1. Introduction
Mature ovulated frog eggs are arrested in metaphase of the second meiotic division by high activity of the key meiotic regulators, the maturation promoting factor (MPF) and the cytostatic factor (CSF) [
1]. The meiotic metaphase arrest prevents cell cycle progression and parthenogenesis prior to fertilization. While awaiting fertilization, meiotically arrested eggs grow older and experience various injuries resulting in the loss of their quality. The stress- and age-acquired damage leads to decreased rates of fertilization, polyspermy, parthenogenesis, and abnormal development of embryos. Poor quality of oocytes and eggs is considered to be a main cause of infertility and abnormal embryo development in different animals, including mammals [
2,
3].
As a consequence of progressive quality worsening, ovulated frog and mammalian eggs can be successfully fertilized only within several hours to days following ovulation. It was reported that in the absence of fertilization, ovulated mammalian eggs gradually deteriorate in the process of postovulatory aging, undergo fragmentation and eventually degrade by apoptosis [
4,
5]. Furthermore, unfertilized eggs of the African clawed frog
Xenopus laevis, which are widely used in cell cycle and reproductive studies due to their high biochemical and cytological tractability, spontaneously activate, exit the meiotic metaphase arrest, and degrade by a well-defined apoptotic process, both in external aquatic environments and in the genital tract, within 48 hours after ovulation [
6,
7]. It is widely recognized that spontaneous activation, which promotes exit from the meiotic metaphase arrest, makes successful fertilization in different species impossible [
8,
9].
The intracellular pathways involved in the spontaneous activation of metaphase II-arrested eggs are poorly understood. It was suggested that this process might employ a calcium-dependent mechanism in mammalian eggs [
8,
10,
11]. Artificial elevation of intracellular calcium is known to cause parthenogenetic activation of eggs from different species. However, spontaneous activation may also engage calcium-independent mechanisms. It was found that aged mouse and pig eggs have decreased activities of major CSF and MPF components [
12,
13]. Furthermore, it was demonstrated that apoptosis is triggered by progressive inactivation of the MAP kinase in aging unfertilized sea urchin eggs [
14,
15]. Thus, the gradual decrease in the content and/or activity of the key meiotic regulators was speculated to cause the meiotic exit in the absence of intracellular calcium signal [
9].
Presently, physiological inducers of spontaneous egg activation remain unidentified. It was suggested that oxidative stress might cause this process, leading to expedited aging and deterioration of postovulatory oocytes [
16]. Indeed, hydrogen peroxide was shown to trigger Src kinase- and calcium-dependent activation of
Xenopus frog eggs [
17]. The study also reported that prolonged treatment with hydrogen peroxide leads to excessive egg activation, i.e., overactivation, which gives rise to a very distinctive and easily identified egg phenotype. Fast and irreversible cortical contraction, lipofuscin accumulation, depletion of intracellular ATP, decline in mitochondrial membrane potential, decrease in the content of soluble cytoplasmic protein were found to occur in the frog eggs overactivated by strong oxidative stress. These intracellular events developed in the absence of caspase activation [
18,
19]. It was concluded that the eggs overactivated by strong oxidative stress die by a distinctive calcium-dependent non-apoptotic mechanism [
19].
Markedly, overactivation can occur spontaneously in the absence of activation stimuli, albeit with a low frequency, in the populations of naturally spawned frog eggs. This phenomenon is viewed as a pathological and uncontrollable process that makes eggs fertilization impossible. The cytological and biochemical events of spontaneous overactivation are not characterized. The present study focuses on the spontaneous overactivation of naturally spawned, as well as in vitro-matured, unfertilized Xenopus frog eggs. We demonstrate here that the main cytological and biochemical events of spontaneous overactivation mirror those of the oxidative stress-induced overactivation. We also report here that egg overactivation can occur in the frog’s genital tract and further propose that mechanical stress may be a key factor causing egg overactivation during natural spawning in frogs. Finally, the overactivation-induced loss of plasma membrane integrity strongly suggests that overactivated eggs die by necrosis.
2. Materials and Methods
2.1. Reagents
Water-soluble progesterone (PG), anesthetic MS-222, and ATP Bioluminescence Assay Kit CLS II were purchased from Sigma (St. Louis, MO, USA). Human chorionic gonadotropin (hCG) was from Teikoku Zoki (Tokyo, Japan) and collagenase (280 U/mg) was obtained from Wako (Osaka, Japan). Fluorogenic caspase-3 substrate IV was purchased from Calbiochem (La Jolla, CA, USA), and Apo-ONE homogenous caspase 3/7 assay was from Promega (Madison, WI, USA). Polyclonal anti-cyclin B2 antibody was ordered from Santa Cruz (Santa Cruz Biotechnology, Dallas, TX), biotinylated anti-rabbit IgG was from Vector Laboratories (Burlingame, CA, USA). Polyclonal anti-MAPK and anti-pMAPK antibodies were from Cell Signaling (Beverly, MA, USA). The Streptavidin Biotin Complex Peroxidase Kit and protein assay CBB were from Nacalai Tesque (Kyoto, Japan). Bioluminescent ApoSENSOR ADP/ATP ratio assay kit was purchased from BioVision (Mountain View, CA). Other chemicals were obtained from Wako and Nacalai Tesque.
2.2. Animals and cells
Water-soluble Adult wild-type female frogs Xenopus laevis were purchased from Shimizu (Kyoto, Japan) and maintained in dechlorinated water at the ambient temperature of 21–23°C. The experiments with the animals were conducted according to the Kyoto Sangyo University Animal Experimentation Regulations under the permission N 2018-20. The experiments with oocytes and eggs were carried out at the ambient temperature of 21–23°C.
Egg ovulation was induced by injection of 500 U/animal of human chorionic gonadotropin in the dorsal lymph sac of female frogs. Eggs were collected by squeezing the abdominal parts of the animals in about 12 hours after injection and kept in OR-2 buffer at the ambient temperature. Immature oocytes were isolated as detailed previously [
6]. Briefly, the frogs were anesthetized in 2 mg/ml solution of MS-222, then the ovaries were surgically removed and placed into OR-2 solution containing 82.5 mM NaCl, 2.5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM Na2HPO4, 5 mM HEPES, pH 7.6. The ovaries were manually dissected into clumps of 50–100 oocytes and extensively washed with OR-2 solution. Oocytes were treated with 5 mg/ml collagenase in OR-2 at 21°C for 3 hours by shaking at 60 rpm, extensively washed in OR-2 solution and left for stabilization over 4 hours. Undamaged defolliculated oocytes of stage VI, ranged in size from 1.2 to 1.3 mm, were manually selected and used in experiments.
