1. Introduction
Breast cancer (BC) is the most common malignancy and the leading cause of cancer mortality in women. Since the mid-2000s female BC incidence rates have gradually increased about 0.5% per year 1. Obesity is associated with various types of cancer, including elevated BC risk and mortality 2, 3. Two-thirds of US adults are overweight or obese, with a higher prevalence in women and disproportionately higher rates among minorities 4. The World Health Organization (WHO) defines obesity as having a Body Mass Index (BMI) of more than 30 kg/m2 5. Obesity and weight loss have potent effects on the gut microbiome, impacting microbially-derived metabolites such as bile acids that may affect cancer progression 2, 6. In fact, it has been demonstrated that BC patients have lower circulating primary bile acids compared to controls 6, 7. We posited that this lack of primary bile acids could be reversed and that activating pathways pharmacologically to mimic higher primary bile acids may be beneficial. To date, the role of microbially derived metabolites in BC has not been well investigated 2. Therefore, further investigation is needed to understand the influence that bile acid signaling plays on BC progression.
Cholic acid (CA) and chenodeoxycholic acid (CDCA) are two major primary bile acids 6, 8. Primary bile acids can be converted into secondary bile acids through dehydroxylation and deconjugation of the 7α or 7β-hydroxyl groups 6, 8. In humans, key secondary bile acids include lithocholic acid (LCA) and deoxycholic acid (DCA) 6. Primary bile acids function as signaling molecules primarily through activating the major bile acid receptor (BAR) called farnesoid X receptor (FXR or NR1H4), a nuclear receptor 6, 8-10. Previous work has suggested antitumorigenic role for FXR in liver, bladder, and colorectal cancer 11-14. Interestingly, we report that BC patients with high FXR expression have greater overall survival uniquely in ER- tumors and the basal-like subtype, but not in the less aggressive luminal A BC subtype or ER+ tumors, suggesting great potential for targeted approaches for subtypes in need of therapeutic advances. Thus, we investigated bile acid receptor signaling using established bile acid receptor mimetic agonists. We demonstrated that agonism of FXR by obeticholic acid (OCALIVA, INT-747, or “OCA”) potently blunted tumor progression in a pre-clinical model. Furthermore, in vitro OCA significantly blunted cancer cell proliferation, migration, and induced cell death. Interestingly, agonism of another main BAR, the membrane receptor called G protein-coupled bile acid receptor 1 (GPBAR1 or TGR5) 6, 8 failed to demonstrate anti-cancer effectiveness. Thus, FXR agonism uniquely demonstrated anticancer efficacy. Results presented herein suggest that bile acids or BAR signaling may be beneficial in BC treatment. It is possible that bile acids elevated by bariatric surgery may be one mechanism mediating improved cancer outcomes. Given that OCA is FDA approved for primary biliary cholangitis (PBC), incorporation of OCA into studies of cancer risk and as a therapeutic may be a readily translated goal for future clinical studies.
2. Materials and Methods
2.1. Reagents
All reagents were procured from Sigma-Aldrich (St. Louis, MO), except where specifically mentioned. Obeticholic acid or OCALIVA (“OCA”, INT-747, Cat. No.: HY-12222, MedChemExpress, Monmouth, NJ), TGR5 agonist (“INT-777”, Cat. No.: HY-15677, MedChemExpress) and Paclitaxel (“Ptax”, Cat. No.: N88686, AstaTech, Inc., Bristol, PA; Cat.:HY-B0015, MedChemExpress) were purchased from AstaTech for
in vivo work and MedChemExpress for
in vitro use. Primary antibodies were purchased from Abcam, Invitrogen, and Proteintech, polyclonal-rabbit GPCR TGR5 (AB72608, Abcam, Waltham, Boston), polyclonal-rabbit NR1H4/FXR (AB235094, Abcam), polyclonal-rabbit FXR (PA5-40755, Invitrogen, Waltham, MA), and loading control monoclonal-mouse GAPDH (60004-1-Ig, Proteintech
®, Rosemont, IL).
Supplemental Table S1 (ST1) includes all reagents.
2.3. Proliferation Assay
Cell proliferation of human SUM159 and MDA-MB-231 and murine E0771 BC cells was detected using the IncuCyte S3 live-cell analysis instrument (Sartorius AG, Gottingen, DE). SUM159 and MDA-MB-231 cells were seeded into 96-well plates at a density of 1,250 cells per well with 100 µL of complete medium. E0771 cells were seeded into 96-well plates at a density of 1,000 cells per well with 100 µL of complete medium. Following an overnight incubation for cell adhesion, 10µL of FXR or TGR5 agonists (INT-747/OCA and INT-777, respectively) were introduced in a dose-dependent manner, with concentrations of 1 µM, 10 µM, 50 µM, & 100 µM, alongside a positive chemotherapy control Paclitaxel (Ptax) at indicated concentrations. Cell proliferation measurements were captured every 6 hrs up to 72 hrs, assessing cell confluency (%) as the primary parameter. Experiments are representative of N=3-4 biological replicates and include N=4-8 technical replicates. When included, representative images are shown, and videos are available in
supplemental materials.
2.4. Cell Viability Assay
Cellular viability was assessed using a Vybrant (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) MTT cell proliferation assay kit (Molecular Probes, Eugene, OR) at 64 hours. 12 mM of MTT stock solution was prepared by adding sterile PBS and 10 µL of the solution was added to each well. Plates were incubated at 37⁰C for 4 hrs. 100 µL of SDS-HCl solution was then added to each well and the plates incubated at 37⁰C for another 4 hrs. Absorbance readings were measured at 570 nm on a Cytation 5 cell imaging multimode reader (BioTek, Winooski, VT). Experiments are representative of N=3 biological replicates and include N=4 technical replicates.
2.5. Migration Assay
The migration capability was studied using a wound or scratch assay on the IncuCyte S3 live-cell analysis instrument. MDA-MB-231 triple negative BC (TNBC) cells were plated in 96-well plates at 7,500 cells per well, respectively. Once cells grew to 90% confluence, the scratch was conducted using the IncuCyte wound maker (Sartorius AG, Gottingen, DE). Wells were washed twice with complete growth medium. The cells remained in low serum (1% FBS) and were treated with 0 (DMSO), 10, 50, and 100 µM of FXR agonist OCA or 0 (DMSO), 1, 10, 50, and 100 µM TGR5 agonist INT-777. The plates were analyzed in the IncuCyte S3 Live Cell Imager for 66-78 hrs. Relative Wound Density (%) was recorded every 6 hrs to endpoint. Experiments are representative of N=3 biological replicates and include N=4-8 technical replicates.
