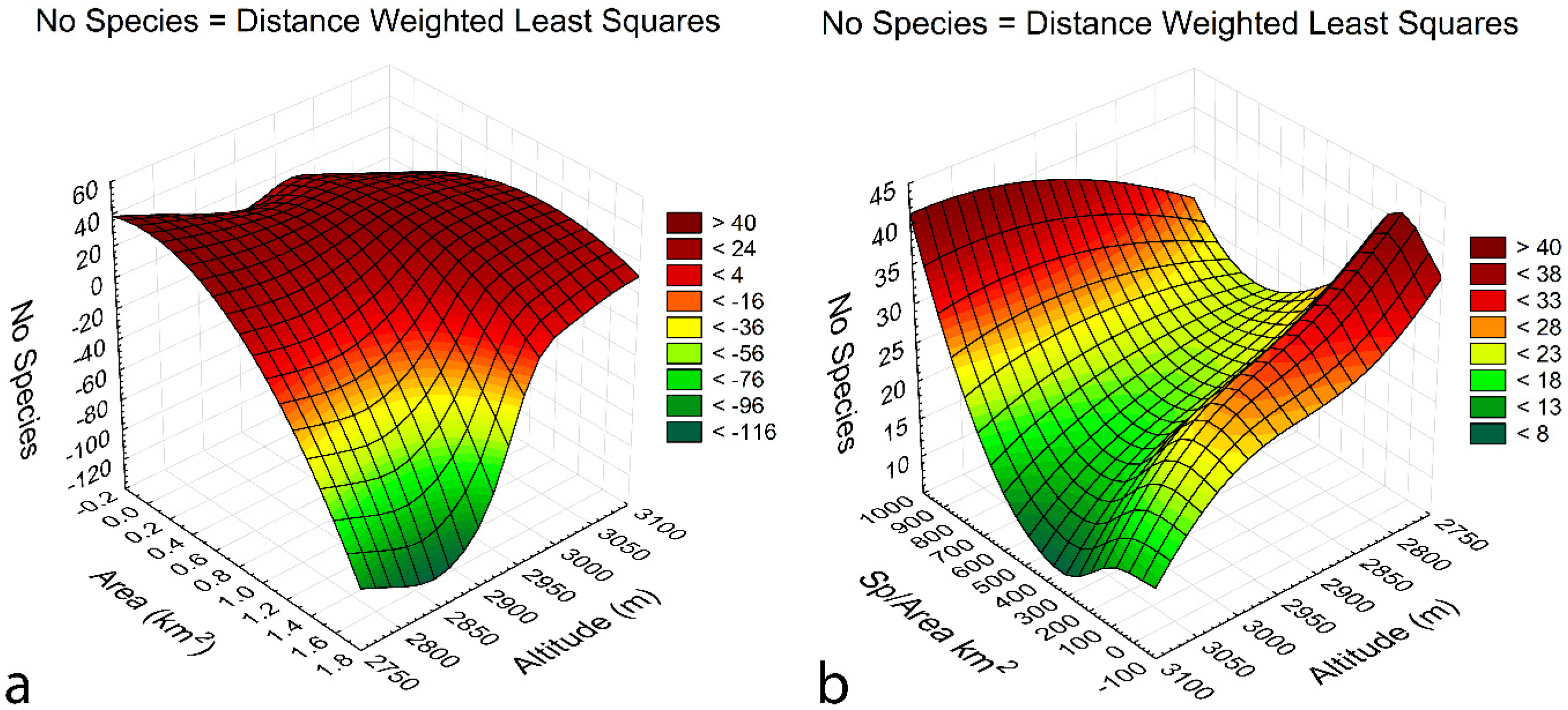
3.5. Species-environment relationships
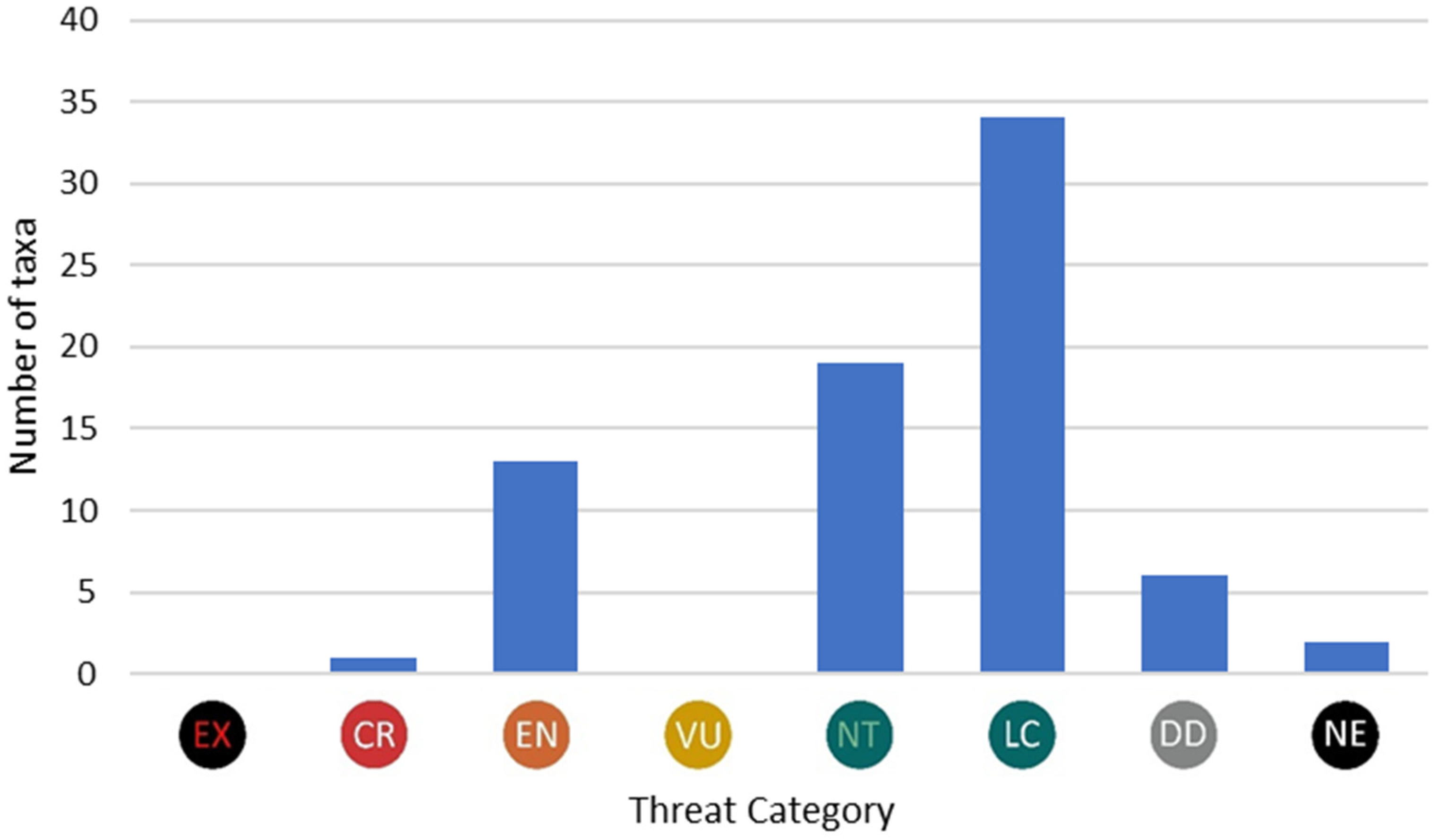
3.6. Species in Threat Categories
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Taxa | H | F | Red List Category | IUCN Category | KPL-1 | KPL-2 | KPL-3 | KPL-4 | KPL-5 | VKL | KRDL | KVL | BDL | AL-1 | AL-2 | ML | TSL-1 | TSL-2 | AP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Achnanthes sp. | 1 | VR | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Achnanthidium minutissimum (Kützing) Czarnecki | 1,2,3 | R | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Amphora ovalis (Kützing) Kützing | 1,2,3 | C | 9 | LC | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| Aulacoseira ambigua (Grunow) Simonsen | 1 | VR | 9 | LC | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aulacoseira valida (Grunow) Krammer | 1,2,3 | VR | 5 | EN | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Caloneis silicula (Ehrenberg) Cleve | 1 | VF | 9 | LC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Campylodiscus bicostatus W.Smith ex Roper | 1 | VR | 7 | NT | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chamaepinnularia hassiaca (Krasske) Cantonati & Lange-Bertalot | 1 | VR | - | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Cocconeis lineata Ehrenberg | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Craticula cuspidata (Kutzing) D.G.Mann | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclotella bodanica var. lemanica (O.Müller ex Schroter) Bachmann | 1,2,3 | VR | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cyclotella distinguenda Hustedt | 1 | VR | 9 | LC | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cyclotella meneghiniana Kützing | 1 | VR | 7 | NT | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Cymbella aspera (Ehrenberg) Cleve | 1,3 | VR | - | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Cymbella cistula (Ehrenberg) O.Kirchner | 1,2,3 | C | - | - | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| Cymbella cymbiformis C.Agardh | 1 | VR | 4 | EN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | 1,2,3 | F | 9 | LC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| Diatoma vulgaris Bory | 1,2 | C | 9 | LC | 0 | 2 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | 1,2,3 | VF | 9 | LC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Diploneis elliptica (Kützing) Cleve | 1,2,3 | F | 7 | NT | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Diploneis oblongella (Nägeli ex Kützing) A.Cleve | 1 | VR | 8 | DD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Diploneis petersenii Hustedt | 1 | VR | - | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Discostella stelligera (Cleve & Grunow) Houk & Klee | 1 | R | 9 | LC | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Encyonema minutum (Hilse) D.G.Mann | 1,2,3 | VF | 9 | LC | 2 | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Encyonema silesiacum (Bleisch) D.G.Mann | 1 | R | 9 | LC | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| Epithemia adnata (Kützing) Brébisson | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Eunotia arcus Ehrenberg | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| *Eunotia cristagalli Cleve | 1 | VR | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Eunotia mucophila (Lange-Bertalot, Nörpel-Schempp & Alles) Lange-Bertalot | 1,3 | C | 5 | EN | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Eunotia paludosa Grunow | 2 | VR | - | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Eunotia praerupta Ehrenberg | 1,2,3 | F | 0 | NE | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Fragilaria gracilis Østrup | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Fragilaria rumpens (Kützing) G.W.F.Carlson | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Frustulia crassinervia (Brébisson ex W.Smith) Lange-Bertalot & Krammer | 1 | R | 7 | NT | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frustulia saxonica Rabenhorst | 1 | VR | 7 | NT | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frustulia vulgaris (Thwaites) De Toni | 3 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Frustulia sp. | 1 | VR | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Gomphonella olivacea (Hornemann) Rabenhorst | 1 | F | 9 | LC | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 |
