1. Introduction
Obesity, as defined by the World Health Organization (WHO), is an unhealthy condition caused by abnormally excessive accumulation of fat in adipose tissue [
1,
2]. Body mass index (BMI) is a person’s weight (kg) divided by the square of their height (m). Individuals with BMI ranging between 25 and 30 are overweight and obese [
1,
2,
3]. According to the WHO obesity report, 1.9 billion adults aged above 18 years are overweight and 600 million are obese, making it a global problem [
4,
5]. According to the National Health and Nutrition Examination Survey in Korea, it has been reported that the prevalence of obesity among adults aged 19 or older is estimated to be approximately 40% for men and 26% for women. By 2030, its prevalence is expected to reach approximately 62% and 37% in women [
6].
The primary cause of obesity is excessive accumulation of body fat resulting from caloric intake over the body's energy consumption [
7]. Adipocytes function as endocrine organs by regulating several metabolic processes that contribute to physiological regulation. These cells release a range of hormones, including leptin and adiponectin, that play essential roles in the maintenance of metabolic homeostasis [
8]. Excessive accumulation of lipids in adipocytes leads to loss of function, such as intracellular endoplasmic reticulum stress and mitochondrial dysfunction, leading to insulin resistance and inflammatory response, and the risk of complications associated with obesity, such as hypertension, cardiovascular disease, cancer, and fatty liver [
9,
10].
Lipid accumulation occurs due to increased triglyceride production and adipocyte differentiation during adipogenesis [
11]. Lipid accumulation controls the size of lipid droplets by increasing the cell number or proliferation [
12]. Obesity-related
in vitro studies of adipogenesis frequently use the mouse embryonic fibroblast 3T3-L1 preadipocyte cell line [
13]. This cell line can differentiate into adipocytes when treated with [3-isobutyl-1-methylxanthine (IBMX), dexamethasone (Dex), and insulin] (MDI), which stimulate complex signaling pathways and various transcription factors [
14,
15]. The various transcription factors include CCAAT/enhancer-binding protein α (C/EBP-α), nuclear receptor peroxisome proliferator-activated receptor-γ (PPAR-γ), and adipocyte fatty acid-binding protein (aP2) [
16]. During the latter stage of differentiation, the levels of C/EBP-α increase along with PPAR-γ, leading to the activation of adipogenesis-inducing genes, including aP2 [
17,
18]. The expression levels of these factors were markedly increased in differentiated adipocytes [
19]. Regulation of the expression of genes associated with adipocyte differentiation is a crucial strategy for the prevention and treatment of obesity [
20]. Therefore, therapeutic agents demonstrating efficacy in promoting adipocyte differentiation and inhibiting adipogenesis have been developed. Currently, the drugs targeting obesity include orlistat, topiramate, sibutramine, rimonabant, and phenylpropanolamine. However, these drugs have side effects, including insomnia, anorexia, gastrointestinal disorders, increased blood pressure, and heart disease [
21]. To address this concern, active research has focused on developing treatments for obesity using safer natural products or phytochemicals with fewer side effects [
22].
Leaf mustard (
Brassica juncea) is a cruciferous vegetable that is widely cultivated in Korea and Japan and has long been used as an ingredient in kimchi in Korea [
23]. The nutritional components of
Brassica juncea are rich in various free sugars, fatty acids, amino acids, vitamins, and minerals, and it has been reported to possess excellent cell-based antioxidant activity [
24]. Additionally, glucosinolates and isothiocyanate compounds. such as allyl isothiocyanate and sinigrin from
Brassica juncea, are known to have various health and functional effects, including anti-cancer [
25,
26,
27]. Sinigrin has been reported to have anticancer, anti-inflammatory, antibacterial, antifungal, and antioxidant effects. Our previous study reported that
Brassica juncea extract (BJE), extracted under optimized sinigrin extraction conditions, has anti-obesity effects
in vitro [
28,
29,
30]. Therefore, following previous studies, we investigated the mechanism of how BJE exerts its anti-obesity effect
in vivo and demonstrated its value as a natural product with anti-obesity efficacy.
2. Materials and Methods
2.1. Chemicals and Standards
Trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA), phosphate-buffered saline (PBS), penicillin-streptomycin (P/S), bovine serum (BS), Dulbecco's modified Eagle's medium (DMEM) with high glucose, and fetal bovine serum (FBS) were purchased from Gibco (Gaithersburg, MD, USA). The primary and secondary antibodies specific for PPAR-γ, C/EBP-α, aP2, acetyl-CoA carboxylase (ACC), phospho-ACC (p-ACC), carnitine palmitoyltransferase-1 (CPT-1), peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA), and Cell Signaling Technology, Inc. (Danvers, MA, USA). Insulin, Dex, IBMX, and sinigrin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
2.2. Standardized BJE Preparation
Brassica juncea was provided by STR Biotech Co., Ltd. (Chuncheon, Korea). In our previous study, we developed a standardized extraction method through systematic experimentation based on extraction time, extraction solvent type, and extraction temperature. Briefly, we adopted a standardized experimental method using 60% ethanol at 70 ℃ as the extraction solvent and the extraction time was 3 h [
30]. The raw materials used in the experiment were thoroughly washed to remove foreign substances and freeze-dried (Ilshin, Seoul, Korea). They were then ground and homogenized for use in the experiment. Ethanol (60%) was added to 40 kg freeze-dried
Brassica juncea. After reflux extraction of
Brassica juncea at 70 °C for 3 h, the extraction mixture was filtered through 0.2-μm filter paper (Whatman, Maidstone, England). The filtered extract was concentrated under reduced pressure using a vacuum evaporator (Eyela, Tokyo, Japan) at 50 °C and lyophilized to obtain a BJE powder. The prepared sample was stored at −20 °C and used after being suspended in a cell culture medium or sterile distilled water (DW). The sinigrin content in BJE was measured using HPLC. The instruments used for analysis were a Shimadzu LC system and Waters Photodiode Array Detector (Waters Corporation, Milford, MA, USA).
Table 1 lists the conditions used in this analysis. The column used for analysis was CAPCELL PAK C
18 UG120 (4.6 × 250 mm, 5.0 μm; Waters Corporation, Milford, MA, USA).
2.3. Cell culture and XTT assay
Mouse-derived 3T3-L1 pre-adipocyte cells were obtained from the American Type Culture Collection (CL-173, ATCC, Manassas, VA, USA). 3T3-L1 adipocytes were seeded on each plate at a concentration of 2 × 106 cells/mL according to the purpose of the experiment and incubated in DMEM containing 10% BS and 1% P/S until 100% confluence. Following 2 d of confluence, adipocyte differentiation was induced using differentiation inducing cocktail containing 1 μg/mL insulin, 1 μM Dex, 0.5 mM IBMX, DMEM, 10% FBS, and 1% P/S. After 2 d, the culture medium was replaced with DMEM containing 10% FBS and 1 μg/mL insulin. The culture medium was replaced with DMEM supplemented with 10% FBS and 1 μg/mL insulin every 2 d. BJE and Garcinia cambogia extracts (Gar) were added to the cells 2 d after reaching 100% confluence and treated continuously during differentiation. The total differentiation period was 6 d. 3T3-L1 adipocytes were seeded in a 96-well plate and on the 6 d of differentiation, a working solution prepared by mixing 1 ml of 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) reagent and 20 μL of phenazine methosulfate reagent, was added to each well at 20% of the medium volume.
