Submitted:
25 January 2024
Posted:
26 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Ribosome Structure and Functions
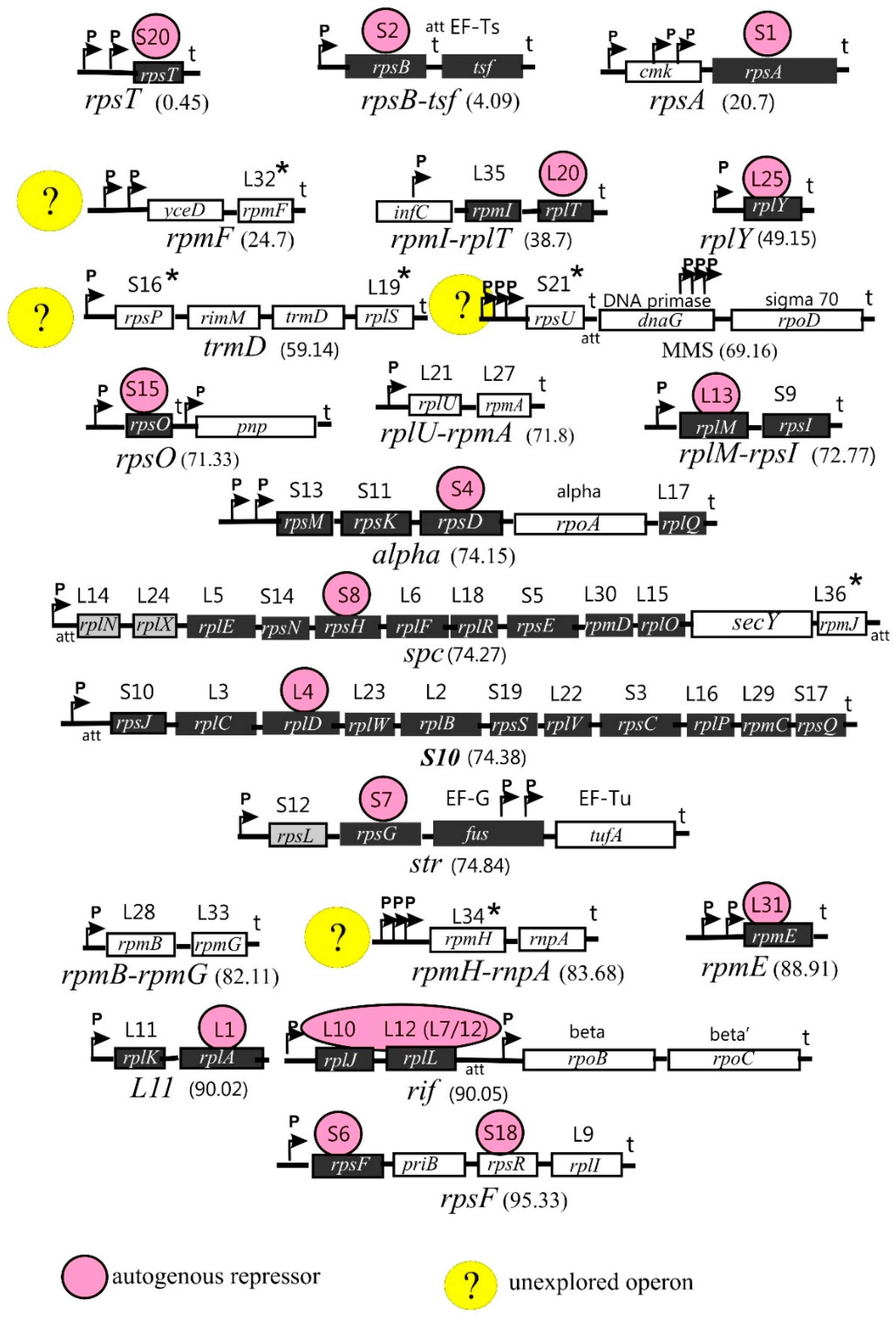
1.2. Arrangement of the r-Protein Genes on Bacterial Chromosome
2. Moonlighting r-Proteins of the 30S Ribosomal Subunit
2.1. Multiple Activities of bS1
2.1.1. Structure and Unique Features of bS1
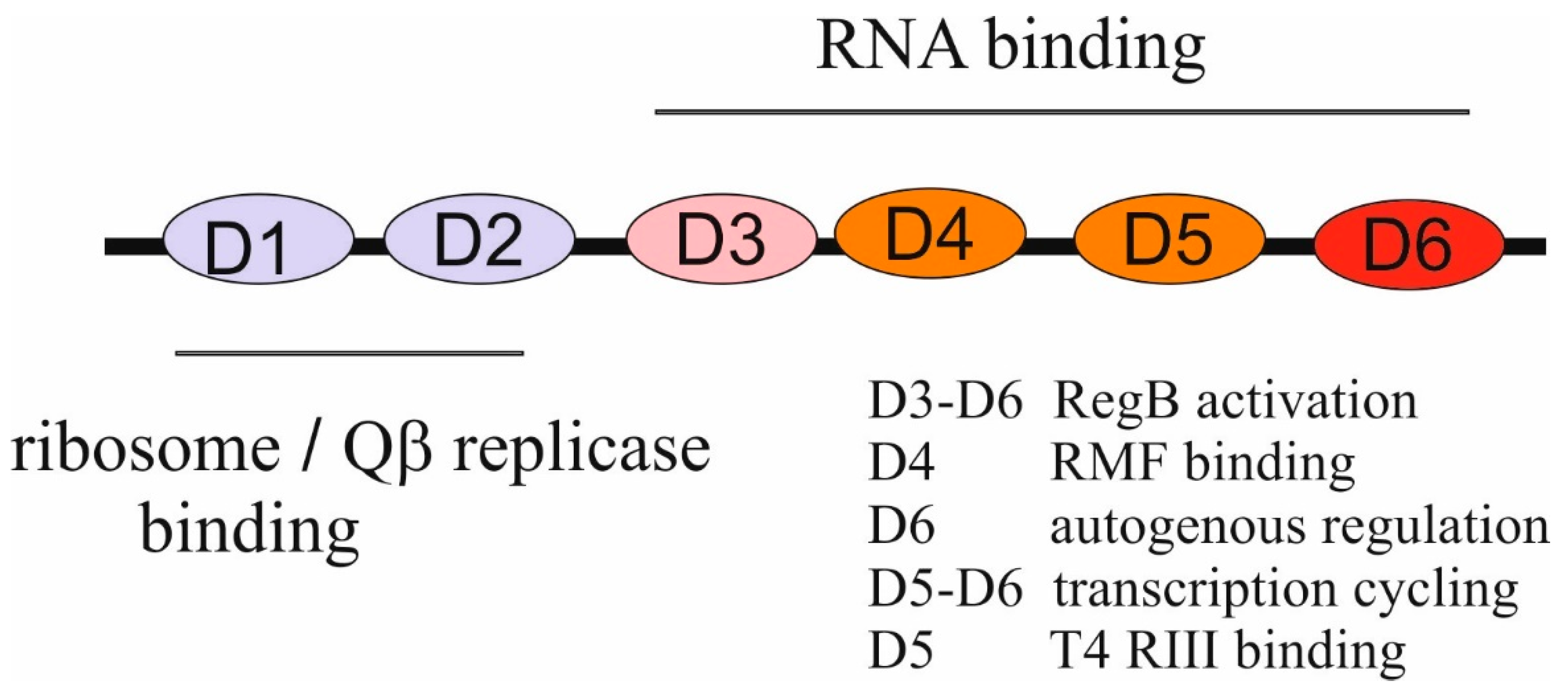
2.1.2. Functions of bS1 in Translation, Translational Control, Transcription, and RNA Decay
2.1.3. bS1 and Trans-Translation
2.1.4. Functions of bS1 during Infections with Bacteriophages
2.2. Functions of uS2 beyond the Ribosome
2.3. uS4, an Essential r-Protein Functioning in Ribosome Biogenesis, Translation, and Transcription
2.4. Ribosomal Proteins bS6 and bS18 Act in Tandem
2.5. A Key Primary Assembly r-Protein uS7 Is Bifunctional
2.6. uS8 Regulates the Longest spc Operon
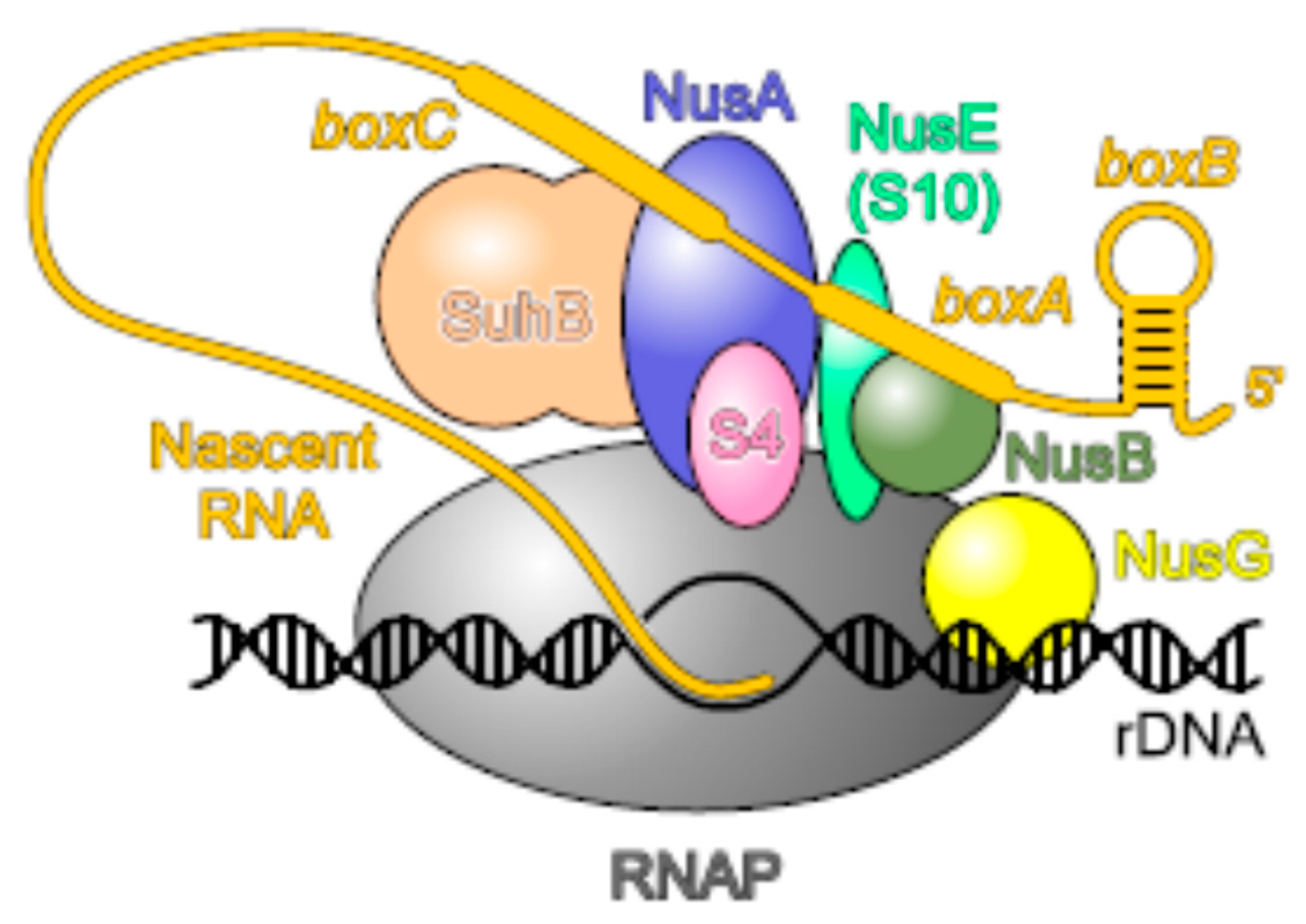
2.7. uS10, an Essential Player in Transcription-Translation Coupling and Transcription Antitermination
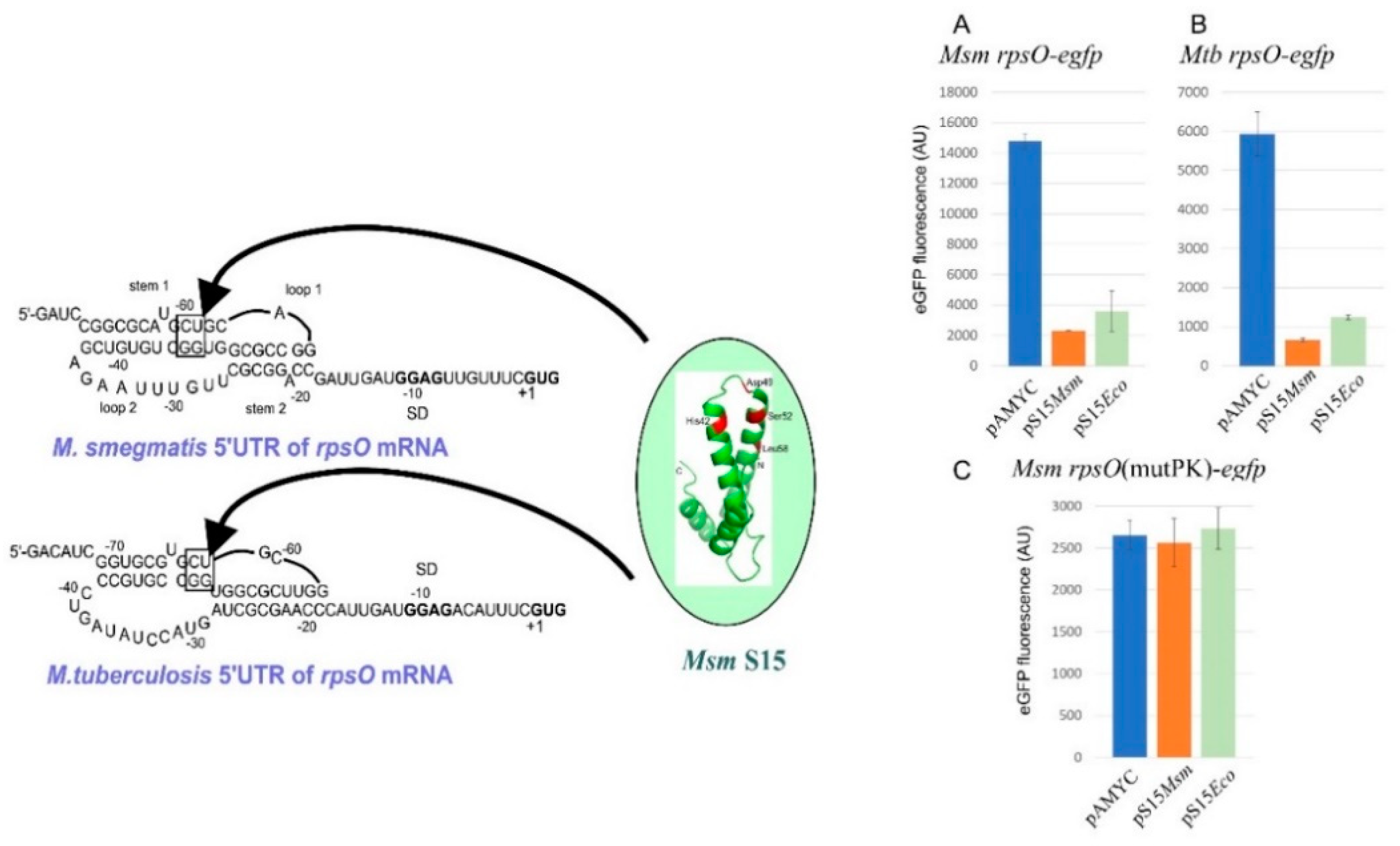
2.8. uS15, a Translational Auto-Repressor in Various Bacterial Phyla
2.9. bS20, a Curious Case of a Regulatory Protein
2.10. bS21 and Heterogeneity of Ribosome Population
3. Multifunctional Proteins of the 50S Ribosomal Subunit
3.1. uL1 as a Widespread Autogenous Repressor
3.2. Multiple Activities of uL2
3.3. uL4 Is Multifunctional
3.4. bL7/12 and uL10 Form Pentameric Complex Both on and beyond the Ribosome
3.5. Ribosomal and Extraribosomal Functions of bL9
3.6. uL13, a Novel Autogenous Repressor
3.7. bL20, an Autogenous Repressor in E. coli and B. subtilis
3.8. Autogenous Regulation of bL25
3.9. Dual activity of bL31 and Its Paralog
4. Non-Specific Activities of Bacterial r-Proteins
4.1. Antimicrobial Activity
4.2. Ribosomal Proteins as Chaperons
4.3. Interactions of r-Proteins with DNA
Concluding Remarks
Funding
References
- Aseev, L.V.; Boni, I.V. Extraribosomal functions of bacterial ribosomal proteins. Mol. Biol. 2011, 45, 739–750. [Google Scholar] [CrossRef]
- Watson, Z.L.; Ward, F.R.; Méheust, R.; Ad, O.; Schepartz, A.; Banfield, J.F.; Cate, J.H. Structure of the bacterial ribosome at 2 Å resolution. Elife 2020, 9, e60482. [Google Scholar] [CrossRef]
- Steitz, T.A. A structural understanding of the ribosome. Nature Rev. Mol. Cell Biol. 2008, 9, 242–253. [Google Scholar] [CrossRef]
- Selmer, M.; Dunham, C.M.; Murphy, F.V. 4th; Weixlbaumer, A.; Petry, S.; Kelley, A.C. 4th; Weixlbaumer, A.; Petry, S.; Kelley, A.C.; Weir, JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science 2006, 313, 1935–1942. [Google Scholar] [CrossRef]
- Gao, Y.G.; Selmer, M.; Dunham, C.M.; Weixlbaumer, A.; Kelley, A.C.; Ramakrishnan, V. The structure of the ribosome with elongation factor G trapped in the posttranslocational state. Science 2009, 326, 694–699. [Google Scholar] [CrossRef]
- Zhou, J.; Lancaster, L.; Donohue, J.P.; Noller, H.F. How the ribosome hands the A-site tRNA to the P site during EF-G-catalyzed translocation. Science 2014, 345, 1188–1191. [Google Scholar] [CrossRef]
- Sharma, M.R.; Manjari, S.R.; Agrawal, E.K.; Keshavan, P.; Koripella, R.K.; Majumdar, S.; Marcinkiewicz, L.; Lin, Y.P.; Agrawal, R.K.; Banavali, N.K. The structure of a hibernating ribosome in a Lyme disease pathogen. Nat. Commun. 2023, 14, 6961. [Google Scholar] [CrossRef]
- Morgan, C.E.; Huang, W.; Rudin, S.D.; Taylor, D.J.; Kirby, J.E.; Bonomo, R.A.; Yu, E.W. Cryo-electron microscopy structure of the Acinetobacter baumannii 70S ribosome and implications for new antibiotic development. mBio. 2020, 11, e03117-119. [Google Scholar] [CrossRef]
- Kushwaha, A.K.; Bhushan, S. Unique structural features of the Mycobacterium ribosome. Prog. Biophys. Mol. Biol. 2020, 152, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.E. Origin and evolution of the ribosome. Cold Spring Harb. Perspect. Biol 2010, 2, a003483. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.S.; Gulen, B.; Norris, A.M.; Kovacs, N.A.; Bernier, C.R.; Lanier, K.A.; Fox, G.E.; Harvey, S.C.; Wartell, R.M.; Hud, N.V.; Williams, L.D. History of the ribosome and the origin of translation. Proc. Natl. Acad. Sci. U S A 2015, 112(50), 15396–15401. [Google Scholar] [CrossRef] [PubMed]
- Tirumalai, M.R.; Rivas, M.; Tran, Q.; Fox, G.E. The peptidyl transferase center: a window to the past. Microbiol. Mol. Biol. Rev 2021, 85, e001042. [Google Scholar] [CrossRef] [PubMed]
- Noller, H.F. Evolution of protein synthesis from an RNA world. Cold. Spring Harb. Perspect. Biol. 2012, 4, a003681. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J. C.; Petrov, A. S. , Frenkel-Pinter, M.; Penev, P. I.; Williams, L. D. Root of the tree: the significance, evolution, and origins of the ribosome. Chem. Rev. 2020, 120, 4848–4878. [Google Scholar] [CrossRef]
- Bose, T.; Fridkin, G.; Davidovich, C.; Krupkin, M.; Dinger, N.; Falkovich, A.H.; Peleg, Y.; Agmon, I.; Bashan, A.; Yonath, A. Origin of life: protoribosome forms peptide bonds and links RNA and protein dominated worlds. Nucleic Acids Res. 2022, 50(4), 1815–1828. [Google Scholar] [CrossRef]
- Ishihama, Y.; Schmidt, T.; Rappsilber, J.; Mann, M.; Hartl, F.U.; Kerner, M.J.; Frishman, D. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics 2008, 9, 102. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutantsmutants: the Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar]
- Akanuma, G.; Nanamiya, H.; Natori, Y.; Yano, K.; Suzuki, S.; Omata, S.; Ishizuka, M.; Sekine, Y.; Kawamura, F. Inactivation of ribosomal protein genes in Bacillus subtilis reveals importance of each ribosomal protein for cell proliferation and cell differentiation. J. Bacteriol. 2012, 194, 6282–6291. [Google Scholar] [CrossRef]
- Bubunenko, M.; Baker, T.; Court, D.L. Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J. Bacteriol. 2007, 189, 2844–2853. [Google Scholar] [CrossRef]
- Goodall, E.C.A.; Robinson, A.; Johnston, I.G.; Jabbari, S.; Turner, K.A.; Cunningham, A.F.; Lund, P.A.; Cole, J.A.; Henderson, I.R. The essential genome of Escherichia coli K-12. mBio 2018, 9, e02096-17. [Google Scholar] [CrossRef]
- Ban, N.; Beckmann, R.; Cate, J.H.; Dinman, J.D.; Dragon, F.; Ellis, S.R.; Lafontaine, D.L.; Lindahl, L.; Liljas, A.; Lipton, J.M.; McAlear, M.A.; Moore, P.B.; Noller, H.F.; Ortega, J.; Panse, V.G.; Ramakrishnan, V.; Spahn, C.M.; Steitz, T.A.; Tchorzewski, M.; Tollervey, D.; Warren, A.J.; Williamson, J.R.; Wilson, D.; Yonath, A.; Yusupov, M. A new system for naming ribosomal proteins. Curr. Opin. Struct. Biol. 2014, 24, 165–169. [Google Scholar] [CrossRef]
- Zengel, J.M.; Lindahl, L. Diverse mechanisms for regulating ribosomal protein synthesis in Escherichia coli. Prog. Nucleic Acid Res. Mol. Biol. 1994, 47, 331–370. [Google Scholar] [CrossRef]
- Galperin, M.Y.; Wolf, Y.I.; Garushyants, S.K.; Vera Alvarez, R.; Koonin, E.V. Nonessential ribosomal proteins in bacteria and archaea identified using COGs. J. Bacteriol. 2021, 203, e00058-21. [Google Scholar] [CrossRef]
- Nomura, M.; Yates, J.L.; Dean, D.; Post, L.E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein mRNA. Proc. Natl. Acad. Sci. U S A 1980, 77, 7084–7088. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Gourse, R.; Baughman, G. Regulation of the synthesis of ribosomes and ribosomal components. Ann. Rev. Biochem. 1984, 53, 75–117. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.M. rRNA Mimicry in RNA regulation of gene expression. Microbiol. Spectr 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Aseev, L.V.; Koledinskaya, L.S.; Boni, I.V. V. Regulation of ribosomal protein operons rplM-rpsI, rpmB-rpmG, and rplU-rpmA at the transcriptional and translational levels. J. Bacteriol. 2016, 198, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.R. Structure and functions of ribosomal protein S1. Prog. Nucleic Acid Res. Mol. Biol. 1983, 28, 101–1142. [Google Scholar] [CrossRef] [PubMed]