| Gomphonema parvulum (Kützing) Kützing | 1,2,3 | R | 9 | LC | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 |
| Gomphonema truncatum Ehrenberg | 1,2,3 | R | 9 | LC | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Gyrosigma acuminatum (Kützing) Rabenhorst | 1,3 | F | 9 | LC | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Hannaea arcus (Ehrenberg) R.M.Patrick | 1,2,3 | C | 7 | NT | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Hantzschia amphioxys (Ehrenberg) Grunow | 1 | R | 9 | LC | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Iconella capronii (Brébisson & Kitton) Ruck & Nakov | 1,2 | VF | - | EN | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Iconella spiralis (Kützing) E.C.Ruck & T.Nakov | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Iconella tenera (W.Gregory) Ruck & Nakov | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Meridion circulare (Greville) C.Agardh | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Navicula cryptocephala Kützing | 1 | VF | 9 | LC | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| Navicula cryptotenella Lange-Bertalot | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Navicula phyllepta Kützing | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Navicula radiosa Kützing | 1,2 | F | 9 | LC | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Navicula rhynchocephala Kützing | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Neidium ampliatum (Ehrenberg) Krammer | 1 | R | 7 | NT | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Neidium bisulcatum (Lagerstedt) Cleve | 1 | VR | 4 | EN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neidium iridis (Ehrenberg) Cleve | 1 | VR | 3 | CR | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Odontidium mesodon (Kützing) Kützing | 1,2 | R | 9 | LC | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Orthoseira dendroteres (Ehrenberg) Genkal & Kulikovskiy | 1 | C | - | DD | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Orthoseira roeseana (Rabenhorst) Pfitzer | 1 | VR | - | DD | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Pinnularia aestuarii Cleve | 1 | VR | - | - | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia appendiculata (C.Agardh) Schaarschmidt | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Pinnularia balatonis (Pantocsek) F.W.Mills | 1 | VR | - | - | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia borealis Ehrenberg | 1 | C | 7 | NT | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Pinnularia brebissonii (Kützing) Rabenhorst | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Pinnularia interrupta W.Smith | 1 | VF | - | - | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 |
| Pinnularia lata (Brébisson) W.Smith | 1 | VR | 4 | EN | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia major (Kützing) Rabenhorst | 1,2,3 | VF | - | - | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pinnularia mesogongyla Ehrenberg | 1 | VR | 8 | DD | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia microstauron (Ehrenberg) Cleve | 1 | VR | 7 | NT | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia microstauron var. nonfasciata Krammer | 1 | VR | - | EN | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pinnularia rupestris Hantzsch | 2 | VR | 5 | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Pinnularia viridis (Nitzsch) Ehrenberg | 1,3 | C | 8 | DD | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Planothidium distinctum (Messikommer) Lange-Bertalot | 1 | VR | - | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 |
| Psammothidium helveticum (Hustedt) Bukhtiyarova & Round | 1 | R | 9 | LC | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Rhopalodia gibba (Ehrenberg) O.Müller | 1,2 | R | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 |
