1. Introduction
Extracellular vesicles (EVs) are pivotal conduits for intercellular communication, profoundly influencing multicellular organisms' physiological and pathological processes [
1]. These vesicles, encompassing both exosomes and macrovesicles, serve as cellular couriers, shuttling complex cargoes of proteins, lipids, metabolites, and various nucleic acids, including mRNA, microRNA, and long non-coding RNA [
2]. The molecular payload, encapsulated within the protective lipid bilayer of EVs, is meticulously tailored to reflect the donor cells' health and activity status, thereby transferring materials and functional information [
2,
3]. The targeting of specific recipient cells allows EVs to deliver signals that can alter gene expression, modulate cellular behavior, and orchestrate a coordinated response among cells in diverse biological contexts [
2,
3]. With their inherent ability to navigate through the extracellular milieu and deliver their cargo precisely, EVs are increasingly recognized as important players in maintaining homeostasis and influencing the progression of diseases [
4,
5,
6,
7], marking them as a focal point for therapeutic innovation and a deeper understanding of cellular communication networks.
Hypertension, a critical risk factor for cardiovascular diseases [
8,
9], is influenced by a complex interplay of factors, notably high dietary salt intake [
10]. Elevated salt intake activates immune responses and sparks inflammatory processes in key brain regions associated with cardiovascular control, such as the hypothalamic paraventricular nucleus (PVN) [
11,
12]. The ensuing neuroinflammation disrupts neurotransmitter balance critical for blood pressure regulation, contributing to the pathogenesis of hypertension [
13,
14]. This condition is further exacerbated by inflammatory mediators like cytokines and chemokines, which emerge from salt-induced neuroinflammation, heightening sympathetic nervous system activity (SNA) and reducing baroreflex sensitivity, thus fostering hypertension [
14,
15,
16,
17]. A pivotal consequence of increased salt consumption is the surge in the generation of reactive oxygen species (ROS) within the brain, straining the brain's antioxidant defenses and leading to oxidative stress. This oxidative stress is a critical perpetrator in the cascade of events that exacerbate hypertension, as it activates pro-inflammatory pathways and boosts SNA [
18,
19,
20,
21,
22], which are recognized contributors to elevated blood pressure [
23]. The lamina terminalis (LT), with its constituent structures—the subfornical organ, the organum vasculosum of the LT, and the median preoptic nucleus—and its lack of a complete blood-brain barrier (BBB), is particularly susceptible to circulating factors such as ions, hormones, and cytokines. It is crucial in body fluid homeostasis and cardiovascular regulation [
24,
25]. It has been observed that pro-inflammatory cytokines are elevated in the LT of rats subjected to a high-fat diet and Ang II infusion, highlighting the significance of cytokine-mediated impairment of LT functions and the overactivation of its neural circuitry during hypertensive conditions [
26].
Based on compelling evidence, we hypothesize that EVs originating from the brains of hypertensive rats contribute to neuroinflammation and oxidative stress, playing a role in the onset of hypertension. To test our hypothesis, we examined the impact of brain derived EVs from hypertensive Dahl Salt Sensitive (DSS) rats on neuroinflammation and oxidative stress, comparing them to EVs from normotensive Sprague Dawley (SD) rats. Specifically, we investigated how these EVs influence cytokine and chemokine levels, as well as ROS and other oxidative stress indicators in both neuronal cultures and specific brain regions linked to blood pressure control. Our study may potentially uncover new targets for high blood pressure treatment.
2. Materials and Methods
2.1. Animals
Adult SD and DSS rats were obtained from Charles River Laboratories (Wilmington, MA, USA) and utilized in our breeding colony to generate offspring. The rats were housed under controlled conditions, with an ambient temperature ranging from 20 to 24°C and a 12-hour light-dark cycle. The rats were provided ad libitum access to food and water throughout the experimental period. The rats were used for intracerebroventricular (ICV) injection, brain-derived EV isolation, and primary neuronal cell preparation; all procedures were described below. All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Michigan Technological University.
2.2. Isolation of brain-derived EVs
Six-week-old male DSS rats were given a high-salt diet (4% NaCl) to develop hypertension [
11], while age-matched SD rats received a regular salt diet (0.4% NaCl) as a control group. Following a minimum six-week dietary regimen, the animals were euthanized for brain extraction. The whole brain except for the cerebellum was used to isolate EVs using a revised established protocol [
27,
28]. Brain tissues were sectioned into small pieces and incubated in Gibco Hibernate E medium at a ratio of approximately 0.2 grams of tissue to 2 mL of medium per well in a 6-well plate. The tissue was then dissociated to a uniform size of about 2x2x2 mm. To this, collagenase D and DNase I were introduced to achieve concentrations of 2 mg/mL and 40 U/mL, respectively, in each well. The plate was incubated at 37°C for 30 minutes with gentle shaking at 70 rpm. Post-incubation, protease, and phosphatase inhibitors were added before the tissue was strained through a 70 μm cell strainer into a new 50 mL tube. Subsequent differential centrifugation steps were conducted at 4°C (300 xg for 10 minutes, 2000 xg for 20 minutes, and 10,000 xg for 30 minutes). The resulting supernatants were passed through a 0.22 μm filter to exclude larger particles. These filtered supernatants were overlaid onto a 30% sucrose cushion and ultracentrifuged at 100,000 xg for 90 minutes at 4°C. Pellets were collected and washed with Dulbecco’s phosphate-buffered saline (DPBS) with ultracentrifugation at 100,000 xg for 90 minutes again at 4°C. The final pellets obtained were resuspended in 50 μL of ice-cold DPBS, enhanced with protease and phosphatase inhibitors, to prepare for downstream applications.
2.3. Scanning Transmission Electron Microscopy (STEM) Analysis
EVs in a 30 μL aliquot were combined with an identical volume of 2% paraformaldehyde (PFA) and left to interact for 5 minutes. Then, 5 μL of this mixture was placed onto the carbon-coated side of a grid and allowed to settle for 1 minute. The grid was then blotted gently to dry using filter paper. For negative staining, uranyl acetate (UA) was employed. Freshly prepared 2% UA solution, 3 μL in volume, was applied to the grid and allowed to remain for 30 seconds before blotting away the excess solution with filter paper. After repeating the staining procedure, the grid was left to dry in the air overnight. The dry samples were subsequently examined under a FEI 200kV Titan Themis STEM.
2.4. Dynamic Light Scattering (DLS) Analysis
For particle size determination, a 10 μl volume of the resuspended EV pellet was diluted in DPBS to a protein concentration of 0.01 μg/μl. Approximately 800 μl of this dilution was placed into a low-volume cuvette. The Malvern Zetasizer Nano series was utilized to assess the size distribution of the particles. Cuvettes with lids were inverted before measurements to ensure uniformity and prevent sedimentation of larger particles. Each dilution was analyzed in triplicate to ensure the accuracy of the results.
2.5. Western Blot Analysis
The presence of specific proteins in EVs was evaluated through Western blot analysis. The EVs were prepared by mixing with RIPA lysis buffer containing 0.5% phenylmethylsulfonyl fluoride (PMSF) and subjected to sonication in three 5-second bursts. The mixture was then incubated on ice for 15 minutes, pipetting at 5-minute intervals to assist lysis. Protein concentrations were quantified using the Bradford reagent. Proteins (40 to 100 μg) were then separated on an SDS-PAGE gel using an electric field (initially 80 mV for 30 minutes, followed by 120 mV for 1.5 to 2.5 hours). After electrophoresis, proteins were transferred to a nitrocellulose membrane with a trans-blot turbo transfer system. The membrane was blocked with 5% milk in Tris-buffered saline containing 0.1% Tween® 20 (TBST) and then incubated with primary antibodies including mouse anti- ALG-2 interacting protein X (mouse-anti-Alix, 1:200 dilution, Santa Cruz Biotechnology), mouse anti- tumor susceptibility 101 (mouse anti-TSG101, 1:200 dilution, Santa Cruz Biotechnology), mouse anti- Golgi matrix protein 130 kD (mouse anti-GM130, 1:200 dilution, Santa Cruz Biotechnology) and rabbit anti-CD9 (1:1000 dilution, Cell Signaling) at 4°C overnight. The membranes were washed thrice with PBS, each for 5 minutes, followed by an hour's incubation with horseradish peroxidase (HRP)-conjugated secondary anti-bodies. Chemiluminescent detection was achieved using SuperSignal West Dura Extend-ed Duration Substrate and imaged using a Bio-Rad gel imaging system. Primary antibodies are listed in
Table S1.
2.6. Fluorescent labeling of brain-derived EV
Following established protocols, brain-derived EVs were labeled with rhodamine-based fluorophores [
29]. The labeled EVs (Rho-EVs) underwent incubation with primary neuronal cultures. The uptake and localization of these Rho-EVs were examined at intervals of 3, 24, 48, and 72 hours using confocal microscopy (Olympus FV1000). Hoechst stain (0.1 g/mL) was applied to the neuronal cultures 10-15 minutes before imaging to visualize cell nuclei.
2.7. Intracerebroventricular (ICV) Injections of EVs
The procedures for ICV injections adhered to previously published protocols [
30,
31]. Rats under anesthesia with 2.5% isoflurane in O2 received an injection of 8 µg of EV protein from either DSS or SD rats into the right lateral ventricle. Stereotaxic coordinates guided the injections: 0.8-0.9 mm posterior to bregma, 1.4-1.8 mm lateral to the midline, and 3.2-3.8 mm below the dural surface. Injections were administered at a flow rate of 1 µL/min using an UltraMicroPump3 (World Precision Instruments). Following the procedure, the animals were euthanized with an excess of isoflurane. Their brains were immediately frozen in liquid nitrogen and stored at -80°C for subsequent mRNA analysis.
