Submitted:
25 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
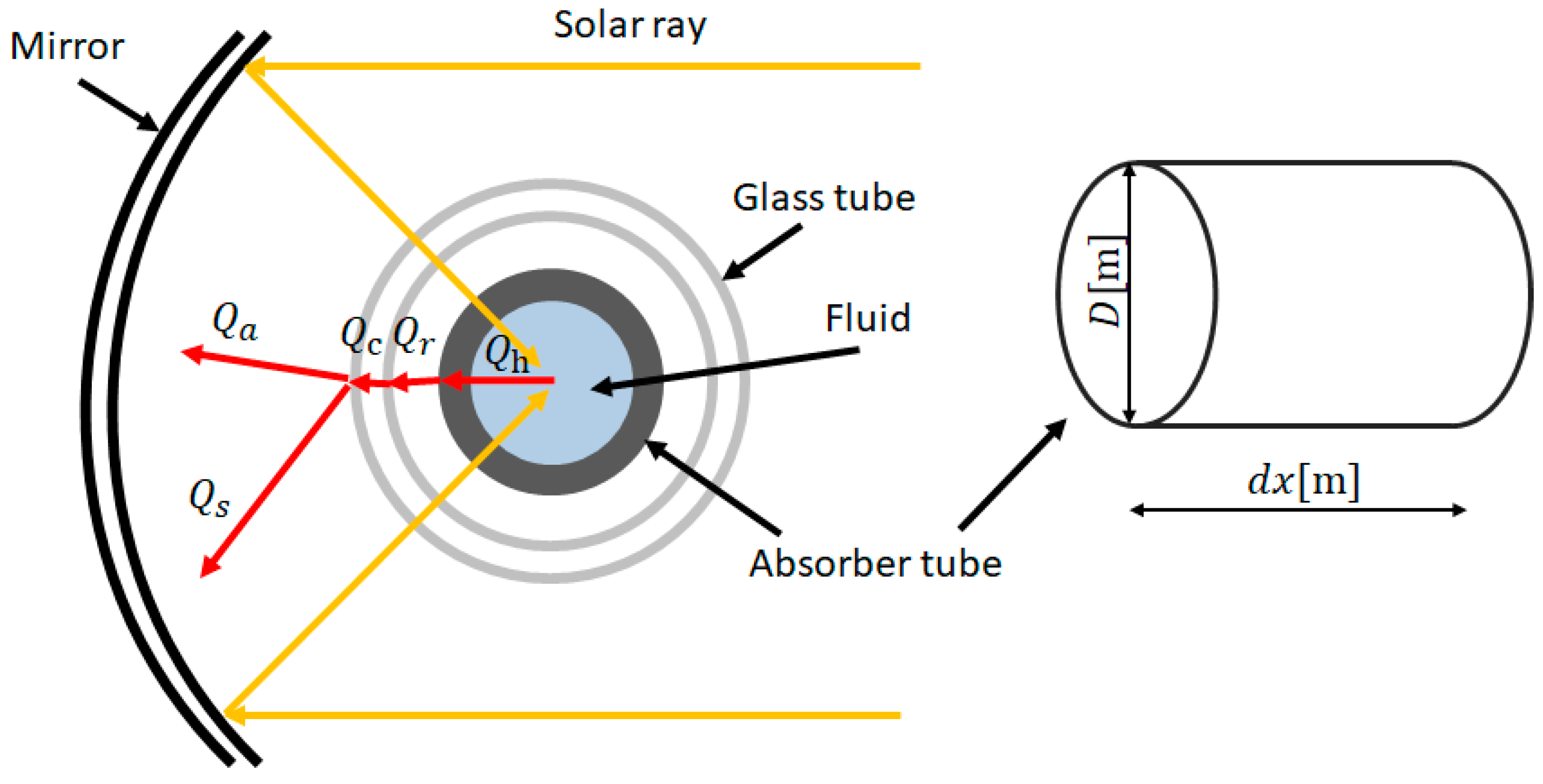
2. Heat Transfer Model for Solar Collector Proposed
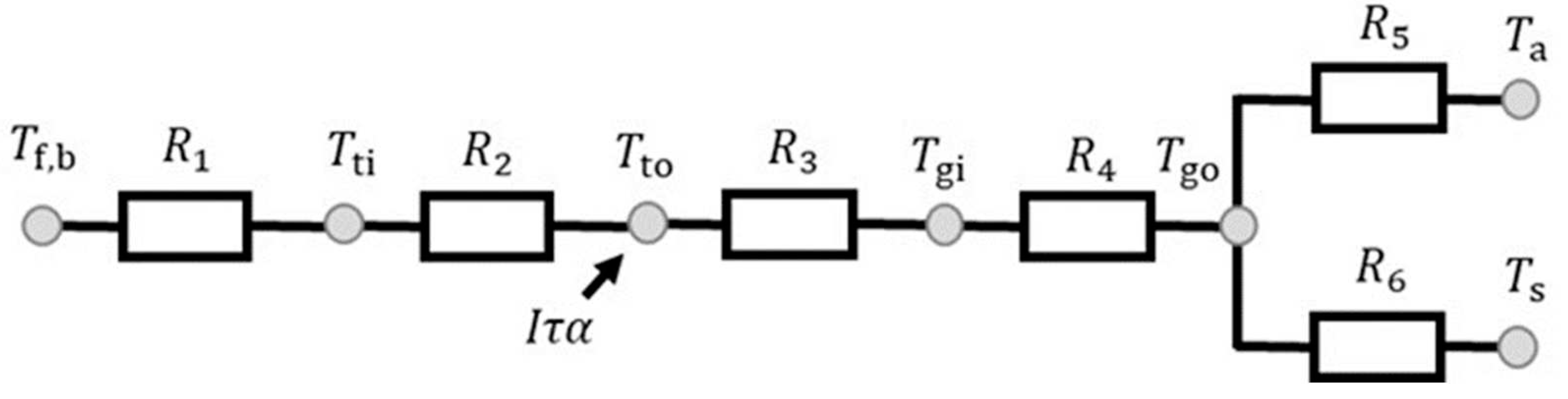
2.1. Govering Equation
2.2. Estimation of Heat Transfer Coefficient
- (i)
