1. Introduction
Marine bioinvasions are to date recognized as a global threat to the integrity of natural ecosystems and the activities of human populations; accordingly, they are acknowledged as one of the top conservation concerns worldwide [
1]. Increasing efforts are devoted to horizon scanning exercises, allowing an early detection of potentially invasive species (IAS hereafter) and, in turn, the implementation of rapid eradication procedures [
2,
3,
4,
5]. Indeed, effective eradication of marine IAS has only been achieved when species were detected early, and management responded rapidly [
6]. However, mitigating the effect of successful bioinvasions through complete eradication is problematic in marine habitats due to their high environmental connectivity [
7]. In contrast, functional eradication i.e. the reduction of population densities below levels that impacts on the native ecosystem are considered acceptable, is to date acknowledged as an appropriate and effective control strategy to manage marine IAS and to mitigate the negative effects on the delivery of goods and services of invaded ecosystems [
8,
9]. This is particularly true when bioinvaders are species of economic interest, so that functional eradication can be combined with commercial exploitation [
10,
11,
12].
The Atlantic blue crab
Callinectes sapidus (Rathbum,1896) (Decapoda, Brachyura, Portunidae) is native to the western coasts of the Atlantic Ocean with a distribution ranging from Nova Scotia to northern Argentina [
13,
14,
15]. The species was introduced in European waters during the first half of the XXth century [
16] and, to date, it can be found almost ubiquitously in the Mediterranean and Black Sea, as well as along the European and African Atlantic coasts [
15,
17]. The invasive success of
C. sapidus relates with its r-selected life history traits of high fecundity, dispersal capacity and fast growth, together with its broad environmental tolerance, large body size and aggressive behavior [
16]. Noticeably, negative impacts on fishing activities have been repeatedly highlighted due to e.g., consumption of commercial fish caught in traps and damage of nets, especially where established population of
C. sapidus are present [
18,
19]. Moreover, long-term impacts in invaded areas have been observed, related with declines in abundance of native species, and suggesting that enduring negative effects may occur also at relatively low blue crab densities [
20].
In native areas, the blue crab is a valuable shellfish product, supporting important commercial and recreational fisheries [
21,
22]. Noticeably, since the beginning of the nineteenth century, in the eastern United States hard-shell blue crab production has been paralleled by aquaculture activities dedicated to soft-shell crabs [
23]. The term soft-shell crab (SSC hereafter) refers to the state of any crab that has just completed the ecdysis, replacing the exoskeleton with a new decalcified and soft one. Hard-shelled crabs are characterized by a low meat yield (generally between 20 and 25%), while SCC can be consumed as a whole with an almost 100% meat yield, making them a much-appreciated and highly valued delicacy [
23,
24,
25]. In the Mediterranean Sea, hard-shell commercial fisheries have also developed in Turkey [
26] and northern Greece [
27,
28], increasing the economic interest towards this species across invaded Mediterranean countries. On the other hand, artisanal fisheries along the Adriatic coasts are currently struggling to find a solution to cope with the presence of established populations of the crab (
https://www.catchupfish.it/prodotti-tecnici/).
A possible solution to increase the commercial interest in blue crabs from invaded areas and ensure their sustainable use is by generating higher added value and diversify the market demand by developing SSC production [
29]. Here, we focused on the blue crab population in the Lesina Lagoon (Apulia Region, SE Italy), where the occurrence of the species was first recorded in 2007 and repeatedly confirmed in the following years [
30,
