Preprint
Review
Tomato and Pepper Seeds as Pathways for the Dissemination of Phytopathogenic Bacteria: A Constant Challenge for the Seed Industry and the Sustainability of Crop Production
Altmetrics
Downloads
154
Views
158
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Alerts
Abstract
The seed industry plays a crucial role in the global food production, but it faces a persistent challenge in ensuring the health and quality of seeds, particularly those of tomato and pepper, which represent key seed commodities on the global market. Seeds can serve as potential pathways for the introduction and dissemination of seed-borne bacteria, which may have devastating effects on crop yield, farmers’ remunerability, and food security. Therefore, fungicides and other anti-microbial compounds are extensively used to disinfect the seeds, thus increasing the input of chemicals in the agri-enviroment. In this review, we address aspects that connect disease epide-miology with seed infection and health, including seed contamination, endophytic colonization, and seed-borne infections. We focused on the main bacterial diseases affecting tomato and pepper by discussing their official seed testing methods as requirements supporting a smooth seed trade. Moreover, we present a survey on the past and recent innovations for seed treatments, focusing on sustainable disinfection methods. Therefore, this review will be a short, but indispensable guide for seed technologists and pathologists involved in the production of high-quality seeds, providing indications and suggestions to contrast seed-borne pathogens dissemination, avoid international controversies and complaints by phytosanitary authorities, extension services and farmers.
Keywords:
Subject: Environmental and Earth Sciences - Sustainable Science and Technology
1. Introduction
“Seeds are a basic input for all crop production: all farmers need good seed, irrespective of their farming systems and the markets that they focus on” [1]. The seed sector is probably the most crucial and critical agricultural input that is fundamental for growers to produce crops for food, feed and non-food uses. Indeed, with the global population expected to reach 9.8 billion by 2050, access to quality seeds is critical for food security and nutrition, as it is often stressed by FAO. The seed industry plays a pivotal role in ensuring crop productivity by strengthening the seed value chain and implementing good seed availability worldwide in terms of improved, well-adapted, productive, nutritious, and resilient genotypes: this appears fundamental in food insecure parts of the world, but also in high income countries, to ensure a correct remuneration to dedicated farmers. According to the ISPM 5 [2], seed (in the botanical sense) is a commodity intended for planting [2], therefore living material that farmers may use to produce a crop plants, more frequently vegetables or cereals, but also ornamentals and weed species to be used in gardens, parks, and other public green. Seed is a true global commodity: in 2021, the market for commercial seeds grew by 7.1% over 2020, reaching an estimated value of $47,242 million [3], with a crucial role in promoting food security: indeed, almost 90% of the world’s food crops are grown from seeds [4]. In 2020, vegetable seeds had a market share of almost 17% in global seed sales of the seed industry, therefore, compared with other seed segments (e.g. maize, soybean and cotton), the vegetable seed market is highly fragmented [5].
As for other propagation materials, the international movement of seeds is thoroughly regulated: the main justification for such rules is to avoid the movement of plant pests that are associated with seeds from a region where they are established to areas afar that are still pest-free. Therefore, the FAO standard on the international movement of seeds was approved and published to provide guidance to national plant protection organisations (NPPOs) in identifying, assessing, and managing the pest risk associated with international seed trade [4].
Tomato, and pepper to a lesser extent, can be also considered global seed commodities: for instance, the global tomato seeds market is valued at $1.33 billion in 2023 and expected to be worth $1.78 billion by 2027 [6]. Very frequently, trade and plant health are tightly connected, when it comes to phytosanitary issues: for instance, in case of tomato, breeding parental lines is done in one European country (e.g. The Netherlands), whereas production of basic seeds is done in a second European country (e.g. Spain). Then, basic seeds are treated and manufactured in different European areas or countries to provide seeds to China, where the production of hybrid seeds is commonly done. From China, large seed lots are again shipped to Europe for treatment as commercial hybrid seeds; finally, commercial packaging, final sale and use may happen in the Americas [7]) (Figure 1).
Seeds, as any other plant material in trade, may be associated with one or more plant pests: therefore, seeds are known pathways for the introduction and spread of pests into new territories, when and wherever suitable hosts and environmental conditions are available. Tomato and pepper seeds are recognised pathways for a large number of pests and pathogens, both regulated and non-regulated. In the European Union, a notification and rapid alert system dealing with interceptions of pests (EUROPHYT, https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en) is in place since several years and is essential for the implementation of preventative measures based on robust and up-to-date data from trade in plants, included seeds. For instance, in 2022, 48 interception of tomato seeds infected by the Tomato Brown Rugose Fruit Virus (ToBRFV) were notified to the European Commission, whereas in 2019 such notifications were only 2. The known seed-associated and seed-transmitted bacteria affecting tomato and pepper seeds are five: the Gram positive Clavibacter michiganensis subsp. michiganensis (Cmm), and four Gram negative Xanthomonads: Xanthomonas euvesicatoria pv. euvesicatoria (Xee), X. e. pv. perforans (Xep), X. hortorum pv. gardneri (Xhg) and X. vesicatoria (Xv). An additional one, Clavibacter capsici (basonym C. michiganensis subsp. capsici; Cc) has been recently reported to affect pepper and not tomato [9]. Finally, Pseudomonas syringae pv. tomato (Pst), is a seed-borne tomato pathogen, but it has never been reported to affect pepper. Apart from the latter, all other bacteria are regulated in the European Union; the status of C. capsici is still to be defined, since it’s a very recent finding and, presumably, will be regulated as well.
2. Seed endophytes: recruitment and dynamics
Endophytic communities are defined as populations of microorganisms that establish themselves within the internal tissues of plants, without causing any apparent harm to the host plant [10]. Nevertheless, this is a debated definition as, theoretically, the microbiota within an apparently healthy plant could consist of a mix of mutualistic, commensal, and latent pathogenic strains [11]. These communities consist of a wide range of taxa, including fungi and bacteria, and have been studied in various plant species and various plant parts, from roots to any aerial organs, with the aim of understanding their interactions with their host. Seed associated microbial communities represent the initial inoculum source for the plant microbiota and assist seed conservation, germination, and seedling development through the production of suitable metabolites that are made available during the early stages of seed revitalisation [12]. Most endophytes appear to originate from the plant rhizosphere [13]. Initially, the germinating seeds absorb water that is available in the sowing bed and start to excrete some exudates that may attract bacteria from the surrounding, particularly from the spermosphere and the rhizoplane; such bacteria may enhance plant growth and vigour [14]. Microorganisms may also be transferred from plants' vegetative parts to the seeds [15] or through the male gametophyte and, as seed develops inside the fruit, it colonises the embryo and then the surrounding endosperm [16]. Additionally, plants recruit endophytes from their phyllosphere as well and, moreover, microbial epiphytes are also recruited horizontally, therefore moving from plant to plant during particular weather conditions like, e.g. rains or showers [17]. Finally, some endophytes may be vertically transmitted through the seed [18].
Seeds represent a significant phase in the endophyte biology, and specific microbial communities (core seed microbiome) can persist there for years in a dormant state, when the appropriate conditions are met; subsequently, such communities will again develop into the new plant originating from the germinating seed [14]. Endophytes found in different parts of the plant, including roots, stems, leaves, flowers, fruits, and seeds, may show a different taxonomic structure and a different functional behaviour [19]: some of them have been shown to exert beneficial effects on certain plant species, whereas they may exhibit a pathogenic behaviour towards other plant species. Therefore, the pathogenicity of some endophytes can be influenced by a number of biotic and environmental factors [20,21]. In general, endophytes play an important role in plant health and may positively influence plant growth and productivity and, particularly, numerous studies revealed that endophytic microorganisms play a crucial role in controlling plant pathogens [22]. Recent studies revealed that endophytes also play a crucial role in plant disease management by assisting in nutrient availability and uptake, enhancing stress tolerance and providing disease resistance [23]. Therefore, most of the published research focuses on the taxonomic identification and characterisation of putative beneficial microbes colonising the seeds, in order to: (i) describe the seed-associated microbiota; (ii) highlight the significance of seed microbiota in seed quality; (iii) describe the plant colonisation and the vertical transmission of seed endophytes; (iv) verify the application potential of selected beneficial microbes [24].
3. Phytopathogenic bacteria in tomato and pepper seeds
Tomato and pepper are affected by several phytopathogens, mainly viruses and fungi; a few phytopathogenic bacteria are also known to cause important diseases and may be regarded as a global threat for the seed industry, since most of them are seed transmitted. Two Gram positive germs are a true challenge, Cmm and Cc: the first may affect both tomato and pepper, whereas the latter is specifically associated with pepper [30]. Additionally, five Gram negative bacteria are also known to be associated to tomato and/or pepper; whereas Pst is specific to tomato, the four xanthomonads show a very different behaviour towards their respective hosts: Xv, Xep are pathogenic to tomato only, whereas Xhg and Xee may affect both tomato and pepper [31]. Other phytopathogenic bacteria may infect tomato and pepper, the most important of which are Ralstonia solanacearum and R. syzygii subsp. indonesiensis [32]: despite their importance as causal agents of destructive diseases, they are not known to be seed-transmitted. To understand the routes of seed infection by the phytopathogenic bacteria, the diseases caused by both Clavibacters, Pst and the Xanthomonads are briefly described.
3.1. Clavibacter michiganensis subsp. michiganensis (Smith) Davis et al. and Clavibacter capsici (Oh et al.) Li et al.
Clavibacter michiganensis subsp. michiganensis (Smith) (Cmm) is a highly destructive Gram-positive bacterium, with an economic relevance in terms of losses on tomato cultivations worldwide. Cmm is the causal agent of the bacterial canker and can produce significant yield losses and economic damage to the affected crops: indeed, during severe epidemics, up to 93% of plant death, and approximately 50% decrease in average fruit weight are reported [33,34]. Cmm is considered a high-risk pathogen and is included in the A2 list of quarantine organisms by the European and Mediterranean Plant Protection Organization (EPPO) [35]. Similarly, Cc causes the bacterial canker of pepper, as initially reported by [36] in Korea. Both pathogenic Clavibacters invade and proliferate within the xylem vessels of their respective hosts, causing a characteristic symptom of browning along the internal vasculature and the gradual degradation of vascular tissues. Cmm and Cc are vascular pathogens that systemically colonise the xylem of tomato or pepper plants, leading to a severe disease known as bacterial canker. The infection causes several characteristic symptoms, including unilateral leaf wilting, marginal leaf necrosis, stem cankers and, ultimately, plant death [34,36,37,38]. Both bacteria are commonly recognised as being seed transmitted, both internally in the seed and on the seed surface. Therefore, seed infection and colonisation are essential aspects of the epidemiology of bacterial canker in tomato and pepper. Despite the rate of seed transmission of the disease is quite low [39], recent studies revealed that, under favourable conditions, one infected seed in 10,000 can give rise to devastating epidemics [40,41]. When infected seeds are sown, the bacterium can move systemically through the emerging seedlings, leading to bacterial canker symptoms as the plants grow [42]. Other sources of primary inoculum are represented by infected plant debris that remains in the field after harvesting; therefore, plantlets may become infected once transplanted into a field where infected debris are present and phytopathogenic bacteria are still viable. Theoretically, secondary inocula may be produced along the growing season, when conducive conditions favour pathogen evasion and dissemination through rains or sprinkler irrigation: in such case, phytopathogenic bacteria may penetrate their respective hosts through stomata and fruit lenticels. Fruit infection is visible when typical bird’s eye spots are developing on the berries. Finally, Cmm can be easily horizontally transmitted when tomato plants are trimmed and/or pinched off: indeed, trimming blades or even operators’ hands can be easily contaminated by a single infected plant and, therefore, spread the inoculum to further plants during such agronomic practice.
Tomato seed contamination by Cmm may occur systemically, through the vascular tissue, or by its penetration through fruit lenticels. Cmm multiply and colonises the xylem vessels acropetally, thus reaching the fruit clusters and penetrating the developing fruits through the pedicels, moving until the placental tissue. Alternatively, Cmm entry through fruit stomata causes the development of circular lesions in the exo- and mesocarp where the bacteria multiply; such bacteria may then reach the seeds through the endocarp or the locular cavity when the tomatoes ripen, thus infecting the seed testa [43]. The bacterium is highly tolerant to desiccation and can survive on/in seeds for years [44].
3.2. Pseudomonas syringae pv. tomato (Okabe) Young, Dye & Wilkie
Pseudomonas syringae pv. tomato (Pst) is a Gram-negative bacterium and the causal agent of the bacterial speck of tomato. Pst is considered one of the most significant and widespread bacterial pathogens affecting tomato plants [45]. The bacterial speck is a parenchymatic disease and causes significant economic losses for tomato growers worldwide, as it can reduce fruit yield and quality [46]. Pst penetrates its host plant mainly through stomata and lenticels and forms necrotic spots on the sepals, necrosis along the pedicel and colonises symptomless flowers of different tomato varieties [47]. Typical symptoms of the bacterial speck consist in small and dark lesions on leaves, usually surrounded by a yellow halo, and necrotic spots along stems and on fruits, provoked by a coronatine toxin; from necrotic lesions the germs can evade the plant as bacterial exudates and spread around. The disease can lead to yield reductions as a result from defoliation, flower abortion and fruit lesions [48,49]. The damages caused by Pst can be remarkable in nurseries, greenhouses, and fields during warm and humid conditions [50]. Pst can survive in various environments, such as in plant debris, soil and, as an epiphyte, on leaf surfaces of the host plants or even weeds [51]: indeed, the bacterial speck is a polycyclic disease and the several secondary inocula provide an excellent means of pathogen spread in the field. While surviving in various environments for extended periods, the bacterium can act as a source of inoculum for new infections and continue its life cycle among susceptible tomato plants [52]. This bacterium is a seed-borne pathogen that primarily survives on the seed surface, attached to the desiccated pulp or internally within the seed: therefore, the bacterium is spread over long distances by infected seeds [53,54,55].
3.3. Xanthomonads: Xanthomonas vesicatoria (Doidge) Vauterin, Hoste, Kersters & Swings, X. euvesicatoria pv. euvesicatoria (Jones et al) Constantin et al.; X. euvesicatoria pv. perforans (Jones et al.) Constantin et al., and X. hortorum pv. gardneri (Jones et al.) Morinière et al.
