1. Introduction
England and Wales started screening for isovaleric acidemia (IVA) in 2015, with Scotland and Northern Ireland following suit in 2017 and 2020 respectively. The screening algorithm is based on the analysis of isovalerylcarnitine (C5i) by flow injection analysis tandem mass spectrometry (FIA-MS/MS), on a dried blood spot specimen collected on day five of life. Initial identification of a condition suspected IVA result is based on a single defined cut-off-value (COV) for C5i. C5i has three common isobars, valerylcarnitine, 2-methylbutyrylcarnitine and pivaloylcarnitine (C5p), which cannot be distinguished by FIA-MS/MS. C5p can be present in blood due to maternal use of pivalic ester pro-drugs e.g., pivmecillinam, or pivalic acid derivatives used as emollients in some creams, including nipple balms used by breastfeeding mothers. As such, the occurrence of false positive (FP) results due to interference from C5p is well documented [
1,
2,
3]. However, when the UK first started screening for IVA, use of pivmecillinam was thought to be uncommon hence FP results were not expected to be an issue. It quickly became evident that this was not the case [
4] and further investigation found that the number of prescriptions for pivmecillinam issued by GP practices in England had increased five-fold between July 2012 and July 2016 [
5]. This coincides with Public Health England recommending the drug as an alternative therapy when there is widespread bacterial resistance to ampicillin, amoxicillin and trimethoprim [
4]. Geographical variation in prescribing patterns is evident, with higher usage presumably correlating with areas of increased antimicrobial resistance [
5].
The aim of this study was to determine whether the FP rate for IVA could be reduced by using Precision Newborn Screening via Collaborative Laboratory Integrated Reports (CLIR). The performance and outcomes of the UK’s current IVA screening algorithm, which uses a single defined COV of 2.0 µmol/L, were compared with CLIR post-analytical clinical decision support software.
2. Materials and Methods
An eight-year, retrospective review of condition suspected results for IVA was performed. Information was obtained on all babies referred via the UK newborn screening program, between January 2015 and December 2022, with an initial condition suspected result for IVA: date of specimen, NHS number; laboratory identifier; initial C5C result, C5 isobars result; mutation analysis; outcome; additional information relating to antibiotic use. Data were analyzed using GraphPad Prism v10.0.2.
A short questionnaire designed to obtain additional information on clinical outcomes was distributed to each of the Newborn Screening Clinical Services. Clinicians were asked to classify each case as ‘asymptomatic’ or ‘clinically affected’. Each case was also classified as severe (classical IVA) or attenuated phenotype (mild IVA) using criteria described previously [
6]. A copy of the questionnaire is provided in
Supplementary material S1.
A retrospective evaluation of Precision Newborn Screening via CLIR was undertaken. Post-analytical tools were configured for the UK location (GBR). Reference case data, true positive (TP) and FP case data were submitted to CLIR to provide initial location specific data. Case data included: age at time of specimen collection; birth weight; gestational age; sex; analyte concentration (methionine, total leucine, phenylalanine, tyrosine, C5C, octanoylcarnitine, decanoylcarnitine, glutarylcarnitine, thyroid stimulating hormone and immunoreactivetrypsin). Post-analytical tools were configured for the GBR location, and a single condition tool (SCT) was created. Quantification of the utility of CLIR in correctly identifying FP IVA cases was determined by submission of additional FP cases to CLIR and subsequent analysis with the SCT, and a dual scatter plot (DSP) designed to discriminate FP results from confirmed cases of IVA. Permission was obtained from the Antenatal & Newborn Screening Research and Innovation Development Advisory Committee for the retrospective evaluation of CLIR. All case data was anonymized prior to submission.
