1. Introduction
Lower urinary tract symptoms (LUTS) are common and bothersome in both men and women.[
1,
2] The pathophysiology of female LUTS is complex and may involve bladder, urethral, and pelvic floor dysfunctions. However, clinical symptoms are unreliable in the diagnosis of lower urinary tract dysfunction (LUTD) in women.[
3] Videourodynamic studies (VUDS) could provide a deep understanding of LUTS and accurately diagnose LUTD in female patients.[
4,
5] Due to the nature of invasive procedures, VUDS should be the second-line investigation after initial diagnosis and treatment based on symptoms or noninvasive tests fail. A comprehensive systemic review of the biomarkers associated with LUTS was recently published.[
6] It analyzed multiple candidate biological pathways and suggested future research into biomarker-based precision medicine, which has so far remained elusive.
Bladder urothelial dysfunction and increased suburothelial inflammation were investigated in a variety of LUTD, including IC/BPS[
7], overactive bladder (OAB)[
8], bladder outlet obstruction, and various bladder dysfunctions[
9], which appeared to reflect their underlying pathophysiology. Recently, a significant association between oxidative stress and LUTS was reported.[
10] The levels of advanced glycation end products and 8-hydroxy-2-deoxyguanosine (8-OHdG) in the urine were higher in severe LUTS patients. Controlling oxidative stress was thought to be a new therapeutic strategy for treating chronic ischemia-induced bladder dysfunction.[
11] Both bladder inflammation and tissue hypoxia with oxidative stress were common pathophysiological bladder features in LUTD, and the roles of urine inflammatory and oxidative stress biomarkers in these LUTD were gradually revealed.[
6,
12,
13]
OAB and interstitial cystitis/bladder pain syndrome (IC/BPS) in female patients commonly share indistinct symptoms that confuse clinical physicians, although their core symptoms for diagnosis are different. According to one recent research, the urine cytokine profiles of IC/BPS and OAB patients differed significantly from those of controls.[
14] Furthermore, a novel pilot diagnostic algorithm, using MIP-1β as the initial screening test for diseased patients, IL-10 as a confirmation test for OAB, and eotaxin, CXCL10, and RANTES as confirmation tests for IC/BPS, was developed with acceptable diagnostic rates. Another research found that female patients with dysfunctional voiding (DV) had significantly higher urine 8-OHdG, IL-1β, IL-8, and brain-derived neurotrophic factor (BDNF) levels than controls.[
15] Based on these findings, the clinical application of urine biomarkers in females with LUTD is expected in the future.
We hypothesized that different LUTDs would have distinct protein profiles and biochemical contents due to their different pathophysiologies and intrinsic bladder conditions. Bladder inflammation and tissue hypoxia with oxidative stress were significant pathophysiological bladder features in LUTD. This study investigated urine inflammatory and oxidative stress biomarker profiles in females with LUTD, including detrusor overactivity (DO), DV, and IC/BPS, and developed a urine biomarker-based decision tree model for prediction.
2. Materials and methods
2.1. Patients and investigation of the clinical characteristics
From February 2015 to March 2021, we enrolled 31 DO, 45 DV, and 114 IC/BPS female patients at the Department of Urology of a single medical center. All patients received VUDS with the indication of refractory LUTS, and their respective LUTD confirmed. The diagnostic details of VUDS were interpreted according to the International Continence Society’s terminology.[
16] The following parameters of VUDS were recorded: first sensation of bladder filling (FSF), cystometric bladder capacity (CBC), detrusor voiding pressure (Pdet), maximal urinary flow rate (Qmax), corrected maximal urinary flow rate (cQmax, defined as Qmax/√CBC), voided volume (Vol), post-void residual volume (PVR), and voiding efficacy (VE, defined as Vol/CBC).
The inclusion criteria for DO, DV, and IC/BPS patients were similar to our previous studies.[
14,
15] DO patients were medically refractory OAB patients with DO evidence in VUDS. Patients diagnosed with DV had an open bladder neck but a narrow membranous urethra or pelvic floor muscle level on real-time fluoroscopy, increased external urethral sphincter electromyography activities, and a low Qmax during voiding, without a history of neurological disease. The diagnostic criteria for IC/BPS are based on the proposed guidelines of the European Society for the Study of Interstitial Cystitis.[
17] All enrolled IC/BPS patients received cystoscopic hydrodistention and were classified as European Society for the Study of Interstitial Cystitis type 1 or 2 (i.e., without or with glomerulations detected during hydrodistention).