| Stauroneis anceps Ehrenberg | 1,2,3 | F | 7 | NT | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | 1 | VR | 7 | NT | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stauroneis smithii Grunow | 1 | VR | 9 | LC | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Staurosira construens Ehrenberg | 1 | VR | 7 | NT | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Staurosirella pinnata (Ehrenberg) D.M.Williams | 1 | VR | 0 | NE | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella angusta Kützing | 1 | F | 9 | LC | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 |
| Surirella minuta Brébisson ex Kützing | 1 | VR | 9 | LC | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Surirella roba Leclercq | 1 | VR | 5 | EN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tabellaria flocculosa (Roth) Kützing | 1,3 | R | 9 | LC | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Ulnaria ulna (Nitzsch) Compère | 1,2,3 | R | 8 | DD | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 0 |
| Taxa | Hab | T | Oxy | Sal | pH | D | S | Aut-Het | Tro |
|---|---|---|---|---|---|---|---|---|---|
| Achnanthes sp. | - | - | - | - | - | - | - | - | - |
| Achnanthidium minutissimum (Kützing) Czarnecki | P-B | eterm | st-str | i | ind | es | 0.95 | ate | e |
| Amphora ovalis (Kützing) Kützing | B | temp | st-str | i | alf | sx | 1.50 | ate | e |
| Aulacoseira ambigua (Grunow) Simonsen | P | temp | st-str | i | alf | sp | 1.70 | ate | om |
| Aulacoseira valida (Grunow) Krammer | P-B | - | - | i | alf | es | 1.30 | ate | om |
| Caloneis silicula (Ehrenberg) Cleve | B | warm | st | i | ind | sp | 1.30 | ats | om |
| Campylodiscus bicostatus W.Smith ex Roper | B | - | - | mh | alb | - | - | ats | e |
| Chamaepinnularia hassiaca (Krasske) Cantonati & Lange-Bertalot | B | temp | st-str | hb | acf | es | 1.00 | ats | ot |
| Cocconeis lineata Ehrenberg | P-B | temp | st-str | i | alf | sx | 1.20 | ate | e |
| Craticula cuspidata (Kutzing) D.G.Mann | B | temp | st-str | i | alf | es | 2.45 | - | me |
| Cyclotella bodanica var. lemanica (O.Müller ex Schroter) Bachmann | P | - | - | i | ind | - | - | - | - |
| Cyclotella distinguenda Hustedt | P | - | str | hl | alf | - | 1.30 | - | om |
| Cyclotella meneghiniana Kützing | P-B | temp | st-str | hl | alf | sp | 2.80 | hne | e |
| Cymbella aspera (Ehrenberg) Cleve | B | - | st-str | i | neu | es | 0.30 | ats | e |
| Cymbella cistula (Ehrenberg) O.Kirchner | B | - | st-str | i | alf | sx | 1.20 | ats | e |
| Cymbella cymbiformis C.Agardh | B | temp | st-str | i | alf | sx | 2.00 | ats | om |
| Cymbopleura naviculiformis (Auerswald ex Heiberg) Krammer | B | temp | st-str | i | ind | - | - | - | - |
| Diatoma vulgaris Bory | P-B | temp | st-str | i | alf | - | 2.40 | - | - |
| Didymosphenia geminata (Lyngbye) Mart.Schmidt | B | - | st-str | i | ind | - | 2.00 | - | - |
| Diploneis elliptica (Kützing) Cleve | B | temp | str | i | alf | es | - | - | - |
| Diploneis oblongella (Nägeli ex Kützing) A.Cleve | B | - | st-str | i | ind | - | - | - | - |
| Diploneis petersenii Hustedt | B | - | str | i | ind | - | - | - | - |
| Discostella stelligera (Cleve & Grunow) Houk & Klee | P-B | temp | st-str | i | ind | - | - | - | - |
| Encyonema minutum (Hilse) D.G.Mann | B | temp | st-str | i | ind | sx | 1.50 | ats | - |
| Encyonema silesiacum (Bleisch) D.G.Mann | B | temp | st-str | i | ind | - | - | - | - |
| Epithemia adnata (Kützing) Brébisson | B | temp | st-str | i | alb | - | 1.20 | - | - |
| Eunotia arcus Ehrenberg | B | temp | st-str | i | acf | sx | 0.40 | ats | ot |
| *Eunotia cristagalli Cleve | P-B | - | st-str | i | acf | - | 1.00 | - | ot |
| Eunotia mucophila (Lange-Bertalot, Nörpel-Schempp & Alles) Lange-Bertalot | P-B | temp | st-str | hb | acf | - | - | - | - |
| Eunotia paludosa Grunow | B | - | str | hb | acf | sx | 0.50 | ats | ot |
| Eunotia praerupta Ehrenberg | P-B | cool | st-str | hb | acf | - | 0.30 | - | - |
| Fragilaria gracilis Østrup | P-B | temp | str | i | ind | es | 1.55 | hne | - |
| Fragilaria rumpens (Kützing) G.W.F.Carlson | P-B | eterm | st-str | i | ind | - | 2.00 | ats | e |