2.8. mRNA level measurement using real-time PCR
The measurement of mRNA levels for select genes was performed in neuronal cultures and specific brain regions (PVN and LT areas) utilizing real-time PCR, as detailed in earlier studies [
30,
31]. RNA was isolated using the RNeasy Mini kit per the manufacturer's protocol. Between 200-400 ng of RNA from each sample was reverse transcribed to synthesize cDNA, which was then used as a template in Real-Time PCR assays. The expression levels of cytokines including tumor necrosis factor alpha (TNFα), interleukin-1 beta (IL-1β) and interleukin 6 (IL-6), chemokines including C-C motif chemokine ligand 2 (CCL2), CCL5 and CCL12, and the nuclear factor NF-kB subunit NF-kB1, inducible nitric oxide synthase (iNOS), NADPH oxidase subunits cytochrome b-245, alpha polypeptide and beta polypeptide (CYBA and CYBB), as well as indicators of neuronal activity including FBJ osteosarcoma oncogene and Fos-like antigen 1 (c-FOS and FOSL1), were quantified. TaqMan-specific primers and probes were employed for these assessments, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as a normalization reference. Primers listed in
Table S1 were purchased from ThermoFisher Scientific.
2.9. Mitochondrial reactive oxygen species (mtROS) measurement
Mitochondria-targeting fluorescent probes (MitoProbe) [
32] were employed to determine ROS levels in the mitochondria of primary brain neuronal cultures and brain tissue. During
in vitro experiments, primary neurons, aged 7 to 10 days in culture, were incubated with MitoProbe (1μM) after either a direct application or a fixation with 4% PFA for 30 minutes, followed by a co-stained with Hoechst for 15 minutes. After incubation, the cells were cleansed with PBS and analyzed using confocal microscopy to evaluate mtROS levels.
For in vivo measurements, rats received ICV injections of EVs. Three hours after the EV administration, MitoProbe was injected into the same brain region. Six hours after the initial EV injections, an overdose of isoflurane was administered for euthanasia, followed by transcardial perfusion with cold PBS and then 4% PFA in 1×PBS. The brains were then extracted, fixed in 4% PFA overnight and preserved in 30% sucrose in 1×PBS until the tissue descended to the container's bottom. Subsequently, the brains were cryo-sectioned for immunofluorescence analysis.
2.10. Immunofluorescence analysis
The process for immunofluorescence began with brains being embedded in O.C.T. compound (Sakura Finetek) and then cryo-sectioned into 20 µm thick coronal sections that included the PVN and LT regions. Following previously established protocols, brain sections were initially rinsed three times in PBS for 10 minutes each, permeabilized in cold methanol for 10 minutes, and then given a PBS wash. The sections were subsequently blocked with 5% horse serum for one hour. Incubation followed with primary antibodies: rabbit anti-neuronal nuclei (rabbit anti-NeuN, 1:300 dilution), mouse anti-glial fibrillary acidic protein (mouse anti-GFAP, 1:300 dilution), or rabbit anti-ionized calcium binding adaptor molecule 1 (rabbit anti-Iba1, 1:500 dilution) in PBS containing 0.5% Triton X-100 and 5% horse serum, maintained for 24 hours at 4°C. The following day, the sections underwent three further PBS washes, each lasting 10 minutes, before incubation with secondary antibodies—Alexa Fluor 488 donkey anti-rabbit IgG or Alexa Fluor 488 donkey anti-mouse IgG—for one hour at room temperature. Subsequent washes in PBS were performed before the sections were mounted in Vectorshield (Vector Labs, Burlingame, CA, United States). Fluorescent images were captured using confocal microscopy (Olympus FV1000).
2.11. Statistical Analysis
Statistical comparisons were conducted using Prism 9 (GraphPad), with all data presented as mean ± SEM. The student’s t-test or one-way ANOVA was applied to assess statistical significance. Post hoc analysis with Tukey’s multiple comparison test was used to evaluate differences between groups in the case of one-way ANOVA. The total fluorescent area was quantified using ImageJ software. A p-value of less than 0.05 was considered indicative of statistical significance.
4. Discussion
Our current study embarked on a quest to decode the influence of brain-derived EVs on the progression of hypertension, focusing on their ability to modulate neuroinflammation and the generation of ROS in primary neuronal cultures and brain regions critical for cardiovascular regulation. The investigation unraveled three pivotal findings:
- (1)
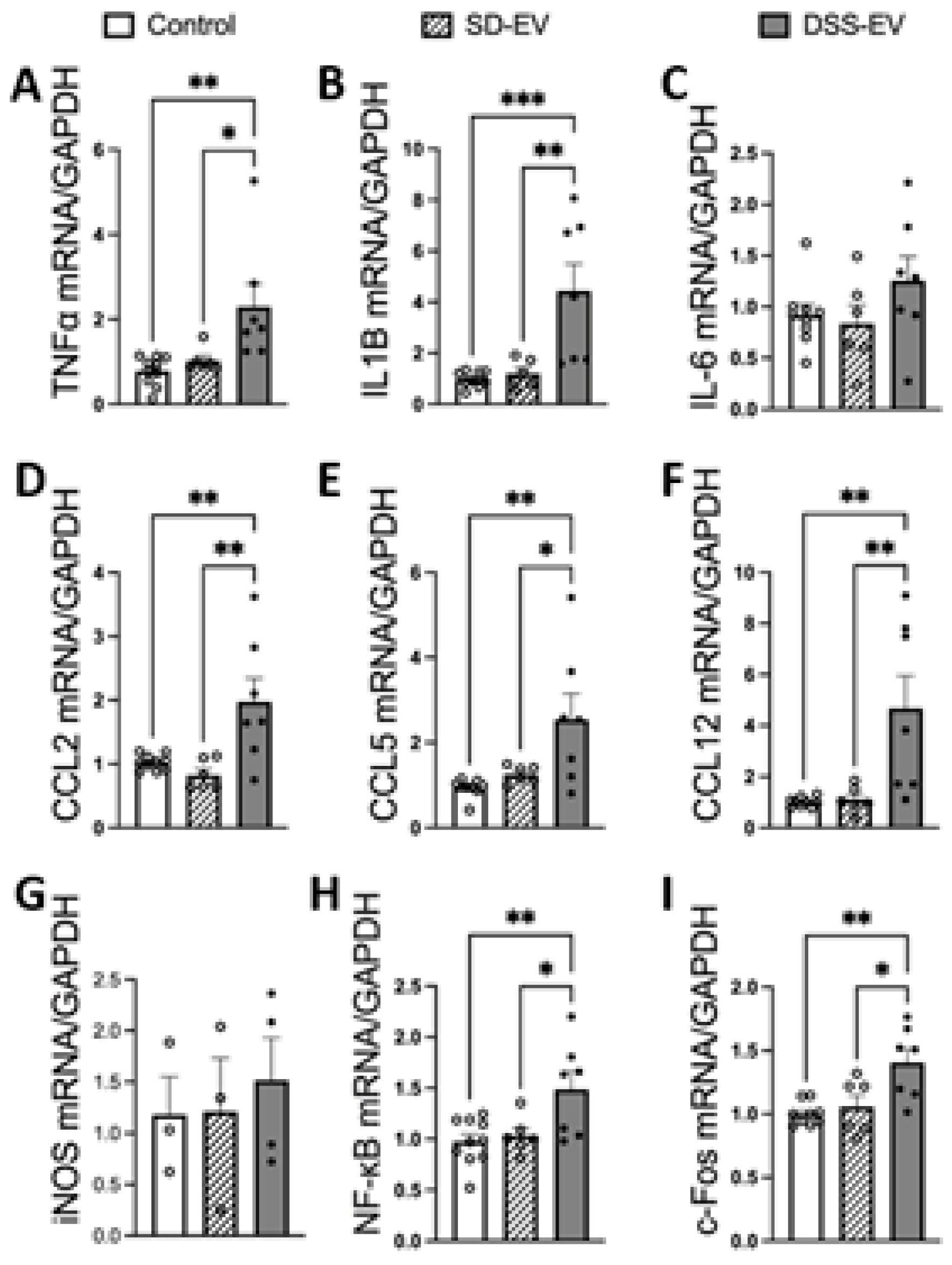
EVs isolated from the brains of hypertensive DSS rats were identified to significantly increase PIC levels, such as TNF-α and IL-1β, in cultured neurons. This suggests that the EVs can carry and transfer inflammatory signals, potentially leading to a heightened inflammatory state in the recipient cells. The activation of NF-κB signaling pathways and elevation of chemokines like CCL2, CCL5, and CCL12, as well as the amplification of neuronal activity evidenced by the c-Fos marker, were also noted. Furthermore, an increase in ROS within these neurons was observed, hinting at the pro-oxidative effects of the EVs.
- (2)
The administration of EVs from hypertensive rats to normotensive SD rats resulted in similar inflammatory and oxidative responses within the PVN and LT—brain regions that are instrumental in managing cardiovascular functions. This reaction was characterized by elevated levels of proinflammatory markers, neuronal activity as indicated by Fosl1, and mitochondrial ROS production.
- (3)
A pronounced rise in ROS production, specifically in neurons located within the PVN and LT, was induced by the hypertensive rat brain-derived EVs, highlighting the targeted nature of EV-related alterations within these crucial neural structures.
These findings underscore the critical role of EVs, including exosomes and larger vesicles, in cellular communication. They serve as vehicles for biological signals that can alter cellular functions, illuminating their potential role in the pathogenesis of hypertension. The detailed examination of EVs from both normotensive and hypertensive rat models sheds light on their possible contribution to hypertension-related complications.
In this study, we selected the DSS rat strain as the hypertensive model and SD rats as the normal controls. The DSS rats are known for their salt sensitivity and were initially derived from the SD rats, ensuring they share the same genetic background [
33]. This genetic commonality establishes a strong basis for attributing the observed neuroinflammatory and oxidative changes to the effects of brain-derived EVs, thereby reinforcing the relevance and potential involvement of EVs in the development of hypertension.
Our research delves into the role of EVs in the brains of hypertensive rats and their impact on primary neuronal cultures, shedding light on their potential contribution to neuroinflammatory processes. Neuroinflammation often manifests through increased levels of PICs, which are known to be associated with various diseases, including hypertension [
11,
34,
35,
36]. In our present study, EVs from hypertensive rats were found to elevate the mRNA expression of inflammatory markers like TNFα and IL1β, as well as the transcription factor NF-κB in cultured neurons. Given that NF-κB orchestrates the regulation of numerous genes, particularly those related to inflammation, its activation is a clear indicator of an inflammatory state.