Exclusion criteria of enrolled patients included active urinary tract infection, neurogenic disorders (e.g., multiple sclerosis, spinal cord injury, cerebrovascular accidents, and Parkinson’s disease), and history of bladder surgery/or traumatic injury, urinary tract malignancy or tuberculosis, pelvic radiation, or nephrotic or nephritic syndrome, urolithiasis, and impaired renal function (serum creatinine > 2.0 mg/dL).
2.2. Assessment of urine biomarker levels
Urine samples were collected from all enrolled study patients and controls. Urine was self-voided when subjects reported feeling full in the bladder. Before storing the urine samples, we performed urinalysis simultaneously to confirm their infection-free status. In total, 50 mL of urine were immediately placed on ice and transferred to the laboratory for preparation. The samples were centrifuged at 1800 rpm for 10 minutes at 4°C. Moreover, the supernatants were separated into aliquots in 1.5 mL tubes (1 mL per tube) and stored at −80°C. Before further analysis, the frozen urine samples were centrifuged at 12,000 rpm for 20 minutes at 4°C, and the supernatants were used for subsequent measurements.
2.3. Quantification of Inflammatory cytokines
The inflammatory cytokines, chemokines, and neurotrophins investigated in urine samples were similar to those investigated in our previous study.[
18] The targeted analytes in urine were assayed using commercially available microspheres with the Milliplex® Human Cytokine/Chemokine magnetic bead-based panel kit (Millipore, Darmstadt, Germany). A total of 14 targeted analytes included IL-1β, IL-2, IL6, IL-8, IL-10, eotaxin, CXCL10, MCP-1, MIP-1β, RANTES, TNFα, and VEGF measured with the multiplex kit catalog number HCYTMAG-60K-PX30, nerve growth factor (NGF) measured with the multiplex kit catalog number HADK2MAG-61K, and BDNF measured with the multiplex kit catalog number HNDG3MAG-36K. The following laboratory procedures of the quantification of these targeted analytes were performed similarly to those in our previous study.[
18]
2.4. Quantification of Prostaglandin E2 (PGE2)
The level of urine PGE2 was measured using a high-sensitivity ELISA kit (Cayman, MI, USA) according to the manufacturer’s instructions. The detailed procedures followed those reported in a previous study.[
19]
2.5. Quantification of oxidative stress biomarkers
The measurements of 8-OHdG, 8-isoprostane, and total antioxidant capacity (TAC) in urine samples were carried out in accordance with the manufacturer’s instructions (8-OHdG ELISA kit, Biovision, Waltham, MA, USA; 8-isoprostane ELIZA kit, Enzo, Farmingdale, NY, USA; Total Antioxidant Capacity Assay Kit, Abcam, Cambridge, MA, USA). The laboratory procedures followed those reported in a previous study.[
15]
2.6. Statistical analysis
Continuous variables were represented by means ± standard deviations, while categorical variables were represented by numbers and percentages. Outliers were defined as values that were outside the range between the means ± three standard deviations for each biomarker in either the study or the control group, and they were excluded from the development of the decision tree model. Mean differences in clinical data, as well as the levels of urine biomarkers, among groups were analyzed using one-way analysis of variance, and post hoc test was performed via Bonferroni’s correction. All calculations were performed using SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). If the p-value is less than 0.05, the difference is considered statistically significant.
2.7. Establishment of the decision tree model
Following data collection and importation into the software, all analyses were carried out in R language (version 3.5.2), primarily using the party (for decision trees) and randomForest (for random forest) packages, and the decision tree and random forest models were established.[
20,
21] The decision tree model was used to develop a predictive model of LUTD based on predictor variables (urine biomarkers), whereas the random forest model was adopted to evaluate the importance of each urine biomarker. Moreover, the efficacy of the decision tree model was evaluated using accuracy.
2.8. Internal validation of the decision-tree model
Internal validation was performed using the bootstrap method to assess the accuracy and consistency of our prediction models.[
22,
23,
24] Thirty percent of testing data from our study population were sampled and used to evaluate the accuracy of prediction models. This procedure was repeated 1,000 times, with the results used to calculate an unbiased estimate of the model accuracy and a 95% confidence interval (CI).
