1. Introduction
The aging process is a natural occurrence that takes place under normal conditions as well as in certain chronic degenerative diseases [
1]. While studies have demonstrated the effectiveness of bioactive natural peptides in mitigating the effects of aging across diverse models, including cell studies, animal studies, and clinical trials [
2], there is limited information regarding the diversity of anti-aging peptides found in marine organisms. This review addresses this gap by presenting recent insights into the anti-aging potential of bioactive peptides sourced from under-utilized marine resources, examining examples that can scavenge free radicals in vitro, inhibit cell apoptosis, extend the lifespan of fruit flies and roundworms, suppress aging in mice, and have protective effects in aging humans. This review additionally delves into the molecular mechanisms that underly these effects, encompassing unregulated oxidase activity, inhibited cell apoptosis and MMP-1 expression, restored mitochondrial function, and regulated intestinal homeostasis [
3]. Finally, the integration of CRISPR technology with marine collagen holds promise for revolutionary anti-aging treatments by leveraging CRISPR’s precise gene-editing capabilities to target aging-related genes, while marine collagen provides supportive properties for enhanced therapeutic effects in areas like skin elasticity and hydration. The findings lay the groundwork for the development of anti-aging nutraceuticals and cosmetic ingredients derived from peptides within underexplored marine organisms [
1].
The extracellular matrix protein collagen is a naturally abundant biomaterial found in all animals, and its unique biochemical and physical properties make it the perfect candidate for various applications in drug development, biomedicine, and nutrition [
4]. Its high biocompatibility, solubility, and tensile strength are just a few of the characteristics that make collagen an ideal biomaterial for medical use [
4]. Recently, collagen-based products have gained popularity due to their acclaimed anti-aging benefits. Functioning as a crucial structural and connective component in systems like the skin, joints, and bones, collagen helps regulate cell growth, adhesion, and migration [
5]. However, the natural degradation of collagen accelerates with age, which can impact skin elasticity, wound healing, bone density, and even neural function [
6]. Thus, it is important to investigate the beneficial effects of collagen treatments on reversing the effects of old age to live longer, healthier lives [
6]. In particular, the application of marine collagen peptides (MCPs) emerges as a promising yet underexplored avenue, displaying remarkable therapeutic potential in delaying aging according to multiple animals and in vitro studies [
7]. Marine organisms are a rich source of high-quality functional proteins and peptides with diverse molecular structures. Accordingly, countries with extensive coastlines such as China can especially benefit from harvesting these valuable and abundant sources of novel bioactive compounds [
7]. While fish are widely used as food resources, there is limited utilization of marine protein resources from other species such as sea cucumber, sea urchin, mussels, and various kinds of algae. Some marine bioactive peptides have been found to regulate free radical homeostasis both in vitro and in vivo, and have shown anti-aging effects in cell, animal, and human clinical trial models [
7]. In addition, marine peptides are also commonly utilized in cosmeceutical skin products for their anti-aging properties.
Table 1 displays the 5 common types of collagens along their functions. Marine collagen is predominantly type I collagen, which is the primary component of the calcium-depleted tissue of the teeth and bone. It is found in the skin, in tendons, in the vasculature of the lungs, and in the heart [
4].
Table 1 significantly highlights the use of porcine collagen (collagen type I and type III), which is essential in the prevention and treatment of osteoporosis [
4].
Our literature review explores the anti-aging activities of peptides from marine organisms, focusing on their capacity to regulate oxidative stress in diverse models including cells, fruit flies, nematodes, mice, and humans [
1]. In the present study, we conducted a literature search using the keywords "peptide" and "anti-aging" or "anti-aging" from several databases including PubMed, Cochrane Library, Web of Science, and Embase, covering publications from 1956 to 2023 [
1]. The identified studies were screened for their relevance to underused marine resources. By analyzing the findings of these papers, we aim to contribute valuable insights that help enhance the utilization of marine sources for anti-aging applications [
8]. However, limitations regarding the lack of long-term studies may hinder its potential use. Thus, this review highlights the applications and limitations of anti-aging marine collagen research while outlining future directions for this field [
6].
2. Marine Collagen: Effects on Skin Aging
The anti-aging industry is growing rapidly with the release of new supplements and nutraceuticals that promise youthful skin, better joint and bone health, and even stronger immune systems [
9,
10]. Among the most popular products is marine-derived collagen used for skin health and restoration. Several studies have cited the effects of collagen supplementation on skin appearance.
A recent study by Lee et al (2022) investigated the importance of collagen formulation on anti-aging efficacy [
11]. Only compounds with low molecular weights may penetrate the skin barrier, limiting the efficacy of intact collagen application, and oral administration of collagen bioactive peptides is limited by their poor stability and absorption in the gastrointestinal tract [
11]. Thus, to increase absorptive ability, the fish collagen was hydrolyzed into small, bioactive collagen peptides and administered as an oral disintegrating film which allows the collagen to be directly absorbed into the bloodstream [
11]. This type of delivery system avoids the systemic metabolism of the active compounds, keeping the active drug concentration high.