Our previous research established that TNFα could intensify the inflammatory response in neurons [
37], a finding reinforced by our current study. Neurons are thus implicated as both sources and targets of neuroinflammation in hypertensive conditions, responding to external hypertensive stimuli, in this case, brain-derived EVs. Moreover, EVs have been known to carry or present cytokines on their surface, including TNFα, IL1β, and IL6 [
38]. This aligns with findings in clinical settings, such as in patients with myocardial infarction, where EV-associated cytokines have shown dysregulation [
39].
While our current work did not determine the exact source of central cytokines, further research is necessary to elaborate. The primary neuronal cultures used were derived from various brain regions, including the hypothalamus and cortex. To pinpoint the brain areas most affected by EVs, we examined the inflammatory responses in two hypertension-relevant regions, the PVN and LT, after introducing EVs intracranially. Consistently, we observed that EVs from hypertensive rats induced a significant increase, or at least a rising trend, in cytokine and chemokine levels in these regions compared to controls. This aligns with previous observations that a high-salt diet increases cerebrospinal fluid sodium levels and that certain cytokines are elevated in hypertensive conditions [
11]. Notably, injections of PICs into the PVN have been shown to raise blood pressure and SNA, implicating these cytokines in hypertension development [
40].
Our present study discovered a marked increase in IL1β and a notable rising trend in IL6 in the LT, characterized by its highly permeable blood-brain barrier. This suggests that neuroinflammation in the LT during hypertension may be mediated, at least in part, by brain-derived EVs. Given the LT's susceptibility to circulating cytokines, it is plausible that these cytokines could affect its functions, a hypothesis supported by models indicating that the LT's neural response to cytokines contributes to hypertension development [
41,
42]. These findings suggest that brain-derived EVs may serve as conduits for neuroinflammation, potentially amplifying the inflammatory environment within the brain, which is associated with the onset and progression of hypertension.
Our investigation expanded to the impact of EVs on chemokine expression, where we discovered pronounced increases in the mRNA levels of CCL2, CCL5, and CCL12 in primary neuronal cultures exposed to EVs from hypertensive DSS rats. Notably, in brain regions such as the PVN and the LT, we detected either significant enhancements or an upward trend in the mRNA levels of these chemokines when SD rats received treatments with EVs sourced from hypertensive DSS counterparts. These findings are pivotal due to the established role of these chemokines in hypertension.
The chemokine CCL2 is crucial in guiding immune cells like neutrophils, monocytes, and T cells to inflammation sites by interacting with specific chemokine receptors. The link between high CCL2 levels and the severity of hypertension-related organ damage has been supported by correlations found in human studies [
43]. Furthermore, experiments blocking CCR2, the receptor for both CCL2 and CCL12, demonstrated a reduction in blood pressure in animal models of hypertension [
44].
CCL5 is another critical chemokine, serving as a strong attractant for monocytes and T cells. Increased levels of CCL5 have been documented in both the central nervous system and peripheral organs such as the kidneys in hypertensive rats, implying a systemic response that could exacerbate hypertension [
45,
46,
47].
The elevated mRNA levels of CCL2 in neurons treated with EVs from hypertensive rats suggest that these vesicles may carry factors that induce an inflammatory response. Since CCL2 is involved in recruiting inflammatory cells, its increased expression could lead to enhanced inflammation within the brain, contributing to the pathophysiology of hypertension. The fact that higher CCL2 levels are associated with more severe organ damage in humans with hypertension underscores the clinical relevance of our findings.
The heightened expression of CCL5 in response to EVs from hypertensive rats highlights a potential mechanism by which hypertension can alter immune cell migration and infiltration in the brain and other organs. Given that CCL5 expression is upregulated in hypertensive rats' central nervous system and kidneys, it's plausible that this chemokine could contribute to the maintenance and progression of high blood pressure through its chemotactic activities. The observed increase in CCL12 mRNA and the known effects of blocking CCR2 present a strong case for these molecules as therapeutic targets. By interfering with CCR2, the EV-mediated propagation of hypertension could be mitigated, which could reduce blood pressure and associated damage.
Our observations indicate a substantial role for brain-derived EVs in mediating chemokine-driven inflammatory responses, implicating them in the recruitment and activation of immune cells that potentially exacerbate neuroinflammation. This could have far-reaching implications for understanding the pathogenesis of hypertension and open the door to novel therapeutic strategies targeting EVs and their inflammatory cargo.
Our research further delves into the contribution of EVs to ROS generation. When produced excessively, ROS can eclipse the body's antioxidant defenses, prompting oxidative stress—a pivotal factor in the onset of hypertension [
48]. Our findings highlight that exposure to EVs from the brains of hypertensive DSS rats significantly escalates CYBA mRNA levels in primary neuronal cultures. This suggests that EVs may activate oxidative stress by engaging the NADPH oxidase system, a known pathway for ROS production. While mitochondria are recognized as the primary sites for ROS generation, our assays of mtROS through fluorescence intensity reinforce the premise that DSS-EVs amplify mtROS production.
To substantiate the link between EVs and oxidative stress
in vivo, we administered ICV injections of DSS-EVs into normotensive SD rats. Post-injection, we observed a pronounced rise in iNOS mRNA within the PVN and a surge in mtROS fluorescence in both the PVN and LT. Our data pinpoint neurons as the predominant ROS generators in response to EV exposure. This is a reasonable observation considering that neurons, with their high metabolic demand, possess an abundance of mitochondria, which, under stress conditions, can leak electrons that react with oxygen to form ROS. Therefore, neuronal cells are particularly susceptible to shifts in redox states and can significantly contribute to ROS production. This discovery is crucial because it directly connects neuroinflammation, oxidative stress, and hypertension. Both neuroinflammation and oxidative stress are known to amplify SNA, which is a major driving force in the development of hypertension. ROS and inflammatory mediators can disturb neurotransmitter equilibrium and autonomic regulation, increasing SNA [
20,
49]. In line with these findings, our experiments showed that treatment with DSS-EVs increased the expression of c-Fos, a marker of neuronal activation, in primary neuron cultures. We also noted higher levels of FOSL1, another indication of neuronal activity, in the PVN and LT regions. Such neuronal activation is a precursor to raised blood pressure and the advancement of hypertension.
6. Limitations
Our study presents compelling evidence that brain-derived EVs from hypertensive DSS rats induce neuroinflammation and increase ROS production, potentially contributing to hypertension development. However, some considerations merit attention. The primary neuronal cultures used encompassed diverse brain regions, limiting our ability to discern region-specific responses to EVs. Additionally, our study didn't directly assess the origin of central cytokines, suggesting a need for a more detailed exploration to elucidate EVs' mechanistic pathways in hypertension. Differentiating systemic versus regional effects of cytokines and addressing the blood-brain barrier's influence in areas like LT pose challenges.
Furthermore, our investigation, though comprehensive, could benefit from more specificity in understanding the roles of PICs, chemokines, and ROS in hypertension development. Long-term effects of EV exposure on neuronal function and blood pressure regulation were not explored, leaving room for understanding the chronic impacts of EVs over time.
Finally, our study lacks interventions to modulate observed EV-mediated effects. Future research incorporating such interventions could offer more conclusive insights into the therapeutic potential of targeting EVs in hypertension, guiding innovative therapeutic directions. Ongoing efforts aim to address these gaps, fostering a more comprehensive understanding of EVs' roles in hypertension and inspiring therapeutic advancements.
Author Contributions
X.C. and X.Y. contributed equally. Conceptualization, X.C. and Z.S.; methodology, X.C., X.Y., L.G., Q.C., L.B. and Z.S.; data curation, X.C., X.Y., and Z.S.; software, X.C.; validation, X.C. and Z.S; investigation, X.C., X.Y., L.B. and Z.S.; writing—original draft preparation, X.C., L.B. and Z.S.; writing—review and editing, X.C., X.Y., L.B. and Z.S.; supervision, Q.C., L.B. and Z.S.; funding acquisition, L.B. and Z.S. All authors have read and agreed to the published version of the manuscript.
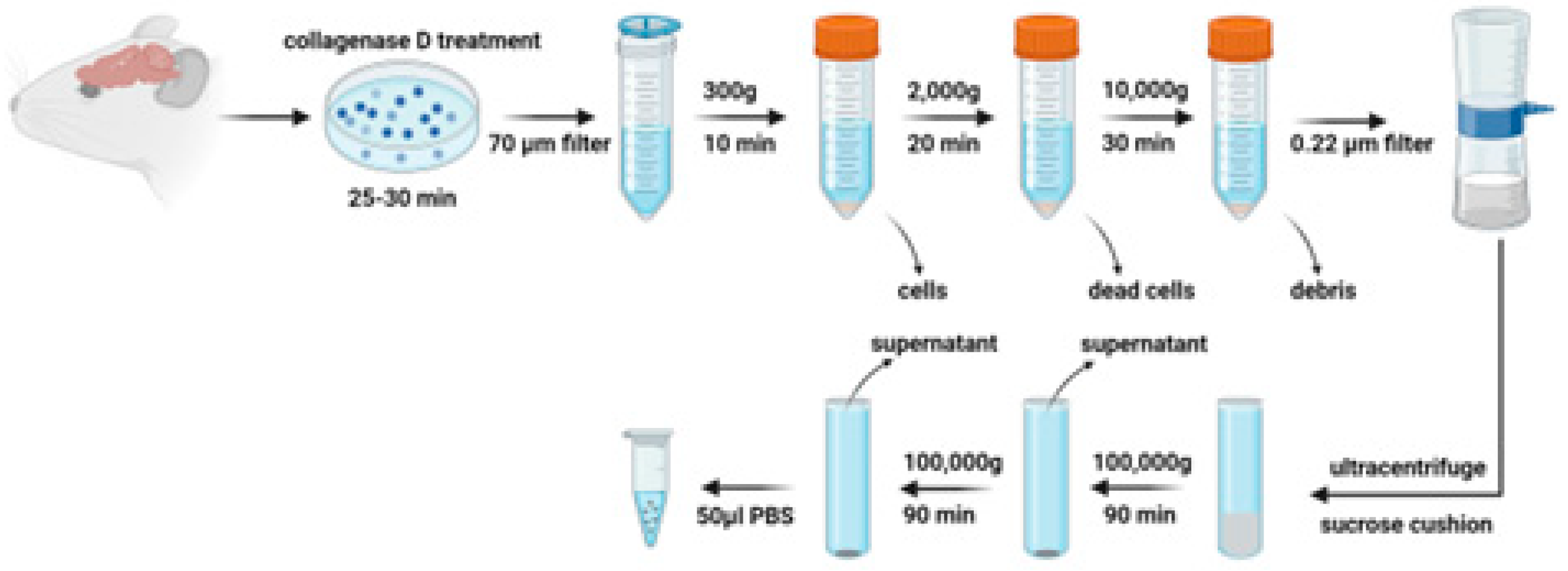
Figure 1.
