Submitted:
22 January 2024
Posted:
25 January 2024
You are already at the latest version
Abstract
Keywords:
INTRODUCTION
METHODS
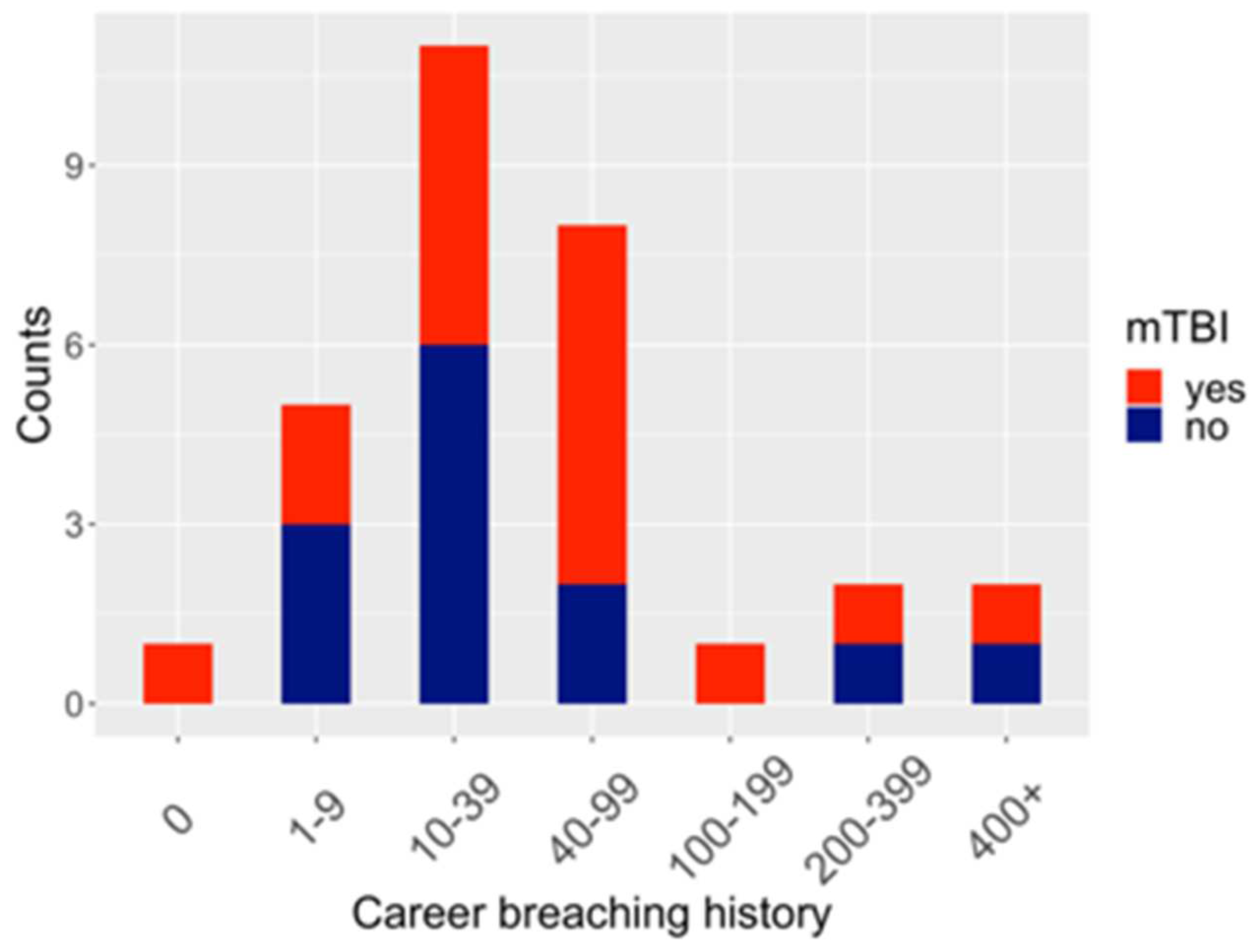
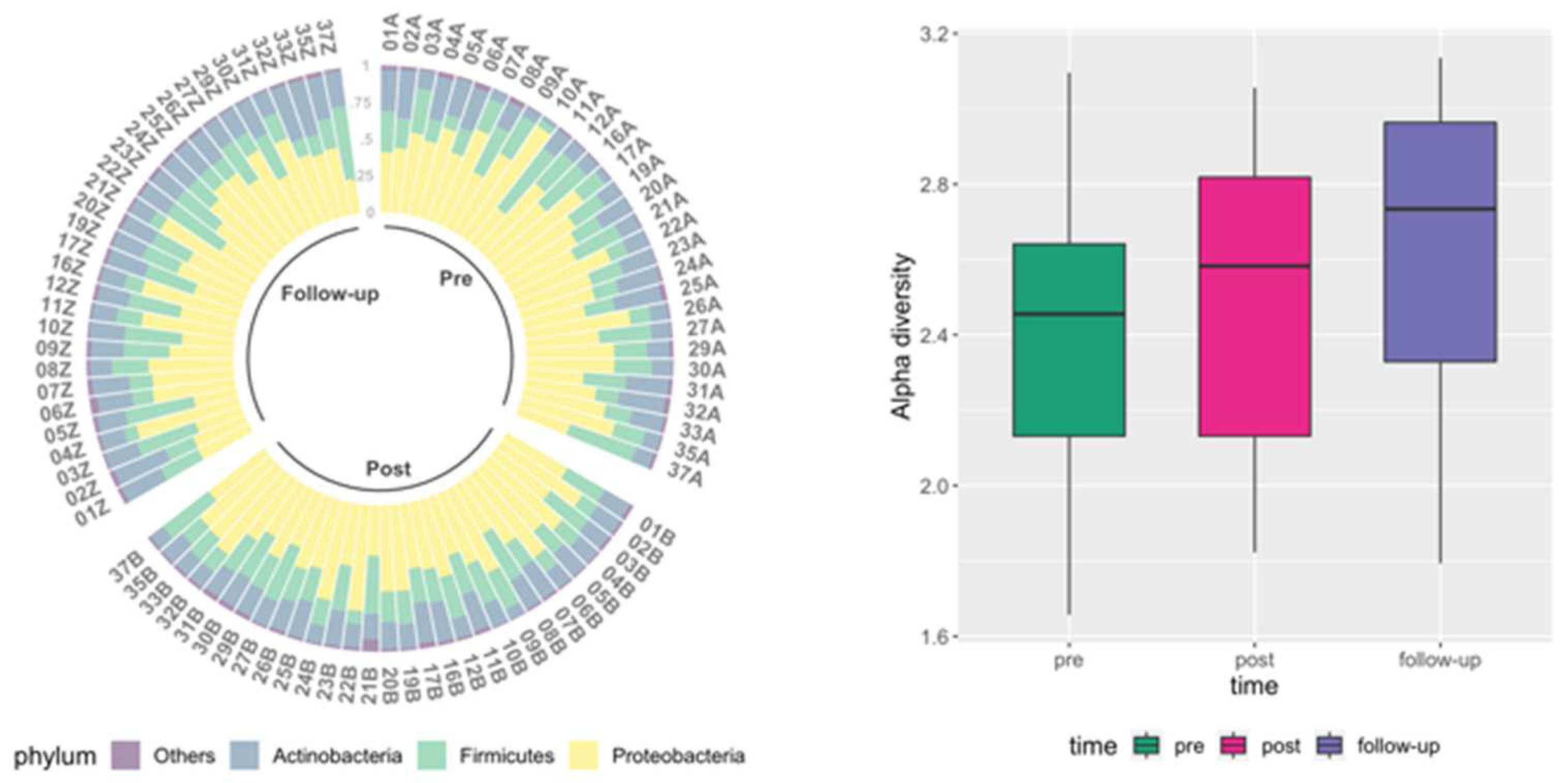
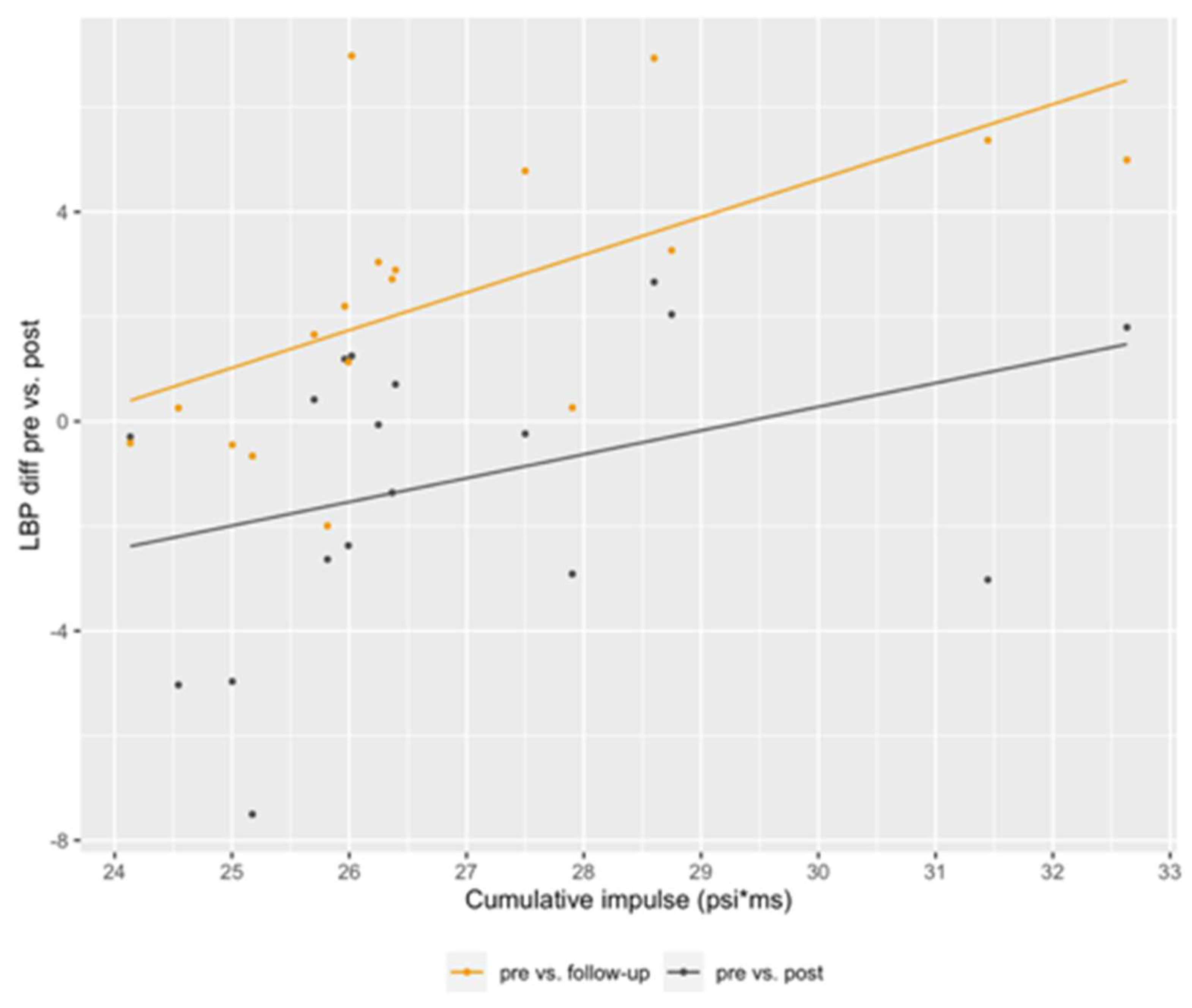
RESULTS
DISCUSSION
Supplementary Materials
Acknowledgements
Conflicts of Interest
Disclaimer
References
- Regasa, L.E.; Agimi, Y.; Stout, K.C. Traumatic brain injury following military deployment: evaluation of diagnosis and cause of injury. The Journal of Head Trauma Rehabilitation 2019, 34, 21-29. [CrossRef]
- Zhu, C.S.; Grandhi, R.; Patterson, T.T.; Nicholson, S.E. A review of traumatic brain injury and the gut microbiome: insights into novel mechanisms of secondary brain injury and promising targets for neuroprotection. Brain sciences 2018, 8, 113. [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. Journal of Clinical Investigation 2021, 131, e143777. [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S.; et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proceedings of the National Academy of Sciences 2009, 106, 16799-16804, doi:doi:10.1073/pnas.0906773106.
- Fasano, A.; Not, T.; Wang, W.; Uzzau, S.; Berti, I.; Tommasini, A.; Goldblum, S.E. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000, 355, 1518-1519. [CrossRef]
- Wang, W.; Uzzau, S.; Goldblum, S.E.; Fasano, A. Human zonulin, a potential modulator of intestinal tight junctions. Journal of cell science 2000, 113, 4435-4440. [CrossRef]