- The mass flow rate of the heat transfer fluid (m) is 0.05 kg/s.
- (ii)
- The distance between absorber and glass tube is 1/10 D.
- (iii)
- Tfb, in is 283 K.
- (iv)
- Ts equals to Ta.
- (v)
- The thickness of absorber and glass tube is 0.005 m and 0.010 m, respectively.
- (vi)
- (vii)
- Tti equals to Tto.
- (viii)
- Tgi equals to Tgo, which is 373 K.
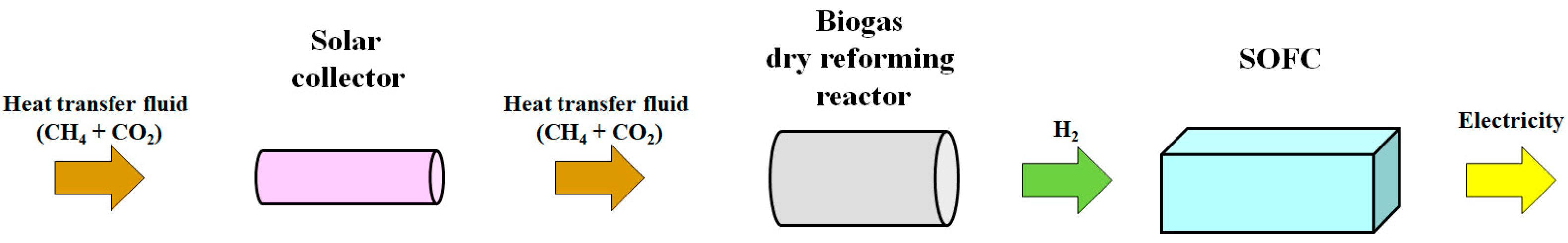
3. Proposed Combined Energy System
4. Results and Discussion
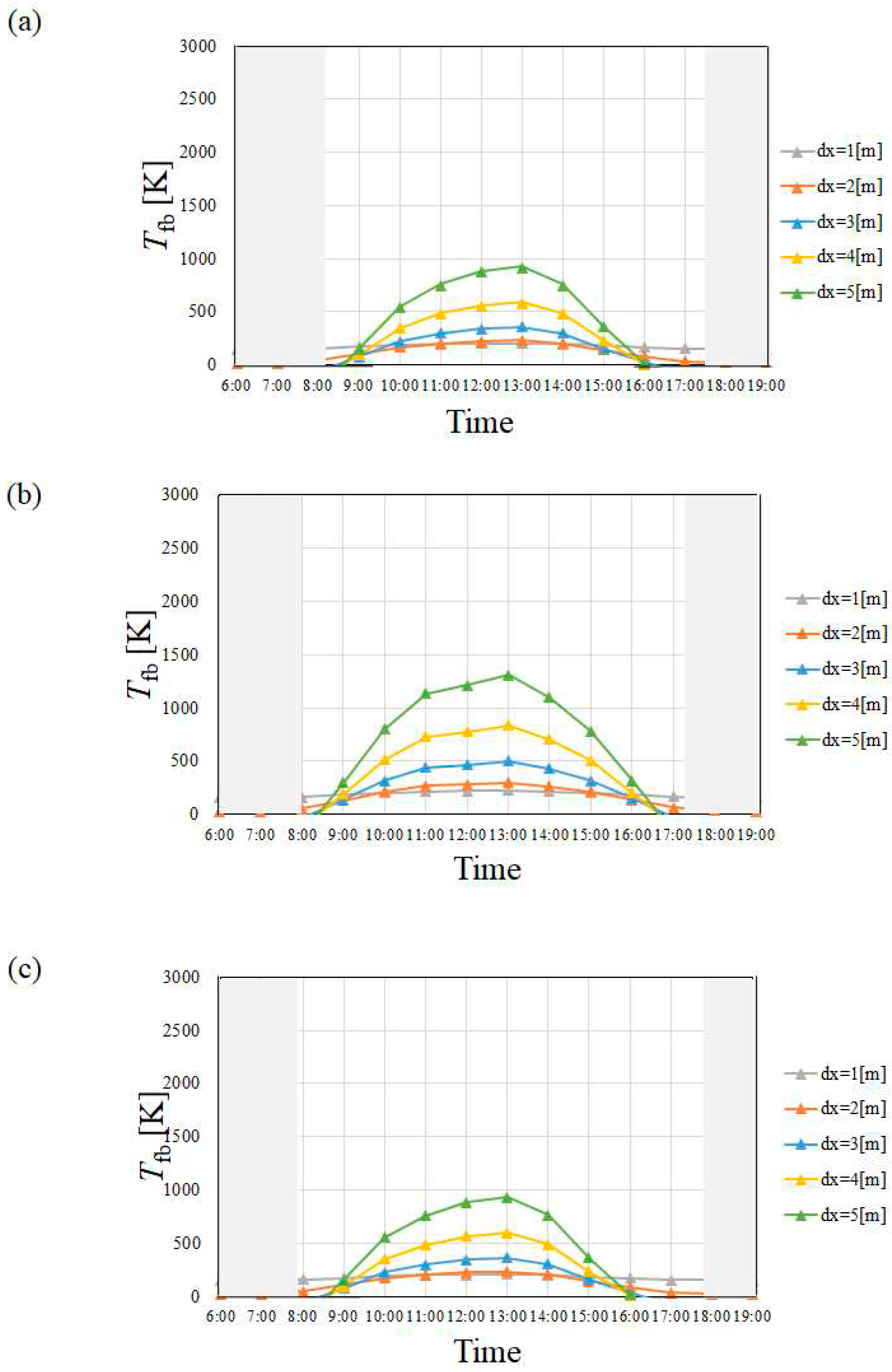
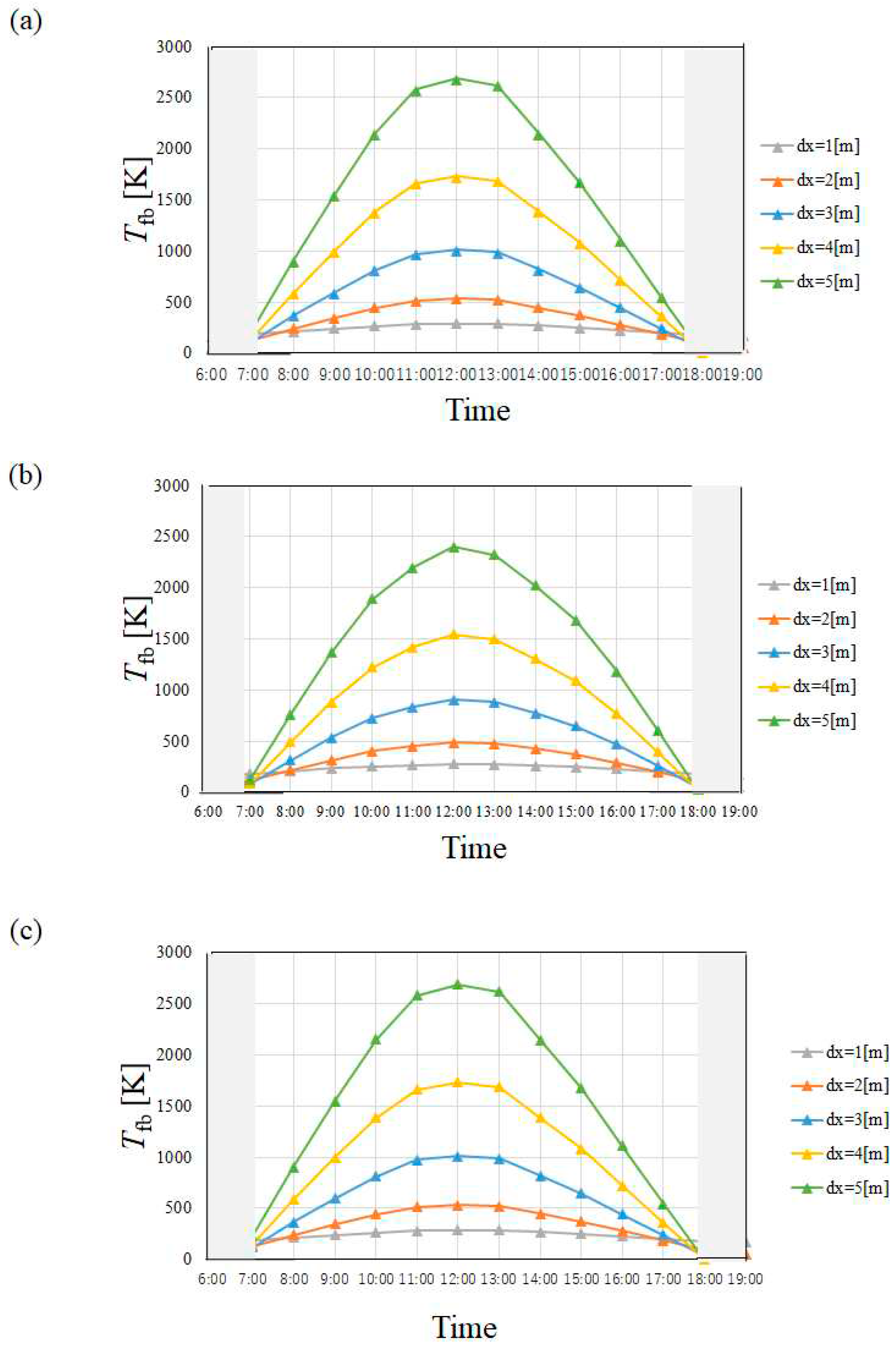
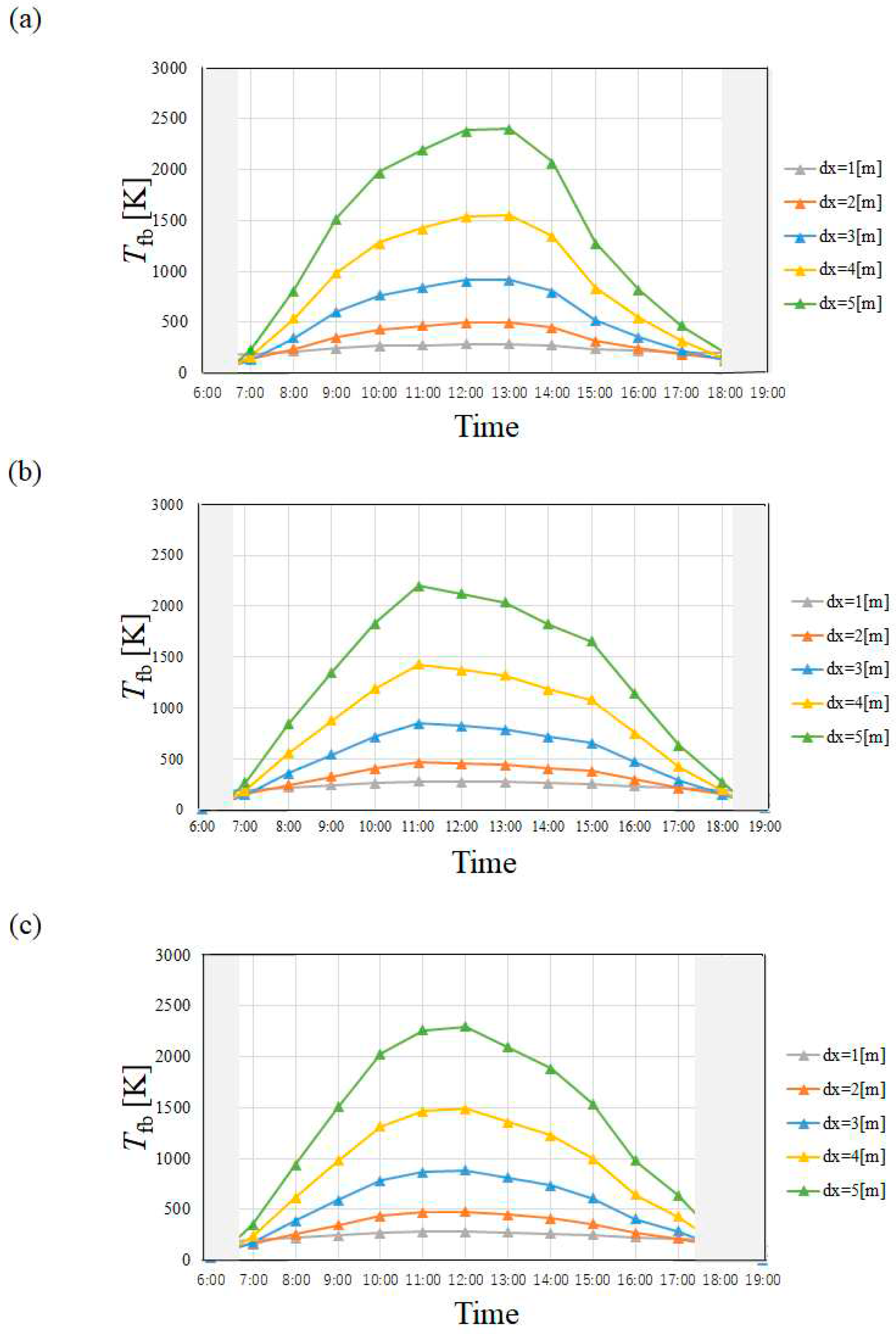