After applying the film for 12 weeks, the authors concluded that fish-derived collagen administered as an oral disintegrating film was effective at significantly increasing type I collagen expression and absorption, reducing skin wrinkle depth and number, and increasing skin elasticity and density in women ages 20 to 60 years old [
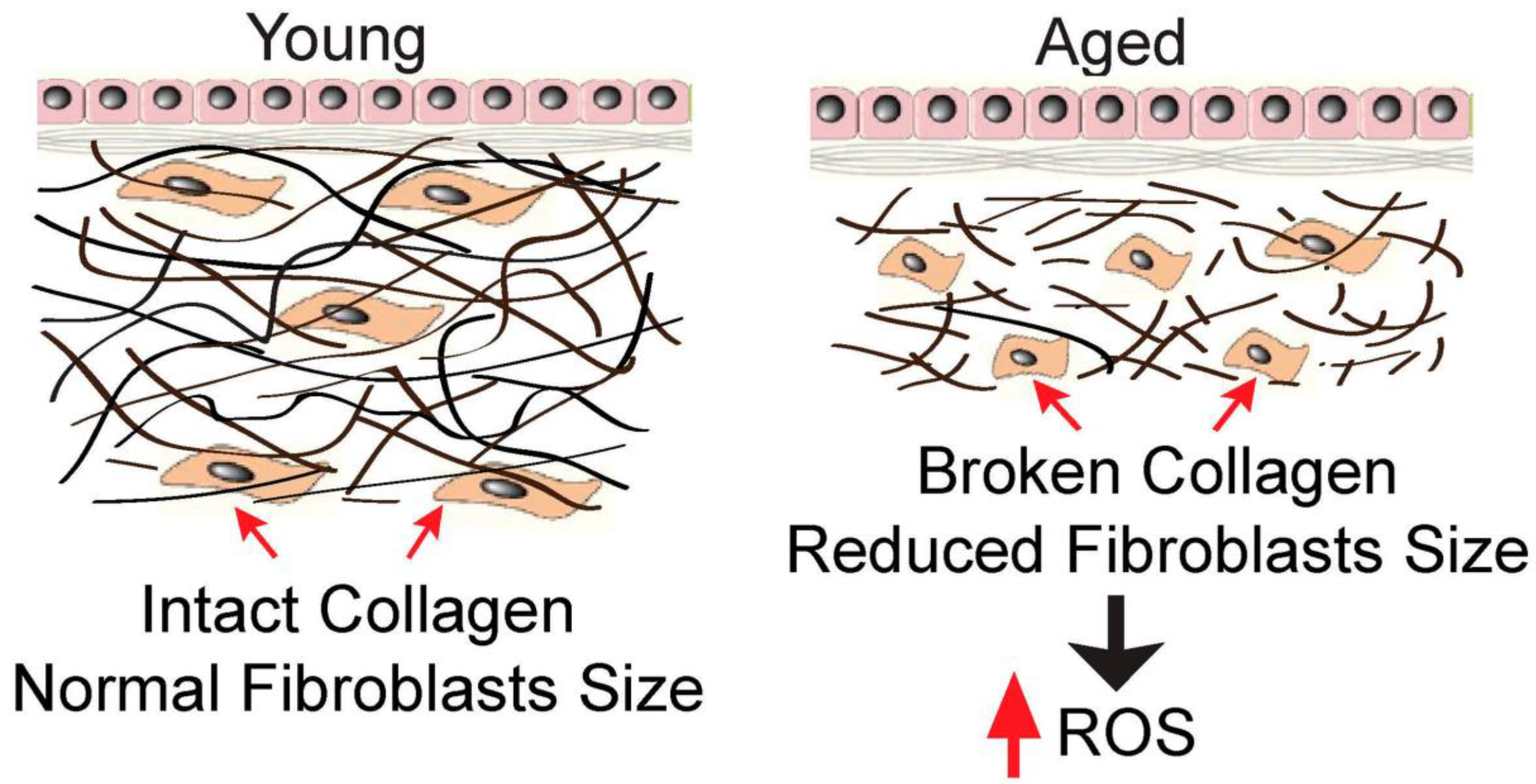
11]. As evident in
Figure 1 [
12], as an individual ages, a number of changes occur in the density and structure of collagen fibres [
12].
Figure 1 [
12] displays a decline in the number of collagen fibrils and the size of the fibroblast cells as the skin ages, emphasizing the importance of collagen fibrils in the maintenance of cell size and skin elasticity for healthy skin. The aged human skin is observed
in vivo which is one of the driving forces of oxidative stress and increased reactive oxygen species (ROS) levels in aged skin [
12].
3. Marine Bioactive peptides: Antioxidant and Anti-Carcinogenic roles
On top of providing valuable sources of nitrogen and amino acids, many bioactive MCPs have demonstrated powerful antioxidant, anti-microbial, and immunomodulatory effects. Several studies have investigated the potential role of MCPs in slowing down senescence and consequently delaying the onset of free radical damage, metabolic and cellular dysfunction, and other genetic changes that majorly increase the risk of developing chronic diseases and disabilities with age [
9]. The active peptide products isolated from fish, sea cucumber, urchins, muscles, and other marine life have shown the potential to lower oxidative stress, inhibit cellular senescence, and extend lifespans in multiple animal studies of ageing [
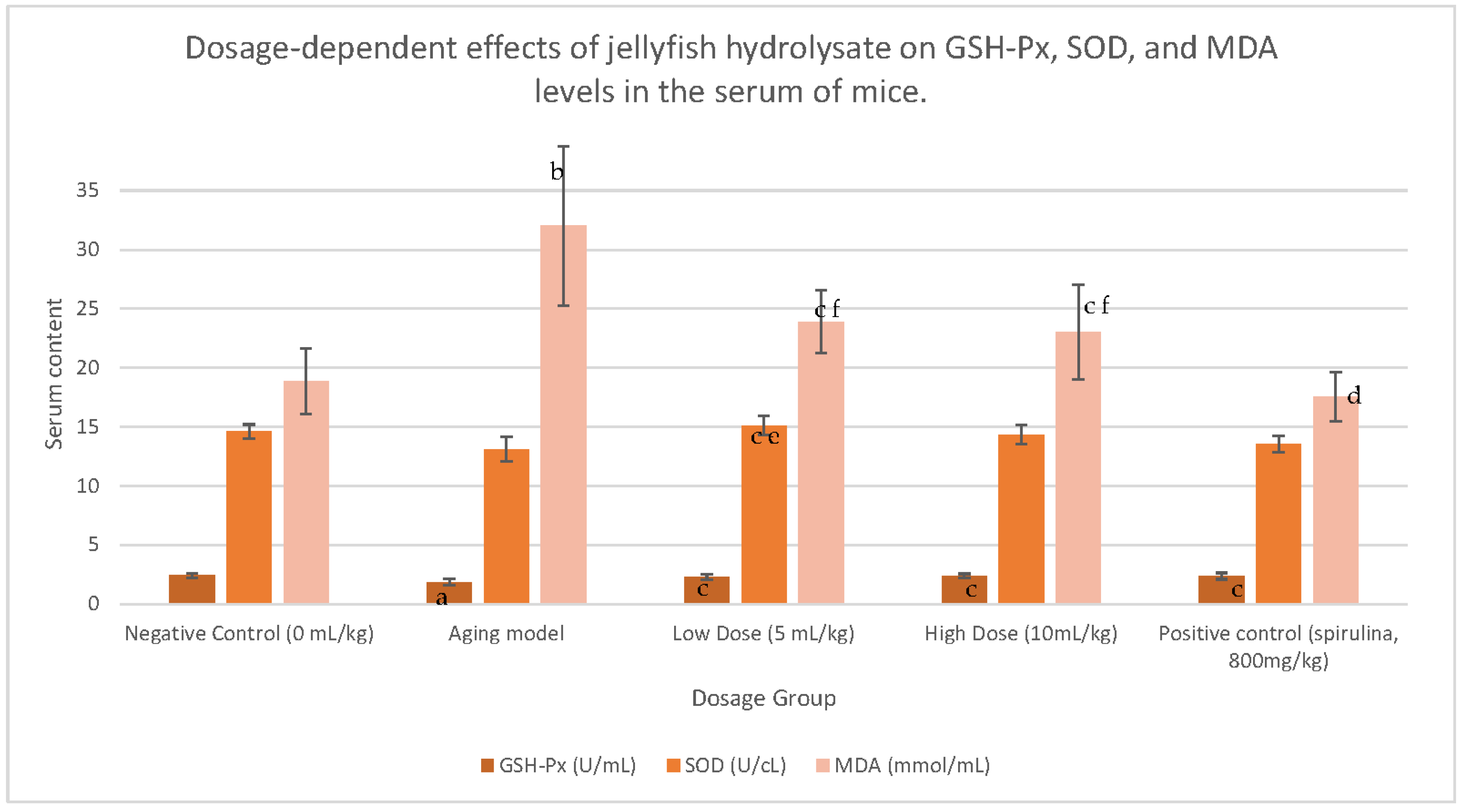
9]. For example, one study found that jellyfish collagen hydrolysate (JCH) improved the exercise tolerance of mice in a dose-dependent manner after 6 weeks [
14]. In the same study, the authors used d-galactose to induce the ageing process in mice, then investigated the effect of JCH on oxidative stress by measuring malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activity [
14]. MDA is a product of lipid peroxidation, which increases with age, whereas SOD and GSH-Px are enzymatic antioxidant systems that neutralize free radicals implicated in the ageing process, wherein activity declines with age [
15]. Significantly, 6-week administration of JHC inhibited the decrease in GSH-Px/SOD activity and the increase in MDA seen in the ageing model [
14]. These results are displayed in
Figure 2 [
14], showcasing the powerful in vivo antioxidant capacity of marine peptides and demonstrating their benefit in anti-aging products.