In vitro oocyte maturation was induced by addition of 5 mM PG and monitored by the appearance of a white spot on the animal hemisphere of oocytes. To obtain crude cytosolic fractions, eggs were homogenized by pipetting in tenfold volume of cold OR-2 buffer containing protease inhibitors APMSF and leupeptin and then centrifuged at 10,000 rpm, 4°C, for 10 min. Supernatant fractions were collected and stored on ice until following biochemical analysis.
2.3. Microscopic observations
Observation and imaging of
Xenopus eggs were carried out using SZX16 stereo zoom microscope (Olympus, Japan) equipped with high-frame digital microscope CCD camera DP73, CCD interface U-TV0.5XC-3, wide-angle objective SDF PLAPO 1xPF. Slide glasses and cover slips for microscopy were purchased from Matsunami Glass (Osaka, Japan). The CellSens Standard software (Olympus) was used for image acquisition. Acquired images were further processed with the ImageJ software of the National Institute of Health [
20] freely available at
https://imagej.nih.gov/ij/.
2.4. Mechanical stress
Mature ovulated eggs surrounded by a jelly layer were subjected to repeated pipetting through a 1 ml plastic pipette tip. The tips for pipetting were cut off at the end and tempered softly in the open fire to attain the size of tip opening that mildly strained the eggs in the process of pipetting. The treated eggs were kept in OR-2 solution and observed for the appearance of morphological features of overactivation over 2 hours.
2.5. Measurements of intracellular ATP and ATP/ADP ratio
Measurement of intracellular ATP and ADP/ATP ratio was carried out using the ATP Bioluminescence Assay Kit CLS II and ApoSENSOR assay kit according to the manufacturer’s manuals. Egg crude cytosolic fractions were obtained as described in
Section 2.2. One-𝜇l fraction aliquots were taken into 100-𝜇l bioluminescence assays. Intensity of luminescence was quantified using the GeneLight GL-220 portable luminometer (Microtec, Funabashi, Japan) within one minute after initiation of luciferase reaction by sample addition.
2.6. Immunoblotting
To monitor cyclin B2 content and MAPK phosphorylation status, crude cytosolic fractions of oocytes and eggs were heated at 95°C for 5 min in the presence of SDS-sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% sucrose, 0.01% BPB, 100 mM DTT). Protein samples were separated by SDS PAGE using 10% polyacrylamide gels and transferred to PVDF membranes using a semidry blotting device from BioRad. The membranes were blocked with T–TBS buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) containing 3 mg/ml bovine serum albumin and incubated at room temperature for 2 hrs with a 200-fold diluted anti-cyclin B2 antibody, 100-fold diluted anti-phospho MAPK, or 200-fold diluted anti-MAPK antibodies. The membranes were extensively washed with T-TBS buffer and treated with a 1000-fold diluted biotinylated anti-rabbit IgG, then with peroxidase-conjugated streptavidin, according to the manufacturer's manual for the Streptavidin Biotin Complex Peroxidase Kit. The immune complexes were detected by color development catalyzed by peroxidase in the presence of hydrogen peroxide and diaminobenzidine tetrahydrochloride.
2.7. Other methods
Protein content in egg cytosolic fractions was determined with the CBB protein assay. Sample absorbance was measured using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Bovine serum albumin was employed as a calibration standard. Caspase activity assay was performed using caspase-3 or caspase 3/7 substrates as described previously [
6]. Quantified data in figures are presented as means ± SD values of four to six replicates. The experiments were repeated with separate batches of eggs obtained from at least three different animals.
4. Discussion
In the present study we investigated a minor subpopulation of overactivated eggs consistently observed amongst spawned frog eggs. Several types of cells can clearly be distinguished by their appearance in aging populations of ovulated unfertilized Xenopus frog eggs. They include mature fertilization-competent eggs, activated eggs with the contracted pigment layer, decoloring apoptotic eggs, and intensively white overactivated eggs [
18]. Normally, the proportion of overactivated eggs is quite low in natural egg populations; it rarely exceeds several percent [
18]. Although the natural triggers of egg overactivation are unknown, it was demonstrated previously that a strong oxidative stress is capable of inducing overactivated phenotype. The hallmark biochemical events of the oxidative stress-induced overactivation have been recently characterized [
18,
19]. Now, one of the main findings of our present study asserts that the major events of spontaneous overactivation, such as irreversible cortical contraction, increase in the egg size, depletion of intracellular ATP, increase in the intracellular ADP/ATP ratio, and degradation of M phase-specific cyclin B2, are largely identical with the previously described events of the oxidative stress-induced process. In addition, similarly to the oxidative stress-induced overactivation, the events of spontaneous overactivation manifest in the eggs within just one hour in the absence of caspase activation. Furthermore, it was found that the same events unfold in the eggs overactivated by a mechanical stress (
Figure 4,
Figure 5 and
Figure 6). These findings suggest that the identical physiological scenario develops in eggs overactivated by various means.
Then, what kind of scenario is this? The finding that ATP is acutely depleted in overactivated eggs strongly implicates necrosis. It was previously proposed that intracellular levels of ATP determine a distinctive way of cell death by apoptosis or necrosis [
21] and further demonstrated that ATP depletion can alter the mode of cell death [
22,
23]. Several studies revealed that necrosis develops in cells treated with drugs or subjected to electric shock under conditions of intracellular ATP depletion [
24,
25,
26]. The phenomenon of ATP depletion was universally observed in the eggs overactivated by oxidative stress [
18] or mechanical stress (
Figure 4 and
Figure 5), as well as in the eggs overactivated spontaneously outside of a frog’s body (
Figure 1 and
Figure 2) or in the genital tract (
Figure 7). The fast depletion of intracellular ATP is one of the hallmarks of classical necrosis that distinguishes it from other types of cell death [
27]. By comparison, the decrease in intracellular ATP occurs quite late in apoptosis, because high ATP levels are necessary to maintain this process [
27,
28]. In particular, apoptosome assembly, which is responsible for caspase activation, requires cytochrome C and ATP/dATP binding. Notably, the events of egg overactivation occur in the absence of caspase activation (
Figure 1,
Figure 2,
Figure 4 and
Figure 7), and they unfold much faster than the hallmark events of the classical apoptotic process described previously in Xenopus eggs [
6,
7]. Similarly to apoptosis, autophagy, the other major mechanism of programmed cell death, which is activated in response to cellular damage, also requires ATP at different steps of this process [
29].