2.4. Oil Red O staining
After a 6-d differentiation period, the culture medium was removed. The differentiated 3T3-L1 cells were washed twice with PBS and then 500 μL of a 10% formalin solution was added to fix them for 1 h at room temperature. The formalin was removed, and the cells were washed with 60% isopropanol solution to completely dry them. Lipids that accumulated in the dried cells were stained with a pre-prepared Oil Red O (ORO) working solution (ORO:DW = 6:4) for 1 h. The cells were washed thrice with DW and dried. ORO bound to the accumulated lipids in the cells was eluted using 100% isopropanol and the absorbance was measured at 490 nm using a microplate reader.
2.5. Animal Experiment
Four-week-old male C57BL/6J mice were purchased from DBL Inc. (Incheon, Korea) and acclimated to a climate-controlled room (temperature, 24 ± 5 ℃; relative humidity, 55 ± 5%) with free access to water and food under 12/12-h light/dark cycles for 1 week. Mice were randomly divided into five groups: group 1, normal-fat diet (CON); group 2, 60% kcal high-fat diet (HFD; D12492; Research Diet, Inc., New Brunswick, NJ, USA); group 3, HFD + Gar 50 mg/kg/day; group 4, HFD + BJE 400 mg/kg/day; and group 5, HFD + BJE 800 mg/kg/day. All groups except the CON group were fed a HFD to induce obesity, and the CON group was supplied a general diet with 10% fat of total calories. For oral administration, BJE and GAR were dissolved in distilled water and administered once daily for 6 weeks. Food intake was measured as the difference between the amount of food served and the remaining amount, and the food efficiency ratio (FER) was calculated using the following formula: FER = body weight gain (g)/food intake (g). At the end of the breeding period, mice were anesthetized with isoflurane (Hwaseong, Korea) after fasting for 12 h, and tissue and blood samples were collected. The dissected epididymal adipose tissue, liver, spleen, and kidneys were washed with normal saline and weighed. This study was approved by the Institutional Animal Care and Use Committee of the Kangwon National University (approval number: KW-221104-2).
2.6. Biochemical analysis
Blood samples were collected in lithium heparin tubes using gel separators (Microtainer PSTTM tubes; Becton Dickinson, Franklin Lakes, NJ, USA). After centrifugation at 12,000 × g and 4 °C for 10 min, the plasma was isolated. Plasma levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total cholesterol (TC), and total triglycerides (TG) were measured using an automatic biochemical analyzer (Hitachi-720, Hitachi Medical, Japan).
2.7. Histological analysis
The epididymal adipose tissue was partially excised and fixed in 10% paraformaldehyde. The fixed epididymal adipose tissue was sequentially dehydrated with a graded series of ethanol solutions using a tissue processor (TP1020; Leica Biosystems, Nussloch, Germany) and paraffin (Rotary Microtome RM2255; Leica Biosystems). Paraffin blocks were cut to a thickness of 6 μm, slides were prepared, and stained with hematoxylin and eosin (H&E). The stained adipose tissue was observed through an optical microscope (ECLIPSE Ni-U; Nikon, Melville, NY, USA).
2.8. Western blot analysis
Adipocytes and epididymal adipose tissue were dissolved in lysis buffer (comprising 0.1% SDS, 1 mM pepstatin, 150 mM sodium chloride, 50 mM Tris-HCl, 0.25% sodium deoxycholate, 1% Nonidet P-40, and 1 mM phenylmethanesulfonyl fluoride) and reacted at 4 °C for 30 min. The cell lysates were centrifuged at 12,000 × g and 4 °C for 20 min. The protein concentrations of the resulting cell lysates were determined using the Bradford protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Equal quantities of proteins were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel (SDS–PAGE) and transferred electrically to a polyvinylidene difluoride membrane (0.2 μM Immun-Blot PVDF membrane; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Tris-buffered saline containing 5% bovine serum albumin was used to inhibit nonspecific binding. SuperSignal West Pico Chemiluminescence (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to detect the target proteins after treatment with primary and secondary antibodies. Western blot bands were observed using the ChemiDoc image software version 5.2.1 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
2.9. Statistical Analysis
The results are expressed as mean ± standard deviation (SD). All statistical analyses were performed using SPSS software (version 24.0; SPSS Inc., Chicago, IL, USA). Statistical significance was determined using Duncan's multiple range test or Dunnett's test.
3. Results
3.1. Sinigrin content in standardized BJE
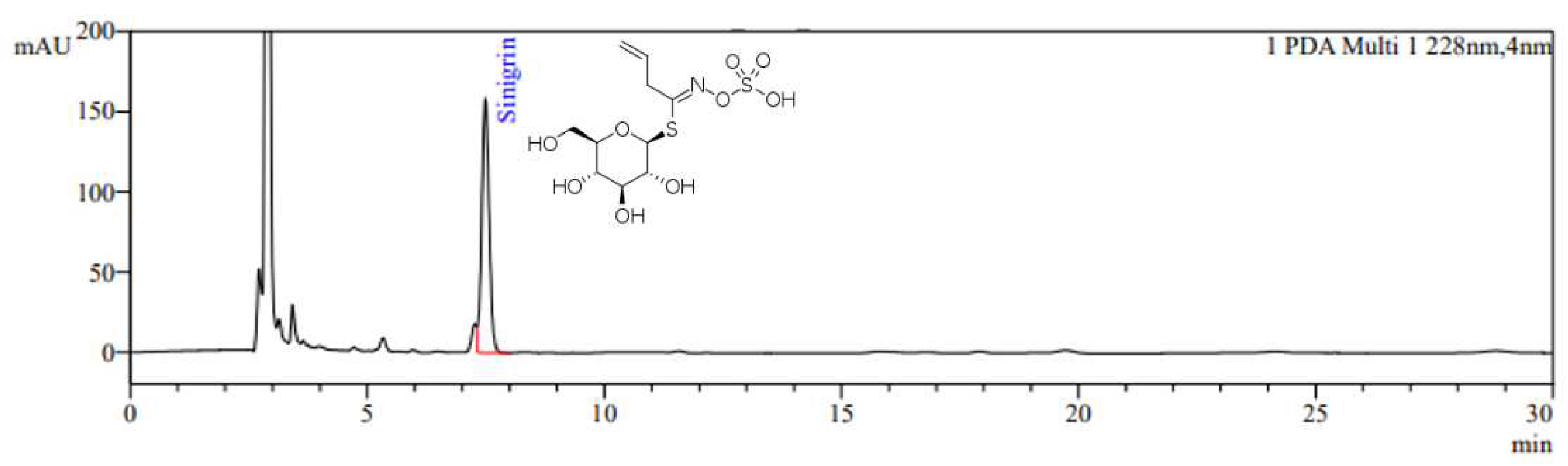
The HPLC chromatogram of BJE is shown in
Figure 1. Sinigrin was detected at a retention time of 7.4 min, and the sinigrin content in BJE was quantified as 18.0 mg/g.