Similarly, Liang et al (2010) discovered that rats fed chum salmon MCPs over the course of their lifespan showed increased GSH-Px and SOD enzymatic activity compared to control rats; however, this change was only significant in rats older than 12 months [
15]. Further on, the observed age-related MDA increase was attenuated in MCP-treated rats, suggesting that MCPs can interfere with the cellular and physiological effects of ageing by exerting antioxidant effects. Significantly, this study also demonstrated that MCP-treated rats on average had longer life spans and better survival outcomes after 28 months [
15]. MCP treatment also delayed tumour growth, decreased tumour size and number and lowered the incidence of deaths from tumours compared to control after 16 months [
15]. Genetic mutations increase in frequency with age, predisposing the cells to various oncogenetic processes; thus, MCP may act in a protective, anti-carcinogenic capacity to slow the progression of aging-related diseases such as cancer. Taken together, these findings suggest that marine collagen may exert anti-fatigue and antioxidant capabilities that interfere with the aging process, leading to longer, healthier lives [
6]. Other examples of bioactive peptides are bio active mussel polypeptide (MP) and fish protein hydrolysate (FPC), immunomodulatory foods that can enhance non-specific host defence mechanisms. To test this, mice were given FPC for 7 days which showed an activation of the immune response [
1]. Another element of collagen is RSH, which helps cells modulate antioxidant and neurotrophic pathways to improve memory, hormonal function and anti-coagulant activity [
1]
4. Marine Collagen in Tissue Engineering for Anti-aging
Marine collagen is recognized for its bioactive properties and is used in skin tissue engineering due to its water solubility, metabolic compatibility, and accessibility. It has shown effectiveness in healing skin injuries of varying severity and in delaying aging processes, promoting keratinocyte and fibroblast migration, and vascularization of the skin. In animal model studies, marine collagen from different species has shown promising results in skin tissue healing [
16,
17]. Treatments using marine collagen have led to increased deposition of granulation tissue, enhanced re-epithelialization, stimulated neoangiogenesis, and improved the morphological aspect of wounds [
17]. These findings underscore its potential in tissue engineering and wound healing applications [
16,
17]. The marine environment has been a significant source of biological macromolecules for developing tissue-engineered substitutes with wound-healing properties. These molecules play a key role in enhancing the wound-healing process and are crucial in advancing wound-care management [
10]. Moreover, studies on collagen-derived peptides from the marine sponge
Chondrosia reniformis reveal their antioxidant activity, ability to stimulate cell growth, and protection against UV-induced cell death [
10]. These peptides have shown no toxicity in cell lines, and their significant ROS scavenging activity indicates their potential in drug and cosmetic formulations, especially for damaged or photoaged skin repair [
10].
Marine collagen’s role in bioprinting and scaffold development is pivotal for tissue regeneration. Marine collagen, particularly type I, is ideal for creating 3D bioprinted structures due to its biocompatibility, biodegradability, and low immunogenicity [
18,
19]. These structures mimic the natural extracellular matrix of human tissues, which is essential for effective tissue regeneration. In tissue engineering scaffolds, marine collagen offers a structure that supports cell attachment, proliferation, and differentiation, key factors for tissue repair and regeneration [
19]. These properties of marine collagen are particularly significant in anti-aging applications, as they support the growth and repair of various tissues, including skin, bone, and cartilage. As aging is associated with the degradation of these tissues, marine collagen scaffolds can be used to replace or support damaged tissues, thereby counteracting some of the effects of aging. Ongoing research is optimizing marine collagen properties for bioprinting and scaffold design, enhancing its mechanical strength, stability, and compatibility with human tissues. The development of hybrid scaffolds, combining marine collagen with other biomaterials, is also an area of interest to improve functionality and efficacy in tissue regeneration [
13,
19,
20,
21].
5. The Integration of CRISPR Technology with Marine Collagen
The integration of CRISPR technology with marine collagen is an innovative area of research, combining the genetic editing capabilities of CRISPR with the beneficial properties of marine-derived collagen. Marine collagen has shown promise as a biomaterial in various applications, including wound healing, skin anti-aging, and bone regeneration. Its biocompatibility makes it an excellent candidate for tissue engineering and regenerative medicine. On the other hand, CRISPR technology offers a groundbreaking approach to gene editing, allowing for precise modifications at the DNA level. This technology has been making strides in various medical applications, including the development of more refined editing techniques like base and prime editing. These newer methods aim for uniform and predictable gene-editing results while minimizing potential risks associated with traditional CRISPR-Cas9 techniques, such as the creation of double-strand DNA breaks [
22,
23,
24].
The integration of these two fields could lead to revolutionary anti-aging treatments. For instance, CRISPR technology could be employed to target and modify specific genes associated with aging and skin degeneration. By precisely editing these genes, it might be possible to slow down or reverse certain aging processes at a molecular level. Meanwhile, marine collagen could play a supportive role in this integration. Its ability to enhance cell viability and support tissue regeneration could be crucial in facilitating the effectiveness of CRISPR-mediated gene edits. For example, in a scenario where CRISPR is used to edit genes related to skin elasticity, marine collagen could provide the necessary extracellular matrix support, enhancing the overall therapeutic effect.