Even more straightforward evidence for the necrotic process in the overactivated frog eggs comes from the finding that ATP and ADP are rapidly released from these cells [
Figure 6]. This fact indicates that plasma membrane integrity is compromised in the overactivated eggs. It was reported that ATP can be released from cells in response to various cell-death inducing conditions, such as hypoxia, cytotoxic agents, hypertonic shock, plasma membrane damage, etc. [
26,
30,
31,
32,
33]. The membrane wounding was found to trigger robust ATP release in sea urchin embryo [
34]. Notably, in our study, overactivated frog eggs were found to lose their ATP not only because of the leakage, but also due to intracellular conversion of ATP to ADP, as witnessed by the increased ADP/ATP ratio in these cells (
Figure 6).
Importantly, although the rapture of the plasma membrane identifies conventionally the necrotic cell death, it is arguable that egg overactivation represents a classical scenario of accidental cell death that was defined recently as “virtually instantaneous and uncontrollable form of cell death corresponding to the physical disassembly of the plasma membrane caused by extreme physical, chemical or mechanical cues” by the Nomenclature Committee on Cell Death [
35]. First, while extremely fast and robust, egg overactivation is not exactly instantaneous - morphological and biochemical changes induced by overactivation develop gradually over time. Thus, the physical damage of the plasma membrane does not lead to immediate massive release of the intracellular content, and the eggs maintain their form and size for some time. Second, it seems that infringement of the plasma membrane can be caused not only by the “extreme cues”, but also by micro perturbations of the intracellular or extracellular environment, as it evidently takes place in the case of spontaneous overactivation. Third, egg overactivation is not an entirely uncontrollable event, as it can be modulated to some extent by calcium chelators, as demonstrated previously [
19]. Finally, egg overactivation develops progressively in a sequential manner, suggesting that it can be a physiological form of regulated and ordered cell death.
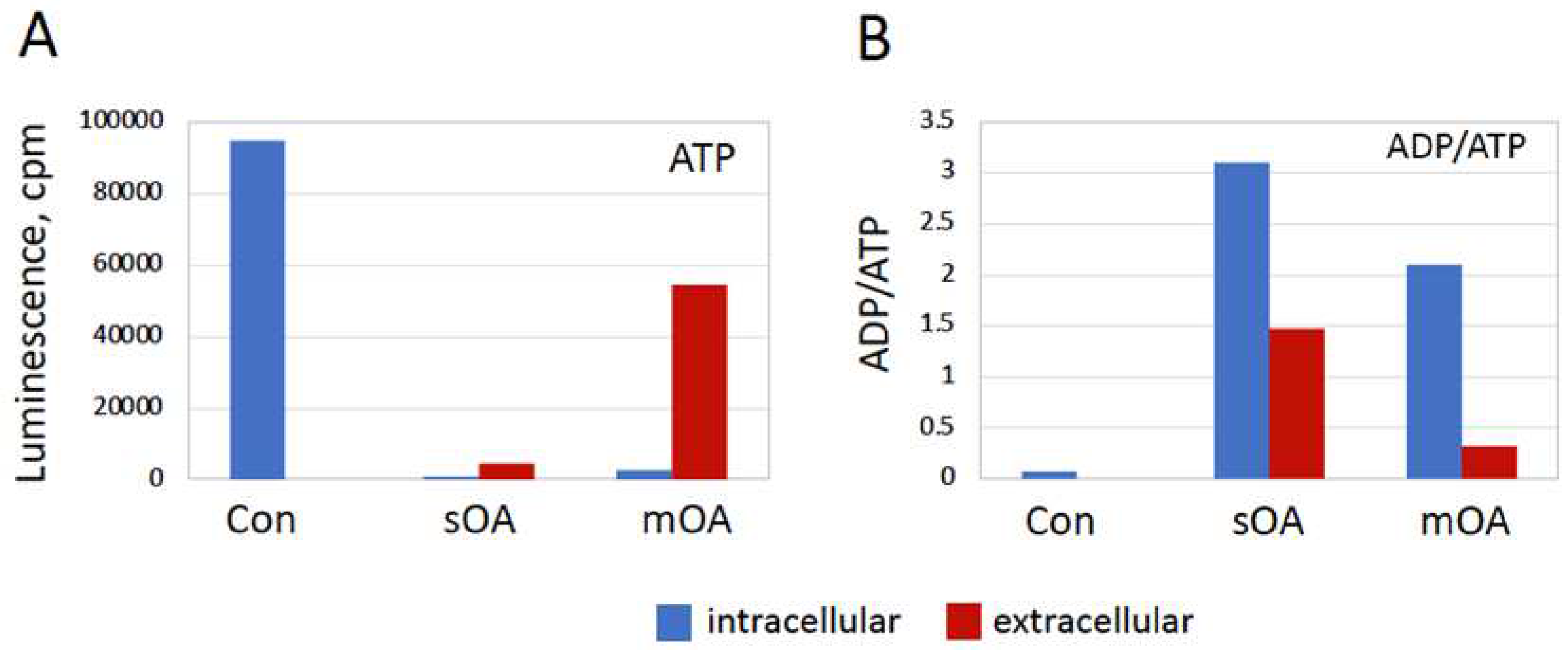
Notably, there still exist some differences between spontaneous and stress-induced overactivation. For instance, the amount of leaked ATP is greater, and the ADP/ATP ratio of both extracellular and intracellular content is lower in the eggs overactivated by mechanical stress, as compared to spontaneously overactivated eggs (
Figure 6). These facts can be explained by a greater damage to the plasma membrane caused by mechanical stress, leading to a faster leakage of ATP before its conversion to ADP by intracellular enzymes. Furthermore, although cyclin B is degraded both in eggs overactivated by mechanical stress and spontaneously overactivated eggs, MAPK becomes dephosphorylated only in the latter case (
Figure 3 and
Figure 5). This discrepancy can also be explained by the different degree of plasma membrane damage in the cells overactivated by different means. Cyclin degradation is initiated by calcium transient upon egg overactivation [
19], and it occurs before downregulation of the MAPK pathway in activated Xenopus eggs [
36,
37]. A relatively mild breach of the plasma membrane in spontaneously overactivated eggs keeps the positive feedback between MPF and CSF [
38] operational for long enough time to bring about CSF inactivation and MAPK dephosphorylation. However, more severe damage of the membrane in stress-overactivated eggs disrupts this feedback rapidly, making MAPK dephosphorylation impossible. Thus, different severity of the plasma membrane damage can account for the observed minor differences between spontaneous and stress-induced overactivation.
