Submitted:
24 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
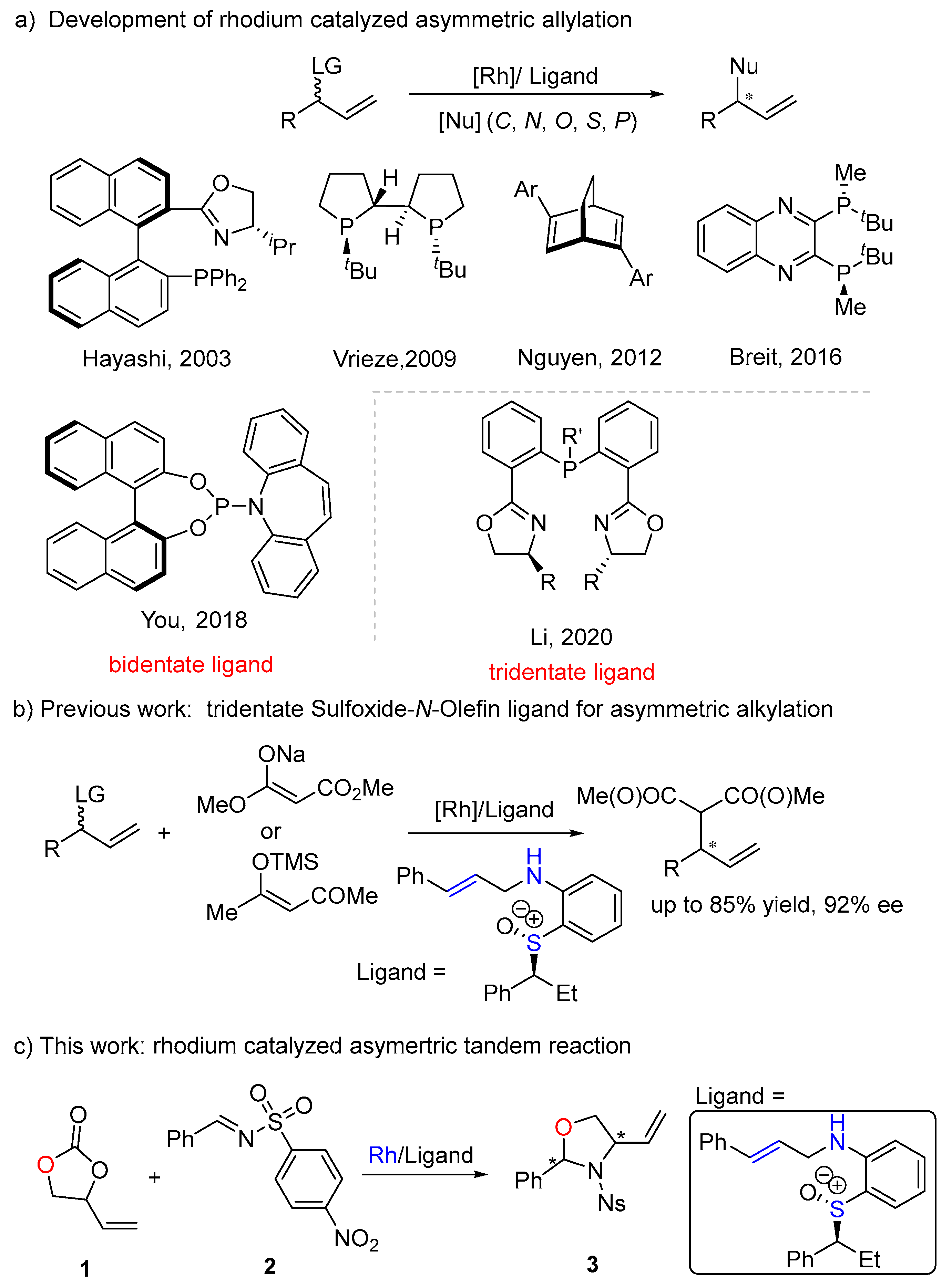
1. Introduction
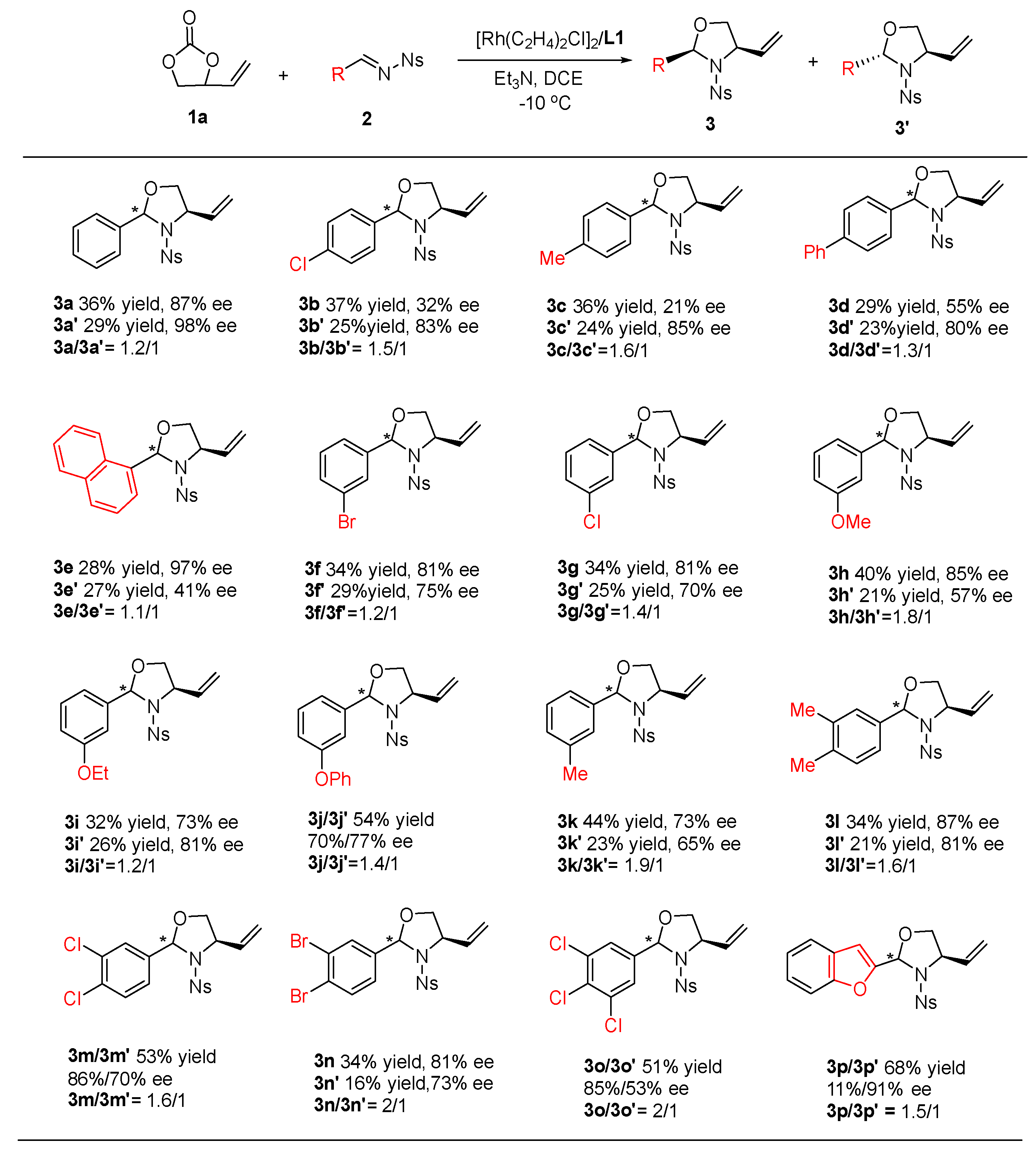
2. Results and Discussion
3. Materials and Methods
3.1. Reagents and General Methods
3.2. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Tsuji, J.; Minami, I.; Shimizu, I. Allyation of Carbonucleophiles with Allylic Carbonates under Neutral Conditions Catalyzed by Rhodium Complexes. Tetrahedron Lett. 1984, 25, 5157–5160. [Google Scholar] [CrossRef]
- Evans, P.A.; Nelson, J.D. Regioselective Rhodium-Catalyzed Allylic Alkylation with a Modified Wilkinson’s Catalyst. Tetrahedron Lett. 1998, 39, 1725–1728. [Google Scholar] [CrossRef]
- Evans, P.A.; Nelson, J.D. Conservation of Absolute Configuration in the Acyclic Rhodium-Catalyzed Allylic Alkylation Reaction: Evidence for an Enyl (σ + π) Organorhodium Intermediate. J. Am. Chem. Soc. 1998, 120, 5581–5582. [Google Scholar] [CrossRef]
- Turnbull, B.W.H.; Evans, P.A. Asymmetric Rhodium-Catalyzed Allylic Substitution Reactions: Discovery, Development and Applications to Target-Directed Synthesis. J. Org. Chem. 2018, 83, 11463–11479. [Google Scholar] [CrossRef]
- Thoke, M.B.; Kang, Q. Rhodium-Catalyzed Allylation Reactions. Synthesis 2019, 51, 2585–2631. [Google Scholar] [CrossRef]
- Hayashi, T.; Okada, A.; Suzuka, T.; Kawatsura, M. High Enantioselectivity in Rhodium-Catalyzed Allylic Alkylation of 1-Substituted 2-Propenyl Acetates. Org. Lett. 2003, 5, 1713–1715. [Google Scholar] [CrossRef]
- Vrieze, D.C.; Hoge, G.S.; Hoerter, P.Z.; Van Haitsma, J.T.; Samas, B.M. A Highly Enantioselective Allylic Amination Reaction Using a Commercially Available Chiral Rhodium Catalyst: Resolution of Racemic Allylic Carbonates. Org. Lett. 2009, 11, 3140–3142. [Google Scholar] [CrossRef] [PubMed]
