1. Introduction
Developmental processes that impact the quality and availability of water in hyper-arid regions is an overriding concern when assessing environmental impact of any proposal to develop resources [
1]. Determining the character of water chemistry and identifying the relative importance of both natural and anthropogenic factors in the contamination of water resources in such regions is a global priority, especially with predicted climate change impacts [
2]. This is particularly important on the arid and mineral-rich western flank of the Andes, where two thirds of Peru’s population share less than 2% of the country’s water and where the world’s largest copper deposits reside [
3,
4,
5,
6]. Increasing pressure from mining on water resources poses a serious challenge to the ecology of these regions and regularly produces social conflict [
7]. The projected nine-fold expansion of copper production in southern Peru, already the second largest producer after Chile, can only accentuate these challenges. Since trace metals are a major factor in the toxicity of contaminated water [
8,
9], mapping their presence in potentially affected river systems and understanding their baseline geochemistry is an important preparatory challenge. The direct impacts of increased mining can be partly mitigated by re-routing rivers using canals and diverting waste water to tailings dams, but the increase in mining inevitably increases the human population in remote mineral-rich regions, with a concomitant increase in urban and agricultural development, both of which are often associated with contamination from trace-metal and persistent residues [
7,
10,
11,
12].
Trace metal contamination in rivers systems is, in part, the product of natural acid rock drainage (ARD), in which the oxidation of sulfide-bearing minerals increases the acidity of run-off waters and enhances the further chemical breakdown of the underlying lithology. Hydrological systems dominated by acidic waters show increased trace metal mobilisation and metal-hydroxide precipitation [
13]. The downstream eco-toxicity of these contaminants depends on their bio-availability and consequent accumulation in the food chain [
14,
15]. Polymetallic mining is a well-documented cause of increased ARD where it is more commonly known as acid mine drainage (AMD). Sulfide minerals and ores, in which the dominant metal ion can be iron, zinc, copper or nickel, are commonly associated with AMD. Chalcopyrite (a copper-iron-sulfide) is the most commonly mined copper bearing ore, and it is frequently found alongside other sulfides, making copper mines particularly prone to AMD [
7,
11,
16,
17]. In geologically active regions such as the Andes, ARD as a consequence of acidic epithermal waters entering the river catchment systems, lead to elevated chemical weathering rates and increased addition of ions to the rivers, the exact chemistry dependent upon the geology of the source region [
18]. In combination, the effect of geology and anthropogenic elevation of weathering at disturbed mine sites in regions such as southern Peru may make water resources unsuitable for human consumption and agricultural irrigation, and therefore, water quality needs careful monitoring. Pesticide and fertilzer effluents and industrial and urban waste waters are also well-documented sources of trace-metal contamination. Arsenic is used in herbicides and pesticides; cadmium in batteries and plastics; chromium in dyes and tanning processes; lead in batteries, wiring and cabling; mercury and manganese in pesticides and batteries; and zinc is found in pharmaceuticals, dyes and batteries. All of these elements can leach into the wider environment from agricultural and urban run-off. The uptake of trace metals depends upon soil characteristics, plant species and the metal concerned [
11,
19]. The trophic transfer of trace metals, even at low concentrations, is known to have mutagenic effects [
20].
In addition to the more global anthropogenic and natural geogenic factors that contribute to the determination of water quality, there are factors specific to areas in which mining and catchment transfer projects have been developed [
16,
21]. The Torata river sub-catchment and lower reaches of the Moquegua river drainage basin represent a region in which both a landscape-scale mine and a regional-scale catchment transfer project could reasonably be thought to impact catchment dynamics, including water resource quality and availability. (
Figure 1) The exploitation of mineral resources increasingly necessitates environmental impact statements (EIS) with concomitant impact mitigation strategies which may become advisory or statutory obligations [
22]. Development of landscape-scale mines increasingly requires such mitigation strategies to avoid the otherwise inevitable environmental consequences and political issues, although these may not be properly identified in many impact assessment processes [
7,
23,
24,
25,
26]. The development of the Cuajone, Quellaveco and Toquepala mines in southern Peru have all involved the implementation of significant impact mitigation, with both river channel and waste material diversion with a complex network of sealed and open channels, tunnels and existing river channels [
22]. Waste materials from the Cuajone and Quellaveco mines do not enter the greater Moquegua river drainage basin but are transferred to the tailings dams at Quebrada Honda (17 28 19.52S, 70 41 51.85W) at 1135m asl and Cortaderas 2 (17 12 02.49S, 70 41 51.85W) at 3086m asl, all of which can be readily seen on satellite imagery. Additionally, a tunnel diverting Torata river channel flow to the north of the principal mine pit at Cuajone was constructed to ensure that water quality from the headwaters is not affected by mine activity. Water scarcity in the region, combined with population growth and agricultural development has increased the need for water resources and driven the development of the catchment transfer Pasto Grande project. This a project transfers water from the headwaters of the Tambo river into the greater Moquegua river catchment in 262km of open channels, tunnels and existing river channels, that include the Sajena, Torata and Moquegua rivers. The long term impacts of this transfer on catchment ecology, hydrological dynamics and water chemistry have not been studied. Very few studies have been carried out on the impacts of catchment transfer projects but they are widely considered to have drastic impacts on hydrological regimes, water chemistry and aquatic biota [
21]. The effect of the Pasto Grande project is the subject of further study.
As such, the Torata river was selected for several significant reasons: (i) the presence of the landscape-scale Cuajone copper mine, owned by Southern Copper, located in the headwaters, and the proposed expansion of mining in the region, gives the opportunity to look at the efficiency of water management measures associated with large-scale mining infrastructure; (ii) there is a global need for detailed studies of the controls on water chemistry in hyper-arid regions to guide resource management; (iii) the nature of the topography provides an ideal context in which to identify the sequential impacts of trace metal dispersion and concentration; (iv) the presence of two well spaced urban settlements at 2200m asl (Torata) and 1400m asl (Moquegua), allowing the identification of distinct chemical signals; and (v) the presence of two distinct and well separated agricultural areas associated with the above settlements
Figure 1.
The general aim of this paper is to establish geogenic and anthropogenic determinants of water chemistry in the Torata river sub-catchment and lower Moquegua river, lying in a hyper-arid region in southern Peru. The catchment is periodically affected by El Niño–Southern Oscillation (ENSO) events with Pacific-derived moisture and regional atmospheric instability producing heavy precipitation and catastrophic flooding in the Moquegua region, as in 1998 [
27] and more recently in 2016 [
28].
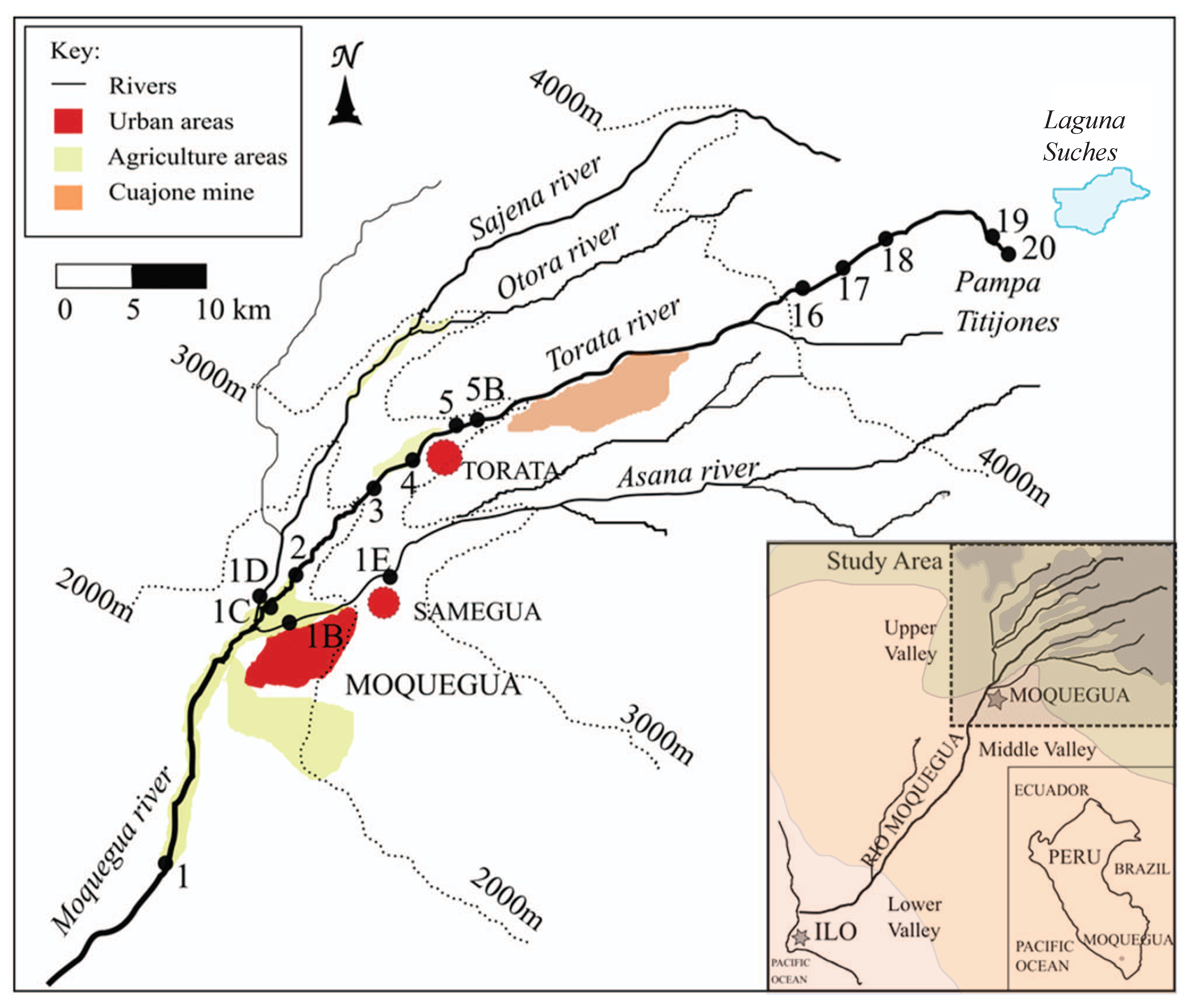
Topographic conditions in the region provide an ideal transect from the high Andes to the Pacific coast with the Torata river and the lower Moquegua river crossing four distinct geological units, passing the landscape-scale Cuajone mine, the urban settlements of Torata and Moquegua and two agricultural areas (
Figure 1). A sample-sites network was established throughout the Torata river sub-catchment, above and below the Cuajone copper mine, below the village of Torata and associated agricultural areas. Additionally, sites were adopted above and below the city of Moquegua and along the lower Moquegua river to the Pacific ocean. We describe the spatial and temporal variation in the chemistry of the river and its main tributaries, and use this to investigate the various contributors to the chemistry. Spatial patterns can be linked to processes specific to the upper, middle and lower catchments, particularly the underlying geology, any influence from mining and related acid rock interactions, agriculture, and hydro-fluvial processes such as ground flow, surface run off, distance from the ocean and elevation. Secondly, we discuss temporal patterns, linked to dry/wet seasons and El Niño and how these affect the baseline. Where the data allow we have indicated qualitative source apportionment based on concentration data. In addition to the overall aim we have, where the data allow, indicated qualitative source apportionment based on concentration data. In this we have attempted to achieve four specific objectives: 1) To identify the impact of the Cuajone mine on the geochemistry of the Torata river. 2) To identify and describe the hydro-chemical impact of the rural settlement of Torata and associated agricultural lands on the Torata river. 3) To identify and describe the hydro-chemical impact of the urban settlement of Moquegua on the Moquegua river. 4) To identify the the hydro-chemical impacts of the agricultural area lying along the lower Moquegua river valley.
2. Materials and Methods
2.1. Study Area
Hyper-arid regions are characterised by long periods of very low precipitation [
18]. In the Peruvian Coastal Desert, the development of hyper-aridity co-evolved with the emergence of the episodic El Niño phenomena, creating a stable xeric environment that supports little vegetation, but which is affected by periodic catastrophic flooding and erosion [
18,
27,
30]. The World’s largest supergene copper deposits are also located in this hyper-arid zone, stretching from southern Peru to northern Chile [
31,
32]. Currently there are three landscape-scale copper mines in the region; the Cuajone mine, the newly developed Quellaveco mine and the Toquepala mine. The study area focuses on the Torata river in the Moquegua drainage. This river rises in the headwater bofedales at 4590m asl at Pampa Titijones, and flows to the Cuajone copper mine at 3500m asl, before descending through the settlement of Torata, approximately 11km below the mine at 2200m asl. The channel then passes through a narrow canyon to join the Sajena river and, subsequently, the Moquegua river at 1290m asl below the city of Moquegua. Thereafter, the river flows through a narrow, intensively farmed, floodplain before reaching the Pacific Ocean at Ilo.
2.2. Study Sites
The sampling network was designed to cover the principal tributaries in the Torata subcatchment and lower Moquegua river catchment. Data discussed here are from 16 sites within the catchment, 11 along the Torata river, four lying at or below the confluence of the Torata river with the Moquegua and Sajena rivers, and one site close to the ocean (
Figure 1). Precise GPS locations and elevations of the sites are given in
Table 1. Site 0A is in the arid tropical zone, near Ilo on the coast. Site 1 is located in the upper tropics, 20 km below both the city of Moquegua and an extensive area of irrigated agriculture. The sites 1B, 1C and 1D are located at the confluence of the Sajena, Torata and Moquegua rivers. Site 1E, lies above all urban development and records the quality of the upper Asana River. Site 2, lies above all agricultural influence from the Moquegua city district, emerging from a canyon that starts in the Torata district. Sites 3 and 4 lie below the small rural town of Torata, and sites 5 and 5B above Torata, with 5B located immediately beneath the Cuajone mine. Directly above the Cuajone mine are sites 16, 17 and 18, and finally, sites 19 and 20 represent the source bofedale (peat wetland) and spring respectively.
2.3. Sample Collection
Three field investigations were undertaken: January 2017 (denoted 17-R), July 2017 (denoted 17-D) and January 2018 (denoted 18-R). The 17-R sampling campaign followed directly after an El Niño episode in which there was considerable flooding in the lower catchment. Despite this, it did not rain during the 17-R visit. During 17-D, the weather was dry and had been dry since the 17-R visit. Visit 18-R was nominally during the wet season but data collection preceded the onset of rains in the headwaters and as such many of the rivers retained dry season characteristics. Field visits were arranged to collect water quality data in-situ and water samples for later analysis. Prior to sample and data collection all field equipment was calibrated at the Universidad Nacional Autonoma de Moquegua (UNAM) laboratories. Owing to weather conditions and logistical constraints, it was not possible to collect samples at all sites on all field visits.
2.3.1. Water Quality Parameters and River Data Collection
At each site the following data were collected: GPS coordinates, atmospheric and water temperature, altitude, dissolved oxygen (DO mg/l and %), pH, conductivity, total dissolved solids (TDS) and oxygen reduction potential (ORP). A simple assessment of nearby land usage was noted.
2.3.2. Water Sample Collection
At each site, 1 litre composite samples were collected in pre-washed polyethylene bottles. Sub samples for Mercury (Hg) analysis were preserved with sulphuric acid and potassium dichromate to pH < 2, and samples for cyanide () analysis were preserved with sodium hydroxide to pH > 12. Samples for total metal analysis were decanted into 50 ml tubes and preserved to pH < 2 with HNO3 (99.9% trace metal basis) in the UNAM laboratory. All samples were packed in cool boxes for transport and stored at 4°C until analysis.