3.2. Effects of BJE on the Viability and Lipid Accumulation of 3T3-L1 Cells
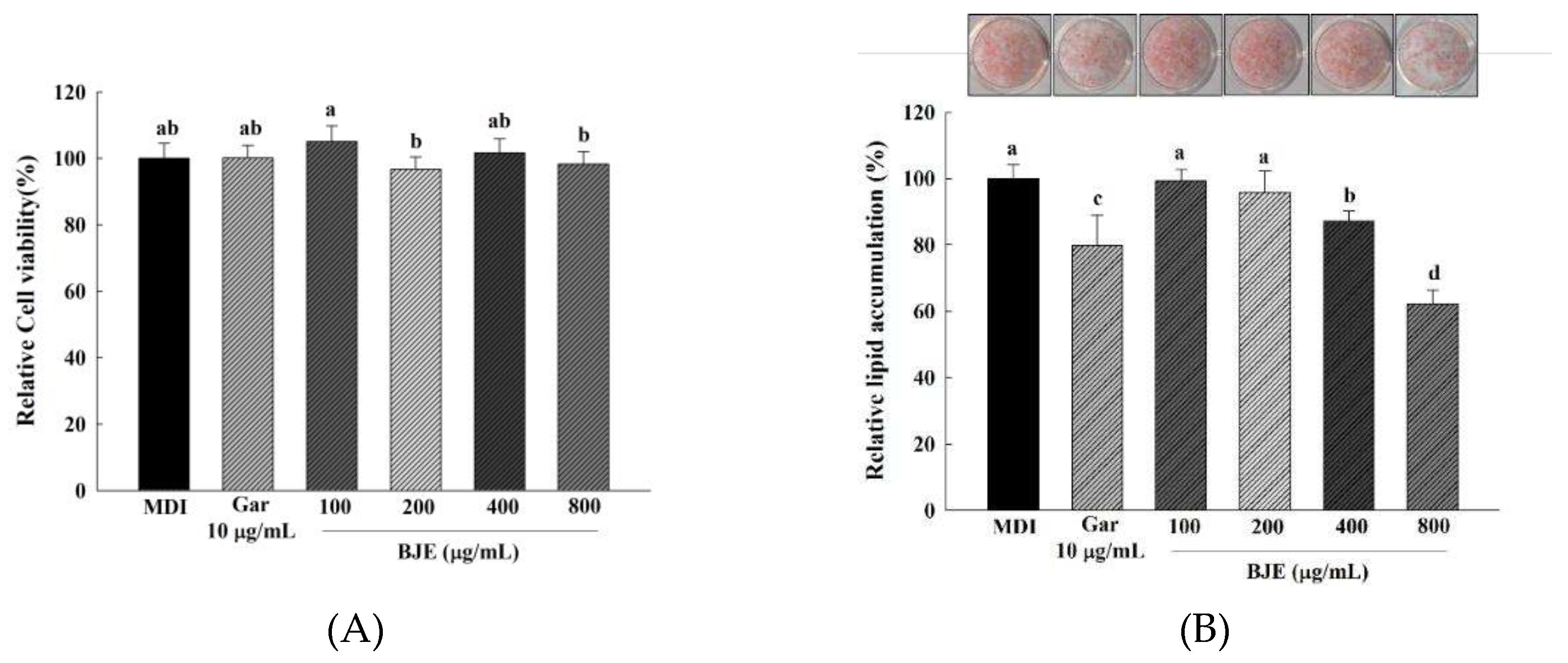
The effect of BJE on cell viability during the induction of 3T3-L1 preadipocyte differentiation is shown in
Figure 2A. The cells of the BJE treatment group (100–800 μg/mL) did not show significant difference compared to the control group; therefore, it was confirmed that there was no cytotoxicity. Therefore, this study attempted to evaluate the anti-obesity characteristics of inhibiting adipocyte differentiation by treating BJE at a concentration of 100–800 μg/mL. The effect of BJE on lipid accumulation in adipocytes was measured using ORO staining. After inducing the differentiation of 3T3-L1 cells for 6 d, lipid accumulation was confirmed by BJE treatment (
Figure 2B). Among BJE concentrations of 100, 200, 400, and 800 μg/mL, it was confirmed that lipid accumulation was significantly decreased only at 400 and 800 μg/mL. Additionally, lipid accumulation significantly decreased as the BJE concentration increased from 400 to 800 μg/mL.
3.3. Effect of BJE on the Expression of Proteins Related to Adipogenesis, Lipid synthesis, Fatty Acid Oxidation, and Heat Generation in 3T3-L1 Cells
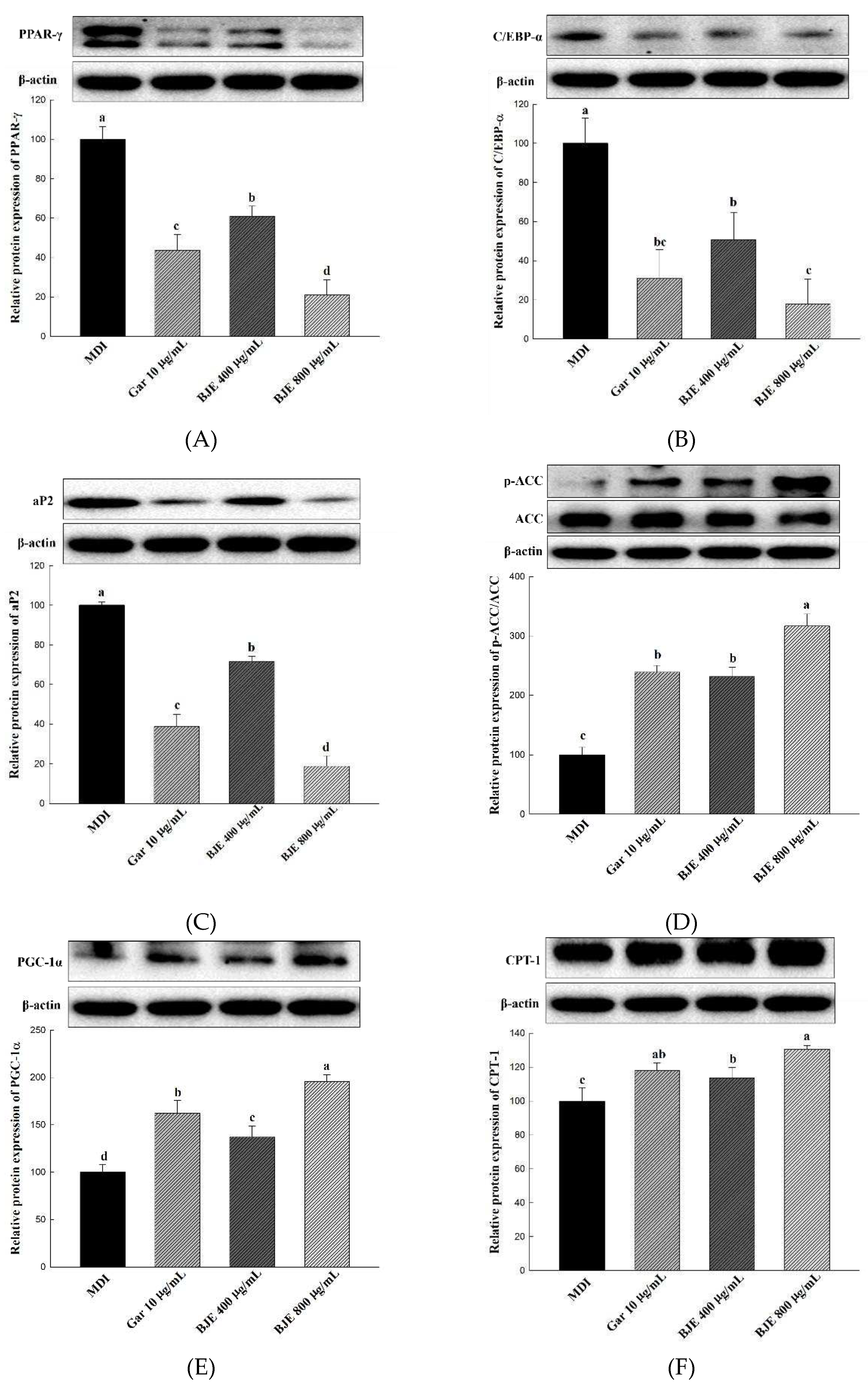
The change in protein expression levels of key adipogenesis-related genes, including PPAR-γ, C/EBP-α, and aP2, was assessed to identify the mechanism of action of BJE in inhibiting lipid accumulation (
Figure 3A-C). As a result, the expression levels of C/EBP-α, PPAR-γ, and aP2 were significantly downregulated in the cells of the group treated with the BJE extract. To investigate the effect of BJE on the expression of lipid synthesis-related proteins, the expression levels of p-ACC/ACC were measured (
Figure 3D). In the present study, BJE effectively inhibited lipid synthesis by increasing p-ACC/ACC expression in cells. To investigate the effects of BJE on the expression of proteins associated with lipolytic effects, CPT-1 and PGC-1α levels were measured (
Figure 3E,F). In the BJE-treated groups, the expression levels of proteins related to lipolytic effects were significantly higher than those in the control group.