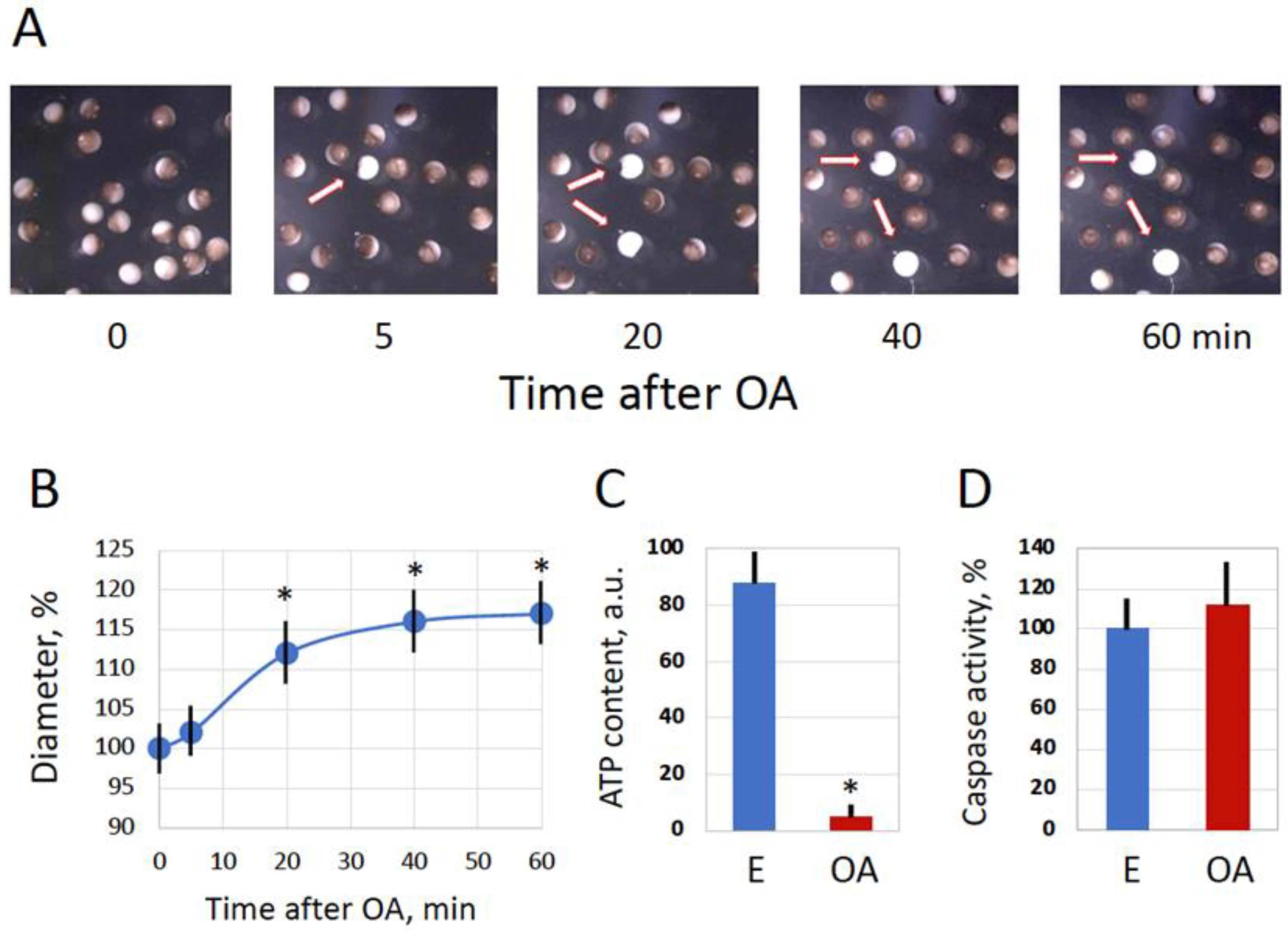
Figure 1.
Spontaneous overactivation of naturally laid Xenopus frog eggs. Phenotypical changes in a subpopulation of naturally laid Xenopus eggs are shown in panel A. Time “0” refers to the time of egg deposition; arrows in the panels point to spontaneously overactivated eggs. The progressive increase in the diameter of overactivated eggs and evaluation of intracellular ATP in normal (E) and overactivated (OA) eggs are presented in panels B and C, respectively. Quantification of caspase activity is shown in panel D. OA eggs in panels C and D were analyzed in one hour after the visually identified beginning of overactivation. Bars in panels represent SD values of the means. More than five cells were analyzed in the panels at each time point. Asterisks in panels B and C indicate statistical difference from control eggs (p<0.05).
Figure 1.
Spontaneous overactivation of naturally laid Xenopus frog eggs. Phenotypical changes in a subpopulation of naturally laid Xenopus eggs are shown in panel A. Time “0” refers to the time of egg deposition; arrows in the panels point to spontaneously overactivated eggs. The progressive increase in the diameter of overactivated eggs and evaluation of intracellular ATP in normal (E) and overactivated (OA) eggs are presented in panels B and C, respectively. Quantification of caspase activity is shown in panel D. OA eggs in panels C and D were analyzed in one hour after the visually identified beginning of overactivation. Bars in panels represent SD values of the means. More than five cells were analyzed in the panels at each time point. Asterisks in panels B and C indicate statistical difference from control eggs (p<0.05).