2.4. Analysis of Water Samples
Chemical analyses for 44 components including major cations, major anions, trace metals, nutrients and biological components (see
Table 2) were conducted by Northumbrian Water Scientific Services (Newcastle, UK), Greenwich University (Kent, UK) and the University of Cambridge (Cambridge, UK) laboratories. Samples for metal analysis were treated to acid digestion at 105°C and analysed by ICP-MS (7500ce, Agilent, UK). The anions were measured using ion chromatography (Dionex IC with Ionpac analytical column¸ Thermo Scientific, UK) and nitrates were measured using discrete automated colorimetry (Aquakem 600, Thermo Scientific, UK). Hg was measured using atomic florescence (Millenium Merlin, PS Analytical, LOD: 0.003
g/l) and total cyanide (CN) was measured using a segmented flow analyser (Skalar, Nederland, LOD: 0.02 mg/l). Total phosphorous (TP) was measured by ICP-OES (iCap 6500, Thermo Scientific, UK) and total nitrogen (TN) was measured using chemiluminescence (Shimadzu TOC-V, UK). Chemical oxygen demand (COD) was measured by digestion followed by colorimetry (Merck COD cell test kit, Germany, COD 5 – 80 mg/l).
E.coli and coliform were quantified using multiple-tube fermentation techniques. All analytical methods were carried out according to EPA and ISO protocols in quality-controlled labs. All samples were analysed in duplicate for precision, and certified reference standards and internal calibration were used for accuracy measurements [
33].
2.5. Statistical Analysis
Cluster analysis (CA) and principal component analysis (PCA) were used to quantify the relative dominance of various physico-chemical components of the river water with the aim of discriminating the main controls over the river quality. These methods are widely used in the analysis of correlations in multivariate data [
9,
16]. Both CA and PCA were applied to the z-score normalised value of the measured parameters [
34]. z-score normalisation facilitates discussion of correlations between parameters with vastly different magnitudes or amplitudes of fluctuations. CA provides a framework for creating subsets (clusters) of samples with high similarities. The internal structure of the data is ordered according to a dissimilarity measure, grouping similar samples. The analysis presented here (
Figure 7 and
Figure 8), uses Ward’s hierarchical agglomerative CA method, together with a Euclidean dissimilarity measure [
9,
35,
36]). This method finds the two closest samples (the data points with the smallest sum of squares of Euclidean distance), producing a secondary pseudo-sample. It then iterates this procedure until all the samples have been grouped, recording the dissimilarity distances,
, where
i indicates the
link. In the CA dendrograms shown in this paper, the dissimilarity distance was reported as a fraction of the largest distance:
. The cophenetic correlation coefficient (CCC) was used to measure how well the cluster dendrograms preserve the pairwise dissimilarity distances between original, un-clustered, data [
37]. A CCC of 100% corresponds to a dendrogram that perfectly preserves the original distances.
CA was employed in two ways in this study. Q-mode CA was used to find how the sample sites cluster with respect to the chemical parameters. This reveals spatial correlations of parameters over the set of sample sites. R-mode CA was used to find how the parameters cluster with respect to the sample sites. Clusters of similar parameters indicate that underlying factors affect them alike [
38].
PCA is a methodology for the dimensional reduction of the number of parameters needed to describe a set of data [
34,
39]. The analysis begins with the calculation of the eigenvectors of the covariance matrix of the data, and the arrangement of them in descending order with respect to decreasing eigenvalues. If some eigenvalues are significantly larger (above some threshold) than the others, their corresponding eigenvectors are called “principal components”. By picking out the principal components with the largest eigenvalues, it is possible to describe the covariance of the data to a level given by the ratio between the sum of their eigenvalues and the sum of all the eigenvalues. In this paper, PCA was employed on clusters with similarly behaving parameters to understand the intra-cluster correlation.
2.6. Contextual Materials and Analyses
In addition to the above methodologies applied to our sample site data, we also refer to data collected under our longer-term campaign, and data collated from published papers and technical reports on water management in the region. This provides a useful context in which to fully understand both the geogenic and anthropogenic determinants of water quality and availability, while underlining the need for further studies.
3. Results
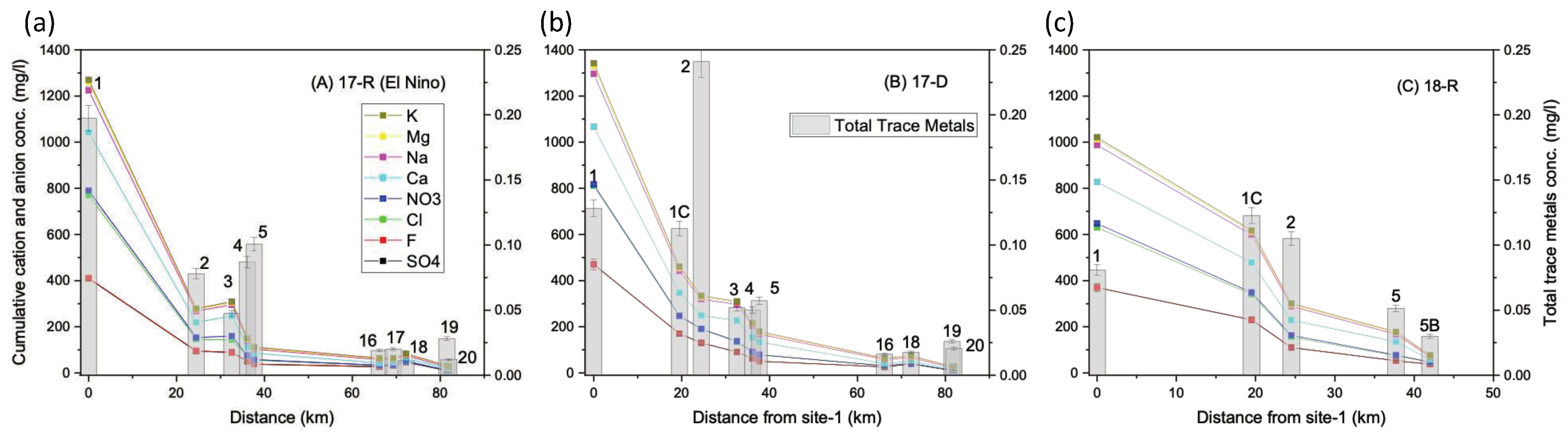
Water chemistry data from the three field seasons (17-R, 17-D and 18-R) are presented in
Figure 2. Measured chemical parameters with their minimum and maximum values are in
Table 2. The following sections discuss the temporal and spatial variations in river water chemistry during the three sampling campaigns.
3.1. Water Quality Parameters and Major Ion Composition
3.1.1. Headwater Sites Above the Cuajone Mine (Sites 16, 17, 18, 19, 20)
These sites were neutral or slightly alkaline (pH 7.84 to 9.45) with most sites recording a pH suitable for aquatic life. Site-19 showed the most alkaline conditions with pH values up to 9.45 and water temperature readings
C higher than ambient temperature. This site is a bofedal (Andean peat wetland) just below the source spring, site-20, and water was collected at midday in 17-R but early morning in 17-D. Both dissolved oxygen (DO) levels and pH at this site were higher at midday (12.4 mg/l and pH 9.45 in 17-R) than in the early morning (6.62 mg/l and pH 8.52 in 17-D) and, when taken together, are consistent with photosynthetic activity consuming CO
2 [
40,
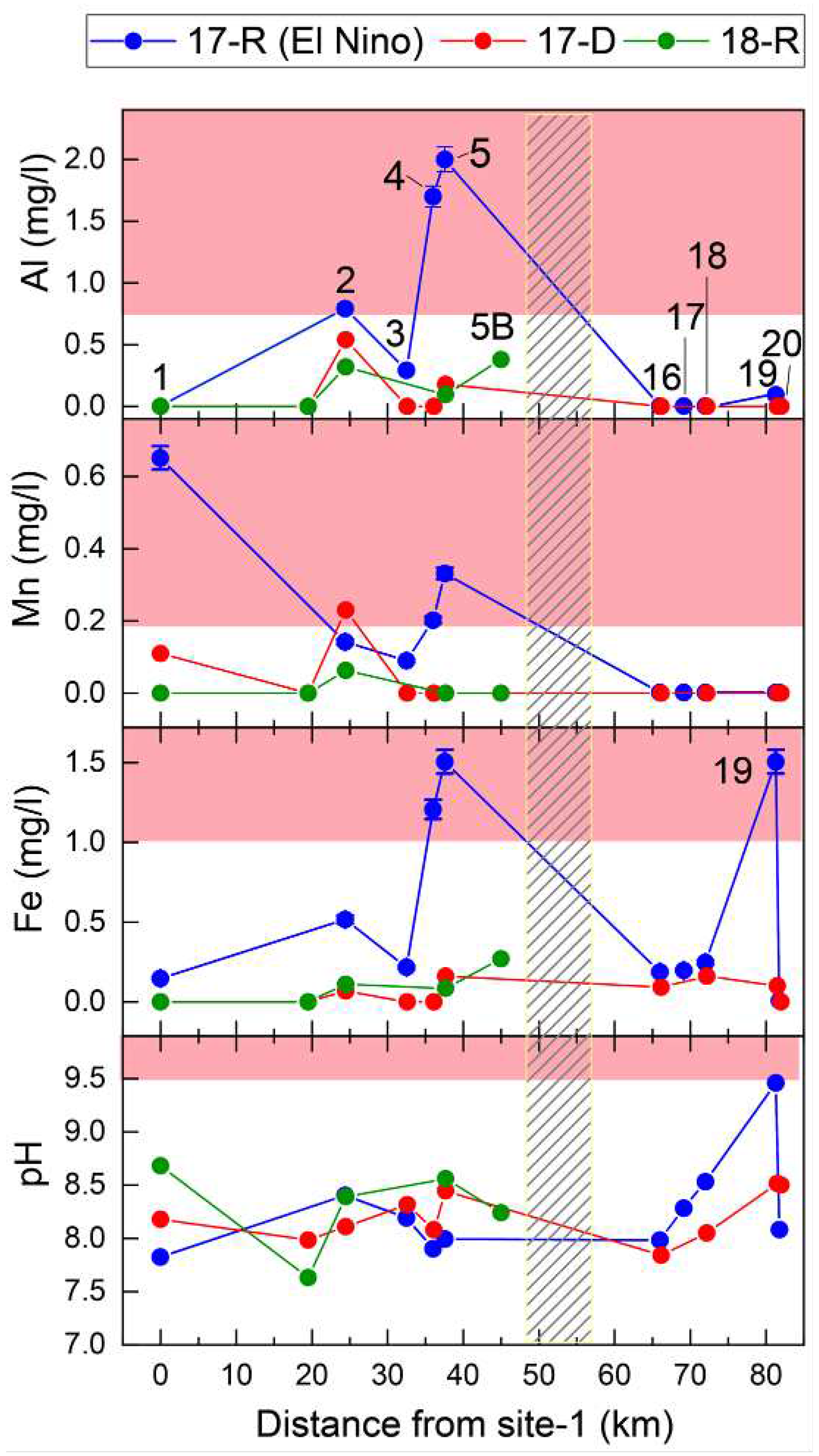
41] and adding free oxygen to the water during daylight hours. However, vegetative growth was more evident during the warmer wetter season (17-R) than the drier colder season (17-D), as to be expected, and therefore increased photosynthesis in season 17-R may also have contributed to the higher pH and DO levels. Similarly, the high quantity of decomposing organic matter related to the rich floral composition of the bofedal, explains why the highest COD measurements in the Torata river (17 mg/l) system were observed at this site.
The headwater sites have the lowest concentrations of major cations (Na
+≳ Ca
2+≫ Mg
2+, K
+) and anions (
≫ Cl
−> F
−,
), with total concentrations in all ions increasing downstream (
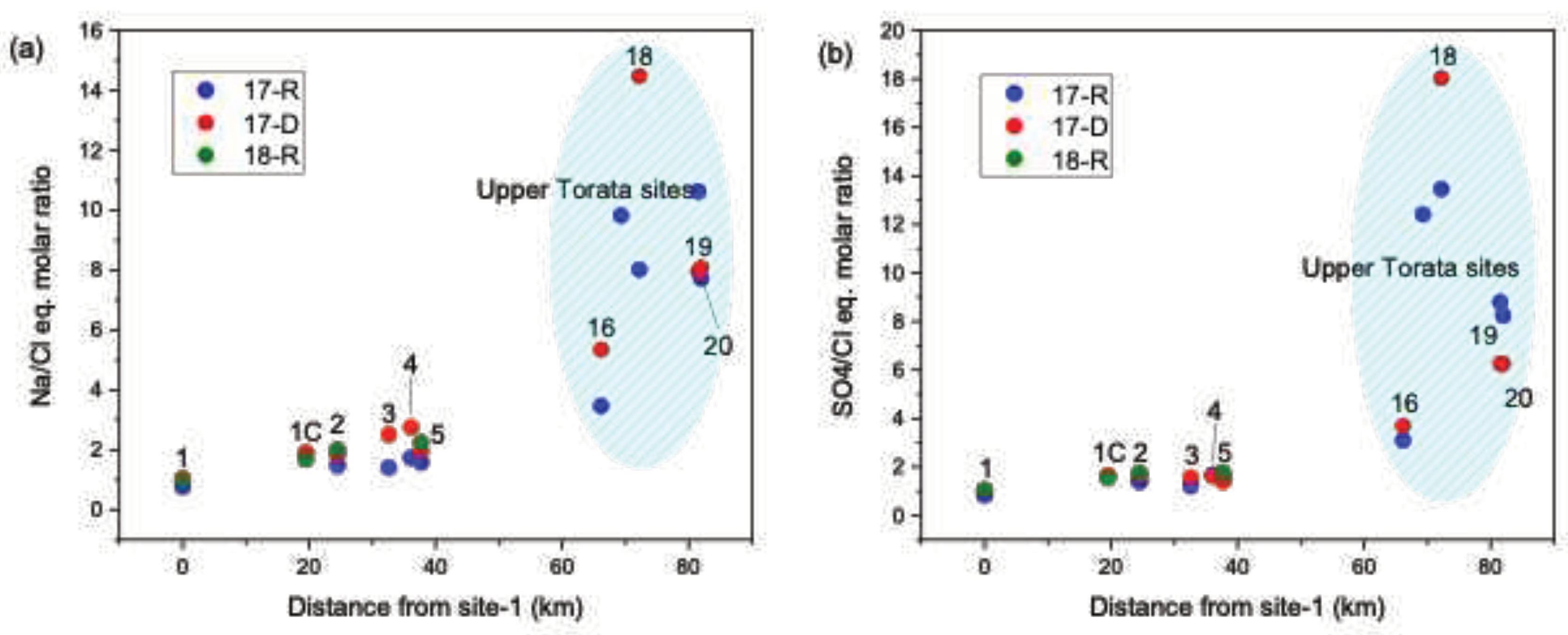
Figure 2) as expected. Na/Cl equivalent molar ratios at the headwater sites,
Figure 3a, were significantly higher (10.6 - 3.4, 17-R, 14.4 - 5.3 17-D) when compared to sites downstream of the Cuajone mine (2.2 - 1.4 17-R, 2.7 - 1.9 17-D), with the highest Na/Cl ratios recorded at site-19 (the bofedal, 10.6 during 17-R) and site-18 (14.4, during 17-D).
/Cl
− equivalent molar ratios are also significantly higher in the headwater sites, with site-18 recording the highest values (13.45 – 17-R, 18.02 – 17-D). Na/Cl equivalent molar ratio can be used as an indicator of ground water contributions to water bodies [
42]. High Na/Cl ratio is an indication of a significant contribution from mineral-rich ground water sources rather than precipitation, which usually has Na/Cl ratios between 0.8 – 1.0 [
42,
43]. The high Na/Cl and
/Cl
− ratios, when combined with the higher water temperature, are consistent with a geothermal contribution to source waters.
Following the El Niño, the bofedal, site-19, had the highest Fe and Al concentrations of the headwater sites (1.5 mg/l and 0.09 mg/l in 17-R respectively,
Figure 4). This Fe concentration exceeds the safe limit of 1 mg/l for aquatic life [
44]. Both the high pH and the organic processes driving reducing conditions in the waters of the bofedal encourage dissolution of iron, and aluminium solubility also increases with higher pH and in the presence of sulphate in the water.