3.4. Effects of BJE on Adipose Tissue Structure, Tissue Weight, and Body Weight in HFD-induced Obese C57BL/6J mice
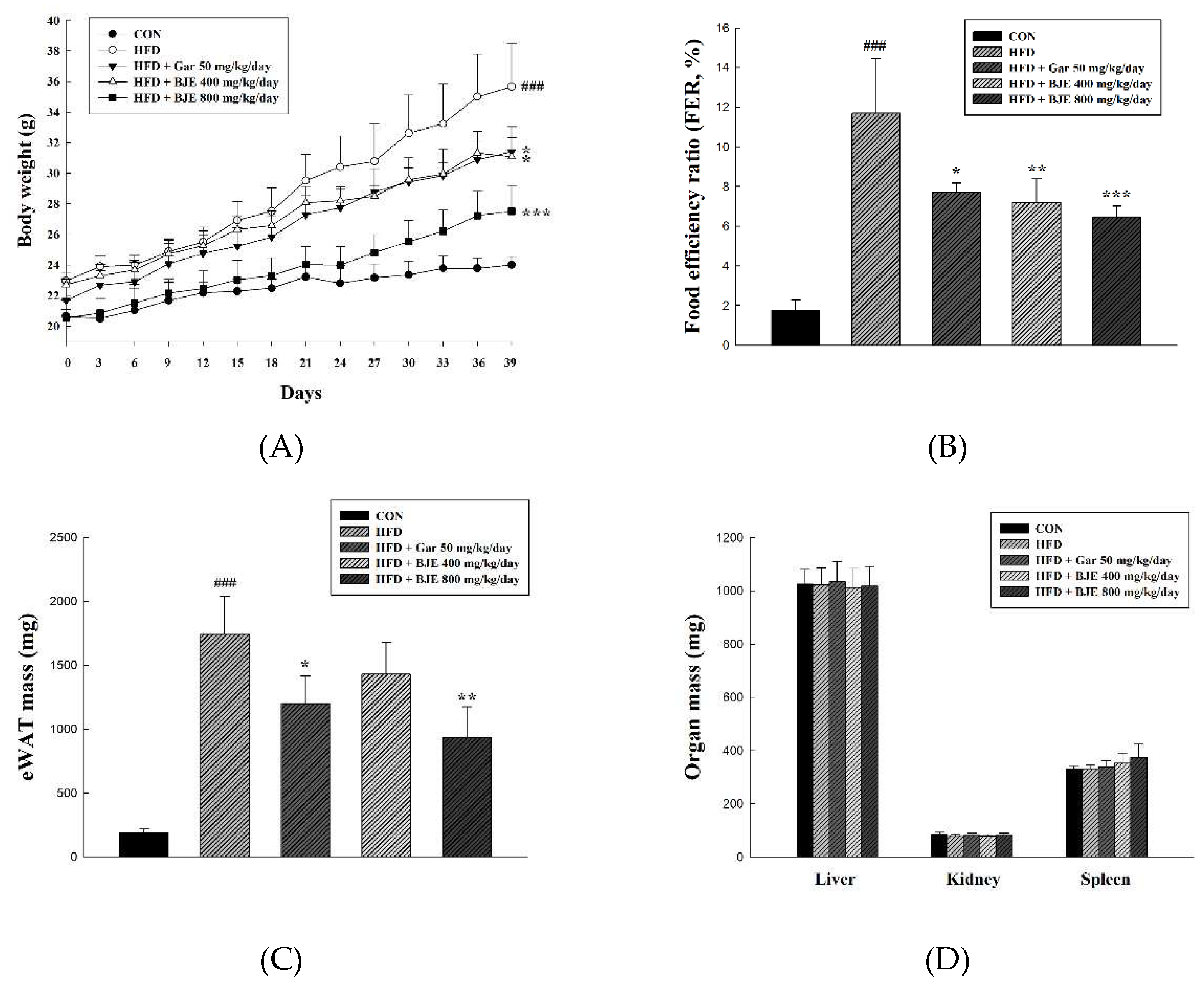
To investigate the anti-obesity effect of BJE, HFD-induced obese mice were administered Gar 50 mg/kg/day (HFD + Gar 50 mg/kg/day), BJE 400 mg/kg/day (HFD + BJE 400 mg/kg/day), or BJE 800 mg/kg/day (HFD + 800 mg/kg/day) for 6 weeks, and body weight was recorded weekly (
Figure 4A). HFD + Gar 50 mg/kg/day was used as a positive control. As expected, HFD successfully induced obesity in C57BL/6J mice. The mice in the HFD group had significantly higher body and epididymal adipose tissue weights than those in the CON group. However, treatment with Gar (50 mg/kg/day) and BJE (800 mg/kg/day) significantly decreased body weight and epididymal adipose tissue weight in HFD-induced obese mice (
Figure 4A,B). There were no significant differences in the weights of the other tissues between the experimental groups (
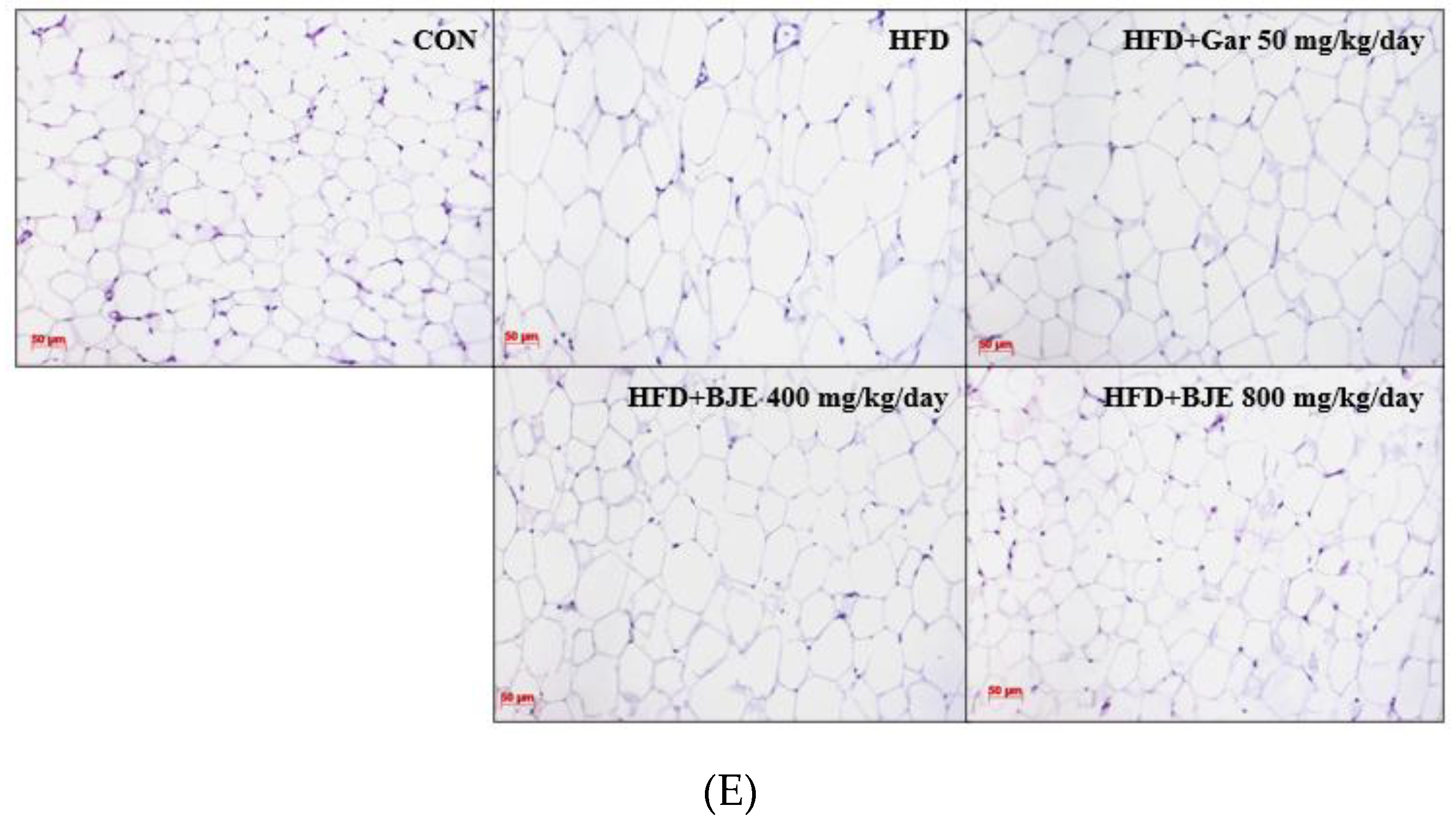
Figure 4D). Additionally, histological analysis showed that adipocytes were significantly large in the HFD group and that HFD-induced changes in adipocyte size were significantly reversed by Gar and BJE (
Figure 4E). Taken together, our results clearly demonstrate that BJE exerts a strong anti-obesity effect in HFD-induced obese mice.