3. Results
3.1. Retrospective review of condition suspected IVA results
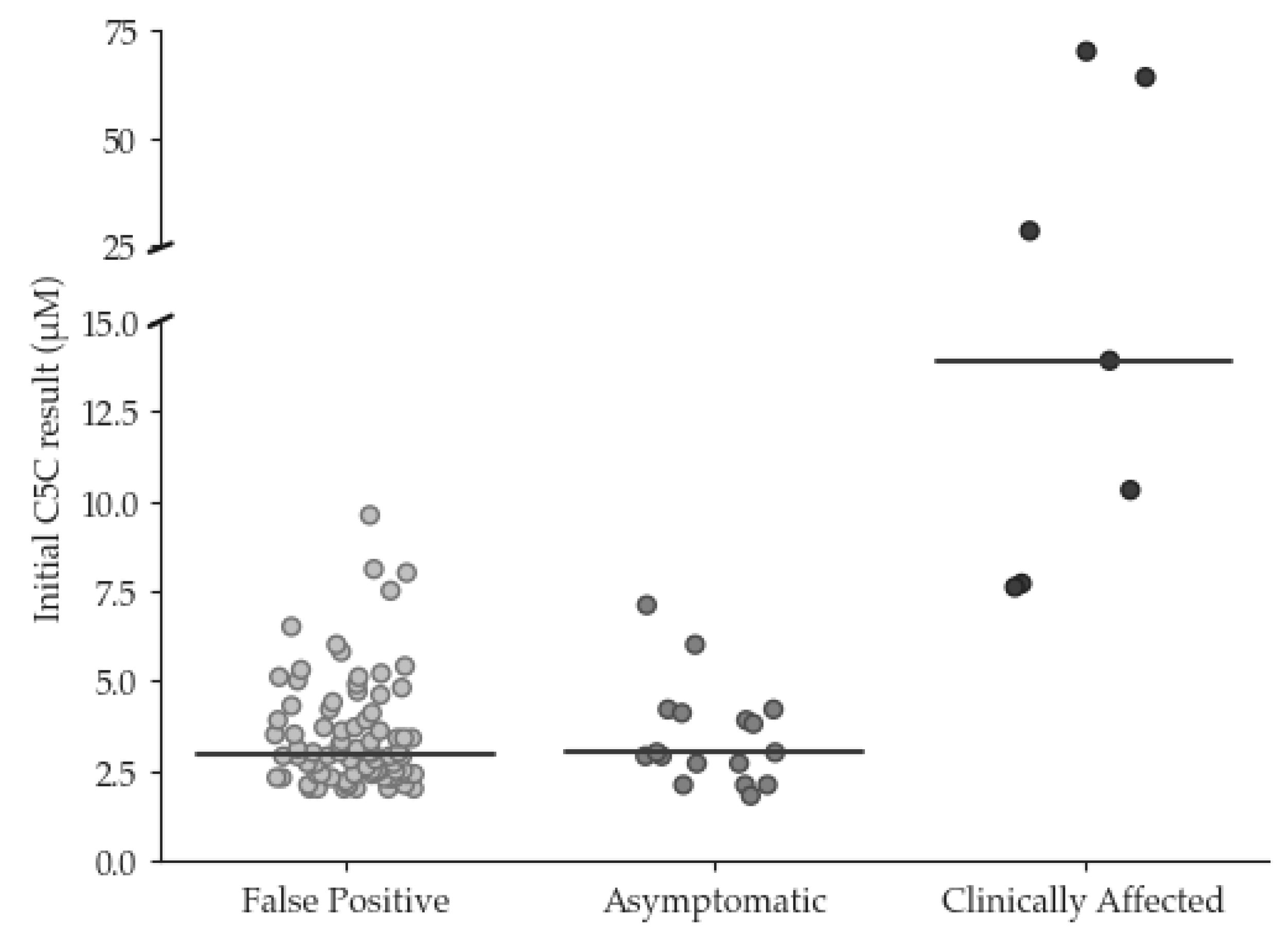
Between January 2015 and December 2022, 109 babies with condition suspected results for IVA were identified. Of these, 24 were TP cases and 84 were FP cases. One ‘other condition suspected’ case was removed from subsequent data analysis. The mean (median, range) C5C result for the TP cases was 10.9 µmol/L (4.0, 1.8 - > 70). Of the TP cases, seven were c.941C>T homozygous, six were compound heterozygous, two of which were c.941C>T compound heterozygous, and seven cases were homozygous other. The remaining four were c.941C>T heterozygous that had been classified as TP cases on the basis of increased urinary isovalerylglycine.
The mean (median, range) C5C result for the FP cases was 3.5 µmol/L (2.9, 2.0 - 9.6). The incidence of FP cases was approximately 0.0015%. The initial C5C results for the TP and FP cases are summarised in
Figure 1. Pivalate interference had been confirmed in 67/84 FP cases by C5 isobar analysis [
4]. Although 17/84 cases did not have isobar analysis performed, 9/17 had documented evidence of maternal pivampicillin use. The remaining 8 cases had unremarkable urine organic acid and bloodspot acylcarnitine profiles.