3.1.2. Foothill Sites Below the Cuajone mine (Sites 5B, 5, 4, 3, 2, 1B, 1C, 1D, 1E)
pH measurements and field observations at sites below the Cuajone mine did not show evidence of AMD. pH values at sites 5, 5B, below the Cuajone mine, ranged from 7.98 – 8.56, little different from site 16 above the mine where pH ranged from 7.84 – 7.97. A healthy riparian eco-system dominates the valley at this altitude. This highly-sensitive environmental indicator suggests that the water quality in this area is good. Foothill sites-2, 5, 5B recorded elevated Al, Fe and Mn concentrations compared to other sites, and levels were particularly high at these sites and site-4 during 17-R, after the El Niño episode. Cretaceous (91-65 Ma) volcanic and sedimentary rocks, with Eocene intrusions (55-45 Ma), underlie the area (
Figure 1) and represent the oldest geological units in the sample area. Chemical weathering leads to formation of clay minerals and insoluble oxides which are relatively high in Al and Fe. Changes in run off rate will have elevated the amount of very fine particulate matter in the rivers and the observed levels of iron and aluminium probably reflect this. The Asana river (sites-1B and 1E) also shows relatively higher concentrations of these elements than equivalent sites on the Torata and Sajena rivers.
Figure 4 shows the spatial distribution of Al, Fe, Mn along the Torata and Moquegua rivers for the three seasons. Interestingly, although Al and Mn levels were not detectable and Fe only found in moderate concentrations (0.18 – 0.24 mg/l) above the Cuajone mine (sites 16-18), all three metals were found in higher concentrations below the mine (site-5, Al, Fe and Mn, 2.0, 1.5 and 0.33mg/l, respectively). USEPA guidelines stipulate that recoverable Al exceeding 0.087 mg/l (pH 6.5 – 9.0) and Fe exceeding 1 mg/l is unsuitable for aquatic life and the secondary drinking water standards specify that Al, Fe and Mn exceeding, 0.2, 0.3 and 0.05 mg/l, respectively, is undesirable in drinking water. Mn concentrations exceed the safe limits for irrigation use (0.2 mg/l). Although these metals are associated with both AMD and neutral mine drainage (NMD) [
14,
15,
45,
46,
47], the concentrations detected were lower than those normally found under AMD or NMD conditions [
48,
49,
50]. Under alternating flooding and draining conditions such as produced during an El Niño event and following dry seasons, changes in the pH of the water will greatly affect Al, Mn and Fe solubility via influencing either reductive dissolution or carbonate formation [
51]. Therefore, it seems that seasonal variability controls the concentrations recorded in the data, and suggests that these metal concentrations probably derive from surface run-off, soil erosion and throughflow during the recent El Niño event [
52,
53,
54]. Observed concentrations were thus considered to be the result of differential weathering of the underlying bedrock and trace metal mobilisation during the episodic rainfall during the recent El Niño event rather than mine leachate.
The metal concentrations decrease downstream from site-5, increase again at site-2 irrespective of the season (
Figure 4). Such an increase in metal concentration could reflect evaporation as the river passes through the desert landscape, however the concentrations of conservative ions such as Cl
− did not show a similar spike, pointing to other origins. Cation enrichment from atmospheric aerosols is a possibility. Regional high pressure and sinking air in the foothills concentrates atmospheric aerosols which then settle out in the lower sections of the Moquegua, Sajena and Torata rivers, possibly affecting water chemistry. However, data from other studies [
55] shows the river chemistry here is more significantly determined by contaminated groundwater. The aquifer is recharged by filtration of surface waters through the porous alluvial deposits of the Moquegua valley floor. The chemistry of these surface waters south of the city provide numerous pollutants from agriculture and the city itself. Therefore, the elevated cations (and bicarbonate) in the river at this altitude, are most likely due to recycled contaminated surface waters through the porous alluvial deposits.
Nitrate () concentration in water can be used to assess chemical addition to the river from local agriculture. We observed raised concentrations, in the wet seasons 17-R and 18-R (0 - 16.64 mg/l and 0 – 7.9 mg/l), compared to the dry season 17-D (0 – 3.4 mg/l). The highest concentrations were recorded at site-3, located in the agricultural area downstream of Torata. The concentration drops at site-2 after the river has passed through 8km of desert canyon. Interestingly, the concentration at site-1C in the farmland around Moquegua, is similar to that at site 2 in both 17-D and 17R, possibly deriving from the greater emphasis on arable versus pastoral farming in this region. concentrations are controlled by increased surface run-off and leaching during the wet season, and relative concentration is linked to land use, being elevated in areas used for arable farming.
3.1.3. Sites Downstream of Moquegua City (Sites 1 and 0A)
The lower stretches of the Moquegua/Osmore river below Moquegua city record slightly alkaline (site-1, pH 7.81 – 8.68) conditions during all seasons. Water composition at site-1 (20km outside Moquegua and below an extensive area of more intensively farmed land) was dominated by Ca
2+ (180 – 250 mg/l), Na
+ (160 - 230 mg/l),
(370 - 470 mg/l) and Cl
− (260 - 360 mg/l). The concentrations of Na
+,
and Cl
− were above or close to unsafe levels for drinking water (EPA and WHO standards, 200 mg/l, 250 mg/l respectively) for all seasons. The tributaries (sites 1B, 1C, 1D, 1E) feeding into the river above the confluence have lower concentrations and slightly more alkaline pH values (e.g. site 1B – 9.09, site 1E – 8.10). At sites 1C (Torata river) and 1D (Sajena river), above the confluence with the Sajena and Torata rivers, metal concentrations were below EPA and WHO safe limits set for drinking water, although relative concentrations are seasonally affected, and Moquegua sites 1B and 1E (Asana river) recorded lower cation and anion concentrations, in all seasons. Mn concentrations at site-1, during both wet and dry seasons (0.65 mg/l and 0.11 mg/l, respectively) are also higher than those recorded from the tributaries (see
Figure 4): site-1B (0.064 mg/l), site-1D (< 0.05 mg/l) and site-1C (< 0.05 mg/l) with the exception of data from 18-R, when Mn concentration was 0.12 mg/l at site-1D, showing a significantly elevated contribution of this metal from the Sajena river. These results suggest that water from the Torata, Sajena and Asana/Moquegua tributaries does not significantly contribute to the elevated cation and anion concentrations at sites 1 and 0A, and the data is consistent with an extraneous source for the elevated chemical levels measured at site 1.
Cl
− concentration was above that considered safe for aquatic life over long periods of time during all seasons (chronic, >230 mg/l) and for irrigation of sensitive crops such as avocados [
56], a common crop in the Moquegua region. The highest K
+ concentration (8.7 – 11.0 mg/l) for all study sites was also found at site-1. As a result of these high concentrations of cations and anions, specific electrical conductivity (EC, 2040 – 2711
S/cm) and total dissolved solids (TDS, 1020 – 1833 ppm) at site-1 were close to or above the maximum limits set by Peruvian water authority for agricultural use (category 3: 2500
S/cm and 1800 ppm, respectively) [
57]. EC and TDS values exceeded the safe limits for agriculture use in the dry season 17-D.
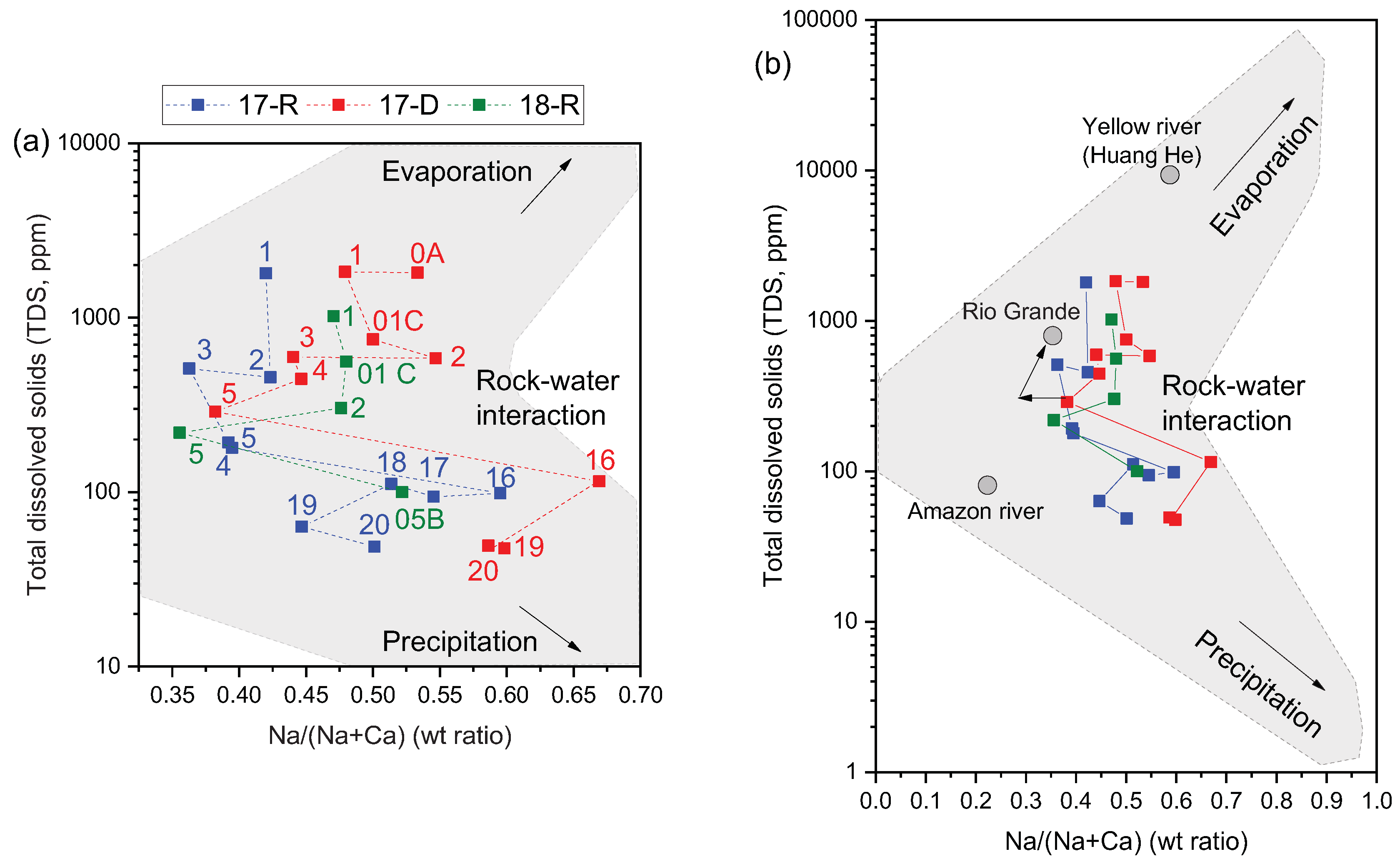
In general, cation and anion concentrations will gradually increase downstream along river systems due to accumulation from natural mineral weathering, evaporation and ground water discharge. However, the concentrations at site-1 were 2-3 times higher than those recorded at sites-1C and 2 in all seasons (
Figure 2: line graphs) which suggests an additional process causing increased concentration. It is unlikely that evaporation alone (see
Figure 5) could account for this dramatic increase so it is likely to be a result of changes in natural mineral weathering or the composition of groundwater discharge. Softer, more friable, sedimentary rocks of the Moquegua Group, which are more susceptible to physical and therefore chemical erosion, underlie the land between sites 1 and 2, in contrast to the more competent volcanic extrusive units found above Moquegua. Site 1 also lies downstream of the agricultural land below Moquegua. It is not possible to separate the relative influence of these two factors with the current data, but given the low precipitation rates (and therefore reduced weathering potential), such a dramatic increase in concentration probably reflects the influence of urban and agricultural activities in the lower valley.
, Cl
− and K
+ were among the main contaminants at site-1 and they are commonly found in agricultural fertilizers, in the form of potassium sulphate (K
2SO
4, 50% K and 17% S), potassium chloride (KCl, 60% K) and magnesium potassium sulphate (MgK(SO
4), 22% S) [
60]. These anions may also derive from urban waste [
61].
Site-1 also showed the highest
concentrations in the study site network (19.65 mg/l 17-R, 17.7 mg/l 18-R, 6.1 mg/l 17-D).
concentrations from the tributaries were much lower throughout the sampling campaign (site-1D, 2.2 - 2.4 mg/l; 1B and 1E 0-0.96 - 0-1.5 mg/l).
is highly soluble and commonly associated with the use of agricultural fertilizers and domestic waste [
9]. To analyse the effect of the latter, samples from site-1 were further analysed for
E.coli and thermal coliform, which are good indicators of sewage contamination. Both
E.coli and thermal coliform were found to be below detection levels (<1.8 MPN) indicating little or no domestic sewage enters the river above site-1. Commonly used nitrogen-based fertilizers in the Moquegua district [
60] are: urea (CO(NH
2)
2, which has the highest nitrogen content (46% N), ammonium nitrate (NH
4NO
3, 33%N) and ammonium sulphate ((NH
4)
2SO
4, 21% N). Another nitrogen-phosphoric fertilizer, commonly used is monoammonium phosphate (NH
4H
2PO
4, 10% N and 48% P), also a source of highly soluble phosphate [
60]. Similar increases in total nitrogen (TN) and total phosphate (TP) were observed at site-1 (
,
), where aquatic plant growth was prolific. Correspondingly, an increase in Mn concentration at this site could also arise from agriculture since Mn is less readily complexed by organic ligands, resulting in high mobility in agriculture arras [
62].
These data and the discussion above are consistent with urban effluent and agricultural run-off and leaching, especially during the wet season, as a major source of raised cationic and anionic concentrations at sites downstream of both the city of Moquegua and the associated downstream industrial agriculture area. However,
Figure 2 shows that there are no significant correlations between the trace metals and cation-anion concentrations, suggesting that the actual system is complex with multivariate determination.
3.1.4. Endmember Controls on Water Chemistry in the Moquegua river Catchment
Water chemistry in rivers is controlled by precipitation, absorption, evaporation, crystallization and weathering. The Gibbs plot of TDS against the weight ratio of Na
+/(Na
+ + Ca
2+) is a useful tool to identify these mechanisms in a river system [
59,
63,
64]. The Gibbs plot for the Torata river sites is shown in
Figure 5 (a). This shows that, irrespective of season, site-16 was the most Na-abundant (0.59 – 0.67 Na/Ca ratio) and site-5 was the most Ca-abundant (0.35 – 0.39 Na/Ca ratio) while the TDS remains between 98 – 115 mg/l at site-16 and 179 – 288 mg/l at site-5. This indicates a major change in water chemistry determinants between site-16 and site-5. The Gibbs plot confirms the dominance of precipitation and silicate weathering at headwater sites on water chemistry, and the change from Na abundance to Ca abundance reflects the change in underlying geology between sites 16 and 5. TDS naturally increases downstream and site-1 records concentration levels a factor of 1000 higher than the rest of the river system in all seasons. Evaporation becomes increasingly important in the lower parts of the river system but the data indicate that both agricultural leachates and evaporation play significant roles in the chemistry of the lower catchment.
3.2. Trace Metals in the Moquegua River System
Total concentration of trace metals (Cd, Cu, Co, Cr, Pb, Li, Mo, Ni, Se, U, V, Zn) along the Torata and Moquegua rivers for each season are shown in
Figure 2 (bar charts). Samples were analysed for trace metals and other compounds associated with AMD and the natural geochemistry of the region (
Table 2). Trace metals can have significant health impacts and are therefore of particular interest. Water analysis showed that Hg, Bi, Th and Te concentrations in all water samples were below detection limits (<0.06, < 2, <4 and <1
g/l, respectively). The study also confirmed that cyanide (CN) remains below detection limits for all study sites (< 20
g/l). In all water samples, Be, Cd, Co, Cr, Cu, Li, Pb, Mo, Ni, Se, U, V and Zn concentrations were well below safe limits for drinking and irrigation purposes, established by USEPA, WHO, FAO and the Peruvian water authority [
44,
57,
65,
66]. Generally, the concentrations of trace metals increase downstream. In our study catchment Be, Co, Pb, Se, U and Zn were only detectable below the Cuajone mine.