Xanthomonads are Gram negative, yellow-pigmented bacteria affecting a wide host of plants around the world. The bacterial spot is an economically significant plant disease affecting tomato and different types of peppers. The disease is caused by four distinct bacteria belonging to the Xanthomonas genus: Xanthomonas vesicatoria, X. euvesicatoria pv. euvesicatoria, X. euvesicatoria pv. perforans, X. hortorum pv. gardneri. Xanthomonads cause necrotic lesions on leaves with polygonal shape, surrounded by a chlorotic tissue; symptoms on fruits are scab-like, raised, whitish lesions, which leads to their decreased market value [56]. Xanthomonads may enter their host plants primarily through stomata and lenticels, but also wounds (e.g. trimming/pinching lesions or hail wounds) can occasionally provide penetration sites into the host plants. Infection by Xanthomonads can cause yield losses of up to 50%. Barak et al. [57] and it has been reported that very few infected plants can lead to severe disease outbreaks [58,59]. The bacterial spot is primarily a parenchymatic disease; therefore, the germs are not supposed to efficiently colonise the xylem vessels and use them to move acropetally/basipetally within the host plants. Xanthomonads are considered seed-borne, or seed-associated pathogens, and the infected seeds are major means for long-distance dispersal of the diseases. Seeds are associated/contaminated by Xanthomonads, where bacteria can be deposited on the seed surface mixed with the infected pulp; internal seed infections are also reported [60]. Xanthomonads can survive on/in stored seed for up to 10 years [54,56]. Indeed, infested tomato and pepper seeds can serve as a significant source of primary inoculum in transplants and fruit production systems [61,62]. These pathogens can also be transmitted to healthy plants as secondary inocula through various agricultural practices, e.g. irrigation, pinching, contaminated tools, and other means [63]. Giovanardi et al. [64] showed that a seed contamination level higher than 100 CFU g-1 is needed for a disease bacterial spot outbreak caused by Xee. Nevertheless, due to the polycyclic nature of the disease, it is important to emphasise that the threshold level may be variable, considering that pepper-growing areas have quite different climatic conditions and/or different agronomic practices (e.g. higher seeding rates). However, the long-distance spread of the bacterial spot in tomatoes and peppers Xanthomonas spp. can be also associated with the trade of infected transplants harbouring latent infections [65].
4. How phytopathogenic Clavibacters, Pst and Xanthomonads colonise tomato and pepper seeds
Seeds are home to the most diverse microbiota and the composition of their microbial communities is determined by plant genotype, environmental conditions, and agricultural practices [66]. In general, microbes can be transmitted to and colonise their host plants horizontally, via the environment from a suitable source (e.g. another host plant), or vertically, from within the parent plant to the offspring via the seed [67]. Phytopathogenic bacteria may be transmitted to and colonise their specific host plants in the same way and, as plant endophytes, they may colonise seeds in their different parts, including the embryo, and their precise localisation in seeds is related to their epidemiology, biology, penetration route and colonisation pattern [68]. The knowledge of the precise location of phytopathogenic bacteria in seed is pivotal during the selection of an appropriate method for seed disinfection: for instance, phytopathogenic bacteria colonising the seed externally may be easily removed by a washing in a disinfecting solution; this sanitation approach is useless, when the pathogens are located in the endosperm or the embryo. In general, bacteria can colonise seeds horizontally from the external environment via flowers or fruits: this pathway requires that the pathogen is able to produce secondary inocula, which are dispersed in the field, e.g. by wind-driven rain droplets or sprinkler irrigation. This is the case of Pst and Xanthomonads that enter the developing fruits through stomata or wounds and infect the pulp. More rarely this happens with Clavibacters, which are vascular pathogens that evade their hosts far more rarely than Pst and Xanthomonads, thus producing secondary inocula only under highly conducive agri-environmental conditions (e.g. extreme warm and humid conditions, favouring stem cankers development with subsequent disruption of tissues). Then, germs are able to penetrate their respective hosts through fruit lenticels, colonise the pulp extensively, and reach the seeds inside the endocarp; in such cases, phytopathogenic bacteria are mostly located above or under the seed coat and, rarely, in the endosperm [69,70]. Developing seeds can be colonised vertically, from the parent plant: for Clavibacters, plant to seed movement usually happens through the colonisation of vegetative parts of the plants to the developing seed via vascular connections to the endosperm and the embryo [43,71]. Theoretically, phytopathogenic bacteria may infect the seed through contaminated pollen: this is considered both horizontal and vertical transmission. Pollen, as pathway for fruit and seed infections, has been described in a few pathosystems [72,73]; in particular, Dutta et al. [74], described the ingress of Acidovorax citrulli into watermelon seeds by the pollen germ tubes through the stigmas, thus resulting in the colonisation of ovules. Regretfully, dedicated studies have never been attempted for the tomato/pepper – phytopathogenic bacteria pathosystems.
Summarising, Cmm and Cc are typically systemic bacteria, causing vascular diseases [38], Pst is a recognised parenchymatic bacterium, therefore with a limited vascular movement [75]. Similarly, phytopathogenic Xanthomonads, despite the development of symptoms frequently resembling those of Pst, have a parenchymatic colonisation pattern, but a limited vascular movement is also reported [76]. Therefore, the plant colonisation pattern described for the different phytopathogenic bacteria, from the penetration site to the seeds, is pivotal for understanding how and where the seeds are colonised.
Then, seeds represent the main source of primary bacterial inoculum in tomato and pepper seedlings production facilities: the bacteria are introduced in the glasshouses by infected seeds or, alternatively, disseminated by water, as in case of Pst and Xanthomonads (in case of poor hygienic conditions). Commercial cultivation of tomatoes and peppers starts with transplanting plantlets in open fields or in protected environments (e.g. tunnels, glasshouses): in such cases, transplants represent the main source of primary inoculum, through contaminated water, plant residues from a previous crop, or reservoir plants may provide an additional source of primary inoculum. Therefore, to ensure the highest phytosanitary standards along the tomato/pepper production chains, the key issue is phytosanitary certification of seeds (mandatory in case of regulated pathogens) and the possible analysis of transplants [56].
5. Detection of phytopathogenic bacteria in tomato and pepper seeds
5.1. Direct isolation on agar media
Direct isolation is a common detection technique in phytobacteriology that is applied to plant samples in order to multiply the target bacteria on a solid medium, thus making them visible in the form of a colony. This technique is highly valuable in diagnosing plant bacterial diseases and allows the identification and characterisation of specific bacterial strains. Various media have been developed to support the growth of specific types of bacterial strains while inhibiting the growth of others; they are based on knowledge of the nutritional requirements and physiological tolerances of the target bacterium. For direct isolation, semi-selective media are preferred, to exclude the growth of unwanted microorganisms, as fungi or saprophytes [83]: for instance, Wilbrink’s medium [84], NSCAA[85], MXV [86] are three of numerous agar media commonly used for the isolation of Xanthomonads. Performance criteria for bacteria isolation from seeds have been assessed and, depending on media and seed sample, the detectable concentrations of the target bacteria may vary from a few dozen germs up to approximately 104 CFU ml-1 [56]. Differently, for the direct isolation of Cmm, two different agar media, CMM1T and SCMF, performed much better than the previous ones, with an analytical sensitivity around 25 CFU ml-1 of final concentrate [30].
Thus, the direct isolation permits the recovery and growth of living germs that could be sub-cultured to obtain an axenic culture suitable for microbial identification. Moreover, the bacterial culture can be stored at -80°C for further studies or strain characterization.
5.2. Serological detection
The serological methods in plant bacteriology diagnostics were set up, approved by the phytosanitary authorities and applied worldwide from the late ‘70s to the ‘90s of the last century, when reliable molecular methods were not available, to speed up the analyses and increase their specificity from both symptomatic and asymptomatic plant material, included seeds. The most popular serological methods applied are the indirect ImmunoFluorescence Antibody Staining (IFAS), with a sensitivity threshold between 103–104 CFU ml-1, the Enzyme-Linked ImmunoSorbent Assay (ELISA), with a sensitivity threshold approximately at 104 CFU ml-1, the Dot-Blot Immunobinding Assay (DIBA), the Fluorescent In Situ Hybridization (FISH) and the more recently implemented – from half ‘90s to half of the 2000s – the Immuno Magnetic Separation (IMS). Interestingly, after the development and implementation of PCR, these immunological assays were studied in combination with molecular methods (i.e. PCR-FISH, PCR-ELISA, PCR-IMS) to improve the detection threshold up to few colonies per ml [87]. Nowadays, serological methods are basically used to confirm or confute the results of direct isolation or the response of molecular methods. Cross reactions are the main concern of serological methods, especially ELISA: indeed, unknown seed endophytes or saprobes may share same epitopes, or antigenic determinants, with Clavibacters or Xanthomonads [88,89]. This problem should be tackled by using a second detection method that is based on a different scientific principle, as previously highlighted. Cmm was one of the numerous bacterial pathogens, which was used to produce monoclonal antibodies, aiming its detection in seed samples [87]. Moreover, an IMS method was set up and coupled with an enrichment step, which allowed Cmm detection at low concentration (approx. 10 CFU ml-1), similarly to pathogen concentration that can be found in naturally infected tomato seeds (approx. 10 - 102 CFU ml-1) [56,90,91]. Serological methods for the detection of the Xanthomonas species for tomato and pepper are present just in earlier literature; monoclonal antibodies were produced for the detection of Xanthomonas campestris pv. vesicatoria, the older name that comprised the nowadays three species of the Xanthomonas genus causing bacterial spot in tomato and pepper. In that paper, the specificity assay failed by reacting with Pseudomonas gardneri, now called Xanthomonas hortorum pv. gardneri. Officially, EPPO Bulletin suggests IF or ELISA assays on bacterial axenic cultures as well, as identification methods, and not only on seed sample extracts as optional screening tests [56,92,93]. The NSHS protocol, the only official protocol for the detection and identification of Pst, allows the culture identification through biochemical methods and pathogenicity assay (NSHS So. 3.1, 2001; https://seedhealth.org/files/2022/03/So-3.1-Pseudomonas-syringae-pv-tomato-%E2%80%93-tomato-word.doc.pdf). In the recent past, monoclonal antibodies were obtained and used towards the lipopolysaccharide O-chains of Pseudomonas pathovars; the MAb Ps4e reacted against the Pst strain IPGR 140, but also reacted on other 9 pathovars, clearly showing its unspecificity [94].
5.3. Molecular detection
From the early ‘90s, at the beginning of the PCR era up to date, molecular methods have significantly increased the specificity and sensitivity of pathogen detection. Molecular detection methods sped up the analytical procedures that should ensure the phytosanitary quality of seed and, contemporarily, they gave the possibility to significantly increase the number of samples to be analysed: this appears particularly important for seed companies involved in the international trade of seeds. Available PCR-based detection methods are numerous nowadays, from the conventional end-point PCR to the qPCR, to the Loop-mediated isothermal amplification (LAMP) and to the last tool available nowadays, the Digital Droplet PCR (ddPCR). These faster methods are usually combined with pathogen extraction methods based on the use of commercial kits that have improved and standardised the yield and quality of the nucleic acids.
The phytosanitary quality of seeds may be checked at the origin, e.g. by the producing company, and/or at destination, e.g. by an accredited diagnostic laboratory in the importing country. Whereas the producing company usually analyses their own seeds prior to additional manipulations (e.g. addition of dyes, fungicide treatments, addition of inert pelleting material), commercial seeds lots to be sampled and analysed by labs in the importing countries frequently find “treated” seeds; it has been noted that any material added to commercial seeds prior to packaging may have a negative impact on the yield and quality of nucleic acids and may strongly inhibit any PCR reaction. Therefore, a nucleic acid cleaning step is highly recommended after the extraction. In general, molecular methods are used twice along the flow charts: as preliminary assay on the seed extract and as a test to confirm pathogen identity after isolating the putative target bacteria.
For the molecular detection of any phytogenic bacteria possibly present in tomato and pepper seeds only one sample is required for extraction: the same seed extract is then analysed, following the respective procedures, to check the possible presence of Clavibacters, Xanthomonads and Pst; then, there is no need to prepare multiple extracts, thus speeding up the phytosanitary analyses [30,56]. For the detection of Cmm in tomato seeds, molecular methods are officially validated and recently updated. Several PCR adapted protocols are available through probes designed on several conserved regions (e.g. 16S-23S rDNA). Starting from the DNA of Cmm axenic culture, end-point duplex-PCR protocol [95] can be applied and it is suggested also by EPPO [30]; a duplex-qPCR method on Cmm pure culture is also suggested [96]. Although the end-point PCR method may detect low numbers of Cmm germs (approx. 102 CFU g-1 seed) with high specificity, the PCR-based detection from seed extracts is still to be validated [64]. The more updated ISHI-Veg method suggests a multiplex-qPCR protocol that was adapted (with endogenous control primers) from different molecular protocols [96,97]: this protocol may be reliably applied on crude seed extracts and proved its high specificity and sensitivity. Nevertheless, direct isolation and a PCR test on axenic cultures is mandatory to identify and confirm the isolates as Cmm. At present, this validated molecular method is the most recent and reliable tool for Cmm detection directly on seed extracts and it is also approved by NSHS (So 2.1). A tool for the identification of Cmm colonies is the barcoding on a specific 350 bp region in 16S rDNA gene, but more rapidly, a Rep-PCR can be enough to identify the isolates in an axenic culture [30,98]. A LAMP PCR for the detection of Cmm directly on seed extracts was set up by using primers designed on the gene micA, coding a toxin towards the other subspecies of C. michiganensis [99]. This assay allows the detection of Cmm up to 104 CFU g-1 seed. The study compared the detection ability of the LAMP method with Immunostrip: both were able to detect Cmm in the seed samples. In a previous work, the same authors set up the LAMP method on pure Cmm cultures and, then, they compared the results with the available methods: in some cases, the conventional and the qPCR failed the detection; on the contrary, LAMP PCR detected Cmm in all the samples prepared, also the avirulent strains [100]. Among the new molecular techniques requiring a validation, Morcia et al. [101] set up a ddPCR, conceptually similar to qPCR - but more sensitive - which is able to detect Cmm (and the Ralstonia solanacearum species complex, Rssc) directly from symptoms and from stems; nevertheless, the authors did not evaluate the robustness of such method directly on seeds. The primer for the ddPCR were designed on 16S-23S region and the did not amplify other bacterial pathogens of tomato, as the Xanthomonads or Pst. For the detection of Viable But Not Culturable (VBNC) Cmm strains, a study was carried out by improving a qPCR [102].
