1. Introduction
Fibromyalgia is often accompanied by widespread musculoskeletal pain, resulting in fatigue, sleep disturbance, memory loss, and psychiatric problems. Classic fibromyalgia symptoms often begin after an event and progressively accumulate without eliciting occurrence. Fibromyalgia often occurs in women who also have tension headaches, irritable bowel syndrome, anxiety, and depression (1-3). Currently, there is no cure for fibromyalgia, medication can only manage symptoms. Nutrients, exercise, meditation, yoga, and relaxation may help (4-6). Fibromyalgia may result from repeated nerve stimulation, also called central sensitization, or changes in overexpression of neurotransmitters or neuromodulators in the brain. These phenomena may result from genetic mutations, infections, and psychological stress. Fibromyalgia affects about 1–8% of the population (7-9). The American College of Rheumatology reorganized the diagnostic principles for fibromyalgia. They announced widespread pain index (WPI) and symptom severity scale (SS) to evaluate fibromyalgia. The WPI assesses 19 common pain points over the last two 2 weeks and the SS estimates the degree of fatigue, waking, and cognitive symptoms. Fibromyalgia pain is defined by a WPI ≥ 7 and SS ≥ 5 or WPI 3–6 and SS ≥ 9 over the last three months. Duloxetine (Cymbalta), milnacipran (Savella), and pregabalin (Lyrica) are current FDA approved treatments for fibromyalgia. Gabapentinoids, sedatives, selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and tricyclic compounds are also utilized for fibromyalgia, though having several side effects. The economic impact of fibromyalgia is high and the precise cost drivers of healthcare spending for symptoms (10-12).
Transient receptor potential vanilloid 1 (TRPV1) channels act as detectors of several microenvironmental signals such as mechanical, thermal, pain, vision, or stress and they can be widely stimulated by several related inputs. These ion channels, permeable to Na+ and Ca2+, are found in several tissues, typically on the cell membrane (13-15). Accordingly, due to the crystal structure of TRPV1. TRPV was selected for drug development and implied in curing many diseases. TRPV1 modulation has been clinically investigated for different indications, especially for pain treatment, as these channels are main detectors and transducers of painful signal from the peripheral to the central nervous system. Numerous potent TRPV1 agonists and antagonists have been used in clinical trials to cure visceral, inflammatory, and neuropathic pain (16-17). However, the therapeutic effect was unsatisfactory, causing several side effects. TRPV1 is considered a critical brain inflammatory sensor and pain biomarker in the mouse brain (19). Triggering TRPV1 can increase the level of mitogen-activated protein kinase (MAPK), which is involved in pain development. MAPK has three major subfamilies: extracellular signal-regulated protein kinase (ERK), p38, and c-Jun N-terminal kinase/stress-activated protein kinase (JNK) (20). TRPV1 downstream PI3K-Akt-mTOR axis was also indicated to be involved in the modulation of pain sensation (21).
Eicosapentaenoic acid (EPA) is a long-chain omega-3 polyunsaturated fatty acid mostly found in cold-water fish. EPA has beneficial effects on heart disease, high triglycerides, hypertension, and inflammation. EPA is an essential nutrient with anti-inflammatory (22), antidepression (23), neuroprotective (24), and cardioprotective effects (25). Even through EPA is used as a dietary supplement or nutrient-based medicine, its detail mechanisms are still unclear. EPA was also reported to have anti-inflammation and immune function in the brain. In addition, docosahexaenoic acid (DHA) is the abundant in the brain and responding for neurotransmission, neuroplasticity and neuroprotection. Recent article indicated lower level of EPA in patients with depression suggesting that EPA play a crucial role in the pathogenesis of depression. The findings provide further support for using EPA as an alternative therapy for depression (26-28). Further, low levels of EPA have been found in patients with depression, indicating that EPA plays an essential role in the pathogenesis of depression (29, 30). Thus, EPA has potential as an alternative therapy for depression without side effects (31-33). Further, EPA has been shown to relieve chronic pain and depression in mouse models. EPA can reduce chronic pain and depression through regulation of interleukins (21).
In the current study, we investigated the role of EPA on fibromyalgia pain and the underlying mechanisms in a mouse model, which remain unknown. We found augmented expression and localization of TRPV1 and associated kinases in a mouse model of fibromyalgia. These increased levels of signaling mediators were found in the mouse thalamus, somatosensory cortex (SSC), anterior cingulate cortex (ACC), medial prefrontal cortex (mPFC), and cerebellum. Furthermore, On the contrary, CB1 has a different decreasing trend. These effects were reversed by EPA administration. Thus, our results demonstrate the positive effects of EPA in a mouse fibromyalgia pain model and advise the clinical use of EPA.
2. Materials and Methods
Animals and EPA administration
We used 27 female C57BL/6 mice (8–12 weeks old) in this study (BioLASCO, Taipei, Taiwan Co., Ltd., Taipei, Taiwan). Mice underwent a 12 h light–dark cycle with food and water ad libitum. We used a statistic method to calculate the sample size. Nine mice in each group were considered as the minimum number required for a significance alpha level of 0.05 and a power of 80%. Mouse use was allowed by the Institute of Animal Care and Use Committee of China Medical University (Permit no. CMUIACUC-2023-071), Taiwan, following the Guide for the use of Laboratory Animals (National Academy Press). Mice were randomly grouped into three groups: normal mice (Group 1: Normal), fibromyalgia mice (Group 2: FM), and fibromyalgia mice treated with EPA group (Group 3: FM + EPA). The research was designed to euthanize the mice at the end of day 5. Nociceptive behaviors were evaluated at day 0, day 3, and day 5. EPA (300 mg/kg/day) was orally administered daily during the treatment period on day 3–5 (21).
Pain behavior test
Mechanical and thermal nociceptive behaviors were evaluated three times on day 0, day 3, and day 5 before and after fibromyalgia pain induction. All mice were placed in the behavior examination room over 30 min to calm down. The behavior was analyzed only when the mice were quiet without sleeping or grooming. We first verified the von Frey filament examination, mechanical pain was tested by calculating the strength of replies to stimuli within 3 performances via electronic von Frey test (IITC Life Science Inc., USA). Rodents were placed to a steel mesh (75 × 25 × 45 cm) and isolated with a plastic cage (10 × 6 × 11 cm). Rodents were confirmed by filament examination at central zone of the right back paw. The forces were calculated as gram and were documented when the mice withdraw their paw. Besides, the Hargreaves’ test was achieved to measure thermal nociceptive behavior by calculating the time to thermal stimuli with 3 presentations (IITC Life Sciences, SERIES8, Model 390G). The animals were engaged in a cage on top of a glass area. All rodents were placed to the experimental room, and were enriched to the condition over 30 min.
Western blot analysis
The whole thalamus, mPFC, SSC, ACC, and cerebellum tissues were excised to extract proteins. Tissues were placed on ice and stored at −80ºC until protein extraction. Total proteins were homogenized in cold radioimmunoprecipitation lysis buffer containing 50 mM Tris-HCl pH 7.4, 250 mM NaCl, 1% NP-40, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 0.02% NaN3, and 1x protease inhibitor cocktail (AMRESCO). The extracted proteins were subjected to 8% SDS-Tris glycine gel electrophoresis and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked with 5% nonfat milk in Tris Buffered Saline with Tween (TBST) buffer (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20), incubated with a primary antibody in TBS-T with 1% bovine serum albumin (BSA) for 1 h at room temperature. Peroxidase-conjugated anti-rabbit antibody, anti-mouse antibody, or anti-goat antibody (1: 5,000) was used as appropriate secondary antibody. The bands were visualized using an enhanced chemiluminescent substrate kit (PIERCE) with LAS-3000 Fujifilm (Fuji Photo Film Co., Ltd.). Where applicable, the image intensities of specific bands were quantified using NIH ImageJ software (Bethesda, MD, USA). β-actin or α-tubulin we used as internal control.
Immunofluorescence
Mice were euthanized using a 5% isoflurane and intracardially perfused with normal saline followed by 4% paraformaldehyde. The brain was immediately dissected and postfixed with 4% paraformaldehyde at 4ºC for 3 days. The tissues were placed in 30% sucrose for cryoprotection overnight at 4ºC. The brain was embedded in an Optimal cutting temperature compound and rapidly frozen using liquid nitrogen before storing the tissues at −80ºC. Frozen segments were cut into 20 mm sections using a cryostat and instantaneously placed on glass slides. The samples were fixed with 4% paraformaldehyde and incubated with a blocking solution, consisting of 3% BSA, 0.1% Triton X-100, and 0.02% sodium azide, for 1 h at room temperature. After blocking, the samples were incubated with the primary antibody (1:200, Alomone), TRPV1, and Iba1, prepared in 1% BSA solution at overnight. Then, samples were incubated with the secondary antibody (1:500), 488-conjugated AffiniPure donkey anti-rabbit IgG (H + L), 594-conjugated AffiniPure donkey anti-goat IgG (H + L), and peroxidase-conjugated AffiniPure donkey anti-mouse IgG (H + L) for 2 h at room temperature before being fixed with coverslips for immunofluorescence visualization.
Statistical analysis
Statistical analysis was performed using the SPSS statistic program. All statistic data are presented as the mean ± standard error (SEM). A Shapiro–Wilk test was performed to test data normality. Differences among all groups were tested using an Analysis of Variance (ANOVA) test followed by a post hoc Tukey’s test. P < 0.05 was considered statistically significant.