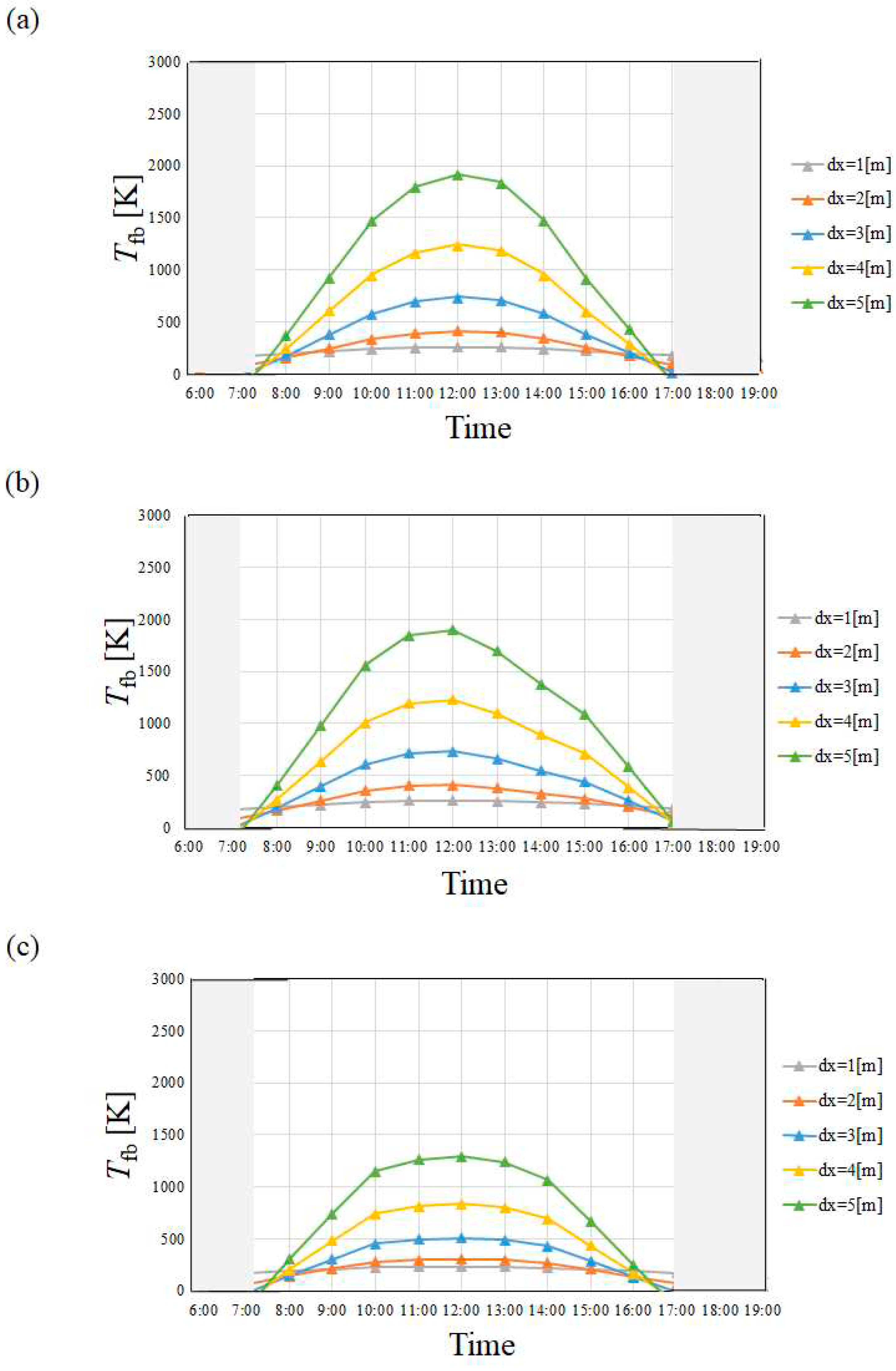
4.1. Temperature of Heat Transfer Fluid
3.2. Amount of H2 Produced from Biogas Dry Reforming and Power Generated by SOFC
4. Conclusions
- Tfb in April and July is higher than that in January and October irrespective of city since I in April and July is higher than that in January and October.
- Tfb increases with the increase in dx. However, this study has decided that the optimum and maximum dx should be 4 m irrespective of city since this study assumed to use stainless steel as the material to make the absorber tube.