- Hajnsdorf, E.; Boni, I.V. Multiple activities of RNA-binding proteins S1 and Hfq. Biochimie 2012, 94, 1544–1553. [Google Scholar] [CrossRef] [PubMed]
- Salah, P.; Bisaglia, M.; Aliprandi, P.; Uzan, M.; Sizun, C.; Bontems, F. Probing the relationship between Gram-negative and Gram-positive S1 proteins by sequence analysis. Nucleic Acids Res. 2009, 37, 5578–5588. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, M.A.; Fricke, J.; Pedersen, S. Ribosomal protein S1 is required for translation of most, if not all, natural mRNAs in Escherichia coli in vivo. J. Mol. Biol. 1998, 280, 561–569. [Google Scholar] [CrossRef]
- Boni, I.V.; Artamonova, V.S.; Dreyfus, M. The last RNA-binding repeat of the Escherichia coli ribosomal protein S1 is specifically involved in autogenous control. J. Bacteriol. 2000, 182, 5872–5879. [Google Scholar] [CrossRef]
- Duval, M.; Korepanov, A.; Fuchsbauer, O.; Fechter, P.; Haller, A.; Fabbretti, A.; Choulier, L.; Micura, R.; Klaholz, B.P.; Romby, P.; et al. Escherichia coli ribosomal protein S1 unfolds structured mRNAs onto the ribosome for active translation initiation. PLoS Biol. 2013, 11, e1001731. [Google Scholar] [CrossRef]
- Bycroft, M.; Hubbard, T.J.P.; Proctor, M.; Freund, S.M.V.; Murzin, A.G. The solution structure of the S1 RNA binding domain: a member of an ancient nucleic acid-binding fold. Cell 1997, 88, 235–242. [Google Scholar] [CrossRef]
- Byrgazov, K.; Grishkovskaya, I.; Arenz, S.; Coudevylle, N.; Temmel, H.; Wilson, D.N.; Djinovic-Carugo, K.; Moll, I. Structural basis for the interaction of protein S1 with the Escherichia coli ribosome. Nucleic Acids Res. 2015, 43(1), 661–673. [Google Scholar] [CrossRef]
- D'Urso, G.; Chat, S.; Gillet, R.; Giudice, E. Structural insights into the binding of bS1 to the ribosome. Nucleic Acids Res. 2023, 51, 3410–3419. [Google Scholar] [CrossRef]
- Beckert, B.; Turk, M.; Czech, A.; Berninghausen, O.; Beckmann, R.; Ignatova, Z.; Plitzko, J.M.; Wilson, D.N. Structure of a hibernating 100S ribosome reveals an inactive conformation of the ribosomal protein S1. Nat. Microbiol. 2018, 3(10), 1115–1121. [Google Scholar] [CrossRef]
- Boni, I.V.; Isaeva, D.M.; Musychenko, M.L.; Tzareva, N.V. Ribosome-messenger recognition: mRNA target sites for ribosomal protein S1. Nucleic Acids Res. 1991, 19, 155–162. [Google Scholar] [CrossRef]
- Tzareva, N.V.; Makhno, V.I.; Boni, I.V. Ribosome-messenger recognition in the absence of the Shine-Dalgarno interactions. FEBS Lett. 1994, 337, 189–1894. [Google Scholar] [CrossRef] [PubMed]
- Komarova, A.V.; Tchufistova, L.S.; Supina, E.V.; Boni, I.V. Protein S1 counteracts the inhibitory effect of the extended Shine-Dalgarno sequence on translation. RNA 2002, 8, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Komarova, A.V.; Tchufistova, L.S.; Dreyfus, M.; Boni, I.V. AU-rich sequences within 5 ' untranslated leaders enhance translation and stabilize mRNA in Escherichia coli. J. Bacteriol. 2005, 187, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.; Wieden, H.J. The prokaryotic activity of the IGR IRESs is mediated by ribosomal protein S1. Nucleic Acids Res. 2022, 50, 9355–9367. [Google Scholar] [CrossRef]
- Beck, H.J.; Moll, I. Leaderless mRNAs in the Spotlight: Ancient but Not Outdated! Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ringquist, S.; Jones, T.; Snyder, E.E.; Gibson, T.; Boni, I.; Gold, L. High-affinity RNA ligands to Escherichia coli ribosomes and ribosomal protein S1: comparison of natural and unnatural binding sites. Biochemistry 1995, 34, 3640–3648. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.E.; Chatterjee, S.; Daher, M.; Walter, N.G. Protein unties the pseudoknot: S1-mediated unfolding of RNA higher order structure. Nucleic Acids Res. 2020, 48, 2107–2125. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Vanderpool, C.K. Translation inhibition from a distance: The small RNA SgrS silences a ribosomal protein S1-dependent enhancer. Mol. Microbiol. 2020, 114, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.Q.; Chen, Z.Q.; Dong, Y.Q.; You, D.; Zhou, Y.; Ye, B.C. Selective recruitment of stress-responsive mRNAs to ribosomes for translation by acetylated protein S1 during nutrient stress in Escherichia coli. Commun Biol. 2022, 5, 892. [Google Scholar] [CrossRef] [PubMed]
- Boni, I.V.; Artamonova, V.S.; Tzareva, N.V.; Dreyfus, M. Non-canonical mechanism for translational control in bacteria: synthesis of ribosomal protein S1. EMBO J. 2001, 20, 4222–4232. [Google Scholar] [CrossRef] [PubMed]
- Tchufistova, L.S.; Komarova, A.V.; Boni, I.V. A key role for the mRNA leader structure in translational control of ribosomal protein S1 synthesis in γ-proteobacteria. Nucleic Acids Res. 2003, 31, 6996–7002. [Google Scholar] [CrossRef]
- Sukhodolets, M.V.; Garges, S. Interaction of Escherichia coli RNA polymerase with the ribosomal protein S1 and the Sm-like ATPase Hfq. Biochemistry 2003, 42(26), 8022–8034. [Google Scholar] [CrossRef]
- Sukhodolets, M.V.; Garges, S.; Adhya, S. Ribosomal protein S1 promotes transcriptional cycling. RNA 2006, 12, 1505–1513. [Google Scholar] [CrossRef]
- Delvillani, F.; Papiani, G.; Dehò, G.; Briani, F. S1 ribosomal protein and the interplay between translation and mRNA decay. Nucleic Acids Res. 2011, 39(17), 7702–7715. [Google Scholar] [CrossRef] [PubMed]
- Briani, F.; Curti, S.; Rossi, F.; Carzaniga, T.; Mauri P, Dehò, G. Polynucleotide phosphorylase hinders mRNA degradation upon ribosomal protein S1 overexpression in Escherichia coli. RNA 2008, 14(11), 2417–2429. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Vicente, A.M.; Hardwick, S.W.; Alvelos, D.M.; Mazzon, R.R.; Luisi, B.F.; Marques, M.V. Association of the cold-shock DEAD-box RNA helicase RhlE to the RNA degradosome in Caulobacter crescentus. J. Bacteriol. 2017, 199, e00135-17. [Google Scholar] [CrossRef] [PubMed]
- Janssen, B.D.; Hayes, C.S. S. The tmRNA ribosome-rescue system. Adv. Protein Chem. Struct. Biol. 2012, 86, 151–191. [Google Scholar] [CrossRef] [PubMed]
- Keiler, K.C.; Feaga, H.A. Resolving nonstop translation complexes is a matter of life or death. J. Bacteriol. 2014, 196, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Keiler, K.C. Mechanisms of ribosome rescue in bacteria. Nat. Rev. Microbiol. 2015, 13, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Saguy, M.; Gillet, R.; Skorski, P.; Hermann-Le Denmat, S.; Felden, B. Ribosomal protein S1 influences trans-translation in vitro and in vivo. Nucleic Acids Res. 2007, 35, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- McGinness, K.E.; Sauer, R.T. Ribosomal protein S1 binds mRNA and tmRNA similarly but plays distinct roles in translation of these molecules. Proc. Natl. Acad. Sci. USA, 2004, 101, 13454–13459. [Google Scholar] [CrossRef]
- Qi, H.; Shimizu, Y.; Ueda, T. Ribosomal protein S1 is not essential for the trans-translation machinery. J. Mol. Biol. 2007, 368, 845–852. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, X.; Jiang, X.; Yuan, H.; Lee, J.S.; Barry, C.E. 3rd; Wang, H.; Zhang, W.; Zhang, Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science 2011, 333, 1630–1632. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Bi, J.; Cai, Q.; Liao, X.; Li, W.; Guo, C.; Zhang, Q.; Lin, T.; Zhao, Y.; Wang, H.; Liu., J.; Zhang, X.; Lin, D. Structural basis for targeting the ribosomal protein S1 of Mycobacterium tuberculosis by pyrazinamide. Mol. Microbiol. 2015, 95, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Dillon, N.A.; Peterson, N.D.; Feaga, H.A.; Keiler, K.C.; Baughn, A.D. Anti-tubercular activity of pyrazinamide is independent of trans-translation and RpsA. Sci. Rep. 2017, 7, 6135. [Google Scholar] [CrossRef] [PubMed]
- Wahba, A.J.; Miller, M.J.; Niveleau, A.; Landers, T.A.; Carmichael, G.G.; Weber, K.; Hawley, D.A.; Slobin, L.I. Subunit I of Q beta replicase and 30 S ribosomal protein S1 of Escherichia coli. Evidence for the identity of the two proteins. J. Biol. Chem. 1974, 249, 3314–3316. [Google Scholar] [CrossRef]