2.6. Western Immunoblot
Cancer cell lines were cultivated at subconfluency and lysed using RIPA lysis buffer (Cat. No: 20188, EMD Millipore Corp, Burlington, MA) as in previous work 16-18. The cells were subsequently freeze-thawed and vortexed in 1X RIPA lysis buffer, followed by centrifugation at 12,000 RPM for 10 minutes at room temperature. The protein supernatant was collected, and its concentration was determined using the Rapid Gold BCA protein assay kit (Cat.no: A53225; PierceTM, Appleton, WI). Eighty µg of protein from each cell lysate was loaded onto a 12% SDS-PAGE gel. Protein separation was achieved at 120 Volts for 45 minutes, and the protein was transferred to a PVDF membrane (Invitrogen) using overnight wet transfer at 4°C. Immunoblotting was performed using polyclonal anti-rabbit FXR and TGR5 for mouse and human proteins at a ratio of 1:2000 overnight and GAPDH was used as the loading control at a ratio of 1:5000 for 1 hr.
2.7. Xenograft model
The UTHSC Animal Care and Use Committee (IACUC) approved all protocols and methods following protocol #23-0432. All animals in the study were kept on a 12-hr light/dark cycle and fed a standard breeder’s chow diet. MDA-MB-231 cells were harvested in serum-free DMEM media and suspended into equal volumes of Matrigel (Product No. 354234, Corning®, Tewksbury, MA) for orthotopic mammary injection in N=30 female NSG mice (12 - 13 weeks old). Five x 105 cells were injected in 100 µl into the 4th right mammary gland at a final Matrigel concentration of 4.5 mg/mL. Injected mice were palpated three times weekly until the tumor grew to a palpable size of 80-150 mm3 in volume using a digital caliper. Mice with equivalent tumor size were randomly separated into 3 groups and that included vehicle (40% DMSO & 60% polyethylene glycol (PEG), N=8), OCA (30 mg/kg, N=7), or chemotherapy paclitaxel (“Ptax”,10 mg/kg, N=6) as a positive control. Animals treated with vehicle or OCA were gavaged daily, and Ptax was administered thrice a week intraperitoneally until the endpoint. Tumor size was calculated using the formula (width2 x length/2) 16, 17, 19. Body weights were recorded every other day. Mice were sacrificed at endpoint. Tumors were excised for ex vivo measurement of tumor volume and weight, pictures, and halved, and snap frozen in liquid nitrogen.
2.8. In Silico Analysis
The Kaplan Meier plotter (kmplot.com 20) database was used to investigate the association between gene expression levels of NR1H4 (FXR) with improved survival in patients with different BC subtypes using RNAseq data.
2.9. Statistical analysis
Values were presented using GraphPad Prism 10.0.2 as Mean ± Standard Error of the Mean (SEM) from at least three independent biological replicate experiments as noted above. Significance was assessed by (Student’s t-test) or One-Way analysis of variance (ANOVA) method in GraphPad Prism and represented with asterisks (*) in the figures indicated as p>0.05*, p>0.01**, p>0.001***, and p>0.0001****.
3. Results
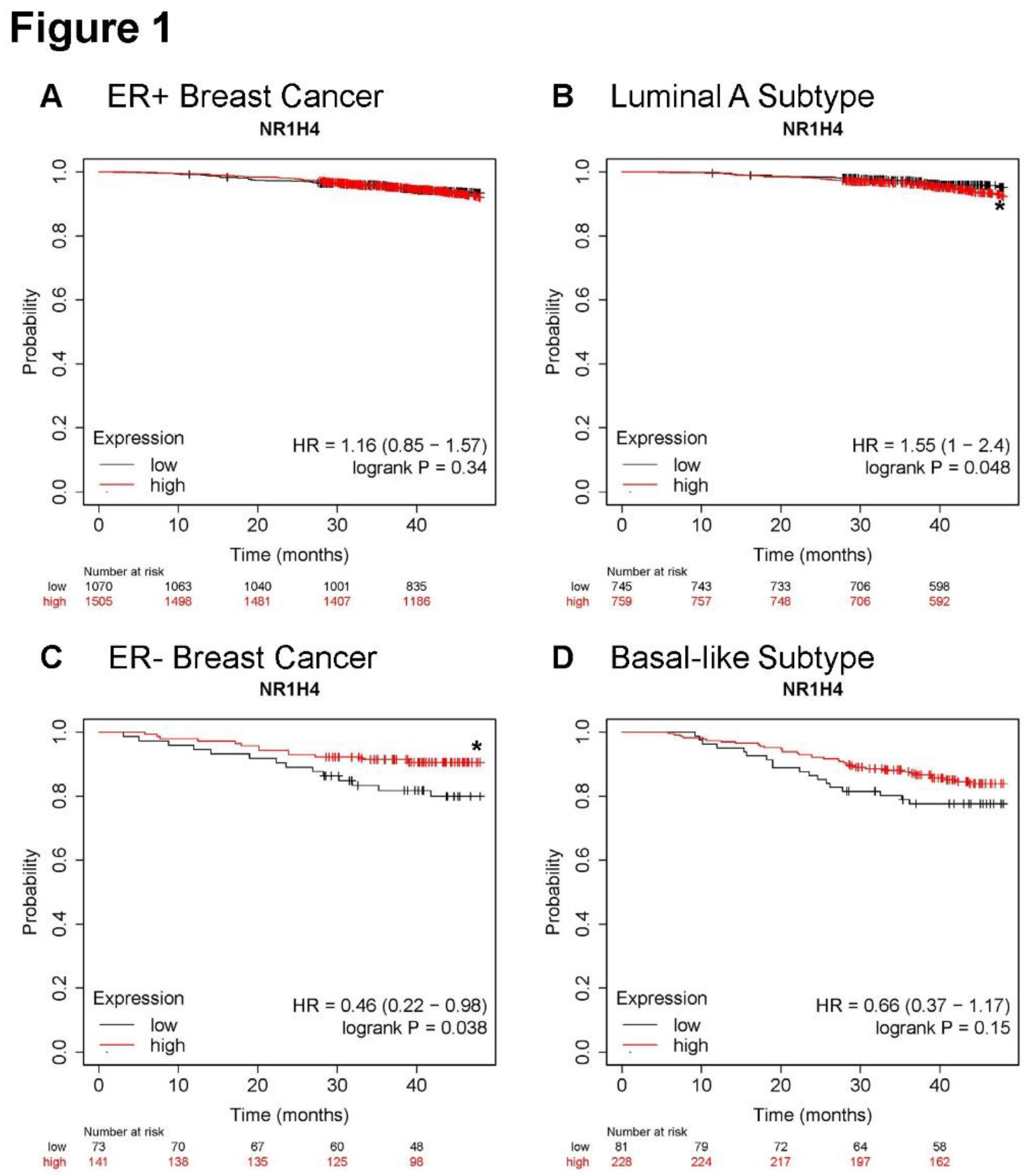
3.1. Higher NR1H4 expression is associated with greater survival in patients with ER- and basal-like BC subtypes
Overall survival was analyzed in KMPlotter using RNAseq data
21. Expression of FXR (
NR1H4) did not alter overall survival in patients with ER+ tumors (
Figure 1A). Likewise, greater
NR1H4 expression in the luminal A subtype, the most prevalent and least aggressive subtype, reduced survival slightly but significantly (
Figure 1B). However, in patients with ER- tumors with higher expression of
NR1H4 significantly associated with improved overall survival (
Figure 1C) with similar protection in patients with tumors of the basal-like subtype, typically TNBC (
Figure 1D). Taken together, in patients with the most aggressive subtypes including ER- and basal-like BC, greater expression of
NR1H4 improved survival.