Schematic diagram of brain-derived extracellular vesicles (EV) isolation Rat brain tissue pieces is first incubated with collagenase D and DNase I to release EVs embedded in the extracellular matrix. A combination of centrifugation, filtration, sucrose cushion ultracentrifugation and additional ultracentrifugation is performed to isolate brain-derived EVs.
Figure 1.
Schematic diagram of brain-derived extracellular vesicles (EV) isolation Rat brain tissue pieces is first incubated with collagenase D and DNase I to release EVs embedded in the extracellular matrix. A combination of centrifugation, filtration, sucrose cushion ultracentrifugation and additional ultracentrifugation is performed to isolate brain-derived EVs.
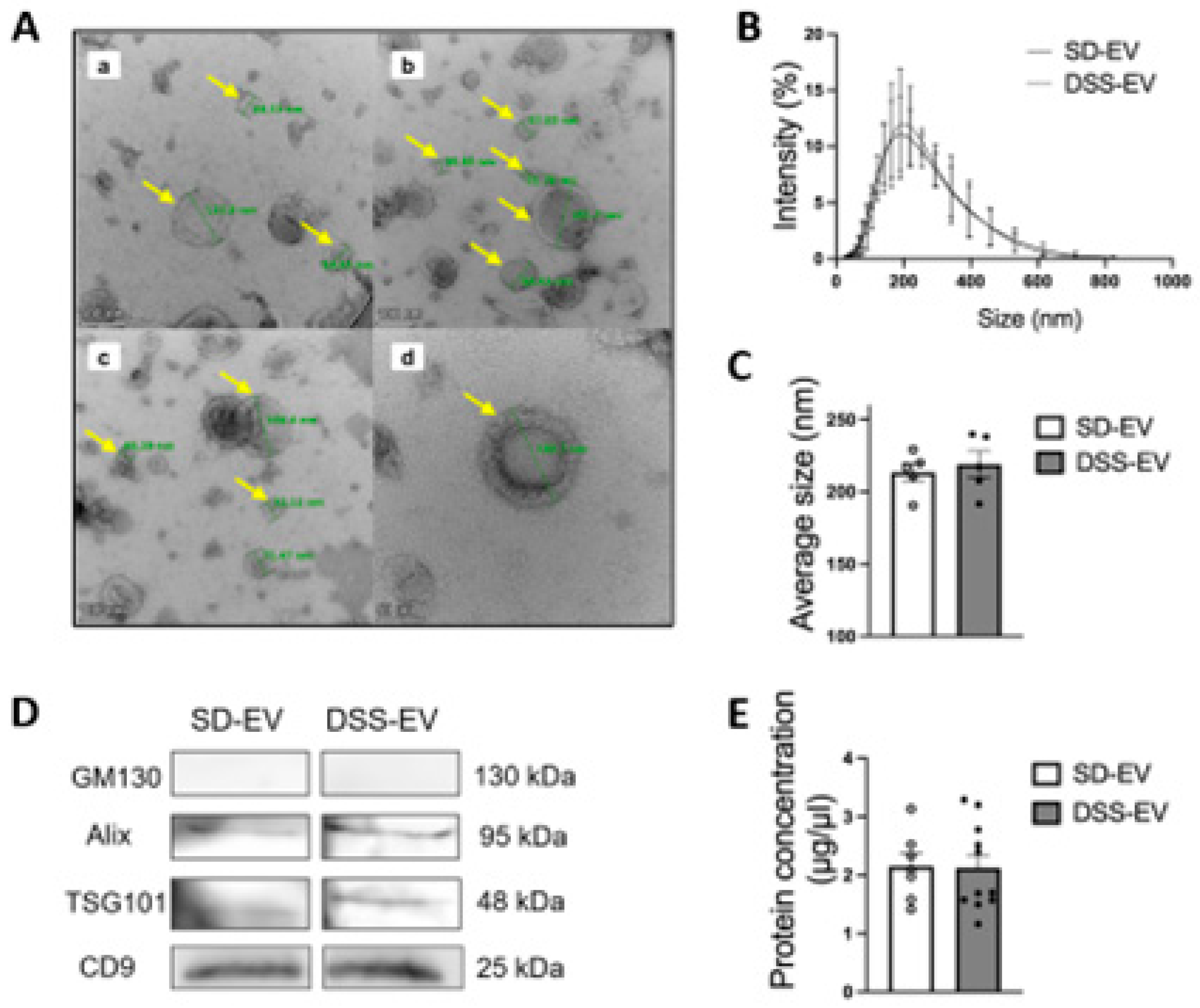
Figure 2.
Identification of brain-derived EVs from Sprague Dawley (SD) and Dahl Salt Sensitive (DSS) rats (A) Representative electron micrograph of brain-derived EVs with transmission electron microscopy; Selected EVs are indicated by yellow arrows and their diameters are measured in green line. Scale bar is 100nm in (a-c) and 50nm in (d). (B-C) Comparison of size of DSS-EV (dashed line) and SD-EV (solid line) with dynamic light scattering; Summary data (C) comparing the average size of DSS-EV with SD-EV; (D) Western blots of EV protein markers (ALIX, TSG101 and CD9) in both groups; GM130 referred as a negative control; (E) Protein concentration of brain-derived EVs in SD and DSS rats. Graphs indicate mean ± SEM.
Figure 2.
Identification of brain-derived EVs from Sprague Dawley (SD) and Dahl Salt Sensitive (DSS) rats (A) Representative electron micrograph of brain-derived EVs with transmission electron microscopy; Selected EVs are indicated by yellow arrows and their diameters are measured in green line. Scale bar is 100nm in (a-c) and 50nm in (d). (B-C) Comparison of size of DSS-EV (dashed line) and SD-EV (solid line) with dynamic light scattering; Summary data (C) comparing the average size of DSS-EV with SD-EV; (D) Western blots of EV protein markers (ALIX, TSG101 and CD9) in both groups; GM130 referred as a negative control; (E) Protein concentration of brain-derived EVs in SD and DSS rats. Graphs indicate mean ± SEM.
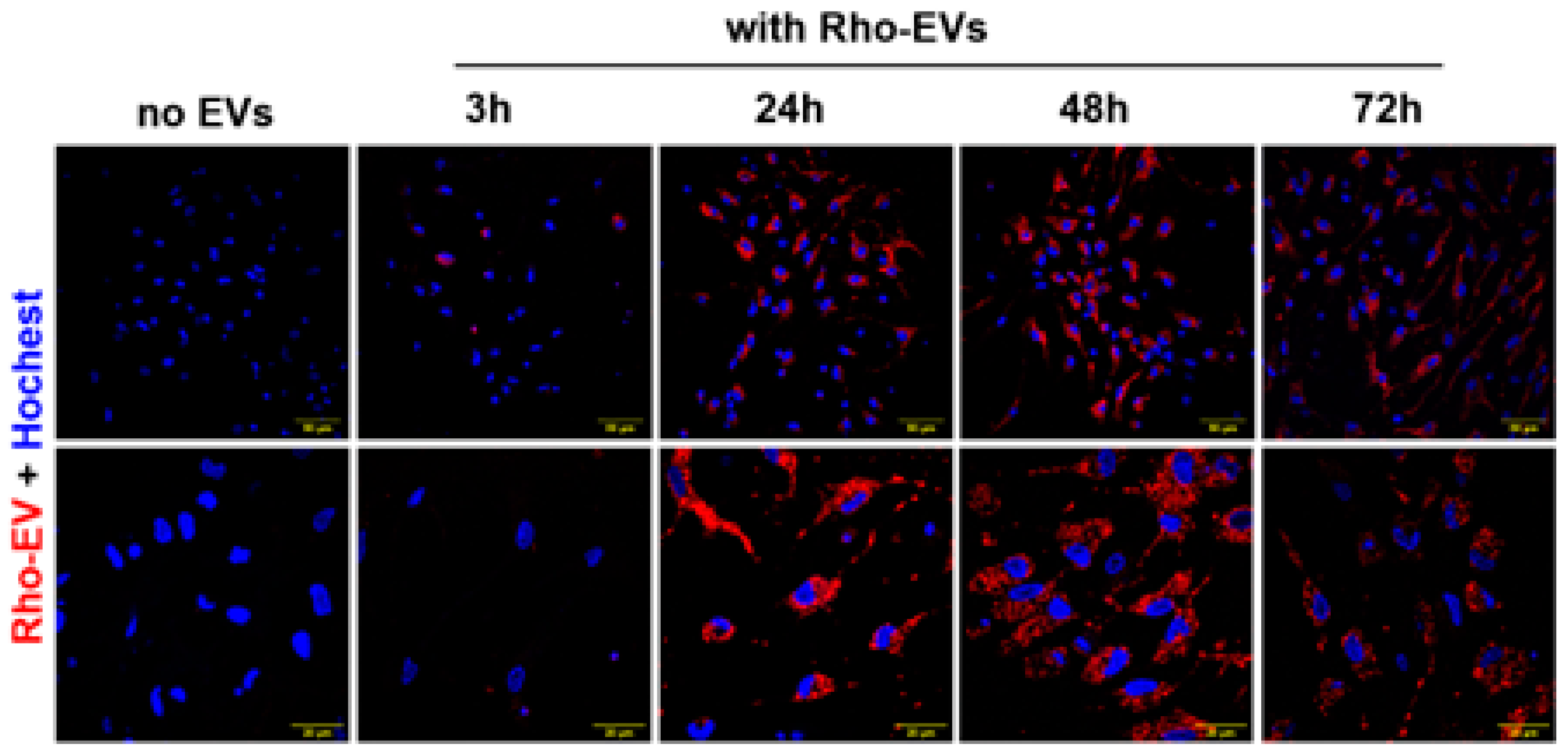
Figure 3.