- Tobias, P.S.; Soldau, K.; Ulevitch, R.J. Isolation of a lipopolysaccharide-binding acute phase reactant from rabbit serum. J Exp Med 1986, 164, 777-793. [CrossRef]
- Schumann, R.R.; Leong, S.R.; Flaggs, G.W.; Gray, P.W.; Wright, S.D.; Mathison, J.C.; Tobias, P.S.; Ulevitch, R.J. Structure and function of lipopolysaccharide binding protein. Science 1990, 249, 1429-1431. [CrossRef]
- Avila-Calderón, E.D.; Ruiz-Palma, M.d.S.; Aguilera-Arreola, M.G.; Velázquez-Guadarrama, N.; Ruiz, E.A.; Gomez-Lunar, Z.; Witonsky, S.; Contreras-Rodríguez, A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Frontiers in Microbiology 2021, 12. [CrossRef]
- Ryu, J.-K.; Kim, S.J.; Rah, S.-H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.-O.; Park, B.S.; Yoon, T.-Y.; Kim, H.M. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38-50. [CrossRef]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Experimental & molecular medicine 2013, 45, e66-e66. [CrossRef]
- Findley, M.K.; Koval, M. Regulation and roles for claudin-family tight junction proteins. IUBMB Life 2009, 61, 431-437. [CrossRef]
- Olde Loohuis, L.M.; Mangul, S.; Ori, A.P.; Jospin, G.; Koslicki, D.; Yang, H.T.; Wu, T.; Boks, M.P.; Lomen-Hoerth, C.; Wiedau-Pazos, M. Transcriptome analysis in whole blood reveals increased microbial diversity in schizophrenia. Translational psychiatry 2018, 8, 1-9. [CrossRef]