For both wet and dry seasons, a notable increase in total trace metal (Cd, Cu, Co, Cr, Pb, Li, Mo, Ni, Se, U, V, Zn) concentration was recorded below the Cuajone mine, with a 5-fold increase in the wet season and a 3.5-fold increase in the dry season measured between site-16 and site-5. The highest total metal concentration at site-5 was recorded after the El Niño episode (96 g/l, 17-R). At site-5, significant levels of Li were recorded in 17-D and 18-R (33, 20 g/l) while Cu was dominant in 17-R (53 g/l: maximum recorded level in this study at any site, but still within safe limits). Pb was detected only at site-5 and site-4 in 17-R (2.9 – 3.9 g/l). These trace metals occur naturally in river waters arising from typical Andean geological units but their concentration is generally increased by AMD. However, apart from a slight peak at sites 4 and 5 after the El Niño episode in 17-R, trace metal concentrations are not dramatically elevated above site 2 but follow a natural concentration profile concurrent with the cations and anions.
Spatially, total trace metal concentration peaked below Moquegua at site-1 during 17-R and at site-2 during 17-D. Trace metals are used in a wide variety of agricultural chemicals and domestic goods and, as such these peaks may derive from increased run-off and domestic waste in the lower valley, or increased weathering processes and the dominance of evaporation in the hyper-arid climes of the tropics. It is also true that trace metals can be easily transported by Fe, Mn and humic substances [
53,
67] which were found abundantly throughout the sample-site network. The trace metals data back up the conclusions from the major cations and anions above – that the dominant endmember in the lower catchment is the agricultural and urban runoff.
It should be noted that the hardness of water, determined by Ca
2+ and Mg
2+ ion concentrations, has a major influence on the toxicity of trace metals [
68,
69]. Water samples from the Torata headwaters (sites 16-20) showed limited hardness (17 – 44 mg/l CaCO3) while at lower elevations (sites-5 to 1C) water was permanently hard (75 – 378 mg/l CaCO3). Irrespective of the season, below Moquegua, water became extremely hard (548 – 764 mg/l CaCO
3). Soft water (<17 mg/l CaCO
3) is generally associated with increased trace metal ecotoxicity [
68,
69,
70]. High levels of water hardness in the region almost certainly reduce the bioavailability, and therefore ecotoxicity, of trace metals in the Moquegua region. The elevated hardness could be an artefact of hyper aridity.
3.3. Arsenic in the Torata and Lower Moquegua River System
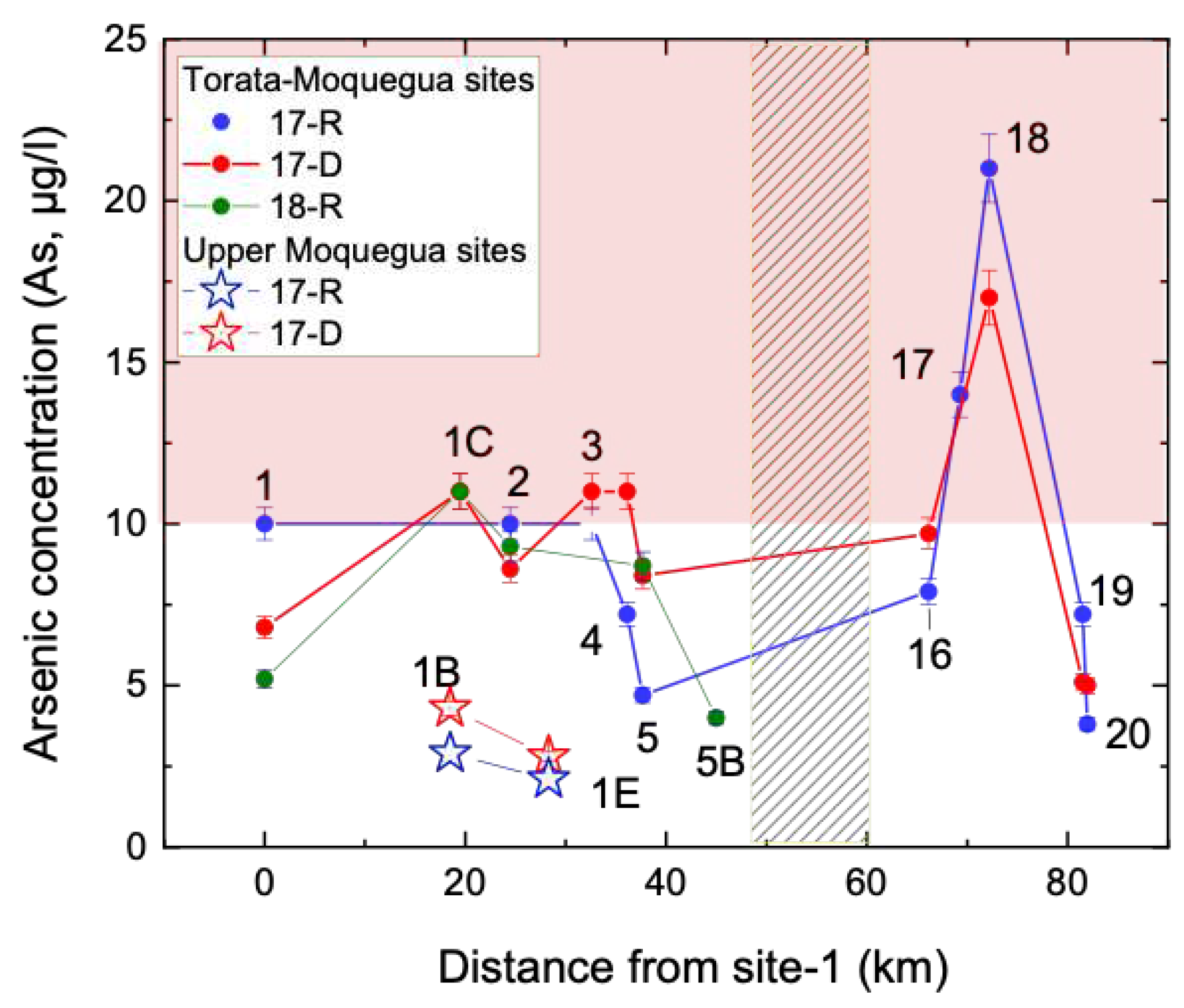
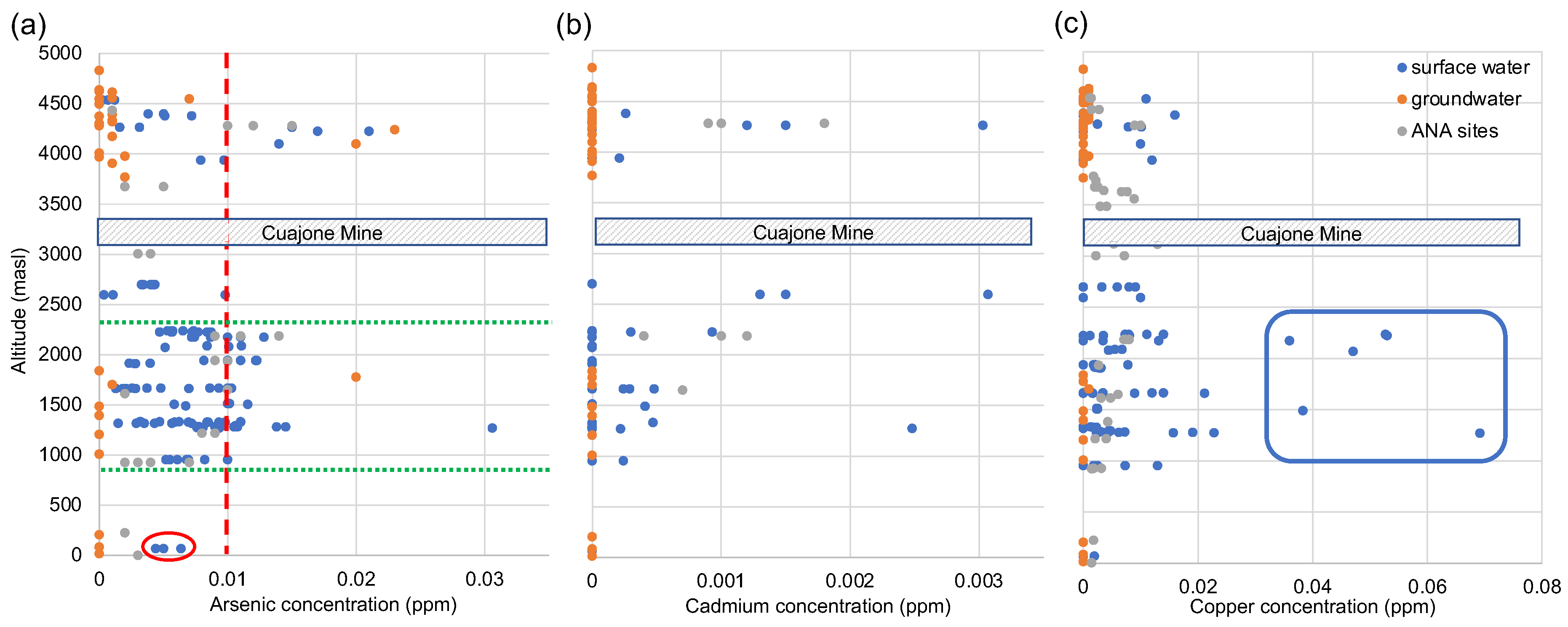
Arsenic (As) concentrations are shown in
Figure 6. As levels along the Torata river were frequently above recommended levels for human consumption and irrigated agriculture (10
g As/l, WHO and USEPA standards). The highest As concentrations were recorded at upper Torata valley sites 17 and 18 (14 - 21
g As/l) where aresenic containing sulfidic minerals deposited by epithermal and hydrothermal processes associated with the active arc are more prevalent. Site-1D along the Sajena river also recorded elevated As in 17-D (11
g As/l). By contrast, the Asana river sites 1B and 1E showed consistently low concentrations of As (2.1 – 4.3
g As/l) for both 17-D and 17-R seasons. The highest As concentration observed in the Moquegua river, below the confluence of the Sajena, Asana and Torata rivers, was at site-1 during 17-R (10
g As/l) which followed extensive flooding owing to the 2017 El Niño episode.
Generally, Peruvian rivers are known to contain As arising from geological sources [
71]. The high Andean sites in the study area (16-20), are underlain by relatively young volcanoclastic deposits that are undergoing low temperature hydrothermal alteration and localised sulfidisation. Previous studies have reported that As is predominantly mobilised from such rocks and their weathering products [
71]. Therefore, the elevated As levels found in the upper Torata sites are most likely to be geogenic in origin.
The dominant minerals found at the Cuajone Copper Mine are sulfides, chiefly chalcopyrite (CuFeS
2), chalcosine (Cu
2S), molybdenite (MoS
2) and pyrite (FeS
2), as well as arsenic-bearing minerals, including enargite (Cu
3AsS
4) and tennantite (Cu
12As
4S
13) [
72]. These arsenic-bearing minerals, frequently associated with copper-porphyry deposits and generally marginal to the main orebody, develop during sulfide mineralization [
72]. Mineralisation is very localised, concentrated within a 3km alternation halo, and does not extend significantly beyond this. It has been shown that weathering of porphyry Cu deposits and associated As-bearing minerals results in the dispersion of As into the groundwater and surface water bodies, depending on the redox state and pH of the system [
73]. Site 5B sits just below the mine and does not show elevated levels of arsenic. However, sites further downstream show variably elevated arsenic levels depending on the season, consistent with remobilisation via groundwater and periods of increased run off and recharge through the shallow aquifer.
It is therefore likely, that the pattern of arsenic concentrations observed,
Figure 6, reflects both local geochemistry and groundwater dispersion.