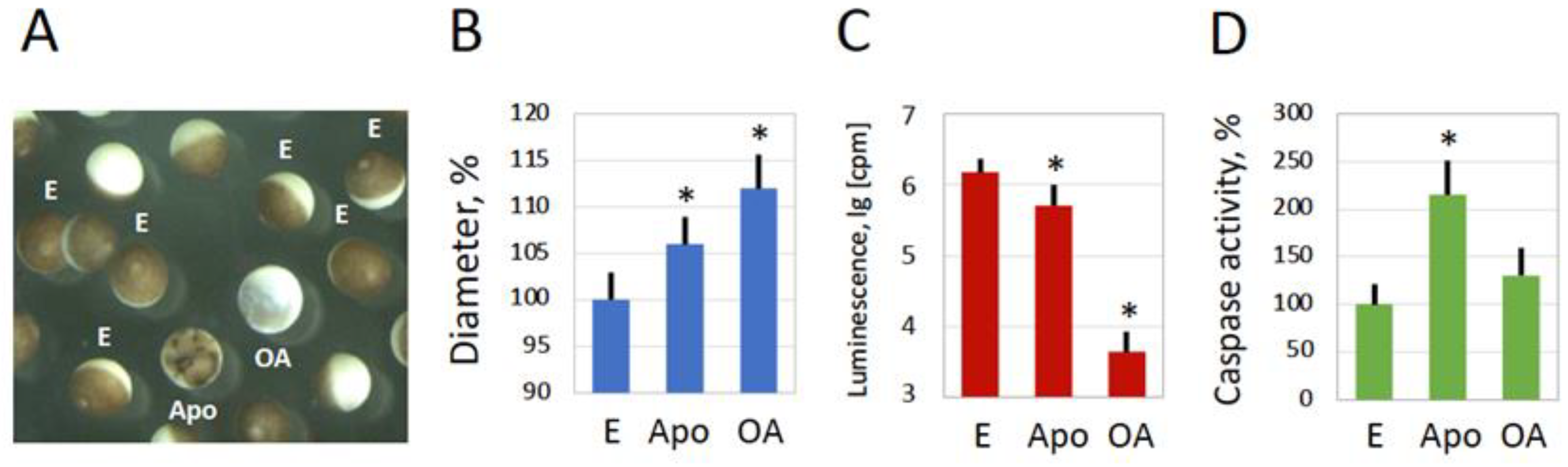
Figure 2.
Spontaneous overactivation of in vitro-matured frog eggs. Morphological types of metaphase-arrested (E), overactivated (OA), and apoptotic (Apo) eggs observed in a subpopulation of defolliculated in vitro matured Xenopus eggs in 24-30 hours after hormone administration are highlighted in panel A. Measurements of egg diameter and intracellular ATP content are presented in panels B and C, respectively. Quantification of caspase activity in the analyzed cell types is presented in panel D. Overactivated eggs were analyzed in one hour after the visually identified beginning of overactivation, metaphase-arrested and apoptotic eggs - at 24-30 hours after hormone administration. Bars in panels represent SD values of means. Asterisks in panels B, C and D indicate statistical difference from control eggs (p<0.05). More than five cells of each morphological type were analyzed in panels B, C and D.
Figure 2.
Spontaneous overactivation of in vitro-matured frog eggs. Morphological types of metaphase-arrested (E), overactivated (OA), and apoptotic (Apo) eggs observed in a subpopulation of defolliculated in vitro matured Xenopus eggs in 24-30 hours after hormone administration are highlighted in panel A. Measurements of egg diameter and intracellular ATP content are