- Wagner, A.; Weise, L.I.; Mutschler, H. In vitro characterization of the MS2 RNA polymerase complex reveals host factors that modulate emesviral replicase activity. Commun. Biol. 2022, 5(1), 264. [Google Scholar] [CrossRef]
- Takeshita, D.; Yamashita, S.; Tomita, K. Molecular insights into replication initiation by Qβ replicase using ribosomal protein S1. Nucleic Acids Res. 2014, 42, 10809–10822. [Google Scholar] [CrossRef]
- Gytz, H.; Mohr, D.; Seweryn, P.; Yoshimura, Y.; Kutlubaeva, Z.; Dolman, F.; Chelchessa, B.; Chetverin, A.B.; Mulder, F.A.; Brodersen, D.E.; Knudsen, C.R. Structural basis for RNA-genome recognition during bacteriophage Qβ replication. Nucleic Acids Res. 2015, 43(22), 10893–10906. [Google Scholar] [CrossRef] [PubMed]
- Vasilyev, N.N.; Kutlubaeva, Z.S.; Ugarov, V.I.; Chetverina, H.V.; Chetverin, A.B. Ribosomal protein S1 functions as a termination factor in RNA synthesis by Qβ phage replicase. Nat. Commun. 2013, 4, 1781. [Google Scholar] [CrossRef] [PubMed]
- Kutlubaeva, Z.S.; Chetverina, H.V.; Chetverin, A.B. The contribution of ribosomal protein S1 to the structure and function of Qβ replicase. Acta Naturae. 2017, 9, 24–30. [Google Scholar] [CrossRef]
- Venkatesh, T.V.; Radding, C.M. Ribosomal protein S1 and NusA protein complexed to recombination protein β of phage λ. J. Bacteriol. 1993, 175, 1844–1846. [Google Scholar] [CrossRef]
- Ruckman, J.; Ringquist, S.; Brody, E.; Gold, L. The bacteriophage T4 regB ribonuclease. Stimulation of the purified enzyme by ribosomal protein S1. J. Biol. Chem. 1994, 269, 26655–26662. [Google Scholar] [CrossRef]
- Durand, S.; Richard, G.; Bisaglia, M.; Laalami, S.; Bontems, F.; Uzan, M. Activation of RegB endoribonuclease by S1 ribosomal protein requires an 11 nt conserved sequence. Nucleic Acids Res. 2006, 34, 6549–6560. [Google Scholar] [CrossRef]
- Bisaglia, M.; Laalami, S.; Uzan, M.; Bontems, F. Activation of the RegB endoribonuclease by the S1 ribosomal protein is due to cooperation between the S1 four C-terminal modules, in a substrate-dependent manner. J. Biol. Chem. 2003, 278, 15261–15271. [Google Scholar] [CrossRef]
- Aliprandi, P.; Sizun, C.; Perez, J.; Mareuil, F.; Caputo, S.; Leroy, J.L.; Odaert, B.; Laalami, S.; Uzan, M.; Bontems, F. S1 ribosomal protein functions in translation initiation and ribonuclease RegB activation are mediated by similar RNA-protein interactions: An NMR and SAXS analysis. J. Biol. Chem. 2008, 283, 13289–13301. [Google Scholar] [CrossRef]
- Uzan, M. RNA processing and decay in bacteriophage T4. Prog. Mol. Biol. Transl. Sci. 2009, 85, 43–89. [Google Scholar]
- Juškauskas, A.; Zajančkauskaitė, A.; Meškys, R.; Ger, M.; Kaupinis, A.; Valius, M.; Truncaitė, L. Interaction between phage T4 protein RIII and host ribosomal protein S1 inhibits endoribonuclease RegB activation. Int. J. Mol. Sci. 2022, 23, 9483. [Google Scholar] [CrossRef]
- Wolfram-Schauerte, M.; Pozhydaieva, N.; Grawenhoff, J.; Welp, L.M.; Silbern, I.; Wulf, A.; Billau, F.A.; Glatter, T.; Urlaub, H.; Jäschke, A.; Höfer, K. A viral ADP-ribosyltransferase attaches RNA chains to host proteins. Nature 2023, 620, 1054–1062. [Google Scholar] [CrossRef]
- Kaminishi, T.; Wilson, D.N.; Takemoto, C.; Harms, J.M.; Kawazoe, M.; Schluenzen, F.; Hanawa-Suetsugu, K.; Shirouzu, M; Fucini, P.; Yokoyama, S. A snapshot of the 30S ribosomal subunit capturing mRNA via the Shine-Dalgarno interaction. Structure 2007, 15, 289–297. [Google Scholar] [CrossRef]
- Yusupova, G.; Jenner, L.; Rees, B.; Moras, D.; Yusupov, M. Structural basis for messenger RNA movement on the ribosome. Nature 2006, 444, 391–394. [Google Scholar] [CrossRef]
- Culver, G.M. Assembly of the 30S ribosomal subunit. Biopolymers 2003, 68(2), 234–249. [Google Scholar] [CrossRef]
- Brodersen, D.E.; Clemons, W.M. Jr; Carter, A.P.; Wimberly, B.T.; Ramakrishnan, V. Crystal structure of the 30 S ribosomal subunit from Thermus thermophilus: structure of the proteins and their interactions with 16S RNA. J. Mol. Biol. 2002, 316(3), 725–768. [Google Scholar] [CrossRef]
- Aseev, L.V.; Levandovskaya, A.A.; Tchufistova, L.S.; Skaptsova, N.V.; Boni, I.V. A new regulatory circuit in ribosomal protein operons: S2-mediated control of the rpsB-tsf expression in vivo. RNA 2008, 14, 1883–1894. [Google Scholar] [CrossRef]
- Aseev, L.V.; Levandovskaya, A.A.; Skaptsova, N.V.; Boni, I.V. Conservation of regulatory elements controlling the expression of the rpsB-tsf operon in γ-proteobacteria. Mol. Biol. 2009, 43, 101–107. [Google Scholar] [CrossRef]
- Fu, Y.; Deiorio-Haggar, K.; Anthony, J.; Meyer, M.M. Most RNAs regulating ribosomal protein biosynthesis in Escherichia coli are narrowly distributed to Gammaproteobacteria. Nucleic Acids Res. 2013, 41, 3491–3503. [Google Scholar] [CrossRef]
- Aseev, L.V.; Chugunov, A.O.; Efremov, R.G.; Boni, I.V. A single missense mutation in a coiled-coil domain of Escherichia coli ribosomal protein S2 confers a thermosensitive phenotype that can be suppressed by ribosomal protein S1. J. Bacteriol. 2013, 195, 95–104. [Google Scholar] [CrossRef]
- Mustoe, A.M.; Lama, N.N.; Irving, P.S.; Olson, S. W/; Weeks, K.M. RNA base-pairing complexity in living cells visualized by correlated chemical probing. Proc. Natl. Acad. Sci. USA. 2019, 116, 24574–24582. [Google Scholar] [CrossRef]
- Qi, Y.; Rao, J.; Shen, W. , Li, J.; Zeng, W.; Zheng, S.; Liu, S.; Li,Y.; Wang, B.; Wu, F.; Li,Y.; Li, Y. Identification of a ribosomal protein RpsB as a surface-exposed protein and adhesin of Rickettsia heilongjiangensis. Biomed. Res. Int. 2019, 2019, 9297129. [Google Scholar] [CrossRef]
- Chaiden, C.; Jaresitthikunchai, J.; Phaonakrop, N.; Roytrakul, S.; Kerdsin, A.; Nuanualsuwan, S. Peptidomics analysis of virulent peptides involved in Streptococcus suis pathogenesis. Animals (Basel) 2021, 11. [Google Scholar] [CrossRef]
- Prakash, C.; Pandey, M.; Talwar, S.; Singh, Y.; Kanojiya, S.; Pandey, A.K.; Kumar, N. Extra-ribosomal functions of Mtb RpsB in imparting stress resilience and drug tolerance to mycobacteria. Biochimie 2020, 177, 87–97. [Google Scholar] [CrossRef]
- Mayerle, M.; Woodson, S.A. Specific contacts between protein S4 and ribosomal RNA are required at multiple stages of ribosome assembly. RNA, 2013, 19, 574–585. [Google Scholar] [CrossRef]
- Rodgers, M.L.; Woodson, S.A. Transcription increases the cooperativity assembly. Cell 2019, 179, 1370–1381.e12. [Google Scholar] [CrossRef]
- Duss, O.; Stepanyuk, G.A.; Puglisi, J.D.; Williamson, J.R. Transient protein-RNA interactions guide nascent ribosomal RNA folding. Cell 2019, 179, 1357–1369.e16. [Google Scholar] [CrossRef]
- Rodgers, M.L.; Sun, Y.; Woodson, S.A. Ribosomal Protein S12 hastens nucleation of co-transcriptional ribosome assembly. Biomolecules 2023, 13, 951. [Google Scholar] [CrossRef]
- Kamath, D.; Allgeyer, B.B.; Gregory, S.T.; Bielski, M.C.; Roelofsz, D.M.; Sabapathypillai, S.L.; Vaid, N.; O'Connor, M. The C-terminus of ribosomal protein uS4 contributes to small ribosomal subunit biogenesis and the fidelity of translation. Biochimie 2017, 138, 194–201. [Google Scholar] [CrossRef]
- Agarwal, D.; Kamath, D.; Gregory, S.; O'Connor, M. Modulation of decoding fidelity by ribosomal proteins S4 and S5. J. Bacteriol. 2015, 197, 1017–1025. [Google Scholar] [CrossRef]
- Takyar, S.; Hickerson, R.P.; Noller, H.F. mRNA helicase activity of the ribosome. Cell 2005, 120, 49–58. [Google Scholar] [CrossRef]
- Jinks-Robertson, S.; Nomura, M. Ribosomal protein S4 acts in trans as a translational repressor to regulate expression of the alpha operon in Escherichia coli. J. Bacteriol. 1982, 151, 193–202. [Google Scholar] [CrossRef]
- Thomas, M.S.; Bedwell, D.M.; Nomura, M. Regulation of alpha operon gene expression in Escherichia coli. A novel form of translational coupling. J. Mol. Biol. 1987, 196, 333–345. [Google Scholar] [CrossRef]