3. Results
The clinical characteristics and VUDS parameters of DO, DV, and IC/BPS patients are shown in
Table 1. DO patients were significantly older than DV and IC/BPS patients (63.9 ± 9.0 vs. 53.2 ± 14.2, 54.6 ± 12.4,
p < 0.001). The mean overactive bladder symptom score in DO patients was 9.4 ± 3.2. In DO and DV patients, the International Prostate Symptom Scores were 11.4 ± 5.8 and 15.1 ± 9.9, respectively. In IC/BPS patients, the mean O'Leary–Saint symptom score was 20.6 ± 7.9, with a mean VAS of 4.2 ± 2.6 and a maximal bladder capacity under anesthesia of 717.8 ± 179.1 mL. Patients in all three groups had distinct VUDS parameters.
The urine biomarker profiles among different female LUTD groups are shown in
Table 2. For each targeted analyte, the number of outliers within groups ranged from 0 to 1 in DO patients, from 0 to 2 in DV patients, and from 0 to 4 in IC/BPS patients. Different female LUTD groups had significantly different urine inflammatory and oxidative stress biomarker profiles, including IL-1β, IL-2, IL-8, IL-10, eotaxin, CXCL10, MIP-1β, RANTES, TNFα, VEGF, NGF, BDNF, 8-isoprostane, and TAC.
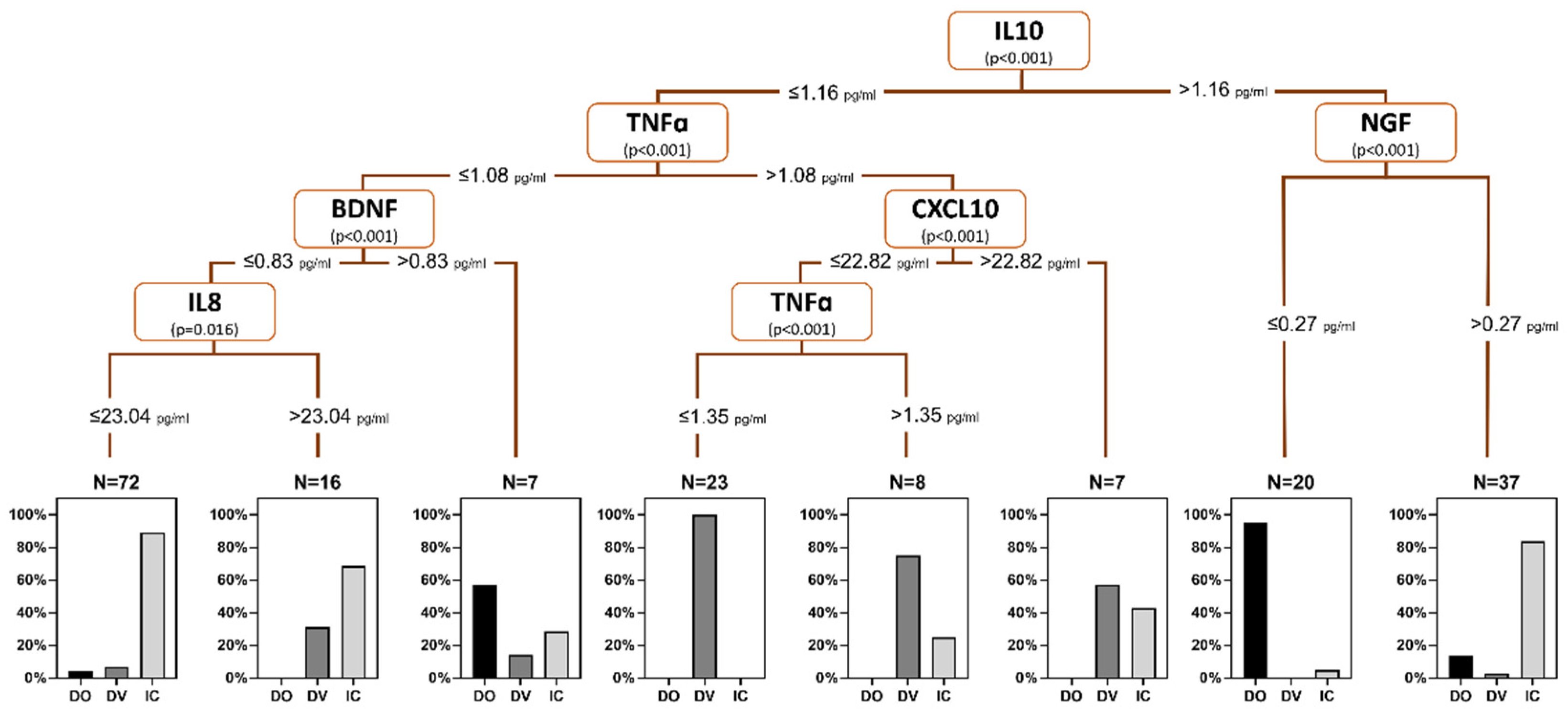
Based on the significant differences in urine biomarker profiles among female LUTD groups, a urine biomarker-based decision tree model was built using the levels of IL-10 (root node, cutoff value 1.16 pg/mL), TNFα (cutoff value 1.08 pg/mL), BDNF (cutoff value 0.83 pg/mL), IL-8 (cutoff value 23.04 pg/mL), CXCL10 (cutoff value 22.82 pg/mL), and NGF (cutoff value 0.27 pg/mL) (