- Atallah, T.; Blankespoor, R.L.; Homan, P.; Hulderman, C.; Samas, B.M.; Van Allsburg, K.; Vrieze, D.C. Substituent Effects on the Amination of Racemic Allyl Carbonates Using Commercially Available Chiral Rhodium Catalysts. Tetrahedron Lett. 2013, 54, 5795–5798. [Google Scholar] [CrossRef]
- Arnold, J.S.; Nguyen, H.M. Rhodium-Catalyzed Dynamic Kinetic Asymmetric Transformations of Racemic Tertiary Allylic Trichloroacetimidates with Anilines. J. Am. Chem. Soc. 2012, 134, 8380–8383. [Google Scholar] [CrossRef]
- Arnold, J.S.; Cizio, G.T.; Heitz, D.R.; Nguyen, H.M. Rhodium-Catalyzed Regio- and Enantioselective Amination of Racemic Secondary Allylic Trichloroacetimidates with N-Methyl Anilines. Chem. Commun. 2012, 48, 11531–11533. [Google Scholar] [CrossRef]
- Arnold, J.; Nguyen, H. Rhodium-Catalyzed Asymmetric Amination of Allylic Trichloroacetimidates. Synthesis 2013, 45, 2101–2108. [Google Scholar] [CrossRef]
- Arnold, J.S.; Mwenda, E.T.; Nguyen, H.M. Rhodium-Catalyzed Sequential Allylic Amination and Olefin Hydroacylation Reactions: Enantioselective Synthesis of Seven-Membered Nitrogen Heterocycles. Angew. Chem. Int. Ed. 2014, 53, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Breit, B. Rhodium-Catalyzed Dynamic Kinetic Asymmetric Allylation of Phenols and 2-Hydroxypyridines. Chem. Eur. J. 2016, 22, 14655–14663. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-B.; Zhang, X.; Tu, H.-F.; You, S.-L. Regio- and Enantioselective Rhodium-Catalyzed Allylic Alkylation of Racemic Allylic Alcohols with 1,3-Diketones. J. Am. Chem. Soc. 2018, 140, 7737–7742. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-B.; Ghorai, S.; Huang, W.; Li, C. Rh(I)/Bisoxazolinephosphine-Catalyzed Regio- and Enantioselective Allylic Substitutions. ACS Catal. 2020, 10, 4491–4496. [Google Scholar] [CrossRef]