31]. To date, blue crabs represent a predominant component of the by-catch in the basin and the species is perceived as a nuisance by anglers, given its low market price and limited interest from local processing companies. Specifically, the main objectives of the present study were to:
- i)
explore for the first time in Europe the suitability of a pond system to produce SSC; to this end, the flow-through or recirculating cultivation facilities in use in native areas to rear pre-molt individuals of
C. sapidus (locally known as peelers), were taken as a model [
24];
- ii)
investigate the molting process in blue crabs from the Lesina Lagoon, and verify if the population is characterized by a predictable molting pattern similar to that observed in native areas.
Indeed, in recent years a number of investigations have focused on the biology and ecology of
C. sapidus in Mediterranean waters, in terms of distribution [
15,
18,
32] population structure and dynamics [
33] and trophic habits ([34-37
]]. Noticeably, no efforts have been made to provide detailed information on this crucial phase of the biological cycle of the species in invaded areas.
2. Materials and Methods
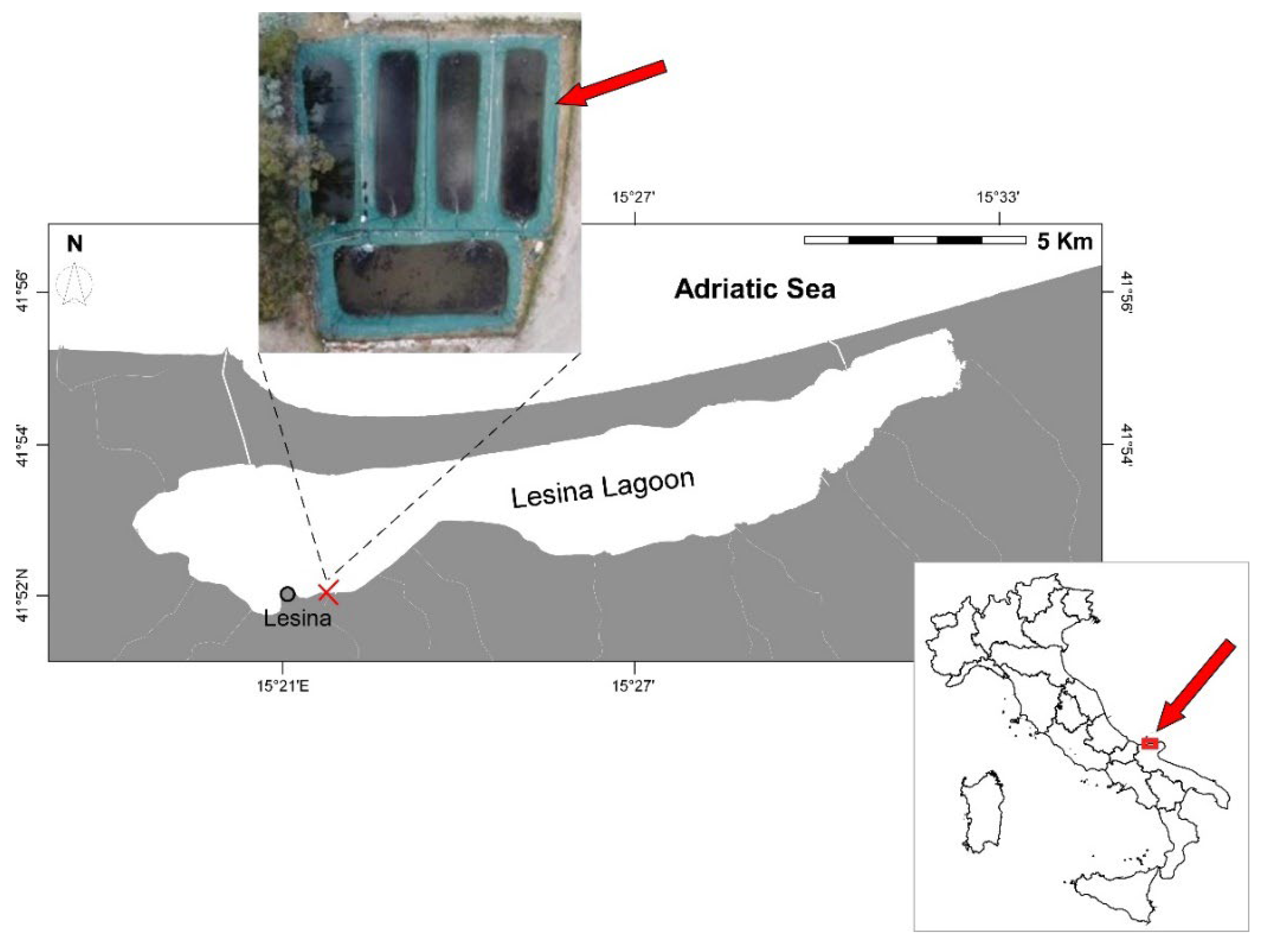
The Lesina Lagoon (41.88°N and 15.45°E;
Figure 1) is a microtidal shallow (0.7-1.5 m) lagoon located along the south-western Adriatic coasts of Italy. It has a total water surface of 51.4 km2 separated from the Adriatic Sea by a sandy isthmus about 18 km and connected to it by two tidal channels: Acquarotta to the West, and Schiapparo to the East [
31,
38].
The lagoon's main hydrological features, temperature and salinity follow a seasonal trend, with minimum values in winter and maximum values in summer [
39]. Anglers operate in the lagoon using mainly fixed nets, known locally as “paranze”. These are nylon seines with a 6-mm mesh placed perpendicularly to the shores, which convey the prey into fyke-nets with a 4 to 6 -mm mesh installed at regular intervals along them [
40].
The trial was performed in a pond located in a semi-enclosed facility located on the southern banks of the Lesina Lagoon close to the IRBIM-CNR Institute. The facility was constituted by a system of four interconnected artificial ponds (
Figure 1) waterproofed by polyethylene sheets and an average area of 100 m
2 and a depth of 1.5 m deep (
Figure 1). The water circulation was open, and directly connected with the lagoon by means of a flow-through system connected to an underwater electric pump (Wilo-Emu FA). Taking as a reference published information on farms producing soft-shell portunid crabs in Americas and Asia [
24] a floating boxes system was built up inside one of the ponds. These devices avoid cannibalism, since each crab was kept in small individual polyethylene cages (30 x 40 x 30 cm) immersed close to the water surface and supported by a floating system anchored to the side of the pond (
Figure 2).
The trial took place in July 2020 as preliminary studies on blue crab’s population dynamics in the basin showed abundance peaks of catches during warmer months, as well as a higher abundance of individuals in the pre-molt phase (Cilenti, personal observation).
Figure 2.
Floating-boxes system anchored to the side of the pond (A) and detail (B) of the polyethylene cages deployed to keep the individuals of Callinectes sapidus.
Figure 2.
Floating-boxes system anchored to the side of the pond (A) and detail (B) of the polyethylene cages deployed to keep the individuals of Callinectes sapidus.
80 randomly-chosen specimens of
C. sapidus captured using fyke-nets and gillnets by local anglers were transferred using aerated tanks to the laboratories of the IRBIM-CNR Institute of Lesina, where they were inspected. Only 50 specimens showed intact appendages; accordingly, they were selected and divided in three groups based on pre-molt signs. Although there are several macroscopic indicators of the pre-molt phase [
41], specimens’ selection was carried out by inspecting the coloration of the line along the inside edge of the last flattened section of the pereiopods, where the new shell formation is more visible.