Figure 1). This model demonstrated an overall accuracy rate of 85.3%, with individual accuracy rates of 74.2%, 73.3%, and 93.0 % for DO, DV, and IC/BPS, respectively.
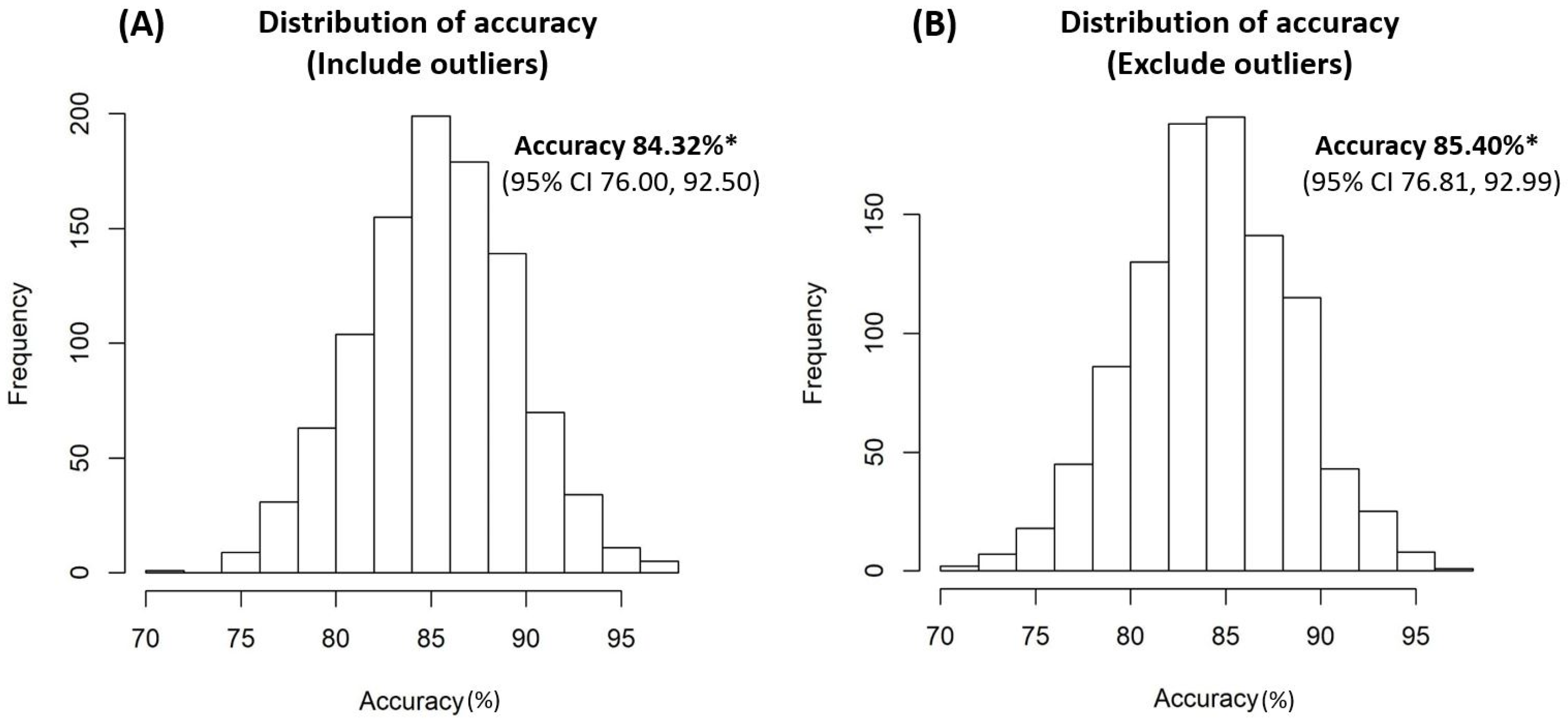
Internal validation using the bootstrap method revealed that the accuracy rates of the model were 84.32% (95% CI 76.00, 92.50) and 85.40% (95% CI 76.81, 92.99) from the sampling data of the entire study patients, including and excluding outliers, respectively (
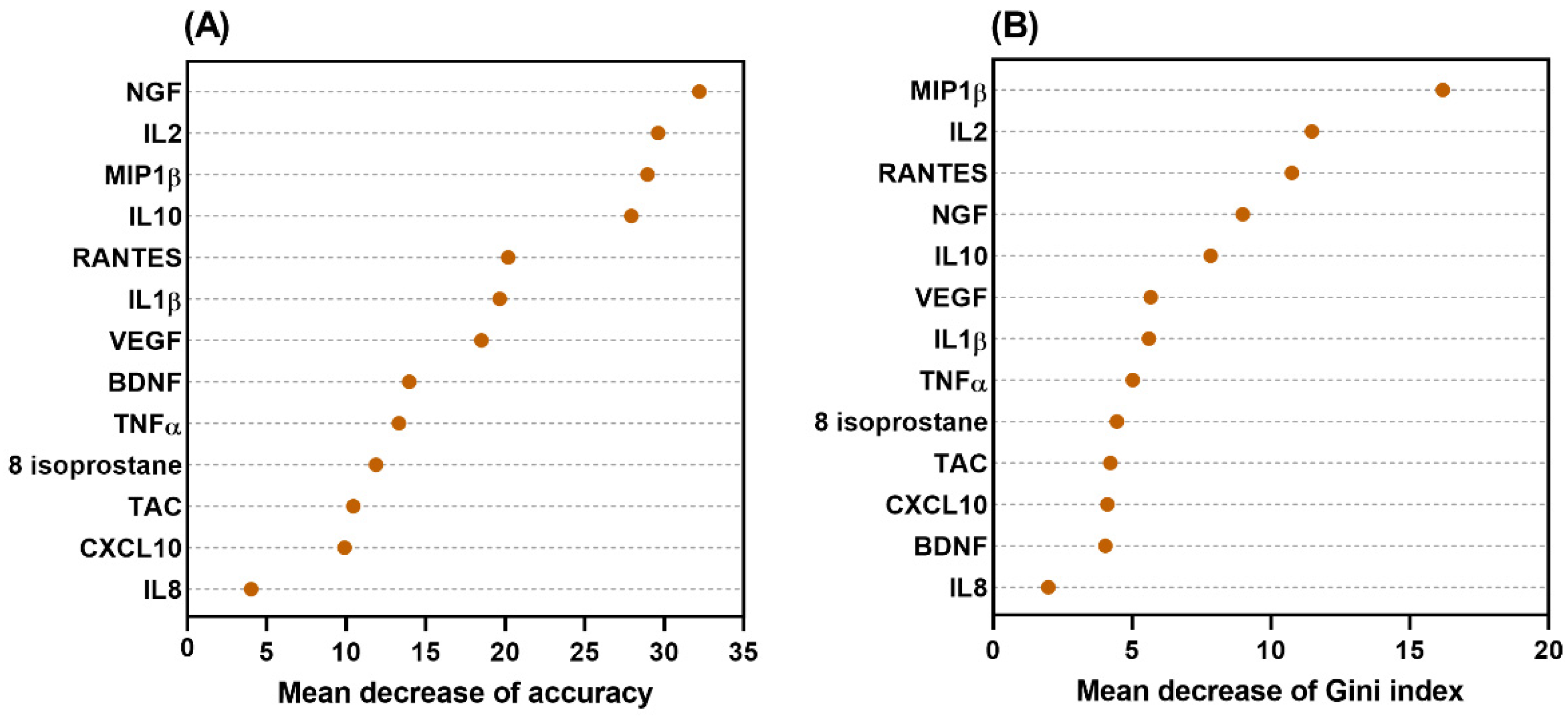
Figure 2). The significance and importance of all selected nodes in this decision tree model were supported by the random forest models (
Figure 3).