- Tang, C.K.; Draper, D.E. Unusual mRNA pseudoknot structure is recognized by a protein translational repressor. Cell 1989, 57, 531–536. [Google Scholar] [CrossRef]
- Spedding, G.; Draper, D.E. Allosteric mechanism for translational repression in the Escherichia coli alpha operon. Proc. Natl. Acad. Sci. U S A 1993, 90, 4399–4403. [Google Scholar] [CrossRef]
- Schlax, P.J.; Xavier, K.A.; Gluick, T.C.; Draper, D.E. Translational repression of the Escherichia coli alpha operon mRNA: importance of an mRNA conformational switch and a ternary entrapment complex. J. Biol. Chem. 2001, 276, 38494–38501. [Google Scholar] [CrossRef]
- Meek, D.W.; Hayward, R.S. Nucleotide sequence of the rpoA-rplQ DNA of Escherichia coli: a second regulatory binding site for protein S4? Nucleic Acids Res. 1984, 12(14), 5813–5821. [Google Scholar] [CrossRef]
- Grundy, F.J.; Henkin, T.M. M. The rpsD gene, encoding ribosomal protein S4, is autogenously regulated in Bacillus subtilis. J. Bacteriol. 1991, 173, 4595–4602. [Google Scholar] [CrossRef]
- Grundy, F.J.; Henkin, T.M. Characterization of the Bacillus subtilis rpsD regulatory target site. J. Bacteriol. 1992, 174, 6763–6770. [Google Scholar] [CrossRef]
- Torres, M.; Condon, C.; Balada, J.M.; Squires, C.; Squires, C.L. Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination. EMBO J. 2001, 20, 3811–3820. [Google Scholar] [CrossRef]
- Huang, Y.H.; Said, N.; Loll, B.; Wahl, M.C. Structural basis for the function of SuhB as a transcription factor in ribosomal RNA synthesis. Nucleic Acids Res. 2019, 47, 6488–6503. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Hilal, T.; Loll, B.; Bürger, J.; Mielke, T.; Böttcher, C.; Said, N.; Wahl, M.C. Structure-based mechanisms of a molecular RNA polymerase/chaperone machine required for ribosome biosynthesis. Mol. Cell. 2020, 79, 1024–1036.e5. [Google Scholar] [CrossRef]
- Drabinska, J.; Steczkiewicz, K.; Kujawa, M.; Kraszewska, E. Searching for biological function of the mysterious PA2504 protein from Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 9833. [Google Scholar] [CrossRef]
- Matelska, D.; Purta, E.; Panek, S.; Boniecki, M.J.; Bujnicki, J.M.; Dunin-Horkawicz, S. S6:S18 ribosomal protein complex interacts with a structural motif present in its own mRNA. RNA 2013, 19, 1341–1348. [Google Scholar] [CrossRef]
- Agalarov, S.C.; Sridhar Prasad, G.; Funke, P.M.; Stout, C.D.; Williamson, J.R. Structure of the S15, S6, S18-rRNA complex: assembly of the 30S ribosome central domain. Science 2000, 288(5463), 107–113. [Google Scholar] [CrossRef]
- Fu, Y.; Deiorio-Haggar, K.; Soo, M.W.; Meyer, M.M. Bacterial RNA motif in the 5' UTR of rpsF interacts with an S6:S18 complex. RNA 2014, 20, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Babina, A.M.; Soo, M.W.; Fu, Y.; Meyer, M.M. An S6:S18 complex inhibits translation of E. coli rpsF. RNA 2015, 21, 2039–2046. [Google Scholar] [CrossRef]
- Pletnev, P.I.; Nesterchuk, M.V.; Rubtsova, M.P.; Serebryakova, M.V.; Dmitrieva, K.; Osterman, I.A.; Bogdanov, A.A.; Sergiev, P.V. Oligoglutamylation of E. coli ribosomal protein S6 is under growth phase control. Biochimie 2019. 167, 61–67. [CrossRef]
- Grenga, L.; Little, R.H.; Chandra, G.; Woodcock, S.D.; Saalbach, G.; Morris, R.J.; Malone, J.G. Control of mRNA translation by dynamic ribosome modification. PLoS Genet. 2020, 16, e1008837. [Google Scholar] [CrossRef]
- Thompson, C.M.A.; Little, R.H.; Stevenson, C.E.M.; Lawson, D.M.; Malone, J.G. Structural insights into the mechanism of adaptive ribosomal modification by Pseudomonas RimK. Proteins 2023, 91(3), 300–314. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hackert, E.; Hendrickson, W.A. Promiscuous substrate recognition in folding and assembly activities of the trigger factor chaperone. Cell 2009, 138, 923–934. [Google Scholar] [CrossRef]
- Li, Z.; Wu, D.; Zhan, B.; Hu, X.; Gan, J.; Ji, C.; Li, J. Structural insights into the complex of trigger factor chaperone and ribosomal protein S7 from Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 2019, 512, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nomura, M. Post-transcriptional regulation of the str operon in Escherichia coli. Structural and mutational analysis of the target site for translational repressor S7. J. Mol. Biol. 1994, 235, 125–139. [Google Scholar] [CrossRef]
- Robert, F.; Brakier-Gingras, L. Ribosomal protein S7 from Escherichia coli uses the same determinants to bind 16S ribosomal RNA and its messenger RNA. Nucleic Acids Res. 2001, 29, 677–682. [Google Scholar] [CrossRef]
- Saito, K.; Mattheakis, L.C.; Nomura, M. Post-transcriptional regulation of the str operon in Escherichia coli. Ribosomal protein S7 inhibits coupled translation of S7 but not its independent translation. J. Mol. Biol. 1994, 235, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Golovin, A.; Spiridonova, V.; Kopylov, A. Mapping contacts of the S12-S7 intercistronic region of str operon mRNA with ribosomal protein S7 of E. coli. FEBS Lett. 2006, 580(25), 5858–5862. [Google Scholar] [CrossRef]
- Meng, B.Y.; Shinozaki, K.; Sugiura, M. Genes for the ribosomal proteins S12 and S7 and elongation factors EF-G and EF-Tu of the cyanobacterium, Anacystis nidulans: structural homology between 16S rRNA and S7 mRNA. Mol. Gen. Genet. 1989, 216, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, L.; Zimmermann, R.A. The binding site for ribosomal protein S8 in 16S rRNA and spc mRNA from Escherichia coli: minimum structural requirements and the effects of single bulged bases on S8-RNA interaction. Nucleic Acids Res. 1994, 22, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Olins, P.O.; Nomura, M. Translational regulation by ribosomal protein S8 in Escherichia coli: structural homology between rRNA binding site and feedback target on mRNA. Nucleic Acids Res. 1981, 9(7), 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Cerretti, D.P.; Mattheakis, L.C.; Kearney, K.R.; Vu, L.; Nomura, M. Translational regulation of the spc operon in Escherichia coli. Identification and structural analysis of the target site for S8 repressor protein. J. Mol. Biol. 1988, 204(2), 309–329. [Google Scholar] [CrossRef]
- Mattheakis, L.C.; Nomura, M. . Feedback regulation of the spc operon in Escherichia coli: translational coupling and mRNA processing. J. Bacteriol. 1988, 170, 4484–4492. [Google Scholar] [CrossRef]
- Mattheakis, L.; Vu, L.; Sor, F.; Nomura, M. Retroregulation of the synthesis of ribosomal proteins L14 and L24 by feedback repressor S8 in Escherichia coli. Proc. Natl. Acad. Sci USA 1989, 86(2), 448–452. [Google Scholar] [CrossRef]
- Gregory, R.J.; Cahill, P.B.F.; Thurlow, D.L.; Zimmermann, R.A. Interaction of Escherichia coli ribosomal protein S8 with its binding sites in ribosomal RNA and messenger RNA. J. Mol. Biol. 1988, 204, 295–307. [Google Scholar] [CrossRef]
- Merianos, H.J.; Wang, J.; Moore, P.B. The structure of a ribosomal protein S8/spc operon mRNA complex. RNA 2004, 10, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.D.; Watkins, T.; Lindahl, L.; Zengel, J.M. Regulation of ribosomal protein synthesis in Vibrio cholerae. J. Bacteriol. 2004, 186, 5933–5937. [Google Scholar] [CrossRef] [PubMed]
- Henkin, T.M.; Moon, S.H.; Mattheakis, L.C.; Nomura, M. Cloning and analysis of the spc ribosomal protein operon of Bacillus subtilis: comparison with the spc operon of Escherichia coli. Nucleic Acids Res. 1989, 17, 7469–7486. [Google Scholar] [CrossRef]
- Shajani, Z.; Sykes, M.T.; Williamson, J.R. Assembly of bacterial ribosomes. Annu Rev. Biochem. 2011, 80, 501–526. [Google Scholar] [CrossRef]
- Proshkin, S.; Rahmouni, A.R.; Mironov, A.; Nudler, E. Cooperation between translating ribosomes and RNA polymerase in transcription elongation. Science 2010, 328(5977), 504–508. [Google Scholar] [CrossRef]