- Maheshwari, P.; Eslick, G.D. Bacterial infection and Alzheimer’s disease: a meta-analysis. Journal of Alzheimer’s Disease 2015, 43, 957-966. [CrossRef]
- Biometrics, B. Blast Gauge®.
- Cicerone, K.D.; Kalmar, K. Persistent postconcussion syndrome: The structure of subjective complaints after mild traumatic brain injury. The Journal of head trauma rehabilitation 1995, 10, 1-17. [CrossRef]
- King, N.S.; Crawford, S.; Wenden, F.J.; Moss, N.E.G.; Wade, D.T. The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. Journal of Neurology 1995, 242, 587-592. [CrossRef]
- Aguet, F.; Brown, A.A.; Castel, S.E.; Davis, J.R.; He, Y.; Jo, B.; Mohammadi, P.; Park, Y.; Parsana, P.; Segrè, A.V.; et al. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204-213. [CrossRef]
- Carr, W.; Polejaeva, E.; Grome, A.; Crandall, B.; LaValle, C.; Eonta, S.E.; Young, L.A. Relation of Repeated Low-Level Blast Exposure With Symptomology Similar to Concussion. The Journal of Head Trauma Rehabilitation 2015, 30. [CrossRef]
- Vartanian, O.; Rhind, S.G.; Nakashima, A.; Tenn, C.; Lam, T.K.; Shiu, M.; Caddy, N.; King, K.; Natale, A.; Jetly, R. Blast effects on post-concussive and mental health outcomes: Data from Canadian Armed Forces breachers and snipers. Journal of Military, Veteran and Family Health 2022, 8, 82-96. [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15-21. [CrossRef]
- Lab, H. FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 20 March 2024).