- Tfb in Yamagata city in January and October, i.e. winter and autumn was lower than that in Kofu city and Nagoya city especially, which is influenced by the tendency of I strongly, not ua. On the other hand, Tfb was almost the same in April and July, i.e. spring and summer, irrespective of city.
- The amount of produced H2 as well as the power generated by SOFC in spring and summer were higher compared to the other seasons irrespective of city.
- The highest available households number per month found in this study was 4.7 in June in Kofu city as well as June and July in Yamagata city. To increase the households number, some measures, e.g. increasing m should be further studied.
Author Contributions
Funding
Conflicts of Interest
References
- Agency for Natural Resources and Energy, Energy White Paper 2023. Available online: https://www.ebecgi.meti.go.jp/about/whitepaper/2023/pdf/ (accessed on 23 January 2024).
- International Energy Agency, World Energy Outlook 2022. Available online: https://www.jea.org/reports/world-energy-outlook-2022/outlook-for-electricity (accessed on 23 January 2024).
- Jelle, B. P. Building integrated photovoltaics: a concise research pathways. Energies 2016, 9. [Google Scholar] [CrossRef]
- World Bioenergy Association, Global Bioenergy Statistics. Available online: https://worldbioenergy.org/uploads/221223%20WBA%20GBS202022.pdf (accessed on 23 January 2024).
- Mao, C.; Chen, S.; Shang, K.; Liang, L.; Ouyang, J. Highly active Ni-Ru bimetallic catalyst integrated with MFI zeolite-loaded cerium zirconium oxide for dry reforming of methane. ACS Applied Material & Interfaces 2022, 14, 47616–47632. [Google Scholar] [CrossRef]
- Nishimura, A.; Sato, R.; Hu, E. An energy production system powered by solar heat with biogas dry reforming reactor and solid oxide fuel cell. Smart Grid and Renewable Energy 2023, 14, 85–106. [Google Scholar] [CrossRef]
- Zhang, T.; Tang, X. Y.; Yang, W. W.; Ma, X. Comprehensive performance study on reflux solar methanol stream reforming reactor for hydrogen production. International Journal of Hydrogen Energy 2023, 48, 879–893. [Google Scholar] [CrossRef]
- Lu, B.; Liu, T.; Yan, X.; Zheng, Z.; Liu, Q. A new solar mid-and-low temperature receiver/reactor with linear Fresnel reflector. Applied Thermal Engineering 2023, 226. [Google Scholar] [CrossRef]
- Sarabchi, N.; Yari, M.; Mahmoudi, S. M. S. Exergy and exergoeconomic analysis of novel high-temperature proton exchange membrane fuel cell based combined cogeneration cycles, including methanol steam reformer integrated with catalytic combustor or parabolic trough solar collector. Journal of Power Sources 2021, 485. [Google Scholar] [CrossRef]
- Cheng, Z. D.; Leng, Y. K.; Men, J. J.; He, Y. L. Numerical study on a novel parabolic trough solar receiver-reactor and a new control strategy for continuous and efficient hydrogen production. Applied Energy 2023, 261. [Google Scholar] [CrossRef]
- Cheng, Z. D.; Men, J. J.; He, Y. L.; Tao, Y. B.; Ma, Z. Comprehensive study on novel parabolic trough solar receiver-reactors for hydrogen production. Renewable Energy 2019, 143, 1766–1781. [Google Scholar] [CrossRef]
- Wang, H.; Liu, M.; Kong, H.; Hao, Y. Thermodynamic analysis on mid-low temperature solar methane steam reforming with hydrogen permeation membrane reactors. Applied Thermal Engineering 2019, 152, 925–936. [Google Scholar] [CrossRef]
- Zhao, Q.; Su, B.; Wang, H.; He, A.; He, R.; Kong, H. Mid/low-temperature solar hydrogen generation via dry reforming of methane enhanced in a membrane reactor. Energy Conversion and Management 2021, 240. [Google Scholar] [CrossRef]
- Fuqiang, W.; Lin, J.; Ziming, C.; Huaxu, L.; Jianyu, T. Combination of thermodynamic analysis and regression analysis for steam and dry methane reforming. International Journal of Hydrogen Energy 2019, 44, 15795–15810. [Google Scholar] [CrossRef]
- Rathod, V. P.; Shete, J.; Bhale, P. V. Experimental investigation on biogas reforming to hydrogen rich syngas production using solar energy. International Journal of Hydrogen Energy 2016, 41, 132–138. [Google Scholar] [CrossRef]
- Graph to Chart, The Annual Duration of Sunshine for Prefectural Capital Cities in Japan. Available online: https://graphtochart.com/japan/world-yearly-sunshine_hour2.php (accessed on 23 January 2024).