Another potential approach to integrating CRISPR with marine collagen could focus on enhancing skin hydration and barrier function. This would involve using CRISPR to edit genes crucial for maintaining skin moisture, such as those involved in hyaluronic acid synthesis. Concurrently, marine collagen could be developed as a topical delivery system for CRISPR components, leveraging its skin absorption properties and biocompatibility. This could involve encapsulating CRISPR components (like Cas9 and guide RNA) within marine collagen-based nano-carriers that can penetrate the skin layers.
While direct research on the integration of CRISPR technology with marine collagen in anti-aging is still in its infancy, the combination of CRISPR’s precision in genetic editing and marine collagen’s supportive properties presents a highly promising avenue. Future research in this area could lead to innovative and effective anti-aging therapies, potentially revolutionizing the way we approach aging and skin health.
6. Marine Collagen Use: The Pros and Cons
The extracellular matrix (ECM) plays a fundamental role in ensuring cell integrity and aiding in various cell functions, such as proliferation, differentiation, migration, and adhesion [
20]. Marine organisms (
Figure 3), including fish, jellyfish, sponges, and other invertebrates, provide a valuable source of collagen that is free from religious restrictions and animal pathogens. This type of collagen is metabolically compatible and has advantages over other sources [
20]. Fish skin is often used to extract type I collagen because it is abundant and not suitable for industrial use. Overall, marine sources of collagen are a safe, convenient, and promising option. The combination of biomaterials and single gene delivery has shown promising potential for tissue engineering. Studies have found that marine collagen from organisms like fish, jellyfish, and sponges can promote wound healing, enhance blood circulation, and prevent infection [
20]. Additionally, marine collagen has anti-aging properties that have been demonstrated in mice with osteoporosis [
20]. It can increase bone mineral density, protect against bone loss and osteoarthritis, induce plastic differentiation, and even improve skin elasticity while slowing the aging process [
20]. Finally, drug delivery and immobilization are two ways marine collagen is used within the human body [
20]. Some of the advantages of using marine collagen over other sources of collagen, such as popularly used bovine or porcine collagen. Collagen is a topic that is talked about many for its advantages and disadvantages. One of the key advantages of collagen is resource abundance, as marine collagen is derived from the massive amounts of marine waste produced by the fishing industry, helps reduce environmental contamination while reducing waste, providing high yields and cheap production. Marine collagen also presents with a higher biocompatibility/no disease transmission risk - as marine collagen is free of the same pathogen transmission risk that mammalian collagen confers, in which case incidences of bovine spongiform encephalopathy (BSE) from the transmission of prions were observed, marine collagen can be seen as safer [
25]. Safety is among the primary considerations when evaluating the advantages and disadvantages of marine collagen. As discussed, marine collagen proves to be a safer alternative to land animal-derived collagen in terms of reduced transmission of disease. However, marine collagen sources, such as fish and marine sponges, carry the possibility of allergens and toxicity [
26]. The level of allergenicity varies depending on the type of fish or fish product from which the collagen is derived [
26]. Likewise, the threat of toxicity is more apparent among certain sources such as jellyfish and sponges [
26]. To reduce the likelihood of adverse effects on human usage and consumption, the extraction and purification of marine collagen must comply with regulations that ensure the safety and consistency of such products [
26]. The anti-aging effect of marine collagen can be evident throughout the body both externally and internally. Externally, through reversing the aging effects of the skin, and internally, through regulating bone health, tissue regeneration, and dietary and metabolic processes. Together, these effects facilitate improved overall health, skin appearance, and well-being.
6.1. Hydration
Hyaluronic acid plays an important role in skin moisture retention, and aging skin experience [
28]. Collagen hydrolysates, restore the production of hyaluronic acid which is rich in proline-hydroxyproline when administered orally. It has been shown that oral collagen improves skin hydration. Fish collagen is found to be the optimal source, with diverse amino acid compositions and high bioavailability [
28]. Marine collagen and collagen peptides, especially from fish, have demonstrated significant effects on skin hydration. Limited research on chicken-derived collagen suggests potential benefits, but more studies are needed for conclusive results [
29].
6.2. Elasticity
The primary collagen in the skin is Type I collagen, leading to structural integrity and strength. Elastin and microfibrils in the elastic fabric network relay elasticity and resilience to the skin. Consuming oral collagen has been expressed to improve skin elasticity, resulting in increased levels of type I collagen [
28]. Numerous studies exhibit positive effects on skin elasticity, including improvements in surface elasticity [
28]. Collagen peptides have been found to increase collagen content and improve skin laxity in a variety of animal and human studies. There are limitations to this research however, like contrast in the duration and forms of amounts, small sample size, and relying on skin elasticity measurements that were self-reported [
30].
While marine collagen unveils multiple advantages, it also introduces detrimental disadvantages. Cross-linking methods can be physical treatments like UV irradiation and gamma irradiation treatment, which results in a stable collagen matrix with no cytotoxicity [
29]. Fishing industries in North America are highly productive as they produce an ample amount of waste during processing that gets repurposed for marine collagen extraction and development, hence decreasing environmental pollution and reducing costs [
16,
31]. Marine-derived peptides also have better chemical and physical/mechanical durability compared to mammalian counterparts [
16].
7. Collagen Peptides: Promising for Osteoporosis Treatment and Prevention
Marine collagen biopharmaceuticals play a crucial role in encouraging cartilage regeneration, contributing to enhanced skin and bone health [
20]. Osteoarthritis (OA) is a condition caused by irreversible cartilage degradation, characterized by joint pain and stiffness. Exposure of subchondral bone further diminishes the quality of life for those with OA [
20]. Nonetheless, research has demonstrated that marine collagen may promote cartilage regeneration by inducing chondrogenic differentiation [
20]. Studies by Raabe et al. have demonstrated that the growth factor TGFB1 and hydrolyzed fish collagen can promote the synthesis of collagen fibers and proteins, and that fish collagen in particular can cause chondrogenic differentiation [
20]. The study investigated the effects of three collagen hydrolysates on fish skin and cartilage chondrocytes [
20]. In one experiment, the levels of collagen I and collagen II were modified by collagen hydrolysate at concentrations of 0.5, 50, and 100 µg/mL [
20]. Collagen use also resulted in a decrease in the expression of protein markers like Cox2, Mmp103, and Adamts5. These indicators have been linked to the development of OA [
20].