3. Results
3.1. EPA attenuated the intermittent cold stress-induced fibromyalgia pain in mice
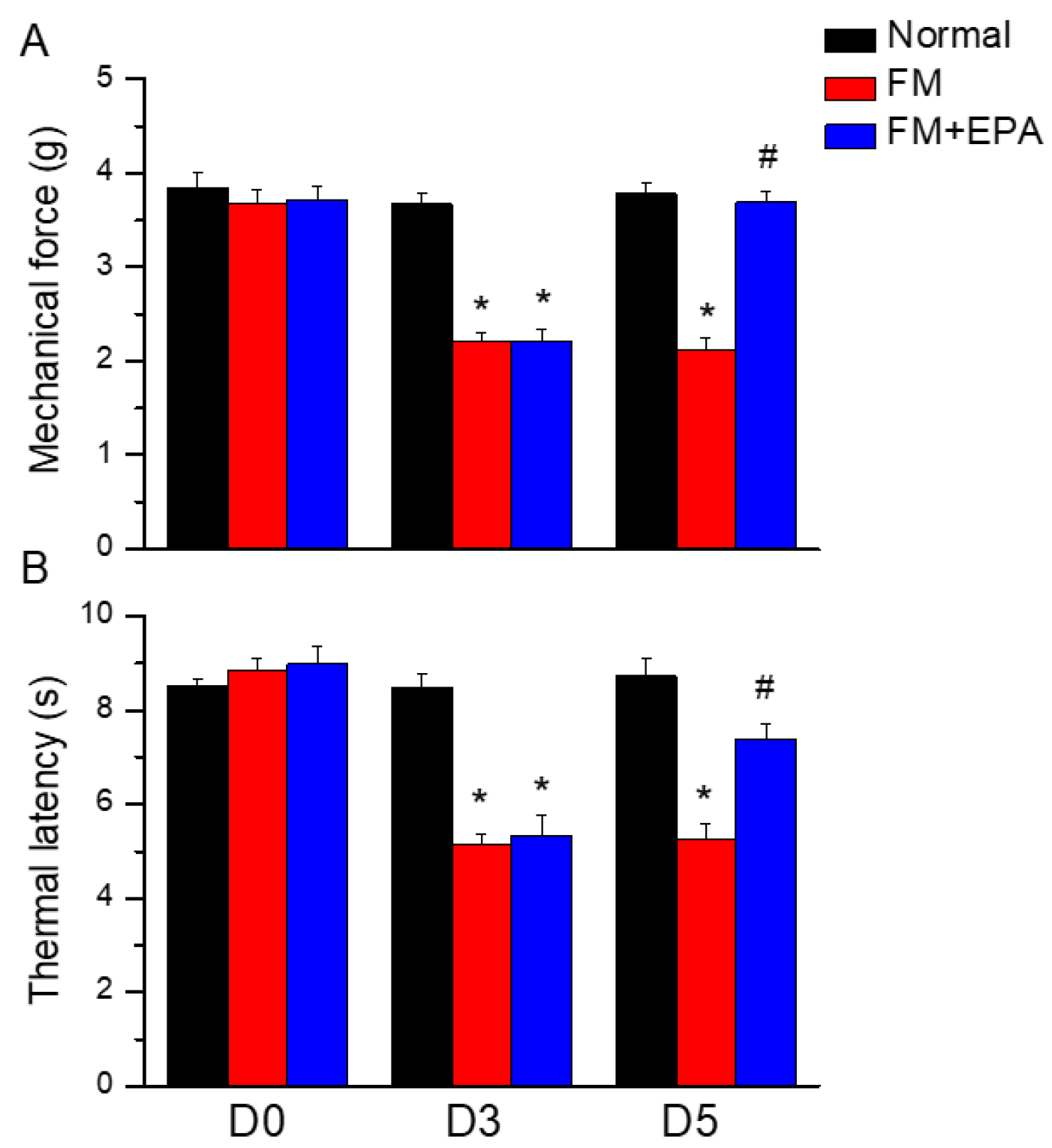
To address if EPA has analgesic effects on mouse fibromyalgia pain, we performed The von Frey and Hargraves’ tests to detect mechanical and thermal hyperalgesia. After inducing the mice fibromyalgia model for three days, their mechanical or thermal hyperalgesia was measured. The mechanical thresholds in the fibromyalgia group, which underwent cold stress induction, were significantly lower than those of the normal group (
Figure 1A, red column, day 5: 2.13 ± 0.12 g, *
P < 0.05, n=9). EPA mitigated the mechanical hypersensitivity by increasing the withdrawal threshold measured by the von Frey test (
Figure 1A, blue column, day 5: 3.69 ± 0.13 g, #
P < 0.05, n=9). In the Hargreaves’ test, cold stress significantly decreased the thermal latency compared to the normal group, suggesting the successful induction of fibromyalgia pain. In contrast, EPA administration alleviated the thermal hyperalgesia by increasing the thermal latency (
Figure 1B, *
P < 0.05, n=9).
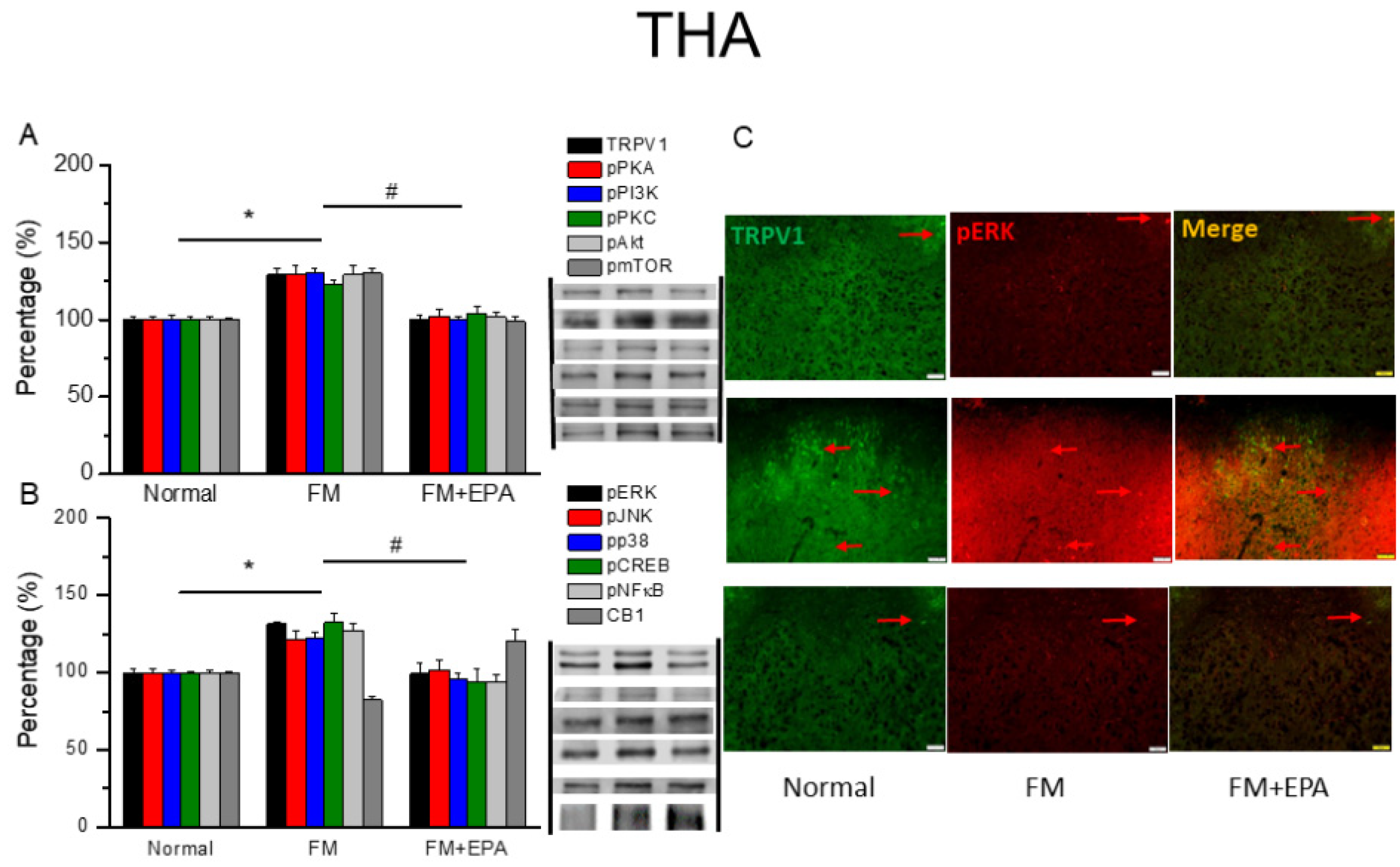
3.2. EPA relieved fibromyalgia pain through regulation of TRPV1 signaling pathway in the mouse thalamus
Central sensitization is involved in hyperalgesia from nociceptor signals in the peripheral region. We used Western blot to detect TRPV1 expression in the mouse thalamus, a crucial brain area for ascending pain processing. Induction of fibromyalgia pain via cold stress reliably increases TRPV1 expression in the thalamus (
Figure 2A, black columns, *
P < 0.05, n = 6), while EPA suppressed this effect after three intakes (
Figure 2A, black columns, #
P < 0.05, n = 6). We next considered the expression of downstream protein kinases involved in conducting pain signals, such as phosphorylated PKA, PI3K, and PKC. Fibromyalgia pain significantly increased the expression of those kinases in comparison to the normal group (
Figure 2A, *
P < 0.05, n = 6). This increase decreased with EPA administration (
Figure 2A, #
P < 0.05, n = 6). In addition, mice with fibromyalgia pain showed dramatical increases in pAkt and the pmTOR axis. Furthermore, pERK, pp38, pJNK protein levels increased in the thalamus of fibromyalgia mice (
Figure 2A, *
P < 0.05, n = 6), such upregulation was reduced by EPA administration (
Figure 2A, #
P < 0.05, n = 6). After the impression of protein kinases activation, the transcription factors including pCREB and pNF-κB therefore initiated the gene transcription. pCREB and pNF-κB expression increased after fibromyalgia pain induction in the thalamus. EPA intake significantly decreased this upregulation. Using immunofluorescent staining, we next determined lower expression and colocalization of TRPV1 and pERK in the normal mouse thalamus than in that of mice with fibromyalgia. EPA treatment inhibited this increase (
Figure 2C, green, red, or yellow color, n = 3).