For the detection of Xanthomonads, two duplex end-point PCR methods are suggested, both together, to cover all pathogen species and pathovars causing the bacterial spot [56]. The method is specific, using probes designed on sequences from an unknown fragment obtained by the Amplified Fragment Length Polymorphism (AFLP), but it cannot be directly applied on seed extracts because of its high detection threshold (approx. 105-106 CFU ml-1). However, it is suggested and adapted by EPPO [56] to perform it on Xanthomonas spp. axenic cultures [103]; in addition, the primers to detect Xhg can fail on Iranian strains, questioning the usability of these primers in all geographic areas [65]. The ISHI-Veg method, even if more updated, suggests two multiplex qPCR methods (without references) on axenic cultures as well. In both official protocols, the direct isolation and the PCR on the axenic culture can validate the analysis; moreover, though it is just recommended, a pathogenicity assay may be done to confirm the isolate identity. Although not yet validated, a multiplex qPCR was also developed, aiming at the detection of the whole set of Xanthomonas spp. that are described as the causal agents of the bacterial spot [104]; the primers were designed on the hrpB7 gene of the Type III Secretion System (T3SS), and they were highly specific but, again, the method was not tested on seed extracts. Moreover, an isothermal reaction, through a Recombinase Polymerase Amplification assay (RPA) was also developed and implemented by using primers designed on the hrpB (or hrcN) gene and evaluated for its use in the field (in situ) on symptomatic plants; such technique showed a sensitivity threshold of about 106 CFU ml-1, which is acceptable during the analysis of symptomatic tissues, though the primers for Xep did not detect their bacterial target [105].
Pst detection and identification lack validated molecular methods and official guidelines for a diagnostic analysis: the reason is that this organism is not regulated in large part of the world (EPPO Global Database, https://gd.eppo.int/). Nevertheless, a few methods based on end-point PCR and qPCR are reliable and available; in particular, Zaccardelli et al. [106] designed primers on the hrpZ gene and developed a specific assay to be used on pure cultures, symptomatic material, symptomless transplants and experimentally contaminated seeds. The calculated detection threshold evaluated on crude extracts of leaves and seeds resulted in approx. 105 and 106 CFU g-1 for leaves and seed, respectively. A qPCR for Pst detection was studied using a molecular beacon (MB) PCR by Fanelli and collaborators [107]; the method, using primers designed from a RAPD unknown fragment, reached a sensitivity threshold of approx. 102 CFU ml-1, both in a pure culture suspension and in seed extracts. Seeds may contain bacteria in their Viable But Not Culturable (VBNC) state [108]: this mainly because of the long quiescent phase, or because sanitation treatments that temporarily block the culturability of bacteria. Therefore, the isolation might result in the underestimation of bacterial contamination or, possibly, to a false negative detection. This impacts on the evaluation of the efficacy of disinfection methods, where it is not generally possible to discriminate dead from viable germs. Then, molecular methods could be improved by adding propidium monoazide (PMA) as was performed for Cmm, where the authors set up a new qPCR method to detect VBNC Cmm in seed samples [102]. Recently, a qPCR amended with PMA was also set up for the detection of Xanthomonads in tomato seed batches [109].
6. Seed treatments
Seed is a recognised and very efficient pathway for the introduction and dissemination of several pests and pathogens [110] and this is particularly true for tomato, as the most important vegetable [111]. Therefore, to ensure their phytosanitary quality and allow their safe trade worldwide, seeds should be treated (or disinfected or sanitised) to reduce the presence of pathogens to a minimum [44]. An excellent example of hygiene in tomato seed production and pathogen control of Cmm is published by the international business chain system, GSPP, to prevent tomato seed to be infected by Cmm GSPP [112]. Therefore, seed is commonly treated/disinfected before commercialization or movement to ensure the production of good seedlings/transplants, to minimise yield losses, to maintain and improve crop quality, and to avoid the spread of harmful organisms [113]. However, the production of healthy high value tomato and pepper seeds should consider all the phytosanitary challenges. Indeed, seed production with healthy stock seeds tested negative for seed borne pathogens and in confined production areas can promote quality and healthy seeds [112]. These production strategies can be also used to reduce the input of copper-based compounds in the field during the cropping season, thus allowing a more sustainable pest management. In fact, bacterial diseases are difficult to manage, once affecting the crops, partly because there are few effective pesticides [114,115]. Copper (Cu) is indeed a fundamental tool in conventional and Integrated Pest Management (IPM) farming systems, despite its several limitations in the European Union [116]. These limitations on Cu-based pesticides are applied for copper, as a heavy metal, accumulate in the environment (soil and water bodies) and, therefore, may have a deleterious impact on biodiversity. Moreover, the presence of copper-resistant or copper-tolerant phytopathogens, such as Cmm [117], Xanthomonas species [118,119] and Pst [120] is currently raising great concern and is making the control of these bacterial diseases quite cumbersome, once they are established in the field. As a matter of fact, the best way to ensure a fair and safe international trade of seed commodities, to avoid the burden to increase the use of copper-based pesticides, and to allow a sustainable and remunerative crop management and yield, is making “clean” seeds available to farmers. This goal may be reached by applying a set of disinfection methods along several, different sanitation procedures. Physical, chemical, or biological methods for seed treatment have been proposed, from time to time, for eliminating or reducing the bacterial seed-borne inoculum (Table 1), as a primary management strategy to prevent disease outbreaks or epidemics [121].
6.1. Tomato and pepper seeds extraction procedures
Botanically, tomatoes and peppers are berries but, though belonging to the same Solanaceae family, they show quite different fruit morphology and structure, and such features impact on methods and procedures to extract and obtain seeds. Basically, tomato seeds are attached to the placental tissue surrounded by a thick layer of jelly polysaccharides and immersed in the locular cavity that, in ripe fruits, is filled by such polysaccharide matrix. In peppers, seeds are also protruding into the locular cavity, but without such a surrounding polysaccharide matrix and, additionally, the locular cavity is and remains empty in ripe fruits. Therefore, after opening ripe peppers (manually or mechanically) seeds are easily detached from the placenta, whereas in tomatoes the cutting or breaking of fruits results in the production of a juicy mass containing the seeds. Harvested peppers can be kept at room temperature to increase seed maturity; later, the fleshy walls are cut and seeds are manually scraped off. In case of large batches, unscraped cores or small whole fruits are covered with water and broken up with a mixing blade; then, mature seeds precipitate and the broken pulp, together with the immature seeds, remain floating in the water and can be discarded. Mature seeds are then rinsed and dried at room temperature under circulating air flow [122].
Large scale production of basic, certified or commercial tomato seeds is, in general, fully mechanised; the ripe (frequently overripe) fruits are mechanically harvested and passed into a crusher that squashes the fruits into a mixture of pulp, juice and gelatinous seeds. The mixture is then passed into a revolving cylindrical screen that separates the juice and the seeds from the remaining fruit debris through an appropriate mesh. Juice and seeds are collected into separate containers and the gelatinous seeds are then separated from the remaining pulp and cleaned from the polysaccharide gel by (i) fermentation or by (ii) adding 10% solution of sodium carbonate or, alternatively, by (iii) addition of hydrochloric acid. After such extraction treatments, seeds are immediately washed and, finally, dried at room temperature under circulating air flow [123].
As previously described, phytopathogenic bacteria affecting tomato and pepper can be present on the seed coat, as well as within the innermost layer of the seed coat, where they are difficult to eradicate by treatments (i.e. chemicals) without damaging the embryo. These sources of infection have been the focus of numerous attempts to devise effective seed treatments for the elimination of bacterial pathogens.
Seed extraction from fruits is the initial challenging step during the seed production chain, where possible cross contaminations of seed borne pathogens may happen [124,125]. Indeed, bacterial spot (Xanthomonas spp.), bacterial speck (Pst) and bacterial canker (Cmm) produce lesions on the surface of fruits, and bacteria growing inside these lesions can contaminate seeds during the seed extraction process [63]. Then, through seed extraction, bacteria can easily contaminate the seed externally; indeed, tomato seeds have numerous epidermal cells that form hairs and crevices on the seed coat that may provide areas and niches in which the pathogen may reside and, later, evade disinfection treatments [126]. Then, the jellified, pectinaceous material surrounding seeds, covering the bacteria and protecting them once the seed has dried, are commonly removed through different manual or mechanical handling, such as fermentation, acid extraction, or pectinase addition to the washing solutions; those procedures are then followed by mechanical washing, drying, and winnowing the inert material from the seed [127,128]. Another important goal of seed extraction is to facilitate the separation of seeds from the surrounding pulp to increase their nutritive value, germination performance, seed quality parameters [129] and, finally, act as a seed sanitation/disinfection step.
Natural fermentation of tomato pulp at room temperature for 24-72 hours and manual seed extraction are commonly used for obtaining seeds [71], and such procedure has been found to decrease the proportion of infected seeds by Cmm in seed lots since many years [130]; regretfully, such an approach is not efficiently applicable for large scale seed production [129]. Nonetheless, Jones et al. [114] reported and stressed that tomato seed extraction by fermentation or acid extraction efficiently reduced the level of seed contamination by killing Xanthomonads. This was confirmed by Giovanardi et al. [64], who proved that the natural fermentation of tomato pulp obtained from symptomatic fruits generated healthy seeds that tested negative by agar plating and standard molecular assays [56]. Similar results, but referred to Pst, were reported by Chambers and Merrimans [131], where the phytopathogenic bacteria were isolated from tomato seeds mechanically extracted from symptomatic fruits, but not from seeds extracted from the pulp mass after either a natural or an acid fermentation process. Pradhanang and Collier [132] compared different fermentation techniques (i.e. natural fermentation and acid extraction, where pulp soaked in an equal volume of 5% HCl solution for 7 to 10 minutes, followed by immediate seed wash), and showed that this fermentation step was effective in decreasing the bacterial inoculum, but still not reliable by itself for completely eliminate Cmm, unless it is followed by a HCl treatment of dry seeds. More recently, Degwale et al. [129] compared natural fermentation to a HCl-based treatment of tomato seeds: their results highlighted the potential of acid extraction in 2% HCl for 60 min to control seed-borne pathogens, as confirmed by the absence of any detectable microbial epiphytes.
For pepper, due to the anatomical and physiological characteristics of fruits and seeds, the most common seed extraction methods manual or mechanical, with the possible use of a disinfectant during the extraction [133]. However, literature is very poor, or even absent, on the possible impact of the different extraction methods on bacterial seedborne pathogens affecting pepper seeds.
6.2. Seed sanitation methods and procedures
The use of “clean” seeds (e.g. sanitised and/or certified) is the most important preventive measure to control seed transmitted diseases, particularly those caused by phytopathogenic bacteria: this approach is considered the simplest, yet the cheapest and safest method of managing different seed-associated pests [113]. The need and necessity to provide clean seeds to the growers led to decades of research on developing and implementing seed treatments [134,135,136]. Nonetheless, the efficacy of currently available treatments for seed-borne bacteria (e.g. thermotherapy, use of chemicals, application of bio-inoculants) are often partially effective, depending on the location of the pathogen on or in the seed; therefore, the control of seed-transmitted bacterial diseases is still a challenge in both organic and conventional production [137]. Moreover, the application of any seed treatment should take into proper account the methods and procedures used for seed extraction and the specific conditions required to keep the embryo vitality and the quality of seeds; these are fundamental aspects in crop production, since uniform seed germination and high seedlings vigour contribute to the successful crop establishment and performance [138]. Finally, treatments should not be applied to pelleted seed, or to seeds that have been previously treated, or to old or poor-quality seed, because germination can be significantly reduced by the treatment and/or the pathogen may not be eliminated completely.
6.2.1. Chemical seed treatments
Chemical-based seed treatment methods, such as the application of generic disinfectants or bactericides, are frequently used to reduce the incidence (or the level of contamination) of pathogens in seeds [139]. Their application and efficacy for controlling bacterial pathogens are well known since decades [140]. Chemical treatments are often simpler and more convenient to use, though care must be taken to use the minimum concentration of chemical(s) required for the minimum exposure time, as these can also decrease seed vitality [141]. Chemical seed treatments are effective and commonly used for seedborne fungal diseases as well [142]. For the elimination of phytopathogenic bacteria associated to seeds, the most used chemicals for treatment are sodium hypochlorite in an aqueous solution (diluted NaHCl, or chlorine, or bleach) and different acids [134,143]: these were found to be efficient in disinfecting tomato seed from Cmm [144,145]. The use of chlorine for the disinfection of tomato and pepper seed is common, but often the used methods do not consider additional complex factors, which can affect results and compromise seed quality (e.g. the presence of a mass of organic matter, like the tomato pulp). One of the best sources for information on using chlorine as a disinfectant is “White’s Handbook of Chlorination and Alternative Disinfectants” a 1062-page compilation of chlorine information [146].
The application of diluted NaHCl in tomato seed treatments can vary from 0.5% to 20% (v/v) in a water mixture and the duration of soaking is from 2 to 40 min [54,147]. In all cases, the seeds should be thoroughly rinsed in water after soaking to minimise any negative impact on seed vitality [148]. A chlorine concentration equivalent to less than 1% bleach can kill bacterial spores within 5 to 10 minutes [149], therefore, since Clavibacters and Xanthomonads are non-sporigenic germs the same concentration is efficiently applied as disinfectant. Finally, McFarquhar [150] showed the potential of diluted NaHCl in disinfecting artificially contaminated pepper seeds by Xee.
Inorganic and organic acids are the most popular in organic seed production for their effectiveness as sanitation treatments for both tomato and pepper [151]. The hydrochloric acid (HCl) is also commonly used as a sanitising chemical in seed production [144]. In fact, tomato seed can be treated with a diluted solution of HCl at different concentrations (i.e. 1 to 5%) and for different soaking times (i.e. 1 to 60 minutes), to eliminate seed-borne bacterial pathogens such as Xanthomonas spp., Pst and Cmm [140,145].
Other than HCl, acetic and peracetic (peroxyacetic) acids [141,152], and hydrogen peroxide are often used in combination in commercial formulation (e.g. Tsunami 100; Oxidate 2.0), were found to reduce pepper (Xanthomonads) and tomato (Cmm, Pst and Xanthomonads) pathogens by 80.3% and 99%, respectively [149,153].