8. Collagen as a Biomaterial for Tissue Engineering
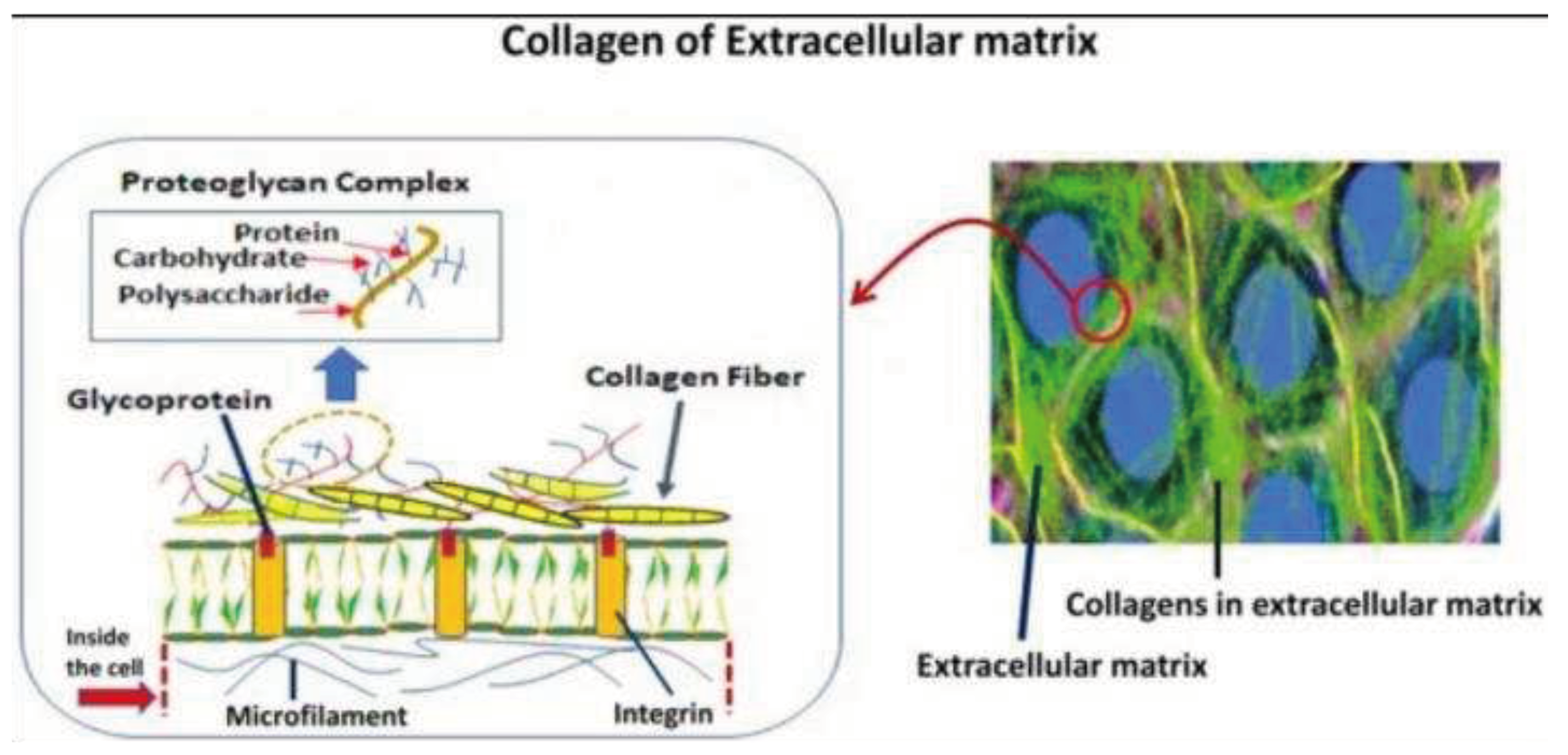
One of the most common and prominent biomaterials in tissue engineering and regenerative medicine is collagen, such as collagen proteins in the extracellular matrix of marine invertebrates (
Figure 4). Although fish collagen peptides (FCP) have been used as a dietary supplement, little is known about how they affect cellular function in the human body [
32]. Extracellular matrix (ECM) replacements can significantly affect cell proliferation and function based on recent research [
3,
33]. These extracellular matrixes, however, are mainly used in a general sense and are not yet tailored to certain cell types [
33]. This paper focuses on ECM-based coating substrates tailored to the individual needs of skin, skeletal muscle, and liver cell cultures [
33]. With ongoing advancements, neural tissue engineering (NTE) indicates significant potential to treat a number of debilitating neurological illnesses [
4]. For NET design strategies that facilitate axonal growth and neural and non-neural cell differentiation, choosing the best scaffolding material is essential. As the nervous system is naturally resistant to regeneration, collagen is often used in NTE applications [
4]. It can function with neurotrophic factors, neural growth inhibitors, and other compounds that promote neural growth [
4]. It can also be used for neural repair and thus mitigate neurodegenerative diseases that come with age [
4]. Time and time again it has become clear that ECM is a powerful structure that influences the cells in contact with it [
34]. A poor prognosis has been linked to the composition and collagen density of the tumour-specific extracellular matrix (ECM) in a number of cancer forms. The cause of this correlation is still mainly a mystery [
34]. Collagen can stimulate the development and migration of cancer cells, although collagen has been found in recent research to influence the activity and phenotype of T cells and tumour-associated macrophages (TAMs), two types of immune cells that infiltrate tumours [
34].
