Submitted:
23 January 2024
Posted:
24 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and methods
Data selection and sample size
AI system
Study design
Data analysis
Statistical analysis
Results
Primary endpoint: Comparison of the performances of the 3 AI algorithms
Discussion
Conclusion
List of abbreviations
| AI | Artificial intelligence |
| BI-RADS | Breast Imaging-Reporting And Data System |
| CAD | computer-aided detection system |
| CE | European conformity |
| FFDM | Full-field digital mammography |
| IRB | Institutional review board |
| NPV | Negative predictive value |
| PPV | Positive predictive value |
| Se | Sensitivity |
| Sp | Specificity |
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast. déc 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Broeders, M.; Moss, S.; Nyström, L.; Njor, S.; Jonsson, H.; Paap, E. The impact of mammographic screening on breast cancer mortality in Europe: a review of observational studies. J. Med. Screen. 2012, 19 (Suppl. S1), 14–25. [Google Scholar] [CrossRef]
- SPF (santé publique France). Éthique et dépistage organisé du cancer du sein [Internet]. [cité 18 juill 2023]. Available online: https://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Ethique-et-depistage-organise-du-cancer-du-sein-Synthese.
- SPF. Sensibilité et spécificité du programme de dépistage organisé du cancer du sein à partir des données de cinq départements français, 2002-2006. Numéro thématique. Dépistage organisé du cancer du sein. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein/sensibilite-et-specificite-du-programme-de-depistage-organise-du-cancer-du-sein-a-partir-des-donnees-de-cinq-departements-francais-2002-2006.-nume. (accessed on 27 February 2023).
- Paci, E. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J. Med. Screen. 2012, 19 (Suppl. S1), 5–13. [Google Scholar] [CrossRef] [PubMed]
- Elmore, J.G.; Jackson, S.L.; Abraham, L. and al. Variability in interpretive performance at screening mammography and radiologists' characteristics associated with accuracy. Radiology 2009, 253, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Miglioretti, D.L.; Smith-Bindman, R.; Abraham, L. and al. Radiologist characteristics associated with interpretive performance of diagnostic mammography. J. Natl. Cancer Inst. 2007, 99, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Giess, C.S.; Wang, A.; Ip, I.K.; Lacson, R.; Pourjabbar, S.; Khorasani, R. Patient, radiologist, and examination characteristics affecting screening mammography recall rates in a large academic practice. J. Am. Coll. Radiol. 2019, 16, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Barlow, W.E.; Chi, C.; Carney, P.A. and al. Accuracy of screening mammography interpretation by characteristics of radiologists. J. Natl. Cancer Inst. 2004, 96, 1840–1850. [Google Scholar] [CrossRef]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A. and al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J. Natl. Cancer Inst. 2019, 111, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Freeman, K.; Geppert, J.; Stinton, C. and al. Use of artificial intelligence for image analysis in breast cancer screening programmes: systematic review of test accuracy. BMJ 2021, 374, n1872. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.A.; Chazard, E.; Poncelet, E. and al. Impact of artificial intelligence in breast cancer screening with mammography. Breast Cancer 2022, 29, 967–977. [Google Scholar] [CrossRef]
- Rodríguez-Ruiz, A.; Krupinski, E.; Mordang, J.J.; Schilling, K.; Heywang-Köbrunner, S.H.; Sechopoulos, I.; Mann, R.M. Detection of breast cancer with mammography: effect of an artificial intelligence support system. Radiology 2019, 290, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, A.T.; Lim, V.; Vu, H.X. and al. Improved cancer detection using artificial intelligence: a retrospective evaluation of missed cancers on mammography. J. Digit. Imaging 2019, 32, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Balleyguier, C.; Arfi-Rouche, J.; Levy, L.; Toubiana, P.R.; Cohen-Scali, F.; Toledano, A.Y.; Boyer, B. Improving digital breast tomosynthesis reading time: a pilot multi-reader, multi-case study using concurrent computer-aided detection (CAD). Eur J Radiol 2017, 97, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; An, J.K.; Woo, J.J.; Kwak, H.Y. Comparison of diagnostic performance in mammography assessment: radiologist with reference to clinical information versus standalone artificial intelligence detection. Diagnostics (Basel) 2022, 13, 117. [Google Scholar] [CrossRef]
- Salim, M.; Wåhlin, E.; Dembrower, K. and al. External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol. 2020, 6, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- ACR (American college of Radiology). Breast Imaging Reporting & Data System [Internet]. [cité 29 mars 2023]. Available online: https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads.