4. Discussion
To our knowledge, this is the first study to demonstrate an internally validated urine biomarker-based decision tree model for predicting female LUTD, including DO, DV, and IC/BPS. Bladder inflammation and tissue hypoxia with oxidative stress were considered as important and common pathophysiological bladder features in different LUTD in females. However, the critical differences among these diseases were not clearly established. This study revealed that the urine inflammatory and oxidative stress biomarker profiles of these diseases differed. We developed a urine biomarker decision tree model that predicts the different LUTD in females with high accuracy, as supported by the internal validation results. Developing a urine biomarker-based decision tree model is a first step toward translating the urine biomarker profiles into clinical practice. It is a significant step forward in the advancement of precision medicine in LUTD.
With an overall accuracy rate of 85.3%, our urine biomarker-based decision tree model selected IL-8, L-10, TNFα, CXCL10, BDNF, and NGF as the nodes to diagnose the different LUTD in females, including DO, DV, and IC/BPS. IL-8 is a chemoattractant of neutrophils and T cells[
25], that can regulate angiogenesis by directly enhancing the survival and proliferation of endothelial cells.[
26] In redox signaling and oxidative stress, both IL-8 and TNFα are important released pro-inflammatory cytokines.[
27] In a rat study of chronic bladder ischemia, both IL-8 and TNFα levels in the bladder tissue were elevated.[
28] Neurotrophins, such as NGF and BDNF, play a role in neural control and sensory function in the urinary bladder and are known as biomarkers in both OAB and IC/BPS.[
29] The higher the value of the mean decrease in accuracy or mean decrease in the Gini index, the higher the importance of the variable in the model. The data from random forest models demonstrated that all of the selected nodes in this decision tree model were significant and important.
In this study, urine biomarker profiles of DO, DV, and IC/BPS were distinct, but it was difficult to distinguish these groups by the analysis of single analyte. The previously reported diagnostic algorithm has acceptable diagnostic rates of 68.4%, 73.3%, and 60%, respectively, for distinguishing controls, IC/BPS, and OAB patients.[
14] The decision tree model is a very popular machine learning algorithm. A decision tree algorithm can be used to solve both regression and classification problems, and it has the advantages of being easy to understand, easy to interpret, and easy to visualize. This urine biomarker-based decision tree, which uses urine levels of inflammatory and hypoxia-related cytokines, as well as neurotrophins, may provide a superior overall accuracy rate of 85.3% in diagnosing DO, DV, and IC/BPS in female patients. The internal validation results revealed similarly high accuracy rates, demonstrating the discrimination and consistency of our prediction model. After importing more data, the decision tree model for predicting LUTD will be more accurate and reliable, more pathophysiological, and more applicable.
This study had a number of limitations. To begin, this urine biomarker-based-decision tree model was developed using existing data from urine biomarker profiles at our institution. Although this decision tree model was internally validated, it will require external validation in the future. Second, all of the enrolled female LUTD patients were medically refractory, and the accuracy rate may drop or differ when this model is applied to the female general population with LUTS. Moreover, a more comprehensive model is needed. Third, the shortcomings of urine biomarkers included intra-individual variations and the presence of extreme values. The extreme values in each study group were less than 5%, and we developed this decision tree with the exclusion of the extreme values. Under internal validation, the accuracy rate slightly reduced from 85.40% to 84.32 % when the data were sampled from the entire study patients with outliers excluded versus without outliers excluded. This suggested that this model was still reliable.