- Schmieder, R.; Edwards, R. Quality control and preprocessing of metagenomic datasets. Bioinformatics 2011, 27, 863-864. [CrossRef]
- Wood, D.E.; Lu, J.; Langmead, B. Improved metagenomic analysis with Kraken 2. Genome biology 2019, 20, 1-13. [CrossRef]
- Weber, N.S.; Gressitt, K.L.; Cowan, D.N.; Niebuhr, D.W.; Yolken, R.H.; Severance, E.G. Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophr Res 2018, 197, 465-469. [CrossRef]
- Giron, L.B.; Dweep, H.; Yin, X.; Wang, H.; Damra, M.; Goldman, A.R.; Gorman, N.; Palmer, C.S.; Tang, H.-Y.; Shaikh, M.W.; et al. Plasma Markers of Disrupted Gut Permeability in Severe COVID-19 Patients. Front Immunol 2021, 12. [CrossRef]
- McKenna, Z.; Houck, J.; Ducharme, J.; Li, Z.; Berkemeier, Q.; Fennel, Z.; Wells, A.; Mermier, C.; Deyhle, M.; Laitano, O. The effect of prolonged interval and continuous exercise in the heat on circulatory markers of intestinal barrier integrity. European Journal of Applied Physiology 2022, 122, 2651-2659. [CrossRef]
- Scheffler, L.; Crane, A.; Heyne, H.; Tönjes, A.; Schleinitz, D.; Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Frontiers in endocrinology 2018, 9, 22. [CrossRef]
- Xu, J.; Tanaka, H.; Shoyama, Y. One-step immunochromatographic separation and ELISA quantification of glycyrrhizin from traditional Chinese medicines. Journal of Chromatography B 2007, 850, 53-58. [CrossRef]
- Altamura, S.; Kopf, S.; Schmidt, J.; Müdder, K.; da Silva, A.R.; Nawroth, P.; Muckenthaler, M.U. Uncoupled iron homeostasis in type 2 diabetes mellitus. Journal of Molecular Medicine 2017, 95, 1387-1398. [CrossRef]
- Team, R.C. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www. R-project.org/ 2013.
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal statistical society: series B (Methodological) 1995, 57, 289-300. [CrossRef]
- McMahon, P.J.; Hricik, A.; Yue, J.K.; Puccio, A.M.; Inoue, T.; Lingsma, H.F.; Beers, S.R.; Gordon, W.A.; Valadka, A.B.; Manley, G.T. Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. Journal of neurotrauma 2014, 31, 26-33. [CrossRef]
- McInnes, K.; Friesen, C.L.; MacKenzie, D.E.; Westwood, D.A.; Boe, S.G. Mild Traumatic Brain Injury (mTBI) and chronic cognitive impairment: A scoping review. PloS one 2017, 12, e0174847. [CrossRef]
- LaValle, C.R.; Carr, W.S.; Egnoto, M.J.; Misistia, A.C.; Salib, J.E.; Ramos, A.N.; Kamimori, G.H. Neurocognitive performance deficits related to immediate and acute blast overpressure exposure. Frontiers in neurology 2019, 10, 949. [CrossRef]
- Karl, J.P.; Hatch, A.M.; Arcidiacono, S.M.; Pearce, S.C.; Pantoja-Feliciano, I.G.; Doherty, L.A.; Soares, J.W. Effects of psychological, environmental and physical stressors on the gut microbiota. Frontiers in microbiology 2018, 2013. [CrossRef]
- Fasano, A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clinical Gastroenterology and Hepatology 2012, 10, 1096-1100. [CrossRef]
- Tripathi, A.; Lammers, K.M.; Goldblum, S.; Shea-Donohue, T.; Netzel-Arnett, S.; Buzza, M.S.; Antalis, T.M.; Vogel, S.N.; Zhao, A.; Yang, S. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proceedings of the National Academy of Sciences 2009, 106, 16799-16804. [CrossRef]
- Caviglia, G.P.; Dughera, F.; Ribaldone, D.G.; Rosso, C.; Abate, M.L.; Pellicano, R.; Bresso, F.; Smedile, A.; Saracco, G.M.; Astegiano, M. Serum zonulin in patients with inflammatory bowel disease: a pilot study. Minerva Medica 2019, 110, 95-100. [CrossRef]
- Camara-Lemarroy, C.R.; Silva, C.; Greenfield, J.; Liu, W.-Q.; Metz, L.M.; Yong, V.W. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Multiple Sclerosis Journal 2020, 26, 1340-1350. [CrossRef]
- Gokulakrishnan, K.; Nikhil, J.; Vs, S.; Holla, B.; Thirumoorthy, C.; Sandhya, N.; Nichenametla, S.; Pathak, H.; Shivakumar, V.; Debnath, M. Altered Intestinal Permeability Biomarkers in Schizophrenia: A Possible Link with Subclinical Inflammation. Annals of Neurosciences 2022, 29, 151-158. [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; De Vos, W.M. Homeostasis of the gut barrier and potential biomarkers. American Journal of Physiology-Gastrointestinal and Liver Physiology 2017, 312, G171-G193. [CrossRef]
- Coe, N.R.; Bernlohr, D.A. Physiological properties and functions of intracellular fatty acid-binding proteins. Biochimica et biophysica acta 1998, 1391, 287-306. [CrossRef]
- Zimmerman, A.; Veerkamp, J. New insights into the structure and function of fatty acid-binding proteins. Cellular and Molecular Life Sciences CMLS 2002, 59, 1096-1116. [CrossRef]
- Chmurzyńska, A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. Journal of applied genetics 2006, 47, 39-48. [CrossRef]
- Grootjans, J.; Thuijls, G.; Verdam, F.; Derikx, J.P.; Lenaerts, K.; Buurman, W.A. Non-invasive assessment of barrier integrity and function of the human gut. World Journal of Gastrointestinal Surgery 2010, 2, 61. [CrossRef]