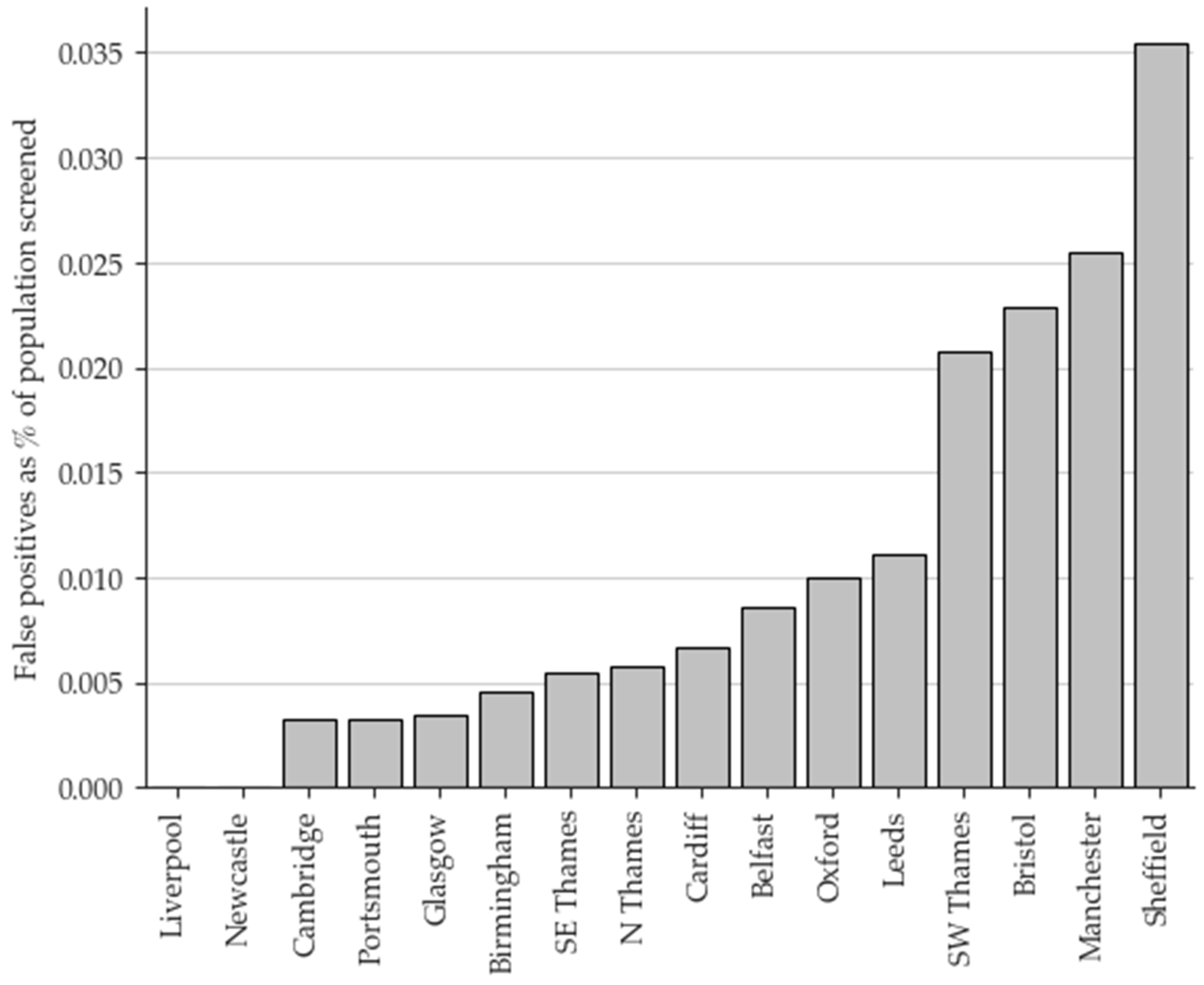
The overall FP rate for the eight-year period was 78%. Geographical variation in the FP rate was evident as illustrated in
Figure 2. Of all the FP results, 26.7% occurred in just one laboratory, and 55.8% occurred in just three. Conversely, two laboratories have not had a FP case to date.