3.5. Effect of BJE on Plasma Biochemical Indexes in HFD-induced Obese C57BL/6J mice
Blood was collected from all experimental animals, serum was separated, and biochemical changes in the plasma were observed. To confirm the effects of BJE on fatty liver disease, changes in ALT and AST levels were measured. ALT and AST levels were higher in the HFD group than those in the CON group, and both factors were significantly decreased in all BJE treatment groups compared to the HFD group. In the HFD + Gar 50 mg/kg/day group, ALT and AST levels decreased compared to those in the HFD group, but the difference was not significant; therefore, BJE suppressed hepatotoxicity caused by fatty liver more effectively than Gar. The plasma changes induced by BJE were confirmed by observing TC and TG levels. In the HFD group, TC levels were significantly increased compared to those in the CON group and decreased in the BJE group compared to those in the HFD group; however, this difference was significant only in the HFD + 800 mg/kg/day group. TG levels in the HFD group were also higher than those in the CON group and decreased in the BJE group compared to those in the HFD group; however, this difference was significant only in the HFD + 800 mg/kg/day group. Accordingly, based on the measurement of biochemical changes in the plasma, hepatotoxicity and abnormalities in TC and TG levels due to obesity were found to be regulated by BJE (
Table 2).
3.6. Effect of BJE on the Expression of Proteins Related to Adipogenesis, Lipid synthesis, Fatty Acid Oxidation, and Heat Generation in HFD-induced obese mice
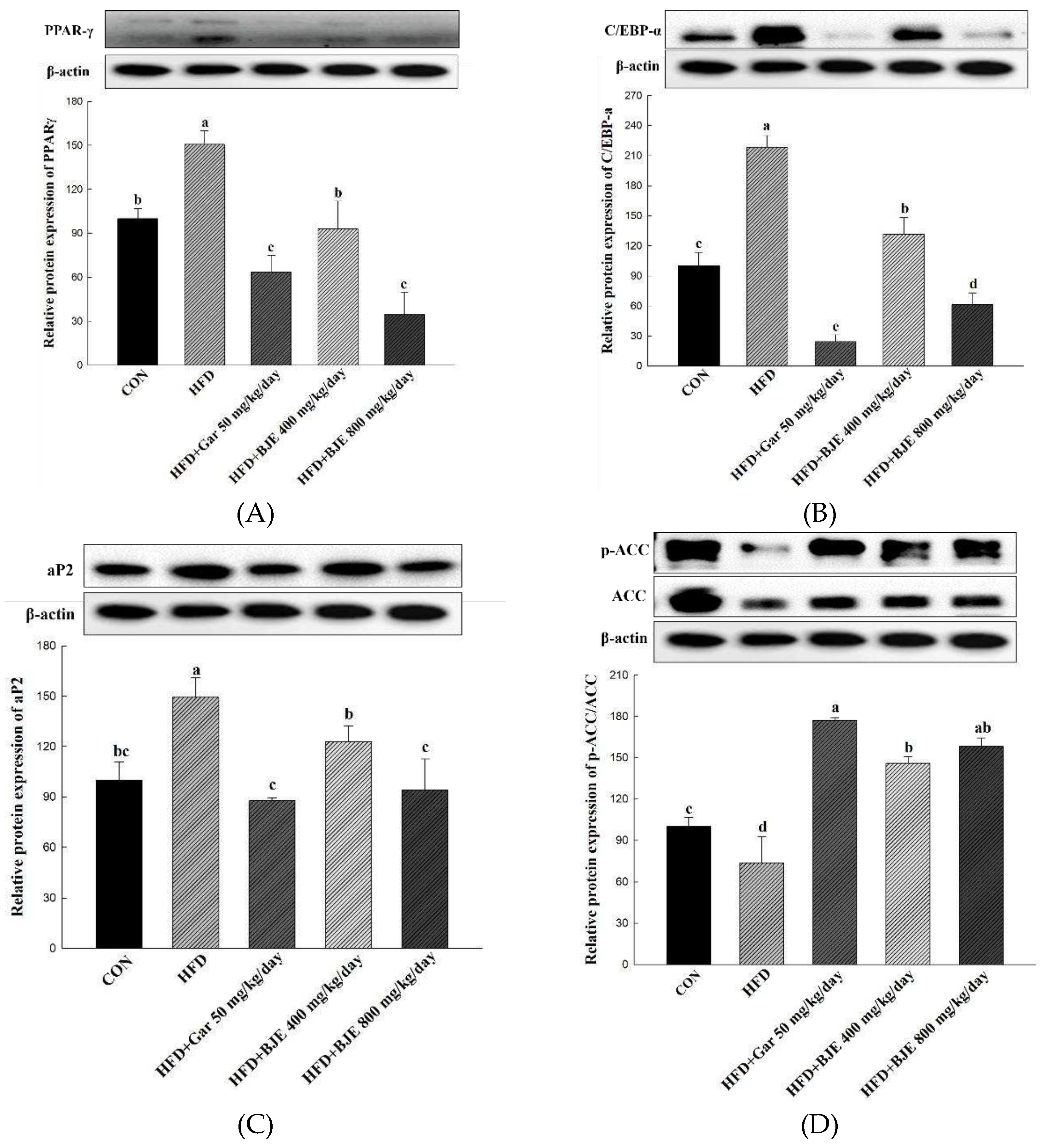
We showed that BJE suppressed adipogenesis by inhibiting the expression of PPAR-γ, C/EBP-α, and ap2 in differentiated 3T3-L1 adipocytes (
Figure 2A-C). In consistent with these findings, the expression of C/EBP-α, PPAR-γ, and ap2 was reduced markedly in the epididymal adipose tissue obtained from BJE groups compared with those from HFD groups (
Figure 5A). Furthermore, to determine whether BJE could also regulate lipid synthesis-related protein expression
in vivo, we measured the expression of p-ACC/ACC in the epididymal tissue. We confirmed that lipid synthesis was suppressed in the BJE group by significantly increasing p-ACC/ACC expression compared with that in the HFD group (
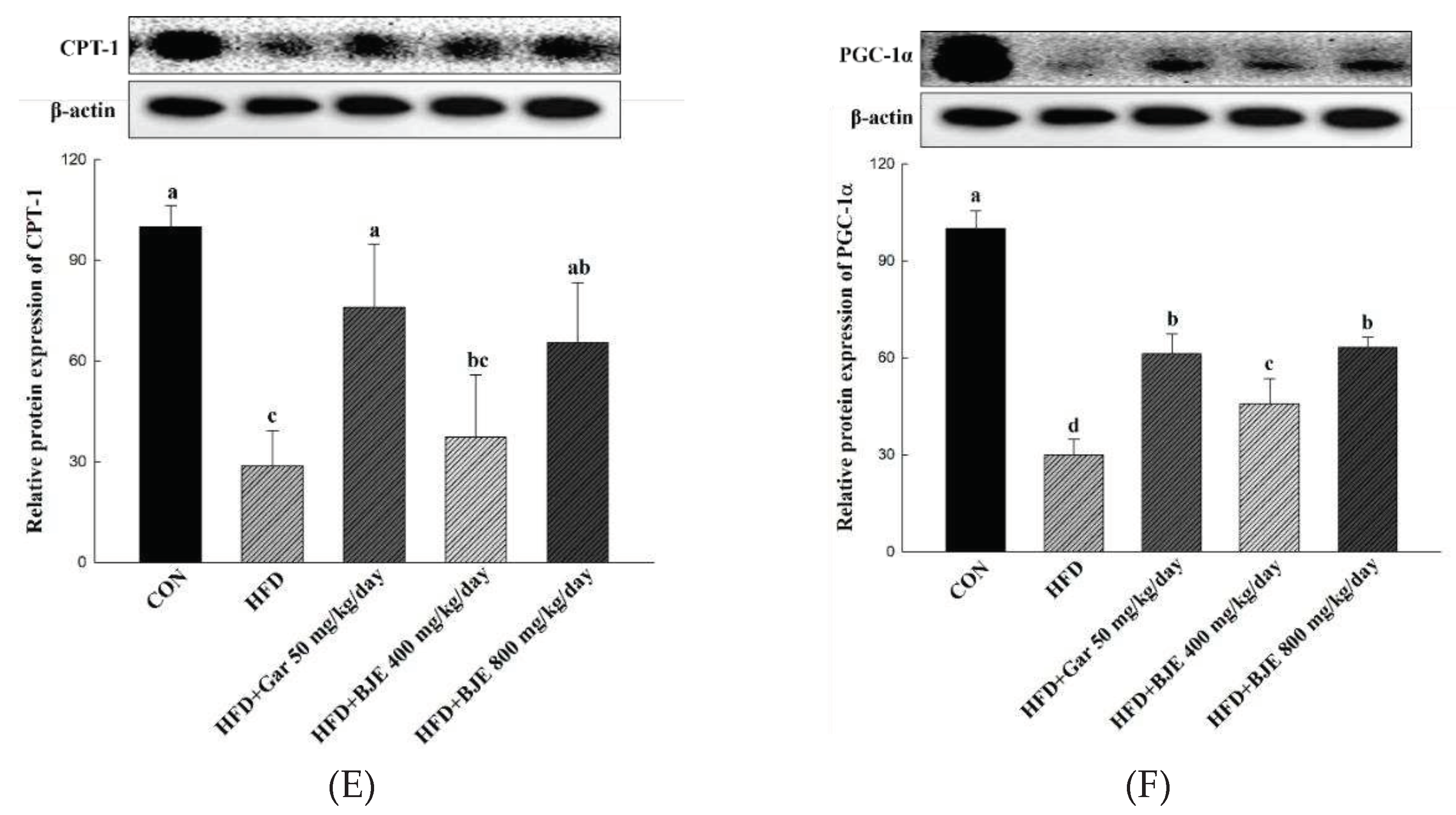
Figure 5B). Finally, the expression of genes related to fatty acid oxidation and heat generation was measured. BJE promoted the expression of PGC-1α and CPT-1
in vivo, which was decreased in the HFD group (
Figure 5C).