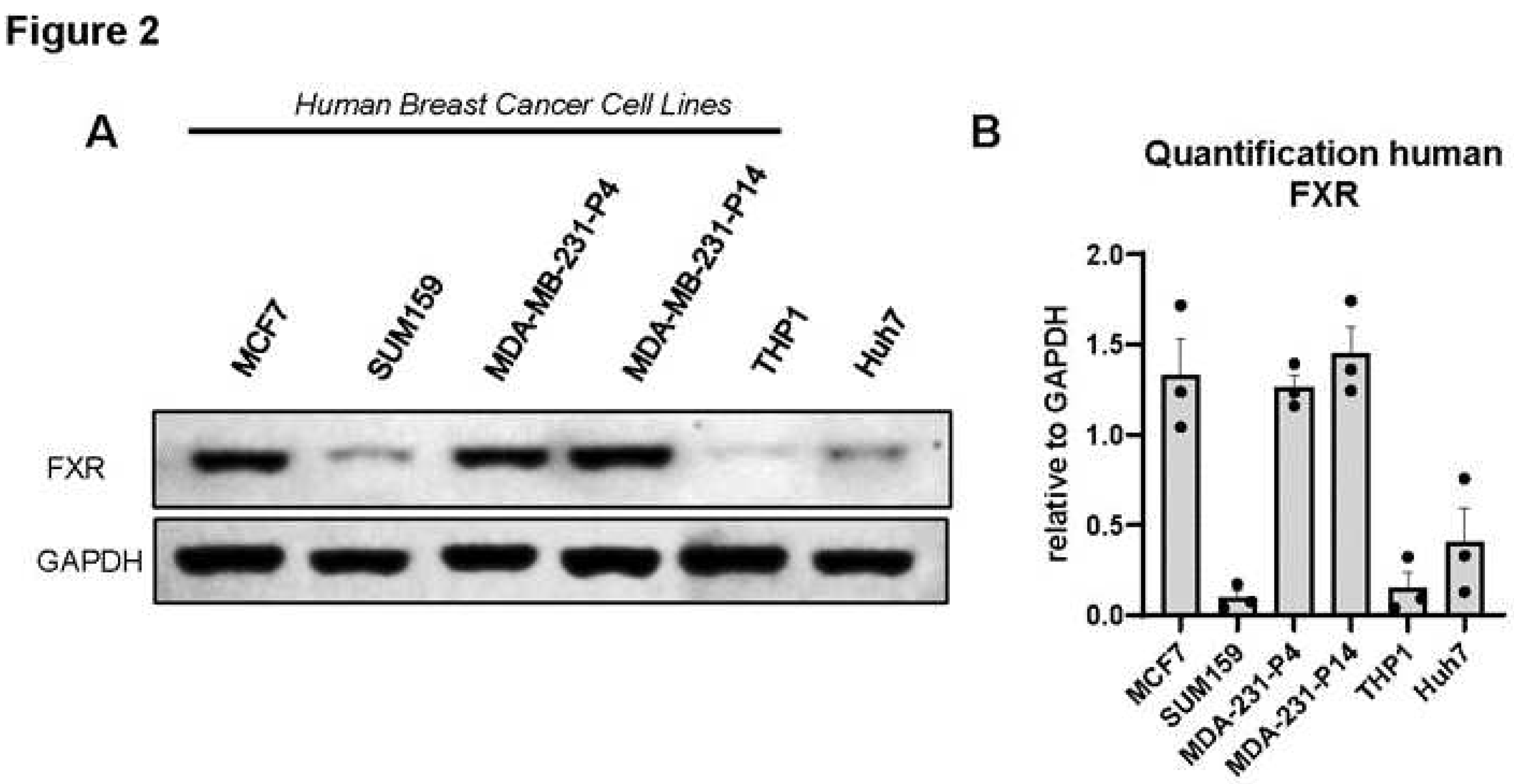
3.2. FXR is expressed in human and murine triple-negative breast cancer cell lines.
Because patients with high expression of
NR1H4 demonstrated greater survival in ER- and basal- like subtypes, which are often TNBC, we aimed to investigate the effects of FXR agonism on TNBC cell lines. We first determined the protein expression of FXR in multiple cell lines. Western immunoblot analysis demonstrated the presence of FXR in human BC cell lines (MDA-MB-231, MCF7, and SUM159). Quantification of triplicate blots demonstrated particularly high levels of FXR protein in MDA-MB-231 (even after high passage) and MCF7 cells with lower levels in SUM159 (
Figure 2A,B). THP1 are a monocytic cell line that express low FXR were included as a negative control, while Huh7 cells are a hepatoma derived hepatocyte line that should express FXR were evaluated as a positive control. Murine E0771 and 4T1 cell lines also express FXR (
Supl Figure S1A,B). In sum, evidence suggests high levels of FXR conserved across multiple BC cell lines.