3.2. Clinical outcome questionnaire
The clinical outcome questionnaire was sent to the Lead Paediatric Metabolic Consultant at the following hospitals which run the Newborn Screening Clinical Services; Birmingham, Bristol, Evelina London, Great Ormond Street, Manchester and Sheffield Children’s Hospitals. Responses were received from 6/6 centers. Of the 24 TP cases, seven were classified as ‘clinically affected’ and 17 were classed as ‘asymptomatic’ (see
Figure 1). Of the ‘clinically affected’ children, 5/7 had experienced further episodes of decompensation since diagnosis and all were being treated with a combination of protein restricted diet and emergency regimen (ER). 2/7 were also receiving glycine supplementation, 2/7 were receiving carnitine supplementation and 3/7 were being supplemented with both. Of the ‘asymptomatic’ children, 15 were being managed on an ER only, 2/15 were receiving an ER with mild protein restriction and carnitine supplementation and 1/2 was also receiving glycine supplementation.
3.3. Evaluation of precision newborn screening
Preliminary data submitted to CLIR included n=288,735 reference cases, n=4 TP IVA cases and n=34 FP cases due to C5p interference. An additional n=50 FP cases were subsequently submitted to CLIR and analysed using the DSP. 3/50 cases were rejected due to missing decanoylcarnitine results. Of the 47 cases remaining, 1/47 was correctly identified as FP, 3/47 were incorrectly classified as TP IVA and 43/47 were classified as ‘indeterminate’. In a screening environment, indeterminate results typically require follow-up, although programs may choose to use this as a group that requires a repeat specimen, rather than referral for confirmatory testing. It may also serve as a useful categorization tool for second tier testing [
7,
8].
4. Discussion
A widely cited quotation draws attention to both the harms and benefits of screening – ‘All screening programmes do harm; some do good as well, and, of these, some do more good than harm at reasonable cost.’ [
9]
The potential harms resulting from newborn screening include both the generation of FP results, with its psychosocial impact on families, and uncertainty in TP cases resulting from a diverse clinical phenotype, making decisions about risk and treatment difficult both for physicians and parents. Furthermore, FP results also have a financial impact, with families making trips to specialist clinical centers which are often not geographically close by. The results from this retrospective study of screening for IVA over eight years in the UK illustrate the practical implications of such disbenefits and the need to continually review and improve current screening practice.