Brain-derived EVs are taken up in primary neuronal cultures. EVs from rat brains were fluorescent labeled (shown in red) and incubated with primary neurons for a series of time- 3h, 24h, 48h and 72h. Subsequently labeled EVs were nuclear stained with Hoechst (shown in blue) and subjected to confocal microscopy. Scale bar of images with low magnification is 50 µm and images with high magnification is 20 µm.
Figure 3.
Brain-derived EVs are taken up in primary neuronal cultures. EVs from rat brains were fluorescent labeled (shown in red) and incubated with primary neurons for a series of time- 3h, 24h, 48h and 72h. Subsequently labeled EVs were nuclear stained with Hoechst (shown in blue) and subjected to confocal microscopy. Scale bar of images with low magnification is 50 µm and images with high magnification is 20 µm.
Figure 4.
Brain-derived EVs from hypertensive DSS rats increase mRNA levels of inflammatory cytokines, chemokines, NF-kB1 and c-Fos in primary neuronal cultures. Primary neurons were incubated with either DSS-EV, SD-EV or PBS control for 24h. mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and c-Fos (I) were semi-quantified by real-time PCR and compared among DSS-EV, SD-EV and PBS control groups using One-way ANOVA test. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
Figure 4.
Brain-derived EVs from hypertensive DSS rats increase mRNA levels of inflammatory cytokines, chemokines, NF-kB1 and c-Fos in primary neuronal cultures. Primary neurons were incubated with either DSS-EV, SD-EV or PBS control for 24h. mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and c-Fos (I) were semi-quantified by real-time PCR and compared among DSS-EV, SD-EV and PBS control groups using One-way ANOVA test. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
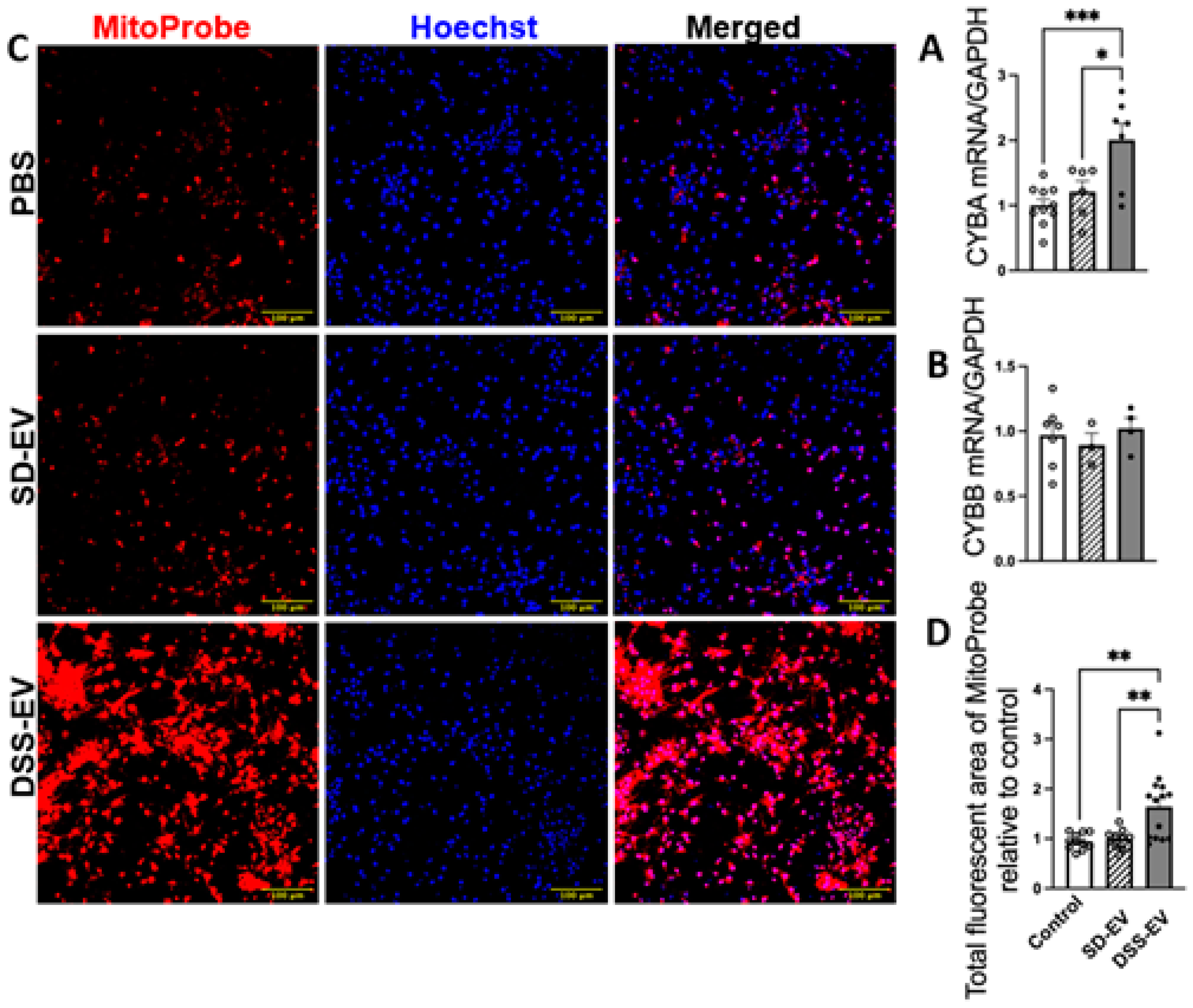
Figure 5.
Brain-derived EVs from hypertensive rats increase mtROS in primary neuronal cultures. Primary neurons were incubated with DSS-EV, SD-EV or PBS control for 24h. The mRNA levels of CYBA (A) and CYBB (B) were compared among DSS-EV, SD-EV and PBS groups and normalized with GAPDH. (C) After 24h, primary neurons were fixed with 4% PFA and stained with Hoechst (shown in blue) and MitoProbe (shown in red) for 30 min. Scale bar of the images is 100 µm. (D)Total fluorescent area of MitoProbe was quantified and normalized to PBS control. Each data point represents a summary data of ROS levels from multiple microscopic views of a cell sample. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
Figure 5.
Brain-derived EVs from hypertensive rats increase mtROS in primary neuronal cultures. Primary neurons were incubated with DSS-EV, SD-EV or PBS control for 24h. The mRNA levels of CYBA (A) and CYBB (B) were compared among DSS-EV, SD-EV and PBS groups and normalized with GAPDH. (C) After 24h, primary neurons were fixed with 4% PFA and stained with Hoechst (shown in blue) and MitoProbe (shown in red) for 30 min. Scale bar of the images is 100 µm. (D)Total fluorescent area of MitoProbe was quantified and normalized to PBS control. Each data point represents a summary data of ROS levels from multiple microscopic views of a cell sample. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
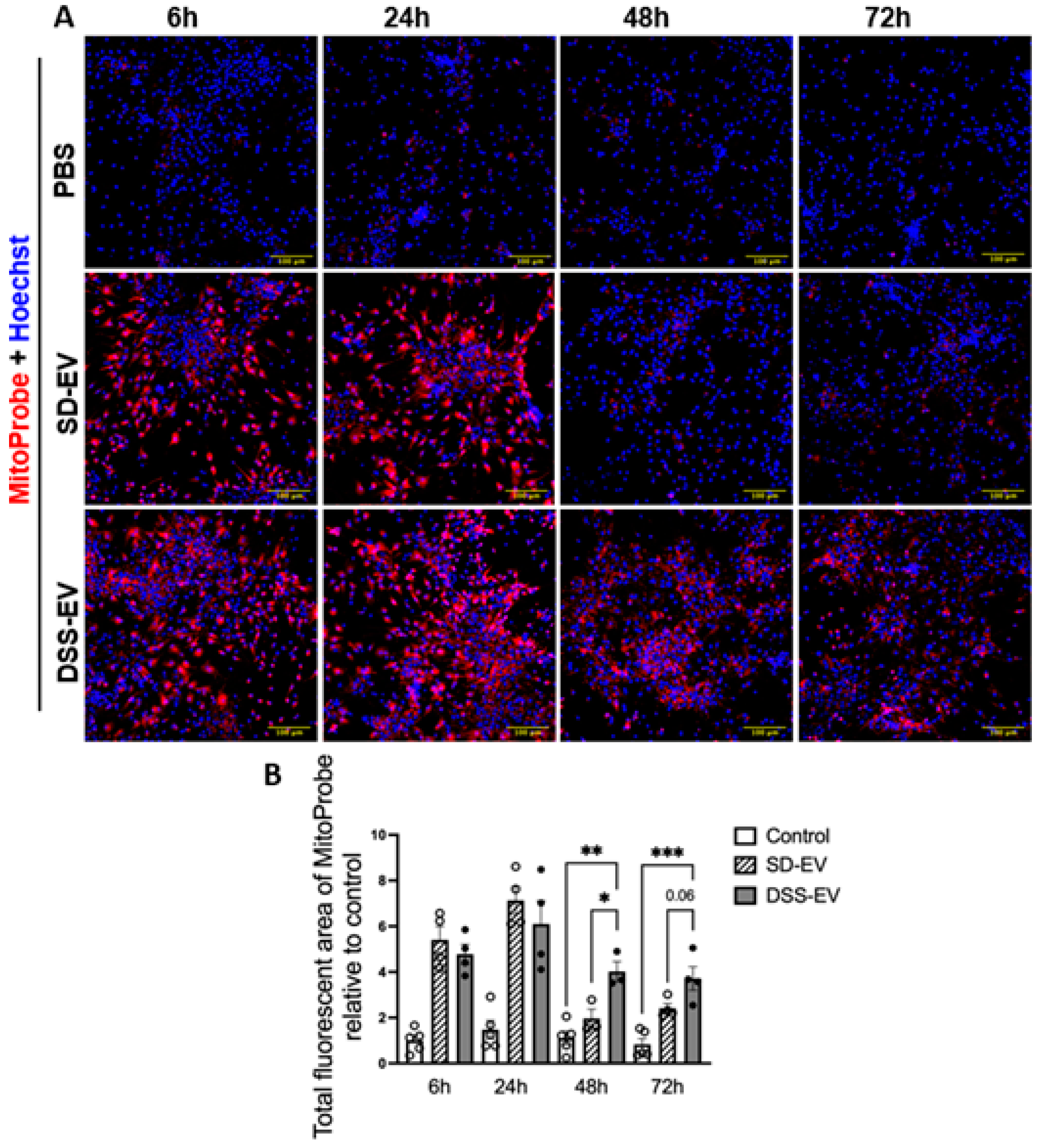
Figure 6.