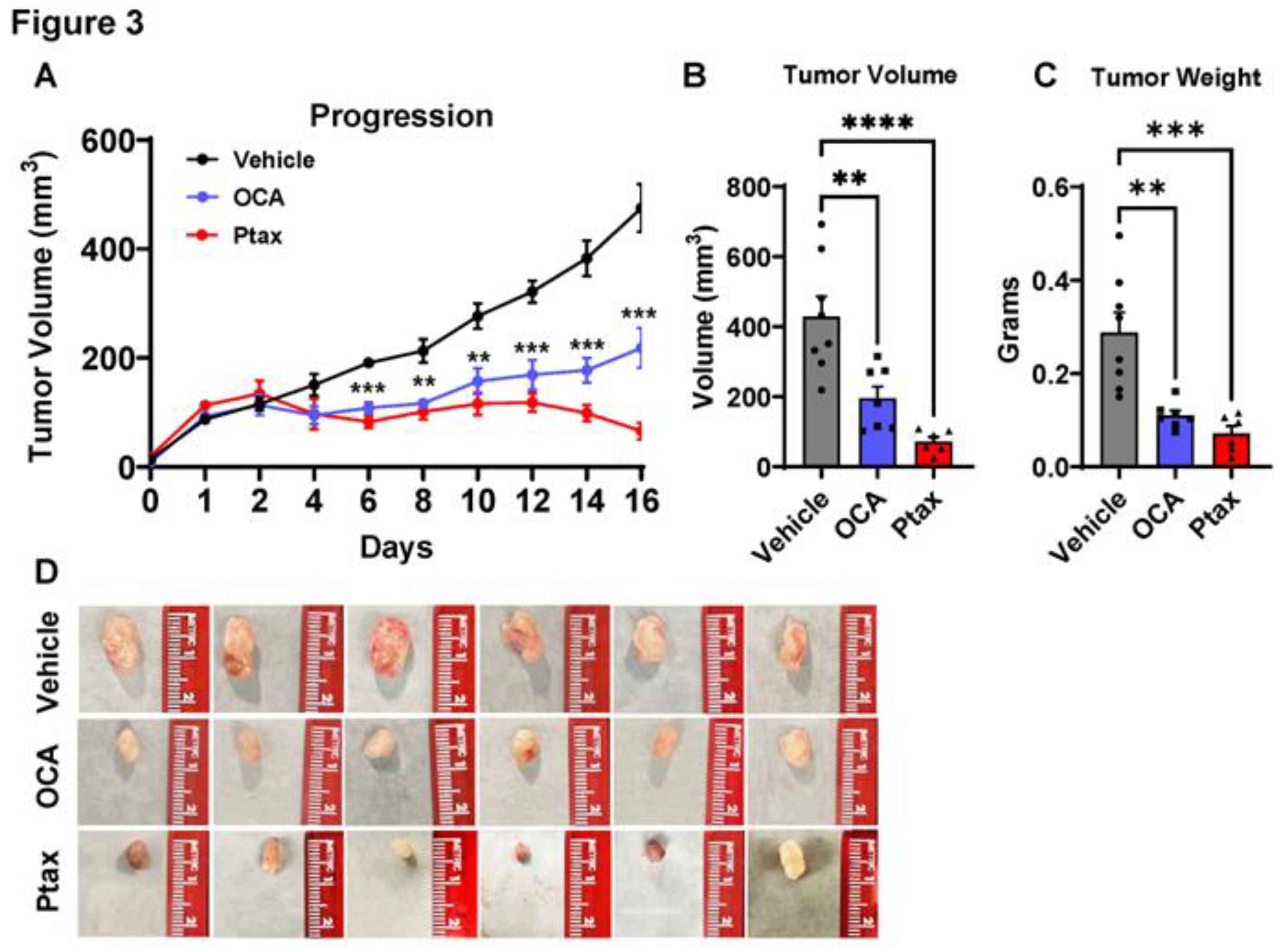
3.3. OCA treatment reduced TNBC tumor growth in xenograft model.
Next, to determine if agonism of FXR could impact tumor progression, we examined OCA treatment in a TNBC xenograft model. Female NSG mice were injected with MDA-MB-231 cells, which resulted in the development of palpable tumors after 36 days. When tumors reached 80mm
3, mice were randomized based on tumor size to treatment group. Vehicle alone or OCA FXR agonist was administered orally once daily at a dose of 30 mg/kg in a vehicle composed of 40% DMSO and 60% PEG. In a third cohort, paclitaxel (Ptax) was injected intraperitoneally three times a week at a dose of 10 mg/kg as a positive control intervention. Body weights were not impacted by vehicle, OCA, or Ptax (
data not shown). OCA treatment significantly reduced breast tumor progression (
Figure 3A) compared to the vehicle group. Ptax reduced tumor progression as expected. Experimental endpoint analyses demonstrated a significant 2.2-fold reduction in tumor volume and 2.6-fold reduction in tumor mass with OCA treatment compared to vehicle controls (
Figure 3B,C). Representative images of excised tumors are shown in
Figure 3D. These findings highlight OCA as a potent therapeutic for inhibiting breast tumor growth
in vivo.