Another common chemical disinfectant for tomato seeds is the trisodium phosphate (TSP). Its application allowed to reduce or even sanitise epiphytic bacterial pathogens through seed soaking in a 10% aqueous solution (v/v) for 15 minutes, followed by rinsing in a cold-water bath for 5 minutes to remove the residual disinfectant prior to seed drying [154].
6.2.2. Physical seed treatments
Physical treatments of tomato and pepper seeds include thermal treatments with hot water, aerated steam, hot dry air, ozonisation and ultraviolet (UV) irradiation. These treatments have a number of advantages over other treatments: in most countries they (i) do not require registration or approval, (ii) have a wide spectrum of activity, (iii) do not leave any toxic or polluting residues, (iv) are considered sustainable and eco-friendly [155].
Phytopathogenic bacteria are, in general, non-sporing, and most of them do not grow at a temperature higher than 41°C: indeed, the most thermophilic phytopathogenic bacteria have an optimum growth temperature between 31-36°C [156]. Temperatures approaching 48-50°C are then very deleterious for non-sporing, phytopathogenic bacteria, which are not able to survive: therefore, the most common temperature range for seed disinfection is between 48-55°C [53,140]. For tomato, no effect on germination was observed when seeds were heat-treated up to 55°C in humid conditions [157].
6.2.2.1. Heat treatments
Heat can be applied to seeds or other plant material as humid treatment (hot water or hot steam) or in dry conditions (ventilated oven), or both. Hot water soaking is an old-age practice, which is usually effective for the sanitation of seeds of different plant species [152,158]. Although theoretically easy to perform, a heat treatment can be difficult to conduct because of the need to fine-tune the temperature required to kill the pathogen(s) without impacting the germination potential, seedling vigour and shelf life of the seed [141,144,159,160]. Moreover, treatment protocols implemented for small seed lots may be difficult or even impractical for large-scale production and there is no guarantee that all the seeds in a large treatment batch will attain the treatment temperature for the recommended duration [161].
Heat treatment may be applied for agricultural commodities by (i) immersion in hot water, (ii) exposure to vapour heat, (iii) exposure to hot dry air. Hot water treatments of seed and plant material are classical thermophysical methods of plant protection and are more eco-friendly and more effective than chemical treatments. In particular, heat can kill bacteria as seed endophytes, whereas chemical treatments are mostly effective against phytopathogens residing on the seed coat or, in a few cases, just below it. A compilation of hot water seed treatment conditions for different vegetables, including tomato and pepper, is publicly available [162]. Several examples of hot water treatment for seed disinfection by bacterial pathogens and their efficacy are listed in Table 1. The main disadvantage of hot water treatments is the need for post treatment drying.
The application of dry heat to seed batches is equally effective as wet heat; interestingly, the increase of temperature in dry conditions is higher than in humid conditions: Marinescu [163] demonstrated that 1 h at 53°C in wet conditions had a potential to disinfect tomato seeds from Cmm equal to 1 h at 80°C in dry air flow. Dry conditions are less dangerous for seeds and heat exposure can be much longer than in humid conditions: indeed, Murata et al. [164] showed that dry heat treatment at 70°C for 4 to 6 days allowed the disinfection of tomato seeds from Cmm. Therefore, also dry heat treatments, which have been developed and implemented several years ago, are common practices applied to solanaceous seeds, as reported by Li et al. [165] and by Akman [166] to control external and internal seed-borne pathogens such as fungi and bacteria.
6.2.2.2. Ozone
Ozone, O3, is a reactive oxygen species (ROS) with a very high antimicrobial potential, and ozonisation is known to be an excellent method for disinfection, thus ensuring food hygiene, sterilisation of utensils, facilities and warehouses, and sanitation of drinkable water [167]. The strong potential of ozone in eliminating or reducing seed-borne bacteria and fungi in seeds has been demonstrated in recent years. Sterilisation performance of O3 and its effects on seed germination closely depend on the concentration and duration of the specific treatment, the type and structure of the seed, as well as the target pathogen [168,169].
The mechanism behind the antimicrobial properties of ozone is attributed to either its strong oxidising capacity, or due to the generation of ROS during its decomposition [170]. Glycoproteins and lipids located in the bacterial and fungal cell membranes are destroyed directly by ozone or its byproducts [171]. The application of ozone to tomato seeds was attempted by Çetinkaya et al. [172]: they demonstrated the efficacy in disinfecting experimentally inoculated seeds with Cmm and Pst by a gaseous ozone treatment at 90 and 120 minutes, without any negative effect on seed germination rate. Therefore, ozonisation might be a very promising treatment, cheap and effective, to eliminate phytopathogenic bacteria without affecting seed quality.
6.2.2.3. UV-C light irradiation
Finally, other methods based on physical principles have been implemented for seed treatment: irradiation by UV-C light has been used as a way to sterilise seed surface [173] and there is evidence that some seed-transmitted fungi may be killed by an appropriate UV-C treatment [174,175]. Regretfully, UV-C irradiation does not have a significant, direct effect on seed endophytes, particularly bacteria, despite some authors reported that an UV-C treatment was able to induce a resistant state in cabbage against Xanthomonas campestris pv. campestris [176] but without showing a direct antibacterial effect against the pathogen. Therefore, such technique does not suit the purpose of sanitising/disinfecting seeds, as a possible pathway for pathogen dissemination.
6.2.3. Microorganisms and Natural Products
Chemical seed dressing against seed-borne pathogens or against soil microorganisms that may affect seed germination and seedlings development is mainly done against fungi, e.g. Fusarium spp. for cereals [177] or Ascochyta pisi for peas [178]. For seed-borne bacteria there are far less options for their control [86]. Effective pesticides for seed dressings, and fungicides among them, are available in the global markets, but the increasing trend and consciousness for a more sustainable agriculture are inducing the policy makers of several countries to revoke such active substances for their intended use. For instance, Thiram, a very popular fungicide that was used for several decades for seed dressing, is now prohibited in the European Union [179].
Nowadays, biological seed treatments represent one of the fastest growing sectors under seed treatment strategies worldwide. Bio-inoculants can protect plants against different seed-borne pathogens through numerous biological mechanisms, such as antibiosis, competitive exclusion or inducing systemic resistance in host plants [180,181,182]. They represent a cost effective, alternative technology to chemical-based plant protection with the potential for a large-scale application by maintaining seed quality and promoting healthy plant growth in sustainable agricultural systems [183]. Thus, the demand for biological solutions for seed treatment is increasing, also in view of consumers’ acceptance for chemical-free food.
Beneficial microorganisms or natural products can be applied through coatings onto the seed surface for the protection of seeds and seedlings from seed-borne and soil-borne pathogens [184]. For seed treatments, various methods are used, such as seed coating, seed pelleting, seed washing, or seed dressing, which usually consists of applications where liquid bio-inoculants (generally supplemented with adhesives) are sprayed onto the seeds either by hand, or rotating drums, or automated seed coaters, followed by drying with forced air using a seed drying equipment.
Recently, the application of bio-inoculants to seeds has increased because of increasing public concern for safety issues and awareness for a healthy environment, sustainable development, and health hazards caused by the excessive use of agrochemicals [185]. However, research on the commercial use of beneficial microorganisms as seed inoculants is limited, and there are only a few examples of their commercial development, implementation and use. Indeed, only few biopesticides formulations for seed coating are currently registered and specifically commercialised in Europe for seed-borne diseases, e.g.: Cerall (Pseudomonas chlororaphis) marketed by Serbios srl, Italy, and Mycostop (Streptomyces griseoviridis K61) marketed by CBC Bioplanet, Italy (http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN). The latter is also true for botanical seed treatments, despite many reports of bactericidal and fungicidal effects of compounds from plants. The reason is possibly due to commercial constraints, like the high development and registration costs in relation to market size [183].
Biopesticides registered for tomato or pepper against seed-borne bacteria, such as Cmm, Pst or Xanthomonads are still few or even absent. In the European Union, the regulatory framework for the registration of microbial biocontrol agents derives from that one currently used for chemical pesticides [186]. Therefore, there are still safety issues to be tackled, e.g. the dynamics and fate of such microbes in the environment, their possible classification as GRAS (Generally Recognised as Safe) (https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras), their impact on biodiversity, etc. The cost of producing a thick dossier including the data required by the regulatory institutions may discourage the registration and marketing of biopesticides.
Nevertheless, different studies have been published reporting the potential of plant extracts or microbial biocontrol agents applied as seed bio-inoculants (Table 1). Among these studies, only a limited number of reports include all scales (e.g. laboratory, greenhouse, and commercial fields), even if different seed sanitation methods and procedures showed to be effective up to 100% in eradicating bacterial pathogens under controlled conditions. For instance, Kasselaki et al. [137] showed that the application of Bacillus sp. as seed bio-inoculant, was able to reduce the presence of Cmm up to 100%. Concerning natural products, Mbega et al. [136] found that 4 plant extracts (i.e. Aloe vera, Coffea arabica, Glycyrrhiza uralensis and Yucca schidigera) out of 84 were able to experimentally disinfect tomato seeds inoculated with Xep in both in vitro and in planta assays.
In other studies, the inconsistency of the seed testing results with the in planta performance can be considered as one of the main challenges for the wide application of seeds coated with bio-inoculant (Table 1). Thus, results that clearly demonstrate and validate the efficacy of the delivery system and the microbial application covering all stages of the process are essential. Among other constraints concerning the microbial application techniques, it may be assumed that seeds can be coated only with a limited amount of inoculant, which might be a limiting factor for such treatment, since a threshold of a mBCA may be needed for a successful biological control. Again, factors influencing microbial viability and survival on seeds are the release of toxic exudates from the seed coat or the incompatibility between the inoculant strains and other seed-applied chemicals [183]. Microbial viability and survival may be improved by the coating materials (e.g. carriers and binders), which could also foster the performance of the target crop [187]. Since different beneficial microorganisms may react differently to coating, the development of coating materials that are compatible with a wide range of bio-inoculants is then crucial to the industry. Finally, the formulation of bio-inoculants should also take into proper consideration the local agriculture conditions and practices (such as soil conditions, use and nature of pesticides applied, fertilisers, and irrigation management) to optimise their performance and survival [183].
7. Conclusions
In this review, we described the epidemiology, seed health assays and treatments of the most harmful seed-borne phytopathogenic bacteria affecting tomato and pepper. These bacteria are responsible for the emergence or re-emergence of disease epidemics, through their movement across international borders and their introduction into new areas, thus limiting crop production and threatening the seed industry worldwide.
According to the Farm to Fork Strategy, part of the European Green Deal, limitations on chemical pesticides foster the importance of an integrated disease management aimed to avoid the spread of these pathogens and to prevent the establishment of trade barriers between countries. Therefore, the implementation by the seed industry of international science-based standards for phytosanitary measures are essential to maintain seed quality and prevent the risk posed by the short to long dissemination of pathogens. Among these standards, seed production strategies (e.g. GSPP), diagnostic protocols for seed analysis and certification, seed extraction and treatments are playing a key role for the seed industry. Moreover, non-chemical seed treatments, which include physical methods and microbial applications, together with the use of other antimicrobial agents of natural origin, like plant extracts, meet the requirements of the new agricultural policies and the market's requests. In fact, the demand for biological seed treatment solutions is increasing in view of consumer acceptance for chemical-free food. Nevertheless, further research and investigations are needed to seek for new solutions for innovative, sustainable and field-applicable Integrated Pest Management (IPM) strategies against tomato and pepper seedborne phytopathogenic bacteria.
Author Contributions
Bekri Xhemali, Davide Giovanardi, Enrico Biondi, Emilio Stefani contributed equally to the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. All authors have read and agreed to the published version of the manuscript.
References
- Louwaars, N.P.; Manicad, G. Seed Systems Resilience—An Overview. Seeds 2022, 1, 340–356. [CrossRef]
- FAO Glossary of Phytosanitary Terms, ISPM 5. Available online: https://www.fao.org/3/mc891e/mc891e.pdf.
- Seed and GM Crop Market Analysis. Available online: https://www.spglobal.com/ratings/en/research-insights/topics/outlook-2023 (accessed on 17 July 2023).
- Dongyu, Q.U. A Statement by FAO Director-General of FAO. Available online: https://www.fao.org/director-general/speeches/detail/en/c/1321173/ (accessed on 4 November 2021).
- Malhotra, B. Global Vegetable Seeds Market Is Increasingly Fragmented and Diversified (accessed on 3 November 2021).
- Tomato Seed Global Market Report; The Business Research Company: Dublin, Ireland, 2023. Available online: https://www.thebusinessresearchcompany.com/report/tomato-seeds-global-market-report.
- The EU Seed Market – Key Facts and Figures. Available online: https://euroseeds.eu/.
- FAO. International Standard for Phytosanitary Measures n°38. International Movement of Seeds, 2018. Available online: http://www.fao.org/3/i7219en/i7219en.pdf.
- Oh, E.J.; Bae, C.; Lee, H.B.; Hwang, I.S.; Lee, H.I.; Yea, M.C.; Yim, K.O.; Lee, S.; Heu, S.; Cha, J.S.; et al. Clavibacter Michiganensis Subsp. Capsici Subsp. Nov., Causing Bacterial Canker Disease in Pepper. International Journal of Systematic and Evolutionary Microbiology 2016, 66, 4065–4070. [CrossRef]
- Wilson, D. Endophyte: The Evolution of a Term, and Clarification of Its Use and Definition. Oikos 1995, 73, 274–276. [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiology and Molecular Biology Reviews 2015, 79, 293–320. [CrossRef]
- Chee-Sanford, J.C.; Williams, M.M.; Davis, A.S.; Sims, J.K. Do Microorganisms Influence Seed-Bank Dynamics? Weed Science 2006, 54, 575–587. [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; van Themaat, E.V.L.; Nahal Ahmadinejad; Ahmadinejad, N.; Federica Assenza; Assenza, F.; Rauf, P.; Huettel, B.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [CrossRef]
- Nelson, E.B. Microbial Dynamics and Interactions in the Spermosphere. Annual Review of Phytopathology 2004, 42, 271–309. [CrossRef]
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology. CRC Press 1996. [CrossRef]
- Malfanova, N.; Lugtenberg, B.J.J.; Berg, G. Molecular Microbial Ecology of the Rhizosphere; Wiley-Blackwell: Hoboken, 2013.