| Frustulia crassinervia (Brébisson ex W.Smith) Lange-Bertalot & Krammer | B | - | str | hb | acf | sx | 0.50 | ats | ot |
| Frustulia saxonica Rabenhorst | B | temp | st-str | hb | acf | - | - | ate | - |
| Frustulia vulgaris (Thwaites) De Toni | P-B | temp | st-str | i | alf | - | 1.00 | - | - |
| Frustulia sp. | - | - | - | - | - | - | - | - | - |
| Gomphonella olivacea (Hornemann) Rabenhorst | B | temp | st-str | i | alf | - | 2.30 | ate | om |
| Gomphonema parvulum (Kützing) Kützing | B | temp | st-str | i | ind | - | 0.70 | ats | ot |
| Gomphonema truncatum Ehrenberg | B | temp | st-str | i | ind | - | 2.00 | - | - |
| Gyrosigma acuminatum (Kützing) Rabenhorst | B | temp | st-str | i | alf | - | - | - | - |
| Hannaea arcus (Ehrenberg) R.M.Patrick | B | temp | str | i | alf | - | - | - | - |
| Hantzschia amphioxys (Ehrenberg) Grunow | B,aer | temp | st-str | i | ind | - | 3.00 | - | me |
| Iconella capronii (Brébisson & Kitton) Ruck & Nakov | P-B,S | - | st | i | ind | sx | 1.00 | ats | e |
| Iconella spiralis (Kützing) E.C.Ruck & T.Nakov | B | - | str | i | alf | - | 1.10 | - | - |
| Iconella tenera (W.Gregory) Ruck & Nakov | P-B | temp | st | i | alf | - | 0.20 | ats | ot |
| Meridion circulare (Greville) C.Agardh | P-B | temp | st-str | i | ind | - | - | - | - |
| Navicula cryptocephala Kützing | P-B | temp | st-str | i | ind | - | 2.40 | - | - |
| Navicula cryptotenella Lange-Bertalot | P-B | temp | st-str | i | ind | - | - | - | - |
| Navicula phyllepta Kützing | B | - | - | hl | - | - | - | - | - |
| Navicula radiosa Kützing | B | temp | st-str | i | ind | sx | - | - | - |
| Navicula rhynchocephala Kützing | B | temp | st-str | hl | alf | - | 1.30 | - | - |
| Neidium ampliatum (Ehrenberg) Krammer | B | temp | st | i | ind | - | - | - | - |
| Neidium bisulcatum (Lagerstedt) Cleve | B | - | st-str | i | ind | - | 1.00 | - | - |
| Neidium iridis (Ehrenberg) Cleve | B | temp | st-str | hb | ind | - | - | - | - |
| Odontidium mesodon (Kützing) Kützing | B | cool | st-str | hb | ind | - | 0.90 | - | - |
| Orthoseira dendroteres (Ehrenberg) Genkal & Kulikovskiy | B,aer | - | - | i | - | es | 1.80 | - | - |
| Orthoseira roeseana (Rabenhorst) Pfitzer | P-B | warm | - | i | ind | - | - | - | om |
| Pinnularia aestuarii Cleve | B | - | - | mh | alf | - | - | - | - |
| Pinnularia appendiculata (C.Agardh) Schaarschmidt | B | - | st-str | i | ind | - | 1.00 | - | ot |
| Pinnularia balatonis (Pantocsek) F.W.Mills | - | - | - | - | - | - | 0.80 | - | - |
| Pinnularia borealis Ehrenberg | B,aer | - | st-str,aer | i | ind | - | 1.00 | - | ot |
| Pinnularia brebissonii (Kützing) Rabenhorst | B | temp | st-str | i | ind | - | 1.00 | - | - |
| Pinnularia interrupta W.Smith | B | - | st-str | i | ind | - | - | - | - |
| Pinnularia lata (Brébisson) W.Smith | P-B | - | str | i | acf | - | 0.30 | - | - |
| Pinnularia major (Kützing) Rabenhorst | B | temp | st-str | i | ind | - | 1.00 | ats | m |
| Pinnularia mesogongyla Ehrenberg | B | - | st | i | ind | sx | 0.20 | ats | ot |
| Pinnularia microstauron (Ehrenberg) Cleve | P-B | temp | st-str | i | ind | - | 0.30 | ats | ot |
| Pinnularia microstauron var. nonfasciata Krammer | B | - | - | - | - | - | - | - | ot |
| Pinnularia rupestris Hantzsch | B | temp | str | i | acf | - | - | - | - |
| Pinnularia viridis (Nitzsch) Ehrenberg | P-B | temp | st-str | i | ind | - | 0.90 | - | ot |
| Planothidium distinctum (Messikommer) Lange-Bertalot | B | - | - | - | - | - | 2.00 | - | o-e |
| Psammothidium helveticum (Hustedt) Bukhtiyarova & Round | B | temp | st-str | hb | alf | es | 2.40 | ate | m |
| Rhopalodia gibba (Ehrenberg) O.Müller | P-B | temp | st-str | i | alf | es | 1.40 | ate | om |
| Stauroneis anceps Ehrenberg | P-B | temp | st-str | i | ind | sx | 1.30 | ats | om |
| Stauroneis phoenicenteron (Nitzsch) Ehrenberg | P-B | temp | st-str | i | ind | - | - | - | - |
| Stauroneis smithii Grunow | P-B | - | st-str | i | alf | - | 1.00 | - | om |
| Staurosira construens Ehrenberg | P-B | temp | st-str | i | alf | - | 1.00 | - | - |
| Staurosirella pinnata (Ehrenberg) D.M.Williams | P-B | temp | st-str | hl | alf | es | 1.10 | ats | om |