In vivo protein expression results are consistent with
in vitro protein expression results.
4. Discussion
Several studies have focused on both anti-obesity drugs and functional foods [
31,
32]. Moreover, many studies have aimed to verify the efficacy of natural substances in functional foods and anti-obesity drugs, with the intention of reducing the various side effects associated with commercially available anti-obesity drugs [
33,
34,
35]. This study was conducted to confirm the effect of
in vivo anti-obesity in an animal model based on the results of
in vitro experiments on the inhibition of lipid accumulation by BJE with an optimized sinigrin content [
30].
The sinigrin content of Brassica juncea extracted using the optimized extraction method was analyzed using HPLC. The sinigrin content of the BJE used in this study was 18.0 mg/g.
3T3-L1 preadipocytes were treated with MDI to induce their differentiation into adipocytes. At the same time, the cytotoxicity of BJE in 3T3-L1 adipocytes was confirmed through XTT analysis, but no cytotoxicity was found at all concentrations investigated (100, 200, 400, and 800 μg/mL). The inhibitory effect of BJE (100, 200, 400, and 800 μg/mL) on adipocyte differentiation was investigated. The morphological characteristics of the cells and stained adipocytes were quantitatively evaluated by ORO staining. This study showed that the number and size of adipocytes and the degree of ORO staining decreased depending on the BJE concentration when compared to the MDI group. The
in vitro anti-obesity mechanism of BJE was investigated via western blotting. C/EBP-α, PPAR-γ, and ap2 are important transcription factors in the differentiation of preadipocytes into mature adipocytes [
37]. In a previous study, BJE was reported to inhibit lipid accumulation by downregulating protein expression of C/EBP-α, PPAR-γ, and ap2, which was consistent with the results of this study [
30]. ACC regulates lipogenic condensation and contributes to the overall regulation of energy metabolism [
38]. It is inactivated by phosphorylation and consequently inhibits the activity of ACC, which means that lipid production can be suppressed, and obesity can be prevented [
39]. BJE inhibited lipid synthesis by inactivating ACC. CPT-1 acts as a key regulator of fatty acid metabolism by regulating malonyl-CoA, the first intermediate in lipid production [
40]. PGC-1 is known to be a major stimulus for mitochondrial production in adipocytes [
41]. Therefore, BJE exerts its anti-obesity effects by regulating CPT-1 and PGC-1 to increase fatty acid oxidation and heat generation.