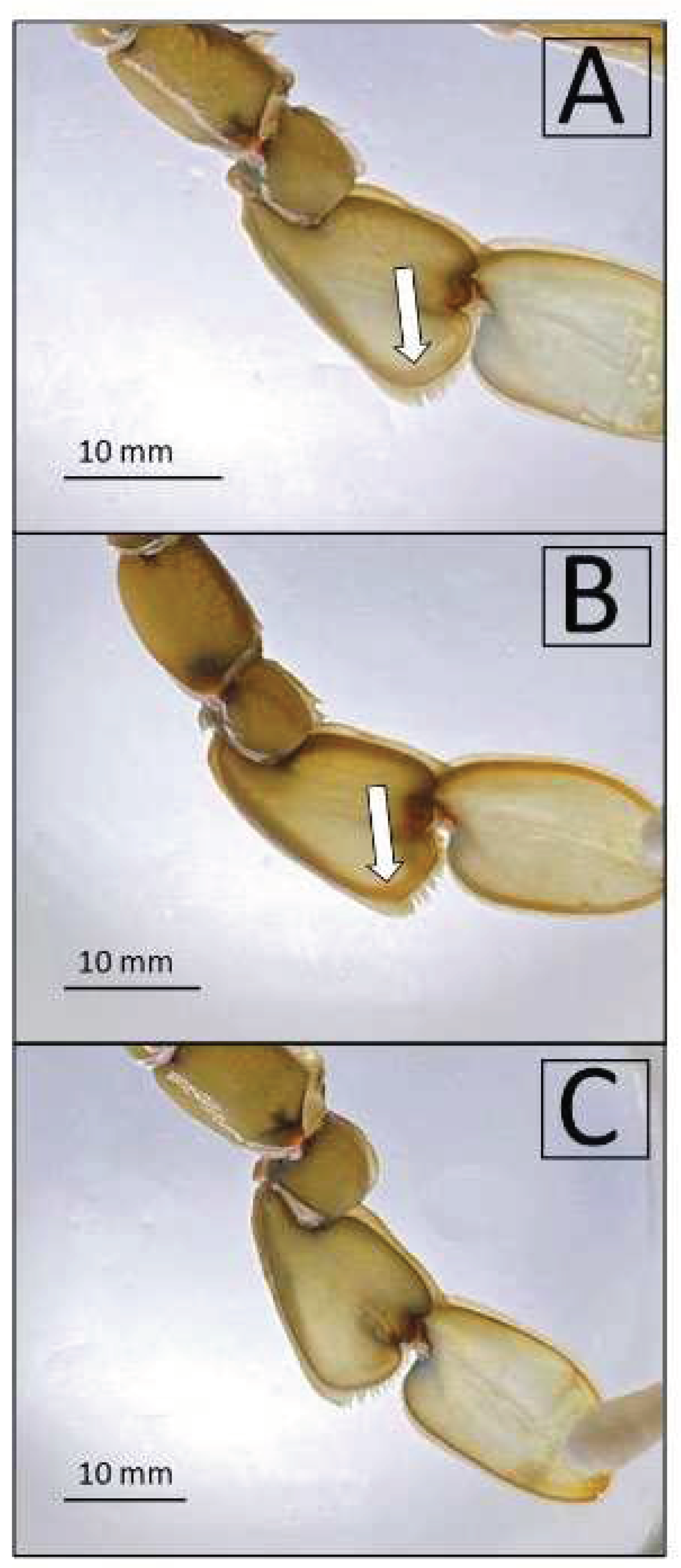
Figure 3.
Presence (A and B) and absence (C) of the macroscopic sign indicators of the pre-moult phase in the inside edge of the last flattened sections of the pereiopod. In figure A the arrow indicates the white line characteristic of an early pre-moult stage, while in figure B the arrow indicates the red line indicative of an advance pre-moult stage and the proximity of ecdysis.
Figure 3.
Presence (A and B) and absence (C) of the macroscopic sign indicators of the pre-moult phase in the inside edge of the last flattened sections of the pereiopod. In figure A the arrow indicates the white line characteristic of an early pre-moult stage, while in figure B the arrow indicates the red line indicative of an advance pre-moult stage and the proximity of ecdysis.
This is the most reliable and widely used method to identify the pre-molt stage [
41]. A white line indicates that the specimen will molt in two weeks; as molting approaches, the color gradually turns to pink and then red, indicating that the crab will molt within one week and one-three days respectively [
41,
42] (
Figure 3). Accordingly, three groups were identified: (1) 20 individuals showing the presence of a white line (W group hereafter), (2) 20 individuals showing the presence of a pink to red line (R group), and (3) 10 individuals showing no line along the edge of the flatten pereiopods (C group). The sex of all individuals was determined by examination of the apron shape; their carapace width (CW) and carapace length (CL) were measured using a caliper (to the nearest 0.1 cm), while their wet weight (WW) was measured using a digital balance (to the nearest 0.1 g). Subsequently, individual crabs were randomly introduced into the polyethylene cages.
The trial lasted 31 days. During the study, water circulation in and out the system was kept continuously active, ensuring an optimal water exchange between the lagoon and the pond. No food were provided, as crabs in the pre-molt stage do not feed [
41]. Cages were inspected three times a day (07:00, 14:00 and 21:00) to record molting or mortality cases. Once molting was observed, crabs were removed from the cage. Pond’s water temperature (°C), salinity (PSU), dissolved oxygen (mg L
-1) and percentage of oxygen saturation (%) were monitored weekly in triplicate using a multi-parametric probe (Hydrolab DS5).
Values in the text are expressed as mean ± 1 SD if not otherwise specified. Data were preliminarily checked for normality (Shapiro-Wilk test) and homoscedasticity (Cochran’s C-test); when necessary, they were square root- or log(x+1)-transformed to meet the required assumptions. Two-way ANOVAs with “sex” (two levels) and “treatment” (three levels) as orthogonal fixed factors were performed to test the differences in biometric parameters (CW, CL, and WW) among the three groups of crabs as affected by sex. Mortality and molting rates were compared using “N-1” χ-square tests as generally recommended [
43,
44]. Given the peculiar characteristics of the data (see Results), a Type III (partial sum of squares) one-way permutational multivariate analysis of variance (PERMANOVA; Anderson, 2005)
with 9,999 unrestricted permutation of raw data was subsequently used to test for among-group differences in the number of days taken by specimens to molt.
3. Results
3.1. Crabs Sex Ratio and Biometry at the Start of the Trial
The overall female-to-male sex ratio of the 50 crab specimens involved in the trial was 0.68:0.32. The sex ratio for the control group was 0.7:03, while for the R and W group was respectively 0.55:0.45 and 0.8:0.2 (
Table 1), yet no significant differences across the three treatments were determined (max χ
2 = 2.76, P = 0.11, 1 d.f. estimated for the comparison Group W vs. Group R).
The overall mean (± 1SD) individual carapace width, length, and wet weight of the specimens from the three treatments were 107.6 ± 13.3 mm, 53.1 ± 5.4 mm, and 100.3 ± 34.3 g. The crabs in the C group had a mean CW, CL, and WW of 110.1 ± 13.8 mm (88 - 126 mm min-max range), 53.3 ± 4.5 mm (46 - 60 mm min-max range), and 110.2 ± 38.9 g (58.1 - 168.1 g min-max range), respectively. In the W group, specimens had a mean CW, CL, and WW of 111.15 ± 15.5 mm (90 - 127 mm min-max range), 54.7 ± 4.9 mm (45 - 63 mm min-max range), and 105.4 ± 29.3 g (60.9 - 152.1 g min-max range), respectively.
Table 1.
Mean individual carapace width (CW), carapace length (CL), and wet weight (WW) the crab specimens included in the different treatments at the start of the trial. Standard deviations are reported in round brackets, while the number of specimens for each combination treatment/sex are in square brackets.
Table 1.