- Burmann, B.M.; Schweimer, K.; Luo, X.; Wahl, M.C.; Stitt, B.L.; Gottesman, M.E.; Rösch, P. A NusE:NusG complex links transcription and translation. Science 2010, 328(5977), 501–504. [Google Scholar] [CrossRef]
- Washburn, R.S.; Zuber, P.K.; Sun, M.; Hashem, Y.; Shen, B.; Li, W.; Harvey, S.; Acosta Reyes, F.J.; Gottesman, M.E.; Knauer, S.H.; Frank, J. Escherichia coli NusG links the lead ribosome with the transcription elongation complex. iScience 2020, 23, 101352. [Google Scholar] [CrossRef]
- Saxena, S.; Myka, K.K.; Washburn, R.; Costantino, N.; Court, D.L.; Gottesman, M.E. Escherichia coli transcription factor NusG binds to 70S ribosomes. Mol. Microbiol. 2018, 108, 495–504. [Google Scholar] [CrossRef]
- Bailey, E.J.; Gottesman, M.E.; Gonzalez, R.L. Jr. NusG-mediated coupling of transcription and translation enhances gene expression by suppressing RNA polymerase backtracking. J. Mol. Biol. 2022, 434, 167330. [Google Scholar] [CrossRef]
- Kohler, R.; Mooney, R.A.; Mills, D.J.; Landick, R.; Cramer, P. Architecture of a transcribing-translating expressome. Science 2017, 356, 194–197. [Google Scholar] [CrossRef]
- O'Reilly, F.J.; Xue, L.; Graziadei, A.; Sinn, L.; Lenz, S.; Tegunov, D.; Blötz, C.; Singh, N.; Hagen, W.J. H/; Cramer, P.; Stülke, J.; Mahamid, J.; Rappsilber, J. In-cell architecture of an actively transcribing-translating expressome. Science 2020, 369, 554–557. [Google Scholar] [CrossRef]
- Woodgate, J.; Zenkin, N. Transcription–translation coupling: Recent advances and future perspectives. Mol. Microbiol. 2023, 120, 539–546. [Google Scholar] [CrossRef]
- Johnson, G.E.; Lalanne, J.B.; Peters, M.L.; Li, G.W. Functionally uncoupled transcription-translation in Bacillus subtilis. Nature 2020, 585, 124–128. [Google Scholar] [CrossRef]
- Gottesman, M.E.; Weisberg, R.A. Little lambda, who made thee? Microbiol. Mol. Biol. Rev. 2004, 68, 796–813. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.I.; Schauer, A.T.; Baumann, M.R.; Baron, L.S.; Adhya, S.L. Evidence that ribosomal protein S10 participates in control of transcription termination. Proc. Natl. Acad. Sci. U S A 1981, 78, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Nodwell, J.R; Greenblatt, J. Recognition of boxA antiterminator RNA by the E. coli antitermination factors NusB and ribosomal protein S10. Cell 1993, 72, 261–268. [Google Scholar] [CrossRef]
- Burmann, B.M.; Luo, X.; Rösch, P.; Wahl, M.C.; Gottesman, M.E. Fine tuning of the E. coli NusB:NusE complex affinity to BoxA RNA is required for processive antitermination. Nucleic Acids Res. 2010, 38, 314–326. [Google Scholar] [CrossRef]
- Luo, X.; Hsiao, H.H.; Bubunenko, M.; Weber, G.; Court, D.L.; Gottesman, M.E.; Urlaub, H.; Wahl, M.C. Structural and functional analysis of the E. coli NusB-S10 transcription antitermination complex. Mol. Cell 2008, 32, 791–802. [Google Scholar] [CrossRef]
- 147. Singh, N.; Bubunenko, M.; Smith, C.; Abbott, D.M.; Stringer, A.M.; Shi, R.; Court, D.L.; Wade, J.T. SuhB associates with Nus factors to facilitate 30S ribosome biogenesis in Escherichia coli. mBio 2016, 7, e00114. [Google Scholar] [CrossRef]
- Baniulyte, G.; Singh, N.; Benoit, C.; Johnson, R.; Ferguson, R.; Paramo, M.; Stringer, A.M.; Scott, A.; Lapierre, P.; Wade, J.T. Identification of regulatory targets for the bacterial Nus factor complex. Nat. Commun. 2017, 8, 2027. [Google Scholar] [CrossRef]
- Melamed, S.; Zhang, A.; Jarnik, M.; Mills, J.; Silverman, A.; Zhang, H.; Storz, G. σ28-dependent small RNA regulation of flagella biosynthesis. Elife 2023, 12, RP87151. [Google Scholar] [CrossRef]
- Bubunenko, M.; Korepanov, A.; Court, D.L.; Jagannathan, I.; Dickinson, D.; Chaudhuri, B.R.; Garber, M.B.; Culver, G.M. 30S ribosomal subunits can be assembled in vivo without primary binding ribosomal protein S15. RNA 2006, 12, 1229–1239. [Google Scholar] [CrossRef]
- Philippe C., Eyermann F., Benard L., Portier C., Ehresmann B., Ehresmann C. Ribosomal protein S15 from Escherichia coli modulates its own translation by trapping the ribosome on the mRNA initiation loading site. Proc. Natl. Acad. Sci. USA. 1993, 90, 4394–4398. [CrossRef]
- Ehresmann C., Philippe C., Westhof E., Benard L., Portier C., Ehresmann B. A pseudoknot is required for efficient translation initiation and regulation of the E. coli rpsO gene coding for ribosomal protein S15. Biochem. Cell Biol. 1995, 73, 1131–1140. [CrossRef] [PubMed]
- Bénard L., Mathy N., Grunberg-Manago M., Ehresmann B., Ehresmann C., Portier C. Identification in a pseudoknot of a U.G motif essential for the regulation of the expression of ribosomal protein S15. Proc. Natl. Acad. Sci. USA. 1998, 95, 2564–2567. [CrossRef]
- Serganov A., Ennifar E., Portier C., Ehresmann B., Ehresmann C. Do mRNA and rRNA binding sites of E. coli ribosomal protein S15 share common structural determinants? J. Mol. Biol. 2002, 320, 963–978. [CrossRef] [PubMed]
- Mathy N., Pellegrini O., Serganov A., Patel D.J., Ehresmann C., Portier C. Specific recognition of rpsO mRNA and 16S rRNA by Escherichia coli ribosomal protein S15 relies on both mimicry and site differentiation. Mol. Microbiol. 2004, 52, 661–675. [CrossRef] [PubMed]
- Marzi S., Myasnikov A.G., Serganov A., Ehresmann C., Romby P., Yusupov M., Klaholz B.P. Structured mRNAs regulate translation initiation by binding to the platform of the ribosome. Cell. 2007, 130, 1019–1031. [CrossRef] [PubMed]
- Scott L.G., Williamson J.R. Interaction of the Bacillus stearothermophilus ribosomal protein S15 with its 5′-translational operator mRNA. J. Mol. Biol. 2001, 314, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Scott L.G., Williamson J.R. The binding interface between Bacillus stearothermophilus ribosomal protein S15 and its 5’-translational operator mRNA. J. Mol. Biol. 2005, 351, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Slinger B.L., Meyer M.M. RNA regulators responding to ribosomal protein S15 are frequent in sequence space. Nucleic Acids Res. 2016, 44, 9331–9341. [Google Scholar] [CrossRef]
- Serganov A., Polonskaia A., Ehresmann B., Ehresmann C., Patel D.J. Ribosomal protein S15 represses its own translation via adaptation of an rRNA-like fold within its mRNA. EMBO J. 2003, 22, 1898–1908. [CrossRef]
- Slinger B.L., Deiorio-Haggar K., Anthony J.S., Gilligan M.M., Meyer M.M. Discovery and validation of novel and distinct RNA regulators for ribosomal protein S15 in diverse bacterial phyla. BMC Genom. 2014, 15. [CrossRef]
- Slinger B.L., Newman H., Lee Y., Pei S., Meyer M.M. Coevolution of bacterial ribosomal protein S15 with diverse mRNA regulatory structures. PLoS Genet. 2015, 11, e1005720. [CrossRef]
- Aseev, L.V.; Koledinskaya, L.S.; Bychenko, O.S.; Boni, I.V. Regulation of ribosomal protein synthesis in Mycobacteria: The autogenous control of rpsO. Int. J. Mol. Sci. 2021, 22, 9679. [Google Scholar] [CrossRef]
- Dutca, L.M.; Culver, G.M. Assembly of the 5' and 3' minor domains of 16S ribosomal RNA as monitored by tethered probing from ribosomal protein S20. J. Mol. Biol. 2008, 376, 92–108. [Google Scholar] [CrossRef]
- Hedrick, E.G.; Hill, W.E. Protein S20 binds two 16S rRNA sites as assembly is initiated. J. Mol. Biol. 2010, 401, 493–502. [Google Scholar] [CrossRef]
- Tobin, C.; Mandava, C.S.; Ehrenberg, M.; Andersson, D.I.; Sanyal, S. Ribosomes lacking protein S20 are defective in mRNA binding and subunit association. J. Mol. Biol. 2010, 397, 767–776. [Google Scholar] [CrossRef]
- Wada, A. Analysis of Escherichia coli ribosomal proteins by an improved two-dimensional gel electrophoresis. I. Detection of four new proteins. J. Biochem. 1986, 100, 1583–1594. [Google Scholar] [CrossRef]
- Parsons, G.D.; Donly, B.C.; Mackie, G.A. Mutations in the leader sequence and initiation codon of the gene for ribosomal protein S20 (rpsT) affect both translational efficiency and autoregulation. J Bacteriol. 1988, 170, 2485–2492. [Google Scholar] [CrossRef]