presented in panels B and C, respectively. Quantification of caspase activity in the analyzed cell types is presented in panel D. Overactivated eggs were analyzed in one hour after the visually identified beginning of overactivation, metaphase-arrested and apoptotic eggs - at 24-30 hours after hormone administration. Bars in panels represent SD values of means. Asterisks in panels B, C and D indicate statistical difference from control eggs (p<0.05). More than five cells of each morphological type were analyzed in panels B, C and D.
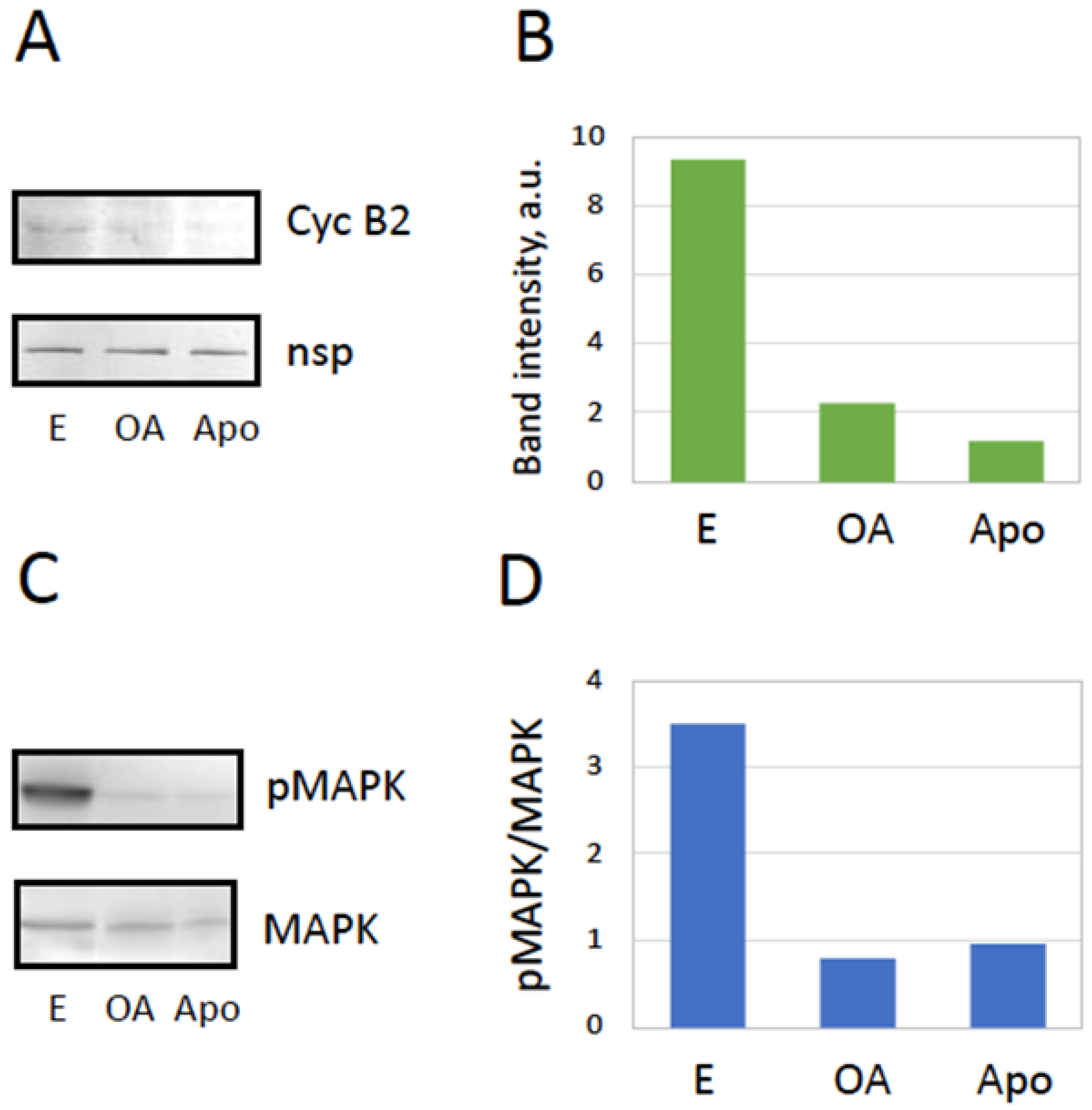
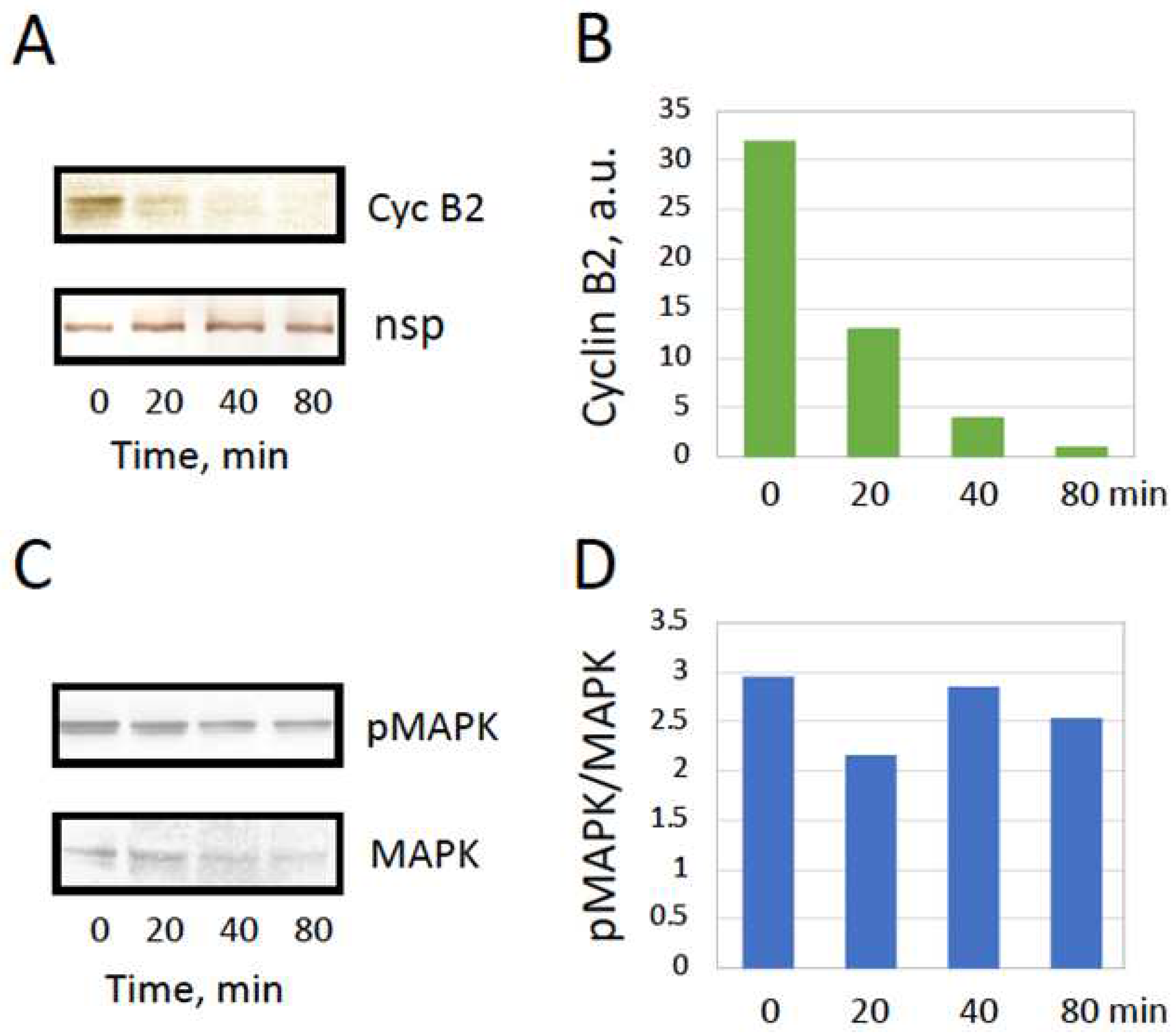
Figure 3.
Meiotic exit after spontaneous overactivation. Evaluation of cyclin B2 contents in metaphase-arrested (E), overactivated (OA), and apoptotic (Apo) eggs is presented in panels A and B, and the phosphorylation state of MAP kinase is assessed in panels C and D. Protein content in the samples was normalized by the intensity of a nonspecific protein band (nsp) detected in the blots. Eggs were matured in vitro in the presence of progesterone and examined in 24-30 hours after hormone administration. The experiment was repeated with three separate batches of eggs and the results of a single-bath experiment are shown. More than five cells of each cell type were subjected to analysis.
Figure 3.