Based on the
in vitro results, an animal study was performed to evaluate the
in vivo anti-obesity effects of BJE. HFD is widely used and accepted in nutritional experiments as a good strategy to induce an overweight status and fat accumulation in animals [
42]. HFD consumption increases body weight and circulating concentrations of TC and TG [
43]. Oral administration of BJE for 6 weeks to HFD-fed mice significantly reduced body weight and decreased plasma TG and TC levels. Additionally, the weight of epididymal adipose tissue and the size of adipocytes decreased. Western blotting was performed to investigate the anti-obesity effects of BJE
in vivo. HFD-induced obesity is associated with high expression of C/EBP-α, PPAR-γ, and ap2 [
44]. C/EBP-α, PPAR-γ, and aP2 showed low expression levels in the epididymal adipose tissue of mice administered orally with BJE. ACC was activated in HFD-fed mice and promoted adipogenesis [
45]. BJE treatment inhibited ACC activation by restoring ACC phosphorylation in the adipose tissue of HFD-fed mice. However, in HFD-fed mice, the expression of PGC-1α, which is involved in heat generation, and CPT-1, which is involved in fatty acid oxidation, was low [
46,
47]. In this study, we investigated whether BJE treatment was associated with the expression of heat generation and fatty acid oxidation-related proteins. Here, we showed that BJE administration induced PGC-1α and CPT-1 expression in HFD-fed obese mice. Therefore, BJE treatment may inhibit lipid accumulation by upregulating heat generation and expression of fatty acid oxidation-related proteins.
Overall, these results confirmed that BJE prevented HFD-induced obesity in C57BL/6J mice. Western blot analysis demonstrated that the anti-obesity effects of BJE involved the inhibition of adipogenesis and lipid synthesis as well as the activation of fatty acid oxidation and heat generation. Therefore, BJE may be a promising source of functional materials for the prevention and treatment of obesity.
5. Conclusions
BJE inhibit lipid accumulation in 3T3-L1 and reduce body and epididymal adipose tissue weight in HFD-induced obese mice by decreasing the expression of adipogenic proteins (C/EBP-α, PPAR-γ, and aP2), phosphorylating the lipid synthesis protein (ACC), and increasing the expression of fatty acid oxidation protein (CPT-1) and heat generation (PGC-1α). Therefore, our data suggest that BJE administration improves HFD-induced obesity by inhibiting adipogenesis and activating heat generation and fatty acid oxidation.
Author Contributions
Project administration, Visualization, Writing - original draft, June seok Lim; Methodology, Project administration, Visualization, Ji-Hyun Im; Investigation, Software. Geon Oh: Investigation, Formal analysis, Xiao Men; Software, Validation, Formal analysis, XioLu-Fu; Resources, Validation, OOO; Conceptualization, Funding acquisition, Supervision, Sun-Il Choi; Conceptualization, Funding acquisition, Supervision, Writing - review & editing, Ok-Hwan Lee. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program (NRF-2021R1A6A1A03044242, 2017R1D1A3B0602846915) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, the BK21 FOUR (Fostering Outstanding Universities for Research) (4299990913942) funded by the Ministry of Education (MOE, Korea).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Kangwon National University. The C57BL/6J mice were maintained in accordance with Kangwon National University Regulations on Animal Experimentation (IACUC approval number: KW-221104-2).
Data Availability Statement
The data presented in this study are available within this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, H.M.; Jo, J.; Park, C.; Choi, B.J.; Lee, H.G.; Kim, K.Y. Epibionts associated with floating Sargassum horneri in the Korea Strait. Algae 2019, 34, 303–313. [Google Scholar] [CrossRef]
- Kim, H.R.; Jung, B.K.; Yeo, M.H.; Yoon, W.J.; Chang, K.S. Inhibition of lipid accumulation by the ethyl acetate fraction of Distylium racemosum in vitro and in vivo. Toxicol. Rep. 2019, 6, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Yang, M.Y.; Chan, K.C.; Chung, P.J.; Ou, T.T.; Wang, C.J. Improvement in high-fat diet-induced obesity and body fat accumulation by a Nelumbo nucifera leaf flavonoid-rich extract in mice. J. Agric. Food Chem. 2010, 58, 7075–7081. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Choi, J.H.; Kim, Y.G.; Lee, C.H. Effect of dietary intake of Salicornia herbacea L. hot water extract on anti-obesity in diet-induced obese rats. J. Korean Soc. Food Sci. Nutr. 2012, 41, 950–956. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Guo, L.; Kang, H.M.; Son, B.G.; Kang, J.S.; Lee, Y.J.; Park, Y.H.; Je, B.I.; Choi, Y.W. Leaves of Cudrania tricuspidata on the shoot positional sequence show different inhibition of adipogenesis activity in 3T3-L1 cells. J. Life Sci. 2021, 31, 209–218. [Google Scholar]
- Lee, Y.S.; Seo, Y.H.; Kim, J.Y. Antiobesity effect of radish leaf extracts on high fat diet-induced obesity in mice. Korean J. Food Sci. Technol. 2022, 54, 297–305. [Google Scholar]
- Greenberg, A.S.; Obin, M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006, 83, 461S–465S. [Google Scholar] [CrossRef]
- Pagliassotti, M.J.; Kim, P.Y.; Estrada, A.L.; Stewart, C.M.; Gentile, C.L. Endoplasmic reticulum stress in obesity and obesity-related disorders: An expanded view. Metabolism 2016, 65, 1238–1246. [Google Scholar] [CrossRef]
- Kim, H.S.; Han, E.J.; Fernando, I.P.S.; Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.J.; Jee, Y.; Kang, S.; Jang, J.; Jang, J.; et al. Anti-allergy effect of mojabanchromanol isolated from Sargassum horneri in bone marrow-derived cultured mast cells. Algal Res. 2020, 48, 101898. [Google Scholar] [CrossRef]
- Kim, G.C.; Kim, J.S.; Kim, G.M.; Choi. S.Y. Anti-adipogenic effects of Tropaeolum majus (nasturtium) ethanol extract on 3T3-L1 cells. Food Nutr. Res. 2017, 61, 1339555. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kang, H. Anti-obesity, and lipid metabolism effects of Ulmus davidiana var. japonica in mice fed a high-fat diet. J Microbiol Biotechnol. 2021, 31, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Nam, W; Nam, S.H.; Kim, S.P.; Levin, C.; Friedman, M. Anti-adipogenic and anti-obesity activities of purpurin in 3T3-L1 preadipocyte cells and in mice fed a high-fat diet. BMC Complement Altern. Med. 2019, 19, 364.