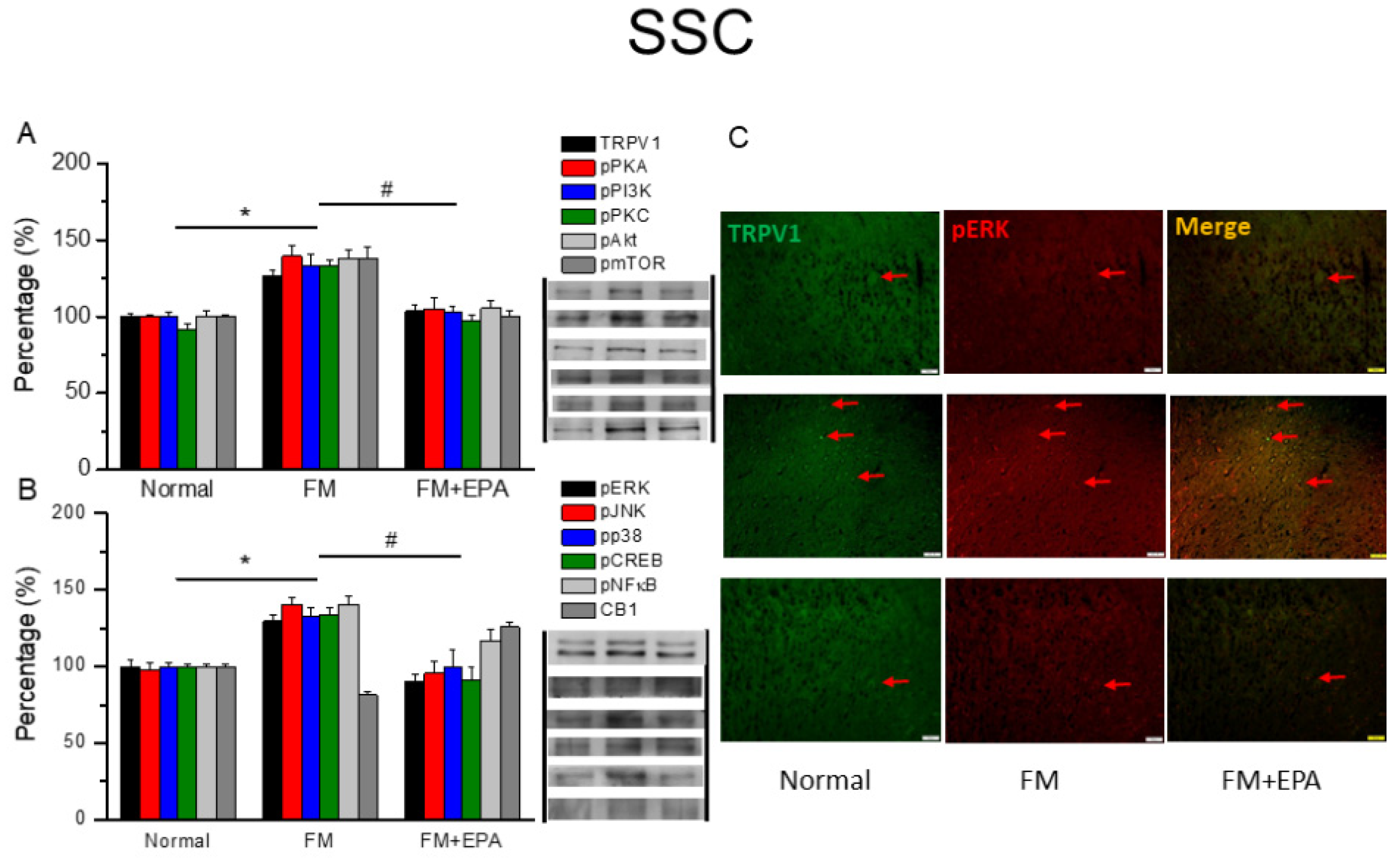
3.3. EPA intake mitigated cold stress-induced fibromyalgia pain in the mouse somatosensory cortex
We next explored the effect of EPA and the TRPV1 pain-signaling pathway in the SSC. TRPV1 expression was potentiated in the mouse SSC after fibromyalgia pain induction (
Figure 3A, *
P < 0.05, n = 6). This overexpression was remarkably alleviated by EPA treatment (
Figure 3A, #
P < 0.05, n = 6). The mouse fibromyalgia model resulted in pPKA, pPI3K, and pPKC overexpression in the SSC; this overexpression decreased by EPA treatment. Similar results were observed for pAkt, pmTOR downstream molecules in the SSC. Furthermore, cold stress increased pERK, pP38, and pJNK levels as well as those of pCREB and pNF-kB in the SSC of the fibromyalgia group (
Figure 3B, *
P < 0.05, n = 6), an effect reversed by EPA treatment (
Figure 3B, #
P < 0.05, n = 6). We next determined lower colocalization of TRPV1 and pERK in the normal mouse SSC than in the fibromyalgia group. This increase was attenuated by EPA treatment (
Figure 3C, green, red, or yellow color, n = 3).
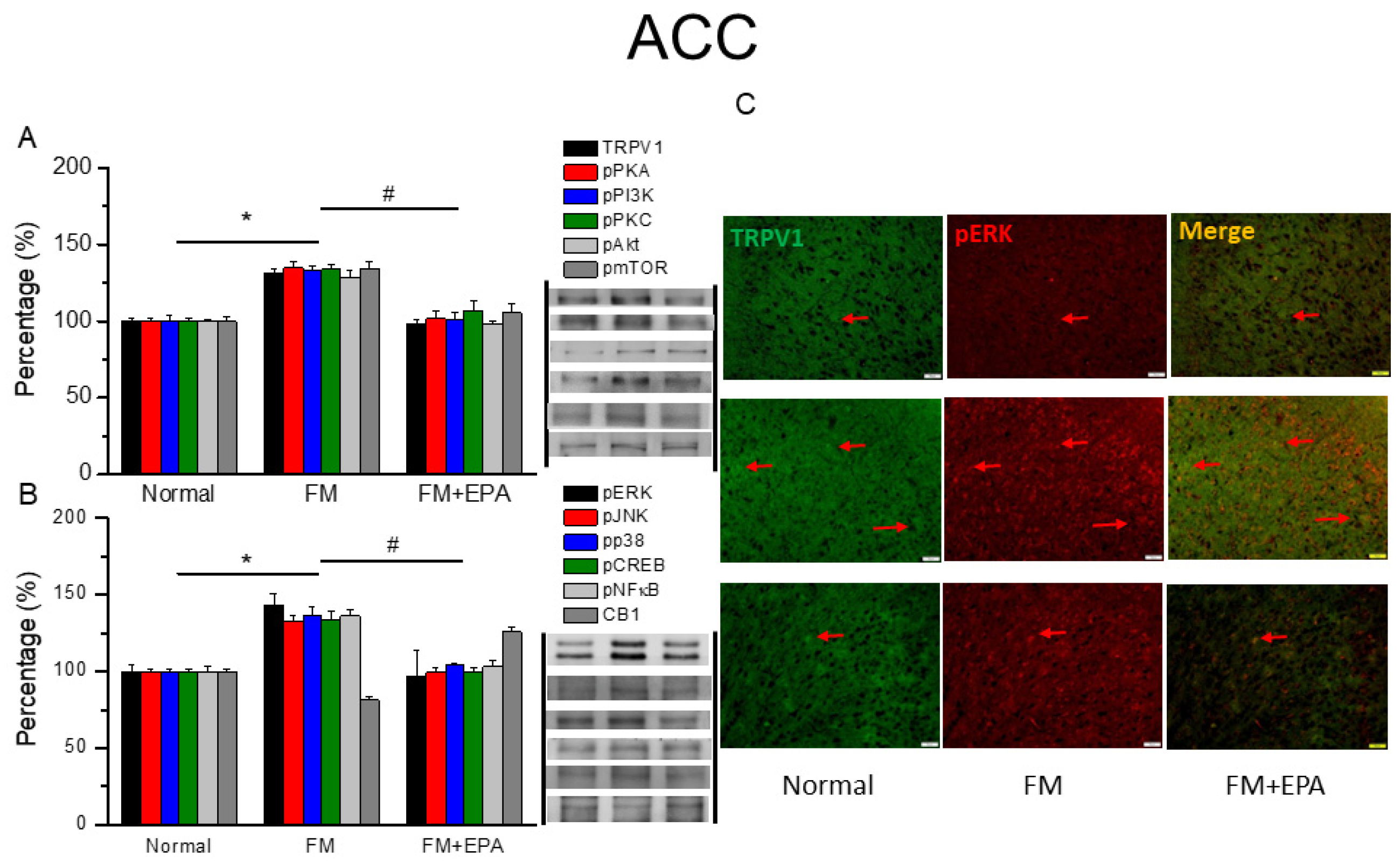
3.4. EPA treatment attenuated fibromyalgia pain through TRPV1 modulation in the ACC
After the final behavioral tests on day 5, we collected the mouse ACC to examine TRPV1 protein expression via Western blotting. Fibromyalgia pain mice had significantly increased TRPV1 levels as well as increased pPKA, pPI3K, and pPKC (
Figure 4A, *
P < 0.05, n = 6). All these effects were decreased by EPA administration (
Figure 2A, #
P < 0.05, n = 6). Mice fibromyalgia pain also increased pAkt and pmTOR expression in the ACC. EPA treatment revealed the analgesic effect accompanied by attenuating the overexpression of pAkt and pmTOR. Furthermore, cold stress initiated mice fibromyalgia simultaneously potentiated pERK, pP38, and pJNK overexpression (
Figure 4A, *
P < 0.05, n = 6), and EPA reversed this phenomenon (
Figure 4B, #
P < 0.05, n = 6). Lastly, the protein level of pCREB and pNF-kB in the ACC displayed a similar response trends (
Figure 4B, *P < 0.05, n = 6). In addition, there was lower colocalization of TRPV1 and pERK in the normal mouse ACC than in that in the fibromyalgia group. This increase was attenuated by EPA treatment (
Figure 4C, green, red, or yellow color, n = 3).