Mean individual carapace width (CW), carapace length (CL), and wet weight (WW) the crab specimens included in the different treatments at the start of the trial. Standard deviations are reported in round brackets, while the number of specimens for each combination treatment/sex are in square brackets.
| Treatment |
Sex |
CW (mm) |
CL (mm) |
WW (g) |
| C group |
F [7] |
108.29 (12.13) |
53.86 (4.85) |
100.54 (27.83) |
| |
M [3] |
114.33 (19.35) |
52.01 (4.01) |
132.71 (58.39) |
| W group |
F [16] |
113.44 (11.87) |
55.06 (4.99) |
108.64 (28.05) |
| |
M [4] |
102.01 (12.08) |
53.01 (4.76) |
92.39 (34.97) |
| R group |
F [11] |
102.45 (11.94) |
50.18 (4.85) |
84.51 (23.59) |
| |
M [9] |
103.22 (14.78) |
52.89 (7.15) |
97.36 (47.08) |
Finally, the CW of crabs in group R ranged between 84 - 128 mm and averaged 102.8 ± 12.9 mm, with a CL ranging between 45 and 66 mm (51.4 ± 6 mm, mean ± 1SD), and a WW comprised between 50.6 and 187.4 g (90.3 ± 35.6 g, mean ± 1SD). In addition, sex-related differences in the body size of crabs in each treatment were observed (
Table 1), yet in general, no significant effects were observed for either the factor “treatment” or “sex” on the variation in body size of the crabs (
Table 2).
3.2. Pond Water Parameters
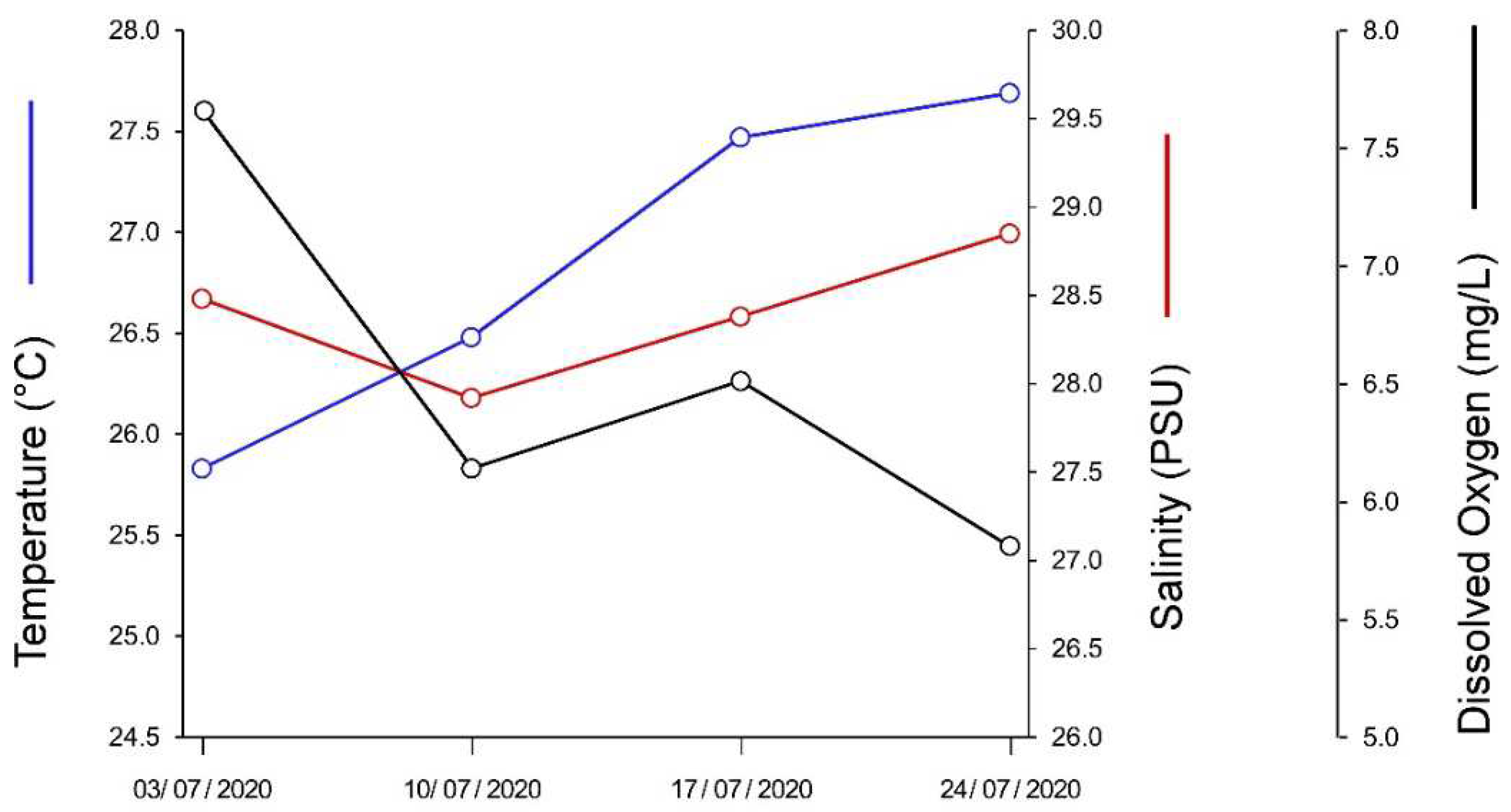
During the trial, the temperature of the water in the pond increased from 25.8 to 27.7°C (
Figure A1 in
Appendix A), paralleled by a decrease in the concentration of the dissolved oxygen concentration from 7.7 to 5.8. Salinity decreased in the first week of the trail from an initial value of 28.5 PSU to 27.9, and then increased up to 28.9 PSU. Despite the observed variations, all water parameters remained in the ranges suggested for optimal maintenance conditions of peelers (
Table 3).
3.3. Crabs Mortality and Molting during the Trial
In total, seven specimens died during the trial (
Table 4), with an overall mortality rate of 14%. No significant differences in mortality rates were observed across treatments (max χ
2 = 0.04,
P = 0.84, 1 d.f. for the comparison Group W vs. Group R). Noticeably, the majority of the mortality events (5) occurred during the first 24 h, and one crab from the R group died during the process of molting after 48 h.