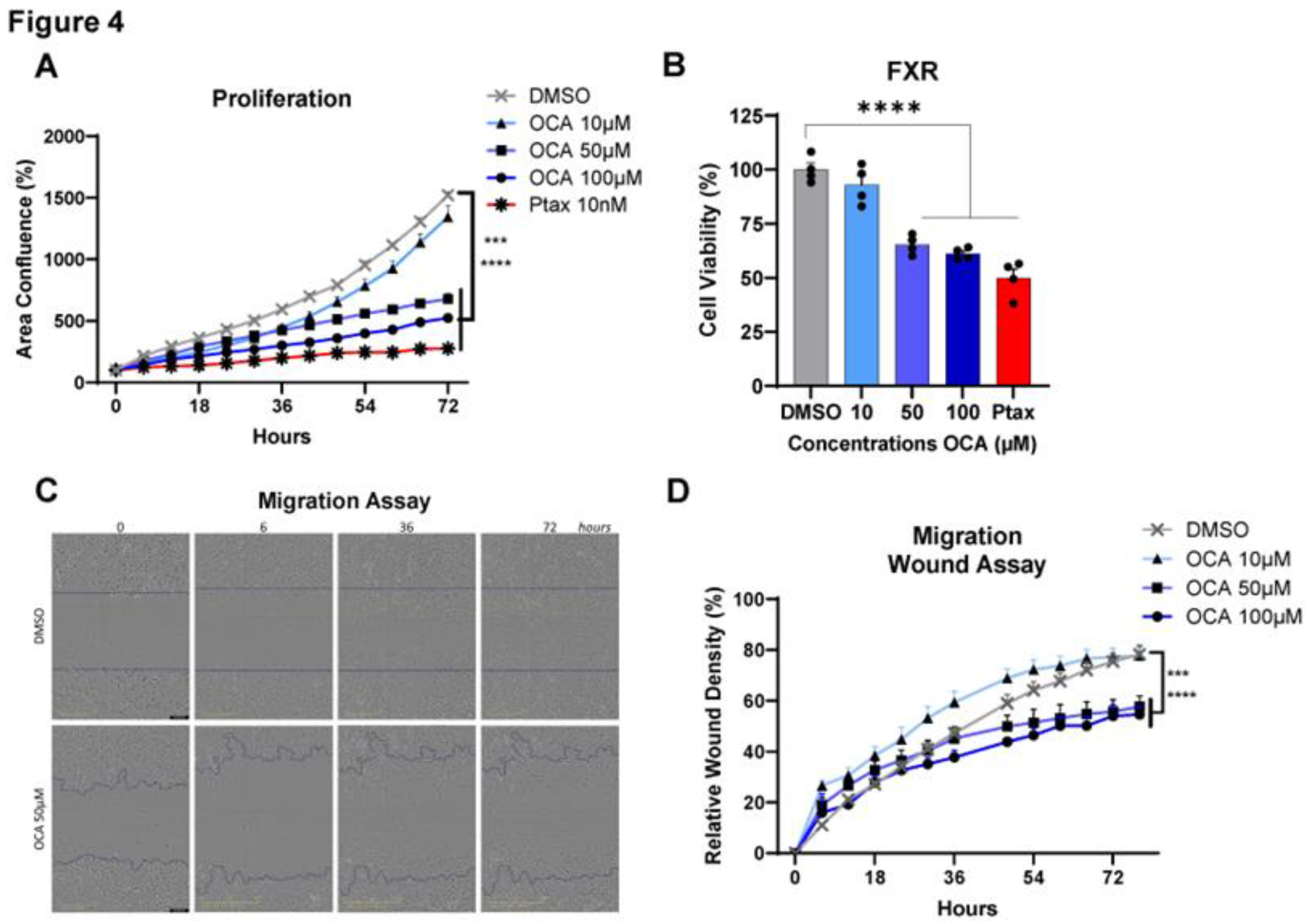
3.4. Agonism of FXR reduces Triple-Negative Breast Cancer cell proliferation, viability, and migration.
To investigate the impacts of OCA on cancer cells, tests including cell proliferation, viability, and migration assays were performed
in vitro by activating FXR over time at concentrations of 0-100 µM. Treatment with OCA demonstrated a potent dose-dependent decrease in MDA-MB-231 BC cell proliferation. Cell proliferation was effectively decreased in OCA-treated cells with doses of 50 and 100 µM, with minimal inhibition quantified with 10 µM (
Figure 4A). Similarly, a consistent reduction in cell viability was measured with higher doses compared to DMSO control and lower doses of OCA and was comparable to the Ptax positive control (
Figure 4B). Cell migration is commonly measured using the wound or scratch assay as a marker of aggressive cancer subtypes to approximate metastasis. Again, migration of MDA-MB-231 cells was not impaired at lower dose of 10 µM but was significantly reduced in cells treated with 50 and 100 µM (
Figure 4C,D, and
videos in Supplemental Videos SV1 and SV2). Analysis of another human TNBC cell line SUM159 showed identical dose and time dependent results on proliferation and viability, with 100 µM OCA having the most potent impacts (
Figure S2A,B). To determine if OCA’s anti-cancer effects were conserved, the murine TNBC cell line E0771 was examined for effects on cell proliferation, viability, and migration. Similar to findings in human cell lines, OCA dose dependently blunted proliferation and viability, with 100 µM leading to excessive cell death (
Figure S1C,D). Migration was also significantly inhibited at 50 µM compared to DMSO controls. Overall, agonism of FXR using a synthetic bile acid mimetic appears to be an effective anti-cancer therapy through reduced cancer cell viability and impaired proliferation and migration.
3.5. TGR5 bile acid receptor agonism failed to reduce Triple-Negative Breast Cancer cell proliferation, viability, and migration.
Lastly, to determine if anti-cancer cell activity was unique to FXR agonism, INT-777, a synthetic agonist of the bile acid receptor TGR5 was also tested for anti-cancer effectiveness. TGR5 is highly expressed in human BC cells (
data not shown). Supplemental Figure 3 (
Figure S3) demonstrates that in human MDA-MB-231 and SUM159 cell lines, TGR5 agonism failed to demonstrate anti-cancer efficacy on cell proliferation, viability, or MDA-MB-231 migration (
Figure S3A–E). Similarly, in murine E0771, INT-777 fails to blunt progression or viability with little impact on migration (
Figure S3G,H). In fact, in some cases, high doses of INT-777 appeared to increase proliferation and migration in human cells above DMSO levels.
4. Discussion
Bile acids have been shown to regulate cancer progression or metastasis in other cancers, but prior to this study, BA-mediated mechanisms had not yet been extensively explored in TNBC 2. The reduction of BC risk after surgery 22 is correlated with elevated bile acid pools observed after bariatric surgery 23, thus revealing possible therapeutic avenues for BC patients related to bile acid signaling. We therefore took advantage of commercially available, and in the case of OCA, FDA approved, synthetic bile acid mimetics. The key findings reported in this study demonstrate a role of bile aid receptor FXR in BC progression. Our data demonstrated that: (i) patients with higher levels of FXR have greater survival, but only in ER- and basal-like subtypes, not ER+ or luminal A subtype; (ii) human and murine BC cell lines express FXR; (iii) the FXR agonist OCA slowed tumor progression in a murine model; (iv) in vitro OCA demonstrated potent dose-dependent inhibition of proliferation and migration, as well as reduced viability. These findings appear unique to FXR agonism because TGR5 agonist INT-777 minimally reduced cell growth and viability and did not impact cell migration. Indeed, others also reported that FXR expression is associated with indicators of better outcomes in BC and is an independent prognostic indicator of survival 24, 25, but these studies did not focus on a subtype specific effect. The striking ER- survival advantage in patients points to the potential for enhanced personalized medicine using BAR agonists. FXR is detected in benign and malignant breast tissue and there it associates with increased apoptosis 26. FXR induces apoptosis in normal and malignant BC cells in vitro 27-29, which is in line with our findings. Taken together, agonism of bile acid receptor FXR may be beneficial to patient survival through direct effects on cancer cell survival and migration. We posited that elevated bile acid metabolites may increase FXR activation potentially through increased apoptosis, reducing breast neoplastic progression.
Microbes may play a central role in cancer biology considering that diversity of the microbiome can be altered by both obesity and cancer2, 30. Microbes can modify levels of their own metabolites and host metabolites, including bile acids 2. Primary (1°) and microbially modified (secondary, 2°) bile acids are not just important in digestion but are bioactive mediators that may signal as paracrine or endocrine factors. Primary bile acids, including cholic acid (cholate, CA), chenodeoxycholic acid (CDCA), and muricholic acid (MCA, in mice), are synthesized from cholesterol via classic and alternative pathways in the liver 6. Primary bile acids are conjugated with glycine (GCA, GCDCA) or taurine (TCA, TCDCA) in the liver before being secreted into the intestine. Certain microbes rich in 7-alpha-hydroxylase, especially Clostridium cluster XIVa and Bacteroides, deconjugate 1° bile acids to allow for 1° to 2° bile acid conversion, which is facilitated by a wide variety of additional microbes. Bile acids may function as potent signaling hormones that regulate energy expenditure, inflammation, and cancer in part through activation of transmembrane or nuclear hormone bile acid receptors. Patients with BC have been reported to have reduced levels of bile acids 31. Interestingly, the presence of bile acids in breast tissue was identified decades ago in humans with evidence that oral bile acid administration preferentially accumulated in the breast 32, 33. Cook et al. identified bile acids in non-human primates that were modified by Western or Mediterranean diet, importantly not reflected in circulating bile acid pools 34. These data point to exciting future studies in the increasingly complex roles for microbes and microbially derived metabolites in cancer risk and progression.