- Santoyo, G. How Plants Recruit Their Microbiome? New Insights into Beneficial Interactions. Journal of Advanced Research 2021. [CrossRef]
- Herre, E.A.; Knowlton, N.; Mueller, U.G.; Stuart A. Rehner; Rehner, S.A. The Evolution of Mutualisms: Exploring the Paths between Conflict and Cooperation. Trends in Ecology and Evolution 1999, 14, 49–53. [CrossRef]
- Brader, G.; Compant, S.; Mitter, B.; Trognitz, F.; Sessitsch, A. Metabolic Potential of Endophytic Bacteria. Current Opinion in Biotechnology 2014, 27, 30–37. [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.J.; Li-J. M.; Sessitsch, A. Ecology and Genomic Insights into Plant-Pathogenic and Plant-Nonpathogenic Endophytes. Annual Review of Phytopathology 2017, 55, 61–83. [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Exploring the Potentialities of Beneficial Endophytes for Improved Plant Growth. Saudi Journal of Biological Sciences 2020, 27, 3622–3633. [CrossRef]
- Ryan, R.P.; Germaine, K.J.; Franks, A.E.; Ryan, D.; Dowling, D.N. Bacterial Endophytes: Recent Developments and Applications. Fems Microbiology Letters 2008, 278, 1–9. [CrossRef]
- Bashir, M.A.; Silvestri, C.; Ahmad, T.; Hafiz, I.A.; Abbasi, N.A.; Manzoor, A.; Cristofori, V.; Rugini, E. Osmotin: A Cationic Protein Leads to Improve Biotic and Abiotic Stress Tolerance in Plants. Plants 2020, 9, 992. [CrossRef]
- Samreen, T.; Naveed, M.; Nazir, M.Z.; et al. Seed Associated Bacterial and Fungal Endophytes: Diversity, Life Cycle, Transmission, and Application Potential. Applied Soil Ecology 2021, 168, 104–161.
- Bergna, A.; Cernava, T.; Rändler, M.; Grosch, R.; Zachow, C.; Berg, G. Tomato Seeds Preferably Transmit Plant Beneficial Endophytes. Phytobiome Journal 2018, 2, 183–193. [CrossRef]
- López, S.M.Y.; Pastorino, G.N.; Franco, M.E.E.; Medina, R.; Lucentini, C. G.; et al. Microbial Endophytes That Live within the Seeds of Two Tomato Hybrids Cultivated in Argentina. Agronomy 2018, 8, 136. [CrossRef]
- Thomas, P.; Shaik, S.P. Molecular Profiling on Surface-Disinfected Tomato Seeds Reveals High Diversity of Cultivation-Recalcitrant Endophytic Bacteria with Low Shares of Spore-Forming Firmicutes. Microbial Ecology 2020, 79, 910–924. [CrossRef]
- Sharma, A.; Kaushik, N.; Sharma, S.; Sharma, A.; Bajaj, A.; Rasane, M.H.; Shouche, Y.S.; Marzouk, T.; Djébali, N. Screening of Tomato Seed Bacterial Endophytes for Antifungal Activity Reveals Lipopeptide Producing Bacillus Siamensis Strain NKIT9 as a Potential Bio-Control Agent. Frontiers in Microbiology 2021, 12, 609482–609482. [CrossRef]
- Yildirim, K.C.; Orel, D.C.; Okyay, H.; Gursan, M.M.; Demir, I. Quality of Immature and Mature Pepper (Capsicum Annuum L.) Seeds in Relation to Bio-Priming with Endophytic Pseudomonas and Bacillus Spp. Horticulturae 2021, 7, 75. [CrossRef]
- Corrigendum - PM 7/42 (3) Clavibacter Michiganensis Subsp. Michiganensis. Eppo Bulletin 2022. [CrossRef]
- PM 7/110 (2) Xanthomonas Spp. (Xanthomonas Euvesicatoria Pv. Euvesicatoria, Xanthomonas Hortorum Pv. Gardneri, Xanthomonas Euvesicatoria Pv. Perforans, Xanthomonas Vesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bulletin 2023. [CrossRef]
- PM 7/21 (2) Ralstonia Solanacearum, R. pseudosolanacearum and R. Syzygii (Ralstonia Solanacearum species Complex). EPPO Bulletin 2018. [CrossRef]
- Sen, Y.; van der Wolf, J.M.; Visser, R.G.F.; van Heusden, A.W.; van Heusden, S. Bacterial Canker of Tomato: Current Knowledge of Detection, Management, Resistance, and Interactions. Plant Disease 2015, 99, 4–13. [CrossRef]
- Peritore-Galve, F.C.; Tancos, M.A.; Smart, C.D. Bacterial Canker of Tomato: Revisiting a Global and Economically Damaging Seedborne Pathogen. Plant Disease 2021, 105, 1581–1595. [CrossRef]
- Osdaghi, E.; Rahimi, T.; Taghavi, S.M.; Ansari, M.; Zarei, S.; Portier, P.; Briand, M.; Jacques, M.-A. Comparative Genomics and Phylogenetic Analyses Suggest Several Novel Species within the Genus Clavibacter, Including Nonpathogenic Tomato-Associated Strains. Applied and Environmental Microbiology 2020, 86. [CrossRef]
- Kyu-Ock Yim; Yim, K.-O.; Hyok-In Lee; Lee, H.-I.; Jung-Hee Kim; Kim, J.-H.; Seungdon Lee; Lee, S.; Jung-Hee Cho; Cho, J.-H.; et al. Characterization of Phenotypic Variants of Clavibacter Michiganensis Subsp. Michiganensis Isolated from Capsicum Annuum. European Journal of Plant Pathology 2012, 133, 559–575. [CrossRef]
- Chalupowicz, L.; Zellermann, E.-M.; Fluegel, M.; Dror, O.; Eichenlaub, R.; Gartemann, K.-H.; Savidor, A.; Sessa, G.; Iraki, N.; Barash, I.; et al. Colonization and Movement of GFP-Labeled Clavibacter Michiganensis Subsp. Michiganensis During Tomato Infection. Phytopathology 2012, 102, 23–31. [CrossRef]
- Nandi, M.; MacDonald, J.; Liu, P.; Weselowski, B.; Yuan, Z.; Yuan, Z.C. Clavibacter Michiganensis Ssp. Michiganensis: Bacterial Canker of Tomato, Molecular Interactions and Disease Management. Molecular Plant Pathology 2018, 19, 2036–2050. [CrossRef]
- Chang, R.J.; Ries, S.M.; Pataky, J.K. Dissemination of Clavibacter Michiganensis Subsp. Michiganensis by Practices Used to Produce Tomato Transplants. Phytopathology 1991, 81, 1276–1281. [CrossRef]
- Jones, J.B.; Zitter, T.A.; Momol, T.M.; Miller, S.A. Compendium of Tomato Diseases and Pests, Second Edition. 2014.
- Gullino, M.L.; et al. Integrated Pest and Disease Management in Greenhouse Crops.; 2nd ed.; Springer International Publishing, 2020.
- Quesada-Ocampo, L.M.; Landers, N.A.; Lebeis, A.C.; Fulbright, D.W.; Hausbeck, M.K. Genetic Structure of Clavibacter Michiganensis Subsp. Michiganensis Populations in Michigan Commercial Tomato Fields. Plant Disease 2012, 96, 788–796. [CrossRef]
- Tancos, M.A.; Chalupowicz, L.; Barash, I.; Manulis-Sasson, S.; Smart, C.D. Tomato Fruit and Seed Colonization by Clavibacter Michiganensis Subsp. Michiganensis through External and Internal Routes. Appl Environ Microbiol 2013, 79, 6948–6957. [CrossRef]
- Rossi, V. Scientific Opinion on the Pest Categorisation of Clavibacter Michiganensis Subsp. Michiganensis (Smith) Davis et Al. EFSA Journal 2014, 12, 1–29. [CrossRef]
- El-Fatah, B. A.; Imran, M.; Abo-Elyousr, K.; Mahmoud, A. Isolation of Pseudomonas Syringae Pv. Tomato Strains Causing Bacterial Speck Disease of Tomato and Marker-Based Monitoring for Their Virulence. Molecular Biology Reports 2023. [CrossRef]
- Wilson, M.; Campbell, H.L.; Ji, P.; Jones, J.B.; Cuppels, D.A. Biological Control of Bacterial Speck of Tomato under Field Conditions at Several Locations in North America. Phytopathology 2002, 92, 1284–1292. [CrossRef]
- Vasileva, K.; Ganeva, D.; Bogatzevska, N. Species Composition of the Bacterial Population Colonizing Tomato Flowers. Bulgarian Journal of Agricultural Science, 2022, 28 (4), 677–690.
- Basim, H.; Basim, E.; Yilmaz, S.; Dickstein, E.R.; Jones, J.B. An Outbreak of Bacterial Speck Caused by Pseudomonas Syringae Pv. Tomato on Tomato Transplants Grown in Commercial Seedling Companies Located in the Western Mediterranean Region of Turkey. Plant Disease 2004, 88, 1050–1050. [CrossRef]
- Orfei, B.; Pothier, J.; Fenske, L.; Blom, J.; Moretti, C.; Buonaurio, R.; Smits, T.H. Race-Specific Genotypes of Pseudomonas Syringae Pv. Tomato Are Defined by the Presence of Mobile DNA Elements within the Genome. Frontiers in Plant Science 2023. [CrossRef]
- Cement, A.; Saygili, H.; Horuz, S.; Aysan, Y. Potential of Bacteriophages to Control Bacterial Speck of Tomato (Pseudomonas Syringae pv. Tomato). Fresenius environmental bulletin 2018, 27, 9366-9373.
- Preston, G.M. Pseudomonas Syringae Pv. Tomato: The Right Pathogen, of the Right Plant, at the Right Time. Molecular Plant Pathology 2000, 1, 263–275. [CrossRef]
- Santamaría-Hernando, S.; López-Maroto, A.; Galvez-Roldán, C.; Munar-Palmer, M.; Monteagudo-Cascales, E.; José-Juan Rodríguez-Herva; Tino Krell; López-Solanilla, E. Pseudomonas Syringae Pv. Tomato Infection of Tomato Plants Is Mediated by GABA and l-Pro Chemoperception. Molecular Plant Pathology 2022, 23, 1433–1445. [CrossRef]
- Devash, Y.; Bashan, Y.; Okon, Y.; Henis, Y. Survival of Pseudomonas Tomato in Soil and Seeds. Journal of Phytopathology 1979, 60, 597–601. [CrossRef]
- Bashan, Y.; Diab, S.; Okon, Y. Survival of Xanthomonas campestris pv. vesicatoria in pepper seeds and roots in symptomless and dry leaves in non-host plants and in the soil. 1982, Plant Soil 68, 161–170. [CrossRef]
- Vinatzer, B.A.; Monteil, C.L.; Clarke, C.R. Population Genomics of Pseudomonas Syringae pv. Tomato to Unravel Emergence and Modes and Routes of Transmission. Acta Hortic. 2015, 1069, 289–292. [CrossRef]
- Scortichini, M.; Stefani, E.; Elphinstone, J.G.; Vlami, M.B. PM 7/110 (1) Xanthomonas Spp. (Xanthomonas Euvesicatoria, Xanthomonas Gardneri, Xanthomonas Perforans, Xanthomona svesicatoria) Causing Bacterial Spot of Tomato and Sweet Pepper. EPPO Bulletin 2013, 43, 7–20. [CrossRef]
- Barak, J.D.; Vancheva, T.; Lefeuvre, P.; Jones, J.B.; Timilsina, S.; Minsavage, G.V.; Gary E. Vallad; Vallad, G.E.; Koebnik, R. Whole-Genome Sequences of Xanthomonas Euvesicatoria Strains Clarify Taxonomy and Reveal a Stepwise Erosion of Type 3 Effectors. Frontiers in Plant Science 2016, 7, 1805–1805. [CrossRef]
- Potnis, N.; Timilsina, S.; Strayer, A.; Shantharaj, D.; Barak, J.D.; Barak, J.D.; Paret, M.L.; Vallad, G.E.; Gary E. Vallad; Jones, J.B. Bacterial Spot of Tomato and Pepper: Diverse Xanthomonas Species with a Wide Variety of Virulence Factors Posing a Worldwide Challenge. Molecular Plant Pathology 2015, 16, 907–920. [CrossRef]
- Timilsina, S.; Jibrin, M.O.; Potnis, N.; Minsavage, G.V.; Kebede, M.; Schwartz, A.R.; Bart, R.; Staskawicz, B.J.; Boyer, C.; Gary E. Vallad; et al. Multilocus Sequence Analysis of Xanthomonads Causing Bacterial Spot of Tomato and Pepper Plants Reveals Strains Generated by Recombination among Species and Recent Global Spread of Xanthomonas Gardneri. Applied and Environmental Microbiology 2015, 81, 1520–1529. [CrossRef]
- Zitter, T.A. Pepper Disease Control It Starts with the Seed; Department of Plant Pathology, Cornell University: Ithaca, NY, USA, 2004; p. 14853.
- Jones, J.B.; Bouzar, H.; Stall, R.E.; Almira, E.C.; Pamela D. Roberts; Roberts, P.D.; B W Bowen; Bowen, B.W.; Bowen, B.W.; Sudberry, J.; et al. Systematic Analysis of Xanthomonads (Xanthomonas Spp.) Associated with Pepper and Tomato Lesions. International Journal of Systematic and Evolutionary Microbiology 2000, 50, 1211–1219. [CrossRef]
- Dutta, B.; Gitaitis, R.D.; S. Ray Smith; Smith, S.; Langston, D.B. Interactions of Seedborne Bacterial Pathogens with Host and Non-Host Plants in Relation to Seed Infestation and Seedling Transmission. PLOS ONE 2014, 9. [CrossRef]
- Ryan, R.P.; Vorhölter, F.-J.; Potnis, N.; Jones, J.B.; Van Sluys, M.-A.; Bogdanove, A.J.; Dow, J.M. Pathogenomics of Xanthomonas: Understanding Bacterium-Plant Interactions. Nature Reviews Microbiology 2011, 9, 344–355. [CrossRef]
- Giovanardi, D.; Biondi, E.; Maja Ignjatov; Ignjatov, M.; Radivoje Jevtić; Jevtic, R.; Stefani, E. Impact of Bacterial Spot Outbreaks on the Phytosanitary Quality of Tomato and Pepper Seeds. Plant Pathology 2018, 67, 1168–1176. [CrossRef]
- Osdaghi, E. Xanthomonas Euvesicatoria Pv. Euvesicatoria (Bacterial Spot of Tomato and Pepper). CABI Compendium 2022. [CrossRef]
- Berg, G.; Jos M. Raaijmakers; Raaijmakers, J.M. Saving Seed Microbiomes. The ISME Journal 2018, 12, 1167–1170. [CrossRef]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [CrossRef]
- Barret, M.; Guimbaud, J.F.; Darrasse, A.; Jacques, M.A. Plant Microbiota Affects Seed Transmission of Phytopathogenic Microorganisms. Molecular Plant Pathology 2016, 17, 791–795. [CrossRef]
- Yoav Bashan; Bashan, Y. Long-Term Survival ofPseudomonas Syringaepv.tomatoandXanthomonas Campestrispv.Vesicatoriain Tomato and Pepper Seeds. Phytopathology 1982, 72, 1143–1144. [CrossRef]
- Bashan, Y.; Bashan, Y. Detection of Cutinases and Pectic Enzymes During Infection of Tomato byPseudomonas Syringaepv.Tomato. Phytopathology 1985, 75, 940. [CrossRef]
- Agarwal, V.K.; Sinclair, J.B. Principles of Seed Pathology. 2nd ed.; Lewis Publisher: Boca Raton, FL, USA, 1997; ISBN 978-0-87371-670-3.