- Donly, B.C.; Mackie, G.A. Affinities of ribosomal protein S20 and C-terminal deletion mutants for 16S rRNA and S20 mRNA. Nucleic Acids Res. 1988, 16(3), 997–1010. [Google Scholar] [CrossRef]
- Burton, Z.F.; Gross, C.A.; Watanabe, K.K.; Burgess, R.R. The operon that encodes the sigma subunit of RNA polymerase also encodes ribosomal protein S21 and DNA primase in E. coli K12. Cell 1983, 32, 335–349. [Google Scholar] [CrossRef]
- Backendorf, C.; Ravensbergen, C.J.; Van der Plas, J.; van Boom, J.H.; Veeneman, G.; Van Duin, J. Basepairing potential of the 3' terminus of 16S RNA: dependence on the functional state of the 30S subunit and the presence of protein S21. Nucleic Acids Res. 1981, 9, 1425–1446. [Google Scholar] [CrossRef]
- Trautmann, H.S.; Ramsey, K.M. A ribosomal protein homolog governs gene expression and virulence in a bacterial pathogen. J. Bacteriol. 2022, 204, e0026822. [Google Scholar] [CrossRef]
- Trautmann, H.S.; Schmidt, S.S.; Gregory, S.T.; Ramsey, K.M. Ribosome heterogeneity results in leader sequence-mediated regulation of protein synthesis in Francisella tularensis. J. Bacteriol. 2023, e0014023. [Google Scholar] [CrossRef]
- Robertson, W.R.; Dowsett, S.J.; Hardy, S.J. Exchange of ribosomal proteins among the ribosomes of Escherichia coli. Mol. Gen. Genet. 1977, 157, 205–214. [Google Scholar] [CrossRef]
- Jha, V.; Roy, B.; Jahagirdar, D.; McNutt, Z.A.; Shatoff, E.A.; Boleratz, B.L.; Watkins, D.E.; Bundschuh, R.; Basu, K.; Ortega, J.; Fredrick, K. Structural basis of sequestration of the anti-Shine-Dalgarno sequence in the Bacteroidetes ribosome. Nucleic Acids Res. 2021, 49, 547–567. [Google Scholar] [CrossRef]
- McNutt, Z.A.; Roy, B.; Gemler, B.T.; Shatoff, E.A.; Moon, K.M.; Foster, L.J.; Bundschuh, R.; Fredrick, K. Ribosomes lacking bS21 gain function to regulate protein synthesis in Flavobacterium johnsoniae. Nucleic Acids Res. 2023, 51(4), 1927–1942. [Google Scholar] [CrossRef]
- Fei, J.; Bronson, J.E.; Hofman, J.M.; Srinivas, R.L.; Wiggins, C.H.; Gonzalez, R. L Jr. Allosteric collaboration between elongation factor G and the ribosomal L1 stalk directs tRNA movements during translation. Proc. Natl. Acad. Sci. U S A. 2009, 106, 15702–15707. [Google Scholar] [CrossRef]
- Trabuco, L.G.; Schreiner, E.; Eargle, J.; Cornish, P.; Ha, T.; Luthey-Schulten, Z.; Schulten, K. The role of L1 stalk-tRNA interaction in the ribosome elongation cycle. J. Mol. Biol. 2010, 402, 741–760. [Google Scholar] [CrossRef]
- Mohan, S.; Noller, H.F. Recurring RNA structural motifs underlie the mechanics of L1 stalk movement. Nat. Commun. 2017, 8, 14285. [Google Scholar] [CrossRef]
- Baughman, G.; Nomura, M. Localization of the target site for translational regulation of the L11 operon and direct evidence for translational coupling in Escherichia coli. Cell 1983, 34(3), 979–988. [Google Scholar] [CrossRef]
- Thomas, M.S.; Nomura, M. Translational regulation of the L11 ribosomal protein operon of Escherichia coli: mutations that define the target site for repression by L1. Nucleic Acids Res. 1987, 15, 3085–3096. [Google Scholar] [CrossRef]
- Köhrer, C.; Mayer, C.; Neumair, O.; Gröbner, P.; Piendl, W. Interaction of ribosomal L1 proteins from mesophilic and thermophilic Archaea and Bacteria with specific L1-binding sites on 23S rRNA and mRNA. Eur. J. Biochem. 1998, 256, 97–105. [Google Scholar] [CrossRef]
- Nevskaya, N.; Tishchenko, S.; Gabdoulkhakov, A.; Nikonova, E.; Nikonov, O.; Nikulin, A.; Platonova, O.; Garber, M.; Nikonov, S.; Piendl, W. Ribosomal protein L1 recognizes the same specific structural motif in its target sites on the autoregulatory mRNA and 23S rRNA. Nucleic Acids Res. 2005, 33, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Nikonov, S.; Nevskaya, N.; Eliseikina, I.; Fomenkova, N.; Nikulin, A.; Ossina, N.; Garber, M.; Jonsson, B.H.; Briand, C.; Al-Karadaghi, S.; Svensson, A.; Aevarsson, A.; Liljas, A. Crystal structure of the RNA binding ribosomal protein L1 from Thermus thermophilus. EMBO J. 1996, 15, 1350–1359. [Google Scholar] [CrossRef]
- Nevskaya, N.; Tischenko, S.; Fedorov, R.; Al-Karadaghi, S.; Liljas, A.; Kraft, A.; Piendl, W.; Garber, M.; Nikonov, S. Archaeal ribosomal protein L1: the structure provides new insights into RNA binding of the L1 protein family. Structure 2000, 8, 363–371. [Google Scholar] [CrossRef]
- Tishchenko, S.; Nikonova, E.; Kljashtorny, V.; Kostareva, O.; Nevskaya, N.; Piendl, W.; Davydova, N.; Streltsov, V.; Garber, M.; Nikonov, S. Domain I of ribosomal protein L1 is sufficient for specific RNA binding. Nucleic Acids Res. 2007, 35, 7389–7395. [Google Scholar] [CrossRef]
- Korepanov, A.P.; Kostareva, O.S.; Bazhenova, M.V.; Bubunenko, M.G.; Garber, M.B.; Tishchenko, S.V. Studying the properties of domain I of the ribosomal protein L1: Incorporation into ribosome and regulation of the L1 operon expression. Protein J. 2015, 34, 103–110. [Google Scholar] [CrossRef]
- Mikhaylina, A.O.; Nikonova, E. Y/; Kostareva, O.S.; Piendl, W.; Erlacher, M.; Tishchenko, S.V. Characterization of regulatory elements of L11 and L1 operons in thermophilic Bacteria and Archaea. Biochemistry (Mosc) 2021, 86(4), 397–408. [Google Scholar] [CrossRef]
- Diedrich, G.; Spahn, C.M.; Stelzl, U.; Schäfer, M.A.; Wooten, T.; Bochkariov, D.E.; Cooperman, B.S.; Traut, R.R.; Nierhaus, K.H. Ribosomal protein L2 is involved in the association of the ribosomal subunits, tRNA binding to A and P sites and peptidyl transfer. EMBO J. 2000, 19, 5241–5250. [Google Scholar] [CrossRef]
- Müller, E.C.; Wittmann-Liebold, B. Phylogenetic relationship of organisms obtained by ribosomal protein comparison. Cell. Mol. Life Sci. 1997, 53(1), 34–50. [Google Scholar] [CrossRef]
- Liu, Q.; Fredrick, K. Intersubunit bridges of the bacterial ribosome. J. Mol. Biol. 2016, 428 10 Pt B, 2146–2164. [Google Scholar] [CrossRef]
- Cooperman, B.S.; Wooten, T.; Romero, D.P.; Traut, R.R. Histidine 229 in protein L2 is apparently essential for 50S peptidyl transferase activity. Biochem. Cell Biol. 1995, 73, 1087–1094. [Google Scholar] [CrossRef]
- Nakagawa, A.; Nakashima, T.; Taniguchi, M.; Hosaka, H.; Kimura, M.; Tanaka, I. The three-dimensional structure of the RNA-binding domain of ribosomal protein L2; a protein at the peptidyl transferase center of the ribosome. EMBO J. 1999, 18(6), 1459–1467. [Google Scholar] [CrossRef]
- Chandler, J.R.; Truong, T.T.; Silva, P.M.; Seyedsayamdost, M.R.; Carr, G.; Radey, M. Bactobolin resistance is conferred by mutations in the L2 ribosomal protein. MBio 2012, 3, e00499–e00512. [Google Scholar] [CrossRef]
- Rippa, V.; Cirulli, C.; Di Palo, B.; Doti, N.; Amoresano, A.; Duilio, A. The ribosomal protein L2 interacts with the RNA polymerase alpha subunit and acts as a transcription modulator in Escherichia coli. J. Bacteriol. 2010, 192(7), 1882–1889. [Google Scholar] [CrossRef]
- Chodavarapu, S.; Felczak, M.M.; Kaguni, J.M. Two forms of ribosomal protein L2 of Escherichia coli that inhibit DnaA in DNA replication. Nucleic Acids Res. 2011, 39(10), 4180–4191. [Google Scholar] [CrossRef]
- Xu, Q.; Chen, H.; Sun, W.; Zhang, Y.; Zhu, D.; Rai, K.R.; Chen, J.L.; Chen, Y. sRNA23, a novel small RNA, regulates to the pathogenesis of Streptococcus suis serotype 2. Virulence 2021, 12(1), 3045–3061. [Google Scholar] [CrossRef]
- O'Connor, M.; Gregory, S.T.; Dahlberg, A.E. Multiple defects in translation associated with altered ribosomal protein L4. Nucleic Acids Res. 2004, 32(19), 5750–5756. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Shamsuzzaman, M.; Kondopaka, M.; Pascual, C.; Zengel, J.M.; Lindahl, L. The extended loops of ribosomal proteins uL4 and uL22 of Escherichia coli contribute to ribosome assembly and protein translation. Nucleic Acids Res. 2016, 44(12), 5798–5810. [Google Scholar] [CrossRef]