Meiotic exit after spontaneous overactivation. Evaluation of cyclin B2 contents in metaphase-arrested (E), overactivated (OA), and apoptotic (Apo) eggs is presented in panels A and B, and the phosphorylation state of MAP kinase is assessed in panels C and D. Protein content in the samples was normalized by the intensity of a nonspecific protein band (nsp) detected in the blots. Eggs were matured in vitro in the presence of progesterone and examined in 24-30 hours after hormone administration. The experiment was repeated with three separate batches of eggs and the results of a single-bath experiment are shown. More than five cells of each cell type were subjected to analysis.
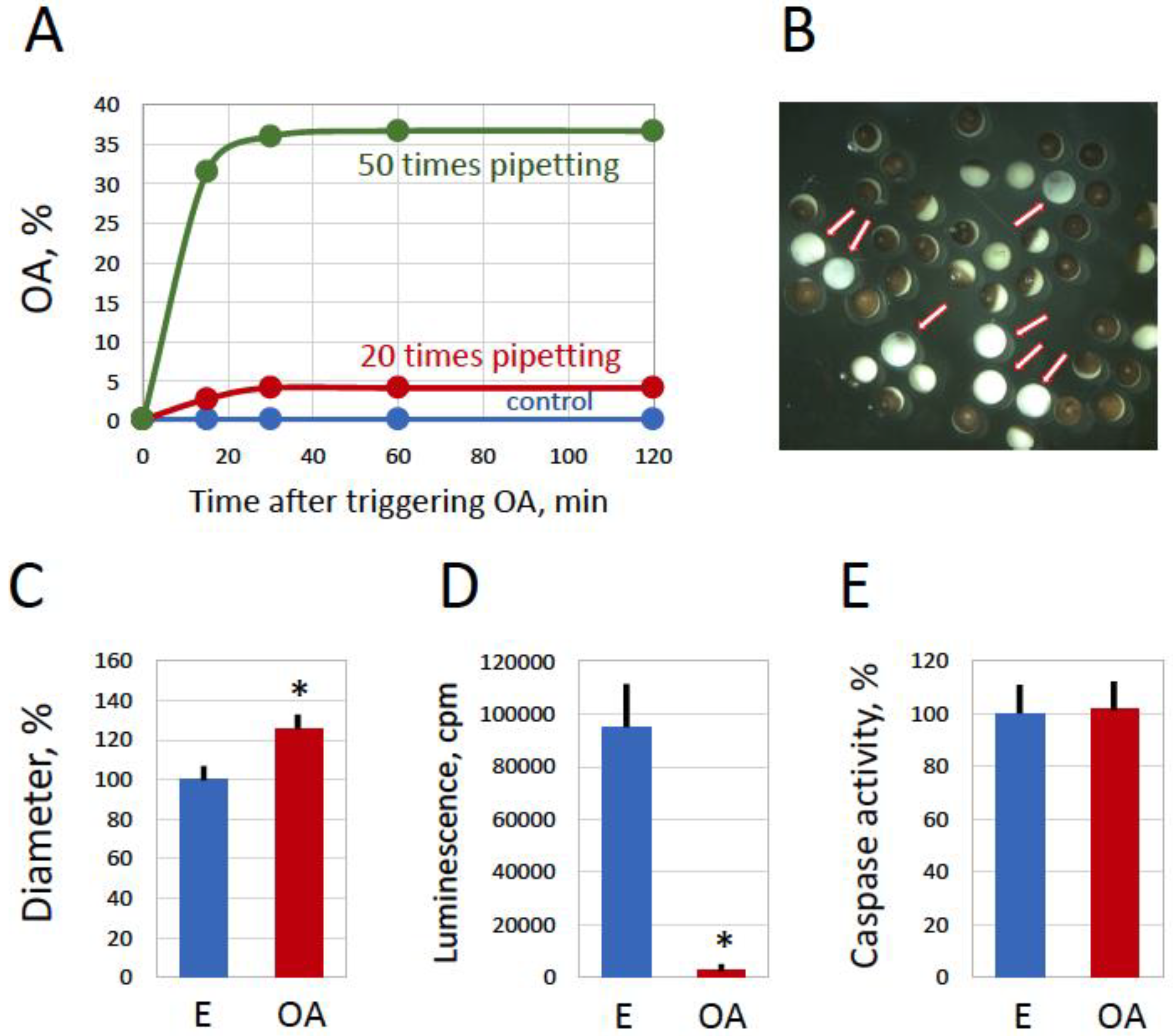
Figure 4.
Mechanical stress is capable of inducing egg overactivation. Overnight-ovulated frog eggs surrounded by a jelly layer were subjected to the repeated pipetting through a cut plastic pipette tip. The emergence of overactivated eggs within 2 hours after pipetting 20 (red line) and 50 (green line) times is presented in panel A. A mixed population of normal and overactivated eggs observed in 2 hours after the pipetting is shown in panel B. Arrows in the panel point to overactivated eggs. Measurements of egg diameter and intracellular ATP content performed in 2 hours after the pipetting are presented in panels C and D, respectively. Panel E shows quantification of caspase activity in the control and overactivated eggs. Bars in panels C, D and E represent SD values of the means. Asterisks in panels C and D indicate statistical difference from control eggs (p<0.05). More than five cells of each morphological type were analyzed in the panels.
Figure 4.
Mechanical stress is capable of inducing egg overactivation. Overnight-ovulated frog eggs surrounded by a jelly layer were subjected to the repeated pipetting through a cut plastic pipette tip. The emergence of overactivated eggs within 2 hours after pipetting 20 (red line) and 50 (green line) times is presented in panel A. A mixed population of normal and overactivated eggs observed in 2 hours after the pipetting is shown in panel B. Arrows in the panel point to overactivated eggs. Measurements of egg diameter and intracellular ATP content performed in 2 hours after the pipetting are presented in panels C and D, respectively. Panel E shows quantification of caspase activity in the control and overactivated eggs. Bars in panels C, D and E represent SD values of the means. Asterisks in panels C and D indicate statistical difference from control eggs (p<0.05). More than five cells of each morphological type were analyzed in the panels.
Figure 5.