- Donati, I.; Fernández, J.A.; Cellini, A.; Buriani, G.; Mauri, S.; Kay, C.; Tacconi, G.; Spinelli, F. Pathways of Flower Infection and Pollen-Mediated Dispersion of Pseudomonas Syringae Pv. Actinidiae, the Causal Agent of Kiwifruit Bacterial Canker. Horticulture research 2018, 5, 56–56. [CrossRef]
- Dutta, B.; Avci, U.; Hahn, S.K.; Hahn, M.G.; Walcott, R. Location of Acidovorax Citrulli in Infested Watermelon Seeds Is Influenced by the Pathway of Bacterial Invasion. Phytopathology 2012, 102, 461–468. [CrossRef]
- Dutta, B.; Ha, Y.; Lessl, J.T.; Avci, U.; et al. Pathways of Bacterial Invasion and Watermelon Seed Infection by Acidovorax Citrulli. Plant Pathology 2015, 64, 537–544. [CrossRef]
- Furci, L.; Pascual-Pardo, D.; Ton, J. A Rapid and Non-Destructive Method for Spatial–Temporal Quantification of Colonization by Pseudomonas Syringae Pv. Tomato DC3000 in Arabidopsis and Tomato. Plant Methods 2021, 17. [CrossRef]
- Jacques, M.-A.; Arlat, M.; Boulanger, A.; et al. Using Ecology, Physiology, and Genomics to Understand Host Specificity in Xanthomonas. Annual Review of Phytopathology 2016, 54, 163–187. [CrossRef]
- Lamichhane, J.R.; Balestra, G.M.; Varvaro, L. Severe Outbreak of Bacterial Canker Caused by Clavibacter Michiganensis Subsp. Michiganensis on Tomato in Central Italy. Plant Disease 2011, 95, 221–221. [CrossRef]
- Dougherty, D.E. Yield Reduction in Tomato Caused by Bacterial Spot and Disease Control with Copper Sprays. In: Proceedings of the Florida State Horticultural Society, 1979; Vol. 91, pp. 291–293.
- Bashan, Y.; Bashan, Y.; Azaizeh, M.; Shaher Diab; Diab, S.; H. Yunis; Yunis, H.; Yaacov Okon; Okon, Y. Crop Loss of Pepper Plants Artificially Infected with Xanthomonas Campestris Pv. Vesicatoria in Relation to Symptom Expression. Crop Protection 1985, 4, 77–84. [CrossRef]
- OECD Seed Schemes. Rules and Regulations; Organization for Economic Co-operation and Development (OECD); 2023; p. 181.
- International Rules for Seed Testing. International Seed Testing Association, Bassersdorf, Switzerland 2023.
- FAO. Instruction to Authors, Diagnostic Protocols for Regulated Pests; International Plant Protection Organization (IPPC) 2016; p. 35.
- Gitaitis, R.D.; Walcott, R. The Epidemiology and Management of Seedborne Bacterial Diseases. Annual Review of Phytopathology 2007, 45, 371–397. [CrossRef]
- Koike, H. The Aluminum-Cap Method for Testing Sugarcane Varieties against Leaf Scald Disease. Phytopathology 1965, 55, 317–319.
- Schaad, N.W.; Franken, A.A.J.M. ISTA Handbook on Seed Health Testing Working Sheet No 50; 2nd ed.; ISTA: Zurich, Switzerland, 1996.
- Sijam, K.; Chang, C.J.; Gitaitis, R.D. A Medium for Differentiating Tomato and Pepper Strains of Xanthomonas Campestris Pv. Vesicatoria. Canadian Journal of Plant Pathology 1992, 14, 182–184. [CrossRef]
- Alvarez, A.M. Integrated Approaches For Detection Of Plant Pathogenic Bacteria And Diagnosis Of Bacterial Diseases. Annu. Rev. Phytopathol. 2004, 42, 339–366. [CrossRef]
- Schaad, N.W. Detection of Seedborne Bacterial Plant Pathogens. Plant Disease 1982, 66, 885–890. [CrossRef]
- Paul Chu; Chu, P.W.G.; P. M. Waterhouse; Waterhouse, P.M.; Robert R. Martín; Martin, R.R.; R. R. Martin; W. L. Gerlach; Gerlach, W.L. New Approaches to the Detection of Microbial Plant Pathogens. Biotechnology & Genetic Engineering Reviews 1989, 7, 45–112. [CrossRef]
- De León, L.; Rodríguez, A.; López, M.M.; Siverio, F. Evaluation of the Efficacy of Immunomagnetic Separation for the Detection of Clavibacter Michiganensis Subsp. Michiganensis in Tomato Seeds. J Appl Microbiol 2008, 104, 776–786. [CrossRef]
- De León, L.; Siverio, F.; Rodríguez, A. Detection of Clavibacter Michiganensis Subsp. Michiganensis in Tomato Seeds Using Immunomagnetic Separation. Journal of Microbiological Methods 2006, 67, 141–149. [CrossRef]
- Van Den Mooter, M.; Swings, J. Numerical Analysis of 295 Phenotypic Features of 266 Xanthomonas Strains and Related Strains and an Improved Taxonomy of the Genus. International Journal of Systematic Bacteriology 1990, 40, 348–369. [CrossRef]
- Bouzar, H.; Jones, J.B.; Stall, R.E.; Hodge, N.C.; Minsavage, G.V.; A. A. Benedict; Benedict, A.A.; Alvarez, A.M. Physiological, Chemical, Serological, and Pathogenic Analyses of a Worldwide Collection of Xanthomonas Campestris Pv. Vesicatoria Strains. Phytopathology 1994, 84, 663–671. [CrossRef]
- Ovod, V.V.; Rudolph, K.; Krohn, K. Serological Classification of Pseudomonas Syringae Pathovars Based on Mononoclonal Antibodies Towards the Lipopolysaccharide O-Chains. J Bacteriol. 1997, 526–531. [CrossRef]
- Pastrik, K.H.; Rainey, F.A. Identification and Differentiation of Clavibacter Michiganensis Subspecies by Polymerase Chain Reaction-Based Techniques. Journal of Phytopathology 1999, 147, 687–693. [CrossRef]
- Oosterhof, J.; Berendsen, S. The Development of a Specific Real-Time TaqMan for the Detection of Clavibacter Michiganensis Subsp. Michiganensis. In: Proceedings of the Phytopathology 2011; Vol. 101.
- Wu, Y.-D.; Chen, L.-H.; Wu, X.-J.; Shang, S.; Lou, J.; Du, L.-Z.; Zhao, Z. Gram Stain-Specific-Probe-Based Real-Time PCR for Diagnosis and Discrimination of Bacterial Neonatal Sepsis. Journal of Clinical Microbiology 2008, 46, 2613–2619. [CrossRef]
- Versalovic, J.; Schneider, M.; De Bruijn, F.J.; Lupski, J.R. Genomic Fingerprinting of Bacteria Using Repetitive Sequence-Based Polymerase Chain Reaction. Methods in molecular and cellular biology 1994, 5, 25–40.
- Yasuhara-Bell, J.; Baysal-Gurel, F.; Miller, S.A.; Alvarez, A.M. Utility of a Loop-Mediated Amplification Assay for Detection of Clavibacter Michiganensis Subsp. Michiganensis in Seeds and Plant Tissues. Canadian Journal of Plant Pathology 2015, 37, 260–266. [CrossRef]
- Yasuhara-Bell, J.; Kubota, R.; Jenkins, D.M.; Alvarez, A.M. Loop-Mediated Amplification of the Clavibacter Michiganensis Subsp. Michiganensis Mica Gene Is Highly Specific. Phytopathology 2013, 103, 1220–1226. [CrossRef]
- Morcia, C.; Piazza, I.; Ghizzoni, R.; Terzi, V.; Carrara, I.; Bolli, G.; Chiusa, G. Molecular Diagnostics in Tomato: Chip Digital PCR Assays Targeted to Identify and Quantify Clavibacter Michiganensis Subsp. Michiganensis and Ralstonia Solanacearum in Planta. Horticulturae 2023, 9, 553. [CrossRef]
- Han, S.; Jiang, N.; Lv, Q.; Kan, Y.; Hao, J.; Li, J.; Luo, L. Detection of Clavibacter Michiganensis Subsp. Michiganensis in Viable but Nonculturable State from Tomato Seed Using Improved qPCR. PLoS ONE, 2018, 13, 5, e0196525. [CrossRef]
- Koenraadt, H.; Van Betteray, B.; Germain, R.; Hiddink, G.; Jones, J.B.; Oosterhof, J. Development Of Specific Primers For The Molecular Detection Of Bacterial Spot Of Pepper And Tomato. Acta Horticulturae 2009, 99–102. [CrossRef]
- Strayer, A.L.; Jeyaprakash, A.; Minsavage, G.V.; Timilsina, S.; Vallad, G.E.; Jones, J.B.; Paret, M.L. A Multiplex Real-Time PCR Assay Differentiates Four Xanthomonas Species Associated with Bacterial Spot of Tomato. Plant Disease 2016, 100, 1660–1668. [CrossRef]
- Strayer-Scherer, A.; Jones, J.B.; Paret, M.L. Recombinase Polymerase Amplification Assay for Field Detection of Tomato Bacterial Spot Pathogens. Phytopathology 2019, 109, 690–700. [CrossRef]
- Zaccardelli, M.; Spasiano, A.; Bazzi, C.; Merighi, M. Identification and in Planta Detection of Pseudomonas Syringae Pv. Tomato Using PCR Amplification of hrpZPst. European Journal of Plant Pathology 2005, 111, 85–90. [CrossRef]
- Fanelli, V.; Cariddi, C.; Finetti-Sialer, M. Selective Detection of Pseudomonas Syringae Pv. Tomato Using Dot Blot Hybridization and Real-time PCR. Plant Pathology 2007, 56, 683–691. [CrossRef]
- Na, J.; Lv, Q.Y.; Xu, X.; Cao, Y.S.; Walcott, R.; J. Q. Li; Li, J.; Luo, L.X. Induction of the Viable but Nonculturable State in Clavibacter Michiganensis Subsp. Michiganensis and in Planta Resuscitation of the Cells on Tomato Seedlings. Plant Pathology 2016, 65, 826–836. [CrossRef]
- Wang, H.; Wagnon, R.; Moreno, D.; Timilsina, S.; Jones, J.; Vallad, G.; Turechek, W.W. A Long-Amplicon Viability-qPCR Test for Quantifying Living Pathogens That Cause Bacterial Spot in Tomato Seed. Plant Disease 2022, 106, 1474–1485. [CrossRef]
- Denancé, N.; Grimault, V. Seed Pathway for Pest Dissemination: The ISTA Reference Pest List, a Bibliographic Resource in Non-vegetable Crops. EPPO Bulletin 2022. [CrossRef]
- Strider, D.L. Bacterial Canker of Tomato Caused by Corynebacterium Michiganense: a Literature Review and Bibliography. Technical Bulletin 1969, 193. Raleigh, NC: North Carolina Agricultural Experiment Station.No. pp 1-110.
- GSPP Standard for Tomato Seed and Young Plant Production Sites (Valid from 1st June 2022) 2022. https://Www.Gspp.Eu/Images/Documents/GSPP_Standard_V3.3.Pdf.
- Management of Seed-Borne Diseases: An Integrated Approach. In: Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R., Gupta, A., Eds.; Springer Singapore: Singapore, 2020; pp. 717–745. ISBN 978-981-329-045-7.
- Jones, J.B.; Pohronezny, K.L.; Stall, R.E.; Jones, J.P. Survival of Xanthomonas Campestris Pv. Vesicatoria in Florida on Tomato Crop Residue, Weeds, Seeds, and Volunteer Tomato Plants. Phytopathology 1986, 76, 430–434. [CrossRef]
- Lo Cantore, P.; Shanmugaiah, V.; Iacobellis, N.S. Antibacterial Activity of Essential Oil Components and Their Potential Use in Seed Disinfection. Journal of Agricultural and Food Chemistry 2009, 57, 9454–9461. [CrossRef]
- European Commission European Commission, 2018. Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018.
- Cooksey, D.A. Genetics of Bactericide Resistance in Plant Pathogenic Bacteria. Annual Review of Phytopathology 1990, 28, 201–219. [CrossRef]
- Jones, J.B.; Lacy, G.H.; Bouzar, H.; Stall, R.E.; Schaad, N.W. Reclassification of the Xanthomonads Associated with Bacterial Spot Disease of Tomato and Pepper. Systematic and Applied Microbiology 2004, 27, 755–762. [CrossRef]
- Shenge, K.C.; Mabagala, R.B.; Mortensen, C.N.; Wydra, K. Resistance of Xanthomonas Campestris Pv. Vesicatoria Isolates from Tanzania to Copper and Implications for Bacterial Spot Management. African Journal of Microbiology Research 2014, 8, 2881–2885. [CrossRef]
- Griffin, K.; Gambley, C.F.; Brown, P.H.; Li, Y. Copper-Tolerance in Pseudomonas Syringae Pv. Tomato and Xanthomonas Spp. and the Control of Diseases Associated with These Pathogens in Tomato and Pepper. A Systematic Literature Review. Crop Protection 2017, 96, 144–150. [CrossRef]
- Van Der Plank, J.E. Plant Diseases: Epidemics and Control. Soil Science 1964, 98, 279. [CrossRef]
- Lordon, M. Sweet Pepper Breeding and Seed Saving Guide, Department of Agriculture: USDA, Minnesota, 2022.