| Surirella angusta Kützing | P-B | temp | st-str | i | alf | - | - | - | - |
| Surirella minuta Brébisson ex Kützing | B | temp | st-str | i | alf | - | - | - | - |
| Surirella roba Leclercq | B | - | str | i | acf | - | - | - | - |
| Tabellaria flocculosa (Roth) Kützing | P-B | eterm | st-str | i | acf | - | 0.30 | - | - |
| Ulnaria ulna (Nitzsch) Compère | P-B | temp | st-str | i | alf | es | 2.40 | ate | e |
| Indicator group | AL-1 | KPL-3 | KPL-4 | KPL-1 | TSL-1 | KPL-2 | ML | TSL-2 | KPL-5 | BDL | KVL | AL-2 | VKL | KRDL | AP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
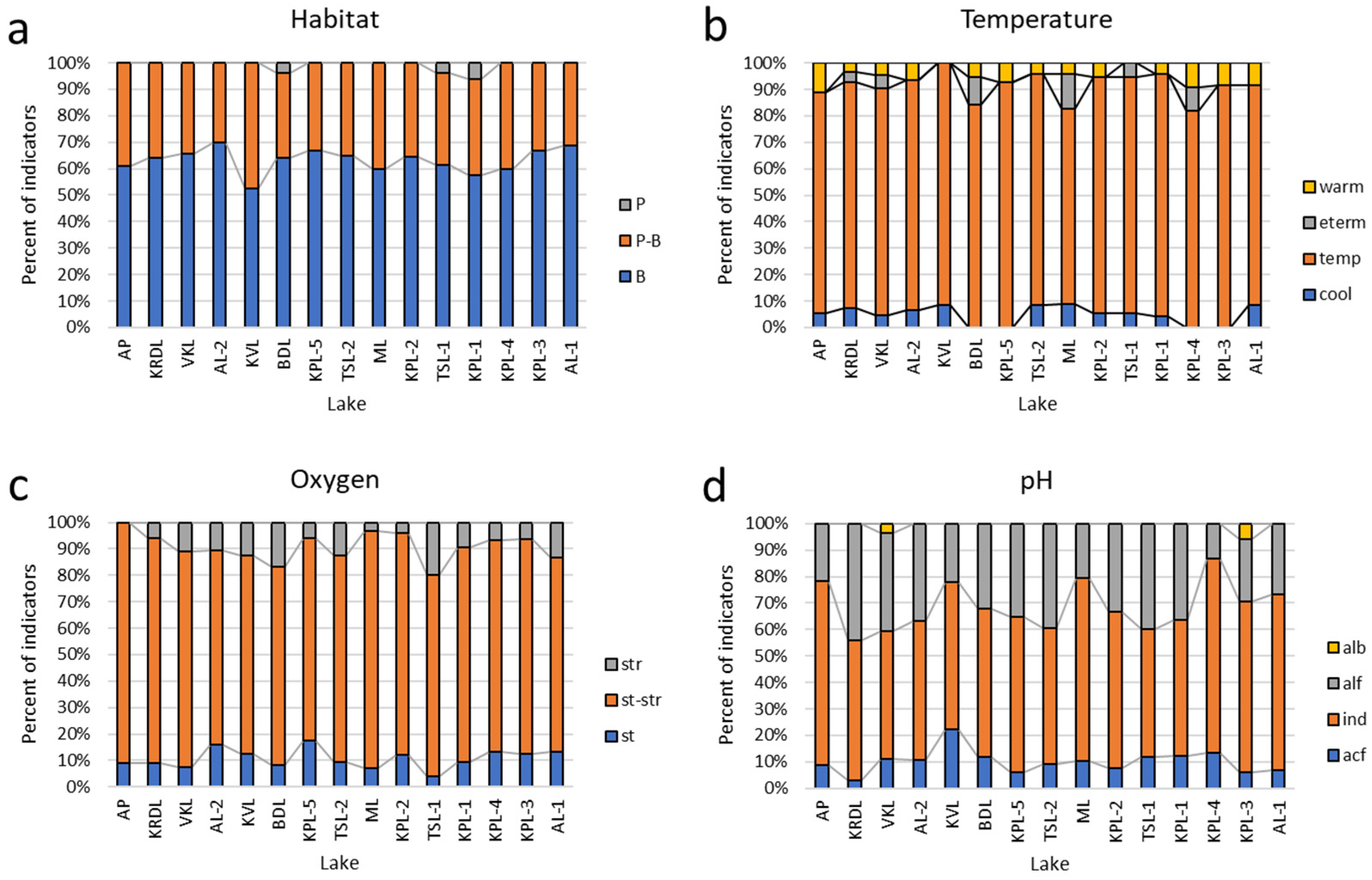
| Habitat | |||||||||||||||
| B | 11 | 12 | 9 | 19 | 16 | 18 | 18 | 22 | 12 | 16 | 10 | 14 | 19 | 23 | 14 |
| P-B | 5 | 6 | 6 | 12 | 9 | 10 | 12 | 12 | 6 | 8 | 9 | 6 | 10 | 13 | 9 |
| P | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Temperature | |||||||||||||||
| cool | 1 | 0 | 0 | 1 | 1 | 1 | 2 | 2 | 0 | 0 | 1 | 1 | 1 | 2 | 1 |
| temp | 10 | 11 | 9 | 22 | 17 | 16 | 17 | 21 | 13 | 16 | 11 | 13 | 18 | 24 | 15 |
| eterm | 0 | 0 | 1 | 0 | 1 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 1 | 1 | 0 |
| warm | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 |
| Oxygen | |||||||||||||||
| st | 2 | 2 | 2 | 3 | 1 | 3 | 2 | 3 | 3 | 2 | 2 | 3 | 2 | 3 | 2 |
| st-str | 11 | 13 | 12 | 26 | 19 | 21 | 26 | 25 | 13 | 18 | 12 | 14 | 22 | 29 | 20 |
| str | 2 | 1 | 1 | 3 | 5 | 1 | 1 | 4 | 1 | 4 | 2 | 2 | 3 | 2 | 0 |
| Salinity | |||||||||||||||
| hb | 1 | 1 | 1 | 4 | 2 | 4 | 5 | 3 | 1 | 1 | 3 | 2 | 2 | 4 | 2 |
| i | 14 | 15 | 14 | 26 | 23 | 23 | 24 | 29 | 16 | 24 | 14 | 18 | 26 | 29 | 21 |
| hl | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 0 |
| mh | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| pH-groups | |||||||||||||||
| acf | 1 | 1 | 2 | 4 | 3 | 2 | 3 | 3 | 1 | 3 | 4 | 2 | 3 | 1 | 2 |
| ind | 10 | 11 | 11 | 17 | 12 | 16 | 20 | 17 | 10 | 14 | 10 | 10 | 13 | 18 | 16 |
| alf | 4 | 4 | 2 | 12 | 10 | 9 | 6 | 13 | 6 | 8 | 4 | 7 | 10 | 15 | 5 |
| alb | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Watanabe | |||||||||||||||
| sx | 3 | 5 | 3 | 6 | 5 | 5 | 6 | 6 | 3 | 7 | 4 | 4 | 7 | 7 | 5 |
| es | 1 | 1 | 1 | 2 | 5 | 3 | 3 | 6 | 2 | 4 | 3 | 4 | 4 | 5 | 2 |
| sp | 1 | 1 | 1 | 3 | 0 | 1 | 1 | 2 | 2 | 1 | 0 | 1 | 1 | 1 | 1 |
| Autotrophy-Heterotrophy | |||||||||||||||
| ats | 5 | 7 | 5 | 9 | 6 | 8 | 8 | 8 | 4 | 7 | 4 | 6 | 6 | 8 | 7 |
| ate | 0 | 0 | 0 | 5 | 4 | 4 | 3 | 4 | 2 | 5 | 2 | 3 | 3 | 5 | 3 |
| hne | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Trophic state | |||||||||||||||
| ot | 1 | 2 | 2 | 4 | 2 | 3 | 2 | 3 | 1 | 1 | 5 | 3 | 3 | 4 | 3 |
| om | 1 | 2 | 2 | 5 | 3 | 5 | 3 | 4 | 2 | 4 | 3 | 3 | 3 | 5 | 5 |
| m | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 2 | 1 |
| me | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| e | 1 | 3 | 1 | 5 | 4 | 2 | 5 | 6 | 3 | 5 | 1 | 2 | 4 | 5 | 3 |
| o-e | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Class of Water Quality | |||||||||||||||
| Class 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Class 2 | 4 | 4 | 6 | 10 | 9 | 10 | 11 | 13 | 3 | 9 | 7 | 8 | 9 | 17 | 5 |
| Class 3 | 4 | 3 | 2 | 8 | 7 | 7 | 5 | 8 | 5 | 5 | 2 | 4 | 7 | 6 | 2 |
| Class 4 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 4 | 1 |
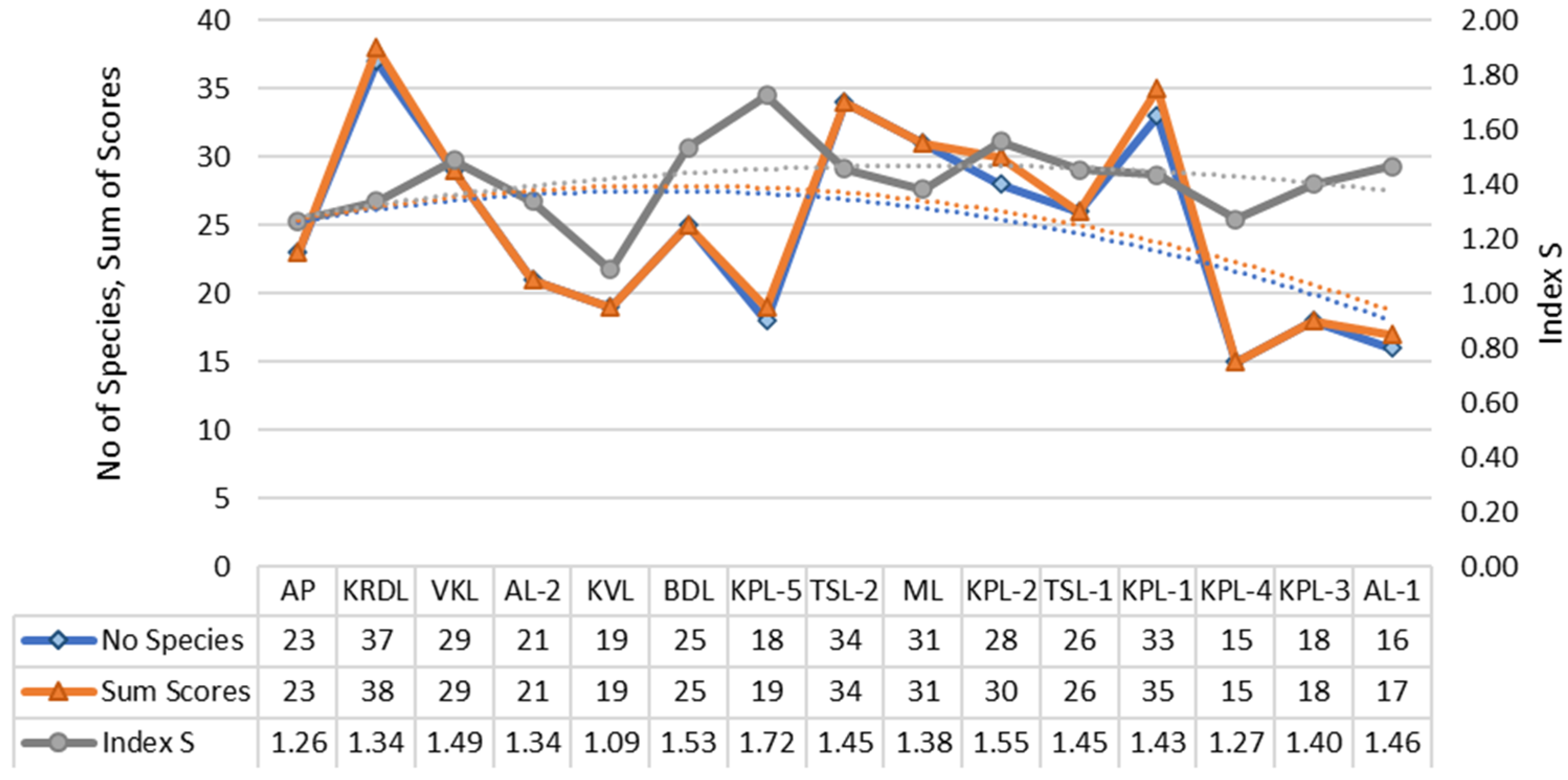
| No of Species | 16 | 18 | 15 | 33 | 26 | 28 | 31 | 34 | 18 | 25 | 19 | 21 | 29 | 37 | 23 |
| Sum of Scores | 17 | 18 | 15 | 35 | 26 | 30 | 31 | 34 | 19 | 25 | 19 | 21 | 29 | 38 | 23 |
| Index S | 1.46 | 1.40 | 1.27 | 1.43 | 1.45 | 1.55 | 1.38 | 1.45 | 1.72 | 1.53 | 1.09 | 1.34 | 1.49 | 1.34 | 1.26 |
References
- Cantonati, M.; Lange-Bertalot, H. Diatom monitors of close-to-pristine, very-low alkalinity habitats: three new Eunotia species from springs in Nature Parks of the southeastern Alps. Journal of Limnology 2011, 70, 209–221. [Google Scholar] [CrossRef]
- Anonymous. Kaçkar Dağları Milli Parkı uzun devreli gelişme planı analitik etüt ve sentez raporu. Doğa Koruma ve Milli Parklar Genel Müdürlüğü, Ankara, 2006.