Patients and pre-clinical models that are obese display microbial alterations compared to lean controls. Obesity leads to poor cancer outcomes for patients. The most effective treatment option for obese patients today is bariatric surgery 3. Recent findings have demonstrated that weight loss by bariatric surgery is not only protective, especially for ER- tumors including TNBC 2, 35, but also improves clinical outcomes 4. Furthermore, we recently demonstrated that weight loss by bariatric surgery decreased TNBC tumor progression in murine models compared to obese controls receiving sham surgery 17. It is possible that bile acids elevated by bariatric surgery may be one mechanism by which improved cancer outcomes are observed in bariatric surgery patients even 5-10 years post-surgery. Though mechanisms underlying bariatric surgery-associated protections are unknown, increasing interest in the field of microbially derived metabolites suggest that cancer could be mediated in part by metabolites such as bile acids and/or agonism of BARs as indicated by work presented herein.
BC subtypes are diagnosed by the presence or absence of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), and tumors that lack all three receptors are triple-negative BC (TNBC) 2. TNBC is a highly aggressive subtype and typically cannot be treated with targeted approaches because most therapies on the market target these three receptors. Considering the limited targeting therapies, patients with TNBC have higher rates of recurrence and metastasis, with poorer overall survival than those with other BC subtypes 36, 37. This gap in clinical care emphasizes the importance of discovering new targeted cancer prevention strategies for TNBC to improve patient survival such as FXR agonism. Analysis of immunotherapy responsiveness across multiple cancers 38 showed that patients with high FXR (NR1H4) performed significantly better with high overall survival after anti-PD-L1 therapy. However, it should be noted that breast cancer is not yet available in that database 38. While chemotherapy is the standard treatment option for TNBC, immunotherapies have recently been approved. Clinically, not every patient responds well to immunotherapy or may have adverse reactions 39. As more TNBC patients receive immunotherapy, it will be interesting to investigate the interaction of bile acids, FXR expression, mutations, or other factors that may mediate response to therapy.
In summary, findings suggest that FXR functions as a tumor suppressor. FXR agonism drastically reduced aggressive cellular cancer dynamics and in vivo demonstrated reduced tumor progression. Taken together, our data provides important support for the potential role of BAR FXR agonism in BC as a potential therapeutic driving tumor suppressor function.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Supplementary Table S1 (Table ST1): Antibodies and reagents; Supplemental Video (SV) Files: Impact of FXR agonism on proliferation. SV1 MDA cells from 0-72 hours with DMSO control or 50µM OCA (SV2). MDA-MB-231 TNBC cell lines were plated in 96 well plates at 1,250 cells per well and IncuCyte Live Cell Imager quantified data at the indicated time points. Cells were treated with 0 or 50 µM of obeticholic acid (OCA, INT-747) in DMSO. Proliferation was measured as area confluence (%). Representative images at time 0, 6, 36, and 72 hours are shown for DMSO and 50µM OCA in Figure 4C. Experiments include n=4 technical replicates, with n=3 biological replicates. Videos are representative of 1 well; Supplementary Figure S1 (Figure S1): Impact of FXR agonism on cell proliferation, viability, and migration in murine cells. A-B. Murine TNBC cell lines E0771 and 4T1 were immunoblotted for FXR with murine (mu) liver as a positive control and human MDA-MB-231 TNBC cells included for comparison. GAPDH was the loading control. Representative images are shown out of N=3 Western immunoblots. B. Images were quantified using ImageJ with the relative intensity of protein expression of FXR normalized to GAPDH loading control with Mean -/+ SEM shown. C-D. E0771 TNBC cell lines were plated in 96 well plates at 1,000 cells per well and IncuCyte Live Cell Imager quantified data at the indicated time points. Cells were treated with increasing concentrations (0-100 µM) of obeticholic acid (“OCA”, INT-747). Paclitaxel (Ptax) was used as a positive control at 1.17 µM. C. Proliferation was measured as area confluence (%). D. Cell viability was quantified by MTT assay at 64-hour timepoint. Statistical analysis was conducted using Fisher’s LSD or One way ANOVA. Experiments include n=4-8 technical replicates and are representative of N=3-4 biological replicates. Data are shown as Means -/+ SEM. **p<0.01, ***p<0.001, ****p<0.0001; Supplemental Figure S2 (Figure S2). Impact of FXR agonism on cell proliferation, viability, and migration in SUM159 cells. A-B. SUM159 TNBC cell lines were plated in 96 well plates at 1,250 cells per well and IncuCyte Live Cell Imager quantified data at the indicated time points. Cells were treated with increasing concentrations (0-100 µM) of obeticholic acid (OCA, INT-747). Paclitaxel (Ptax) was used as a positive control at 10 nM. A. Proliferation was measured as area confluence (%). B. Cell viability was quantified by MTT assay at 64-hour timepoint. Statistical analysis was conducted using Fisher’s LSD or One way ANOVA. Experiments include n=4 technical replicates and are representative of N=3 biological replicates. Data are shown as Means -/+ SEM. *p<0.05, **p<0.01, ***p<0.001; Supplemental Figure S3 (Figure S3). Impact of TGR5 agonism on cell proliferation, viability, and migration in MDA-MB-231, SUM159, and E0771 cells. A-E. MDA-MB-231 and SUM159 TNBC cell lines were plated in 96 well plates at 1,250 cells per well. F-H. Murine E0771 TNBC cell line was plated in 96 well plates at 1,000 cells per well. IncuCyte Live Cell Imager quantified data at the indicated time points. Cells were treated with increasing concentrations (0-100 µM) of TGR5 ligand INT-777. Paclitaxel (Ptax) was used as a positive control at 10nM. A, D, F: Proliferation was measured as area confluence (%). B, E, G: Cell viability was quantified by MTT assay at 64-hour timepoint. Paclitaxel (Ptax) was used as a positive control at 1.17µM. C, H: Cell migration was quantified using the IncuCyte by a migration wound or scratch assay. MDA-MB-231 or Murine E0771 cells were plated in 96 well plates at 7,500 cells per well or 3,000 cells per well, respectively. Quantification of relative wound density (%). Statistical analysis was conducted using Fisher’s LSD or One way ANOVA. Experiments include n=4-8 technical replicates and are representative of N=3-4 biological replicates. Data are shown as Means -/+ SEM. ****p<0.0001.