- Kumar, K. H.; Daabo, A. M.; Karmakar, M. K.; Hirani, H. Solar parabolic dish collector for concentrated solar thermal systems: a review and recommendation. Environmental Science and Pollution Research 2022, 29, 32335–32367. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, S. A. Solar thermal collectors and application. Prog. Energy Combust. Sci. 2004, 30, 231–295. [Google Scholar] [CrossRef]
- Badar, R.; Pedretti, A.; Barbato, M.; Steingeld, A. An air-based corrugated cavity-receiver for solar parabolic trough concentrators. Applied Energy 2015, 138, 337–345. [Google Scholar] [CrossRef]
- Valladares, O. G.; Velazquez, N. Numerical simulation of parabolic trough solar collector: improvement using counter flow concentric circular heat exchangers. International Journal of Heat and Mass Transfer 2009, 52, 597–609. [Google Scholar] [CrossRef]
- Japan Meteorological Agency, Past Meteorological Data Search. Available online: http://www/data/jma.go.jp/obd/stats/etrn/index/php (accessed on 23 January 2024).
- Nishimura, A.; Tanaka, T.; Ohata, S.; Kolhe, M. L. Biogas dry reforming for hydrogen through membrane reactor utilizing negative pressure. Fuels 2021, 2, 191–209. [Google Scholar] [CrossRef]
- Nishimura, A.; Ohata, S.; Okukura, K.; Hu, E. The impact of operating conditions on the performance of a CH4 dry reforming membrane reactor for H2 production. Journal of Energy and Power Technology 2020, 2. [Google Scholar] [CrossRef]
- Kreith, F.; Freider, J. K. Preprints of thermodynamics and heat transfer applied to solar energy. Solar Energy Handbook, 1st ed.; McGraw-Hill, New York, 1981; p. 1.
- NEDO (New Energy and Industry Technology Development Organization), Road Map of 2017 of NEDO Fuel Cell and Hydrogen. Available online. https://www.nedo.go.jp/content/100871873.pdf (23 January 2024).
- The Japan Society of Mechanical Engineers. JSME Heat Transfer Handbook, 1st ed.; Maruzen, Tokyo, 1993; p. 371.
- Power Plan. Available online. https://standard-project.net/energy/stasistis/emegy-consumption-day.html (23 January 2024).
| Property | Value | Information |
| α [-] | 0.94 | - |
| τ [-] | 0.94 | - |
| εt [-] | 0.9 | - |
| c [J/(kg・K)] | 1.335 | for CH4:CO2 = 1.5:1 |
| σ [W/(m2・K4)] | 5.67×10-8 | Stefan-Boltzmann coefficient |
| εg [-] | 0.94 | Glass smooth surface |
| ka [W/(m・K)] | 0.0257 | Surrounding air |
| ρa [kg/m3] | 1.166 | Surrounding air |
| μa [Pa・s] | 1.82×10-5 | Surrounding air |
| Cp,a [J/(kg・K)] | 1006 | Surrounding air |
| kt [W/(m・K)] | 16 | Stainless steel |
| Kg [W/(m・K)] | 1.3 | Quartz glass |
| Time | I [MJ/m2] | ua [m/s] | Ta [K] |
| 6:00 | 0 | 1.5 | 272.6 |
| 7:00 | 0.6 | 1.6 | 272.4 |
| 8:00 | 53.4 | 1.4 | 273.3 |
| 9:00 | 186.2 | 1.6 | 275.0 |
| 10:00 | 316.6 | 1.7 | 276.7 |
| 11:00 | 427.1 | 2.0 | 278.4 |
| 12:00 | 478.4 | 2.5 | 279.8 |
| 13:00 | 472.3 | 2.6 | 280.9 |
| 14:00 | 401.3 | 2.9 | 281.5 |
| 15:00 | 287.0 | 3.1 | 281.6 |
| 16:00 | 157.7 | 2.9 | 281.1 |
| 17:00 | 35.2 | 3.1 | 279.9 |
| 18:00 | 0.1 | 3.1 | 279.9 |
| 19:00 | 0 | 2.5 | 277.9 |
| Time | I [MJ/m2] | ua [m/s] | Ta [K] |
| 6:00 | 0 | 2.1 | 275.4 |
| 7:00 | 0 | 2.2 | 275.3 |
| 8:00 | 43.8 | 2.2 | 275.8 |
| 9:00 | 166.6 | 2.4 | 277.0 |
| 10:00 | 295.3 | 2.7 | 278.4 |
| 11:00 | 379.0 | 3.4 | 279.5 |
| 12:00 | 398.4 | 3.6 | 280.3 |
| 13:00 | 422.5 | 3.8 | 280.6 |
| 14:00 | 369.0 | 3.9 | 281.0 |
| 15:00 | 288.3 | 3.9 | 280.9 |
| 16:00 | 168.7 | 4.0 | 280.4 |
| 17:00 | 46.8 | 3.5 | 279.7 |
| 18:00 | 0.6 | 3.2 | 279.0 |
| 19:00 | 0 | 2.8 | 278.5 |
| Time | I [MJ/m2] | ua [m/s] | Ta [K] |
| 6:00 | 0 | 1.4 | 270.5 |
| 7:00 | 0.2 | 1.4 | 270.4 |
| 8:00 | 37.5 | 1.5 | 270.8 |
| 9:00 | 134.3 | 1.5 | 271.6 |
| 10:00 | 236.3 | 1.6 | 272.5 |
| 11:00 | 289.1 | 1.8 | 272.9 |
| 12:00 | 320.7 | 1.6 | 273.3 |