- Gabashvili, I.S.; Gregory, S.T.; Valle, M.; Grassucci, R.; Worbs, M.; Wahl, M.C.; Dahlberg, A.E.; Frank, J. The polypeptide tunnel system in the ribosome and its gating in erythromycin resistance mutants of L4 and L22. Mol. Cell 2001, 8(1), 181–188. [Google Scholar] [CrossRef]
- Zengel, J.M.; Lindahl, L. Ribosomal protein L4 and transcription factor NusA have separable roles in mediating terminating of transcription within the leader of the S10 operon of Escherichia coli. Genes Dev. 1992, 6, 2655–2662. [Google Scholar] [CrossRef]
- Lindahl, L.; Zengel, J.M. Domain I of 23S rRNA competes with a paused transcription complex for ribosomal protein L4 of Escherichia coli. Nucleic Acids Res. 1993, 21(10), 2429–2435. [Google Scholar] [CrossRef]
- Stelzl, U.; Zengel, J.M.; Tovbina, M.; Walker, M.; Nierhaus, K.H.; Lindahl, L.; Patel, D.J. RNA-structural mimicry in Escherichia coli ribosomal protein L4-dependent regulation of the S10 operon. J. Biol. Chem. 2003, 278, 28237–28245. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.; Shen, P.; Samsel, L.; Liu, R.; Lindahl, L.; Zengel, J.M. Phylogenetic analysis of L4-mediated autogenous control of the S10 ribosomal protein operon. J. Bacteriol. 1999, 181(19), 6124–6132. [Google Scholar] [CrossRef]
- Worbs, M.; Huber, R.; Wahl, M.C. Crystal structure of ribosomal protein L4 shows RNA-binding sites for ribosome incorporation and feedback control of the S10 operon. EMBO J. 2000, 19(5), 807–818. [Google Scholar] [CrossRef]
- Trubetskoy, D.; Proux, F.; Allemand, F.; Dreyfus, M.; Iost, I. SrmB, a DEAD-box helicase involved in Escherichia coli ribosome assembly, is specifically targeted to 23S rRNA in vivo. Nucleic Acids Res. 2009, 37(19), 6540–6549. [Google Scholar] [CrossRef]
- Proux, F.; Dreyfus, M.; Iost, I. Identification of the sites of action of SrmB, a DEAD-box RNA helicase involved in Escherichia coli ribosome assembly. Mol. Microbiol. 2011, 82(2), 300–311. [Google Scholar] [CrossRef]
- Singh, D.; Chang, S.J.; Lin, P.H.; Averina, O.V.; Kaberdin, V.R.; Lin-Chao, S. Regulation of ribonuclease E activity by the L4 ribosomal protein of Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106(3), 864–869. [Google Scholar] [CrossRef]
- Singh, D.; Murashko, O.N.; Lin-Chao, S. Posttranscriptional regulation of tnaA by protein-RNA interaction mediated by ribosomal protein L4 in Escherichia coli. J. Bacteriol 2020, 202, e00799-19. [Google Scholar] [CrossRef]
- Davydov, I.I.; Wohlgemuth, I.; Artamonova, I.I.; Urlaub, H.; Tonevitsky, A.G.; Rodnina, M.V. Evolution of the protein stoichiometry in the L12 stalk of bacterial and organellar ribosomes. Nat. Commun. 2013, 4, 1387. [Google Scholar] [CrossRef]
- Wahl, M.C.; Möller, W. Structure and function of the acidic ribosomal stalk proteins. Curr. Prot. Pept. Sci. 2002, 3, 93–106. [Google Scholar] [CrossRef]
- Diaconu, M.; Kothe, U.; Schlünzen, F.; Fischer, N.; Harms, J.M.; Tonevitsky, A.G.; Stark, H.; Rodnina, M.V.; Wahl, M.C. Structural basis for the function of the ribosomal L7/12 stalk in factor binding and GTPase activation. Cell 2005, 121, 991–1004. [Google Scholar] [CrossRef]
- Carlson, M.A.; Haddad, B.G.; Weis, A.J.; Blackwood, C.S.; Shelton, C.D.; Wuerth, M.E.; Walter, J.D.; Spiegel, P.C. Jr. Ribosomal protein L7/L12 is required for GTPase translation factors EF-G, RF3, and IF2 to bind in their GTP state to 70S ribosomes. FEBS J. 2017, 284, 1631–1643. [Google Scholar] [CrossRef]
- Helgstrand, M.; Mandava, C.S.; Mulder, F.A.; Liljas, A.; Sanyal, S.; Akke, M. The ribosomal stalk binds to translation factors IF2, EF-Tu, EF-G and RF3 via a conserved region of the L12 C-terminal domain. J. Mol. Biol. 2007, 365, 468–79. [Google Scholar] [CrossRef]
- Ge, X.; Mandava, C.S.; Lind, C.; Åqvist, J.; Sanyal, S. Complementary charge-based interaction between the ribosomal-stalk protein L7/12 and IF2 is the key to rapid subunit association. Proc. Natl. Acad. Sci U S A 2018, 115, 4649–4654. [Google Scholar] [CrossRef]
- Johnsen, M.; Christensen, T.; Dennis, P.P.; Fiil, N.P. Autogenous control: ribosomal protein L10-L12 complex binds to the leader sequence of its mRNA. EMBO J. 1982, 1, 999–1004. [Google Scholar] [CrossRef]
- Passador, L.; Linn, T. Autogenous regulation of the RNA polymerase beta subunit of Escherichia coli occurs at the translational level in vivo. J. Bacteriol. 1989, 171(11), 6234–6242. [Google Scholar] [CrossRef]
- Iben, J.R; Draper, D.E. Specific interaction of the L10(L12)4 ribosomal protein complex with mRNA, rRNA, and L11. Biochemistry 2008, 47, 2721–2731. [Google Scholar] [CrossRef]
- Yakhnin, H.; Yakhnin, A.V.; Babitzke, P. Ribosomal protein L10(L12)4 autoregulates expression of the Bacillus subtilis rplJL operon by a transcription attenuation mechanism. Nucleic Acids Res. 2015, 43, 7032–7043. [Google Scholar] [CrossRef]
- Leipuviene, R.; Björk, G.R. Alterations in the two globular domains or in the connecting alpha-helix of bacterial ribosomal protein L9 induces +1 frameshifts. J. Bacteriol. 2007, 189(19), 7024–7031. [Google Scholar] [CrossRef]
- Herr, A.I.; Nelson, C.C.; Wills, N.M.; Gesteland, R.F.; Atkins, J.F. Analysis of the roles of tRNA structure, ribosomal protein L9, and the bacteriophage T4 gene 60 bypassing signals during ribosome slippage on mRNA. J. Mol. Biol. 2001, 309, 1029–1048. [Google Scholar] [CrossRef]
- Soni, D.; Bafana, A.; Gandhi, D.; Sivanesan, S.; Pandey, R.A. Stress response of Pseudomonas species to silver nanoparticles at the molecular level. Environ. Toxicol. Chem. 2014, 33, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Pei, H.; Han, S.; Yang, S.; Lei, Z.; Zheng, J.; Jia, Z. Phosphorylation of bacterial L9 and its functional implication in response to starvation stress. FEBS Lett. 2017, 591(20), 3421–3430. [Google Scholar] [CrossRef]
- Xue, L.; Lenz, S.; Zimmermann-Kogadeeva, M.; Tegunov, D.; Cramer, P.; Bork, P.; Rappsilber, J.; Mahamid, J. Visualizing translation dynamics at atomic detail inside a bacterial cell. Nature, 2022, 610, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, X.; Yin, L.; Liu, Q.; Yu, Z.; Xu, C.; Ma, Z.; Xia, Y.; Shi, J.; Gong, Y.; Bai, F.; Cheng, Z.; Wu, W.; Lin, J.; Jin, Y. RplI interacts with 5' UTR of exsA to repress its translation and type III secretion system in Pseudomonas aeruginosa. PLoS Pathog 2022, 18(1), e1010170. [Google Scholar] [CrossRef] [PubMed]
- Charollais, J.; Pflieger, D.; Vinh, J.; Dreyfus, M.; Iost, I. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 2003, 48, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Mustoe, A.M.; Busan, S.; Rice, G.M.; Hajdin, C.E.; Peterson, B.K.; Ruda, V.M.; Kubica, N.; Nutiu., R.; Baryza, J.L.; Weeks, K.M. Pervasive regulatory functions of mRNA structure revealed by high-resolution SHAPE probing. Cell 2018, 173, 181–195.e18. [Google Scholar] [CrossRef] [PubMed]
- Iost, I.; Jain, C. A DEAD-box protein regulates ribosome assembly through control of ribosomal protein synthesis. Nucleic Acids Res. 2019, 47, 8193–8206. [Google Scholar] [CrossRef]
- Rabuck-Gibbons, J.N.; Popova, A.M.; Greene, E.M.; Cervantes, C.F.; Lyumkis, D.; Williamson, J.R. SrmB rescues trapped ribosome assembly intermediates. J. Mol. Biol. 2020, 432(4), 978–990. [Google Scholar] [CrossRef]
- Dong, X.; Doerfel, L.K.; Sheng, K.; Rabuck-Gibbons, J.N.; Popova, A.M.; Lyumkis, D.; Williamson, J.R. Near-physiological in vitro assembly of 50S ribosomes involves parallel pathways. Nucleic Acids Res. 2023, 51, 2862–2876. [Google Scholar] [CrossRef]
- Guillier, M.; Allemand, F.; Dardel, F.; Royer, C.A.; Springer, M.; Chiaruttini, C. Double molecular mimicry in Escherichia coli: binding of ribosomal protein L20 to its two sites in mRNA is similar to its binding to 23S rRNA. Mol. Microbiol. 2005, 56, 1441–1456. [Google Scholar] [CrossRef]