- Zeissig, S.; Bürgel, N.; Günzel, D.; Richter, J.; Mankertz, J.; Wahnschaffe, U.; Kroesen, A.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 2007, 56, 61-72. [CrossRef]
- Goswami, P.; Das, P.; Verma, A.K.; Prakash, S.; Das, T.; Nag, T.; Ahuja, V.; Gupta, S.D.; Makharia, G.K. Are alterations of tight junctions at molecular and ultrastructural level different in duodenal biopsies of patients with celiac disease and Crohn’s disease? Virchows Archiv 2014, 465, 521-530. [CrossRef]
- Prasad, S.; Mingrino, R.; Kaukinen, K.; Hayes, K.L.; Powell, R.M.; MacDonald, T.T.; Collins, J.E. Inflammatory processes have differential effects on claudins 2, 3 and 4 in colonic epithelial cells. Laboratory investigation 2005, 85, 1139-1162. [CrossRef]
- Thuijls, G.; Derikx, J.P.; de Haan, J.-J.; Grootjans, J.; de Bruïne, A.; Masclee, A.A.; Heineman, E.; Buurman, W.A. Urine-based detection of intestinal tight junction loss. Journal of clinical gastroenterology 2010, 44, e14-e19. [CrossRef]
- Lu, Z.; Ding, L.; Lu, Q.; Chen, Y.-H. Claudins in intestines: Distribution and functional significance in health and diseases. Tissue barriers 2013, 1, e24978. [CrossRef]
- Zweigner, J.; Schumann, R.R.; Weber, J.R. The role of lipopolysaccharide-binding protein in modulating the innate immune response. Microbes and Infection 2006, 8, 946-952. [CrossRef]
- Jensen, S.B.; Latysheva, N.; Hindberg, K.; Ueland, T. Plasma lipopolysaccharide-binding protein is a biomarker for future venous thromboembolism: Results from discovery and validation studies. Journal of Internal Medicine 2022, 292, 523-535. [CrossRef]
- Pugin, J.M.; Schürer-Maly, C.; Leturcq, D.; Moriarty, A.; Ulevitch, R.J.; Tobias, P.S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proceedings of the National Academy of Sciences 1993, 90, 2744-2748. [CrossRef]
- Ferreira, S.; Masi, J.; Giménez, V.; Carpinelli, M.-M.; Laterza, O.; Hermoso, M.; Ortiz-Villalba, J.; Chamorro, M.-E.; Langjahr, P. Effect of gluten-free diet on levels of soluble CD14 and lipopolysaccharide-binding protein in adult patients with celiac disease. Central European Journal of Immunology 2021, 46, 225-230. [CrossRef]
- Myc, A.; Buck, J.; Gonin, J.; Reynolds, B.; Hammerling, U.; Emanuel, D. The level of lipopolysaccharide-binding protein is significantly increased in plasma in patients with the systemic inflammatory response syndrome. Clinical Diagnostic Laboratory Immunology 1997, 4, 113-116. [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The gut microbiome in coronary artery disease and heart failure: Current knowledge and future directions. EBioMedicine 2020, 52. [CrossRef]
- Gonzalez-Quintela, A.; Alonso, M.; Campos, J.; Vizcaino, L.; Loidi, L.; Gude, F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PloS one 2013, 8, e54600. [CrossRef]
- Wang, Z.; Wilson, C.M.; Mendelev, N.; Ge, Y.; Galfalvy, H.; Elder, G.; Ahlers, S.; Yarnell, A.M.; LoPresti, M.L.; Kamimori, G.H.J.J.o.N. Acute and chronic molecular signatures and associated symptoms of blast exposure in military breachers. 2019. [CrossRef]
- Gill, J.; Motamedi, V.; Osier, N.; Dell, K.; Arcurio, L.; Carr, W.; Walker, P.; Ahlers, S.; LoPresti, M.; Yarnell, A. Moderate blast exposure results in increased IL-6 and TNFα in peripheral blood. Brain, behavior, and immunity 2017, 65, 90-94. [CrossRef]
- Edwards, K.A.; Leete, J.J.; Smith, E.G.; Quick, A.; Modica, C.M.; Wassermann, E.M.; Polejaeva, E.; Dell, K.C.; LoPresti, M.; Walker, P. Elevations in tumor necrosis factor alpha and interleukin 6 from neuronal-derived extracellular vesicles in repeated low-level blast exposed personnel. Frontiers in Neurology 2022, 13. [CrossRef]
- Malan-Muller, S.; Valles-Colomer, M.; Foxx, C.L.; Vieira-Silva, S.; van den Heuvel, L.L.; Raes, J.; Seedat, S.; Lowry, C.A.; Hemmings, S.M. Exploring the relationship between the gut microbiome and mental health outcomes in a posttraumatic stress disorder cohort relative to trauma-exposed controls. European Neuropsychopharmacology 2022, 56, 24-38. [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.; Sandhu, K.V.; Bastiaanssen, T.F.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V. The microbiota-gut-brain axis. Physiological reviews 2019. [CrossRef]
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelletti, P.; Ghaemi, A.; Sacco, S.; Togha, M.; Federation, S.o.A.S.o.t.E.H. Gut-brain axis and migraine headache: a comprehensive review. The journal of headache and pain 2020, 21, 1-12. [CrossRef]
- Aamodt, A.; Stovner, L.; Hagen, K.; Zwart, J. Comorbidity of headache and gastrointestinal complaints. The Head-HUNT Study. Cephalalgia 2008, 28, 144-151.