- Welbaum, G. Tomato. Publisher: VCE Publication, Department of Horticulture, Virginia Tech: Blacksburg, VA, 2021. Available at: https://pubs.ext.vt.edu/426/426-418/mobile-version.html.
- Agrios, G.N. Plant Pathology; 5th ed.; Elsevier Academic Press: Amsterdam, 2005.
- Zitter, T.A. Bacterial Diseases of Tomato, Vegetable MD Online; Cornell University: Ithaca, NY, USA, 1985.
- Sauer, D.B.; Burroughs, R. Disinfection of Seed Surfaces with Sodium Hypochlorite. Phytopathology 1986, 76, 745–749. [CrossRef]
- Dhanvantari, B.N. Effect of Seed Extraction Methods and Seed Treatments on Control of Tomato Bacterial Canker. Canadian Journal of Plant Pathology 1989, 11, 400–408. [CrossRef]
- Raval, A; Sasidharan, N.; Rao, K. Effect of seed extraction procedures on seed quality parameters in tomato. Advances in Life Sciences, 2016, 5, 20, 9020-9024.
- Degwale, A.; Tesfa, T.; Meseret B.; Fantaw, S. Seed Extraction Methods Affect the Physiological Quality of Tomato Seed and Developing Seedlings. International Journal of Vegetable Science 2022, 1–9. [CrossRef]
- Ercolani, G.L. Effettività e Misura Della Trasmissione Di Xanthomonas Vesicatoria e Di Corynebacterium Michiganense Attraverso Il Seme Del Pomodoro. Industria Conserve 1968, 43, 14–22.
- Chambers, S.; Merriman, P. Perennation and Control of Pseudomonas Tomato in Victoria. Crop & Pasture Science 1975, 26, 657–663. [CrossRef]
- Pradhanang, P.M.; Collier, G. How Effective Is Hydrochloric Acid Treatment to Control Clavibacter Michiganensis Subsp. Michiganensis Contamination in Tomato Seed. Acta Horticulturae 2009, 81–85. [CrossRef]
- McCormack, J. Pepper Seed Production. 2005. Available online at: https://www.carolinafarmstewards.org/wp-content/uploads/2012/05/PepperSeedProductionVer1_2.pdf.
- Carisse, O.; Ouimet, A.; Toussaint, V.; Philion, V. Philion Evaluation of the Effect of Seed Treatments, Bactericides, and Cultivars on Bacterial Leaf Spot of Lettuce Caused by Xanthomonas Campestris Pv. Vitians. Plant Disease 2000, 84, 295–299. [CrossRef]
- Mbega, E.R.; Mabagala, R.B.; Mortensen, C.N.; Wulff, E.G.; Wulff, E.G. Evaluation of Essential Oils as Seed Treatment for the Control of Xanthomonas Spp. Associated with the Bacterial Leaf Spot of Tomato in Tanzania. Journal of Plant Pathology 2012, 94, 273–281. [CrossRef]
- Mbega, E.R.; Mortensen, C.N.; Mabagala, R.B.; Wulff, E.G. The Effect of Plant Extracts as Seed Treatments to Control Bacterial Leaf Spot of Tomato in Tanzania. Journal of General Plant Pathology 2012, 78, 277–286. [CrossRef]
- Kasselaki, A.-M.; Goumas, D.E.; Tamm, L.; Jacques Fuchs; Fuchs, J.G.; Cooper, J.; Leifert, C. Effect of Alternative Strategies for the Disinfection of Tomato Seed Infected with Bacterial Canker (Clavibacter Michiganensis Subsp Michiganensis). NJAS: Wageningen Journal of Life Sciences 2011, 58, 145–147. [CrossRef]
- Cardarelli, M.; Woo, S.L.; Rouphael, Y.; Colla, G. Seed Treatments with Microorganisms Can Have a Biostimulant Effect by Influencing Germination and Seedling Growth of Crops. Plants 2022, 11, 259. [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting Sustainability of Fungicide Seed Treatments for Field Crops. Plant Disease 2020, 104, 610–623. [CrossRef]
- Shoemaker, P.B.; Echandi, E. Seed and Plant Bed Treatments for Bacterial Canker of Tomato. Plant disease reporter 1976, 60, 163–166.
- van der Wolf, J.M.; Y.E. Birnbaum; Birnbaum, Y.E.; P.S. van der Zouwen; van der Zouwen, P.S.; Groot, S.P.C.; Groot, S.P.C. Disinfection of Vegetable Seed by Treatment with Essential Oils, Organic Acids and Plant Extract. Seed Science and Technology 2008, 36, 76–88. [CrossRef]
- Maude, R.B. Seedborne Diseases and Their Control: Principles and Practice. Publisher: Wallingford, UK, CAB International, 1996, pp. 1–280.
- Chun, S.-C.; Schneider, R.W.; Cohn, M.A. Sodium Hypochlorite: Effect of Solution pH on Rice Seed Disinfestation and Its Direct Effect on Seedling Growth. Plant Disease 1997, 81, 821–824. [CrossRef]
- Fatmi, M.; Schaad, N.W.; Schaad, N.W.; H. A. Bolkan; Bolkan, H.A. Seed Treatments for Eradicating Clavibacter Michiganensis Subsp. Michiganensis from Naturally Infected Tomato Seeds. Plant Disease 1991, 75, 383–385. [CrossRef]
- Thyr, B.D.; Webb, R.E.; Jaworski, C.A.; Toby Ratcliffe; Ratcliffe, T.J. Tomato Bacterial Canker: Control by Seed Treatment. Plant disease reporter 1973, 57, 974–977.
- Black & Veatch Corporation. White’s handbook of chlorination and alternative disinfectants, 5th ed. Publisher: John Wiley & Sons, Inc., Hoboken, NJ, US. 2010; pp. 1–1062.
- Ritchie, D.F.; Averre, C.W. Bacterial Spot of Pepper and Tomato. Phytopathology 1996, 86, 952-958. [CrossRef]
- Khah, E.M.; Passam, H.C. Sodium Hypochlorite Concentration, Temperature, and Seed Age Influence Germination of Sweet Pepper. Hortscience 1992, 27, 821–823. [CrossRef]
- Rutala, W.A.; Weber, D.J. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Infection Control and Hospital Epidemiology 2008, 18, 240–264.
- McFarquhar, J. Organic Seed Treatments for the Reduction of Xanthomonas Euvesicatoria on Tomato Seed. Dissertation MS’s Thesis. University of Georgia (USA), 2015. http://getd.libs.uga.edu/pdfs/mcfarquhar_judith_201505_ms.pdf.
- Le Buanec, B. Organic Seed. APSA; APSA: Seoul, South Korea, 2004.
- Borgen, A.; Bent Nielsen. Effect of seed treatment with acetic acid in control of seed borne diseases. In: Biddle, A. J. (Ed.) Proceedings of the BCPC Symposium No. 76: “Seed Treatment: Challenges & Opportunities”, Farnham, 76, British Crop Protection Council. 2001. http://orgprints.org/1116/1/acidBCPC.htm.
- Mtui, H.D.; Bennett; Maerere, A.P.; Miller, S.A.; Kleinhenz, M.D.; K. P. Sibuga; Sibuga, K.P. Effect of Seed Treatments and Mulch on Seedborne Bacterial Pathogens and Yield of Tomato (Solanum Lycopersicum Mill.) in Tanzania. Journal of Animal and Plant Sciences 2010, 8, 1006–1015.
- Kuhar, T.P.; Rideout, S.L.; Reiter, M.S. Southeastern U.S. Vegetable Crop Handbook. 2021. [CrossRef]
- Scott, G.; Almasrahi, A.; Mansoorkhani, F.M.; M. Rupar; Rupar, M.; M. Rupar; M. Dickinson; Dickinson, M.; Gilbert Shama; Shama, G. Hormetic UV-C Seed Treatments for the Control of Tomato Diseases. Plant Pathology 2019, 68, 700–707. [CrossRef]
- Schaad, N.W. Emerging Plant Pathogenic Bacteria and Global Warming. In: Pseudomonas syringae pathovars and related pathogens–identification, epidemiology and genomics. M. Fatmi, A. Collmer, N. S. Lacobellis, J. W. Mansfield, J. Murillo, N. W. Schaad, and M. Ullrich, Eds. Springer, Dordrecht, Netherlands, 2008, 369–379. [CrossRef]
- Bryan, M.K. Studies on Bacterial Canker of Tomato. Journal of Agricultural Research 1930, 41.
- McGee, D.C. Seed Pathology: Its Place in Modern Seed Production. Plant Disease 1981, 65, 638–642. [CrossRef]
- Goode, M.J.; Sasser, M. Prevention--the Key to Controlling Bacterial Spot and Bacterial Speck of Tomato. Plant Disease 1980, 64, 831–834. [CrossRef]
- Nega, E.; Roswitha Ulrich; Ulrich, R.; S. Werner; Werner, S.; Marga Jahn; Jahn, M. Hot Water Treatment of Vegetable Seed – an Alternative Seed Treatment Method to Control Seed Borne Pathogens in Organic Farming. Journal of Plant Disease and Protection, 2003, 110, 220–234.
- du Toit, L.J.; Pablo Hernandez-Perez; Hernandez-Perez, P. Efficacy of Hot Water and Chlorine for Eradication of Cladosporium Variabile, Stemphylium Botryosum, and Verticillium Dahliae from Spinach Seed. Plant Disease 2005, 89, 1305–1312. [CrossRef]
- McGrath, M.T. Hot Water Seed Treatment Protocols 2013.
- Marinescu, G. Recherches Sur La Desinfection Des Semences de Tomates Contaminees Par Corynebacterium Rnichiganense (E. F. Smith). Jensen. Bull. Acad. Sci. Agric. For. 1975, 5, 101–109.
- Murata, A.; Numata, I. Heat Endurance of Corynebacterium Michiganense and Tomato Seeds to Dry Seeds. In: Proceedings Kanto Pl. Protocol Soc. 1970, 17, 55–56.
- Fu-xin, G. Effect of Dry-Heat Treatment of Different Temperature on Germination of Vegetable Seed. China Vegetables 2011.
- Akman, Z. Comparison of High Temperature Tolerance in Maize, Rice and Sorghum Seeds by Plant Growth Regulators. Journal of Animal and Veterinary Advances 2009, 8, 358–361.
- Pérez-Calvo, M. Sanitation With Ozone. Gases in Agro-Food Processes 2019, 561–567. [CrossRef]
- Mohan, N.; Kirit Patel; Patel, K.; Padmanabhan, K.; S. Ananthi; Ananthi, S. Ozone for Plant Pathological Applications. Ozone-science & Engineering 2005, 27, 499–502. [CrossRef]
- Pascual, A.; Llorca, I.; Albert Canut; Canut, A. Use of Ozone in Food Industries for Reducing the Environmental Impact of Cleaning and Disinfection Activities. Trends in Food Science and Technology 2007, 18. [CrossRef]
- Bocci, V.; Borrelli, E.; Travagli, V.; Zanardi, I. The Ozone Paradox: Ozone Is a Strong Oxidant as Well as a Medical Drug. Medicinal Research Reviews 2009, 29. [CrossRef]
- Cho, M.; Kim, J.Y.; Yoon, J.; Kim, J.H. Mechanisms of Escherichia Coli Inactivation by Several Disinfectants. Water Research 2010, 44, 3410–3418. [CrossRef]
- Çetinkaya, N.; Pazarlar, S.; Paylan, İ.C. Ozone Treatment Inactivates Common Bacteria and Fungi Associated with Selected Crop Seeds and Ornamental Bulbs. Saudi Journal of Biological Sciences 2022, 29, 103480. [CrossRef]
- Czarnek, K.; Tatarczak-Michalewska, M.; Dreher, P.; Rajput, V. D.; Wójcik, G.; Gierut-Kot A.; Szopa, A.; Blicharska, E. UV-C Seed Surface Sterilization and Fe, Zn, Mg, Cr Biofortification of Wheat Sprouts as an Effective Strategy of Bioelement Supplementation. International Journal of Molecular Sciences 2023, 24, 10367–10367. [CrossRef]
- Neelamegam, R.; Sutha, T. UV-C Irradiation Effect on Seed Germination, Seedling Growth and Productivity of Groundnut (Arachis Hypogaea L.). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 430–443.
- Falconí, C.E.; Yánez-Mendizábal, V. Efficacy of UV-C Radiation to Reduce Seedborne Anthracnose (Colletotrichum Acutatum) from Andean Lupin (Lupinus Mutabilis). Plant Pathology 2018, 67, 831–838. [CrossRef]
- Brown, J.E.; Lu, T.Y.; Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Collins, D.J.; Wilson, M.A.; Igwegbe, E.C.K.; Chalutz, E.; et al. The Effect of Low Dose Ultraviolet Light-C Seed Treatment on Induced Resistance in Cabbage to Black Rot (Xanthomonas Campestris Pv. Campestris). Crop Protection 2001, 20, 873–883. [CrossRef]
- Shude, S.P.N.; Yobo, K.S.; Mbili, N.C. Progress in the Management of Fusarium Head Blight of Wheat: An Overview. South African Journal of Science 2020, 116, 1–7. [CrossRef]
- R. B. Maude; Maude, R.B.; Ann M. Kyle; Kyle, A.M. Seed Treatments with Benomyl and Other Fungicides for the Control of Ascochyta Pisi on Peas. Annals of Applied Biology 1970, 66, 37–41. [CrossRef]
- European Commision Commission Implementing Regulation (EU) 2018/1500 of 9 October 2018 Concerning the Non-Renewal of Approval of the Active Substance Thiram, and Prohibiting the Use and Sale of Seeds Treated with Plant Protection Products Containing Thiram, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending Commission Implementing Regulation (EU) No 540/2011 2018.
- Sain, S.K.; Pandey, A.K. Biological Spectrum of Trichoderma Harzianum Rifai Isolates to Control Fungal Diseases of Tomato (Solanum Lycopersicon L.). Archives of Phytopathology and Plant Protection 2016, 49, 507–521. [CrossRef]
- Chandel, S.; Allan, E.J.; Woodward, S. Biological Control of Fusarium Oxysporum f. sp. Lycopersici on Tomato by Brevibacillus Brevis. Journal of Phytopathology 2010, 158, 470–478. [CrossRef]
- Jambhulkar, P.P.; Sharma, P. Promotion of Rice Seedling Growth Characteristics by Development and Use of Bioformulation of Pseudomonas Fluorescens. Indian Journal of Agricultural Sciences 2013, 83.