5. Conclusions
Female DO, DV, and IC/BPS patients had distinct urine inflammatory and oxidative stress biomarker profiles. We developed an internally validated urine biomarker-based decision tree to predict these females with LUTDs with an accuracy rate of 85.3%. In the future, as more patients are enrolled and more urine analytes with significance are selected, a more comprehensive and accurate decision tree to diagnose female LUTD is expected.
Author Contributions
Yuan-Hong Jiang collected and analyzed data and wrote the manuscript. Jia-Fong Jhang analyzed the data and provided critical revision of the manuscript. Jen-Hung Wang established the decision tree model and performed the statistical analysis. Ya-Hui Wu performed the laboratory procedures. Hann-Chorng Kuo conceived and designed the study and critically revised the manuscript.
Funding
This study was funded by the National Science and Technology Council (Taiwan) with grant number NSTC 112-2628-B-303-002-MY2.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board and Ethics Committee of Buddhist Tzu Chi General Hospital (No. IRB107-175-A and No. IRB107-37-A). All procedures were carried out in accordance with the relevant guidelines and regulations.
Informed Consent Statement
We informed all study patients and controls about the risks, rationale, procedures, ethics, and costs of this study, and they provided informed consent.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; Abrams, P. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the EPIC study. Eur Urol 2006, 50, 1306–1314. [Google Scholar] [CrossRef]
- Liu, S.P.; Chuang, Y.C.; Sumarsono, B.; Chang, H.C. The prevalence and bother of lower urinary tract symptoms in men and women aged 40 years or over in Taiwan. J Formos Med Assoc 2019, 118 Pt 1, 170–178. [Google Scholar] [CrossRef]
- Kuo, H.C. Clinical symptoms are not reliable in the diagnosis of lower urinary tract dysfunction in women. J Formos Med Assoc 2012, 111, 386–391. [Google Scholar] [CrossRef]
- Hsiao, S.M.; Lin, H.H.; Kuo, H.C. Videourodynamic Studies of Women with Voiding Dysfunction. Sci Rep 2017, 7, 6845. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Chen, S.F.; Kuo, H.C. Role of videourodynamic study in precision diagnosis and treatment for lower urinary tract dysfunction. Ci Ji Yi Xue Za Zhi 2020, 32, 121–130. [Google Scholar]
- Siddiqui, N.Y.; Helfand, B.T.; Andreev, V.P.; Kowalski, J.T.; Bradley, M.S.; Lai, H.H.; Berger, M.B.; Mueller, M.G.; Bickhaus, J.A.; Packiam, V.T.; Fenner, D.; Gillispie, B.W.; Kirkali, Z. Symptoms of Lower Urinary Tract Dysfunction Research, N. Biomarkers Implicated in Lower Urinary Tract Symptoms: Systematic Review and Pathway Analyses. J Urol 2019, 202, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Shie, J.H.; Kuo, H.C. Higher levels of cell apoptosis and abnormal E-cadherin expression in the urothelium are associated with inflammation in patients with interstitial cystitis/painful bladder syndrome. BJU Int 2011, 108 Pt 2, E136–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Kuo, H.C. Urothelial Dysfunction and Chronic Inflammation in Diabetic Patients with Overactive Bladder. Low Urin Tract Symptoms 2017, 9, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Lee, C.L.; Kuo, H.C. Urothelial Dysfunction, Suburothelial Inflammation and Altered Sensory Protein Expression in Men with Bladder Outlet Obstruction and Various Bladder Dysfunctions: Correlation with Urodynamics. J Urol 2016, 196, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Hatakeyama, S.; Imai, A.; Tanaka, T.; Hagiwara, K.; Konishi, S.; Okita, K.; Yamamoto, H.; Tobisawa, Y.; Yoneyama, T.; Yoneyama, T.; Hashimoto, Y.; Koie, T.; Nakaji, S.; Ohyama, C. Relationship between oxidative stress and lower urinary tract symptoms: Results from a community health survey in Japan. BJU Int 2019, 123, 877–884. [Google Scholar] [CrossRef]