Author Contributions
Conceptualization: SCJ, SES, MSB, LMS, RN, KLC, JFP, LM; Methodology: SCJ, SES, MSB, MK, MEP, BWS, DGG, STG, UT, JRH, SP, RN; Formal analysis: SCJ, SES, MSB, MK, UT, JRT, LM; Investigation: SCJ, SES, MSB, LMS, MK, MEP, BWS, DGG, UT, JRT; Resources: LM, DNH, RN; Writing – original draft: SCJ, SES, LM; Writing – review & editing: All authors; Supervision: DNH, LM; Project administration: SCJ, SES, LM.
Funding
This work was supported by the following grants to faculty and trainees: National Institutes of Health grant NCI R01CA253329 (LM, JFP, KLC), National Institutes of Health grant NCI U01CA272541 (LM, JFP, KLC), DOD BCRP Breakthrough Level 2 award BC190271 (KLC), National Institutes of Health grant NCI R37CA226969 (DNH, LM), National Institutes of Health grant NCI UG1CA233333 (DNH), National Institutes of Health grant NCI CA264021 (DNH), V Foundation (DNH, LM), Transdisciplinary Research on Energetics and Cancer R25CA203650 (LMS), National Institutes of Health NCI F32 CA250192 (LMS), The Obesity Society/Susan G. Komen Cancer Challenge award 2018 (LMS), National Institutes of Health grant NCI R01 CA229164 (RN), W81XWH-21-1-0055 (RN), National Institutes of Health grant NIDDK R01DK127209 (JFP), Tennessee Governor Pediatric Recruitment Grant (JFP), Tennessee Clinical and Translational Science Institute (JFP), National Institute of Health grant NCI F30CA265224 (JRH).
Acknowledgments
We thank the UTHSC Center for Cancer Research Animal Shared Resource.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J Clin 2023, 73(1), 17–48. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.S.; Sipe, L.M.; Pye, M.E.; Davis, M.J.; Pierre, J.F.; Makowski, L. The role of obesity and bariatric surgery-induced weight loss in breast cancer. Cancer Metastasis Rev 2022, 41(3), 673–695. [Google Scholar] [CrossRef]
- Aminian, A.; Wilson, R.; Al-Kurd, A.; Tu, C.; Milinovich, A.; Kroh, M.; Rosenthal, R.J.; Brethauer, S.A.; Schauer, P.R.; Kattan, M.W.; et al. Association of Bariatric Surgery With Cancer Risk and Mortality in Adults With Obesity. Jama 2022, 327(24), 2423–2433. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Kruszon-Moran, D.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Trends in Obesity Among Adults in the United States, 2005 to 2014. JAMA 2016, 315(21), 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease, C. Prevention. Vital signs: binge drinking prevalence, frequency, and intensity among adults - United States, 2010. MMWR Morb Mortal Wkly Rep 2012, 61 (1), 14-19.
- Sipe, L.M.; Chaib, M.; Pingili, A.K.; Pierre, J.F.; Makowski, L. Microbiome, bile acids, and obesity: How microbially modified metabolites shape anti-tumor immunity. Immunol Rev 2020, 295(1), 220–239. [Google Scholar] [CrossRef] [PubMed]
- Mikó, E.; Vida, A.; Kovács, T.; Ujlaki, G.; Trencsényi, G.; Márton, J.; Sári, Z.; Kovács, P.; Boratkó, A.; Hujber, Z.; et al. Lithocholic acid, a bacterial metabolite reduces breast cancer cell proliferation and aggressiveness. Biochim Biophys Acta Bioenerg 2018, 1859(9), 958–974. [Google Scholar] [CrossRef]
- Režen, T.; Rozman, D.; Kovács, T.; Kovács, P.; Sipos, A.; Bai, P.; Mikó, E. The role of bile acids in carcinogenesis. Cell Mol Life Sci 2022, 79(5), 243. [Google Scholar] [CrossRef]
- Chiang, J. Y. L.; Ferrell, J. M. Discovery of farnesoid X receptor and its role in bile acid metabolism. Mol Cell Endocrinol 2022, 548, 111618. [Google Scholar] [CrossRef]
- Schumacher, J. D.; Guo, G. L. Pharmacologic Modulation of Bile Acid-FXR-FGF15/FGF19 Pathway for the Treatment of Nonalcoholic Steatohepatitis. Handb Exp Pharmacol 2019, 256, 325–357. [Google Scholar] [CrossRef]
- Huang, X.; Fan, M.; Huang, W. Pleiotropic roles of FXR in liver and colorectal cancers. Mol Cell Endocrinol 2022, 543, 111543. [Google Scholar] [CrossRef]
- Kao, C. C.; Lai, C. R.; Lin, Y. H.; Chen, T. M.; Tsai, Y. L.; Tsai, W. C.; Ong, T. Y.; Wang, H. H.; Wu, S. T.; Chen, Y. GW4064 inhibits migration and invasion through cathepsin B and MMP2 downregulation in human bladder cancer. Chem Biol Interact 2024, 389, 110869. [Google Scholar] [CrossRef]
- Golonka, R. M.; San Yeoh, B.; Saha, P.; Tian, Y.; Chiang, J. Y. L.; Patterson, A. D.; Gewirtz, A. T.; Joe, B.; Vijay-Kumar, M. Sex dimorphic effects of bile acid metabolism in liver cancer in mice. Cell Mol Gastroenterol Hepatol 2024. [CrossRef]
- Stofan, M.; Guo, G. L. Bile Acids and FXR: Novel Targets for Liver Diseases. Front Med (Lausanne) 2020, 7, 544. [Google Scholar] [CrossRef]
- Pingili, A. K.; Chaib, M.; Sipe, L. M.; Miller, E. J.; Teng, B.; Sharma, R.; Yarbro, J. R.; Asemota, S.; Al Abdallah, Q.; Mims, T. S.; et al. Immune checkpoint blockade reprograms systemic immune landscape and tumor microenvironment in obesity-associated breast cancer. Cell Rep 2021, 35(12), 109285. [Google Scholar] [CrossRef] [PubMed]
- Chaib, M.; Sipe, L. M.; Yarbro, J. R.; Bohm, M. S.; Counts, B. R.; Tanveer, U.; Pingili, A. K.; Daria, D.; Marion, T. N.; Carson, J. A.; et al. PKC agonism restricts innate immune suppression, promotes antigen cross-presentation and synergizes with agonistic CD40 antibody therapy to activate CD8(+) T cells in breast cancer. Cancer Lett 2022, 531, 98–108. [Google Scholar] [CrossRef]
- Sipe, L. M.; Chaib, M.; Korba, E. B.; Jo, H.; Lovely, M. C.; Counts, B. R.; Tanveer, U.; Holt, J. R.; Clements, J. C.; John, N. A.; et al. Response to immune checkpoint blockade improved in pre-clinical model of breast cancer after bariatric surgery. Elife 2022, 11. [Google Scholar] [CrossRef]