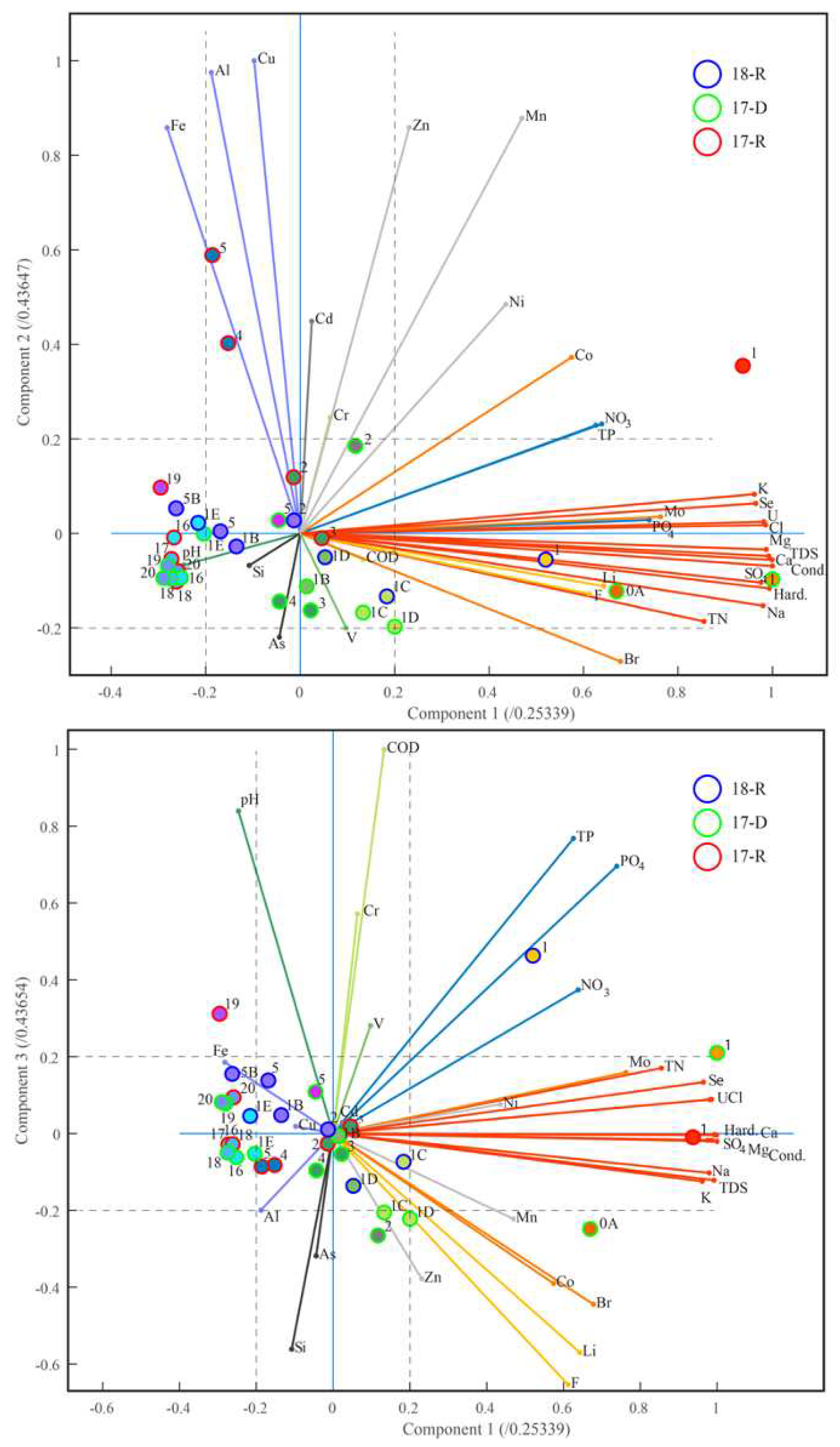
3.4. Multivariate Analysis of Water Parameters
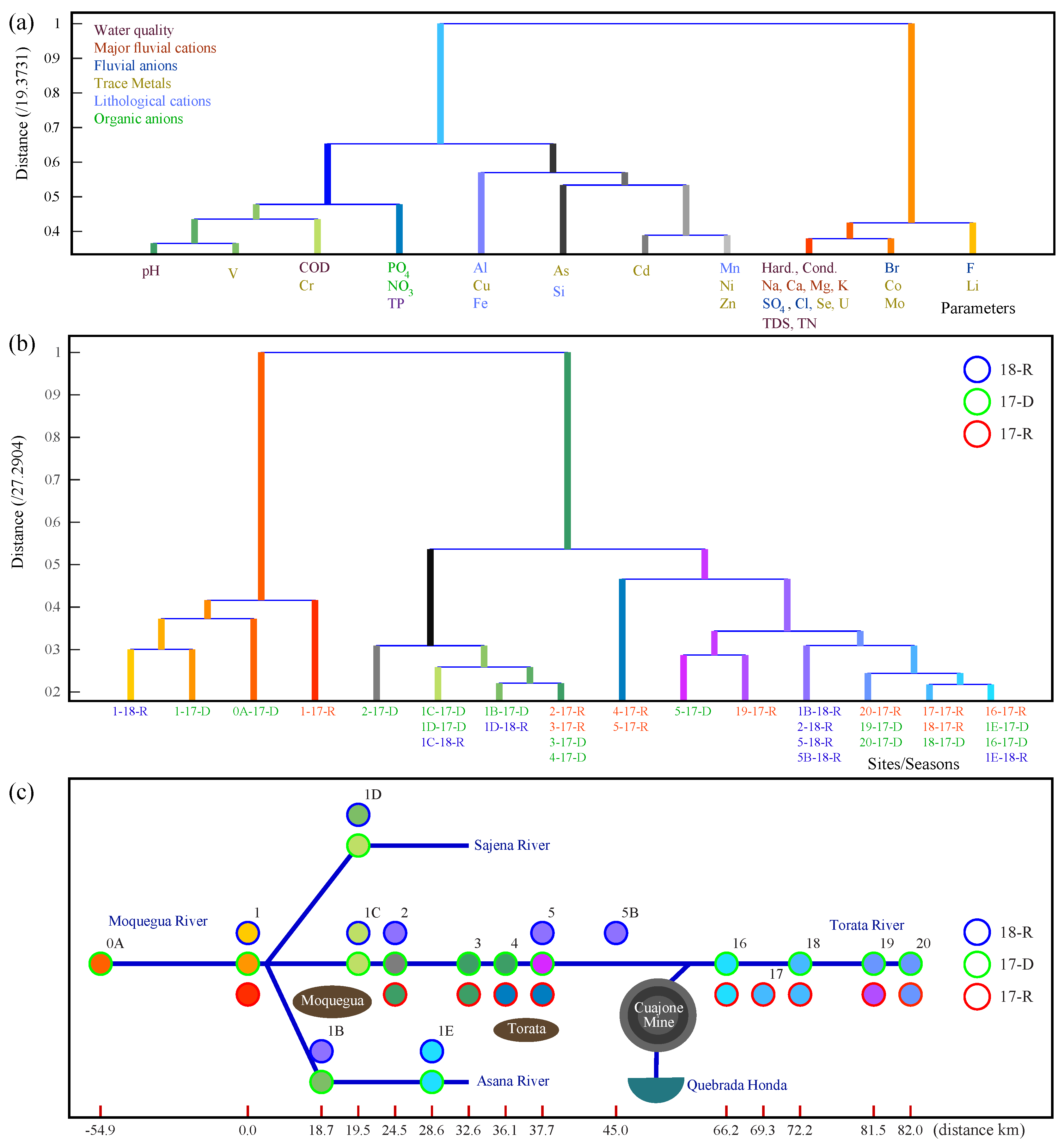
Multivariate analysis was used to give an unbiased assessment of statistical correlations in our data. Correlations between water quality parameters and correlations between sites have been analysed using both CA and PCA. Our analysis took into account the 33 water quality parameters listed in
Table 2. Parameters NO
2, CN, Be, Bi, Te, Th, Hg, Pb were not included because their concentrations were below measurement accuracy. Parameters CO
3, and DO were omitted because the datasets were incomplete.
Figure 7a presents a dendrogram of an R-mode CA for all sites visited in 17-R, 17-D and 18-R. It shows how the parameters cluster according to sites. It was produced with a CCC of 82%. Parameters that have a similar distribution across the sites cluster. At the highest level, we see three clusters. The right cluster contains major ions, and trace metals. These parameters arise from the natural water chemistry and dynamics in combination with the local geology. The left cluster contains nitrates, phosphates, trace metals, pH and COD. These parameters are indicative of agricultural or biological impact. The central cluster contains Al, As, Fe, Si, Mn and trace metals associated with of weathering of geological units with economic potential and / or urban effluent [
74,
75]. Peaks in these elements are also associated with periods of increased turbulence and greater suspended load (see above).
Figure 7b,c present a dendrogram from a Q-mode CA and corresponding Beck map (similar to a subway map with sites as stops) for the same 33 water quality parameters. They show how the sites cluster according to parameters. It was produced with a CCC of 84%. Sites with similar patterns of parameters cluster. At the highest level we see three clusters: the left cluster contains the two lowland sites downstream of Moquegua city, 0A and 1; the central cluster contains foothill sites; and the right cluster mostly contains the highland sites reinforcing the conclusion that chemistry is controlled by geographical location and regional inputs.
PCA analysis carried out on the same data shows that 74% of the variance can be accounted for by the first four components. The first component PC1 accounts for 46% of the variance, PC2 accounts for 12%, PC3 accounts for 9% and PC4 for 7%. Results are shown in
Figure 8a - biplot of PC1 against PC2, and
Figure 8b - PC1 against PC3. The parameter vectors in the biplots are coloured to indicate which parameter cluster they belong to in
Figure 7a. As can be seen in
Figure 8, the right cluster (red, orange; those controlled by natural hydrological processes and underlying geology) aligns well with PC1, the central cluster (light blue, grey; those controlled by the suspended load and increased runoff) aligns with PC2 and the left cluster (green, blue; those controlled principally by anthropogenic activity) aligns with PC3. As and Si align with PC4. Coloured circles are used in the biplot to indicate the site clusters from
Figure 7b,c. The colour of the outer rings indicates the season.
3.4.1. Headwater Sites
In general, the headwater sites 16-20 cluster with site 5 (and site 4 in 17-R) below the Cuajone mine. This precludes chemical addition to the river through mining activity. PC1 and PC2 are low for the headwater sites (in contrast to sites 4 and 5 see below) suggesting the main controls on river chemistry up to this point are hydrological. Contribution from PC3 (chemicals associated with organics) is also low except for site-19, the bofedal, and this is interpreted as being naturally high levels of biogeochemical components in an Andean peatland. Data from the Asana and Sajena rivers was collected during 17-D and site 1E, on the Asana river, also clusters with headwater sites suggesting this river has similar chemistry and dynamics to the Torata river to this point. In 18-R headwater sites were inaccessible.
3.4.2. Foothill Sites
As mentioned above, sites 4 and 5 (5B) (between the mine and Torata) show strong alliance with the headwater sites but share some affinity with sites 2 and 3, below Torata. All four sites showed an average contribution from PC1 as expected from their location in the middle of the river system. During 17-R, Sites (4, 5) showed some contribution from PC2 whereas the headwater sites and sites (2, 3) showed average values. Elevated contribution from PC2 is consistent with increased runoff creating turbulent flow, following El Niño. All foothill sites showed an average value for PC3. Samples collected from the confluence of the Torata with the Osana and Sajena rivers (sites 1B, 1C, 1D) during 17-D, cluster with the other foothill sites (2, 3, 4). These sites showed average values for PC1, PC2 and PC3, consistent with the season being dry and the hydrographic location of these sites. The specific chemistry of the tributary sites in this area is seasonally regulated, sometimes sites share characteristics with the foothill sites and other times share characteristics with headwater sites. This is consistent with variable runoff rates in different seasons changing the exact chemical elements added to the river system at various sites. In general though, hydrographic processes determine the chemistry of the foothill sites (see
Figure 5), and the multivariate analysis for these sites shows that the Cuajone mine had little or no effect on the river system over the observation period. The fact that this period included an El Niño episode shows that the measures to isolate the mine from the Torata river are effective.
3.4.3. Lowland Sites
The lowland sites (sites 1, 0) formed an isolated cluster irrespective of season. PC1 was high in all seasons consistent with these sites being towards the lower reaches of a river running through a hyper-arid zone with high levels of evaporation. In addition, elemental concentrations are elevated by accumulation of dissolved ions through chemical weathering of all three geological zones, from several agricultural regions and the two main urban centres; Torata and Moquegua. In 17-R PC2 was high indicating elevated wash through caused by the El Niño episode. In 17-D and 18-R PC3 was high, consistent with the agricultural activities along the river at that time.
3.5. Analysis of ANA and INGEMMET Data
In
Figure 9 and
Figure 10 we present a collation of data for the Torata river system including our surface water data, available survey data collected by ANA, the National Water Authority in Peru, from 2013 and 2014 [
76,
77,
78] and groundwater analysed by INGEMMET around the same time [
55].
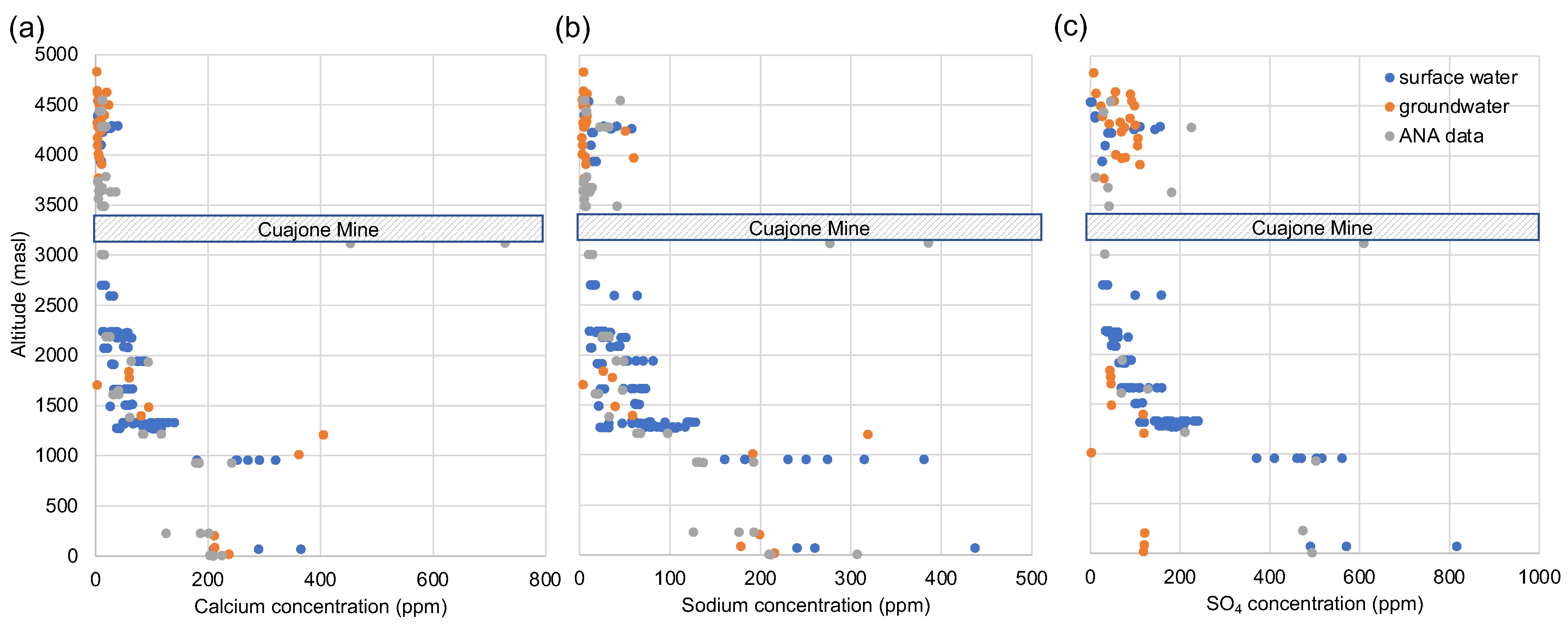
Figure 9 shows the downstream concentration of a selection of major ions (calcium, sodium, sulfate) plotted against altitude. The data show an increase in concentration from source to sink, consistent with conservative behaviour of the ions controlled predominantly by cumulative addition of elements along the water course through weathering, and further concentration through evaporation. Points plotting to the right of the main cluster at just below 1000m asl are surface waters affected by contaminated groundwater (c.f. conclusions drawn by INGEMET [
55]). The Cuajone mine is marked by a grey box with diagonal lines. If the mine had a significant impact on the surface waters we would expect to see a similar deviation of the surface waters to the right in the vicinity of the mine. In fact the two grey data points at 1100m asl, are samples of runoff analysed by ANA from the base of the tailing fan in 2013 and 2014. These data clearly show elevated concentrations of major ions. However, these elevated concentrations are not spatially distributed and data gathered only a few hundred meters from the tailing fan show normal concentrations. The ions are quickly scavenged from the surface water through sorption and precipitation and do not travel far from source.
Figure 10 shows the concentration of arsenic, cadmium and copper plotted against altitude. The concentration of these three trace elements in the water is a major concern for the people who live in the area. Data are not controlled by conservative processes as in the major ions, but are more dispersed. Of note are the digressions to higher concentrations around 4250m asl in all three elements. The higher altitude headwaters are underlain by young volcanic arc units, with very high permeability. There is extensive epithermal and hydrothermal activity associated with the active arc. This allows mobilisation of these cations via natural acidic chemical weathering of the mantle derived volcanic material, and rapid recharge into the surface waters as a result of the highly porous nature of the lithology (c.f. findings by INGEMMET [
55]). The Cuajone mine is once again represented by a grey box with dashed lines. There is no significant increase in concentration of any of the three trace metals at the altitude of the mine. In contrast, the green lines just below 2500 masl and 1000m asl represent the start and end of the irrigated land between Torata and Moquegua. The data in all three plots is very dispersed in this region. Surface waters contaminated by the presence of the urban settlements and agricultural practices, are rapidly mixed into the ground water through the porous alluvial valley fill at this altitude, and recharge leads to increased concentrations in surface waters down stream. The concentrations of cadmium and copper are below guidelines for safe water throughout the river network, both for drinking and use in agriculture. The levels of arsenic are generally below the limits quoted by USEPA guidelines [
65] except a few sites in the headwaters, where aggressive chemical weathering takes place, and also at times of elevated flow following heavy rains and/or flash floods in the agricultural plains.
4. Discussion
Hyper-arid regions rely heavily on river systems as the principal source of water. Changes in both river water availability and chemistry therefore have a direct and immediate impact on dependant systems in such regions. This is particularly true in the Andean transect in this study, where the Moquegua river system passes through the hyper-arid Atacama Desert and is used for human consumption and agricultural and industrial use throughout its length.
The location of the Cuajone mine above Torata required that both channel and waste material diversion mitigation strategies were essential, to avoid impacting water quality in heavily settled areas beneath the mine. To this end a diversionary channel was constructed around the projected mine pit at the Cuajone mine. Additionally, the construction of tunnels and concrete lined channels enables waste materials to be diverted to the tailings dams at Quebrada Honda and Cortaderas 2. The expansion of urban areas and the extension and modernisation of agriculture in the region necessitated the development of a regional irrigation project. The Pasto Grande catchment transfer project diverts water from the Tambo catchment, to the north, to sub-catchment tributaries in the greater Moquegua drainage basin. The catchment transfer project has significantly increased channel flow in the Sajena, Otora, Torata and Moquegua rivers and will undoubtedly add physico-chemical characteristics of the upper Tambo river to the Moquegua-Torata river system. Our data indicate a clear urban and agricultural signal in the lower parts of the Torata and Moquegua river sample sites. While data from Pasto Grande samples sites have not been analysed to identify a clear signal from that source in the lower Moquegua drainage basin.