The lowest frequency of molting events occurred in the C group, where only one crab molted after 17 days from the beginning of the trial. Conversely, the highest frequencies occurred in crabs belonging to groups W and R, with a molt rate of 65.0% and 85%, respectively (
Table 4).
Table 4.
Mortality and molting rates of the individuals held in the floating-boxes system.
Table 4.
Mortality and molting rates of the individuals held in the floating-boxes system.
| Treatment |
Dead |
Molting |
Non-molting |
Days to molting |
| C group |
2 (20%) |
1 (10%) |
7 (70%) |
17 |
| W group |
2 (10%) |
13 (65%) |
5 (25%) |
13.07 ± 6.91 |
| R group |
3 (15%) |
17 (85%) |
0 |
4 ± 1.27 |
Negligible differences were observed between them (χ
2 = 2.08,
P = 0.14, 1 d.f.), yet both treatments showed frequencies significantly higher than that observed in the control group (W group vs. C group: χ
2 = 7.83,
P = 0.005; R group vs. C group: χ
2 = 15.09,
P = 0.0001; 1 d.f. for both tests). In addition, the number of days taken by specimens to molt varied significantly across treatments (one-way PERMANOVA, pseudo-F
2,30 = 25.07,
Pperm = 0.0001). On average, crabs in the R group molted after 4 days, significantly earlier than what observed for both the C and W groups (
Table 4; post-hoc bivariate comparisons: minimum
t = 4.46,
P = 0.032 for the comparison group R vs. group C). In contrast, no significant differences were observed between group C and W (
t = 0.71,
P = 0.78).
4. Discussion
Our study indicated that for
Callinectes sapidus from the Lesina Lagoon the color of the propodus and dactylus of the fifth pereiopod can be successfully used as an indicator of the progress of the molting process. A visual inspection allowed the identification of peelers, and accordingly, to separate individuals in the pre-molt stage from those in the intermolt stage as previously described [
42]. These results are comparable to those observed in a study on molting success conducted in Maryland in a floating system, where the time to molt of peelers showing white and red line on their paddles took respectively 3-10 days and 1-3 days [
42]. The macroscopic sign observed during the initial selection of individuals, as well as the progressive change of the line’s color from white to red, were similar to those previously reported by studies on the determination pre-molt phase of
C. sapidus [
42] as well as of
C. arcuatus and
C. ornatus [
46,
47]. Furthermore, significant differences between the days to molt allowed the differentiation of individuals in the very early phase of the pre-molt stage to those in the advanced phase.
Information on Asian and American soft-shell crab enterprises are rare and data on peelers mortality rates are difficult to obtain [
24,
48]. Nevertheless, the overall mortality rate recorded in this study was lower to that reported by Spitznagel et al. [
49] for eight different culture facilities in the Chesapeake Bay region, where the mean mortality was 21.7 ± 2.8%. Interestingly, our results were closer to that reported for recirculating systems (16.4 ± 3.1%) than for flow-through facilities (32.9 ± 4.3%). Experiments conducted in continuous flow system similar to that used in the present study on
C. danae and
C. exasperatus [
50], reported mortality rates ranging from 37.5 to 60%. A study on the North Carolina (U.S.A.) soft-shell crab industry [
51] estimated that approximately 23% of the crabs placed in shedding systems die within 5 days, highlighting that the stress caused by capture and transport is one of the causes of peelers mortality. Therefore, it is likely that the high death rate observed here within the first 24h might have been determined by the stress induced by capture, handling, and transport from the Lesina Lagoon. The common practice of anglers to keep crabs in waterless containers increases the probability of injuries and physiological stress and may result in immediate or delayed effects on the captured blue crabs, including mortality [
52]. However, excessive stress and consequent mortality can be avoided by keeping the crabs cool and moisten during transport, as highlighted elsewhere [
51].
Noticeably, we showed that only the advanced pre-molt stage testified by the reddening of the line on the paddles can be used as an effective indicator, since crabs characterized by a white line (W group), did not show significant differences in terms of days taken to molt with the control group. This lack of differentiation is likely to have been determined by the relatively low number of specimens involved in the trial, and by the duration of the trial itself. The experiment had to be terminated after one month given the fully summer conditions and the variations observed in the chemical-physical characteristics of the pond water, where temperatures reached values no longer suitable for optimal crabs rearing (
Table 3). The high survival and molting success of our experiment, together with the water parameters in line with those suggested by Malone and Burden [
45], indicated that the semi-closed system equipped with floating boxes can represent a viable semi-culture technology to keep individuals of
C. sapidus under controlled conditions and let them complete the molting process. However, future experimental designs performed in larger ponds, with a higher number of specimens, and with a longer duration, may allow crabs showing no pre-molt signs in control groups to conclude the molting process and have the appropriate statistical power to validate the results of the present study. Yet, we are confident that our investigation provided a preliminary yet robust confirmation that the procedures generally in use to identify and rear
Callinectes sapidus for the production of soft-shell crabs in native areas can be successfully used also in the Mediterranean context.
Our study clearly suggests that the commercial interest in the blue crab in invaded areas may be increased promoting the development of a product similar to the soft-shell crabs provided in the American and Asian markets, where the price of soft-shell crabs can exceed that of a hard-shelled ones by 300% to 400% [
29]. To our knowledge, the only example of soft-shell crabs production in the Mediterranean Sea is represented by the “moleche” industry in Venice Lagoon, where males of the Mediterranean crab
Carcinus aestuarii are seasonally harvested and held in submerged wooden or plastic containers (vieri) until molting [
53,
54]. Moleche represent an important source of income for the fishermen, given that their market price may exceed 80 €/kg [
55]. Taking as a model these already-established circumstances, the introduction in the Mediterranean fishery sector of novel technical procedures and practices for the production of soft-shell blue crabs may represent a cost-effective strategy to encourage the capture and marketing of this invasive species as a high quality and valuable shellfish product, while minimizing control costs and impacts.