9. Applications of Marine Collagen in the Cosmetic Market
The cosmeceutical industry is flooded with a variety of anti-aging products that claim to address wrinkles, fine lines, and other signs of aging through various mechanisms of action. Some of the most popular products on the market include retinoids such as retinol, retinyl esters, and retinaldehyde - vitamin A derivatives known for their ability to stimulate collagen production and promote cell turnover, thereby increasing skin elasticity and reducing the appearance of fine lines and wrinkles [
26]. Alternatively, vitamin C- and vitamin E-based serums provide anti-aging benefits through their powerful antioxidant effects that protect the skin against UV-induced photodamage and oxidative stress [
26]. Vitamin C stimulates collagen synthesis, and both vitamins have anti-inflammatory functions that aid in wound healing. Another widely used anti-aging ingredient is hyaluronic acid, a hydrating glycosaminoglycan (GAG) that can act as a barrier against trans-epidermal water loss to help retain skin moisture and reduce the appearance of fine lines. Other popular products include alpha hydroxy acids (AHAs i.e. lactic acid, glycolic acid, citric acid) and beta hydroxy acids (BHAs i.e. salicylic acid) that exfoliate the skin, promote cell turnover, and facilitate GAG and collagen synthesis to improve skin texture and tone [
26,
27]. Finally, there are numerous bioactive peptide formulations that can help stimulate collagen production and improve skin elasticity [
26]. For example, extracts from brown algae have proven a plentiful resource for anti-inflammatory and antioxidant compounds. Because of their photoprotective properties, these bioactive peptides can be used in cosmetic preparations for anti-aging skincare and sunscreen [
35]. In one study, the brown algae,
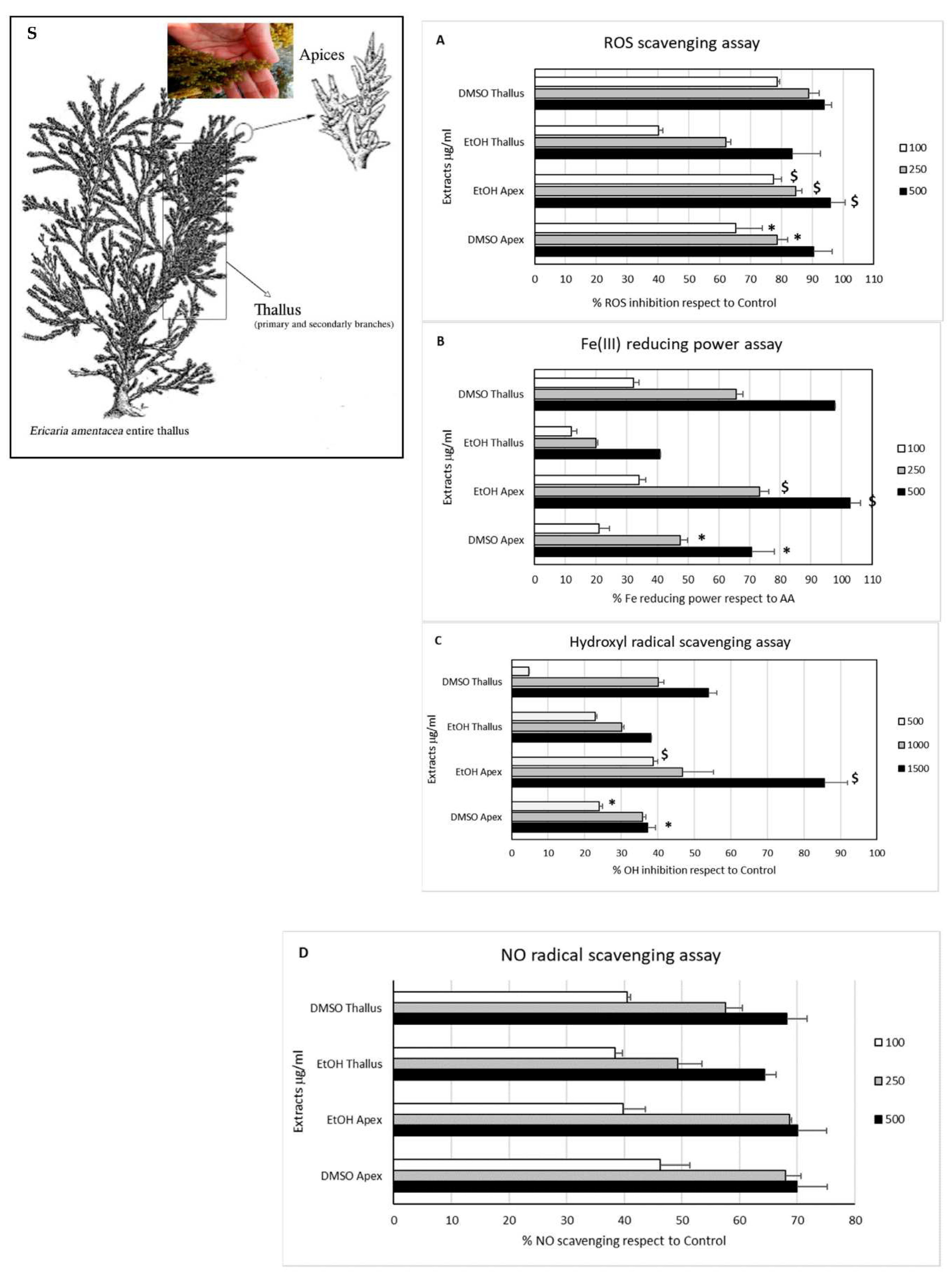
Ericaria amentacea, showed dose-dependent in vitro activity for reducing various markers of oxidative stress, inflammation, and collagen and hyaluronic degradation. The results of various antioxidant assays are displayed in
Figure 5 [
35], demonstrating remarkable potential for anti-aging cosmetic applications.
While these products demonstrate numerous anti-aging benefits, there are associated drawbacks that may limit their efficacy and applicability. For one, prescription-strength retinoid formulations and AHAs may induce adverse reactions such as skin irritation, burning, and dermatitis [
26]. In addition, the oxidation of retinol, vitamin C, and vitamin E over time poses a problem for the stability of the product, which can affect the overall quality and efficacy of the cosmetic preparation [
26]. Further on, in rare cases, topical application of vitamin E has been linked to cases of contact dermatitis, erythema multiforme, and xanthomatous reaction [
26]. As a result, recent trends in the anti-aging industry have demonstrated rising consumer interest in the natural bioactive compounds found in marine collagen rather than synthetic ingredients. A recent study of the Portuguese anti-aging cosmetic market revealed a 27% increase in marine collagen cosmetics from 2011 to 2018, with red algae being the most widely used marine ingredient [
36].