The findings from 108 screen positive cases indicate that 84 were FP (78%) while among the 24 TP cases identified, only seven required classical treatment with protein restriction and supplementary glycine and/or carnitine with 17/24 true positive cases remaining asymptomatic with less onerous interventions.
These results emphasize the responsibility of those conducting screening to continually review and improve the specificity of newborn screening and, where possible, more closely define the prognosis in true positive cases.
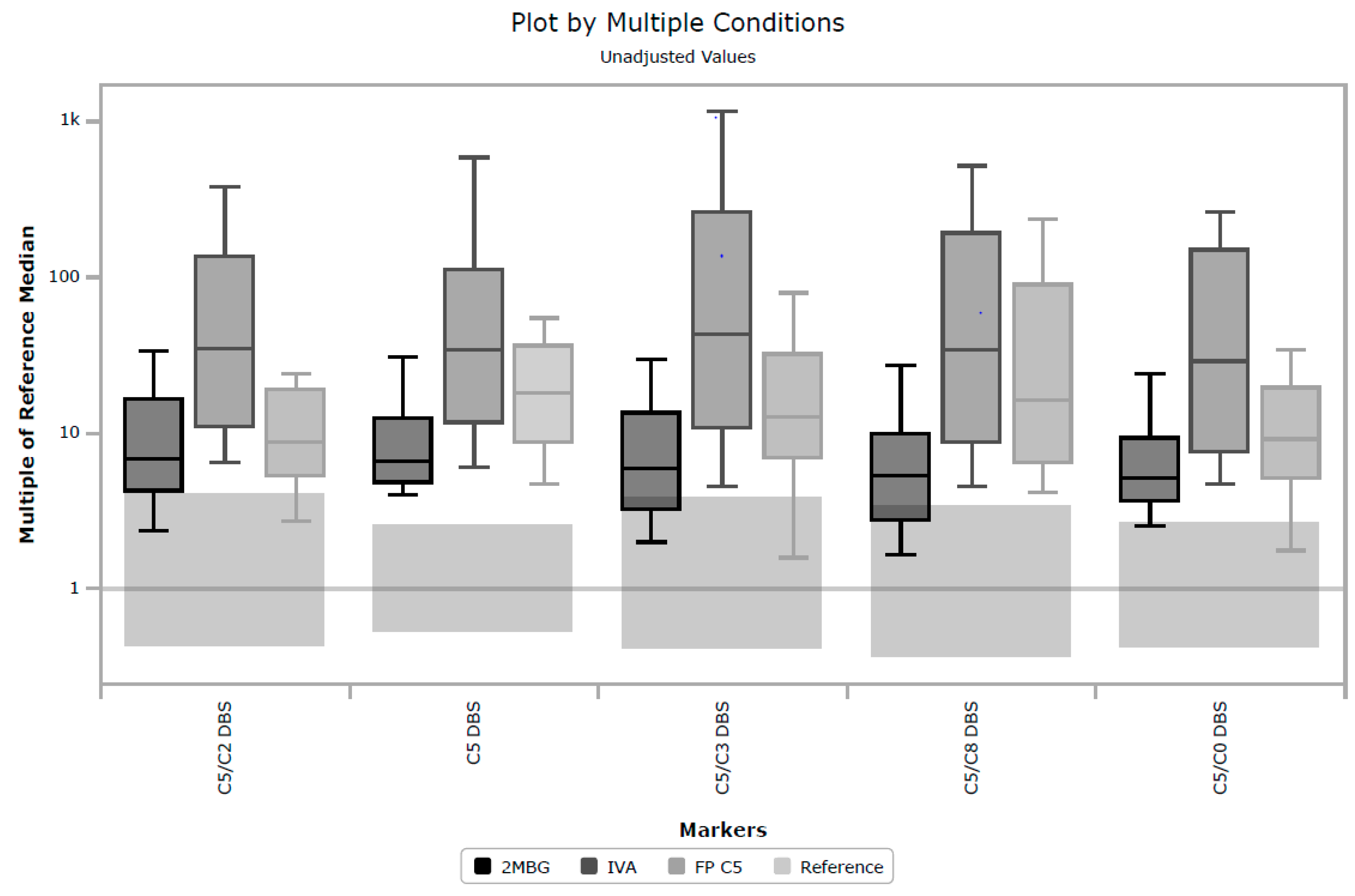
Unfortunately, in a UK context, post-analytical tools in CLIR provided limited utility in eliminating samples known to be FP. There are multiple factors causing this performance, but a key driver to CLIR’s success is taking advantage of multiple analytes and combining small features to discriminate between very similar profiles, for example, 2-methylbutyrylglycinuria, isovaleric acidemia and FP C5 cases.
Figure 3 shows a plot by multiple conditions, based on the cumulative data in CLIR, and illustrates the similarities between these three conditions. The largest elevations in C5 are associated with TP IVA, but milder elevations can be seen in all three conditions. That utility was not able to be fully exploited in this study, due to the limited panel of analytes included in the UK. The shared single condition tool for IVA in CLIR utilizes 21 analytes (combination of amino acids and acylcarnitines), however for the location specific tool created for this study, only eight analytes were available. Furthermore, age at time of specimen collection was only available in whole days, not hours as CLIR is designed to use. Additional case data and further stratification of TP and FP cases, for example, ‘IVA symptomatic’, ‘IVA asymptomatic’ and FP IVA, may allow for the creation of more specifically targeted tools. Post-analytical tools in CLIR can be customized for a specific location, thus a rule to mimic the higher symptomatic IVA cutoff could be included if a specific location were aggressively targeting FP reduction.