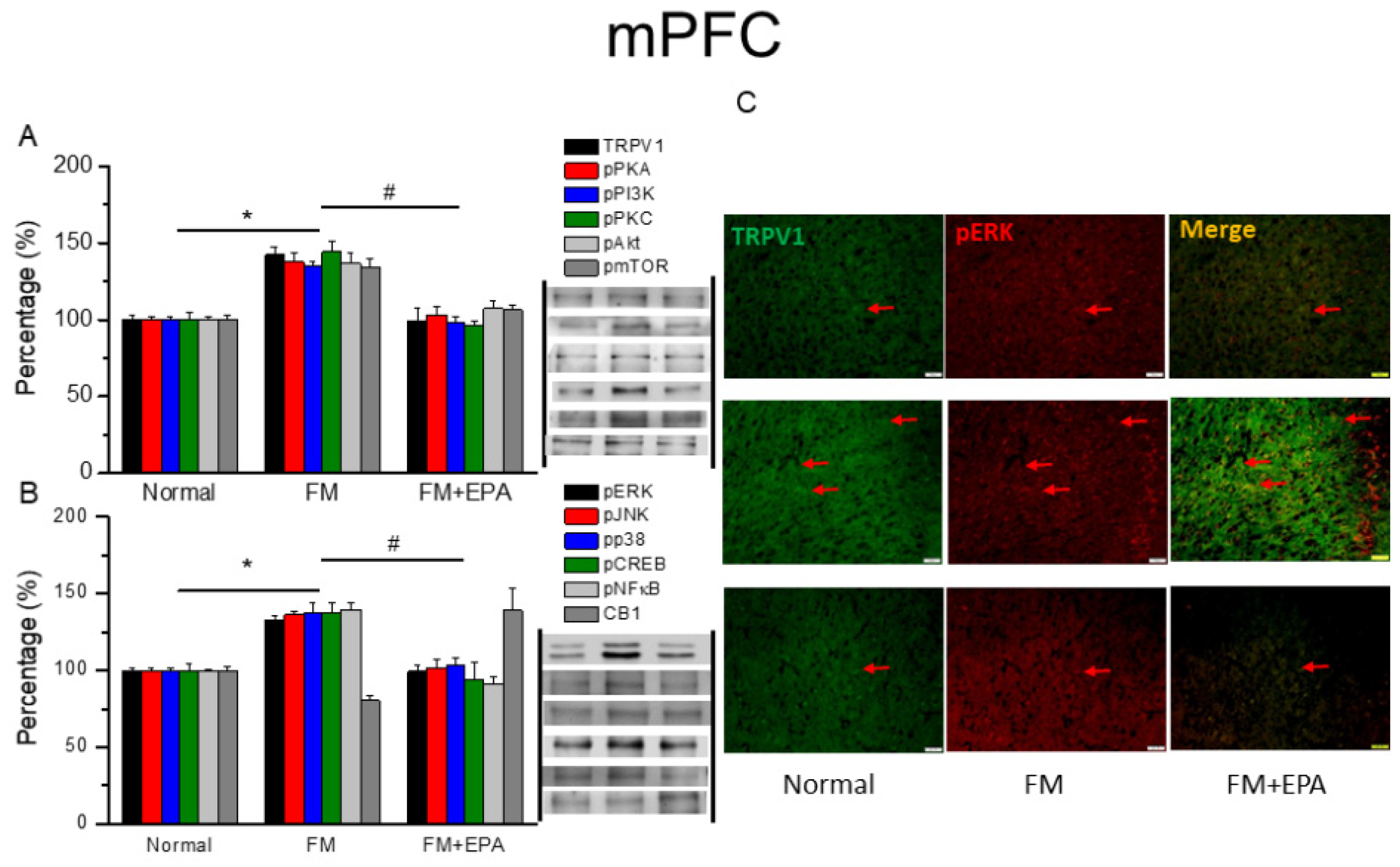
3.5. The increased levels of TRPV1 and associated molecules in the mPFC in fibromyalgia mice decreased with EPA treatment
The mouse mPFC was known involving in fibromyalgia pain through TRPV1 and associated mediators. Fibromyalgia mice showed higher protein levels of TRPV1 (
Figure 5A, *
P < 0.05, n = 6), which EPA administration effectively decreased (
Figure 2A, #
P < 0.05, n = 6). Downstream protein kinases such as pPKA, pPI3K, and pPKC, were simultaneously increased after fibromyalgia pain induction, which were then abrogated by EPA treatment. We further examined the expression of pAkt and pmTOR expression, which were augmented by cold stress-induced pain (
Figure 5A, *
P < 0.05, n = 6), and further reversed by EPA treatment (
Figure 5B, #
P < 0.05, n = 6). EPA treatment also attenuated the overexpression of pERK, pP38, pJNK, pCREB, and pNF-kB. Lower TRPV1 and pERK colocalization was observed in the normal mouse mPFC compared to the fibromyalgia group. This increase was attenuated by EPA treatment (
Figure 5C, green, red, or yellow color, n = 3).