Brain-derived EVs from hypertensive rats increase mtROS in primary neuronal cultures in a time-dependent manner. (A) Primary neurons were incubated with DSS-EV, SD-EV, or PBS for a series of time-6h, 24h, 48h and 72h. After EV incubation, neurons were immediately stained with Hoechst (shown in blue) and MitoProbe (shown in red) for 30 min. Scale bar of the images is 100 µm. (B)Total fluorescent area of MitoProbe was quantified and normalized to control-6h group. Each data point represented a summary data of ROS levels from multiple microscopic views of a cell sample. Graphs indicate mean ± SEM. One-way ANOVA test is performed to compare fluorescence of different groups treated for the same amount of time. * P< 0.05, ** P<0.01, *** P<0.001.
Figure 6.
Brain-derived EVs from hypertensive rats increase mtROS in primary neuronal cultures in a time-dependent manner. (A) Primary neurons were incubated with DSS-EV, SD-EV, or PBS for a series of time-6h, 24h, 48h and 72h. After EV incubation, neurons were immediately stained with Hoechst (shown in blue) and MitoProbe (shown in red) for 30 min. Scale bar of the images is 100 µm. (B)Total fluorescent area of MitoProbe was quantified and normalized to control-6h group. Each data point represented a summary data of ROS levels from multiple microscopic views of a cell sample. Graphs indicate mean ± SEM. One-way ANOVA test is performed to compare fluorescence of different groups treated for the same amount of time. * P< 0.05, ** P<0.01, *** P<0.001.
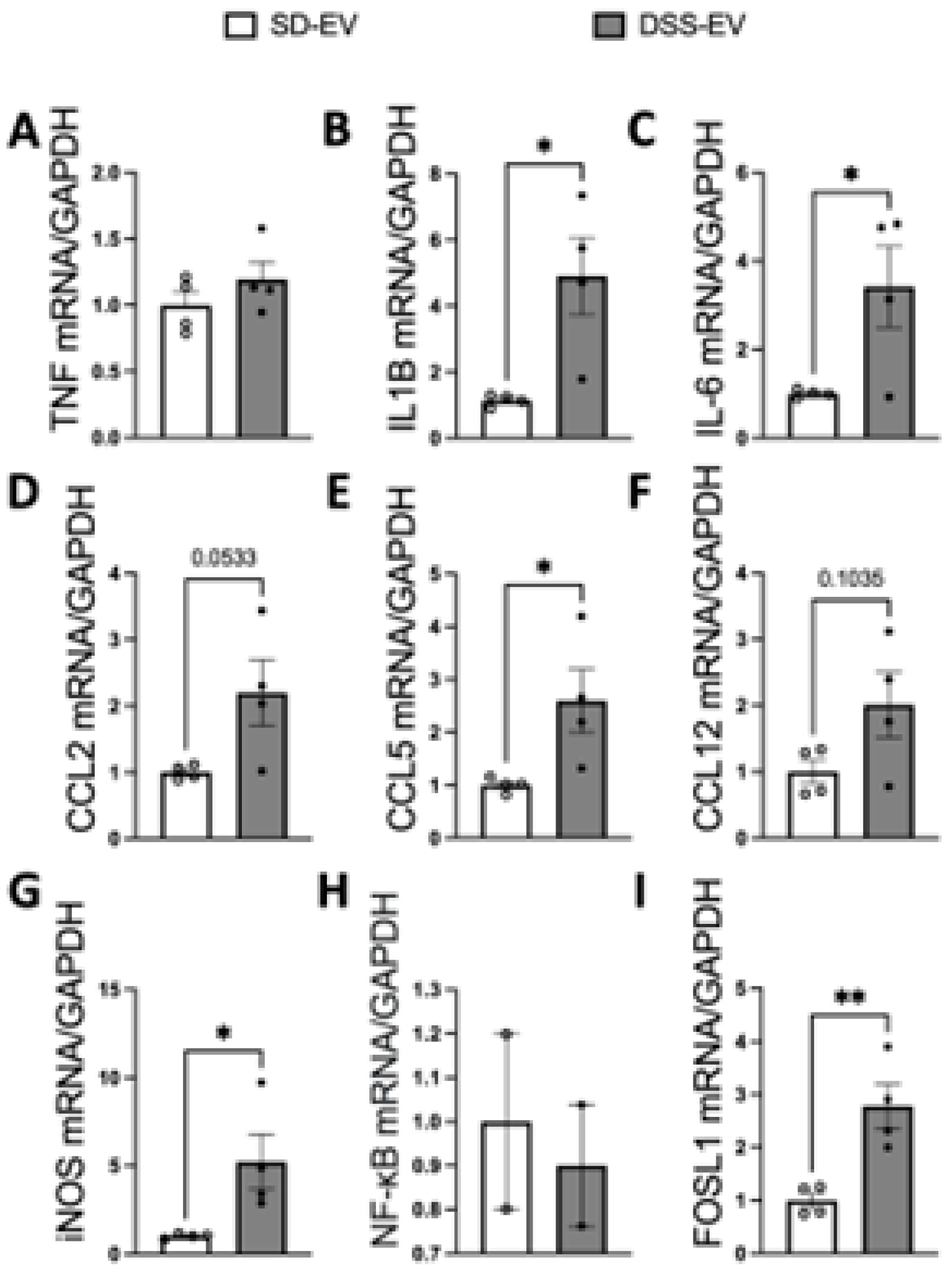
Figure 7.
Brain-derived EVs from DSS hypertensive rats increase mRNA levels of inflammatory cytokines, chemokines and FOSL1 in the PVN of SD rats. SD rats were administrated either DSS-EV (n=4) or SD-EV (n=4) into the right lateral ventricle and brain PVN were punched out 6h after ICV injection. PVN mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and FOSL1 (I) were semi-quantified by real-time PCR and compared between DSS-EV and SD-EV groups. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
Figure 7.
Brain-derived EVs from DSS hypertensive rats increase mRNA levels of inflammatory cytokines, chemokines and FOSL1 in the PVN of SD rats. SD rats were administrated either DSS-EV (n=4) or SD-EV (n=4) into the right lateral ventricle and brain PVN were punched out 6h after ICV injection. PVN mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and FOSL1 (I) were semi-quantified by real-time PCR and compared between DSS-EV and SD-EV groups. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
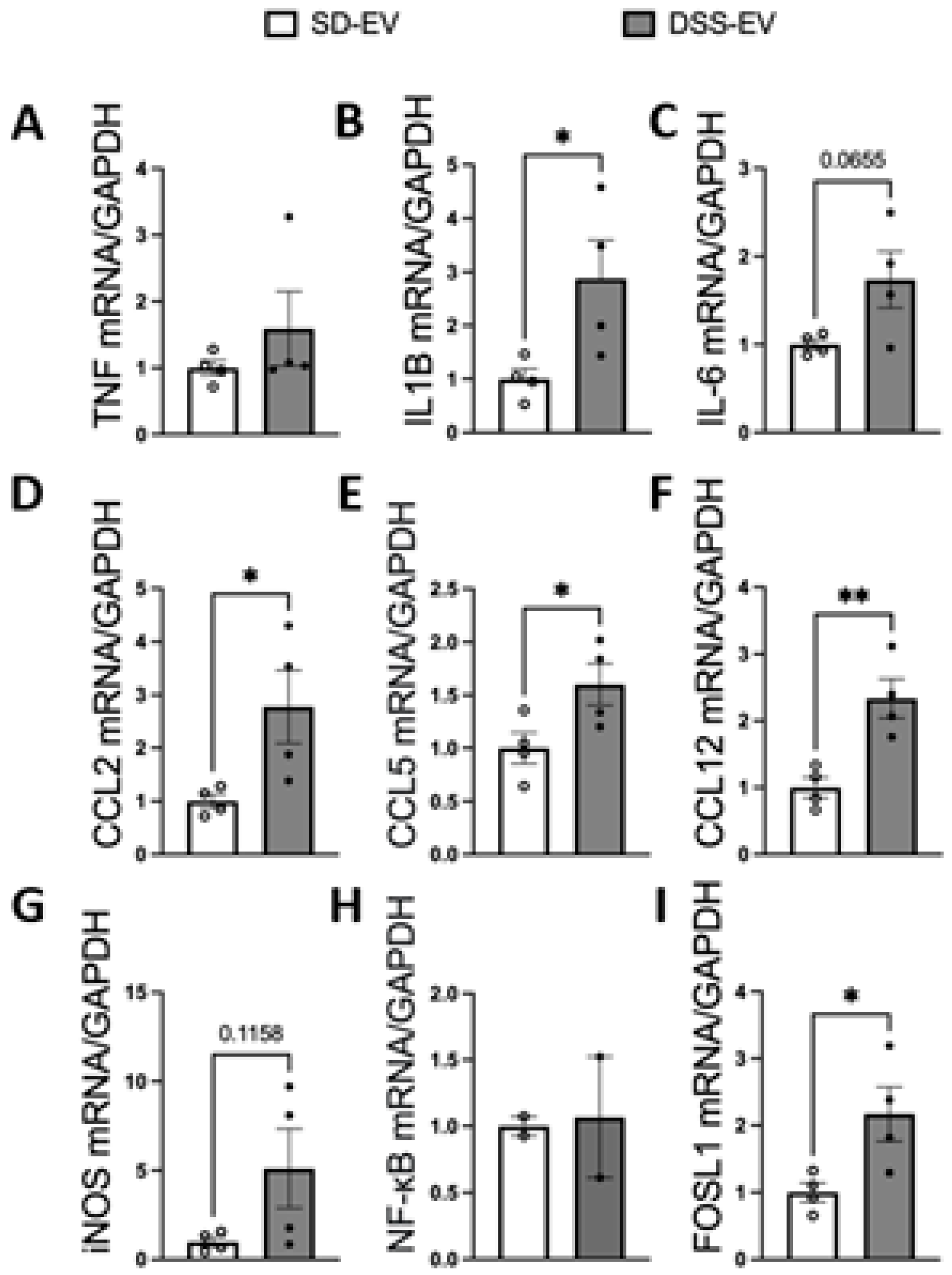
Figure 8.