- Li, K.; Li, C. Enantioselective Synthesis of 3-Allylindolizines via Sequential Rh-Catalyzed Asymmetric Allylation and Tschitschibabin Reaction. Org. Lett. 2020, 22, 9456–9461. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-B.; Sun, M.; Shu, M.; Li, C. Rhodium-Catalyzed Regio- and Enantioselective Allylic Amination of Racemic 1,2-Disubstituted Allylic Phosphates. J. Am. Chem. Soc. 2021, 143, 8255–8260. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Wei, L.; Li, C. Regio- and Enantioselective Allylic Cyanomethylation by Synergistic Rhodium and Silane Catalysis. J. Am. Chem. Soc. 2023, 145, 3897–3902. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Luo, Y.; Liu, M.; Xia, Y.; Li, C. Asymmetric Formal Abnormal Claisen Rearrangement Enabled by Rh-Catalyzed Regio- and Enantioselective Allylic Alkylation. ACS Catal. 2023, 13, 5482–5490. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Q.; Zhu, G.; Zhang, X. Highly Effective NPN-Type Tridentate Ligands for Asymmetric Transfer Hydrogenation of Ketones. Tetrahedron Lett. 1997, 38, 215–218. [Google Scholar] [CrossRef]
- Li, B.; Liu, M.; Ur Rehman, S.; Li, C. Rh-Catalyzed Regio- and Enantioselective Allylic Phosphinylation. J. Am. Chem. Soc. 2022, 144, 2893–2898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, J.; Wang, X.; Zheng, S.-C.; Zhao, X. Hemilabile Tridentate Sulfoxide-N-olefin Hybrid Ligands in Rhodium-Catalyzed Asymmetric Allylic Substitution. Manuscript in submission. [CrossRef]
- Liu, Z.K.; Gao, Y.; Hu, X.Q. Recent Advances in Catalytic Synthesis of Medium-Ring Lactones and their Derivatives. Catal. Sci. Technol. 2021, 11, 6931–6946. [Google Scholar] [CrossRef]
- Khan, A.; Yang, L.; Xu, J.; Jin, L.Y.; Zhang, Y.J. Palladium Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Michael Acceptors: Construction of Vicinal Quaternary Stereocenters. Angew. Chem., Int. Ed. 2014, 53, 11257–11260. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Xing, J.X.; Zhao, J.M.; Kan, Y.H.; Zhang, W.B.; Zhang, Y.J. Palladium-Catalyzed Enantioselective Decarboxylative Cycloaddition of Vinylethylene Carbonates with Isocyanates. Chem. Eur. J. 2015, 21, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Zhao, C.; Zhang, Y.J. Pd-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with 3-cyanochromones. Chem. Commun. 