3.6. Effects of EPA on fibromyalgia related pathways and associated molecules in cerebellum lobules 5-7
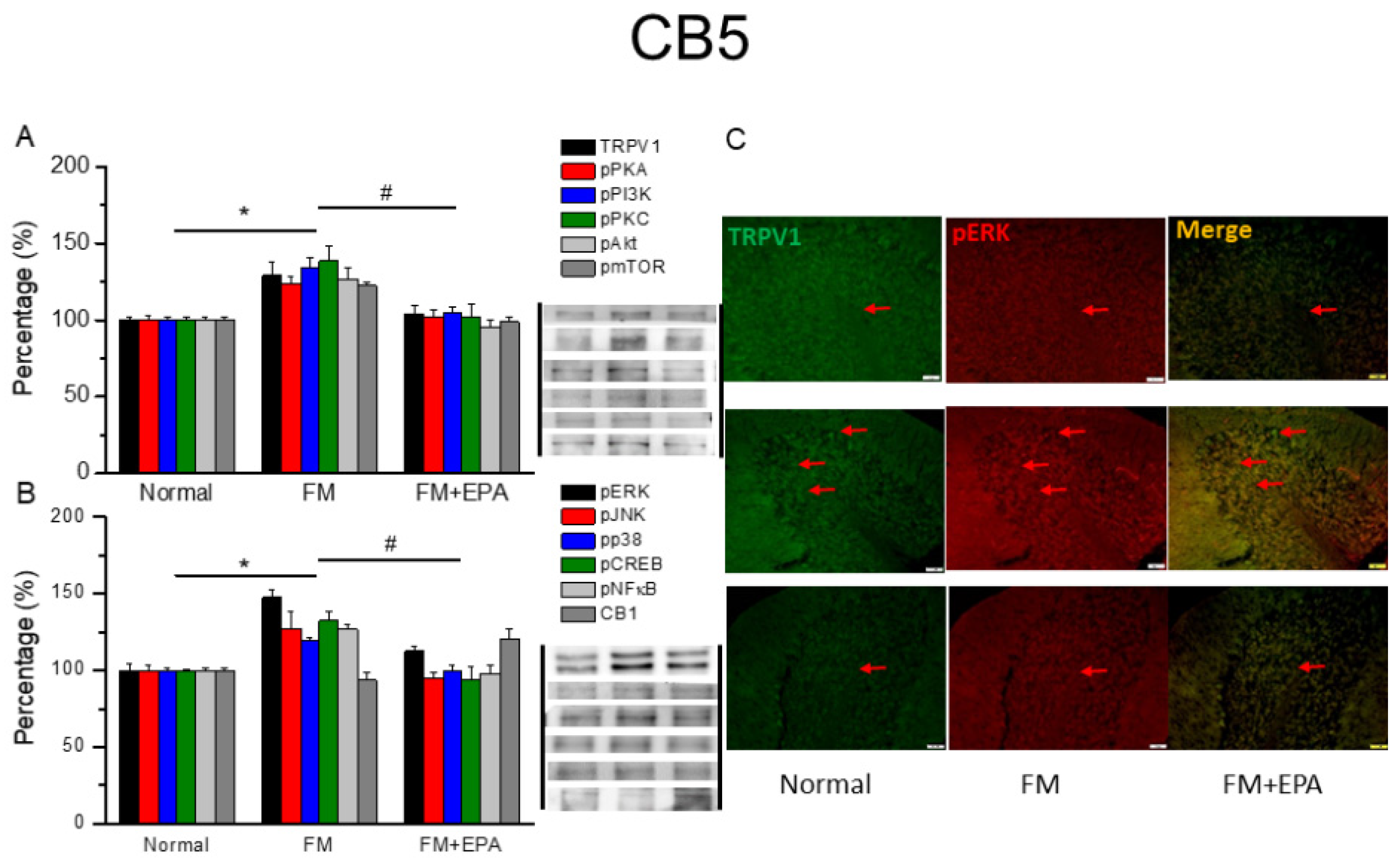
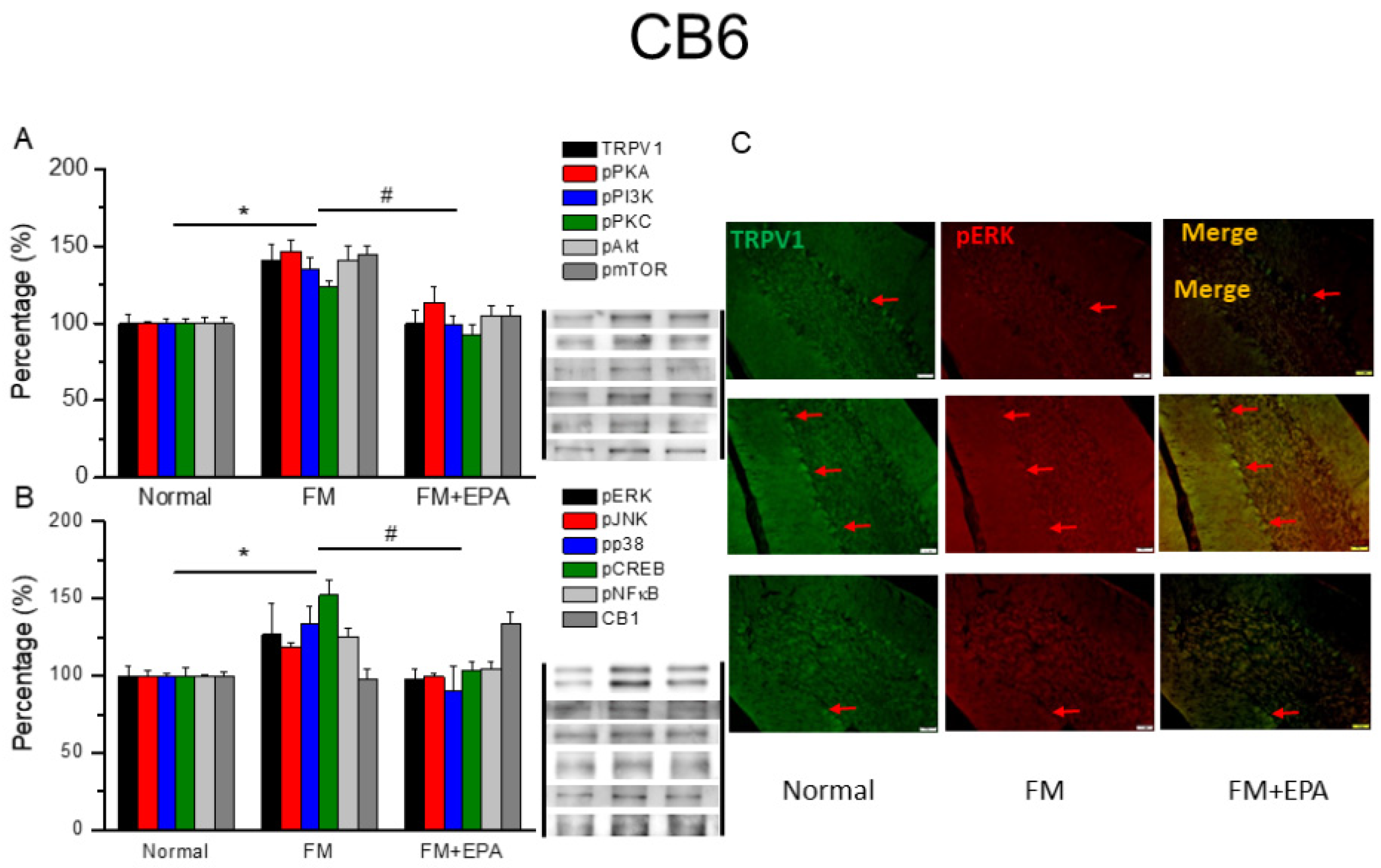
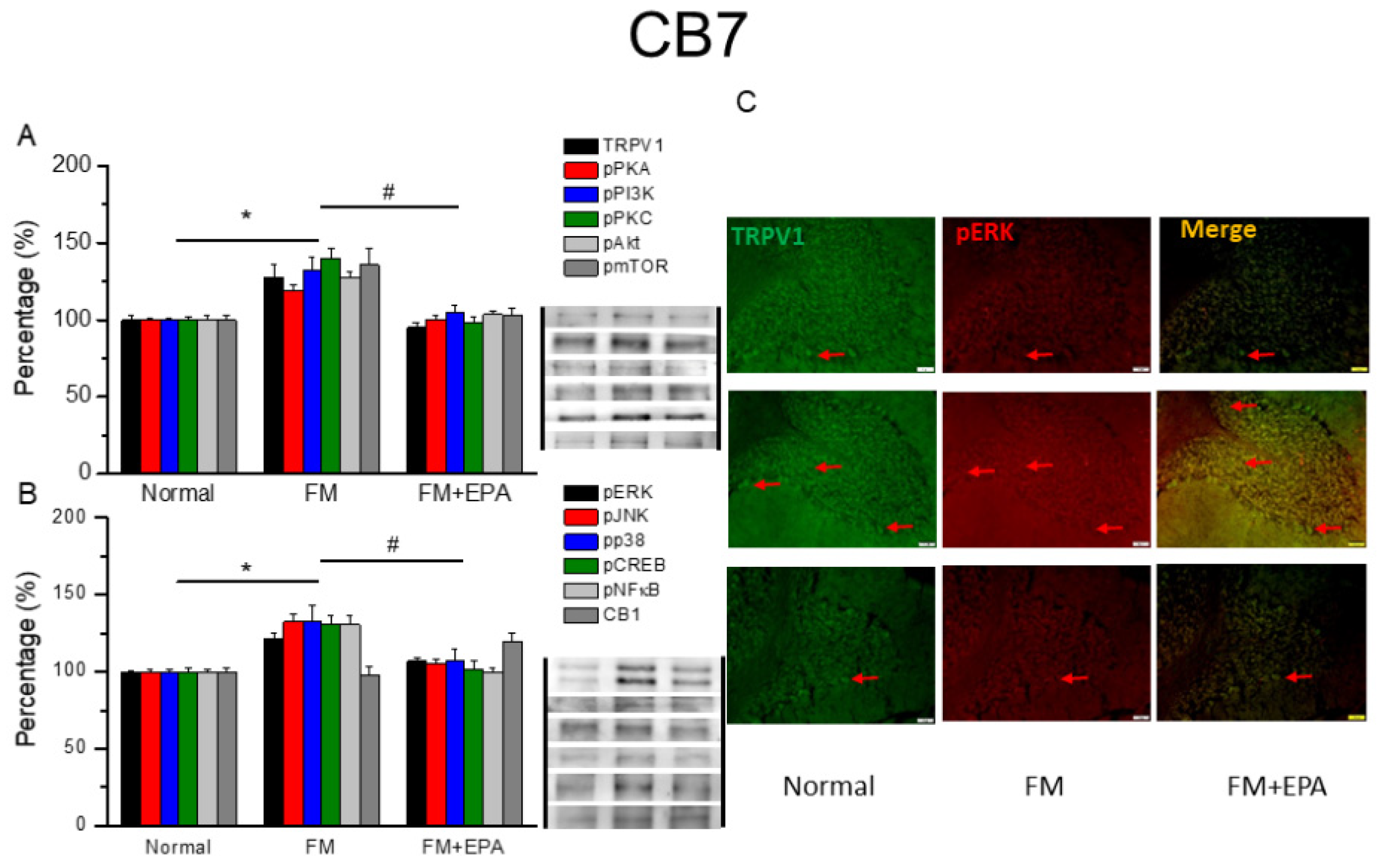
After the original approval of fibromyalgia pain according to the applicable mice behavior data, the mice cerebellum brain regions were sampled in order to discover the linked protein alteration in the mice cerebellum lobules 5-7. The outcome of fibromyalgia on TRPV1 pathway was investigated, and the linked molecular foundations of EPA were then examined. The normal mouse group was taken as the ideal value and normalized to 100%. Totally, the cerebellum lobules 5-7 were determined. TRPV1 expression was significantly higher in the fibromyalgia group (Figure 6-8A, * P < 0.05, n = 6); an increase attenuated by EPA treatment (Figure 6-8A, # P < 0.05, n = 6). In addition, as expected, the participation of pPKA, pPI3K, pPKC, pAkt, and pmTOR, which are the major pathways of MAPK, were all increased in fibromyalgia mice and further reversed by EPA treatment. Lastly, similar tendency was also observed in pERK, pP38, pJNK, pCREB, and pNF-kB (Figures 6-8B). Finally, we observed lower colocalization of TRPV1 and pERK in the normal mouse cerebellum than in the fibromyalgia group. This increase was attenuated by EPA treatment (Figure 6-8C, green, red, or yellow color, n = 3).