Water availability in the Moquegua river system arises from seasonal rains and geothermal sources. In the month prior to the 17-R investigation, an El Niño episode had led to catastrophic flooding and a temporary superabundance of turbid river flow. River courses were altered, farmland destroyed and bridges and roads damaged. However, climatic conditions during the study period were generally dry and the rivers had already returned to dry season flow rates during the sampling campaign. The same was observed for 17-D and 18-R. The cycles of flooding and drought in this region, which disrupt water availability and complicate water resource allocation, are irregular and therefore difficult to predict.
Irregular seasonality affecting water chemistry, through the erosion and concentration of trace metal containing silts, further complicates water resource allocation and treatment. Elevated concentrations of Al in drinking water is often considered to increase the risk of Alzheimer’s disease, with prolonged exposure leading to systematic toxicity and renal failure; increased Fe and Mn is associated with aesthetic problems such as staining of laundry, unpleasant smell and taste [
79,
80]; elevated As concentrations in drinking water is associated with skin, liver and kidney cancers [
81,
82]. This study has shown significant seasonal variability in the levels of Al, Fe, Mn and As at any one site at any one time, making it hard to determine the social and economic cost associated with potentially harmful hydrochemistry.
The study indicates multi-factoral determination of river chemistry at many sites, including more significantly seasonality. This has significant implications for the management of water resources in hyper-arid regions. These areas are particularly prone to episodic and localised precipitation patterns, as well as distinct seasonality which may suffer increased climatic volatility in the future [
83,
84]. The data shows increased mobilisation of trace metals into the river system following episodes of more intense rainfall. These have a relatively short residence time and are scavenged from the water by sorption and precipitation, within a matter of months (17-R c.f. 17-D). However, management of the purity of the water in the lower catchment requires consideration of agricultural practises and supervision of the disposal of anthropogenic waste as these contaminated superficial waters are rapidly recycled into the river through the porous aquifer of the alluvial plain [
55].
The desert micro-habitats in the region, although depauperate, have a high level of biological endemicity [
85]. Hyper-arid environments are particularly sensitive to perturbation and recuperation is slow. Thus, the impacts on biodiversity and ecological integrity from irregular water contamination are disproportionately high; making the monitoring of water quality and control of contamination all the more consequential, for what is a scarce and very limited resource. The impact of elevated metal, metalloid and anion concentrations in this river system on ecologies is not yet known. It is of note that the Peruvian National Water authority (ANA) monitors only 98 of the 159 river catchments identified in Peru. Only 60% of these monitored rivers meet nationally agreed environmental quality standards [
57].
This study finds three distinct geochemical and anthropogenic determinants to the water chemistry of the Torata River system; natural hydrological factors, addition of elements through increased run off and turbulence, and biogeochemical parameters associated with elevated organics, both natural and anthropogenic. Sites (16, 17, 18, 19, 20) fall within the arid temperate zone from 2800 – 4600m asl and are all above the Cuajone mine. Water quality here is determined by geological conditions and weathering processes. Cation, anion and trace metal levels were well below the safe limits for human consumption, with the exception of Arsenic concentrations, which exceeded safe limits for human consumption at sites 17 and 18. This can be attributed to geochemical weathering processes, but water chemistry also suggests the river is fed by Na
+ and
rich ground waters, which can also be a source of As contamination [
71]. Sites 3, 4, 5 and 5B, fall within the arid sub-tropics from 1400-2800m asl below the Cuajone Mine, town of Torata, and its associated agricultural areas. These sites showed moderate water quality with significant seasonal variation. While agricultural leachates are still detectable, naturally weathered contaminant loading is more prevalent. Increased surface runoff following El Niño, led to elevated Al, Fe and Mn in the water, where concentrations were recorded above recommended limits for domestic consumption and agricultural use. An increase in total trace metals, Li, Cu and Pb concentrations was also found below the Cuajone mine, but no indication of AMD was seen. Cation-anion composition at these sites showed water chemistry undergoing a transition between the Cuajone mine and Torata village, in which water composition changes from Na-dominant to Ca-dominant. This reflects a change in dominance from precipitative to evaporative processes in determining the hydrochemistry of the river. Sites 0A, 1, 1B, 1C, 1D, 1E and 2, fall within a hot, hyper-arid desert extending from sea level to 1400 m asl. Sites 1B, 1C, 1D, 1E and 2 show more chemical affinity with the sites in the arid subtropical zone, with water chemistry controlled though natural hydrological processes and minor concentration of some elements during periods of high runoff. Sites-1 and 0A, below Moquegua, exhibited the worst water quality irrespective of season. The data presented here is consistent with elevated levels of these chemicals from urban waste and agricultural leachates rather than natural weathering or hydrological processes. Significant levels of cations (Ca
2+, Na
+, K
+) and anions (
, Cl
−) as well as high EC and TDS levels were recorded. Intermittently, trace metals and metalloids (Mn, As) exceeded guideline values. Water quality in this section of the catchment did not meet strict criteria for safe domestic consumption and, on occasion, agricultural use.
The preceding discussion of chemical analyses and cluster analysis (CA) of site data show that the impact of the Cuajone mine is less significant than agricultural and urban activities in the contamination of this river system. Owing to hyper-aridity in the region, the low weathering regime reduces the mobilization of soil minerals and it is difficult to conclude that ARD occurs to any significant extent in this catchment, or that surface water quality has been greatly affected. Direct contamination as a result of AMD from the mine itself is reduced by the bypass of the Torata river around the Cuajone mine through a system of canals and diversions, and an upstream dam that largely prevents flooding from affecting the lower catchment. However, although AMD from open pit mining may be controlled during operation periods, it can cause long-term environmental problems after closure [
14] and it is therefore important to develop a strategy to maintain good control over the mine drainage and tailing disposal to protect this drainage basin long after mine closure.
The data shows that the impact of agriculture and urban activities are far more influential than mining in determining cation-anion concentrations in the hyper-arid river system in this study. These rivers, in combination with the local climate and alluvial soils, provide ideal conditions for the development of highly-productive small and medium scale agriculture, which has been practised for centuries [
66]. The modernization of agriculture has led to the rapid increase in use of pesticides and fertilizers, which in sensitive environments will have consequences for the quality of water resources and their value for domestic consumption and agricultural irrigation. Additionally, the use of river water and river channels to dispose of urban waste and industrial effluents is clearly deleterious to water quality and environmental integrity. Effective regulation of agrochemical use and tight control of urban and industrial effluent and sewage disposal in Peru will be key for the long-term environmental stability and management of a very scarce resource and therefore the long-term sustainability of development.
5. Conclusions
Our research shows that the geochemistry of the Torata river subcatchment is substantially determined by the underlying geological units and, in combination with weathering processes, characteristic of conditions found along the transect from the high Puna at 4500m asl to the lower coastal deserts. There does appear to be an indication of seasonal variation, perhaps linked to the episodic El Niño phenomena. Our data show that the Cuajone mine does not affect water quality parameters in the Torata river nor significantly alter the geochemistry of the river. We do not have data prior to the onset of mining at the site, and so cannot constrain any quantitative changes to the geological contribution to river chemistry as a result of the mine, but the data are consistent with chemical weathering being the dominant control on the fluvial geochemistry, both above and below the mine site during the period of investigation. It seems, at least in the time period concerned, that mine activity has had no adverse effect on water qualities necessary for aquatic life. Data does indicate a clear signal from the rural settlement of Torata and associated agricultural lands beneath the Cuajone mine. A clear geochemical signal is also discernible below the city of Moquegua in the Moquegua river, and also below the confluence of the Sajena and Torata with the Moquegua river. The data are consistent with contamination by urban and agro-chemical usage in the built up and agricultural areas lying below the city of Moquegua. This is the result of rapid recharge of contaminated surface waters from the urban and agricultural areas through the highly porous alluvial fill in the Moquegua valley. Additionally, significant increases in organic matter and sulphates were identified at El Conde, 18km below the city.
This study represents the time-limited findings of research undertaken from 2015 to 2020 in the Torata and greater Moquegua river systems in southern Peru and offers an insight into the determinants of water quality and availability in hyper-arid catchments in the region and, in furtherance of our understanding of geochemical dynamics in hyper-arid river systems, a unique case study. Our findings do indicate the need for further investigation into the significance of both mine operations and water management strategies, including catchment transfer projects, for water resources and environmental integrity. In southern Peru, this would involve investigating the wider environmental and resource implications associated with landscape-scale mine operations, including impact mitigation strategies employed prior to, during and long after mining operations cease, as well as the impact of the mine activity on groundwater flow and storage, headwater ecology and waste material diversion.
Author Contributions
Conceptualization, DHNP, EPGB, LSV, CHWB; methodology, MH, DHNP, EPGB, EEP, PJN, LSV, CHWB; software, DAS, CHWB; validation, MH, DHNP, EPGB, NI, PJN, LSV, PB, CHWB; formal analysis, MH, DHNP, EPGB, LSV, PAB, CHWB; investigation, MH, DHNP, EPGB, EEP, LSV; resources, DHNP, EEP, DAS, CB; data curation, MH, DHNP, EEP, CHWB; writing—original draft preparation, MH, DHNP, EPGB, CHWB; writing—review and editing, MH, DHNP, EPGB, HVL, NI, PJN, PAB, CHWB; visualization, MH, DHNP, EPGB, HVL, NI, CHWB; supervision, DHNP, EPGB, HVL, CHWB; project administration, EPGB, EEP, CHWB; funding acquisition, EPGB, LSV, CHWB. All authors have read and agreed to the published version of the manuscript
Funding
This research is supported through a collaborative agreement between the National University of Moquegua (UNAM) and the University of Cambridge (grant RG85120).
Data Availability Statement
Acknowledgments
The authors acknowledge the valuable support of Dr. A. Quispe and W. Zeballos at UNAM, Dr. Himantha Cooray for his assistance in developing the Mathematica program for data analysis, and John Forrest for his contribution in arranging fieldwork and Julia Porturas for her administrative support.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| MDPI |
Multidisciplinary Digital Publishing Institute |
| DOAJ |
Directory of open access journals |
| ARD |
acid rock drainage |
| AMD |
acid mine drainage |
| EIS |
Environmental impact statements |
| masl |
meters above sea level |
| 17-R |
January 2017 |
| 17-D |
July 2017 |
| 18-R |
January 2018 |
| UNAM |
Universidad Nacional Autonoma de Moquegua |
| GPS |
Global Positioning System |
| DO |
Dissolved oxygen |
| TDS |
Total dissolved solids |
| ICP-MS |
Inductively coupled plasma mass spectrometry |
| ICP-OES |
Inductively coupled plasma - optical emission spectrometry |
| CN |
cyanide |
| TP |
Total phosphorous |
| TN |
Total nitrogen |
| COD |
Chemical oxygen demand |
| ENSO |
El Niño–Southern Oscillation |
| Ma |
Millions of years |
| CA |
Cluster analysis |
| PCA |
Principal componenet analysis |
| PC |
Principal component |
| EPA |
Environmental Protection Agency |
| ISO |
International Organization for Standardization |
| CCC |
cophenetic correlation coefficient |
| EC |
Electrical conductivity |
| USEPA |
United States Environmental Protection Agency |
| WHO |
World Health Organization |
| FAO |
Food and Agriculture Organization of the United Nations |
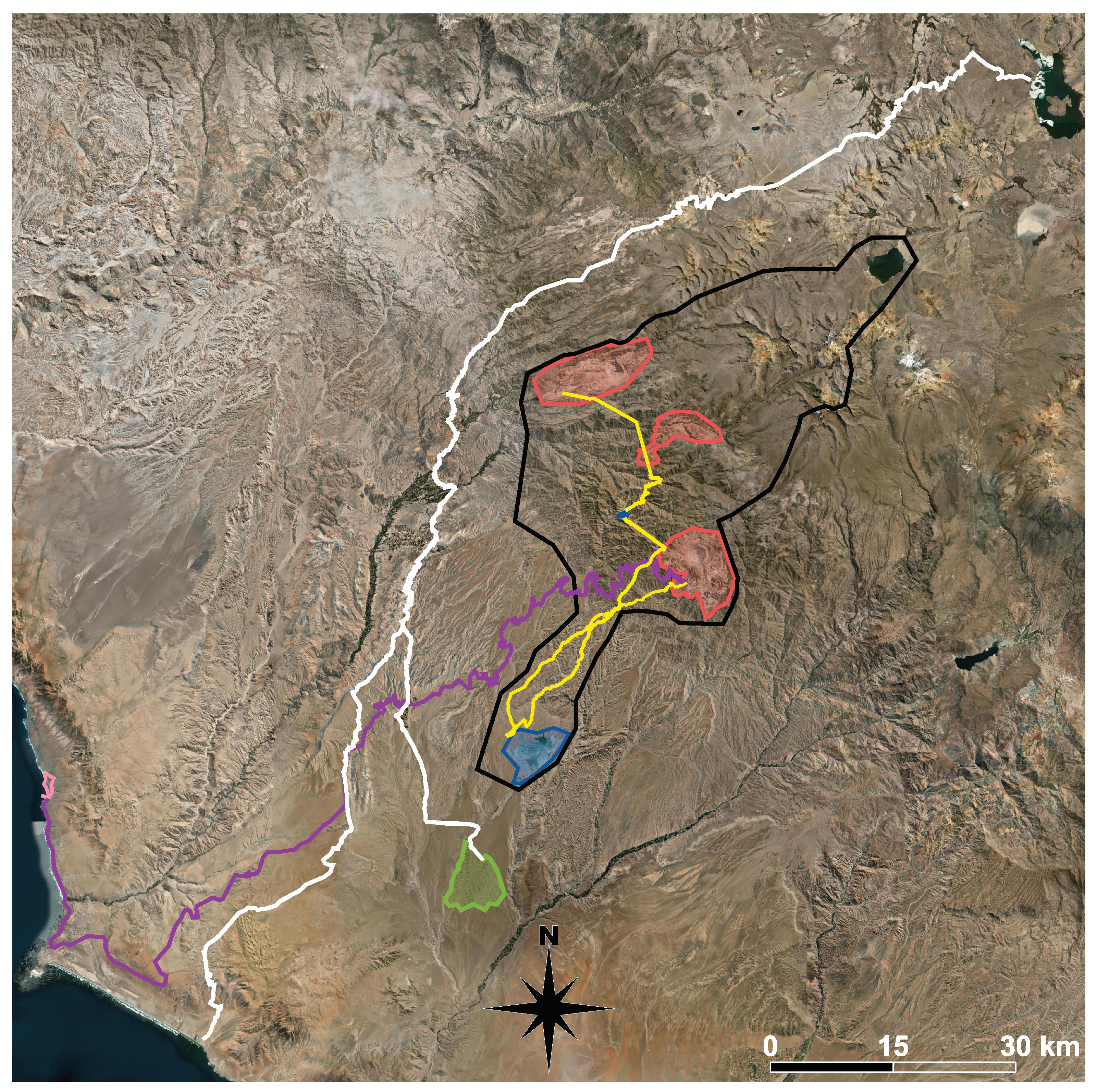
Appendix A. Waste Canal from the Cuajone Mine
Figure A1 shows the study area with mining activities outlined. The canal identified in yellow, collects waste products from the Cuajone mine and carries them out to the Quebrada Honda tailings dam. These channels are made up of a series of tunnels, concrete-lined open channels and existing natural river channels
Figure A1.