5. Conclusions
Invasive alien species are undesired organisms for which eradication and/or prevention of expansion is considered as the main management objective. However, eradication can be costly or unfeasible, depriving local communities of economic benefits, and even produce counterintuitive effects on the integrity of ecosystems [
56]. For instance, the proposed management of the pacific oyster
Crassostrea gigas recommended the sustainable use of this non-native species if individuals are kept in confined cages, while suggested eradication from local invaded marine ecosystems of wild populations [
57]. A comparable scenario is that of the Atlantic blue crab
Callinectes sapidus, representing a highly invasive alien species impacting biodiversity and ecosystem services and threatening the socio-economic sector of fishing of invaded regions, even though in native areas is a commercially important species [
10,
18]. Established blue crab populations in the Mediterranean regions need to be controlled to satisfy the principles of sustainable development that guarantees environmental protection and conservation while ensuring the economic growth of local communities. The present study provided a clear indication that the capture of
C. sapidus specimens in the pre-molt phase together with the development of techniques and practices for their rearing could represent an additional seasonal activity allowing professional anglers to improve their profit while contributing to mitigate the impact on invaded ecosystems.
Author Contributions
Conceptualization, L.C., N.L. and G.M.; investigation, L.C., N.L., A.O.L., D. Li V., and T.S.; methodology, L.C. and N.L.; formal analysis, L.C. and G.M.; data curation, L.C., N.L., and G.M., writing - original draft preparation, L.C. and N.L.; writing – review and editing, G.M.; funding acquisition, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by funding from the Apulia Region through Po-FEAMP Misura 1.44 (Art. 44, par. 1 lett. cdel Reg. UE n. 508/2014) “CatchUpFish - Sviluppo di metodologie innovative per lo sfruttamento sostenibile delle risorse biologiche nella Laguna di Lesina”.
Institutional Review Board Statement
No ethical issues related with the use of animals in the performed analyses were involved.
Informed Consent Statement
Not applicable.
Data Availability Statement
data used in this study are available upon request from the corresponding author. The data are not publicly available due to ongoing comparative analyses.
Acknowledgments
The authors wish to thank for their help during field operations the associations of anglers operating in the Lesina Lagoon.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A
Figure A1.
Variation in mean temperature, salinity, and dissolved oxygen concentration of the pond water during the trial. The standard deviation of the estimations is not included for the sake of clarity.
Figure A1.
Variation in mean temperature, salinity, and dissolved oxygen concentration of the pond water during the trial. The standard deviation of the estimations is not included for the sake of clarity.
References
- IPCC. Summary for Policymakers. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate, IPCC, I., Pörtner, H.O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., et al., Eds.; 2019; p. 36.
- Dehnen-Schmutz, K.; Boivin, T.; Essl, F.; Groom, Q.J.; Harrison, L.; Touza, J.M.; Bayliss, H. Alien futures: what is on the horizon for biological invasions? Divers. Distrib. 2018, 24, 1149–1157. [Google Scholar] [CrossRef]
- Lieurance, D.; Canavan, S.; Behringer, D.C.; Kendig, A.E.; Minteer, C.R.; Reisinger, L.S.; Romagosa, C.M.; Flory, S.L.; Lockwood, J.L.; Anderson, P.J.; et al. Identifying invasive species threats, pathways, and impacts to improve biosecurity. Ecosphere 2023, 14, e4711. [Google Scholar] [CrossRef]
- Reaser, J.K.; Burgiel, S.W.; Kirkey, J.; Brantley, K.A.; Veatch, S.D.; Burgos-Rodríguez, J. The early detection of and rapid response (EDRR) to invasive species: a conceptual framework and federal capacities assessment. Biol. Invasions 2020, 22, 1–19. [Google Scholar] [CrossRef]
- Russell, J.C.; Binnie, H.R.; Oh, J.; Anderson, D.P.; Samaniego-Herrera, A. Optimizing confirmation of invasive species eradication with rapid eradication assessment. J. Appl. Ecol. 2017, 54, 160–169. [Google Scholar] [CrossRef]
- Giakoumi, S.; Katsanevakis, S.; Albano, P.G.; Azzurro, E.; Cardoso, A.C.; Cebrian, E.; Deidun, A.; Edelist, D.; Francour, P.; Jimenez, C.; et al. Management priorities for marine invasive species. Sci. Total Environ. 2019, 688, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Green, S.J.; Grosholz, E.D. Functional eradication as a framework for invasive species control. Front. Ecol. Environ. 2021, 19, 98–107. [Google Scholar] [CrossRef]
- Grosholz, E.; Ashton, G.; Bradley, M.; Brown, C.; Ceballos-Osuna, L.; Chang, A.; de Rivera, C.; Gonzalez, J.; Heineke, M.; Marraffini, M.; et al. Stage-specific overcompensation, the hydra effect, and the failure to eradicate an invasive predator. Proc. Natl. Acad. Sci. USA 2021, 118, e2003955118. [Google Scholar] [CrossRef] [PubMed]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. On the Atlantic blue crab (Callinectes sapidus Rathbun 1896) in southern European coastal waters: Time to turn a threat into a resource? Fish Res 2017, 194, 1–8. [Google Scholar] [CrossRef]
- Cerveira, I.; Baptista, V.; Teodósio, M.A.; Morais, P. What’s for dinner? Assessing the value of an edible invasive species and outreach actions to promote its consumption. Biol. Invasions 2022, 24, 815–829. [Google Scholar] [CrossRef]
- Ens, N.J.; Harvey, B.; Davies, M.M.; Thomson, H.M.; Meyers, K.J.; Yakimishyn, J.; Lee, L.C.; McCord, M.E.; Gerwing, T.G. The Green Wave: reviewing the environmental impacts of the invasive European green crab (Carcinus maenas) and potential management approaches. Environ. Rev. 2022, 30, 306–322. [Google Scholar] [CrossRef]
- Millikin, M.R.; Williams, A.B. Synopsis of biological data on blue crab, Callinectes sapidus Rathbun; FAO Fisheries Synopsis 38: 1984; p. 39.