- Seo, Y.J.; Kim, K.J.; Choi, J.; Koh, E.J.; Lee, B.Y. Spirulina maxima extract reduces obesity through suppression of adipogenesis and activation of browning in 3T3-L1 cells and high-fat diet-induced obese mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Han, J.H.; Yu, K.H.; Hong, M.; Lee, S.Y.; Park, K.H.; Lee, S.U.; Kwon, T.H. Antioxidant and anti-obesity activities of Polygonum cuspidatum extract through alleviation of lipid accumulation on 3T3-L1 adipocytes. J. Microbiol. Biotechnol. 2020, 30, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Lee, S.J.; Kim, S.H.; Jang, S.H.; Won, C.W.; Kim, H.D.; Cho, J.H. Antiobesity activity of Acer tegmentosum Maxim on 3T3-L1 preadipocyte and high-fat diet-induced obese rats. Biol. Pharm. Bull. 2008, 31, 1415–1421. [Google Scholar]
- Makowski, L.; Brittingham, K.C.; Reynolds, J.M.; Suttles, J.; Hotamisligil, G.S. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity: macrophage expression of aP2 impacts peroxisome proliferator-activated receptor γ and IκB kinase activities. J. Biol. Chem. 2005, 280, 12888–12895. [Google Scholar] [CrossRef] [PubMed]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Ho, J.N.; Choi, J.W.; Lim, W.C.; Kim, M.K.; Lee, I.Y.; Cho, H.Y. Kefir inhibits 3T3-L1 adipocyte differentiation through down-regulation of adipogenic transcription factor expression. J. Sci. Food Agric. 2013, 93, 485–490. [Google Scholar] [CrossRef]
- Choi, R.Y.; Lee, H.I.; Yun, K.W.; Ham, J.R.; Lee, M.K. Inhibitory Effects of Aralia elata Sprout Hot-Water Extract on Adipocyte Differentiation and Triglyceride Synthesis in 3T3-L1 cells. J. Korean Soc. Food Sci. Nutr. 2020, 49, 631–637. [Google Scholar] [CrossRef]
- Cha, J.Y.; Nepali, S.; Lee, H.Y.; Hwang, S.W.; Choi, S.Y.; Yeon, J.M.; Song, B.J.; Kim, D.K.; Lee, Y.M. Chrysanthemum indicum L. ethanol extract reduces high-fat diet-induced obesity in mice. Exp. Ther. Med. 2018, 15, 5070–5076. [Google Scholar]
- Goktas, Z.; Zu, Y.; Abbasi, M.; Galyean, S.; Wu, D.; Fan, Z.; Wang, S. Recent advances in nanoencapsulation of phytochemicals to combat obesity and its comorbidities. J. Agric. Food Chem. 2020, 68, 8119–8131. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Han, Y.S. Effect of mustard leaf on quality and sensory characteristics of Kimchi. J. Korean Soc. Food Sci. Nutr. 1994, 23, 618–624. [Google Scholar]
- Kwon, H.Y.; Choi, S.I.; Cho, B.Y.; Choi, S.H.; Sim, W.S.; Han, X.; Jang, G.W.; Choi, Y.E.; Yeo, J.H.; Cho, J.H.; Lee, O.H. Analysis of nutritional components and cell-based antioxidant activity on Brassica juncea cultivated in Jeongseon, South Korea. Korean J. Food Nutr. 2019, 32, 462–472. [Google Scholar]
- Popova, I.E.; Morra, M.J. Simultaneous quantification of sinigrin, sinalbin, and anionic glucosinolate hydrolysis products in Brassica juncea and sinapis alba seed extracts using ion chromatography. J. Agric. Food Chem. 2014, 62, 10687–10693. [Google Scholar] [CrossRef]
- Talalay, P.; Fahey, J.W. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J. Nutr. 2001, 3027S–3033S. [Google Scholar] [CrossRef]
- Blažević, I.; Radonić, A.; Mastelić, J.; Marina, Z.; Skočibušić, M.; Maravić, A. Glucosinolates, glycosidically bound volatiles and antimicrobial activity of Aurinia sinuata (Brassicaceae). Food Chem. 2010, 121, 1020–1028. [Google Scholar] [CrossRef]
- Oh, S.K.; Kim, K.W.; Bea, S.O.; Choi, M.R. Sinigrin content of different parts of Dolsan leaf mustard. Korean J. Food Preserv. 2015, 22, 553–558. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica Juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Choi, S.I.; Han, X.; Men, X.; Jang, G.W.; Choi, Y.E.; Lee, O.H. Antiobesity effect of Brassica juncea cultivated in Jeonseon with optimized sinigrin content using 3T3-L1 adipocytes. J. Food Biochem. 2021, 45, e13650. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Choi, S.I.; Park, H.I.; Choi, S.H.; Sim, W.S.; Yeo, J.H.; Cho, J.H.; Lee, O.H. Comparative analysis of the nutritional components and antioxidant activities of different Brassica juncea cultivars. Foods 2020, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Trigueros, L.; Peña, S.; Ugidos, A.V.; Sayas-Barberá, E.; Pérez-Álvarez, J.A.; Sendra, E. Food Ingredients as Anti-Obesity Agents: A Review. Crit. Rev. Food Sci. Nutr. 2013, 53, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G. The search for compounds that stimulate thermogenesis in obesity management: From pharmaceuticals to functional food ingredients. Obes. Rev. 2011, 12, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.H.; Lee, J.J.; Son, H.K.; Kim, B.H.; Byun, J.; Ha, J.H. Antiobesity Effects of Extract from Spergularia marina Griseb in Adipocytes and High-Fat Diet-Induced Obese Rats. Nutrients 2020, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Kim, H.; Lee, Y.J.; Ku, S.K. Anti-diabetic Obesity Effects of Wasabia Japonica Matsum Leaf Extract on 45% Kcal High-Fat Diet-Fed Mice. Nutrients 2020, 12, 2837. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.W.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Doenjang, A Korean Traditional Fermented Soybean Paste, Ameliorates Neuroinflammation and Neurodegeneration in Mice Fed a High-Fat Diet. Nutrients 2019, 11, 1702. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Park, S.; Jang, S.; Cho, H.H.; Lee, S.; You, S.; Kim, S.H.; Moon, H.S. Cascade regulation of PPARγ2 and C/EBPα signaling pathways by celastrol impairs adipocyte differentiation and stimulates lipolysis in 3T3-L1 adipocytes. Metabolism 2016, 65, 646–654. [Google Scholar] [CrossRef]
- Brownsey, R.W.; Boone, A.N.; Elliott, J.E.; Kulpa, J.E.; Lee, W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006, 24, 223–227. [Google Scholar] [CrossRef]
- Park, Y.H.; An, M.; Kim, J.K.; Lim, Y.H. Antiobesity effect of ethanolic extract of Ramulus mori in differentiated 3T3-L1 adipocytes and high-fat diet-induced obese mice. J. Ethnopharmacol. 2020, 251, 112542. [Google Scholar] [CrossRef]
- Schreurs, M.; Kuipers, F.; Van Der Leij, F.R. Regulatory enzymes of mitochondrial β-oxidation as targets for treatment of the metabolic syndrome. Obes. Rev. 2010, 11, 380–388. [Google Scholar] [CrossRef]
- Gleyzer, N.; Vercauteren, K.; Scarpulla, R.C. Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol. Cell Biol. 2005, 25, 1354–1366. [Google Scholar] [CrossRef]
- Chun, M.R.; Lee, Y.J.; Kim, K.H.; Kim, Y.W.; Park, S.Y.; Lee, K.M.; Kim, J.Y.; Park, Y.K. Differential effects of high-carbohydrate and high-fat diet composition on muscle insulin resistance in rats. J. Korean Med. Sci. 2010, 25, 1053–1059. [Google Scholar] [CrossRef]
- Kalaivanisailaja, J.; Manju, V.; Nalini, N. Lipid profile in mice fed a high-fat diet after exogenous leptin administration. Pol. J. Pharmacol. 2003, 55, 763–769. [Google Scholar] [PubMed]
- Seo, Y.J.; Kim, K.J.; Choi, J.; Koh, E.J.; Lee, B.Y. Spirulina maxima extract reduces obesity through suppression of adipogenesis and activation of browning in 3T3-L1 cells and high-fat diet-induced obese mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yamashita, Y.; Yasuda, M.; Yamamoto, N.; Ashida, H. Ashitaba (Angelica keiskei) extract prevents adiposity in high-fat diet-fed C57BL/6 mice. Food Funt. 2015, 6, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Coulter, A.A.; Bearden, C.M.; Liu, X.; Koza, R.A.; Kozak, L.P. Dietary fat interacts with QTLs controlling induction of PGC-1 alpha and UCP1 during conversion of white to brown fat. Physiol. Genomics 2003, 14, 139–147. [Google Scholar] [CrossRef]
- Tung, Y.C.; Lin, Y.H.; Chen, H.J.; Chou, S.C.; Cheng, A.C.; Kalyanam, N.; Ho, C.T.; Pan, M.H. Piceatannol exerts anti-obesity effects in C57BL/6 mice through modulating adipogenic proteins and gut microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).