- Feret, L.; Bouchez, A.; Rimet, F. Benthic diatom communities in high altitude lakes: a large scale study in the French Alps. Annales de Limnologie - International Journal of Limnology 2017, 53, 411–423. [Google Scholar] [CrossRef]
- Biskaborn, B.K.; Nazarova, L.; Pestryakova, L.A.; Syrykh, L.; Funck, K.; Meyer, H.; Chapligin, B.; Vyse, S.; Gorodnichev, R.; Zakharov, E.; Wang, R.; Schwamborn, G.; Bailey, H.L.; Diekmann, B. Spatial distribution of environmental indicators in surface sediments of Lake Bolshoe Toko, Yakutia, Russia. Biogeosciences 2019, 16, 4023–4049. [Google Scholar] [CrossRef]
- Moser, K.A.; Baron, J.S.; Brahney, J.; Oleksy, I.A.; Saros, J.E.; Hundey, E.J.; Sadro, S.; Kopáček, J.; Sommaruga, R.; Kainz, M.J.; Strecker, A.L.; Chandra, S.; Walters, D.M.; Preston, D.L.; Michelutti, N.; Lepori, F.; Spaulding, S.A.; Christianson, K.R.; Melack, J.M.; Smol, J.P. Mountain lakes: Eyes on global environmental change. Global and Planetary Change 2019, 178, 77–95. [Google Scholar] [CrossRef]
- Falasco, E.; Ector, L.; Ciaccio, E.; Hoffmann, L.; Bona, F. Alpine freshwater ecosystems in a protected area: a source of diatom diversity. Hydrobiologia 2012, 695, 233–251. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, X.; Anderson, N.J.; Ji, J. Diatom Seasonality and Sedimentation in a Subtropical Alpine Lake (Lugu Hu, Yunnan-Sichuan, Southwest China). Arctic, Antarctic, and Alpine Research 2018, 47, 461–472. [Google Scholar] [CrossRef]
- Vinna, L.R.; Medhaug, I.; Schmid, M.; Bouffard, D. The vulnerability of lakes to climate change along an altitudinal gradient. Communications Earth & Environment 2021, 2, 35. [Google Scholar]
- Cantonati, M.; Lange-Bertalot, H. Achnanthidium dolomiticum sp. nov. (Bacillariophyta) from oligotrophic mountain springs and lakes fed by dolomite aquifers. Journal of Phycology 2006, 42, 1184–1188. [Google Scholar] [CrossRef]
- Noga, T.; Kochman, N.; Peszek, L.; Stanek-Tarkowska, J.; Pajaczek, A. Diatoms (Bacillariophyceae) in rivers and streams and on cultivated soils of the Podkarpacie region in the years 2007-2011. Journal of Ecological Engineering 2014, 15, 6–25. [Google Scholar]
- Borics, G.; Görgényi, J.; Grigorszky, I.; László-Nagy, Zs.; Tóthmérész, B.; Krasznai, E.; Várbíró, G. The role of phytoplankton diversity metrics in shallow lake and river quality assessment. Ecol Indic. 2014, 45, 28–36. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P.G. Primary production of the biosphere: integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Sarthou, G.; Timmermans, K.R.; Blain, S.; Tréguer, P. Growth physiology and fate of diatoms in the ocean: a review. J. Sea Res. 2005, 53, 25–42. [Google Scholar] [CrossRef]
- Armbrust, E.V. The life of diatoms in the world’s oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Gnjato, S.; Narancic, B.; Antoniades, D.; Pienitz, R.; Biskaborn, B.K.; Gnjato, R.; Dekić, R. Surface sediment diatom assemblages from four alpine lakes in the Zelengora Mountains (Bosnia and Herzegovina): A Pilot Study. Botanica Serbica 2022, 46, 61–70. [Google Scholar] [CrossRef]
- Şahin, B. A study on the benthic algae of Uzungöl (Trabzon). Turk. Journal of Botany 1998, 22, 171–189. [Google Scholar]
- Şahin, B.; Akar, B.; Bahçeci, İ. Species composition and diversity of epipelic algae in Balık Lake (Şavşat-Artvin, Turkey). Turk. Journal of Botany 2010, 34, 441–448. [Google Scholar] [CrossRef]
- Şahin, B.; Akar, B.; Barinova, S. Algal flora and ecology of the high mountain lakes in the Artabel Lakes Nature Park (Gümüşhane, Turkey), I-Bacillariophyta. International Journal of Advanced Research in Botany (IJARB) 2019, 5, 1–13. [Google Scholar]
- Şahin, B.; Barinova, S. Role of altitude in formation of diatom diversity of high-mountain protected glacier lakes in the Kaçkar Mountains National Park, Rize, Turkey. Environments MDPI 2022, 9, 1–18. [Google Scholar] [CrossRef]
- Kurdoğlu, O. Kaçkar Dağları Milli Parkı ve yakın çevresinin doğal kaynak yönetimi açısından incelenmesi. KTÜ Fen Bilimleri Enstitüsü, Orman Mühendisliği Ana Bilim Dalı, Doktora Tezi, Trabzon, 2002.
- Gürpınar, T. Kuş göçü açısından Türkiye’nin önemi, Türkiye ve Balkan Ülkelerinde Yaban Hayatı. Uluslararası Sempozyumu Bildiriler Kitabı, 1987, 16-20, Eylül, İstanbul.
- Round, F.E. An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. Journal of Ecology 1953, 41, 174–197. [Google Scholar] [CrossRef]
- Sládečková, A. Limnological investigation methods for the periphyton (“Aufwuchs”) community. Botanical Review 1962, 28, 286–350. [Google Scholar] [CrossRef]
- Huber-Pestalozzi, G. Das Phytoplankton des Süßwassers systematic und biologie (Die Binnengewässer, Band XVI), 2, 2, Diatomeen, Stuttgart, E. Schweizerbart’sche Verlagsbuchhandlung (Nagele u. Obermiller), 1942, p. 183. (in German).
- Hustedt, F. Bacillariophyta (Diatomeae) Zweite Auflage. In: Die Süsswasser-Flora Mitteleuropas. Heft 10. (Pascher, A. Eds.), Jena: Verlag von Gustav Fischer, 1930, p. 466 (in German).
- Joh, G. Algal flora of Korea. Volume 3, Number 1. Chrysophyta: Bacillariophyceae: Centrales. Freshwater diatoms I. pp. [1–6], 1-161, figs 1-105. Incheon: National Institute of Biological Resources, 2010.
- Krammer, K.; Lange Bertalot, H. Bacillariophyceae 1. Naviculaceae. In Sǘßwasserflora von Mittelflora, 2/1; G. Fischer: Jena, Germany; Stuttgart, Germany; Lubeck, Germany; Ulm, Germany, 1986; p. 876. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 2. Bacillariaceae, Epithemiaceae, Surirellaceae. In Süsswasserflora von Mitteleuropa, 2/2; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; G. Fischer: Jena, Germany, 1988; p. 596. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 3. Centrales, Fragilariaceae, Eunotiaceae. Susswasserflora von Mitteleuropa, 2/3; Ettl, H., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fisher Verlag: Stuttgart, Germany, 1991; p. 576. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae 4. Achnanthaceae, Kritische Erganzungen zu Navicula (Lineolatae) und Gomphonema. In Die Süsswasserflora von Mitteleuropa; Gustav Fisher Verlag: Stuttgart, Germany, 1991; bd. 2/4, p. 437. [Google Scholar]
- Lange-Bertalot, H.; Hofmann, G.; Werum, M.; Cantonati, M. Freshwater benthic diatoms of Central Europe. Koeltz Botanical Books: Schmitten-Oberreifenberg, Germany, 2017; 942 p.
- Lee, J.H. Algal flora of Korea. Volume 3, Number 8. Chrysophyta: Bacillariophyceae: Pennales: Raphidineae: Naviculaceae. Freshwater diatoms VI. pp. [1–6] 1-56, figs 1-10. Incheon: National Institute of Biological Resources, 2012.
- Lee, J.H. Algal flora of Korea. Volume 3, Number 9. Chrysophyta: Bacillariophyceae: Pennales: Raphidineae: Naviculaceae. Freshwater diatoms VII. Incheon: National Institute of Biological Resources Ministry of Environment, 2012.
- Patrick, R.; Reimer, C.W. The Diatoms of the United States, exclusive of Alaska and Hawaii: Fragilariaceae, Eunotiaceae, Achnanthaceae, Naviculacae. Philadelphia, USA, Academy of Natural Sciences, 1966.