- Johnson, D.S. The savory swimmer swims north: a northern range extension of the blue crab Callinectes sapidus? J. Crust. Biol. 2015, 35, 105–110. [Google Scholar] [CrossRef]
- Mancinelli, G.; Bardelli, R.; Zenetos, A. A global occurrence database of the Atlantic blue crab Callinectes sapidus. Sci. Data 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Nehring, S. Nehring, S. Invasion History and Success of the American Blue Crab Callinectes sapidus in European and Adjacent Waters. In In the Wrong Place - Alien Marine Crustaceans: Distribution, Biology and Impacts, Galil, B.S., Clark, P.F., Carlton, J.T., Eds.; Invading Nature - Springer Series in Invasion Ecology; Springer Netherlands: 2011; Volume 6, pp. 607–624. [CrossRef]
- Chaouti, A.; Belattmania, Z.; Nadri, A.; Serrão, E.; Encarnação, J.; Teodósio, M.; Reani, A.; Sabour, B. The invasive Atlantic blue crab Callinectes sapidus Rathbun, 1896 expands its distributional range southward to Atlantic African shores: first records along the Atlantic coast of Morocco. Bioinvasions Rec. 2022, 11, 227–237. [Google Scholar] [CrossRef]
- Mancinelli, G.; Chainho, P.; Cilenti, L.; Falco, S.; Kapiris, K.; Katselis, G.; Ribeiro, F. The Atlantic blue crab Callinectes sapidus in southern European coastal waters: distribution, impact and prospective invasion management strategies. Mar. Pollut. Bull. 2017, 119, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Clavero, M.; Franch, N.; Bernardo-Madrid, R.; López, V.; Abelló, P.; Queral, J.M.; Mancinelli, G. Severe, rapid and widespread impacts of an Atlantic blue crab invasion. Mar. Pollut. Bull. 2022, 176, 113479. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, J.T.; Verani, J.R.; Nordi, N. Evaluation and management of blue crab Callinectes sapidus (Rathbun, 1896) (Decapoda-Portunidae) fishery in the Estuary of Cananéia, Iguape and Ilha Comprida, São Paulo, Brazil. Braz. J. Biol. 2010, 70, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Woodward, R.T.; Wilberg, M.J.; Tomberlin, D. Management evaluation for the Chesapeake Bay blue crab fishery: an integrated bioeconomic approach. N Am J Fish Manage 2015, 35, 216–228. [Google Scholar] [CrossRef]
- Hungria, D.B.; dos Santos Tavares, C.P.; Pereira, L.Â.; de Assis Teixeira da Silva, U.; Ostrensky, A. Global status of production and commercialization of soft-shell crabs. Aquacult. Int. 2017, 25, 2213–2226. [Google Scholar] [CrossRef]
- Prestes dos Santos Tavares, C.; Silva, U.A.T.; Pereira, L.A.; Ostrensky, A. Systems and techniques used in the culture of soft-shell swimming crabs. Rev Aquacult 2018, 10, 913–923. [Google Scholar] [CrossRef]
- Wickins, J.F.; O'C Lee, D. Crustacean Farming, Ranching and Culture; Wiley-Blackwell: Oxford, UK, 2002; p. 480. [Google Scholar]
- Ayas, D.; Ozogul, Y. The effects of sex and seasonality on the metal levels of different muscle tissues of mature Atlantic blue crabs (Callinectes sapidus) in Mersin Bay, north-eastern Mediterranean. Int J Food Sci Tech 2011, 46, 2030–2034. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C.; Avramoglou, K.; Efstathiadis, J.; Chintiroglou, C. Population structure of the blue crab Callinectes sapidus in Thermaikos Gulf (Methoni Bay). In Proceedings of the 15th Pan-Hellenic Congress of Ichthyologists, Thessaloniki, Greece, 10-13 October 2013; pp. 113–116. [Google Scholar]
- Kevrekidis, K.; Kevrekidis, T.; Mogias, A.; Boubonari, T.; Kantaridou, F.; Kaisari, N.; Malea, P.; Dounas, C.; Thessalou-Legaki, M. Fisheries biology and basic life-cycle characteristics of the invasive blue crab Callinectes sapidus Rathbun in the estuarine area of the Evros River (Northeast Aegean Sea, Eastern Mediterranean). J. Mar. Sci. Eng. 2023, 11, 462. [Google Scholar] [CrossRef]
- Ibarra, L.E.; Olivas, E.; Partida, A.L.; Paredes, D. Generation of added value through the process of Soft Shell Crab: a sustainable development option in the coastal region of Sonora. J Manag Sustain 2015, 5, 57–68. [Google Scholar] [CrossRef]
- Florio, M.; Breber, P.; Scirocco, T.; Specchiulli, A.; Cilenti, L.; Lumare, L. Exotic species in Lesina and Varano lakes: Gargano National Park (Italy). Transit Water Bull 2008, 2, 69–79. [Google Scholar] [CrossRef]
- Cilenti, L.; Pazienza, G.; Scirocco, T.; Fabbrocini, A.; D’Adamo, R. First record of ovigerous Callinectes sapidus (Rathbun, 1896) in the Gargano Lagoons (south-west Adriatic Sea). Bioinvasions Rec. 2015, 4, 281–287. [Google Scholar] [CrossRef]
- Bardelli, R.; Mancinelli, G.; Mazzola, A.; Vizzini, S. The Atlantic blue crab Callinectes sapidus spreading in the Tyrrhenian sea: evidence of an established population in the Stagnone di Marsala (Sicily, southern Italy). Nase More 2023, 70, 177–183. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Antoniadou, C. Abundance and population structure of the blue crab (Decapoda, Portunidae) in Thermaikos Gulf (Methoni Bay), northern Aegean Sea. Crustaceana 2018, 91, 641–657. [Google Scholar] [CrossRef]
- Mancinelli, G.; Glamuzina, B.; Petrić, M.; Carrozzo, L.; Glamuzina, L.; Zotti, M.; Raho, D.; Vizzini, S. The trophic position of the Atlantic blue crab Callinectes sapidus Rathbun 1896 in the food web of Parila Lagoon (South Eastern Adriatic, Croatia): a first assessment using stable isotopes. Mediterr. Mar. Sci. 2016, 17, 634–643. [Google Scholar] [CrossRef]
- Mancinelli, G.; Guerra, M.T.; Alujević, K.; Raho, D.; Zotti, M.; Vizzini, S. Trophic flexibility of the Atlantic blue crab Callinectes sapidus in invaded coastal systems of the Apulia region (SE Italy): a stable isotope analysis. Estuar. Coast. Shelf Sci. 2017, 198, 421–431. [Google Scholar] [CrossRef]