Figure 6.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 5. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 5 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 6.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 5. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 5 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 7.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 6. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 6 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 7.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 6. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 6 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 8.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 7. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 7 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 8.
Changing percentage of TRPV1 and related molecules in the mouse cerebellum 7. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse cerebellum 7 (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
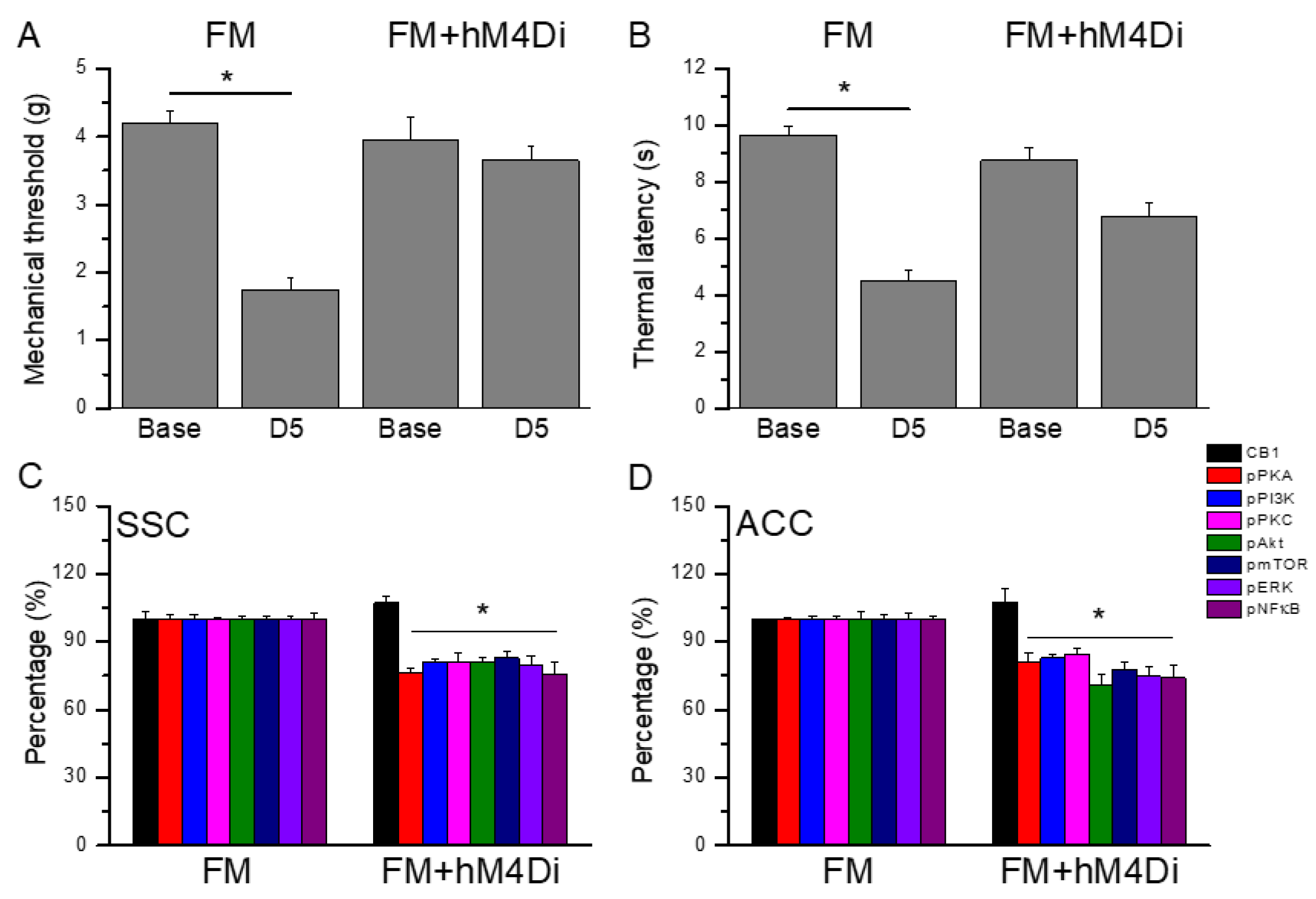
3.7. Chemogenetics inhibition of SSC area significantly alleviated mice fibromyalgia pain
Figure 9A displays a noteworthy FM-induced mechanical hyperalgesia at day 5 after induction (
Figure 7A, n = 6). Besides, chemogenetics blockage of the SSC region also meaningfully diminished mechanical hyperalgesia (
Figure 7A, n = 6). Regarding thermal hyperalgesia, fibromyalgia mice showed substantial thermal hyperalgesia compared to basal condition (
Figure 7B, n = 6). Furthermore, thermal hyperalgesia was released in mice with SSC inhibition after chemogenetics manipulation (
Figure 7B, n = 6). Furthermore, we next showed that CB1 was not changed after chemogenetics treatment. Considerably, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, and pNFkB were reduced after chemogenetics manipulation in both SSC and ACC regions (
Figure 7 C & D, n = 6).
4. Discussion
In the present study, we examined the effect and mechanisms of EPA on mice fibromyalgia pain. We performed cold stress to successfully generate a mouse model of fibromyalgia pain. Mechanical and thermal hyperalgesia can be attenuated by EPA oral administration. Our data showed an increase expression of TRPV1 and related kinases in the mice thalamus, SSC, ACC, mPFC, and cerebellum. The phenomena were diminished by EPA administration. Our data reveal the positive effects of EPA in a mouse fibromyalgia pain model and recommend the clinical use of EPA in fibromyalgia.
EPA, which is one of the omega-3 polyunsaturated fatty acid, is a vital nutrient that has heart-protect, anti-inflammation, neuroprotective, lower triglycerides functions. Although EPA was used as a health medicine or dietary supplement, its molecular target and molecular medicine is still unclear. Kato et al. reported that EPA significantly inhibits vesicular nucleotide transporter (VNUT), a main mediator for releasing adenosine triphosphate. In addition, VNUT–/– mice showed anti-nociceptive effects on both neuropathic and inflammatory pain. EPA administration effectively attenuated both neuropathic and inflammatory pain. Furthermore, the analgesic effect of EPA was blocked by intrathecal injection of purinergic agonists. Thus, VNUT is a cellular target of EPA in both neuropathic and inflammatory pain (34). Further, our results indicated that EPA has analgesic effect on fibromyalgia pain through regulation of TRPV1 and associated molecules.
Perna et al. suggest that DHA and EPA derivate such as resolvins (RvD1, RvD2, and RvE1) are endogenous anti-inflammatory lipid residues with analgesic effects in somatic pain through TRPV1 modulation. In addition, RvD2 attenuated the capsaicin-induced Ca2+ influx of rectal submucosal neurons of patients with irritable bowel syndrome. RvD1, RvD2, and RvE1 diminished histamine-induced TRPV1 activation in peripheral dorsal root ganglion neurons delivering an analgesic effect (35). The current study showed increased protein levels of TRPV1 and related factors in the ascending pain pathway including the thalamus, SSC, ACC, and mPFC in fibromyalgia mice, which EPA administration could decrease. In addition, CB1 receptor was attenuated in this fibromyalgia model suggesting a crucial role in EPA analgesia. These data evidences the possible role of TRPV1 as novel target for treating fibromyalgia pain.
Silva et al. showed that fish oil meaningfully inhibited mechanical and thermal hyperalgesia via decreasing tumor necrosis factor levels in the spinal cord. In addition, fish oil increased the sciatic functional index, electrophysiological recordings, and increased the number of myelinated fibers in the sciatic nerve (36). In addition, Veigas et al. found that mice fed with fish oil had alleviated thermal nociception accompanied by reduced acid-sensing ion channel 1a (ASIC1a), ASIC3, and TRPV1 expression in the dorsal root ganglion (37). Moreover, in another study, EPA increased the proliferation of neural stem cells and the expression of 2-arachidonylglycerol. Pretreatment with cannabinoid 1 or cannabinoid 2 receptor antagonists can further diminish such possessions (38). RvE1 is a derivate from EPA specialized pro-resolving lipid mediator, which has anti-inflammatory and analgesic effects. Suzuki et al. reported that chronic pain significantly induced after spared nerve injury and was further diminished by intracerebroventricular injection of RvE1. The analgesic effect of RvE1 through chemerin receptor ChemR23 (39). Xu et al. indicated that intrathecal pre-treatment of RvE1 prohibited the induction of nerve injury-induced nociception and increase of Iba-1 (microglial marker) and TNF-α in the mice spinal cord. Intrathecal injection of RvE1 at 3 weeks after neuropathic pain induction reliably abridged mechanical allodynia and heat hyperalgesia (40).