| 13:00 | 331.9 | 1.6 | 273.9 |
| 14:00 | 288.6 | 1.6 | 274.2 |
| 15:00 | 186.7 | 1.6 | 274.1 |
| 16:00 | 97.8 | 1.6 | 273.8 |
| 17:00 | 17.0 | 1.9 | 273.1 |
| 18:00 | 0 | 1.7 | 272.6 |
| 19:00 | 0 | 1.6 | 272.4 |
| Kofu city | ||||||||||||
| 7:00 | 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 | 14:00 | 15:00 | 16:00 | 17:00 | 18:00 | |
| Jan | ✓ | ✓ | ✓ | ✓ | ||||||||
| Feb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Mar | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Apr | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| May | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Jun | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Jul | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Aug | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Sep | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Oct | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Nov | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Dec | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Nagoya city | ||||||||||||
| 7:00 | 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 | 14:00 | 15:00 | 16:00 | 17:00 | 18:00 | |
| Jan | ✓ | ✓ | ✓ | ✓ | ||||||||
| Feb | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Mar | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Apr | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| May | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Jun | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Jul | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Aug | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| Sep | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Oct | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Nov | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Dec | ✓ | ✓ | ✓ | ✓ | ||||||||
| Yamagata city | ||||||||||||
| 7:00 | 8:00 | 9:00 | 10:00 | 11:00 | 12:00 | 13:00 | 14:00 | 15:00 | 16:00 | 17:00 | 18:00 | |
| Jan | ||||||||||||
| Feb | ✓ | ✓ | ✓ | |||||||||
| Mar | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| Apr | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||||
| May | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Jun | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Jul | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||
| Aug | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Sep | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| Oct | ✓ | ✓ | ✓ | ✓ | ✓ | |||||||
| Nov | ✓ | ✓ | ||||||||||
| Dec | ||||||||||||
| Kofu city [kg] | Nagoya city [kg] | Yamagata city [kg] | |
| Jan | 29.82 | 29.82 | 0 |
| Feb | 40.40 | 40.40 | 20.20 |
| Mar52.18 | 52.18 | 52.18 | 37.27 |
| Apr | 57.72 | 57.72 | 57.72 |
| May | 59.64 | 52.18 | 52.18 |
| Jun | 64.93 | 57.72 | 61.93 |
| Jul | 52.18 | 59.64 | 67.09 |
| Aug | 59.64 | 74.55 | 52.18 |
| Sep | 50.50 | 50.50 | 50.50 |
| Oct | 44.73 | 52.18 | 37.27 |
| Nov | 36.07 | 36.07 | 36.07 |
| Dec | 37.27 | 29.82 | 0 |
| Kofu city [kWh] | Nagoya city [kWh] | Yamagata city [kWh] | |
| Jan | 551 | 551 | 0 |
| Feb | 746 | 746 | 373 |
| Mar52.18 | 964 | 964 | 689 |
| Apr | 1070 | 1070 | 1070 |
| May | 1100 | 964 | 964 |
| Jun | 1200 | 1070 | 1200 |
| Jul | 964 | 1100 | 1240 |
| Aug | 1100 | 1380 | 964 |
| Sep | 933 | 933 | 933 |
| Oct | 826 | 964 | 689 |
| Nov | 666 | 666 | 267 |
| Dec | 689 | 551 | 0 |
| Kofu city [-] | Nagoya city [-] | Yamagata city [-] | |
| Jan | 1.7 | 1.7 | 0 |
| Feb | 2.6 | 2.6 | 1.3 |
| Mar52.18 | 4.0 | 4.0 | 2.9 |
| Apr | 4.6 | 4.6 | 4.6 |
| May | 4.6 | 4.0 | 4.0 |
| Jun | 4.7 | 4.2 | 4.7 |
| Jul | 3.7 | 4.2 | 4.7 |
| Aug | 4.2 | 4.2 | 3.7 |
| Sep | 4.0 | 4.0 | 4.0 |
| Oct | 3.5 | 4.0 | 2.9 |
| Nov | 2.8 | 2.8 | 1.1 |
| Dec | 2.1 | 1.7 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