Black polygon: area of direct mining and mine related infrastructural impact. Red polygons: upper red polygon = Southern Copper Cuajone mine, middle red polygon = Anglo American Quellaveco mine, lower red polygon = Southern Copper Toquepala mine. Yellow lines: mine waste channels. Blue polygons: Cortaderas 2 and Quebrada Honda tailings dams. Purple line:railway line to Ilo smelter. Pink polygon: Ilo smelter. White lines: Pasto Grande project irrigation canals (tunnels, concrete lined channels and existing river channels). Green polygon: AH Pampa Sitana irrigation project.
Figure A1.
Black polygon: area of direct mining and mine related infrastructural impact. Red polygons: upper red polygon = Southern Copper Cuajone mine, middle red polygon = Anglo American Quellaveco mine, lower red polygon = Southern Copper Toquepala mine. Yellow lines: mine waste channels. Blue polygons: Cortaderas 2 and Quebrada Honda tailings dams. Purple line:railway line to Ilo smelter. Pink polygon: Ilo smelter. White lines: Pasto Grande project irrigation canals (tunnels, concrete lined channels and existing river channels). Green polygon: AH Pampa Sitana irrigation project.
References
- Ling, H.; Zhang, P.; Xu, H.; Zhang, G. Determining the ecological water allocation in a hyper-arid catchment with increasing competition for water resources. Global and Planetary Change 2016, 145, 143–152. [Google Scholar] [CrossRef]
- Zarch, M.A.A.; Sivakumar, B.; Malekinezhad, H.; Sharma, A. Future aridity under conditions of global climate change. Journal of Hydrology 2017, 554, 451–469. [Google Scholar] [CrossRef]
- Clark, A.H.; Farrar, E.; Kontak, D.J.; Langridge, R.J.; Arenas F, M.J.; France, L.J.; McBride, S.L.; Woodman, P.L.; Wasteneys, H.A.; Sandeman, H.A.; others. Geologic and geochronologic constraints on the metallogenic evolution of the Andes of southeastern Peru. Economic Geology 1990, 85, 1520–1583. [Google Scholar] [CrossRef]
- Fraser, B. Water wars come to the Andes. Scientific American 2009, 19, 1–3. [Google Scholar]
- Reich, M.; Palacios, C.; Vargas, G.; Luo, S.; Cameron, E.M.; Leybourne, M.I.; Parada, M.A.; Zúñiga, A.; You, C.F. Supergene enrichment of copper deposits since the onset of modern hyperaridity in the Atacama Desert, Chile. Mineralium Deposita 2009, 44, 497–504. [Google Scholar] [CrossRef]
- Lepage, H.V.; Barnes, E.; Kor, E.; Hunter, M.; Barnes, C.H. Greening and Browning Trends on the Pacific Slope of Peru and Northern Chile. Remote Sensing 2023, 15, 3628. [Google Scholar] [CrossRef]
- Bebbington, A.; Williams, M. Water and mining conflicts in Peru. Mountain research and development 2008, 28, 190–195. [Google Scholar] [CrossRef]
- Ali, M.M.; Ali, M.L.; Islam, M.S.; Rahman, M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environmental Nanotechnology, Monitoring & Management 2016, 5, 27–35. [Google Scholar]
- Varol, M.; Şen, B. Assessment of nutrient and heavy metal contamination in surface water and sediments of the upper Tigris River, Turkey. Catena 2012, 92, 1–10. [Google Scholar] [CrossRef]
- Budds, J.; Hinojosa, L. Restructuring and rescaling water governance in mining contexts: The co-production of waterscapes in Peru. Water alternatives 2012, 5, 119. [Google Scholar]
- Han, Y.S.; Youm, S.J.; Oh, C.; Cho, Y.C.; Ahn, J.S. Geochemical and eco-toxicological characteristics of stream water and its sediments affected by acid mine drainage. Catena 2017, 148, 52–59. [Google Scholar] [CrossRef]
- Tume, P.; González, E.; Reyes, F.; Fuentes, J.P.; Roca, N.; Bech, J.; Medina, G. Sources analysis and health risk assessment of trace elements in urban soils of Hualpen, Chile. Catena 2019, 175, 304–316. [Google Scholar] [CrossRef]
- Olías, M.; Nieto, J.M.; Pérez-López, R.; Cánovas, C.R.; Macías, F.; Sarmiento, A.M.; Galván, L. Controls on acid mine water composition from the Iberian Pyrite Belt (SW Spain). Catena 2016, 137, 12–23. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): causes, treatment and case studies. Journal of cleaner production 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Blowes, D.W.; Ptacek, C.J. Hydrogeochemistry and microbiology of mine drainage: An update. Applied Geochemistry 2015, 57, 3–16. [Google Scholar] [CrossRef]
- Rey, J.; Martínez, J.; Hidalgo, M.; Rojas, D. Heavy metal pollution in the Quaternary Garza basin: A multidisciplinary study of the environmental risks posed by mining (Linares, southern Spain). Catena 2013, 110, 234–242. [Google Scholar] [CrossRef]
- Martin, C.W. Trace metal concentrations along tributary streams of historically mined areas, Lower Lahn and Dill River basins, central Germany. Catena 2019, 174, 174–183. [Google Scholar] [CrossRef]
- Sun, T.; Bao, H.; Reich, M.; Hemming, S.R. More than ten million years of hyper-aridity recorded in the Atacama Gravels. Geochimica et Cosmochimica Acta 2018, 227, 123–132. [Google Scholar] [CrossRef]
- Colica, A.; Benvenuti, M.; Chiarantini, L.; Costagliola, P.; Lattanzi, P.; Rimondi, V.; Rinaldi, M. From point source to diffuse source of contaminants: The example of mercury dispersion in the Paglia River (Central Italy). Catena 2019, 172, 488–500. [Google Scholar]
- Shaban, N.S.; Abdou, K.A.; Hassan, N.E.H.Y. Impact of toxic heavy metals and pesticide residues in herbal products. Beni-suef university journal of basic and applied sciences 2016, 5, 102–106. [Google Scholar] [CrossRef]
- Davies, B.R.; Thoms, M.; Meador, M. An assessment of the ecological impacts of inter-basin water transfers, and their threats to river basin integrity and conservation. Aquatic conservation: Marine and freshwater ecosystems 1992, 2, 325–349. [Google Scholar] [CrossRef]
- Li, F. Documenting accountability: environmental impact assessment in a Peruvian mining project. PoLAR: Political and Legal Anthropology Review 2009, 32, 218–236. [Google Scholar] [CrossRef]
- Bebbington, A.J.; Bury, J.T. Institutional challenges for mining and sustainability in Peru. Proceedings of the National Academy of Sciences 2009, 106, 17296–17301. [Google Scholar] [CrossRef] [PubMed]
- Samimi Namin, F.; Shahriar, K.; Bascetin, A. Environmental impact assessment of mining activities. A new approach for mining methods selection. Gospodarka Surowcami Mineralnymi 2011, 27, 113–143. [Google Scholar]
- Gwimbi, P.; Nhamo, G. Benchmarking the effectiveness of mitigation measures to the quality of environmental impact statements: lessons and insights from mines along the Great Dyke of Zimbabwe. Environment, development and sustainability 2016, 18, 527–546. [Google Scholar] [CrossRef]
- Valladares, L.d.L.S.; Ccamapaza, J.L.; Valencia-Bedregal, R.A.; Borja-Castro, L.E.; Velazquez-Garcia, J.; Perera, D.H.N.; Ionescu, A.; Arvidsson, D.; Barnes, E.P.; Newton, P.; others. Physical and chemical characterization of sediments from an Andean river exposed to mining and agricultural activities: The Moquegua River, Peru. International Journal of Sediment Research 2022, 37, 780–793. [Google Scholar] [CrossRef]
- Magilligan, F.J.; Goldstein, P.S.; Fisher, G.B.; Bostick, B.C.; Manners, R.B. Late Quaternary hydroclimatology of a hyper-arid Andean watershed: Climate change, floods, and hydrologic responses to the El Niño-Southern Oscillation in the Atacama Desert. Geomorphology 2008, 101, 14–32. [Google Scholar] [CrossRef]
- Santoso, A.; Mcphaden, M.J.; Cai, W. The defining characteristics of ENSO extremes and the strong 2015/2016 El Niño. Reviews of Geophysics 2017, 55, 1079–1129. [Google Scholar] [CrossRef]
- Decou, A.; Von Eynatten, H.; Mamani, M.; Sempere, T.; Wörner, G. Cenozoic forearc basin sediments in Southern Peru (15–18 S): Stratigraphic and heavy mineral constraints for Eocene to Miocene evolution of the Central Andes. Sedimentary Geology 2011, 237, 55–72. [Google Scholar] [CrossRef]
- Oerter, E.; Amundson, R.; Heimsath, A.; Jungers, M.; Chong, G.; Renne, P. Early to middle Miocene climate in the Atacama Desert of northern Chile. Palaeogeography, Palaeoclimatology, Palaeoecology 2016, 441, 890–900. [Google Scholar] [CrossRef]
- Clark, A.H.; Tosdal, R.M.; Farrar, E.; Plazolles V, A. Geomorphologic environment and age of supergene enrichment of the Cuajone, Quellaveco, and Toquepala porphyry copper deposits, southeastern Peru. Economic Geology 1990, 85, 1604–1628. [Google Scholar] [CrossRef]
- Masuno, R.K. ; others. Hazard evaluation of the city of Moquegua 2001. [Google Scholar]
- Clesceri, L.S. Standard methods for examination of water and wastewater. American public health association 1998, 9. [Google Scholar]
- OTTO, M. OTTO, M. Multivariate methods. IN: KELLNER, R.; MERMET, JM; OTTO, M.; WIDMER, HM. Analytical chemistry. Weinheim: Wiley-VCH 1998.
- Ward Jr, J.H. Hierarchical grouping to optimize an objective function. Journal of the American statistical association 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Kazi, T.; Arain, M.; Jamali, M.K.; Jalbani, N.; Afridi, H.; Sarfraz, R.; Baig, J.; Shah, A.Q. Assessment of water quality of polluted lake using multivariate statistical techniques: A case study. Ecotoxicology and environmental safety 2009, 72, 301–309. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, pp. 33–40.
- Bhuiyan, M.A.; Islam, M.; Dampare, S.B.; Parvez, L.; Suzuki, S. Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. Journal of hazardous materials 2010, 179, 1065–1077. [Google Scholar] [CrossRef]
- Jolliffe, I.; Lovric, M. International encyclopedia of statistical science. Principa l Component Analysis. Berlin Heidelberg Berlin, Heidelberg: Springer 2011, pp. 1094–6.
- Wurts, W.A.; Durborow, R.M. Interactions of pH, carbon dioxide, alkalinity and hardness in fish ponds 1992.
- Talling, J. The depletion of carbon dioxide from lake water by phytoplankton. The Journal of Ecology 1976, pp. 79–121.
- Möller, D. The Na/Cl ratio in rainwater and the seasalt chloride cycle. Tellus B 1990, 42, 254–262. [Google Scholar] [CrossRef]
- Magaritz, M.; Nadler, A.; Koyumdjisky, H.; Dan, J. The use of Na/Cl ratios to trace solute sources in a semiarid zone. Water Resources Research 1981, 17, 602–608. [Google Scholar] [CrossRef]
- Gorchev, H.G.; Ozolins, G. WHO guidelines for drinking-water quality. WHO chronicle 1984, 38. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Acid mine drainage remediation options: a review. Science of the total environment 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Kalin, M.; Fyson, A.; Wheeler, W.N. The chemistry of conventional and alternative treatment systems for the neutralization of acid mine drainage. Science of the total environment 2006, 366, 395–408. [Google Scholar] [CrossRef]
- Sheoran, A.; Sheoran, V. Heavy metal removal mechanism of acid mine drainage in wetlands: a critical review. Minerals engineering 2006, 19, 105–116. [Google Scholar] [CrossRef]
- Rösner, U. Effects of historical mining activities on surface water and groundwater-an example from northwest Arizona. Environmental Geology 1998, 33, 224–230. [Google Scholar] [CrossRef]
- Hudson-Edwards, K.A.; Schell, C.; Macklin, M.G. Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Applied Geochemistry 1999, 14, 1015–1030. [Google Scholar]
- Miller, J.; Hudson-Edwards, K.; Lechler, P.; Preston, D.; Macklin, M. Heavy metal contamination of water, soil and produce within riverine communities of the Rıo Pilcomayo basin, Bolivia. Science of the total environment 2004, 320, 189–209. [Google Scholar] [CrossRef]
- Pan, Y.; Koopmans, G.F.; Bonten, L.T.; Song, J.; Luo, Y.; Temminghoff, E.J.; Comans, R.N. Influence of pH on the redox chemistry of metal (hydr) oxides and organic matter in paddy soils. Journal of Soils and Sediments 2014, 14, 1713–1726. [Google Scholar] [CrossRef]
- Canfield, D.E. The geochemistry of river particulates from the continental USA: Major elements. Geochimica et Cosmochimica Acta 1997, 61, 3349–3365. [Google Scholar] [CrossRef]
- Tipping, E.; Rey-Castro, C.; Bryan, S.E.; Hamilton-Taylor, J. Al (III) and Fe (III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochimica et cosmochimica acta 2002, 66, 3211–3224. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers: New insights from a new database. Science of the total Environment 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Ng Cutipa, W.L.; Peña Laureano, F.; Acosta Pereira, H. Hidrogeología de la cuenca del río Ilo-Moquegua (13172), región Moquegua [Boletín–H 6] 2019.
- Ayers, R.S.; Westcot, D.W.; others. Water quality for agriculture; Vol. 29, Food and Agriculture Organization of the United Nations Rome, 1985.
- ANA. Modifican los Estándares Nacionales de Calidad Ambiental para Agua y establecen disposiciones complementarias para su aplicación. Decreto Supremo, 015–2015–MINAM, Peru 2015.
- Harrison, R.M.; De Mora, S.J. Introductory chemistry for the environmental sciences; Vol. 7, Cambridge University Press, 1996.
- Xiao, J.; Jin, Z.D.; Zhang, F.; Wang, J. Solute geochemistry and its sources of the groundwaters in the Qinghai Lake catchment, NW China. Journal of Asian Earth Sciences 2012, 52, 21–30. [Google Scholar] [CrossRef]
- PERU, M. Boletín Estadístico de Medios de Producción Agropecuarios.