Brain-derived EVs from DSS hypertensive rats increase mRNA levels of inflammatory cytokines, chemokines and FOSL1 in the LT of SD rats. SD rats were administered either DSS-EV (n=4) or SD-EV (n=4) into the right lateral ventricle and brain LT were punched out 6h after ICV injection. LT mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and FOSL1 (I) were semi-quantified by real-time PCR and compared between DSS-EV and SD-EV groups. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
Figure 8.
Brain-derived EVs from DSS hypertensive rats increase mRNA levels of inflammatory cytokines, chemokines and FOSL1 in the LT of SD rats. SD rats were administered either DSS-EV (n=4) or SD-EV (n=4) into the right lateral ventricle and brain LT were punched out 6h after ICV injection. LT mRNA levels of genes including TNFα (A), IL-1β (B), IL-6 (C), CCL2 (D), CCL5 (E), CCL12 (F), iNOS (G), NF-kB (H) and FOSL1 (I) were semi-quantified by real-time PCR and compared between DSS-EV and SD-EV groups. The mRNA levels were normalized with GAPDH. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01.
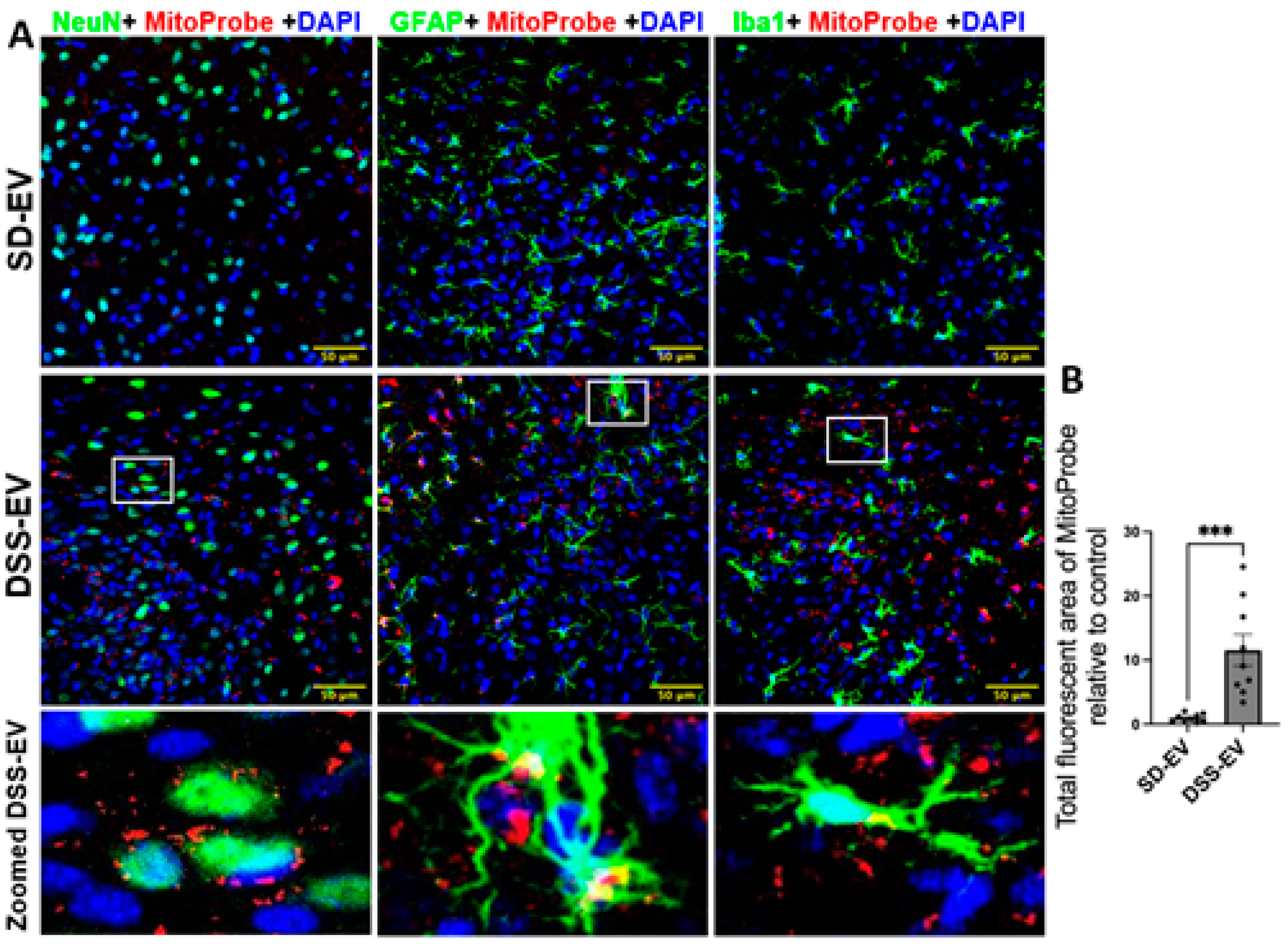
Figure 9.
Brain-derived EVs from hypertensive rats increase mtROS in the PVN of SD rats. (A) Representative images showed PVN ROS levels in different brain cells (NeuN for neurons, GFAP for astrocytes, Iba1 for microglia, all show in green) in SD-EV-treated and DSS-EV-treated rats. Scale bar of the images is 50 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents the average fluorescent area of multiple microscopic views of a PVN region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001. (DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride).
Figure 9.
Brain-derived EVs from hypertensive rats increase mtROS in the PVN of SD rats. (A) Representative images showed PVN ROS levels in different brain cells (NeuN for neurons, GFAP for astrocytes, Iba1 for microglia, all show in green) in SD-EV-treated and DSS-EV-treated rats. Scale bar of the images is 50 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents the average fluorescent area of multiple microscopic views of a PVN region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001. (DAPI: 4′,6-diamidino-2-phenylindole dihydrochloride).
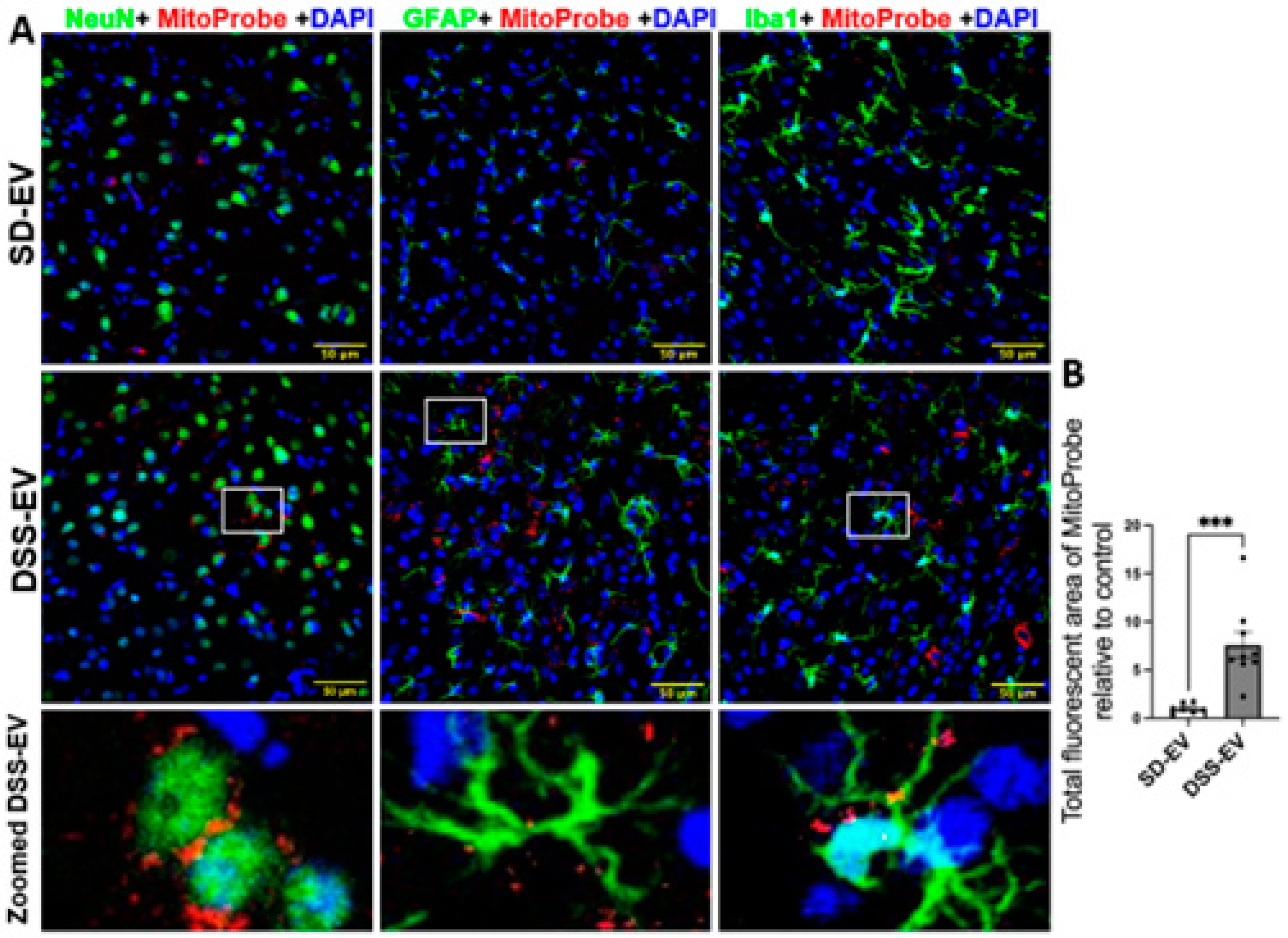
Figure 10.