- Kampouris, T.E.; Porter, J.S.; Sanderson, W.G. Callinectes sapidus Rathbun, 1896 (Brachyura: Portunidae): An assessment on its diet and foraging behaviour, Thermaikos Gulf, NW Aegean Sea, Greece: evidence for ecological and economic impacts. Crustac Res 2019, 48, 23–37. [Google Scholar] [CrossRef]
- Prado, P.; Ibáñez, C.; Chen, L.; Caiola, N. Feeding habits and short-term mobility patterns of blue crab, Callinectes sapidus, across invaded habitats of the Ebro Delta subjected to contrasting salinity. Estuar Coasts 2021. [Google Scholar] [CrossRef]
- Mancinelli, G.; Rossi, L. Indirect, size-dependent effects of crustacean mesograzers on the Rhodophyta Gracilaria verrucosa (Hudson) Papenfuss: evidence from a short-term study in the Lesina Lagoon (Italy). Mar Biol 2001, 138, 1163–1173. [Google Scholar] [CrossRef]
- Roselli, L.; Fabbrocini, A.; Manzo, C.; D'Adamo, R. Hydrological heterogeneity, nutrient dynamics and water quality of a non-tidal lentic ecosystem (Lesina Lagoon, Italy). Estuar. Coast. Shelf Sci. 2009, 84, 539–552. [Google Scholar] [CrossRef]
- Manzo, C.; Fabbrocini, A.; Roselli, L.; D’Adamo, R. Characterization of the fish assemblage in a Mediterranean coastal lagoon: Lesina Lagoon (central Adriatic Sea). Reg. Stud. Mar. Sci. 2016, 8, 192–200. [Google Scholar] [CrossRef]
- Smith, S.G.; Chang, E.C. Molting and Growth. In The Blue Crab: Callinectes sapidus, Kennedy, V.S., Cronin, L.E., Eds.; Maryland Sea Grant College: College Park, Maryland, 2007; pp. 197–255. [Google Scholar]
- Oesterling, M.J. Manual for Handling and Shedding Blue Crabs (Callinectes sapidus); Virgina Sea Grant Program: Virginia Institute of Marine Science, College of William & Mary, Gloucester Point, VA 23062, 1988; p. 102. [Google Scholar]
- Campbell, I. Chi-squared and Fisher–Irwin tests of two-by-two tables with small sample recommendations. Stat. Med. 2007, 26, 3661–3675. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.T.E. The analysis of 2 × 2 contingency tables - Yet again. Stat. Med. 2011, 30, 890–890. [Google Scholar] [CrossRef] [PubMed]
- Malone, R.F.; Burden, D.G. Design of Recirculating Blue Crab Shedding Systems; Louisiana Sea Grant College Program, Center for Wetland Resources: Baton Rouge, LA, 1988; p. 86. [Google Scholar]
- Wehrtmann, I.S.; Mena-Castañeda, D. Molt sign description of the Pacific blue crab Callinectes arcuatus Ordway, 1863 (Decapoda, Portunidae). Nauplius 2003, 11, 135–139. [Google Scholar]
- Prestes dos Santos Tavares, C.; Assis Teixeira da Silva, U.; Pereira, L.A.; Ostrensky, A. Evaluation of different induced molting methods in Callinectes ornatus (Crustacea, Decapoda, Portunidae) as a tool for the commercial production of soft-shell crabs. An Acad Bras Cienc 2021, 93, 1–14. [Google Scholar] [CrossRef]
- Prestes dos Santos Tavares, C.; Zhao, M.; Lopes Vogt, É.; Felipe Argenta Model, J.; Sommer Vinagre, A.; de Assis Teixeira da Silva, U.; Ostrensky, A.; James Schott, E. High prevalence of CsRV2 in cultured Callinectes danae: Potential impacts on soft-shell crab production in Brazil. J. Invertebr. Pathol. 2022, 190, 107739. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, M.I.; Small, H.J.; Lively, J.A.; Shields, J.D.; Schott, E.J. Investigating risk factors for mortality and reovirus infection in aquaculture production of soft-shell blue crabs (Callinectes sapidus). Aquaculture 2019, 502, 289–295. [Google Scholar] [CrossRef]
- Ostrensky, A.; Ventura, R.; Araújo Corrêa, A.M.; Vieira Santos, G.; Castilho-Westphal, G.G. Improving production of soft-shelled swimming crabs: macroscopic signs of molting as a tool for selection and management of Callinectes danae and Callinectes exasperatus (Portunidae, Decapoda, Crustacea). Arch Vet Sci 2015, 20, 122–131. [Google Scholar] [CrossRef]
- Chaves, J.C.; Eggleston, D.B. Blue crab mortality in the North Carolina soft-shell industry: biological and operational effects. J. Shellfish Res. 2003, 22, 241–249. [Google Scholar]
- Guillory, V. A review of incidental fishing mortalities of blue crabs. In Proceedings of the Proceedings of the blue crab mortality symposium, Lafayette, Lousiana; 2001; pp. 28–41. [Google Scholar]
- Pellizzato, M. Pesca ed allevamento di Carcinus aestuarii, Nardo 1847 nel contesto della attivita alieutiche lagunari e delle tradizioni Venete. In Proceedings of the Convegno: La Risorsa Crostacei nel Mediterraneo: Ricerca, Produzione e Mercato, Corte Benedettina, Legnaro, Italy; 2010; pp. 136–145. [Google Scholar]
- Lazzarini, R.; Vendramini, A.; Cruciani, L.; Galvan, T. Moleche in laguna di Venezia: dati di produzione ed efficienza del sistema. Il Pesce 2023, 2, 1–7. [Google Scholar]
- Cataudella, S.; Crosetti, D.; Massa, F. Mediterranean Coastal Lagoons: Sustainable Management and Interactions Among Aquaculture, Capture Fisheries and the Environment; Food and Agricolture Organization of the United Nations: Rome, 2015; Volume 95, p. 293. [Google Scholar]
- Sundet, J.H.; Hoel, A.H. The Norwegian management of an introduced species: the Arctic red king crab fishery. Mar. Policy 2016, 72, 278–284. [Google Scholar] [CrossRef]
- Herbert, R.J.H.; Humphreys, J.; Davies, C.J.; Roberts, C.; Fletcher, S.; Crowe, T.P. Ecological impacts of non-native Pacific oysters (Crassostrea gigas) and management measures for protected areas in Europe. Biodivers. Conserv. 2016, 25, 2835–2865. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).