- Haentjens-Sitri, J.; Allemand, F.; Springer, M.; Chiaruttini, C. A competition mechanism regulates the translation of the Escherichia coli operon encoding ribosomal proteins L35 and L20. J. Mol. Biol 2008, 375, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Choonee, N.; Even, S.; Zig, L.; Putzer, H. Ribosomal protein L20 controls expression of the Bacillus subtilis infC operon via a transcription attenuation mechanism. Nucleic Acids Res. 2007, 35, 1578–1588. [Google Scholar] [CrossRef] [PubMed]
- Babina, A.M.; Parker, D.J.; Li, G.W.; Meyer, M.M. Fitness advantages conferred by the L20-interacting RNA cis-regulator of ribosomal protein synthesis in Bacillus subtilis. RNA 2018, 24, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Jeon, H.; Oh, J.I.; Hwang, J. Overexpressed L20 rescues 50S ribosomal subunit assembly defects of bipA-deletion in Escherichia coli. Front. Microbiol. 2020, 10, 2982. [Google Scholar] [CrossRef] [PubMed]
- Korepanov, A.P.; Gongadze, G.M.; Garber, M.B.; Court, D.L.; Bubunenko, M.G. Importance of the 5 S rRNA-binding ribosomal proteins for cell viability and translation in Escherichia coli. J. Mol. Biol. 2007, 366, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Aseev, L.V.; Bylinkina, N.S.; Boni, I.V. Regulation of the rplY gene encoding 5S rRNA binding protein L25 in Escherichia coli and related bacteria. RNA 2015, 21, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Lilleorg, S.; Reier, K.; Remme, J.; Liiv, A. The intersubunit bridge B1b of the bacterial ribosome facilitates initiation of protein synthesis and maintenance of translational fidelity. J. Mol. Biol. 2017, 429, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Aseev, L.V.; Koledinskaya, L.S.; Boni, I.V. Autogenous regulation in vivo of the rpmE gene encoding ribosomal protein L31 (bL31), a key component of the protein-protein intersubunit bridge B1b. RNA 2020, 26, 814–826. [Google Scholar] [CrossRef]
- Lilleorg, S.; Reier, K.; Pulk, A.; Liiv, A.; Tammsalu, T.; Peil, L.; Cate, J.D.; Remme, J. Bacterial ribosome heterogeneity: changes in ribosomal protein composition during transition into stationary growth phase. Biochimie 2019, 156, 169–180. [Google Scholar] [CrossRef]
- Akanuma, G.; Nanamiya, H.; Natori, Y.; Nomura, N.; Kawamura, F. Liberation of zinc-containing L31 (RpmE) from ribosomes by its paralogous gene product, YtiA, in Bacillus subtilis. J. Bacteriol. 2006, 188(7), 2715–2720. [Google Scholar] [CrossRef]
- Ueta, M.; Wada, C.; Wada, A. YkgM and YkgO maintain translation by replacing their paralogs, zinc-binding ribosomal proteins L31 and L36, with identical activities. Genes Cells. 2020, 25, 562–581. [Google Scholar] [CrossRef]
- Lilleorg, S.; Reier, K.; Volõnkin, P.; Remme, J.; Liiv, A. Phenotypic effects of paralogous ribosomal proteins bL31A and bL31B in E. coli. Sci. Rep. 2020, 10(1), 11682. [Google Scholar] [CrossRef]
- Rasmussen, R.A.; Wang, S.; Camarillo, J.M.; Sosnowski, V.; Cho, B.K.; Goo, Y.A. , Lucks JB, O'Halloran, T.V. Zur and zinc increase expression of E. coli ribosomal protein L31 through RNA-mediated repression of the repressor L31p. Nucleic Acids Res. 2022, 50, 12739–12753. [Google Scholar] [CrossRef]
- Hurtado-Rios, J.J.; Carrasco-Navarro, U.; Almanza-Pérez, J.C.; Ponce-Alquicira, E. Ribosomes: The new role of ribosomal proteins as natural antimicrobials. Int. J. Mol. Sci 2022, 23(16), 9123. [Google Scholar] [CrossRef]
- Pidutti, P.; Federici, F.; Brandi, J.; Manna, L.; Rizzi, E.; Marini, U.; Cecconi, D. Purification and characterization of ribosomal proteins L27 and L30 having antimicrobial activity produced by the Lactobacillus salivarius SGL 03. J. Appl. Microbiol. 2018, 124, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Ma, Z.; Yao, L.; Gao, Z.; Zhang, S. Preserved antibacterial activity of ribosomal protein S15 during evolution. Mol. Immunol. 2020, 127, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Kovacs D, Rakacs M, Agoston B, Lenkey K, Semrad K, Schroeder R, Tompa P. Janus chaperones: assistance of both RNA- and protein-folding by ribosomal proteins. FEBS Lett. 2009, 583, 88–92. [CrossRef]
- Woodgate, R.; Rajagopalan, M.; Lu, C.; Echols, H. UmuC mutagenesis protein of Escherichia coli: purification and interaction with UmuD and UmuD'. Proc. Natl. Acad. Sci. U S A 1989, 86, 7301–7305. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Liang, L.; Su, L.; Wen, A.; Zhou, C.; Feng, Y. Structural basis for regulation of SOS response in bacteria. Proc. Natl. Acad. Sci. U S A 2023, 120(2), e2217493120. [Google Scholar] [CrossRef]
- Katsuya-Gaviria, K.; Paris, G.; Dendooven, T.; Bandyra, K.J. Bacterial RNA chaperones and chaperone-like riboregulators: behind the scenes of RNA-mediated regulation of cellular metabolism. RNA Biol. 2022, 19, 419–436. [Google Scholar] [CrossRef]
- Coetzee, T.; Herschlag, D.; Belfort, M. Escherichia coli proteins, including ribosomal protein S12, facilitate in vitro splicing of phage T4 introns by acting as RNA chaperones. Genes Dev. 1994, 8(13), 1575–88. [Google Scholar] [CrossRef] [PubMed]
- Woodson, S.A.; Panja, S.; Santiago-Frangos, A. Proteins that chaperone RNA regulation. Microbiol Spectr 2018, 6(4), 10.1128/microbiolspec.RWR-0026-2018. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.S.; Bains, J.K; Sreeramulu, S.; Schwalbe, H.; Fürtig, B. Conformational switch in the ribosomal protein S1 guides unfolding of structured RNAs for translation initiation. Nucleic Acids Res. 2018, 46, 10917–10929. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.V.; Hien, E.D.M.; Lafontaine, D.A. Riboswitch regulation mechanisms: RNA, metabolites and regulatory proteins. Biochim. Biophys. Acta Gene Regul. Mech 2020, 1863(3), 194501. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, V.; Qureshi, N.S.; Warhaut, S.; Bains, J.K.; Dietz, M.S.; Heilemann, M.; Schwalbe, H.; Fürtig, B. Switching at the ribosome: riboswitches need rProteins as modulators to regulate translation. Nat. Commun. 2021, 12, 4723. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, P.; Woodson, S.A. S16 throws a conformational switch during assembly of 30S 5' domain. Nat. Struct. Mol. Biol. 2009, 16(4), 438–445. [Google Scholar] [CrossRef] [PubMed]
- Oberto, J.; Bonnefoy, E.; Mouray, E.; Pellegrini, O.; Wikström, P.M.; Rouvière-Yaniv, J. The Escherichia coli ribosomal protein S16 is an endonuclease. Mol Microbiol. 1996, 19, 1319–1330. [Google Scholar] [CrossRef]
- Bonnefoy, E. The ribosomal S16 protein of Escherichia coli displaying a DNA-nicking activity binds to cruciform DNA. Eur. J. Biochem. 1997, 247, 852–859. [Google Scholar] [CrossRef]
- Bowater, R.P.; Bohálová, N.; Brázda, V. Interaction of proteins with inverted repeats and cruciform structures in nucleic acids. Int. J. Mol. Sci. 2022, 23, 6171. [Google Scholar] [CrossRef]
- Zouine, M.; Beloin, C.; Ghelis, C.; Le Hégarat, F. The L17 ribosomal protein of Bacillus subtilis binds preferentially to curved DNA. Biochimie 2000, 82(1), 85–91. [Google Scholar] [CrossRef]
- Exley, R.; Zouine, M.; Pernelle, J.J.; Beloin, C.; Le Hégarat, F.; Deneubourg, A.M. A possible role for L24 of Bacillus subtilis in nucleoid organization and segregation. Biochimie 2001, 83, 269–275. [Google Scholar] [CrossRef]
- Yancey, J.E.; Matson, S.W. The DNA unwinding reaction catalyzed by Rep protein is facilitated by an RHSP-DNA interaction. Nucleic Acids Res. 1991, 19, 3943–3951. [Google Scholar] [CrossRef]
- Wool, I.G. Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 1996, 21, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.R.; McIntosh, K.B. How common are extraribosomal functions of ribosomal proteins? Mol. Cell. 2009, 34, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Lan, T.; Mo, B. Extraribosomal functions of cytosolic ribosomal proteins in plants. Front. Plant. Sci 2021, 12, 607157. [Google Scholar] [CrossRef] [PubMed]
- Reier, K.; Lahtvee, P.J.; Liiv, A.; Remme, J. A conundrum of r-protein stability: Unbalanced stoichiometry of r-proteins during stationary phase in Escherichia coli. mBio 2022, 13, e0187322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