Brain-derived EVs from hypertensive rats increase mtROS in the LT of SD rats. (A) Representative images showed LT ROS levels in different brain cells in SD-EV-treated and DSS-EV-treated rats. Scale bar of the images is 50 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents the average fluorescent area of multiple microscopic views of a LT region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
Figure 10.
Brain-derived EVs from hypertensive rats increase mtROS in the LT of SD rats. (A) Representative images showed LT ROS levels in different brain cells in SD-EV-treated and DSS-EV-treated rats. Scale bar of the images is 50 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents the average fluorescent area of multiple microscopic views of a LT region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
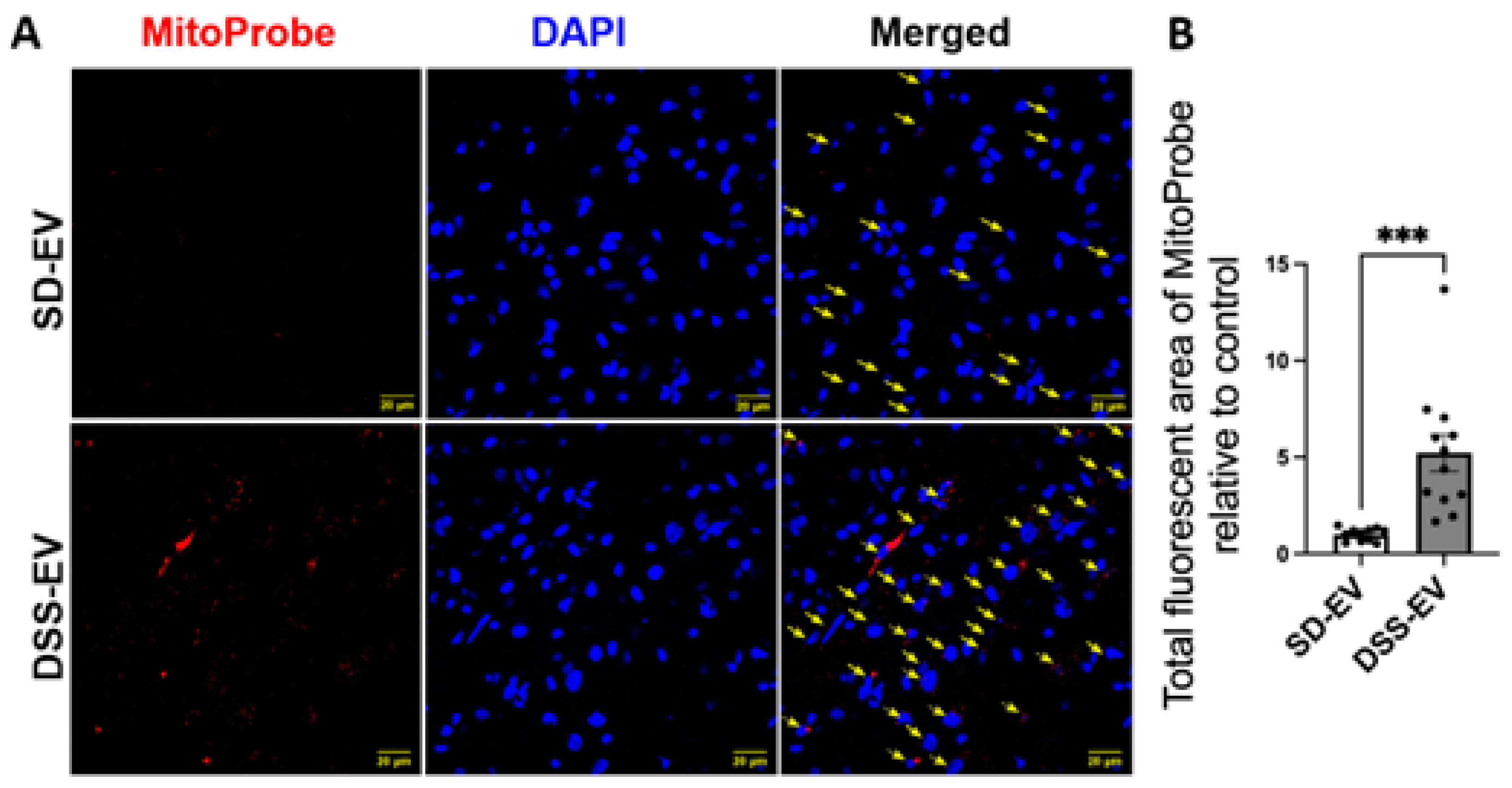
Figure 11.
Brain-derived EVs from hypertensive rats increase mtROS in the PVN of SD rats. (A) Representative images demonstrated ROS fluorescence in the identified PVN region. Yellow arrows indicate mtROS + cells. Scale bar of the images is 20 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents a microscopic view of a PVN region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
Figure 11.
Brain-derived EVs from hypertensive rats increase mtROS in the PVN of SD rats. (A) Representative images demonstrated ROS fluorescence in the identified PVN region. Yellow arrows indicate mtROS + cells. Scale bar of the images is 20 µm. (B) Total fluorescent area of MitoProbe was quantified and normalized to control. Each data point represents a microscopic view of a PVN region. Graphs indicate mean ± SEM. * P< 0.05, ** P<0.01, *** P<0.001.
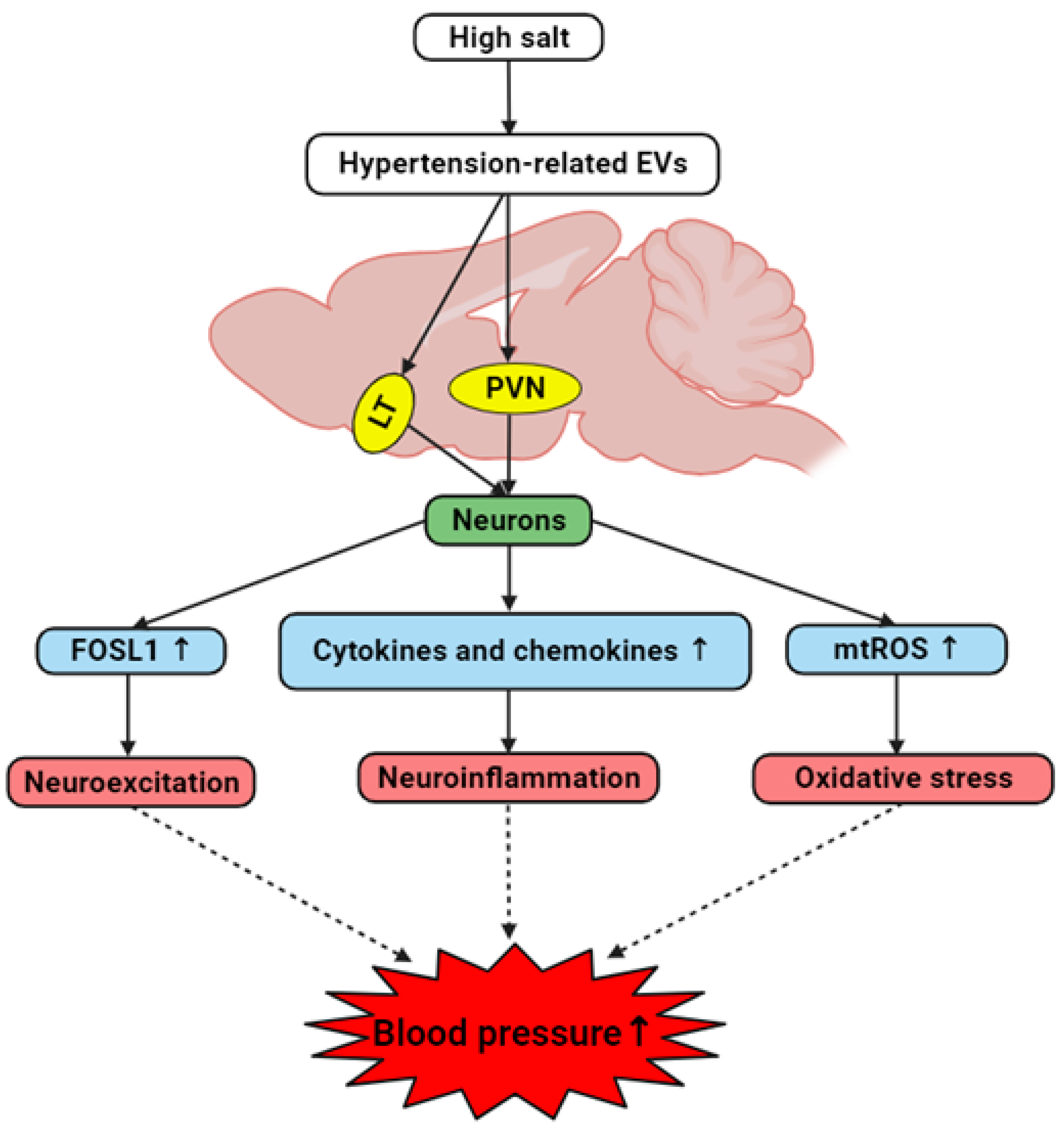
Figure 12.
The hypothesized interplay between brain-derived EVs and hypertension development in hypertensive DSS rats. High salt diet induces the generation of brain-derived EVs containing hypertension-associated factors. These hypertension-related EVs target cardiovascular regions including LT and PVN and trigger neurons to elevate levels of FOSL1, cytokines, chemokines, and mitochondrial ROS. Consequently, this results in neuroexcitation, neuroinflammation and oxidative stress, collectively contributing to the onset and/or progression of hypertension.
Figure 12.
The hypothesized interplay between brain-derived EVs and hypertension development in hypertensive DSS rats. High salt diet induces the generation of brain-derived EVs containing hypertension-associated factors. These hypertension-related EVs target cardiovascular regions including LT and PVN and trigger neurons to elevate levels of FOSL1, cytokines, chemokines, and mitochondrial ROS. Consequently, this results in neuroexcitation, neuroinflammation and oxidative stress, collectively contributing to the onset and/or progression of hypertension.