- Lotter, W.; Diab, A.R.; Haslam, B. and al. Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat. Med. 2021, 27, 244–249. [Google Scholar] [CrossRef] [PubMed]
- McKinney, S.M.; Sieniek, M.; Godbole, V. and al. International evaluation of an AI system for breast cancer screening. Nature 2020, 577, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Schaffter, T.; Buist, D.S.M.; Lee, C.I. and al. Evaluation of combined artificial intelligence and radiologist assessment to interpret screening mammograms. JAMA Netw. Open 2020, 3, e200265. [Google Scholar] [CrossRef] [PubMed]
- Rauscher, G.H.; Murphy, A.M.; Qiu, Q.; Dolecek, T.A.; Tossas, K.; Liu, Y.; Alsheik, N.H. The "sweet spot" revisited: optimal recall rates for cancer detection with 2D and 3D digital screening mammography in the metro Chicago breast cancer registry. AJR Am. J. Roentgenol. 2021, 216, 894–902. [Google Scholar] [CrossRef]
- McDonald, E.S.; Oustimov, A.; Weinstein, S.P.; Synnestvedt, M.B.; Schnall, M.; Conant, E.F. Effectiveness of digital breast tomosynthesis compared with digital mammography: outcomes analysis from 3 years of breast cancer screening. JAMA Oncol. 2016, 2, 737–743. [Google Scholar] [CrossRef]
- Dratsch, T.; Chen, X.; Mehrizi, M.R. and al. Automation bias in mammography: the impact of artificial intelligence BI-RADS suggestions on reader performance. Radiology 2023, 307, e222176. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.D.; Wellman, R.D.; Buist, D.S.; Kerlikowske, K.; Tosteson, A.N.; Miglioretti, D.L. Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern. Med. 2015, 175, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.P.; Samala, R.K.; Hadjiiski, L.M. CAD and AI for breast cancer-recent development and challenges. Br. J. Radiol. 2020, 93, 20190580. [Google Scholar] [CrossRef] [PubMed]
- Sechopoulos, I.; Teuwen, J.; Mann, R. Artificial intelligence for breast cancer detection in mammography and digital breast tomosynthesis: state of the art. Semin. Cancer Biol. 2021, 72, 214–225. [Google Scholar] [CrossRef]
- Dembrower, K.; Crippa, A.; Colón, E.; Eklund, M.; Strand, F. ; ScreenTrustCAD TrialConsortium. Artificial intelligence for breast cancer detection in screening mammography in Sweden: a prospective, population-based, paired-reader, non-inferiority study. Lancet Digit Health, 2023; S2589-7500(23)00153-X. [Google Scholar]
- Lång, K.; Josefsson, V.; Larsson, A.M.; Larsson, S.; Högberg, C.; Sartor, H.; et al. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): a clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. août 2023, 24, 936–44. [Google Scholar]
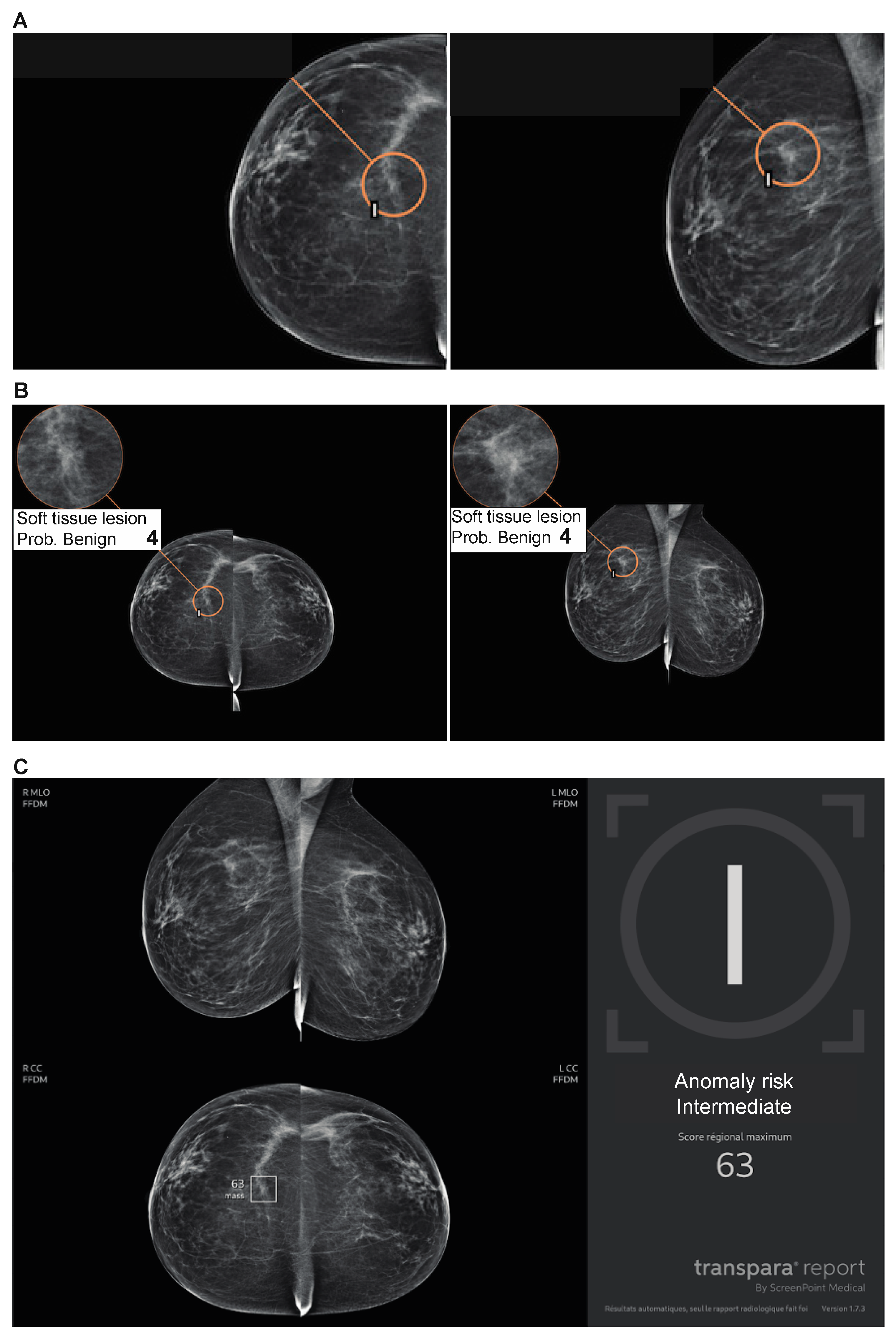
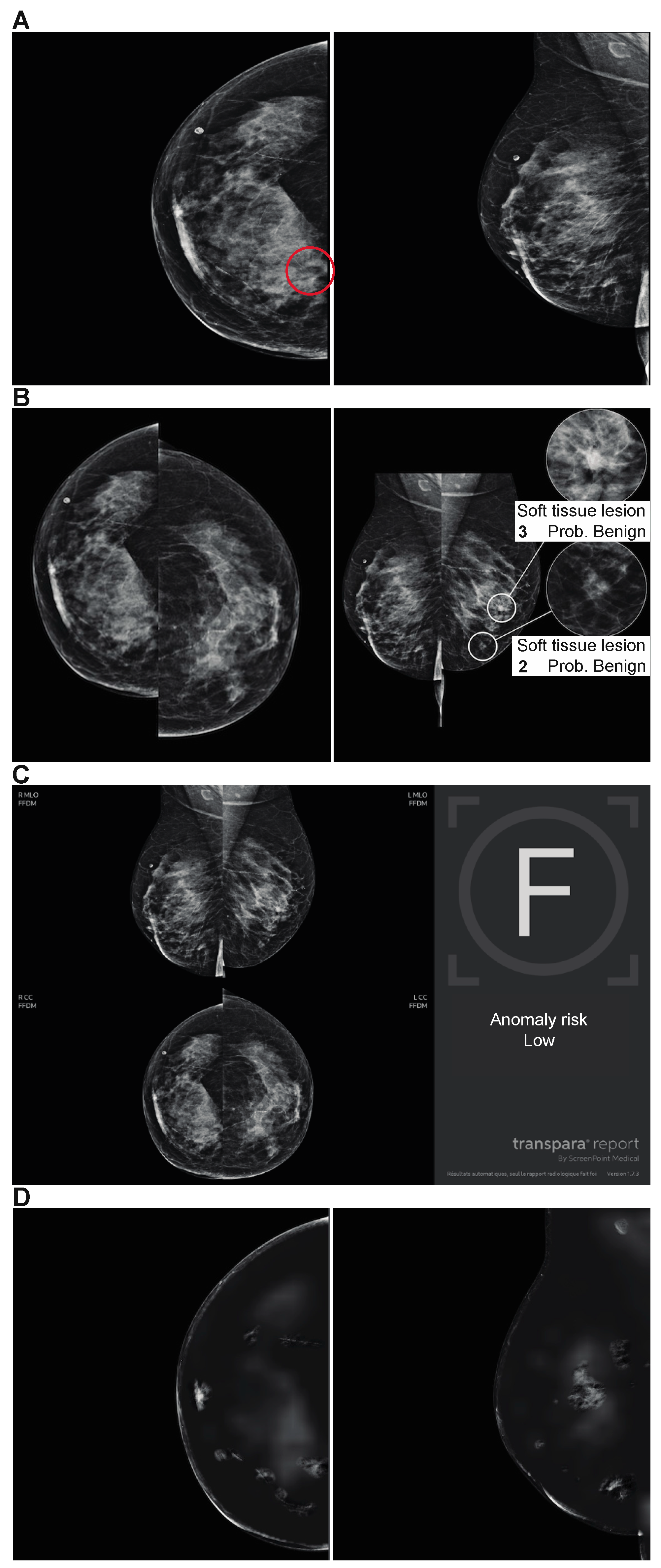
| Variables | N (%) |
|---|---|
| Status | |
| Benign | 505 (80.4) |
| Malignant | 123 (19.6) |
| Location of malignant lesion | |
| Left | 53 (43.1) |
| Right | 36 (29.3) |
| Both | 34 (27.6) |
| Cancer type | |
| Mass | 74 (60.2) |
| Calcification | 32 (26) |