- Nomiya, M.; Andersson, K.E.; Yamaguchi, O. Chronic bladder ischemia and oxidative stress: New pharmacotherapeutic targets for lower urinary tract symptoms. Int J Urol 2015, 22, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Lopes, T.; Cruz, F. Urinary Biomarkers in Overactive Bladder: Revisiting the Evidence in 2019. Eur Urol Focus 2019, 5, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Matsuo, T.; Mitsunari, K.; Asai, A.; Ohba, K.; Sakai, H. A Review of Oxidative Stress and Urinary Dysfunction Caused by Bladder Outlet Obstruction and Treatments Using Antioxidants. Antioxidants (Basel) 2019, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine biomarkers in ESSIC type 2 interstitial cystitis/bladder pain syndrome and overactive bladder with developing a novel diagnostic algorithm. Sci Rep 2021, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Ho, H.C.; Hsu, Y.H.; Kuo, H.C. Diagnostic and prognostic value of urine biomarkers among women with dysfunctional voiding. Sci Rep 2022, 12, 6608. [Google Scholar] [CrossRef] [PubMed]
- Haylen, B.T.; de Ridder, D.; Freeman, R.M.; Swift, S.E.; Berghmans, B.; Lee, J.; Monga, A.; Petri, E.; Rizk, D.E.; Sand, P.K.; Schaer, G.N.; International Urogynecological, A.; International Continence, S. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn 2010, 29, 4–20. [Google Scholar] [CrossRef]
- van de Merwe, J.P.; Nordling, J.; Bouchelouche, P.; Bouchelouche, K.; Cervigni, M.; Daha, L.K.; Elneil, S.; Fall, M.; Hohlbrugger, G.; Irwin, P.; Mortensen, S.; van Ophoven, A.; Osborne, J.L.; Peeker, R.; Richter, B.; Riedl, C.; Sairanen, J.; Tinzl, M.; Wyndaele, J.J. Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: An ESSIC proposal. Eur Urol 2008, 53, 60–67. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine cytokines as biomarkers for diagnosing interstitial cystitis/bladder pain syndrome and mapping its clinical characteristics. Am J Physiol Renal Physiol 2020, 318, F1391–F1399. [Google Scholar] [CrossRef]
- Chen, S.F.; Jiang, Y.H.; Kuo, H.C. Urinary biomarkers in patients with detrusor underactivity with and without bladder function recovery. Int Urol Nephrol 2017, 49, 1763–1770. [Google Scholar] [CrossRef]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. Journal of Computational and Graphical Statistics 2006, 15, 651–674. [Google Scholar] [CrossRef]
- Strobl, C.; Boulesteix, A.-L.; Zeileis, A.; Hothorn, T. Bias in random forest variable importance measures: Illustrations, sources and a solution. BMC Bioinformatics 2007, 8, 25. [Google Scholar] [CrossRef]
- Levine, A.C.; Glavis-Bloom, J.; Modi, P.; Nasrin, S.; Rege, S.; Chu, C.; Schmid, C.H.; Alam, N.H. Empirically Derived Dehydration Scoring and Decision Tree Models for Children With Diarrhea: Assessment and Internal Validation in a Prospective Cohort Study in Dhaka, Bangladesh. Glob Health Sci Pract 2015, 3, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Harrell, F.E. Jr.; Lee, K.L.; Mark, D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996, 15, 361–387. [Google Scholar] [CrossRef]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): The TRIPOD statement. Ann Intern Med 2015, 162, 55–63. [Google Scholar] [CrossRef]
- Tseng-Rogenski, S.; Liebert, M. Interleukin-8 is essential for normal urothelial cell survival. Am J Physiol Renal Physiol 2009, 297, F816–F821. [Google Scholar] [CrossRef]
- Li, A.; Dubey, S.; Varney, M.L.; Dave, B.J.; Singh, R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol 2003, 170, 3369–3376. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr Biol 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Nomiya, M.; Sagawa, K.; Yazaki, J.; Takahashi, N.; Kushida, N.; Haga, N.; Aikawa, K.; Matsui, T.; Oka, M.; Fukui, T.; Andersson, K.E.; Yamaguchi, O. Increased bladder activity is associated with elevated oxidative stress markers and proinflammatory cytokines in a rat model of atherosclerosis-induced chronic bladder ischemia. Neurourol Urodyn 2012, 31, 185–189. [Google Scholar] [CrossRef]
- Ochodnicky, P.; Cruz, C.D.; Yoshimura, N.; Cruz, F. Neurotrophins as regulators of urinary bladder function. Nat Rev Urol 2012, 9, 628–637. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).