An alternative solution to help reduce false positive results due to C5p would be to introduce C5 isobar analysis as a second-tier screening test [
4,
10,
11,
12,
13]. This would successfully address the problem of false positives due to pivalate and prevent the unnecessary referral of these babies. Second-tier testing protocols are becoming increasingly common in screening programs around the world and have enabled additional disorders, e.g., disorders of propionate metabolism, classical homocystinuruia and remethylation disorders, maple syrup urine disease, guanidinoacetate methyl transferase deficiency, to be included in existing programs whilst minimizing FP rates and improving the efficacy of screening [
14,
15,
16,
17,
18]. There are however practical issues to consider in this context as screen positive results for IVA are relatively rare. In the UK there are 16 newborn screening laboratories, and the evidence suggests approximately 14 screen positive cases for IVA per year, this indicates that a typical laboratory would be required to undertake isobar analysis only once per year. Ensuring the quality and robustness of the C5 isobar test, maintaining accreditation and being able to deliver the test when required, at short notice, poses a challenge for several laboratories.
A more immediate solution to mitigate the challenge associated with all laboratories providing second tier testing may be to centralise C5 isobar testing at a small number of centres, although at least two laboratories would be required. This would help maintain a robust service and facilitate continuous competency, external quality assessment and accreditation requirements. However, a drawback of this approach is the resulting delay in clinical referral whilst specimens are transported between laboratories for testing. Fortunately, the initial screening result appears to correlate with disease severity and consequently risk; it is notable that all 17 TP cases with initial C5C concentration < 7.2 µmol/L remained asymptomatic whilst those requiring more intensive care demonstrated initial C5C results > 7.5 µmol/L. This is broadly comparable with finding from the German NBS programme where a review of 84 individuals with IVA confirmed by NBS concluded that an initial C5C concentration <5.6 µmol/L was associated with asymptomatic disease course in most cases [
19].
These findings indicate isobar analysis could be safely conducted in those babies in whom the initial C5C result was less than a conservative COV of 5.0 µmol/L, even though this would introduce a short delay (three to four days) in clinical referral while maintaining immediate referral for those babies whose initial C5C results were over 5.0 µmol/L and likely to be at greater risk. These data suggests that this tiered approach to secondary testing would significantly reduce the burden of FP results when screening for IVA while avoiding a risk of delay for babies whose screening test results indicate that immediate intervention may be warranted.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Example of questionnaire: Supplementary material S1.
Author Contributions
Conceptualization, R.S.C.; methodology, R.S.C.; software, P.H.; data collection, N.F.; T.G.; S.J.M.; J.S.; L.S.; N.T.; H.W.; H.L.; G.P.; M.S.; A.G.; S.S.; M.A.; formal analysis, R.S.C.; investigation, R.S.C..; resources, R.S.C.; J.R.B; data curation, K.H.; writing—original draft preparation, R.S.C., J.R.B, P.H; writing—review and editing, A.C., K.H.; visualization, R.S.C., P.H, K.H.; project administration, R.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the Antenatal & Newborn Screening Research and Innovation Development Advisory Committee, DATE.
Informed Consent Statement
Patient consent was waived - all data was anonymized.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
We acknowledge all staff in UK newborn screening laboratories and the clinicians in Newborn Screening Clinical Centres in the NHS. We also acknowledge Dr Piero Rinaldo for his help and support with CLIR.
References
- Abdenur, J.E.; Chamoles, N.A.; Guinle, A.E.; Schenone, A.B.; Fuertes, A.N. False positive result due to pivaloylcarnitine in a newborn screening programme. J Inherit Metab Dis 1998, 21, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Schoos, R.; de Halleux, V.; Kalenga, M.; Debray, F. Surprising causes of C5-carnitine false positive results in newborn screening. Mol Gen Metab 2014, 111, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Bonham, J.R.; Carling, R.S.; Lindner, M.; Franzson, L.; Zetterstrom, R.; Boemer, F.; Cerone, R.; Eyskens, F.; Vilarinho, L.; Hougaard, D.M.; Schielen, P.C.J.I. Raising awareness of false positive newborn screening results arising from pivalate-containing creams and antibiotics in Europe when screening for isovaleric acidemia. Int Journ Neonatal Screen. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Carling, R.S.; Burden, D.; Hutton, I.; Randle, R.; John, C.; Bonham, J.R. Introduction of a simple second tier screening test for C5 isobars in dried blood spots: Reducing the false positive rate for isovaleric acidemia in expanded newborn screening. J Inherit Meta. Dis 2018, 38, 75–80. [Google Scholar] [CrossRef]
- OpenPrescribing. Available online: https://openprescribing.net/ (accessed on 4th December 2023).