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An Attractive Tool for Sustainable Agriculture. Biotechnology Reports 2023, 37, e00781–e00781. [CrossRef]
- Bejarano, A.; Puopolo, G. Bioformulation of Microbial Biocontrol Agents for a Sustainable Agriculture. Progress in Biological Control 2020, 275–293. [CrossRef]
- Jambhulkar, P.P.; Sharma, P.; Rakesh Yadav; Yadav, R. Delivery Systems for Introduction of Microbial Inoculants in the Field. In: Microbial Inoculants in Sustainable Agricultural Productivity (New Delhi: Springer), 2016, 199–218. [CrossRef]
- Grimaldi, A.; Galy, R. An Overview of European Regulatory of Biopesticides. 4th International Symposium on Biological Control of Bacterial Plant Diseases. J Plant Pathol 2019, 101, 849–883. [CrossRef]
- Rocha, I.; Ma, Y.; Souza-Alonso, P.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating: A Tool for Delivering Beneficial Microbes to Agricultural Crops. Frontiers in Plant Science 2019, 10, 1357–1357. [CrossRef]
- Pyke, N.B.; Milne, K.S.; Neilson, H.F. Tomato Seed Treatments for the Control of Bacterial Speck. New Zealand journal of experimental agriculture 1984, 12, 161–164. [CrossRef]
- Kotan, R.; Dadaşoğlu, F.; Karagöz, K.; Cakir, A.; Hakan Özer; Özer, H.; Saban Kordali; Kordali, S.; Saban Kordali; Cakmakci, R.; et al. Antibacterial Activity of the Essential Oil and Extracts of Satureja Hortensis against Plant Pathogenic Bacteria and Their Potential Use as Seed Disinfectants. Scientia Horticulturae 2013, 153, 34–41. [CrossRef]
- Karabuyuk, F.; Aysan, Y. Aqueous Plant Extracts as Seed Treatments on Tomato Bacterial Speck Disease. Acta Horticulturae 2018. [CrossRef]
- Umesha, S.; Kavitha, R. Prevalence of Bacterial Spot in Tomato Fields of Karnataka and Effect of Biological Seed Treatment on Disease Incidence. Crop Protection 2006, 25, 375–381. [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; de-Bashan, L.E. Protection of Tomato Seedlings against Infection by Pseudomonas Syringae Pv. Tomato by Using the Plant Growth-Promoting Bacterium Azospirillum Brasilense. Applied and Environmental Microbiology 2002, 68, 2637–2643. [CrossRef]
- Kritzman, G. A. Chemi-Thermal Treatment for Control of Seedborne Bacterial Pathogens of Tomato. Phytoparasitica 1993, 21, 101–109. [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Reduction of Bacterial Speck (Pseudomonas Syringae Pv. Tomato) of Tomato by Combined Treatments of Plant Growth-Promoting Bacterium, Azospirillum Brasilense, Streptomycin Sulfate, and Chemo-Thermal Seed Treatment. European Journal of Plant Pathology 2002, 108, 821–829. [CrossRef]
- Sanogo, S.; Clary, M. Bacterial Leaf Spot of Chile Pepper: A Short Guide for Growers 2008. New Mexico State University, College of Agriculture and Home Economics. https://pubs.nmsu.edu/research/horticulture/NMCA30/index.html.
Figure 1.
Trade representation on the movement of vegetable seeds around the globe (adapted from [8]).
Figure 1.
Trade representation on the movement of vegetable seeds around the globe (adapted from [8]).
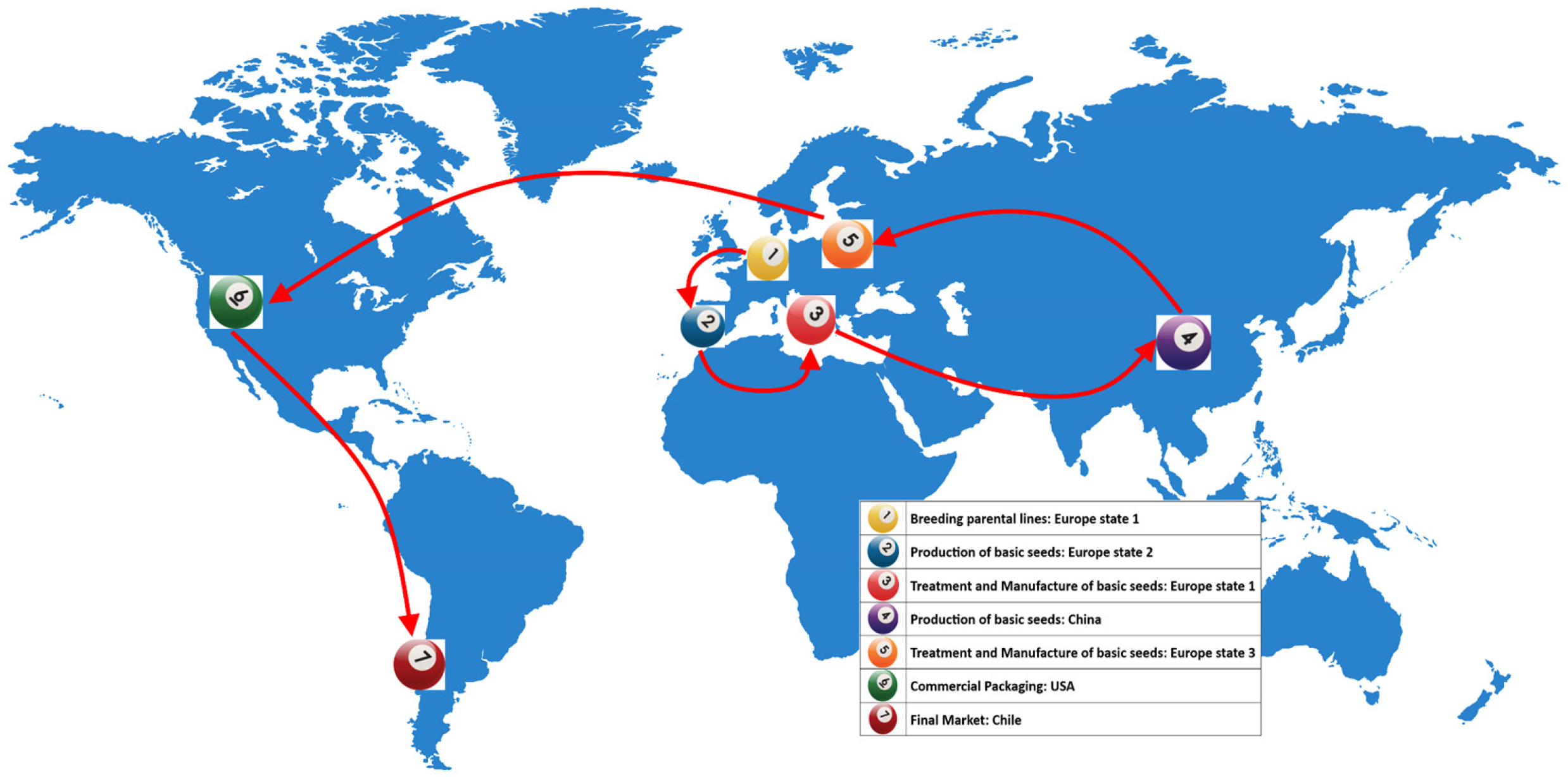
Table 1.
List of physical, biological and chemical treatments for tomato and pepper seeds, and their disinfection efficacy from phytopathogenic bacteria.
Table 1.
List of physical, biological and chemical treatments for tomato and pepper seeds, and their disinfection efficacy from phytopathogenic bacteria.
| Nature of seed treatment | Principle of the method | Substance / Antimicrobial compound | Operating conditions | Target pathogen | Crop plants | Efficacy and additional notes | Reference |
|---|---|---|---|---|---|---|---|
| Physical | Hot water | / | Soaking infected seeds at 53, 54 and 55°C for 10–60 min. | Cmm | Tomato | Partial seed disinfection after 30 min at 53 and 54°C and complete disinfection after 40 min treatments. Germination unaffected up to 55°C for 30 min; | [157] |
| Physical | Hot water | / | Soaking infected seeds at 56°C for 30 min. | Cmm | Tomato | Reduced disease quantity observed in the field; seed germination slightly reduced. | [140] |
| Physical | Hot water | / | Soaking infected seeds at 48°C for 60 min. | Pst | Tomato | No disease observed under greenhouse conditions; seed germination not affected. | [53] |
| Physical | Hot water | / | Soaking infected seeds at 50°C for 30 min. | Pst | Tomato | Pathogen infecting seeds reduced by 93.9%, as evidenced by agar plating. | [188] |
| Physical | Steam-air | / | Treating infected seeds at 55°C for 30 min. | Pst | Tomato | Pathogen infecting seeds reduced by 76.5%, as evidenced by agar plating. | [188] |
| Physical | Dry heating | / | Heating at 70°C for 4 to 6 days. | Cmm | Tomato | Complete seed disinfection, as evidenced by agar plating. | [164] |
| Physical | Ozone | Gaseous O3 | Gaseous O3 treatment at a concentration of 150 mg O3/Nm3 for 90 and 120 min. | Cmm, Pst | Tomato | Complete seed disinfection, as evidenced by agar plating. | [171] |
| Biological | Plant extracts | Plant extracts from Aloe vera, Coffea arabica, Glycyrrhiza uralensis and Yucca schidigera | Soaking infected seeds in 10 % plant extracts on a rotary shaker at 100 rpm overnight at 25°C. | Xep | Tomato | Complete seed disinfection, as evidenced by in vitro and in planta observations; germination performance and seedlings growth increase. | [136] |
| Biological | Plant extracts | Hexane–methanol extracts from Satureja hortensis | Soaking infected seeds in extracts dilutions (2.5, 5, 10, 20 and 40 mg ml−1) on a rotary shaker at 80 rpm for 3 h at 28° C. | Cmm, Xv | Tomato | Disease severity reduced up to 75.6% (Cmm) and 73.4% (Xv) under controlled conditions; germination performance decreased by up to 73.3% | [189] |
| Biological | Plant extracts | Aqueous plant extracts from coriander (Coriandrum sativum), eucalyptus (Eucalyptus sp.), Kastamonu garlic (Allium sativum ‘Kastamonu’), ginger (Zingiber officinale), Istanbul thyme (Origanum vulgare subsp. hirtum) and Izmir thyme (Origanum onites) | Soaking infected seeds in the extract’s dilutions on a rotary shaker at 150 rpm for 30 min. | Pst | Tomato | Disease incidence and severity on seedlings reduced by 63-100% and 57-100%, respectively, under controlled conditions. | [190] |
| Biological | Microorganisms (BCAs) |
Pseudomonas fluorescens | Soaking infected seeds both in (i) a bacterial suspension (1×108 CFU ml-1) and in (ii) a bacterial formulation (2.8 ×108 CFU g-1 in purified talcum powder (25% w/w) and Carboxy methyl cellulose (1% w/w) for 12 h. | Cmm | Tomato | Disease incidence reduced by 24%, as observed in the field. | [191] |
| Biological | Microorganisms (BCAs) |
Azospirillum brasilense | Soaking infected seeds both in a bacterial suspension (1×108 CFU ml-1) at 25±2°C for 1 h. | Pst | Tomato | No disease observed on seedlings under greenhouse conditions; seed germination not affected. | [192] |
| Chemical and Physical | Chemi-thermal Treatment | Cupric acetate (2.0 g L-1). Glacial acetic acid (1.0 ml L-1). Mixed solution of 23.2% pentachloronitrobenzene, and 5.8% 5-methoxy 3(trichloromethyl)-l,2,4-thiadiazole (4.5 ml L-1) Triton x-100 (0.2 ml L-1) |
Soaking infected seeds in the chemical solutions, at a ratio of 1:4 (w/v) for up to 90 min, at increasing temperatures ranging from 22 to 48°C. | Cmm, Xv, Pst | Tomato | Complete seed disinfection after 60 min of chemi-thermal treatment, as evidenced in vitro and in planta under controlled conditions; seed germination and seedlings vigour were not affected. | [193] |
| Chemical and Biological | Acidified nitrite / copper hydroxide / Bacillus spp. strains | Acidified nitrite solution (300 mmol l−1, pH 2). Kocide 101 (copper hydroxide 50% WP) at the rate of 3 g l−1. Bacillus spp. strains |
Soaking infected seeds into prepared solutions for 10 min. | Cmm | Tomato | Complete seed disinfection by copper hydroxide and Bacillus spp.; Seed disinfection by 98% using acidified nitrite solution, as observed under controlled conditions. | [194] |
| Chemical and Physical | Chemical treatment / Hot water |
NaHCl | Not available | Xanthomonads | Pepper | Reduction of bacterial populations on seed surface. | [195] |
| Physical, Chemical and Biological | Hot water, Chemical, Plant extracts | Hot water, NaHCl, Oxidate 2.0 and Thyme oil | Soaking infected seeds in: (i) Hot water: for 10 min at 37°C; 25 min at 50°C and 5 min at 10°C. (ii) NaHCl at 1.05% for 10 min. (iii) Oxidate 2.0 at 0.99% (1:100) dilution for 2 min. (iv) Thyme oil at 0.33% for 30 min |
Xe | Pepper | Complete seed disinfection by hot water and NaHCl; Seed disinfection by 80.3% using Oxidate 2.0 and by 93.9% using thyme oil, as evidenced by agar plating. |
[150] |
| Physical, Chemical | Hot water, Chemical, | NaHCl | Soaking infected seeds in: (i) Hot water: in a shaking water bath for 10 min at 37°C, 25 min at 50°C and 5 min at 10°C, respectively. (ii) NaHCl at 2% for 5 min. (iii) Metalaxyl-M (Ridomil) |
Cmm, Xv, Pst | Tomato | Hot water treatment: reduction of seed contamination by 99%; Chlorine: reduction of seed contamination by 99% (Xv and Pst), and by 97.4% (Cmm) as evidenced by agar plating. No disinfection observed using Metalaxyl-M. | [153] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