- Eugin Simon, S.; Ahmed, U.; Saad, S. M.; Anwar, A.; Khan, K. M.; Tan, E. W.; Tan, K. O. New synthetic phenylquinazoline derivatives induce apoptosis by targeting the pro-survival members of the BCL-2 family. Bioorg Med Chem Lett 2022, 67, 128731. [Google Scholar] [CrossRef]
- Chaib, M.; Holt, J. R.; Fisher, E. L.; Sipe, L. M.; Bohm, M. S.; Joseph, S. C.; Simmons, B. W.; Eugin Simon, S.; Yarbro, J. R.; Tanveer, U.; et al. Protein kinase C delta regulates mononuclear phagocytes and hinders response to immunotherapy in cancer. Sci Adv 2023, 9(51), eadd3231. [Google Scholar] [CrossRef]
- Gyorffy, B.; Lanczky, A.; Eklund, A. C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 2010, 123(3), 725–731. [Google Scholar] [CrossRef] [PubMed]
- Lánczky, A.; Győrffy, B. Web-Based Survival Analysis Tool Tailored for Medical Research (KMplot): Development and Implementation. J Med Internet Res 2021, 23(7), e27633. [Google Scholar] [CrossRef] [PubMed]
- Feigelson, H. S.; Caan, B.; Weinmann, S.; Leonard, A. C.; Powers, J. D.; Yenumula, P. R.; Arterburn, D. E.; Koebnick, C.; Altaye, M.; Schauer, D. P. Bariatric Surgery is Associated With Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women. Ann Surg 2019. [CrossRef] [PubMed]
- Wang, W.; Cheng, Z.; Wang, Y.; Dai, Y.; Zhang, X.; Hu, S. Role of Bile Acids in Bariatric Surgery. Front Physiol 2019, 10, 374. [Google Scholar] [CrossRef]
- Giaginis, C.; Karandrea, D.; Alexandrou, P.; Giannopoulou, I.; Tsourouflis, G.; Troungos, C.; Danas, E.; Keramopoulos, A.; Patsouris, E.; Nakopoulou, L.; Theocharis, S. High Farnesoid X Receptor (FXR) expression is a strong and independent prognosticator in invasive breast carcinoma. Neoplasma 2017, 64(4), 633–639. [Google Scholar] [CrossRef]
- Barone, I.; Vircillo, V.; Giordano, C.; Gelsomino, L.; Gyorffy, B.; Tarallo, R.; Rinaldi, A.; Bruno, G.; Caruso, A.; Romeo, F.; et al. Activation of Farnesoid X Receptor impairs the tumor-promoting function of breast cancer-associated fibroblasts. Cancer Lett 2018, 437, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Swales, K. E.; Korbonits, M.; Carpenter, R.; Walsh, D. T.; Warner, T. D.; Bishop-Bailey, D. The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res 2006, 66(20), 10120–10126. [Google Scholar] [CrossRef] [PubMed]
- Baker, P. R.; Wilton, J. C.; Jones, C. E.; Stenzel, D. J.; Watson, N.; Smith, G. J. Bile acids influence the growth, oestrogen receptor and oestrogen-regulated proteins of MCF-7 human breast cancer cells. Br J Cancer 1992, 65(4), 566–572. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Barone, I.; Vircillo, V.; Panza, S.; Malivindi, R.; Gelsomino, L.; Pellegrino, M.; Rago, V.; Mauro, L.; Lanzino, M.; et al. Activated FXR Inhibits Leptin Signaling and Counteracts Tumor-promoting Activities of Cancer-Associated Fibroblasts in Breast Malignancy. Sci Rep 2016, 6, 21782. [Google Scholar] [CrossRef]
- Giordano, C.; Catalano, S.; Panza, S.; Vizza, D.; Barone, I.; Bonofiglio, D.; Gelsomino, L.; Rizza, P.; Fuqua, S. A.; Ando, S. Farnesoid X receptor inhibits tamoxifen-resistant MCF-7 breast cancer cell growth through downregulation of HER2 expression. Oncogene 2011, 30(39), 4129–4140. [Google Scholar] [CrossRef]
- Sepich-Poore, G. D.; Zitvogel, L.; Straussman, R.; Hasty, J.; Wargo, J. A.; Knight, R. The microbiome and human cancer. Science 2021, 371 (6536). [CrossRef]
- Rezen, T.; Rozman, D.; Kovacs, T.; Kovacs, P.; Sipos, A.; Bai, P.; Miko, E. The role of bile acids in carcinogenesis. Cell Mol Life Sci 2022, 79(5), 243. [Google Scholar] [CrossRef]
- Javitt, N. B.; Budai, K.; Miller, D. G.; Cahan, A. C.; Raju, U.; Levitz, M. Breast-gut connection: origin of chenodeoxycholic acid in breast cyst fluid. Lancet 1994, 343(8898), 633–635. [Google Scholar] [CrossRef]
- Raju, U.; Levitz, M.; Javitt, N. B. Bile acids in human breast cyst fluid: the identification of lithocholic acid. J Clin Endocrinol Metab 1990, 70(4), 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Shively, C. A.; Register, T. C.; Appt, S. E.; Clarkson, T. B.; Uberseder, B.; Clear, K. Y. J.; Wilson, A. S.; Chiba, A.; Tooze, J. A.; Cook, K. L. Consumption of Mediterranean versus Western Diet Leads to Distinct Mammary Gland Microbiome Populations. Cell Rep 2018, 25 (1), 47-56 e43. [CrossRef]
- Feigelson, H. S.; Caan, B.; Weinmann, S.; Leonard, A. C.; Powers, J. D.; Yenumula, P. R.; Arterburn, D. E.; Koebnick, C.; Altaye, M.; Schauer, D. P. Bariatric Surgery is Associated With Reduced Risk of Breast Cancer in Both Premenopausal and Postmenopausal Women. Ann Surg 2020, 272(6), 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kohler, B. A.; Sherman, R. L.; Howlader, N.; Jemal, A.; Ryerson, A. B.; Henry, K. A.; Boscoe, F. P.; Cronin, K. A.; Lake, A.; Noone, A. M.; et al. Annual Report to the Nation on the Status of Cancer, 1975-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst 2015, 107(6), djv048. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K. B.; Neuhausen, S. L.; Robson, M.; Barrowdale, D.; McGuffog, L.; Mulligan, A. M.; Andrulis, I. L.; Spurdle, A. B.; Schmidt, M. K.; Schmutzler, R. K.; et al. Associations of common breast cancer susceptibility alleles with risk of breast cancer subtypes in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res 2014, 16(6), 3416. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S. A.; Fekete, J. T.; Gyorffy, B. Predictive biomarkers of immunotherapy response with pharmacological applications in solid tumors. Acta Pharmacol Sin 2023, 44(9), 1879–1889. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E. O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15 (7). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).