Bagher et al. reported that stimulating the CB1 receptor by orthosteric agonists has significant effects in alleviating the pain and neuroinflammation in chemotherapy-induced peripheral neuropathy (CIPN). They further verified that newly synthesized GAT229, a pure CB1 positive allosteric modulators, in alleviating neuropathic pain and slowing the progression of CIPN. GAT229 also abridged proinflammatory cytokines in the mice dorsal root ganglia neurons through brain-derived neurotrophic factor and the nerve growth factor levels in the mice DRG neurons (41). Thapa et al. determined that painful responses were found following capsaicin stimulation of injured corneas in mice. Corneal neutrophil infiltration was also analyzed. CB1 allosteric ligands reliably alleviated pain responses to capsaicin activation. These nociceptive responses can be further reversed by CB1 antagonist AM251 suggesting the crucial target of CB1 receptor (42). Sierra et al. identified that CB1 receptor and opioid ligands has noteworthy diminution of pain behaviors in CIPN mice better than separate ligands. The tendency can be next blocked by the CB1R-opioid antibody (43). In the present study, we indicated that EPA administration significantly diminished mice fibromyalgia pain. Consistent with our hypothesis, CB1 receptor was decreased in the mice specific regions in fibromyalgia pain mice. Furthermore, these effects can be then reversed by EPA administration. Our information advise that EPA have anti-nociceptive effects associated with CB1 signaling in the mouse brain. Thus, these targets could be potential treatments for fibromyalgia pain.
5. Conclusion
In this research, we evaluated the therapeutic effect of EPA on fibromyalgia pain and its actual cellular mechanisms in a mouse model. We used intermittent cold stress to initiate a mouse fibromyalgia pain model, which mechanical and thermal hyperalgesia were observed via von Frey and Hargraves’ test. Mechanical and thermal pain was reversed by EPA administration. The protein levels of TRPV1 signaling pathway were augmented in the ascending pain pathway, including the thalamus, medial prefrontal cortex, somatosensory cortex, anterior cingulate cortex, and cerebellum. Interestingly, CB1 receptor was reduced in this fibromyalgia mouse model. EPA ingestion meaningfully diminished these phenomena in the aforementioned areas. Chemogenetics technique was performed to show analgesic effect at SSC to ACC circuit through TRPV1 downstream pathway. Therefore, we deliver innovative evidences for the use of EPA in mice fibromyalgia pain model and recommend the clinical use of EPA.
Author Contributions
HY Liao, IH Hsiao, and CM Yen: Conceptualization, Methodology, Software, Data curation, Writing - original draft, Visualization, Investigation. HC Hsu and YW Lin: Supervision, Validation, Writing - review & editing.
Funding
This work was supported by the following grants: NSTC 112-2314-B-039-021, CMUHCH-DRM-112-011, and the "Chinese Medicine Research Center, China Medical University" from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.
Acknowledgments
The authors would like to thank Enago (
www.enago.tw) for the English language review.
Conflicts of Interest
There are no financial or other relationships that might lead to a conflict of interest for all authors.
References
- Gyorfi, M.; Rupp, A.; Abd-Elsayed, A. Fibromyalgia Pathophysiology. Biomedicines 2022, 10(12). [Google Scholar] [CrossRef]
- Kocyigit, B. F.; Akyol, A. Fibromyalgia syndrome: epidemiology, diagnosis and treatment. Reumatologia 2022, 60(6), 413–421. [Google Scholar] [CrossRef]
- Masood, R.; Mandalia, K.; Moverman, M. A.; Puzzitiello, R. N.; Pagani, N. R.; Menendez, M. E.; Salzler, M. J. Patients With Functional Somatic Syndromes-Fibromyalgia, Irritable Bowel Syndrome, Chronic Headaches, and Chronic Low Back Pain-Have Lower Outcomes and Higher Opioid Usage and Cost After Shoulder and Elbow Surgery. Arthroscopy 2023, 39(6), 1529–1538. [Google Scholar] [CrossRef]
- Kadayifci, F.Z.; Bradley, M.J.; Onat, A.M.; Shi, H.N.; Zheng, S. Review of nutritional approaches to fibromyalgia. Nutr Rev 2022, 80, 2260–2274. [Google Scholar] [CrossRef]
- Lazaridou, A.; Koulouris, A.; Devine, J.K.; Haack, M.; Jamison, R.N.; Edwards, R.R.; Schreiber, K.L. Impact of daily yoga-based exercise on pain, catastrophizing, and sleep amongst individuals with fibromyalgia. J Pain Res 2019, 12, 2915–2923. [Google Scholar] [CrossRef]
- Saleh, E.; Yabroudi, M.A.; Al-Wardat, M.; Nawasreh, Z.H.; Almhdawi, K.; Etoom, M. The effectiveness of home-based therapeutic exercises on adults with fibromyalgia: a systematic review and meta-analysis. Int J Rehabil Res 2023, 46, 359–368. [Google Scholar] [CrossRef]
- Berwick, R.; Barker, C.; Goebel, A.; guideline development, g. The diagnosis of fibromyalgia syndrome. Clin Med (Lond) 2022, 22(6), 570–574. [Google Scholar]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol 2020, 16(11), 645–660. [Google Scholar] [CrossRef]
- Siracusa, R.; Paola, R. D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int J Mol Sci 2021, 22(8). [Google Scholar] [CrossRef]
- Galvez-Sanchez, C. M.; Reyes Del Paso, G. A. Diagnostic Criteria for Fibromyalgia: Critical Review and Future Perspectives. J Clin Med 2020, 9(4). [Google Scholar] [CrossRef]
- D'Onghia, M.; Ciaffi, J.; Ruscitti, P.; Cipriani, P.; Giacomelli, R.; Ablin, J. N.; Ursini, F. The economic burden of fibromyalgia: A systematic literature review. Semin Arthritis Rheum 2022, 56, 152060. [Google Scholar] [CrossRef]
- Giorgi, V.; Sirotti, S.; Romano, M. E.; Marotto, D.; Ablin, J. N.; Salaffi, F.; Sarzi-Puttini, P. Fibromyalgia: one year in review 2022. Clin Exp Rheumatol 2022, 40(6), 1065–1072. [Google Scholar] [CrossRef]
- Bujak, J. K.; Kosmala, D.; Szopa, I. M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front Oncol 2019, 9, 1087. [Google Scholar] [CrossRef]
- Iftinca, M.; Defaye, M.; Altier, C. TRPV1-Targeted Drugs in Development for Human Pain Conditions. Drugs 2021, 81(1), 7–27. [Google Scholar] [CrossRef]
- Benitez-Angeles, M.; Morales-Lazaro, S. L.; Juarez-Gonzalez, E.; Rosenbaum, T. TRPV1: Structure, Endogenous Agonists, and Mechanisms. Int J Mol Sci 2020, 21(10). [Google Scholar] [CrossRef]
- Braga Ferreira, L. G.; Faria, J. V.; Dos Santos, J. P. S.; Faria, R. X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur J Pharmacol 2020, 887, 173356. [Google Scholar] [CrossRef]
- Fernandez-Carvajal, A.; Fernandez-Ballester, G.; Ferrer-Montiel, A. TRPV1 in chronic pruritus and pain: Soft modulation as a therapeutic strategy. Front Mol Neurosci 2022, 15, 930964. [Google Scholar] [CrossRef]
- Gladkikh, I. N.; Sintsova, O. V.; Leychenko, E. V.; Kozlov, S. A. TRPV1 Ion Channel: Structural Features, Activity Modulators, and Therapeutic Potential. Biochemistry (Mosc) 2021, 86 Suppl 1, S50–S70. [Google Scholar] [CrossRef]
- Lu, K. W.; Hsieh, C. L.; Yang, J.; Lin, Y. W. Effects of electroacupuncture in a mouse model of fibromyalgia: role of N-methyl-D-aspartate receptors and related mechanisms. Acupunct Med 2017, 35(1), 59–68. [Google Scholar] [CrossRef]
- Yang, J.; Hsieh, C. L.; Lin, Y. W. Role of Transient Receptor Potential Vanilloid 1 in Electroacupuncture Analgesia on Chronic Inflammatory Pain in Mice. Biomed Res Int 2017, 2017, 5068347. [Google Scholar] [CrossRef]
- Lin, Y. W.; Chou, A. I. W.; Su, H.; Su, K. P. Transient receptor potential V1 (TRPV1) modulates the therapeutic effects for comorbidity of pain and depression: The common molecular implication for electroacupuncture and omega-3 polyunsaturated fatty acids. Brain Behav Immun 2020, 89, 604–614. [Google Scholar] [CrossRef]
- Borja-Magno, A.I.; Furuzawa-Carballeda, J.; Guevara-Cruz, M.; Arias, C.; Granados, J.; Bourges, H.; Tovar, A.R.; Sears, B.; Noriega, L.G.; Gomez, F.E. Supplementation with EPA and DHA omega-3 fatty acids improves peripheral immune cell mitochondrial dysfunction and inflammation in subjects with obesity. J Nutr Biochem 2023, 120, 109415. [Google Scholar] [CrossRef]
- Chang, C.H.; Wu, H.C.; Hsieh, Y.R.; Lai, W.D.; Tung, T.H.; Huang, J.J.; Kao, W.Y.; Huang, S.Y. Modulatory effect of n-3 polyunsaturated fatty acids on depressive-like behaviors in rats with chronic sleep deprivation: potential involvement of melatonin receptor pathway and brain lipidome. Food Funct 2023, 14, 5977–5993. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The Polyunsaturated Fatty Acid EPA, but Not DHA, Enhances Neurotrophic Factor Expression through Epigenetic Mechanisms and Protects against Parkinsonian Neuronal Cell Death. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Casula, M.; Olmastroni, E.; Gazzotti, M.; Galimberti, F.; Zambon, A.; Catapano, A.L. Omega-3 polyunsaturated fatty acids supplementation and cardiovascular outcomes: do formulation, dosage, and baseline cardiovascular risk matter? An updated meta-analysis of randomized controlled trials. Pharmacol Res 2020, 160, 105060. [Google Scholar] [CrossRef]