2018, 54, 4708–4711. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Khan, I.; Cheng, J.; Hsueh, Y.J.; Zhang, Y.J. Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with beta-Nitroolefins by Cooperative Catalysis of Palladium Complex and Squaramide. ACS Catal. 2018, 8, 11600–11604. [Google Scholar] [CrossRef]
- Xia, Y.; Bao, Q.F.; Li, Y.; Wang, L.J.; Zhang, B.S.; Liu, H.C.; Liang, Y.M. Ligand-Controlled Regiodivergent Allyl Palladium Catalysis Enables a Switch between [3 + 2] and [3 + 3] Cycloadditions. Chem. Commun. 2019, 55, 4675–4678. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, L.; Yang, Y.W.; Zhang, Z.; Yang, W. Vinylethylene Carbonates as α,β-Unsaturated Aldehyde Surrogates for Regioselective [3 + 3] Cycloaddition. Org. Lett. 2019, 21, 6674–6678. [Google Scholar] [CrossRef]
- Singha, S.; Serrano, E.; Mondal, S.; Daniliuc, C.G.; Glorius, F. Diastereodivergent Synthesis of Enantioenriched α,β-disubstituted γ-butyrolactones via Cooperative N-heterocyclic Carbene and Ir Catalysis. Nat. Catal. 2020, 3, 48–54. [Google Scholar] [CrossRef]
- Xiao, L.; Wei, L.; Wang, C.J. Stereodivergent Synthesis of Enantioenriched γ-Butyrolactones Bearing Two Vicinal Stereocenters Enabled by Synergistic Copper and Iridium Catalysis. Angew. Chem., Int. Ed. 2021, 60, 24930–24940. [Google Scholar] [CrossRef]
- Rong, Z.Q.; Yang, L.C.; Liu, S.; Yu, Z.; Wang, Y.N.; Tan, Z.Y.; Huang, R.Z.; Lan, Y.; Zhao, Y. Nine-Membered Benzofuran Fused Heterocycles: Enantioselective Synthesis by Pd-Catalysis and Rearrangement via Transannular Bond Formation. J. Am. Chem. Soc. 2017, 139, 15304–15307. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.C.; Rong, Z.Q.; Wang, Y.N.; Tan, Z.Y.; Wang, M.; Zhao, Y. Construction of Nine-Membered Heterocycles through Palladium-Catalyzed Formal [5 + 4] Cycloaddition. Angew. Chem., Int. Ed. 2017, 56, 2927–2931. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Gondo, S.; Nagender, P.; Uno, H.; Tokunaga, E.; Shibata, N. Access to Benzo-Fused Nine-Membered Heterocyclic Alkenes with a Trifluoromethyl Carbinol Moiety via a Double Decarboxylative formal Ringexpansion Process under Palladium Catalysis. Chem. Sci. 2018, 9, 3276–3281. [Google Scholar] [CrossRef]
- Zhao, H.W.; Du, J.; Guo, J.M.; Feng, N.N.; Wang, L.R.; Ding, W.Q.; Song, X.Q. Formal [5 + 2] cycloaddition of vinylethylene carbonates to oxazol-5-(4H)-ones for the synthesis of 3,4-dihydrooxepin-2(7H)-ones. Chem. Commun. 2018, 54, 9178–9181. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wu, X.Y.; Wei, Y.; Shi, M. Palladium-Catalyzed Diastereoselective Formal [5 + 3] Cycloaddition for the Construction of Spirooxindoles Fused with an EightMembered Ring. Org. Lett. 2019, 21, 4859–4863. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Li, M.M.; Li, Y.; Lan, Y.; Lu, L.Q.; Xiao, W.J. Enantioselective Trapping of Pd-Containing 1,5-Dipoles by Photogenerated Ketenes: Access to 7-Membered Lactones Bearing Chiral Quaternary Stereocenters. J. Am. Chem. Soc. 2019, 141, 133–137. [Google Scholar] [CrossRef]
- Zeng, R.; Li, J.L.; Zhang, X.; Liu, Y.Q.; Jia, Z.Q.; Leng, H.J.; Huang, Q.W.; Liu, Y.; Li, Q.Z. Diastereoselective Construction of 6,8-Dioxabicyclo [3.2.1]-octane Frameworks from Vinylethylene Carbonates via Palladium Organo Relay Catalysis. ACS Catal. 2019, 9, 8256–8262. [Google Scholar] [CrossRef]
- Wu, H.H.; Fan, X.Z.; Tang, Z.; Zhang, H.; Cai, L.Y.; Bi, X.F.; Zhao, H.W. Palladium-Catalyzed Formal (5 + 6) Cycloaddition of Vinylethylene Carbonates with Isatoic Anhydrides for the Synthesis of MediumSized N,O-Containing Heterocycles. Org. Lett. 2021, 23, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
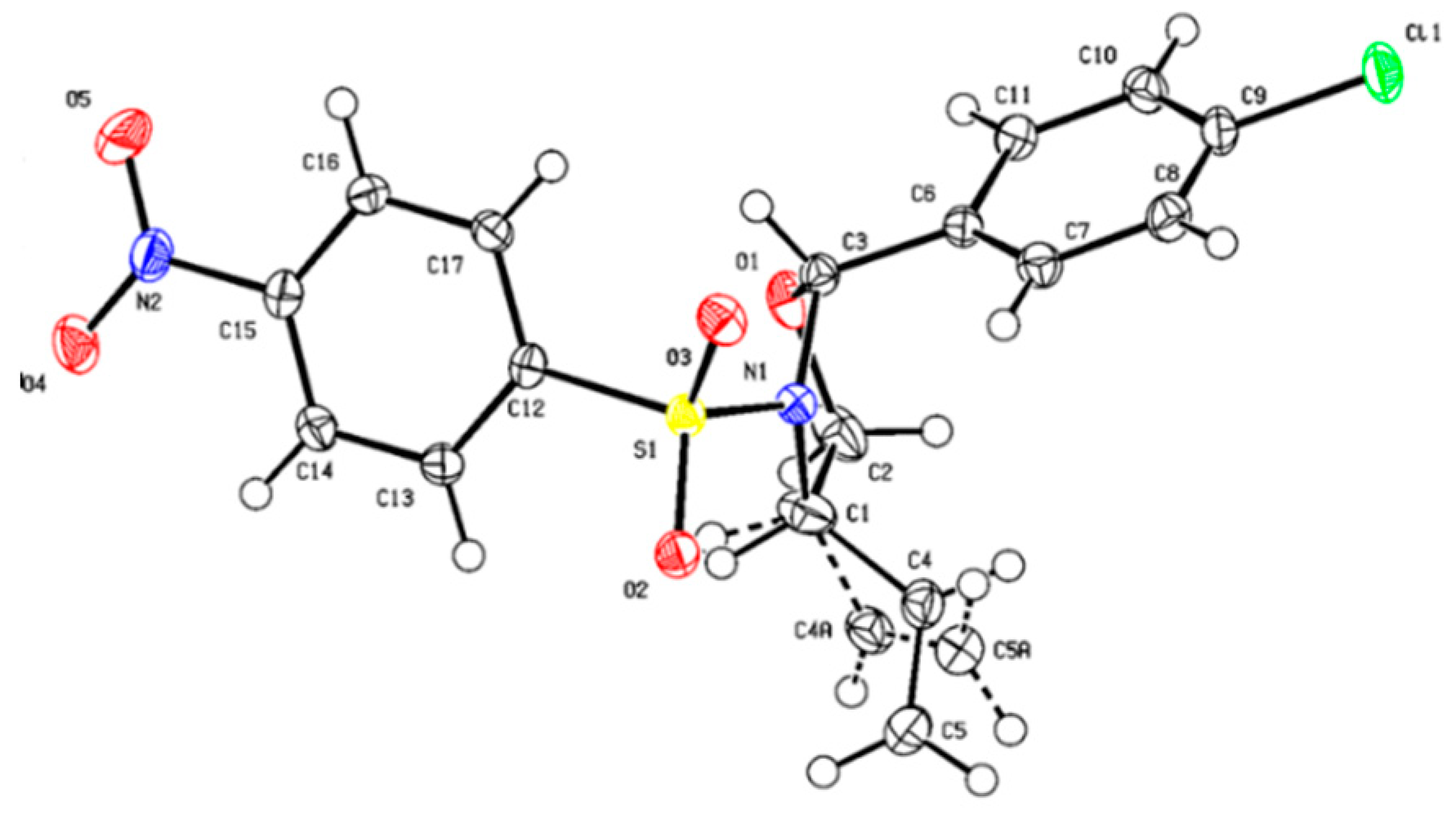
- The Cambridge Crystallographic Data Centre. CCDC 2265381. Available online: https://www.ccdc.cam.ac.uk/.