- Patrick, R.; Reimer, C.W. The Diatoms of the United States Vol 2. Entomoneigaceae, Cymbellaceae, Gomphonemaceae, Epithemiaceae. Philadelphia, USA, Academy of Natural Science, 1975.
- Guiry, M.D.; Guiry, G.M. AlgaeBase World-Wide Electronic Publication. National University of Ireland, Galway. Available online: http://www.algaebase.org (accessed on 15 June 2023).
- Kocataş, A. Ekoloji (Çevre Biyolojisi). İzmir, Türkiye, Ege Üniversitesi Matbaası, 1992.
- Barinova, S. Essential and practical bioindication methods and systems for the water quality assessment. Int. J. Environ. Sci. Nat. Resour. 2017, 2, 1–11. [Google Scholar] [CrossRef]
- Barinova, S.S.; Medvedeva, L.A.; Anissimova, O.V. Diversity of Algal Indicators in Environmental Assessment; Pilies Studio Publisher: Tel Aviv, Israel, 2006. 498 p. (In Russian) [Google Scholar]
- Barinova, S.S.; Bilous, O.P.; Tsarenko, P.M. Algal Indication of Water Bodies in Ukraine: Methods and Prospects; Publishing House of Haifa University: Haifa, Kyiv, Israel, 2019. 367 p. (In Russian) [Google Scholar]
- Wessa, P. Pearson Correlation (v1.0.13) in Free Statistics Software (v1.2.1), Office for Research Development and Education, URL https://www.wessa.net/rwasp_correlation.wasp/. (accessed on 3 January 2024).
- Love, J.; Selker, R.; Marsman, M.; Jamil, T.; Dropmann, D.; Verhagen, J.A.; Ly, A.; Gronau, F.Q.; Smira, M.; Epskamp, S.; et al. JASP: Graphical statistical software for common statistical designs. J. Stat. Softw. 2019, 88, 1–17. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power Press: Ithaca, NY, USA, 2002; 500 p. [Google Scholar]
- Hofmann, G.; Lange-Bertalot, H.; Werum, M.; Klee, R. Rote Liste und Gesamtartenliste der limnischen Kieselalgen (Bacillariophyta) Deutschlands. In Rote Liste der gefährdeten Tiere, Pflanzen und Pilze Deutschlands; Band 7: Pflanzen. Bonn (Bundesamt für Naturschutz). Naturschutz und Biologische Vielfalt, 2018, 70(7), pp. 601–708. https://www.rote-liste-zentrum.de/en/Limnische-Kieselalgen-Bacillariophyta-1772.html.
- IUCN. IUCN Red List Categories and Criteria, Version 3.1, 2nd ed.; IUCN: Gland, Switzerland; Cambridge, UK, 2012; pp. 1–32. [Google Scholar]
- Şahin, B. Kaçkar Dağları Milli Parkı’ndan (Rize/Türkiye) yeni bir diyatome kaydı. Bağbahçe Bilim Dergisi 2022, 9, 41–44. (In Turkish) [Google Scholar]
- Barinova, S.; Gabyshev, V.; Genkal, S. Diversity of Diatom Algae in the Lena Delta Nature Reserve and the Adjacent Territory in the Specific Ecological Factors of the Arctic. Diversity 2023, 15, 802. [Google Scholar] [CrossRef]
- Van Dam, H.; Mertens, A.; Sinkeldam, J. A coded checklist and ecological indicator values of freshwater diatoms from the Netherlands. Neth. J. Aquat. Ecol. 1994, 28, 117–133. [Google Scholar] [CrossRef]
- Wehr, J.D.; Sheath, R.G. Freshwater algae of North America (Ecology and Classification). California, USA, Academic Press, 2003.
- Odum, E.P. Fundamentals of Ecology. Third Edition, W.B. Saunders Co., Philadelphia, 1971; pp. 1–574.
- Bjork, S. Redevelopment of lake system—A case study approach. Ambio 1988, 17, 90–98. [Google Scholar]
- Lotter, A.F.; Pienitz, R.; Schmidt, R. Diatoms as indicators of environmental change near arctic and alpine treeline. In The Diatoms: Application for the Environmental and Earth Sciences, 1st ed.; Stoermer, E.F., Smol, J.P., Eds.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Niyatbekov, T.; Barinova, S. Bioindication of aquatic habitats with diatom algae in the Pamir Mountains, Tajikistan. MOJ Ecol. Environ. Sci. 2018, 3, 117–120. [Google Scholar]
- Barinova, S.; Niyatbekov, T. Comparative analysis of diatom algae diversity in the Pamir Protected Lakes, Tajikistan. Int. J. Adv. Res. Bot. 2019, 5, 1–17. [Google Scholar]
- Protasov, A.; Barinova, S.; Novoselova, T.; Sylaieva, A. The Aquatic Organisms Diversity, Community Structure, and Environmental Conditions. Diversity 2019, 11, 190. [Google Scholar] [CrossRef]
- Hustedt, F. Die Diatomeen flora des Flußsystems der Weser im Gebiet der Hansestadt Bremen. Abh. Naturwiss. Ver. Brem. 1957, 34, 181–440. [Google Scholar]