A potential explanation for the increasing popularity of marine collagen products may lie in the manufacturing, safety, and efficacy advantages that they provide. For one, marine sources are biodiverse, abundant, and easy to cultivate and modulate during their life cycles. These characteristics make it possible to harness the production of specific bioactive compounds involved in collagen synthesis and wound healing [
27,
37]. The highly productive fishing industry in North America produces a lot of waste during processing; as demonstrated by
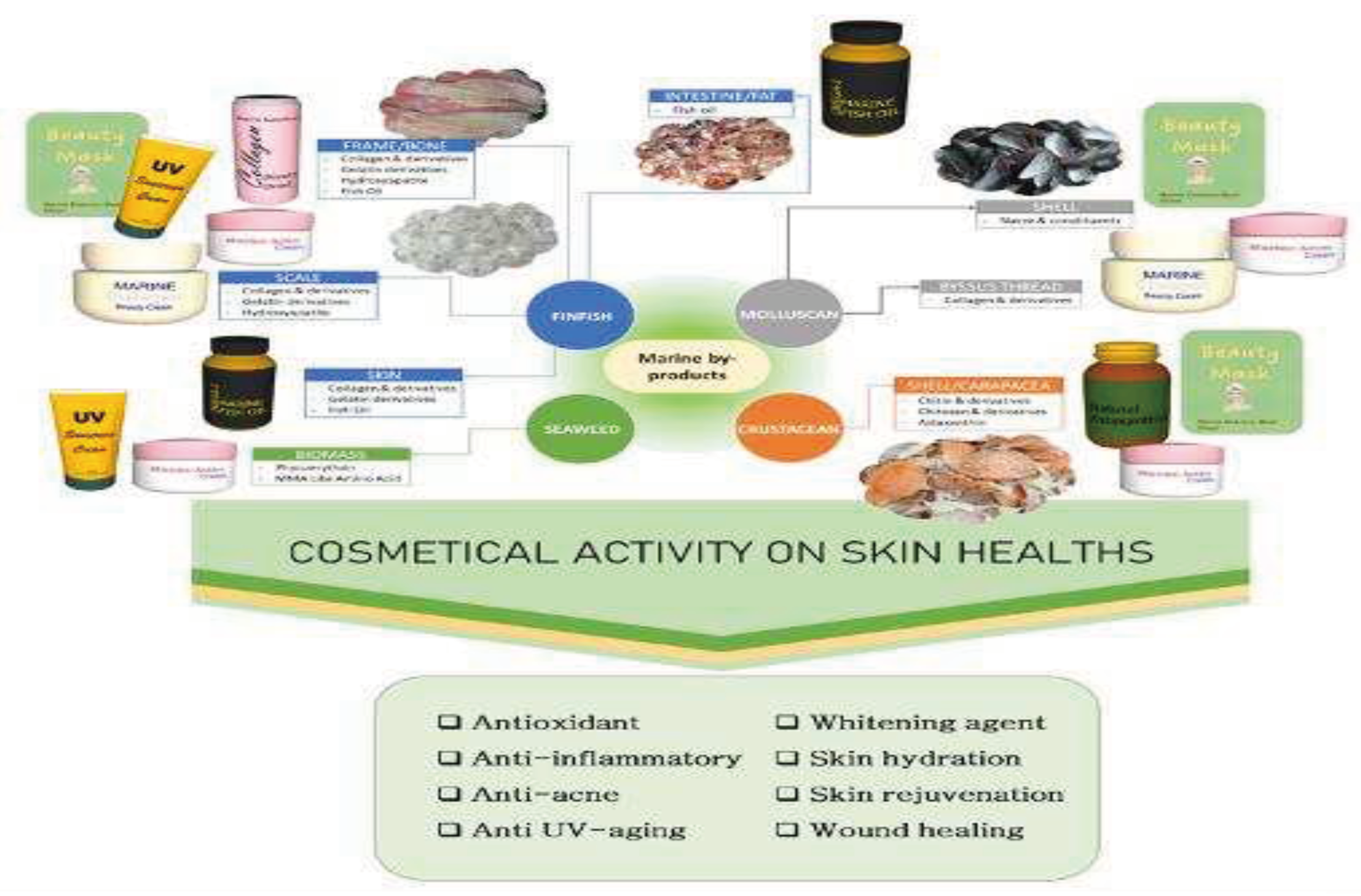
Figure 6 [
27], this marine waste can be repurposed for marine collagen extraction and development, thus reducing environmental pollution and keeping production costs low [
27]. Furthermore, marine collagen is highly bioavailable, considering it is easily absorbed by the body and efficiently utilized for collagen synthesis. Marine collagen also offers several other advantages over mammalian-derived collagen, such as better biocompatibility (less antigenic), better biodegradability and water solubility, and better safety profile (free of the risk of mammalian pathogen transmission) [
36]. Marine-derived peptides also have better chemical and physical/mechanical durability compared to mammalian counterparts [
27].
It is important to note that while marine collagen has a wide range of benefits, the effectiveness of any anti-aging product can vary depending on individual genetic and environmental factors. Thus, other anti-aging products, such as those containing retinoids, hyaluronic acid, antioxidants, and other beneficial compounds may be more effective for specific skin types and concerns.
10. Concluding Remarks
This comprehensive literature review highlights the diverse biomedical anti-aging applications of marine collagen, establishing it as a superior biomaterial in tissue engineering and regenerative medicine. Marine collagen’s unique properties, such as promoting osteogenesis, collagen synthesis, and anti-inflammation, highlight its pivotal roles in accelerating the healing process, preventing signs of aging, and treating degenerative conditions such as osteoporosis. Compared to land animal sources, advantages like metabolic compatibility, safety, and environmental sustainability further position marine collagen as a compelling choice for anti-aging applications. Utilizing readily available marine waste from the fishing industry ensures cost-effective production and addresses environmental concerns. Marine collagen also proves valuable in vascular tissue engineering, demonstrating promise in crafting advanced scaffolds for vascular grafts, enhancing mechanical strength, and fostering vascular endothelial cell development. Ongoing research and innovation efforts focused on marine collagen extraction, processing, and application, as well as synergy with anti-aging CRISPR technology underscore its continued importance in advancing the fields of tissue engineering and biomedicine. The pressing need to discover further anti-aging applications of diverse marine collagen sources emphasizes their significance and utility in marine health and science, and further research efforts should continue to explore this biomaterial’s remarkable therapeutic efficacy and versatility.
Author Contributions
Conceptualization, A.R.; validation, A.R.; writing—original draft preparation, R.R., D.G.P., M.H., S.P., M.A. and A.R.; writing—review and editing, A.R., D.G.P. and R.R.; visualization, D.G.P., R.R, S.P. and A.R; supervision, A.R.; project administration, A.R.; funding acquisition, A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xia, E.; Zhu, X.; Gao, X.; Ni, J.; Guo, H. Antiaging Potential of Peptides from Underused Marine Bioresources. Mar. Drugs 2021, 19, 513. [Google Scholar] [CrossRef] [PubMed]
- Ao, Haiyong., Yang, SHengbing., Nie, Bin’en., Fan, Qiming., Zhang, Quanchao., Zong, Jiajia., Gus, Shegrong., Zheng, Xuebin., Tang, Tingting. Improved antibacterial properties of collagen I/hyaluronic acid/quaternized chitosan multilayer modified titanium coatings with both contact-killing and release-killing functions. J. Mater. Chem. B, 2019,7, 1951-1961.