- Chang, W. C.; So, J.; Lamon-Fava, S. Differential and shared effects of eicosapentaenoic acid and docosahexaenoic acid on serum metabolome in subjects with chronic inflammation. Sci Rep 2021, 11(1), 16324. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Munoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: relevance for major depression and for human hippocampal neurogenesis. Mol Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Lin, P. Y.; Chang, C. H.; Chong, M. F.; Chen, H.; Su, K. P. Polyunsaturated Fatty Acids in Perinatal Depression: A Systematic Review and Meta-analysis. Biol Psychiatry 2017, 82(8), 560–569. [Google Scholar] [CrossRef]
- Bazinet, R. P.; Metherel, A. H.; Chen, C. T.; Shaikh, S. R.; Nadjar, A.; Joffre, C.; Laye, S. Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain Behav Immun 2020, 85, 21–28. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T. R.; Dunlop, B. W.; Kinkead, B.; Schettler, P. J.; Nierenberg, A. A.; Felger, J. C.; Maddipati, K. R.; Fava, M.; Rapaport, M. H. Dose- and time-dependent increase in circulating anti-inflammatory and pro-resolving lipid mediators following eicosapentaenoic acid supplementation in patients with major depressive disorder and chronic inflammation. Prostaglandins Leukot Essent Fatty Acids 2021, 164, 102219. [Google Scholar] [CrossRef]
- Yang, B.; Lin, L.; Bazinet, R. P.; Chien, Y. C.; Chang, J. P.; Satyanarayanan, S. K.; Su, H.; Su, K. P. Clinical Efficacy and Biological Regulations of omega-3 PUFA-Derived Endocannabinoids in Major Depressive Disorder. Psychother Psychosom 2019, 88(4), 215–224. [Google Scholar] [CrossRef] [PubMed]
- Guu, T. W.; Mischoulon, D.; Sarris, J.; Hibbeln, J.; McNamara, R. K.; Hamazaki, K.; Freeman, M. P.; Maes, M.; Matsuoka, Y. J.; Belmaker, R. H.; Jacka, F.; Pariante, C.; Berk, M.; Marx, W.; Su, K. P. International Society for Nutritional Psychiatry Research Practice Guidelines for Omega-3 Fatty Acids in the Treatment of Major Depressive Disorder. Psychother Psychosom 2019, 88(5), 263–273. [Google Scholar] [CrossRef] [PubMed]
- Cussotto, S.; Delgado, I.; Oriolo, G.; Kemper, J.; Begarie, D.; Dexpert, S.; Sauvant, J.; Leboyer, M.; Aouizerate, B.; Martin-Santos, R.; Schaefer, M.; Capuron, L. Low omega-3 polyunsaturated fatty acids predict reduced response to standard antidepressants in patients with major depressive disorder. Depress Anxiety 2022, 39(5), 407–418. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ohsugi, K.; Fukuno, Y.; Iwatsuki, K.; Harada, Y.; Miyaji, T. Vesicular nucleotide transporter is a molecular target of eicosapentaenoic acid for neuropathic and inflammatory pain treatment. Proc Natl Acad Sci U S A 2022, 119(30), e2122158119. [Google Scholar] [CrossRef] [PubMed]
- Perna, E.; Aguilera-Lizarraga, J.; Florens, M. V.; Jain, P.; Theofanous, S. A.; Hanning, N.; De Man, J. G.; Berg, M.; De Winter, B.; Alpizar, Y. A.; Talavera, K.; Vanden Berghe, P.; Wouters, M.; Boeckxstaens, G. Effect of resolvins on sensitisation of TRPV1 and visceral hypersensitivity in IBS. Gut 2021, 70(7), 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Silva, R. V.; Oliveira, J. T.; Santos, B. L. R.; Dias, F. C.; Martinez, A. M. B.; Lima, C. K. F.; Miranda, A. L. P. Long-Chain Omega-3 Fatty Acids Supplementation Accelerates Nerve Regeneration and Prevents Neuropathic Pain Behavior in Mice. Front Pharmacol 2017, 8, 723. [Google Scholar] [CrossRef] [PubMed]
- Veigas, J. M.; Williams, P. J.; Halade, G.; Rahman, M. M.; Yoneda, T.; Fernandes, G. Fish oil concentrate delays sensitivity to thermal nociception in mice. Pharmacol Res 2011, 63(5), 377–82. [Google Scholar] [CrossRef] [PubMed]
- Dyall, S. C.; Mandhair, H. K.; Fincham, R. E. A.; Kerr, D. M.; Roche, M.; Molina-Holgado, F. Distinctive effects of eicosapentaenoic and docosahexaenoic acids in regulating neural stem cell fate are mediated via endocannabinoid signalling pathways. Neuropharmacology 2016, 107, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Otsuka, T.; Hitora-Imamura, N.; Ishimura, K.; Fukuda, H.; Fujiwara, K.; Shuto, S.; Deyama, S.; Minami, M. Resolvin E1 Attenuates Chronic Pain-Induced Depression-Like Behavior in Mice: Possible Involvement of Chemerin Receptor ChemR23. Biol Pharm Bull 2021, 44(10), 1548–1550. [Google Scholar] [CrossRef]
- Xu, Z. Z.; Berta, T.; Ji, R. R. Resolvin E1 inhibits neuropathic pain and spinal cord microglial activation following peripheral nerve injury. J Neuroimmune Pharmacol 2013, 8(1), 37–41. [Google Scholar] [CrossRef]
- Bagher, A.M.; Binmahfouz, L.S.; Shaik, R.A.; Eid, B.G. Cannabinoid receptor 1 positive allosteric modulator (GAT229) attenuates cisplatin-induced neuropathic pain in mice. Saudi Pharm J 2023, 31, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Thapa, D.; Cairns, E.A.; Szczesniak, A.M.; Kulkarni, P.M.; Straiker, A.J.; Thakur, G.A.; Kelly, M.E.M. Allosteric Cannabinoid Receptor 1 (CB1) Ligands Reduce Ocular Pain and Inflammation. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Sierra, S.; Gupta, A.; Gomes, I.; Fowkes, M.; Ram, A.; Bobeck, E.N.; Devi, L.A. Targeting Cannabinoid 1 and Delta Opioid Receptor Heteromers Alleviates Chemotherapy-Induced Neuropathic Pain. ACS Pharmacol Transl Sci 2019, 2, 219–229. [Google Scholar] [CrossRef]
- Ait-Said, F.; Elalamy, I.; Werts, C.; Gomard, M. T.; Jacquemin, C.; Couetil, J. P.; Hatmi, M. Inhibition by eicosapentaenoic acid of IL-1beta-induced PGHS-2 expression in human microvascular endothelial cells: involvement of lipoxygenase-derived metabolites and p38 MAPK pathway. Biochim Biophys Acta 2003, 1631(1), 77–84. [Google Scholar] [CrossRef]
Figure 1.
(A) Mechanical force measured with the von Frey filament test. (B) Thermal latency time via Hargreaves’ test. *Noteworthy difference in comparison to the normal group. #significant differences with the FM group. n = 9.
Figure 1.
(A) Mechanical force measured with the von Frey filament test. (B) Thermal latency time via Hargreaves’ test. *Noteworthy difference in comparison to the normal group. #significant differences with the FM group. n = 9.
Figure 2.
Changing percentage of TRPV1 and related molecules in the mouse thalamus. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse THA (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 2.
Changing percentage of TRPV1 and related molecules in the mouse thalamus. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse THA (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 3.
Changing percentage of TRPV1 and related molecules in the mouse SSC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse SSC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 3.
Changing percentage of TRPV1 and related molecules in the mouse SSC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse SSC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 4.
Changing percentage of TRPV1 and related molecules in the mouse ACC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse ACC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 4.
Changing percentage of TRPV1 and related molecules in the mouse ACC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse ACC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 5.
Changing percentage of TRPV1 and related molecules in the mouse mPFC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse mPFC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 5.
Changing percentage of TRPV1 and related molecules in the mouse mPFC. Western blotting results of (A) TRPV1, pPKA, pPI3K, pPKC, pAkt, and pmTOR. (B) pERK, pJNK, pp38, pCREB, pNF-κB, and CB1 protein levels. *significant difference to normal. #significant changes compared with the FM group. n = 9. (C) Immunofluorescence labeling of TRPV1, pERK, and double staining in the mouse mPFC (green, red, or yellow). Bar, 100 μm. n = 3 in all groups.
Figure 9.
Mice pain behaviors of FM and FM mice treated with chemogenetics method (hM4Di). * P < 0.05 means statistically significant difference (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). Protein intensities of CB1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, and pNFkB were measured in mice (C) SSC and (D) ACC.
Figure 9.
Mice pain behaviors of FM and FM mice treated with chemogenetics method (hM4Di). * P < 0.05 means statistically significant difference (A) Mechanical hyperalgesia (von Frey test). (B) Thermal hyperalgesia (Hargreaves’ test). Protein intensities of CB1, pPKA, pPI3K, pPKC, pAkt, pmTOR, pERK, and pNFkB were measured in mice (C) SSC and (D) ACC.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).