- Hustedt, F. Systematische und Ökologische Untersuchungen über die Diatomeenflora von Java, Bali und Sumatra. Archiv. Hydrobiol. Suppl. 1938, 15, 131–177, 393–506, 638–790, Eratum in Archiv. Hydrobiol. Suppl. 1939, 16, 1–155, 274–394. [Google Scholar]
- Sládeček, V. Diatoms as indicators of organic pollution. Acta Hydrochimica et Hydrobiologica 1986, 14, 555–566. [Google Scholar] [CrossRef]
- Watanabe, T.; Asai, K.; Houki, A. Numerical estimation of organic pollution of flowing water by using the epilithic diatom assemblage – Diatom Assemblage Index (DAIpo). Science of Total Environment 1986, 55, 209–218. [Google Scholar] [CrossRef]
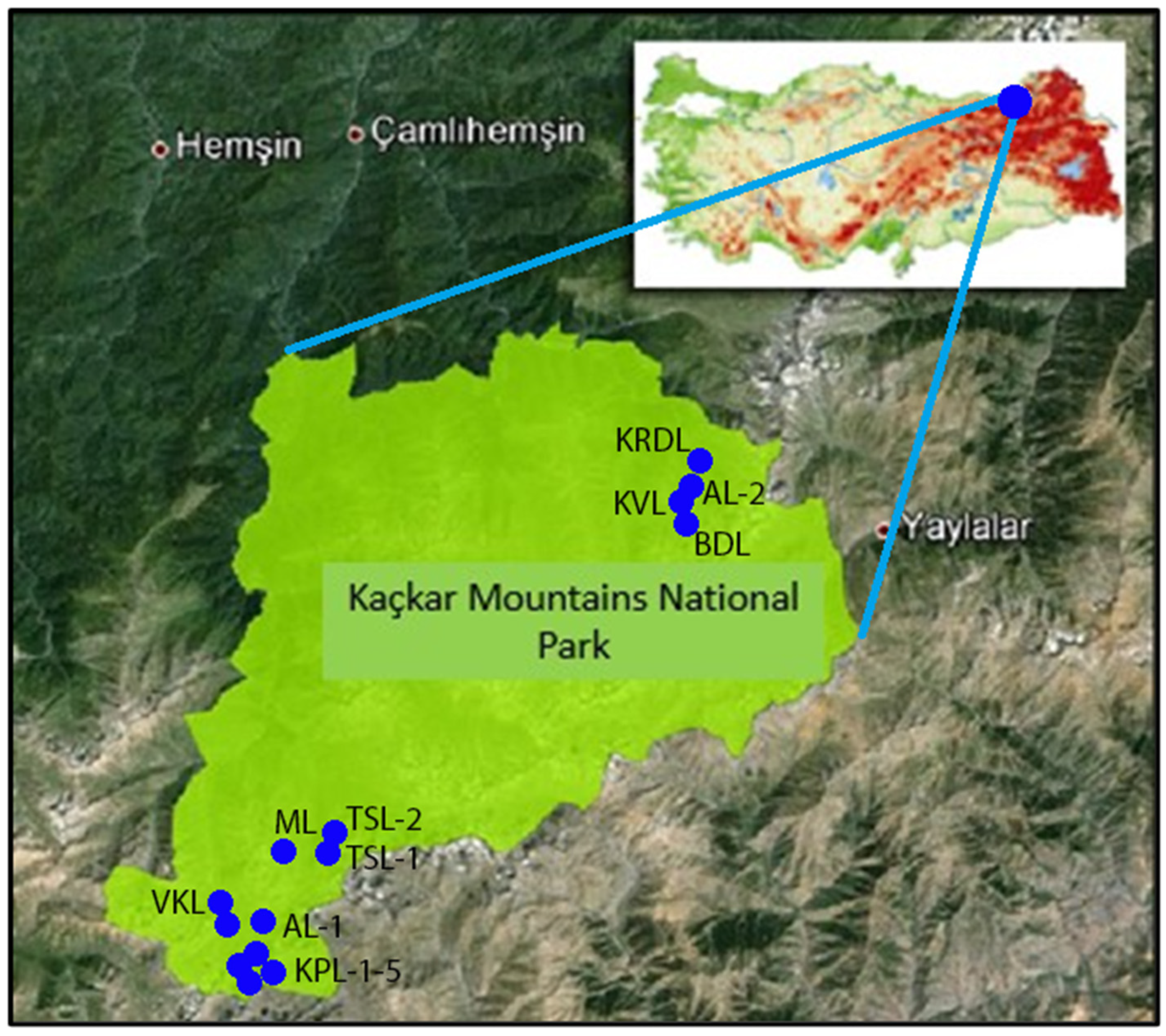
| Lake with abbreviation | Geographic coordinates | Altitude (m) | Area (km2) |
|---|---|---|---|
| Kapılı Lake-1 (KPL-1) | 40o42′56″.59 N; 40o54′51″.71 E | 2980 | 0.71 |
| Kapılı Lake-2 (KPL-2) | 40o43′08″.70 N; 40o54′55″.30 E | 2973 | 0.15 |
| Kapılı Lake-3 (KPL-3) | 40o42′34″.73 N; 40o54′49″.02 E | 3074 | 0.36 |
| Kapılı Lake-4 (KPL-4) | 40o42′43″.97 N; 40o54′47″.24 E | 3028 | 0.05 |
| Kapılı Lake-5 (KPL-5) | 40o42′59″.03 N; 40o54′20″.06 E | 2926 | 0.07 |
| Vercenik Kumlu Lake (VKL) | 40o43′17″.91 N; 40o54′16″.58 E | 2864 | 0.03 |
| Karadeniz Lake (KRDL) | 40o52′39″.19 N; 41o10′ 02″.06 E | 2782 | 0.24 |
| Kavron Lake (KVL) | 40o52′24″.39 N; 41o09′45″.73 E | 2911 | 0.09 |
| Büyük Deniz Lake (BDL) | 40o52′04″.60 N; 41o09′38″.54 E | 2922 | 0.68 |
| Adsız Lake-1 (AL-1) | 40o42′39″.75 N; 40o54′57″.65 E | 3075 | 1.62 |
| Adsız Lake-2 (AL-2) | 40o52′21″.18 N; 41o10′06″.94 E | 2868 | 0.04 |
| Moçar Lake (ML) | 40o44′11″.63 N; 40o56′05″.36 E | 2958 | 0.22 |
| Tatos Sulak Lake-1 (TSL-1) | 40o44′16″.11 N; 40o56′42″.25 E | 2976 | 0.46 |
| Tatos Sulak Lake-2 (TSL-2) | 40o44′25″.50 N; 40o56′51″.18 E | 2940 | 0.17 |
| IUCN Category | IUCN Code | No of Red List Category | Red List Category | Number of taxa |
|---|---|---|---|---|
| EXTINCT | EX | 1 | Extinct or Lost | 0 |
| CRITICALLY ENDANGERED | CR | 2, 3 | Threatened with Extinction, Highly Threatened | 1 |
| ENDANGERED | EN | 4, 5 | Threatened, Threat of Unknown Extent | 13 |
| VULNERABLE | VU | 6 | Extremely Rare | 0 |
| NEAR THREATENED | NT | 7 | Near Threatened | 19 |
| LEAST CONCERN | LC | 9 | Not Threatened | 34 |
| DATA DEFICIENT | DD | 8 | Data Deficient | 6 |
| NOT EVALUATED | NE | 0, 10 | Not established | 2 |
| Parameters | KPL-1 | KPL-2 | KPL-3 | KPL-4 | KPL-5 | VKL | KRDL | KVL | BDL | AL-1 | AL-2 | ML | TSL-1 | TSL-2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 14.2 | 14.0 | 14.5 | 14.0 | 14.8 | 22.5 | 12.5 | 14.7 | 11.2 | 17.6 | 7.1 | 12.2 | 13 | 13 |
| DO (mg L-1) | 8.03 | 8.33 | 8.14 | 8.02 | 8.20 | 8.18 | 8.89 | 8.55 | 8.69 | 7.98 | 8.97 | 9.03 | 9.17 | 8.90 |
| pH | 6.30 | 6.30 | 6.55 | 6.32 | 8.21 | 7.88 | 6.33 | 7.18 | 6.10 | 7.30 | 6.54 | 7.13 | 6.9 | 6.9 |
| Conductivity (μSm cm-1) | 23.4 | 23.2 | 27.1 | 26.0 | 29.30 | 22.9 | 36.8 | 19.9 | 71.4 | 14.1 | 38.9 | 46 | 40 | 40 |
| TDS (mg L-1) | 17.73 | 15.93 | 18.72 | 18.23 | 20.46 | 17.86 | 22.82 | 12.34 | 44.27 | 9.92 | 27.34 | 21.89 | 20.34 | 19.84 |
| Potassium (mg L-1) | 0.37 | 0.37 | 0.50 | 0.40 | 0.47 | - | - | 0.38 | 0.44 | 0.35 | - | - | - | - |
| Total hardness CaCO3 (mg L-1) | 17.96 | 17.64 | 19.94 | 19.70 | 23.60 | - | - | - | 38.84 | - | - | 17.59 | - | - |
| Calcium (mg L-1) | 5.13 | 5.00 | 5.92 | 5.82 | 7.17 | - | - | 4.02 | 12.69 | - | 7.25 | 4.52 | 5.30 | 5.33 |
| Magnesium (mg L-1) | - | - | - | - | 1.38 | - | - | - | 1.73 | - | - | 1.53 | - | - |
| Ammonium (mg L-1) | 0.31 | 0.28 | 0.34 | 0.23 | 0.40 | 0.35 | 0.17 | 0.76 | 0.82 | 0.31 | 0.13 | 0.11 | 0.08 | 0.12 |
| Chlorine (mg L-1) | 2.11 | 1.77 | 2.20 | 2.70 | 1.98 | 1.51 | 7.51 | 2.44 | 1.70 | 2.02 | 7.48 | 7.26 | 7.03 | 7.74 |
| Nitrate (mg L-1) | - | 0.214 | 0.207 | 0.232 | 0.330 | - | 0.575 | - | 0.233 | 0.225 | 0.515 | 0.361 | 0.279 | 0.218 |
| Nitrite (mg L-1) | - | - | - | - | - | - | - | - | - | - | - | 0.020 | 0.020 | - |
| Phosphate (P2O5) (mg L-1) | - | - | - | - | 0.113 | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