| Focal asymmetry | 7 (5.7) |
| Architectural distorsion | 10 (8.1) |
| Cancer BI-RADS score | |
| 2 | 2 (1.6) |
| 3 | 11 (8.9) |
| 4 | 55 (44.7) |
| 5 | 55 (44.7) |
| Breast density | |
| A | 57 (18.2) |
| B | 164 (52.2) |
| C | 90 (28.7) |
| D | 3 (0.96) |
| Breast analysis | AI 1 (%) | AI 2 (%) | AI 3 (%) |
|---|---|---|---|
| SE | 91/123 (74) | 64/123 (52) | 86/123 (69.9) |
| SP | 399/505 (79) | 497/505 (98.4) | 229/505 (45.4) |
| PPV | 64/72 (46.2) | 64/72 (88.9) | 86/362 (23.8) |
| NPV | 399/431 (92.6) | 497/556 (89.4) | 229/266 (86.1) |
| Accuracy | 490/628 (78) | 561/628 (89.3) | 315/628 (50.2) |
| Balanced accuracy | 76.5 | 75.2 | 57.6 |
| Breast analysis (%) | AI 1 | AI 2 | AI 3 | p value | ||
|---|---|---|---|---|---|---|
| AI 1 vs AI 2 | AI 1 vs AI 3 | AI 2 vs AI 3 | ||||
| SE | 74 | 52 | 69.9 | < 0.001 | 0.478 | 0.004 |
| SP | 79 | 98.4 | 45.4 | < 0.001 | < 0.001 | < 0.001 |
| PPV | 46.2 | 88.9 | 23.8 | < 0.001 | < 0.001 | < 0.001 |
| NPV | 92.6 | 89.4 | 86.1 | 0.086 | 0.005 | 0.168 |
| Accuracy | 78 | 89.3 | 50.2 | 0.001 | 0.004 | < 0.001 |
| Threshold | 0.8 | 0.7 | 0.6 | 0.5 | 0.4 |
|---|---|---|---|---|---|
| SE (%) | 52 | 59.4 | 66.7 | 69.9 | 74.8 |
| SP (%) | 98.42 | 96.2 | 91.7 | 86.1 | 70.5 |
| PPV (%) | 89 | 79.4 | 66.1 | 55.1 | 38.2 |
| NPV (%) | 89 | 90.7 | 91.9 | 92.2 | 92 |
| Accuracy (%) | 89.3 | 89 | 86.8 | 83 | 71.3 |
| Balanced accuracy (%) | 75.2 | 77.8 | 79.2 | 78 | 72.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