- Rahman, M.A. Collagen of Extracellular Matrix from Marine Invertebrates and Its Medical Applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. (2021). A Review of the Effects of Collagen Treatment in Clinical Studies. Polymers, 13(22), 3868. [CrossRef]
- 5. Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991, 276,307-13. [CrossRef]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef] [PubMed]
- Aziz, J.; Shezali, H.; Radzi, Z.; Yahya, N.A.; Abu Kassim, N.H.; Czernuszka, J.; Rahman, M.T. Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin. Skin Pharmacol. Physiol. 2016, 29, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Elango, J.; Robinson, J.; Zhang, J.; Bao, B.; Ma, N.; de Val, J.E.M.S.; Wu, W. Collagen Peptide Upregulates Osteoblastogenesis from Bone Marrow Mesenchymal Stem Cells through MAPK- Runx2. Cells 2019, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Yang,H., Zhang, Q., Zhang, B., Zhou, Y., Wang, N., Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs 2023, 21(3), 144. [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar Drugs. 2018 Nov 23;16(12):465. [CrossRef]
- Lee, Y.I.; Lee, S.G.; Kim, E.; Jung, I.; Suk, J.; Kim, J.; Lee, J.H. (2022). Anti-aging effect of an oral disintegrating collagen film: a prospective, single-arm study. International journal of dermatology, 61(1), 54–61. [CrossRef]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Xu, N.; Peng, X.L.; Li, H.R.; Liu, J.X.; Cheng, J.S.; Qi, X.Y.; Ye, S.J.; Gong, H.L.; Zhao, X.H.; Yu, J.; Xu, G.; Wei, D.X. (2021). Marine-Derived Collagen as Biomaterials for Human Health. Frontiers in nutrition, 8, 702108. [CrossRef]
- Jin-Feng, D.; Yan-Yan, L.; Jia-Jie, X.; Xiu-Rong, S.; Xiang, G.; Fu-Peng, Y. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocolloids 2011, 25(5), 1350–1353. [Google Scholar] [CrossRef]
- Liang, J., Pei, X. R., Wang, N., Zhang, Z. F., Wang, J. B., & Li, Y. (2010). Marine collagen peptides prepared from chum salmon (Oncorhynchus keta) skin extend the life span and inhibit spontaneous tumor incidence in Sprague-Dawley Rats. Journal of medicinal food, 13(4), 757–770. [CrossRef]
- Cruz MA, Araujo TA, Avanzi IR, Parisi JR, de Andrade ALM, Rennó ACM. Collagen from Marine Sources and Skin Wound Healing in Animal Experimental Studies: a Systematic Review. Mar Biotechnol (NY). 2021 Feb;23(1):1-11. [CrossRef] [PubMed]
- Chandika P, Ko SC, Jung WK. Marine-derived biological macromolecule-based biomaterials for wound healing and skin tissue regeneration. Int J Biol Macromol. 2015;77:24-35. [CrossRef]
- Liu S, Lau CS, Liang K, Wen F, Teoh SH. Marine collagen scaffolds in tissue engineering. Curr Opin Biotechnol. 2022 Apr;74:92-103. [CrossRef] [PubMed]
- Lim YS, Ok YJ, Hwang SY, Kwak JY, Yoon S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar Drugs. 2019 Aug 10;17(8):467. [CrossRef]
- Barzkar, Noora, et al. ‘Marine Collagen: Purification, Properties and Application’. Frontiers in Marine Science, vol. 10, 2023. Frontiers, https://www.frontiersin.org/articles/10.3389/fmars.2023.1245077.
- Lin Z, Tao Y, Huang Y, Xu T, Niu W. Applications of marine collagens in bone tissue engineering. Biomed Mater. 2021 Apr 1;16(4):042007. [CrossRef]
- Pickar-Oliver, A., Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat Rev Mol Cell Biol 20, 490–507 (2019). [CrossRef]
- Li, T., Yang, Y., Qi, H. et al. CRISPR/Cas9 therapeutics: progress and prospects. Sig Transduct Target Ther 8, 36 (2023). [CrossRef]
- He, S. The first human trial of CRISPR-based cell therapy clears safety concerns as new treatment for late-stage lung cancer. Sig Transduct Target Ther 5, 168 (2020). [CrossRef]
- Whelan, A.; Duffy, J.; Gaul, R.T.; O’Reilly, D.; Nolan, D.R.; Gunning, P.; Lally, C.; Murphy, B.P. Collagen Fibre Orientation and Dispersion Govern Ultimate Tensile Strength, Stiffness and the Fatigue Performance of Bovine Pericardium. J. Mech. Behav. Biomed. Mater. 2019, 90, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin aging & Modern age Anti-Aging Strategies; PharmaTutor; 2019; 7(8); 22-70.
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Yang, R.; Zhang, Z.; Xu, Y.; Han, X.; Wang, J.; Li, Y. Effects of marine collagen peptide on delaying the skin aging. Zhonghua Yu Fang Yi Xue Za Zhi [Chin. J. Prev. Med.] 2008, 42, 235–238. [Google Scholar] [PubMed]
- Pu, S.-Y.; Huang, Y.-L.; Pu, C.-M.; Kang, Y.-N.; Hoang, K.D.; Chen, K.-H.; Chen, C. Effects of Oral Collagen for Skin Anti-Aging: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2080. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of Thermal Properties of Fish Collagen and Bovine Collagen in the Temperature Range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Nagaoka, H.; Terajima, M.; Tsuda, N.; Hayashi, Y.; Yamauchi, M. Effects of Fish Collagen Peptides on Collagen Post-Translational Modifications and Mineralization in an Osteoblastic Cell Culture System. Dent. Mater. J. 2013, 32, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wen-Hui Huang, Sheng-Long Ding, Xi-Yuan Zhao, Kai Li, Hai-Tao Guo, Ming-Zhu Zhang, Qi Gu(2023).Collagen for neural tissue engineering: Materials, strategies, and challenges,Materials Today Bio,20. [CrossRef]
- Romero Askehoi,A., Thorseth, M., Madsen Hargbol, D.,Immune Modulatory Properties of Collagen in Cancer. Front. Immunol., 2021, 12.
- Mirata, S.; Asnaghi, V.; Chiantore, M.; Salis, A.; Benvenuti, M.; Damonte, G.; Scarfì, S. Photoprotective and Anti-Aging Properties of the Apical Frond Extracts from the Mediterranean Seaweed Ericaria amentacea. Mar. Drugs 2023, 21, 306. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.; Ferreira, M.; Magalhães, C.; Lobo, J.M.; Sousa, E; Almeida, I.F. Trends in the use of marine ingredients in anti-aging cosmetics. Algal Research 2021, 55. [CrossRef]
- Fan L, Ren Y, Emmert S, Vučković I, Stojanovic S, Najman S, Schnettler R, Barbeck M, Schenke-Layland K, Xiong X. The Use of Collagen-Based Materials in Bone Tissue Engineering. Int J Mol Sci. 2023 Feb 13;24(4):3744. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).