- Crawford, J.; Liu, S.; Tao, F. Gut microbiota and migraine. Neurobiology of Pain 2022, 11, 100090. [CrossRef]
- Sgro, M.; Ray, J.; Foster, E.; Mychasiuk, R. Making Migraine Easier to Stomach: The Role of the Gut Brain Immune Axis in Headache Disorders. European Journal of Neurology 2023. [CrossRef]
- Mac Donald, C.L.; Barber, J.; Andre, J.; Evans, N.; Panks, C.; Sun, S.; Zalewski, K.; Sanders, R.E.; Temkin, N. 5-Year imaging sequelae of concussive blast injury and relation to early clinical outcome. Neuroimage: clinical 2017, 14, 371-378. [CrossRef]
- Mac Donald, C.L.; Barber, J.; Patterson, J.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Association between 5-year clinical outcome in patients with nonmedically evacuated mild blast traumatic brain injury and clinical measures collected within 7 days postinjury in combat. JAMA network open 2019, 2, e186676-e186676. [CrossRef]
- Hoffman, J.M.; Lucas, S.; Dikmen, S.; Braden, C.A.; Brown, A.W.; Brunner, R.; Diaz-Arrastia, R.; Walker, W.C.; Watanabe, T.K.; Bell, K.R. Natural history of headache after traumatic brain injury. Journal of neurotrauma 2011, 28, 1719-1725. [CrossRef]