- Yang, L.; Khan, A.; Zheng, R.; Jin, L.Y.; Zhang, Y.J. Pd-Catalyzed Asymmetric Decarboxylative Cycloaddition of Vinylethylene Carbonates with Imines. Org. Lett. 2015, 17, 6230–6233. [Google Scholar] [CrossRef]
- Heathcote, D.A.; Patel, H.; Kroll, S.H.B.; Hazel, P.; Periyasamy, M.; Alikian, M.; Kanneganti, S.K.; Jogalekar, A.S.; Scheiper, B.; Barbazanges, M.; Blum, A.; Brackow, J.; Siwicka, A.; Pace, R.D.M.; Fuchter, M.J.; Snyder, J.P.; Liotta, D.C.; Freemont, P.S.; Aboagye, E.O.; Coombes, R.C.; Barrett, A.G.M.; Ali, S. A Novel Pyrazolo[1,5-a]pyrimidine Is a Potent Inhibitor of Cyclin-Dependent Protein Kinases 1, 2, and 9, Which Demonstrates Antitumor Effects in Human Tumor Xenografts Following Oral Administration. J. Med. Chem. 2010, 53, 8508–8522. [Google Scholar] [CrossRef]
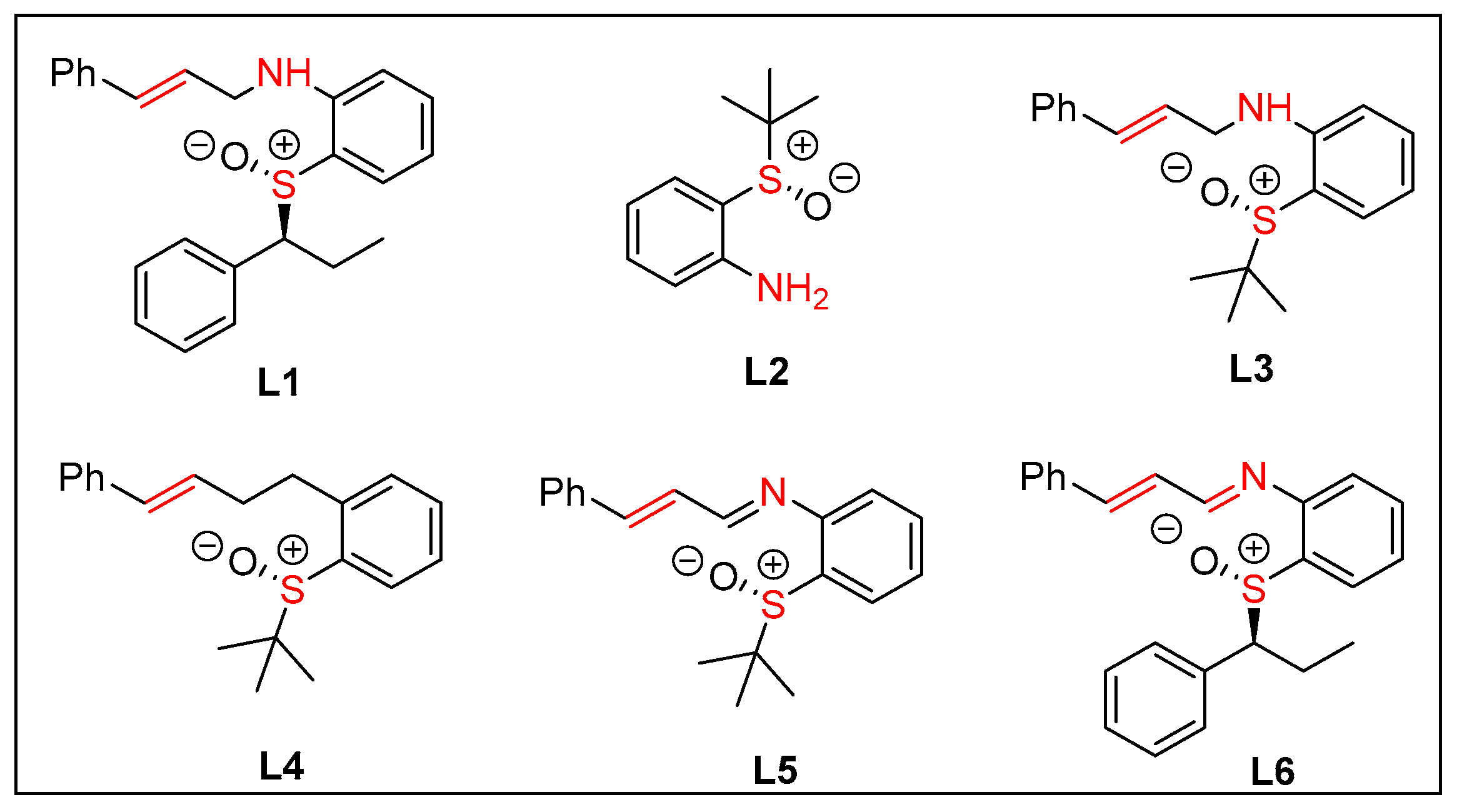
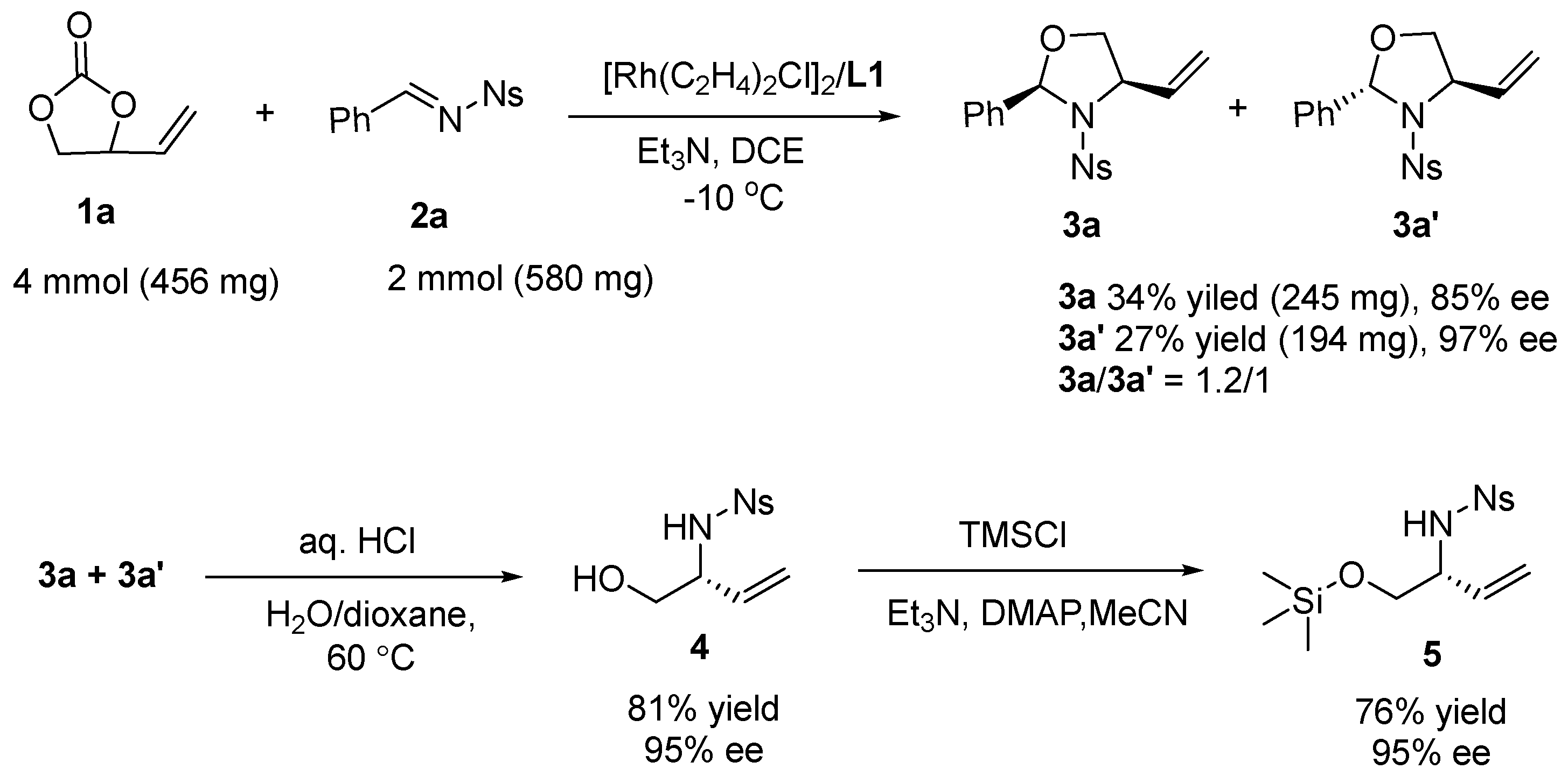