- Mütze, U.; Henze, L.; Gleich, F.; Lindner, M.; Grünert S., C.; Spiekerkoetter, U.; Santer, R.; Blessing, H.; Thimm, E.; Ensenauer, R.; Weigel, J.; et al. Newborn screening and disease variants predict neurological outcome in isovaleric aciduria. J Inherit Metab Dis 2021, 44, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.L.; Marquardt, G.; McHugh, D.M.S.; Currier, R.J.; Tang, H.; Stoway, S.D.; Rinaldo, P. Postanalytical tools improve performance of newborn screening by tandem mass spectrometry. Genet Med 2014, 16, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.D.; Stoway, S.D.; Åhlman, H.; Arora, V.; Caggana, M.; Fornari, A.; Hagar, A.; Hall, P.L.; Marquardt, G.C.; Miller, B.J.; et al. A Novel Approach to Improve Newborn Screening for Congenital Hypothyroidism by Integrating Covariate-Adjusted Results of Different Tests into CLIR Customized Interpretive Tools. Int J Neonatal Screen 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.A.M.; Patnick, J. Blanks R.G. Maximising benefit and minimising harm of screening. BMJ 2008, 336, 480-3. [Google Scholar] [CrossRef] [PubMed]
- Forni, S.; Fu, X.; Palmer, S.E.; Sweetman, L. Rapid determination of C4-acylcarnitine and C5-acylcarnitine isomers in plasma and dried blood spots by UPLC-MS/MS as a second-tier test following flow-injection MS/MS acylcarnitine profile analysis. Mol Genet Metab. 2010, 101, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Cloppenborg, T.; Janzen, N.; Wagner, H.; Steuerwald, U.; Peter, M.; Das, A. Application of a second-tier newborn screening assay for C5 isoforms. JIMD Rep 2014, 13, 23-6. [Google Scholar] [CrossRef]
- Minkler, P.E.; Stoll, M.S.K.; Ingalls, S.T.; Hoppel, C.L. Selective and accurate C5 acylcarnitine quantitation by UHPLC–MS/MS: Distinguishing true isovaleric acidemia from pivalate derived interference. J Chromatogr B 2017, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Murko, S.; Aseman, A.D.; Reinhardt, F.; Gramer, G.; Okun, J.G.; Mütze, U.; Santer, R. Neonatal screening for isovaleric aciduria: Reducing the increasingly high false-positive rate in Germany. JIMD Rep. 2022, 64, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Matern, D.; Tortorelli, S.; Oglesbee, D.; Gavrilov, D.; Rinaldo, P. Reduction of the false-positive rate in newborn screening by implementation of MS/MS-based second-tier tests: The Mayo Clinic experience (2004–2007). J Inherit Metab Dis. 2007, 30, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Oglesbee, D.; Sanders, K.A.; Lacey, J.M.; Magera, M.J.; Casetta, B.; Strauss, K.A.; Tortorelli, S.; Rinaldo, P.; Matern, D. Second-tier test for quantification of alloisoleucine and branched-chain amino acids in dried blood spots to improve newborn screening for maple syrup urine disease (MSUD). Clin Chem 2008, 54, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Calton, L.; Hammond, G.; Brown, H.A.; Wallace, A.M.; Sacchetta, P.; Morris, M. Serum steroid profiling for congenital adrenal hyperplasia using liquid chromatography–tandem mass spectrometry. Clin Chim Acta. 2010, 411, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, G.B.; Ester, M.; Horvath, G.; Karnebeek, C.D.; van Stockler-Ipsirogu, S.; Vallance, H. Integrated Multianalyte Second-Tier Testing for Newborn Screening for MSUD, IVA, and GAMT Deficiencies. J. Inborn Errors Metab Screen. 2016, 4. [Google Scholar] [CrossRef]
- Kilgore, MB.; Platis, D.; Lim, T.; Isenberg, s.; Pickens, C.A.; Cuthbert, c.; Petritis, K. Development of a Universal Second-Tier Newborn Screening LC–MS/MS Method for Amino Acids, Lysophosphatidylcholines, and Organic Acids. Anal Chem 2023, 95, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Mütze, U.; Henze, L.; Schröter, J.; Gleich, F.; Lindner, M.; Grunert, S.C.; et al. Isovaleric aciduria identified by newborn screening: Strategies to predict disease severity and stratify treatment. J Inherit Metab Dis 2023, 46, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).