Cyclin B2 content and MAPK phosphorylation in the eggs overactivated by mechanical stress. Evaluation of cyclin B2 contents (panels A and B) and the phosphorylation state of MAP kinase (panels C and D) in the overactivated eggs analyzed at different times after the visually identified beginning of overactivation are presented. Protein content in the samples was normalized by the intensity of a nonspecific protein band (nsp) detected in the blots. Xenopus eggs were matured in vitro in the presence of progesterone and examined in 12 hours after hormone administration. The experiment was repeated with three separate batches of eggs obtained from different animals and the results of a single-bath experiment are shown. More than five cells were analyzed at each time point.
Figure 5.
Cyclin B2 content and MAPK phosphorylation in the eggs overactivated by mechanical stress. Evaluation of cyclin B2 contents (panels A and B) and the phosphorylation state of MAP kinase (panels C and D) in the overactivated eggs analyzed at different times after the visually identified beginning of overactivation are presented. Protein content in the samples was normalized by the intensity of a nonspecific protein band (nsp) detected in the blots. Xenopus eggs were matured in vitro in the presence of progesterone and examined in 12 hours after hormone administration. The experiment was repeated with three separate batches of eggs obtained from different animals and the results of a single-bath experiment are shown. More than five cells were analyzed at each time point.
Figure 6.
Changes in ATP and ADP contents elicited by egg overactivation. The relative ATP content in the intracellular and extracellular compartments of spontaneously (sOA) and mechanically (mOA) overactivated eggs, as well as in control (Con) untreated eggs, is presented in panel A. The ADP/ATP ratio is shown in panel B. Data for the intracellular and extracellular compartments are presented in blue and red colors, accordingly. All measurements were performed in one hour after the visually identified beginning of overactivation. The experiment was repeated with four different eggs of each cell type (Con, sOA and mOA) and the results of a single-cell experiment are presented.
Figure 6.
Changes in ATP and ADP contents elicited by egg overactivation. The relative ATP content in the intracellular and extracellular compartments of spontaneously (sOA) and mechanically (mOA) overactivated eggs, as well as in control (Con) untreated eggs, is presented in panel A. The ADP/ATP ratio is shown in panel B. Data for the intracellular and extracellular compartments are presented in blue and red colors, accordingly. All measurements were performed in one hour after the visually identified beginning of overactivation. The experiment was repeated with four different eggs of each cell type (Con, sOA and mOA) and the results of a single-cell experiment are presented.
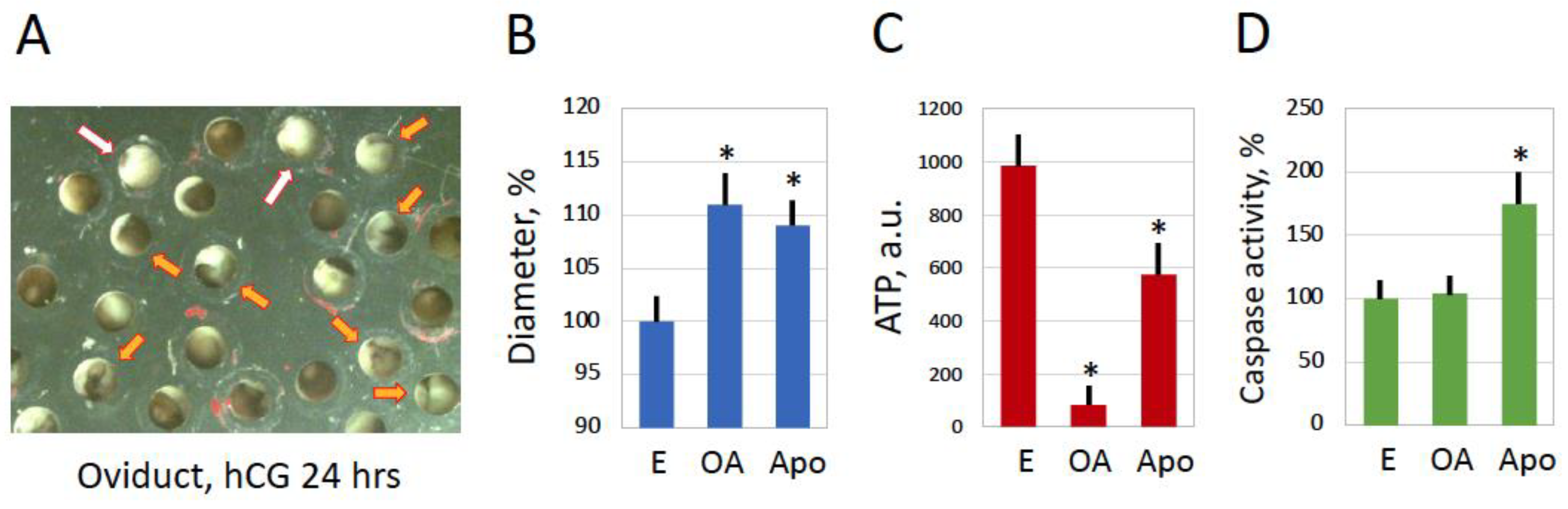
Figure 7.
Occurrence of egg overactivation in the frog’s genital tract. A sub-population of Xenopus eggs retained in the genital tract for 30 hours after hCG injection is shown in panel A. White and orange arrows in the panel point to overactivated (OA) and apoptotic (Apo) eggs, accordingly. Measurements of egg diameter and intracellular ATP content are presented in panels B and C, respectively. Quantification of caspase activity in the analyzed cell types is presented in panel D. Bars in panels B, C and D represent SD values of means. Asterisks in panels B, C and D indicate statistical difference from control eggs (p<0.05). More than ten cells of each morphological type were analyzed in the panels.
Figure 7.
Occurrence of egg overactivation in the frog’s genital tract. A sub-population of Xenopus eggs retained in the genital tract for 30 hours after hCG injection is shown in panel A. White and orange arrows in the panel point to overactivated (OA) and apoptotic (Apo) eggs, accordingly. Measurements of egg diameter and intracellular ATP content are presented in panels B and C, respectively. Quantification of caspase activity in the analyzed cell types is presented in panel D. Bars in panels B, C and D represent SD values of means. Asterisks in panels B, C and D indicate statistical difference from control eggs (p<0.05). More than ten cells of each morphological type were analyzed in the panels.