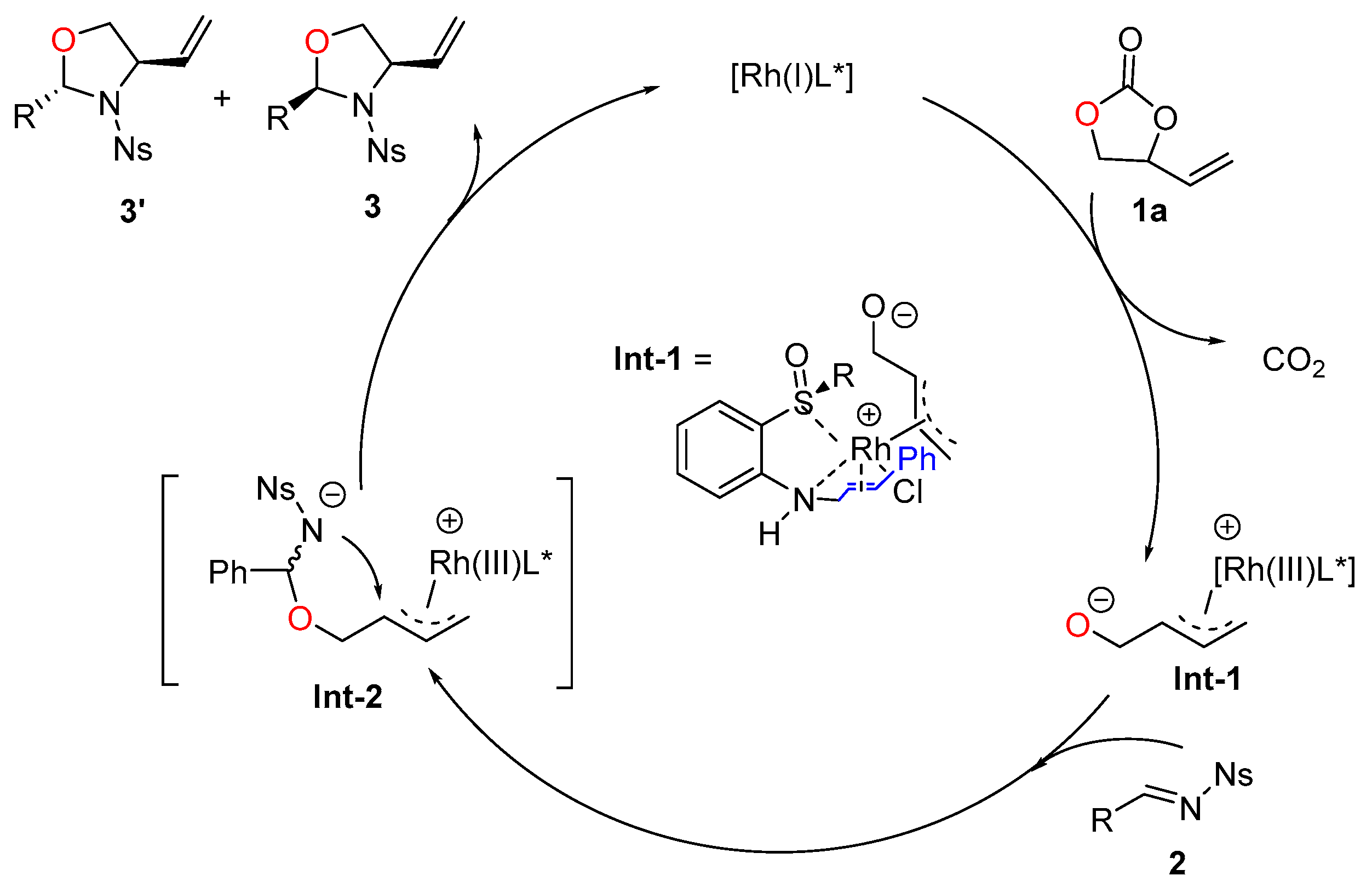
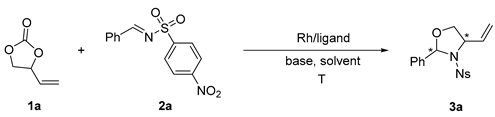
| Entry | Rh | L | Base | Solvent | T (°C) | Yield b(%) | ee c(%) | dr d |
| 1 | [Rh(C2H4)2Cl]2 | L1 | Cs2CO3 | DCM | rt | 32 | 41/37 | 1.1/1 |
| 2 | [Rh(C2H4)2Cl]2 | L1 | Cs2CO3 | Tol. | rt | 9 | 50/51 | 1.8/1 |
| 3 | [Rh(C2H4)2Cl]2 | L1 | Cs2CO3 | THF | rt | 47 | 12/23 | 1.1/1 |
| 4 | [Rh(C2H4)2Cl]2 | L1 | Cs2CO3 | MeCN | rt | 24 | 16/31 | 2/1 |
| 5 | [Rh(C2H4)2Cl]2 | L1 | Cs2CO3 | DCE | rt | 35 | 53/73 | 1.2/1 |
| 6 | [Rh(C2H4)2Cl]2 | L1 | CsF | DCE | rt | 24 | 60/77 | 2/1 |
| 7 | [Rh(C2H4)2Cl]2 | L1 | K2CO3 | DCE | rt | 29 | 31/35 | 2.2/1 |
| 8 | [Rh(C2H4)2Cl]2 | L1 | CsCl | DCE | rt | 19 | 53/51 | 1.5/1 |
| 9 | [Rh(C2H4)2Cl]2 | L1 | Et3N | DCE | rt | 65 | 45/89 | 1.2/1 |
| 10 | [Rh(C2H4)2Cl]2 | L1 | DBU | DCE | rt | 61 | 23/25 | 2/1 |