- Borrok, D.M.; Engle, M.A. The role of climate in increasing salt loads in dryland rivers. Journal of arid environments 2014, 111, 7–13. [Google Scholar] [CrossRef]
- LaZerte, B.D.; Burling, K. Manganese speciation in dilute waters of the Precambrian Shield, Canada. Water Research 1990, 24, 1097–1101. [Google Scholar] [CrossRef]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rammohan, V.; Sahayam, J.D.; Jeevanandam, M. Assessment of groundwater quality and hydrogeochemistry of Manimuktha River basin, Tamil Nadu, India. Environmental Monitoring and Assessment 2009, 159, 341–351. [Google Scholar] [CrossRef]
- EPA, U. National Primary Drinking Water Regulations. Technical Fact 2019. [Google Scholar]
- Vera Delgado, J. The socio-cultural, institutional and gender aspects of the water transfer-agribusiness model for food and water security. Lessons learned from Peru. Food Security 2015, 7, 1187–1197. [Google Scholar] [CrossRef]
- Tessier, A.; Fortin, D.; Belzile, N.; DeVitre, R.; Leppard, G. Metal sorption to diagenetic iron and manganese oxyhydroxides and associated organic matter: narrowing the gap between field and laboratory measurements. Geochimica et Cosmochimica Acta 1996, 60, 387–404. [Google Scholar] [CrossRef]
- Pagenkopf, G.K. Gill surface interaction model for trace-metal toxicity to fishes: role of complexation, pH, and water hardness. Environmental Science & Technology 1983, 17, 342–347. [Google Scholar]
- Pascoe, D.; Evans, S.A.; Woodworth, J. Heavy metal toxicity to fish and the influence of water hardness. Archives of Environmental Contamination and Toxicology 1986, 15, 481–487. [Google Scholar] [CrossRef]
- Zitko, V.; Carson, W. Mechanism of the effects of water hardness on the lethality of heavy metals to fish. Chemosphere;(United Kingdom) 1976, 5. [Google Scholar] [CrossRef]
- Bundschuh, J.; Litter, M.I.; Parvez, F.; Román-Ross, G.; Nicolli, H.B.; Jean, J.S.; Liu, C.W.; López, D.; Armienta, M.A.; Guilherme, L.R.; others. One century of arsenic exposure in Latin America: A review of history and occurrence from 14 countries. Science of the Total Environment 2012, 429, 2–35. [Google Scholar] [CrossRef]
- Schwartz, M.O. Arsenic in porphyry copper deposits: economic geology of a polluting element. International Geology Review 1995, 37, 9–25. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Cameron, E.M. Source, transport, and fate of rhenium, selenium, molybdenum, arsenic, and copper in groundwater associated with porphyry–Cu deposits, Atacama Desert, Chile. Chemical Geology 2008, 247, 208–228. [Google Scholar] [CrossRef]
- Byrne, P.; Reid, I.; Wood, P.J. Sediment geochemistry of streams draining abandoned lead/zinc mines in central Wales: the Afon Twymyn. Journal of soils and Sediments 2010, 10, 683–697. [Google Scholar] [CrossRef]
- Byrne, P.; Taylor, K.G.; Hudson-Edwards, K.A.; Barrett, J.E. Speciation and potential long-term behaviour of chromium in urban sediment particulates. Journal of Soils and Sediments 2017, 17, 2666–2676. [Google Scholar] [CrossRef]
- de agua Moquegua, A.L. Informe del tercer monitoreo participativo de calidad de agua superficial de la cuenca Moquegua-Ilo, 2013.
- de agua Moquegua, A.L. Informe del cuarto monitoreo participativo de calidad de agua superficial de la cuenca Moquegua-Ilo, 2014.
- de agua Moquegua, A.L. Quinto monitoreo participativo de calidad de agua superficial de la cuenca Moquegua-Ilo, 2014.
- Forbes, W.F.; Hill, G.B. Is exposure to aluminum a risk factor for the development of Alzheimer disease?—Yes. Archives of neurology 1998, 55, 740–741. [Google Scholar] [CrossRef]
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain research bulletin 2001, 55, 187–196. [Google Scholar] [CrossRef]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environmental health perspectives 1992, 97, 259–267. [Google Scholar] [CrossRef]
- Davis, M.A.; Signes-Pastor, A.J.; Argos, M.; Slaughter, F.; Pendergrast, C.; Punshon, T.; Gossai, A.; Ahsan, H.; Karagas, M.R. Assessment of human dietary exposure to arsenic through rice. Science of the Total Environment 2017, 586, 1237–1244. [Google Scholar] [CrossRef]
- Insel, N.; Poulsen, C.J.; Ehlers, T.A. Influence of the Andes Mountains on South American moisture transport, convection, and precipitation. Climate Dynamics 2010, 35, 1477–1492. [Google Scholar] [CrossRef]
- Taylor, M.P.; Kesterton, R.G. Heavy metal contamination of an arid river environment: Gruben River, Namibia. Geomorphology 2002, 42, 311–327. [Google Scholar] [CrossRef]
- Arakaki, M.; Cano, A. Floral composition of the Ilo-Moquegua and Lomas de Ilo river basin, Moquegua, Peru. Peruvian journal of biologyía 2003, 10, 5–19. [Google Scholar]
Figure 1.
Map of selected study site locations in the foothill and headwaters. (site-0A, 40 km downstream from site 1 near Ilo, is not shown). Inset shows underlying geological units adapted from Decou
et al. [
29]. Geological lithologies coded by colours: pink- Coastal Batholith (intrusive 145-55 Ma); buff- Moquegua Group (sedimentary 50-4 Ma); green- Cretaceous volcanics and Eocene intrusives; grey- Miocene to recent pyroclastic deposits. Study area shown in dashed box.
Figure 1.
Map of selected study site locations in the foothill and headwaters. (site-0A, 40 km downstream from site 1 near Ilo, is not shown). Inset shows underlying geological units adapted from Decou
et al. [
29]. Geological lithologies coded by colours: pink- Coastal Batholith (intrusive 145-55 Ma); buff- Moquegua Group (sedimentary 50-4 Ma); green- Cretaceous volcanics and Eocene intrusives; grey- Miocene to recent pyroclastic deposits. Study area shown in dashed box.
Figure 2.
Spatial and temporal variation in cations (, , , : line graphs), anions (, , , : line graphs) and total trace metals (, , , , , , , , , U, V, : bar graphs) in water samples from Moquegua river sites. (a) January 2017 (17-R), (b) July 2017 (17-D) and (c) January 2018 (18-R). Error bars represent standard deviation of measurement. Site numbers are given in the labels.
Figure 2.
Spatial and temporal variation in cations (, , , : line graphs), anions (, , , : line graphs) and total trace metals (, , , , , , , , , U, V, : bar graphs) in water samples from Moquegua river sites. (a) January 2017 (17-R), (b) July 2017 (17-D) and (c) January 2018 (18-R). Error bars represent standard deviation of measurement. Site numbers are given in the labels.
Figure 3.
equivalent molar ratio (a) and equivalent molar ratio (b) graphs for Moquegua river system. This shows that upper Torata sites have significantly higher ratios indicating ground water recharge in this region.
Figure 3.
equivalent molar ratio (a) and equivalent molar ratio (b) graphs for Moquegua river system. This shows that upper Torata sites have significantly higher ratios indicating ground water recharge in this region.
Figure 4.
Al, Fe and Mn concentrations and pH, showing elevated wet season metal concentrations in the Torata river below the Cuajone mine. Distance is measured upstream from Site-1. Red shaded regions show the concentration levels above the safe limits for aquatic life. Cuajone mine is located between site-5B and site-16 (vertical shaded region). Error bars represent standard deviation of measurement fluctuation. Labels represent site numbers.
Figure 4.
Al, Fe and Mn concentrations and pH, showing elevated wet season metal concentrations in the Torata river below the Cuajone mine. Distance is measured upstream from Site-1. Red shaded regions show the concentration levels above the safe limits for aquatic life. Cuajone mine is located between site-5B and site-16 (vertical shaded region). Error bars represent standard deviation of measurement fluctuation. Labels represent site numbers.
Figure 5.
: (a) Gibbs plot for the Moquegua river showing changes in water chemistry along the river and (b) compared with other major rivers, Hauang He river, China and Amazon river, rio Grande, USA and Amazon river, South America. Data from [
58,
59].
Figure 5.
: (a) Gibbs plot for the Moquegua river showing changes in water chemistry along the river and (b) compared with other major rivers, Hauang He river, China and Amazon river, rio Grande, USA and Amazon river, South America. Data from [
58,
59].
Figure 6.
Arsenic concentrations in samples from Torata and Moquegua river site. Error bars show the standard deviations of measurement. Red shaded regions show the concentration levels above the safe limits for potable use. Cuajone mine is located between site-5B and site-16 (vertical shaded region).
Figure 6.
Arsenic concentrations in samples from Torata and Moquegua river site. Error bars show the standard deviations of measurement. Red shaded regions show the concentration levels above the safe limits for potable use. Cuajone mine is located between site-5B and site-16 (vertical shaded region).
Figure 7.
(a) Dendrogram showing the Euclidean dissimilarity between the 33 measured water parameters taking 17 Torata-Moquegua sample sites and three seasons 17-R, 17-D and 18-R into account.(b) Dendrogram showing the largest dissimilarity between the 17 Torata-Moquegua sample sites in different seasons taking 33 measured water parameters into account (c) Beck map showing the spatial distribution of site clusters. The colour map is the same as in (b). The colour of the outer ring of each circle identifies the season using the key on the right.
Figure 7.
(a) Dendrogram showing the Euclidean dissimilarity between the 33 measured water parameters taking 17 Torata-Moquegua sample sites and three seasons 17-R, 17-D and 18-R into account.(b) Dendrogram showing the largest dissimilarity between the 17 Torata-Moquegua sample sites in different seasons taking 33 measured water parameters into account (c) Beck map showing the spatial distribution of site clusters. The colour map is the same as in (b). The colour of the outer ring of each circle identifies the season using the key on the right.
Figure 8.
PCA biplots using 33 water parameters measured at 17 Torata-Moquegua sample sites in the three seasons 17-R, 17-D and 18-R. Colours of parameter vectors are taken from
Figure 7a. Colours for site points are taken from
Figure 7b,c. (a) PC1 vs PC2 (b) PC1 vs PC3. Dashed lines at ±0.2 indicate the qualitative boundary used to indicate where components become significant at a site.
Figure 8.
PCA biplots using 33 water parameters measured at 17 Torata-Moquegua sample sites in the three seasons 17-R, 17-D and 18-R. Colours of parameter vectors are taken from
Figure 7a. Colours for site points are taken from
Figure 7b,c. (a) PC1 vs PC2 (b) PC1 vs PC3. Dashed lines at ±0.2 indicate the qualitative boundary used to indicate where components become significant at a site.
Figure 9.
Combined data sources from field work, ANA data and INGEMMET groundwater reports [
55,
76,
77,
78]. Concentration of major ions in the Torata river system.
Figure 9.
Combined data sources from field work, ANA data and INGEMMET groundwater reports [
55,
76,
77,
78]. Concentration of major ions in the Torata river system.
Figure 10.
Combined data sources from field work, ANA data and INGEMMET groundwater report [
55,
76,
77,
78]. Concentration of trace elements in the Torata river system. The red dashed line in panel (a) indicates the safe limit for arsenic. The safety limits for cadmium and copper concentrations do not appear in panels (b) and (c) as they are above every data point. The green dotted lines in panel (a) delimit the area of intensive agricultural land ranging from above Torata to below Moquegua. The red circle in panel (a) identify data taken at Site O. The blue box in panel (c) outline data that were taken after a flash flood event.
Figure 10.
Combined data sources from field work, ANA data and INGEMMET groundwater report [
55,
76,
77,
78]. Concentration of trace elements in the Torata river system. The red dashed line in panel (a) indicates the safe limit for arsenic. The safety limits for cadmium and copper concentrations do not appear in panels (b) and (c) as they are above every data point. The green dotted lines in panel (a) delimit the area of intensive agricultural land ranging from above Torata to below Moquegua. The red circle in panel (a) identify data taken at Site O. The blue box in panel (c) outline data that were taken after a flash flood event.
Table 1.
Selected study sites including, Approximate GPS coordinates, elevation above sea level and distance from site 1.
Table 1.
Selected study sites including, Approximate GPS coordinates, elevation above sea level and distance from site 1.
| Site |
Site name |
GPS coordinates |
Elevation (m) |
Distance from site-1 (km) |
| Sites on Moquegua river (Osmore) |
| 0A |
Lower Moquegua valley -El Algarrobal |
17 37.705’S |
71 17.579’W |
68 |
54.9 |
| 1 |
Middle Moquegua valley - El Conde |
17 20.052’S |
70 59.889’W |
955 |
0 |
| Sites on Torata river |
| 1C |
Above Sajena-Torata river confluence |
17 10.720’S |
70 57.037’W |
1330 |
19.5 |
| 2 |
Puente Estuquina – beginning of agriculture area |
17 09.015’S |
70 55.01’W |
1495 |
24.5 |
| 3 |
Puente Coplay – end of agriculture area |
17 05.807’S |
70 52.726’W |
1941 |
32.6 |
| 4 |
Puente Canilay – downstream of Torata settlement |
17 05.183’S |
70 51.4303’W |
2175 |
36.1 |
| 5 |
Fire station - beginning of agriculture area and upstream of Torata settlement |
17 04.353’S |
70 50.248’W |
2225 |
37.7 |
| 5B |
Middle Torata valley – the highest downstream of Cuajone mine |
17 03.546’S |
70 47.704’W |
2700 |
45 |
| 16 |
Upper Torata valley – Lowest upstream of Cuajone mine |
16 59.019’S |
70 36.392’W |
3939 |
66.2 |
| 17 |
Upper Torata valley - Middle |
16 58.360’S |
70 35.004’W |
4100 |
69.3 |
| 18 |
Upper Torata valley – Highest |
16 57.454’S |
70 33.672’W |
4225 |
72.2 |
| 19 |
Bofedale.at Torata river |
16 57.120’S |
70 29.698’W |
4380 |
81.5 |
| 20 |
Torata river source |
16 57.324’S |
70 29.677’W |
4400 |
82 |
| Sites on Moquegua river (Asana river) |
| 1B |
Below Moquegua city. |
17 11.205’S |
70 57.193’W |
1318 |
18.7 |
| 1E |
Above Moquegua and Samegua. |
17 09.737’S |
70 52.343’W |
1662 |
28.6 |
| Sites on Sajena/Otora river (Huaracane river) |
| 1D |
Before Sajena-Torata confluence |
17 10.690’S |
70 57.052’W |
1440 |
19.5 |
Table 2.
Water parameters and their minimum and maximum values measured during the study. CN – Cyanide, TP Total Phosphorus, TN – Total Nitrogen, Cond – electrical conductivity, TDS – Total Dissolved Solids, Temp – Temperature, DO – Dissolved Oxygen, COD - chemical oxygen demand, Hard. - water hardness. Other symbols take their usual meanings.
Table 2.
Water parameters and their minimum and maximum values measured during the study. CN – Cyanide, TP Total Phosphorus, TN – Total Nitrogen, Cond – electrical conductivity, TDS – Total Dissolved Solids, Temp – Temperature, DO – Dissolved Oxygen, COD - chemical oxygen demand, Hard. - water hardness. Other symbols take their usual meanings.
| Parameter |
min-max |
Parameter |
min-max |
Parameter |
min-max |
| SO4 (mg/l) |
11-490 |
HCO3− (mg/l) |
13.4-350 |
K (mg/l) |
2.1-11 |
| NO2 (mg/l) |
0-0.486 |
Hard. (mg/l CaCO3) |
17.3-764.3 |
Li (g/l) |
0-170 |
| NO3 (mg/l) |
0-19.65 |
Al (mg/l) |
0-2.0 |
Mg (mg/l) |
1.8-36 |
| PO4 (mg/l) |
0-0.57 |
As (g/l) |
2.1-21.0 |
Mn (mg/l) |
0-0.65 |
| CO3 (mg/l) |
0-0 |
Be (g/l) |
0-0.4 |
Mo (g/l) |
0.56-8.3 |
| CN Total (mg/l) |
0-0 |
Bi (mg/l) |
0-0 |
Na (mg/l) |
4.9-240 |
| TP (mg/l as P) |
0-0.41 |
Br (mg/l) |
0-0.82 |
Ni (g/l) |
0-26 |
| TN (mg/l) |
0-8.1 |
Ca (mg/l) |
4.5-251.8 |
Pb (g/l) |
0-3.9 |
| pH |
7.63-9.45 |
Cd (g/l) |
0-0.93 |
Se (g/l) |
0-4.8 |
| Cond. (mS/cm) |
95.4-2711.4 |
Cl (mg/l) |
0-360.3 |
Si (mg/l) |
2.5-25 |
| TDS (mg/l) |
0-1833 |
Cr (g/l) |
0-3.4 |
Te (mg/l) |
0-0 |
| Temp. (°C) |
3.8-29.2 |
Co (g/l) |
0-4 |
Th (g/l) |
0-0.029 |
| DO (mg/l) |
0-12.4 |
Cu (g/l) |
0-53.0 |
U (g/l) |
0-13 |
| COD |
0-27.85 |
F (mg/l) |
0-0.49 |
V (g/l) |
1.9-11 |
| |
|
Fe (mg/l) |
0-1.5 |
Zn (g/l) |
0-55.0 |
| |
|
Hg (mg/l) |
0-0 |
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).