- Walker, W.C.; Marwitz, J.H.; Wilk, A.R.; Ketchum, J.M.; Hoffman, J.M.; Brown, A.W.; Lucas, S. Prediction of headache severity (density and functional impact) after traumatic brain injury: A longitudinal multicenter study. Cephalalgia 2013, 33, 998-1008. [CrossRef]
- Stacey, A.; Lucas, S.; Dikmen, S.; Temkin, N.; Bell, K.R.; Brown, A.; Brunner, R.; Diaz-Arrastia, R.; Watanabe, T.K.; Weintraub, A. Natural history of headache five years after traumatic brain injury. Journal of neurotrauma 2017, 34, 1558-1564. [CrossRef]
- Mac Donald, C.L.; Barber, J.; Jordan, M.; Johnson, A.M.; Dikmen, S.; Fann, J.R.; Temkin, N. Early clinical predictors of 5-year outcome after concussive blast traumatic brain injury. JAMA neurology 2017, 74, 821-829. [CrossRef]
- Carr, W.; Dell, K.; Yanagi, M.; Hassan, D.; LoPresti, M. Perspectives on repeated low-level blast and the measurement of neurotrauma in humans as an occupational exposure risk. Shock waves 2017, 27, 829-836. [CrossRef]
- Caplan, B.; Bogner, J.; Brenner, L.; Carr, W.; Polejaeva, E.; Grome, A.; Crandall, B.; LaValle, C.; Eonta, S.E.; Young, L.A. Relation of repeated low-level blast exposure with symptomology similar to concussion. Journal of Head Trauma Rehabilitation 2015, 30, 47-55. [CrossRef]
- Brenner, L.A.; Forster, J.E.; Stearns-Yoder, K.A.; Stamper, C.E.; Hoisington, A.J.; Brostow, D.P.; Mealer, M.; Wortzel, H.S.; Postolache, T.T.; Lowry, C.A. Evaluation of an immunomodulatory probiotic intervention for veterans with co-occurring mild traumatic brain injury and posttraumatic stress disorder: a pilot study. Frontiers in neurology 2020, 11, 1015. [CrossRef]
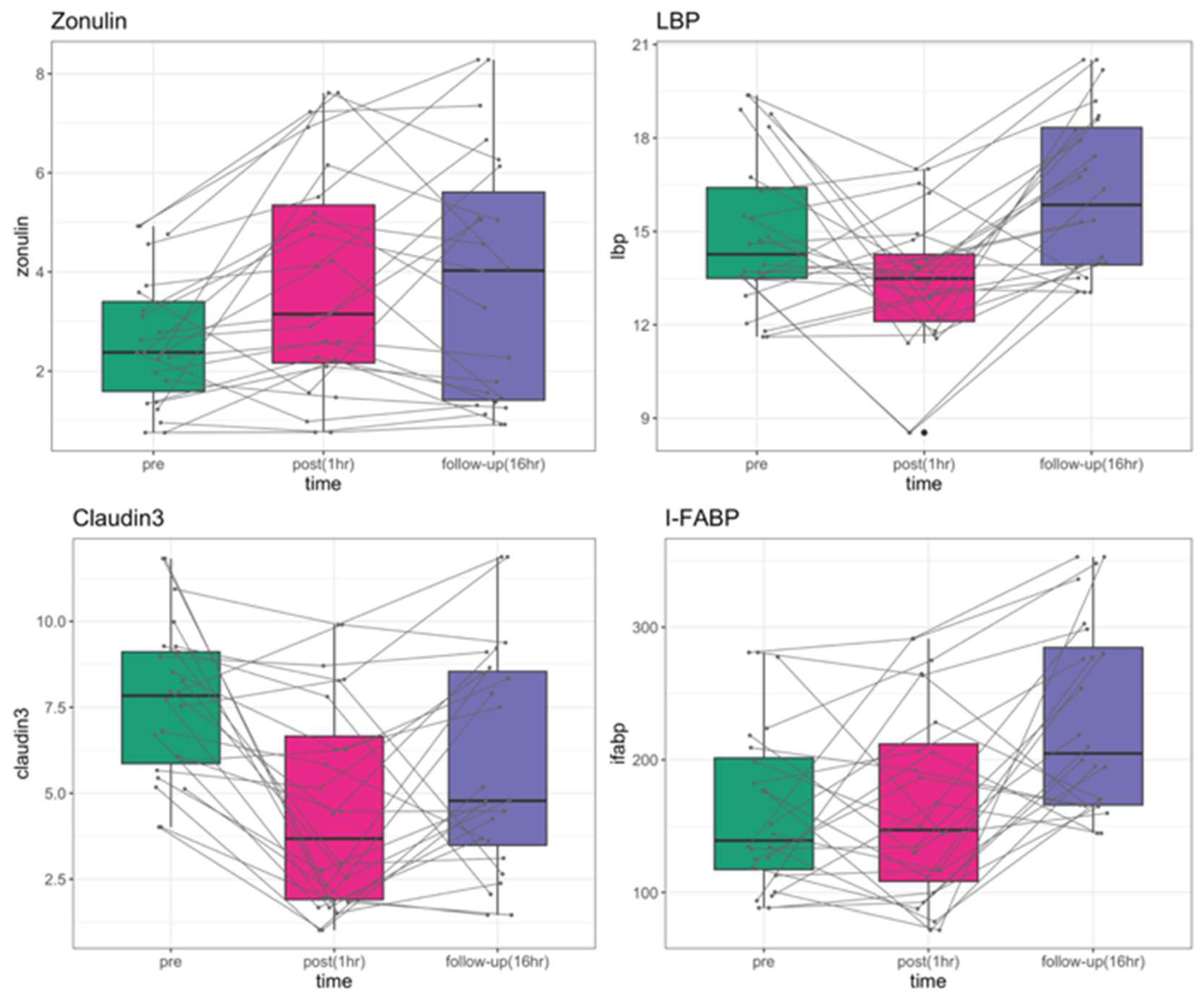
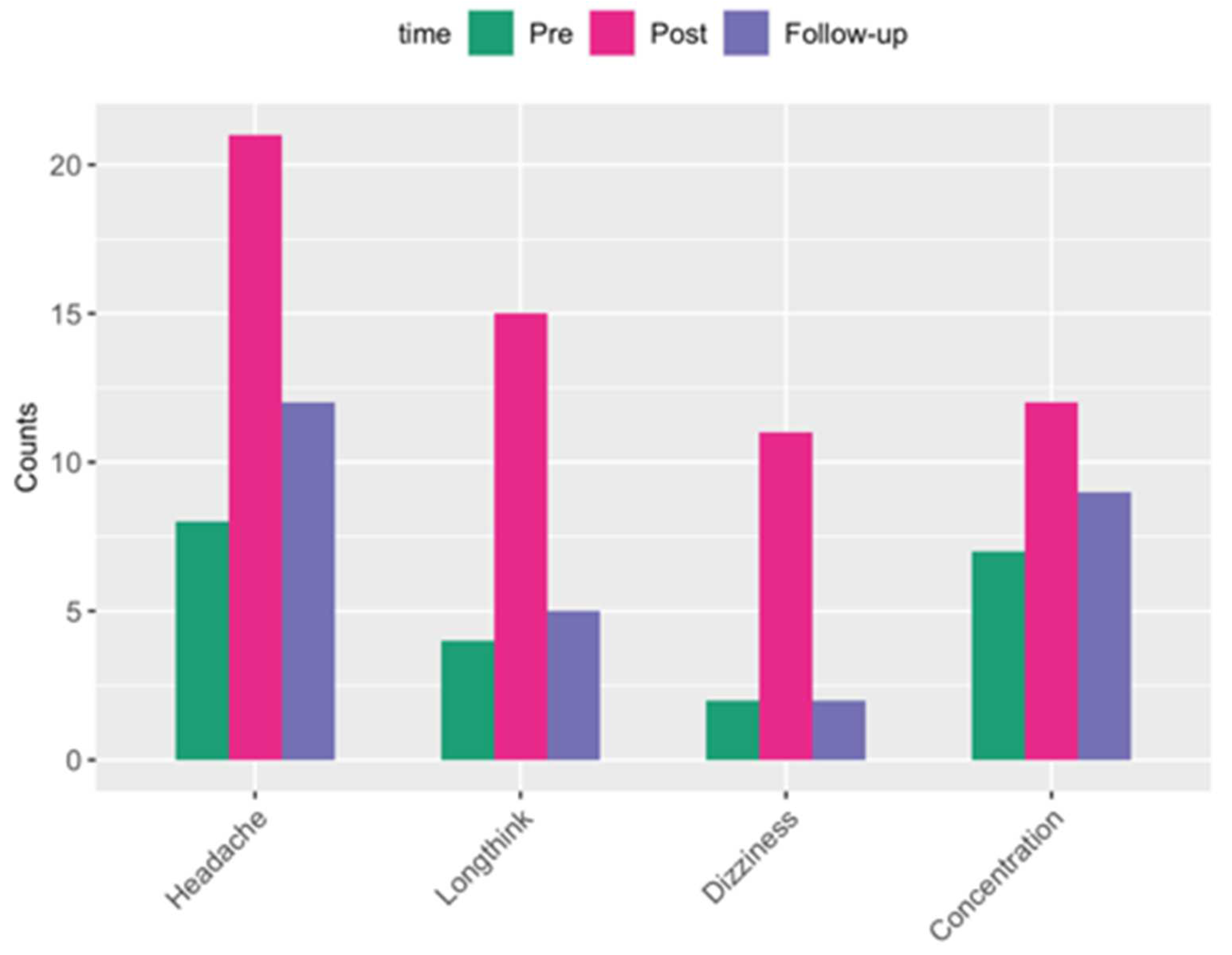
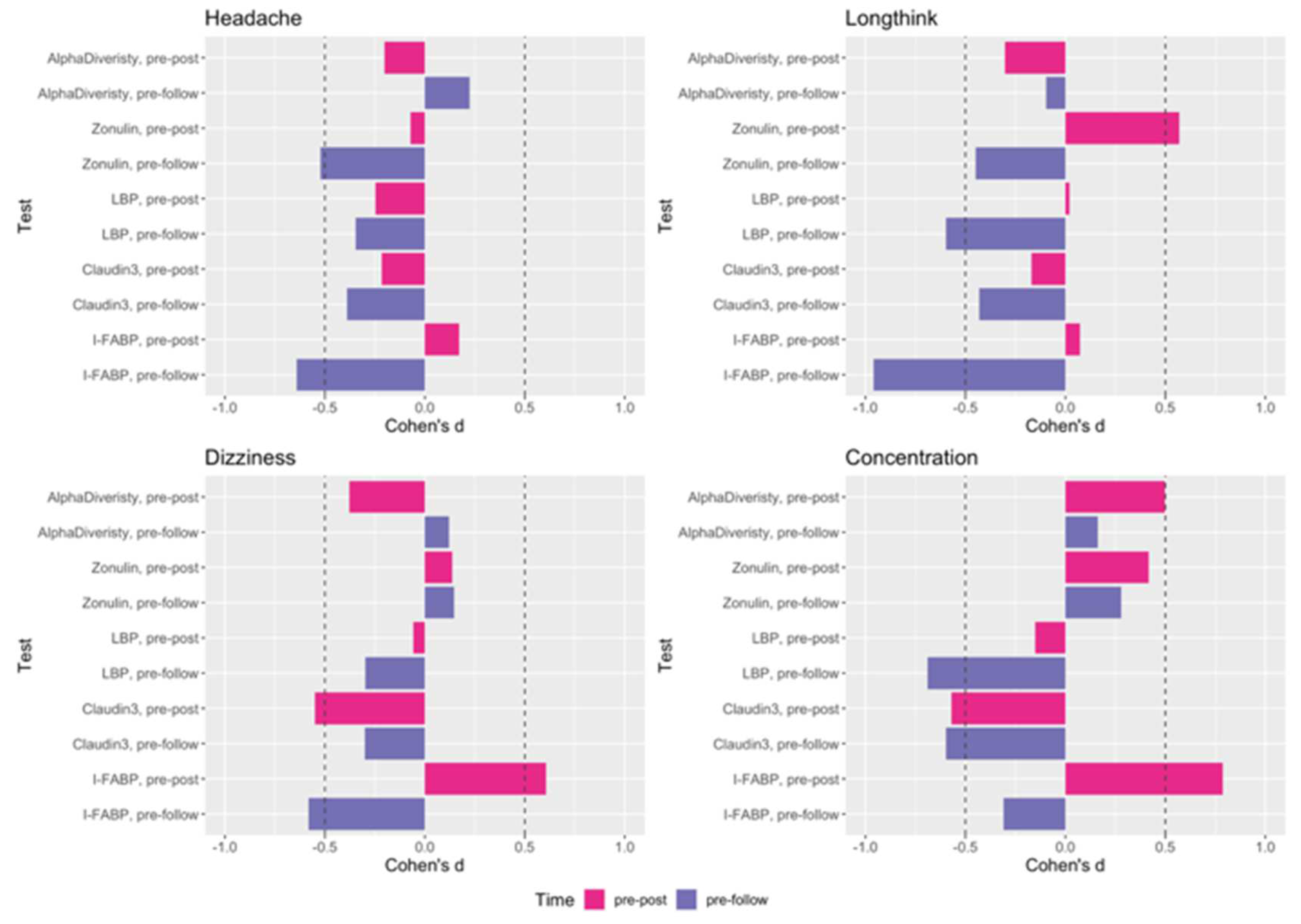
| P-value | Zonulin | LBP | Claudin-3 | I-FABP |
| ANOVA | 0.0018 | 0.0002 | 6.24E-05 | 8.34E-05 |
| pre vs. post | 0.0029 | 0.0222 | 3.8E-05 | 0.7870 |
| pre vs. follow-up | 0.0029 | 0.0513 | 0.0115 | 0.0003 |
| post vs. follow-up | 0.9680 | 0.0001 | 0.0412 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