| 11 | [Rh(C2H4)2Cl]2 | L1 | TMEDA | DCE | rt | 39 | 65/69 | 1.4/1 |
| 12 | [Rh(C2H4)2Cl]2 | L1 | Et3N | DCE | 0 | 65 | 60/88 | 1.2/1 |
| 13 | [Rh(C2H4)2Cl]2 | L1 | Et3N | DCE | -10 | 61 | 81/91 | 1.2/1 |
| 14 | [Rh(C2H4)2Cl]2 | L1 | Et3N | DCE | -25 | 52 | 57/91 | 1.2/1 |
| 15 | Rh(acac)(C2H4)2 | L1 | Et3N | DCE | -10 | 42 | 15/65 | 2/1 |
| 16 | [Rh(cod)Cl]2 | L1 | Et3N | DCE | -10 | nr | - | - |
| 17 e | [Rh(C2H4)2Cl]2 | L1 | Et3N | DCE | -10 | 65 | 87/98 | 1.2/1 |
| 18 | [Rh(C2H4)2Cl]2 | L2 | Et3N | DCE | -10 | 32 | 7/5 | 2/1 |
| 19 | [Rh(C2H4)2Cl]2 | L3 | Et3N | DCE | -10 | 26 | 7/9 | 1.4/1 |
| 20 | [Rh(C2H4)2Cl]2 | L4 | Et3N | DCE | -10 | 25 | rac | 1.7/1 |
| 21 | [Rh(C2H4)2Cl]2 | L5 | Et3N | DCE | -10 | 51 | 59/61 | 1.4/1 |
| 22 | [Rh(C2H4)2Cl]2 | L6 | Et3N | DCE | -10 | 66 | 33/37 | 1.5/1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





