1. Introduction
Human and animal athletes are prone to articular lesions due to trauma or stress induced pathologies. The high impact nature of equine sports leaves their articular cartilage particularly susceptible to damage [
1]. Due to its avascular nature, among other factors, this tissue has little regenerative efficiency[
2]. Traditional treatments predominantly focus on pain and inflammation control, relieving symptoms but having limited ability to correct the underlying pathology, to delay disease progress and to, ultimately, regenerate damaged tissues [
3]. When conservative treatment fails, surgical approaches might be considered. Nevertheless, these different treatments also have limitations: they do not alter disease progression, do not regenerate cartilage and do not enhance organ function [
4]. Surgical treatments, specifically result in inferior quality cartilage (fibrocartilage) formation, donor site morbidity, loss of phenotype from differentiation of primary chondrocytes during expansion, and possible need for open surgery. These techniques are also limited by low tissue availability [
5]. Therefore, there is a need for new treatments with the ability to regenerate tissues and restore function, allowing a return to physical work and athletic performance [
1,
4,
6,
7].
Articular defects might be partial, affecting only articular cartilage, or full-thickness, also reaching subchondral bone. Full-thickness defects may occur in young horses and in mature sport horses, secondarily to stress-related trauma, when cartilage is exposed to excessive loading forces, as equine articular cartilage thickness ranges from 1.5-2mm [
8]. The poor ability of cartilage to self-renew along with its avascular nature, results in the inability of blood clot formation, of progenitor cells in blood to migrate to sites of articular cartilage lesion and, of resident chondrocytes to produce sufficient amount of extracellular matrix (ECM). This poor intrinsic ability to heal, favours the development of osteoarthritis (OA) a common chronic joint disease characterized by pain, deformity, instability, and reduction of motion and function [
6]. The pathologic traits of OA consist of articular cartilage degradation together with subchondral bone thickening, osteophyte formation, synovial inflammation, ligament degeneration and capsule hypertrophy [
9].
Dedicated research in the regenerative medicine field led to the development of new therapeutics for cartilage and bone pathologies, focusing in the modulation and inhibition of disease progress and in the promotion of tissues regeneration, allowing the return to an anatomic and physiological function close to the original tissue properties [
10,
11]. The goal is to reverse the inflammation and catabolic nature of the joint to an anabolic state, allowing cartilage to regenerate.
Implantation of Mesenchymal Stem/Stromal Cells (MSCs) into cartilage defects have shown great promise in both cartilage and subchondral bone repair [
12,
13,
14,
15,
16], migrating to cartilage defects and promoting repair and regeneration [
17,
18]. Currently, bone marrow - MSCs (BM-MSCs), adipose tissue – MSCs (AT-MSCs), synovial membrane - MSCs (SM-MSCs), and umbilical cord stroma - MSCs (UC- MSCs) are the most widely MSCs types used in cartilage tissue engineering. They present advantages in promoting cartilage regeneration and have heterogeneous potential concerning their accessibility/ invasion during harvest, immunogenicity, proliferative, chondrogenic and immunomodulatory abilities [
12]. Synovial MSCs have shown a greater chondrogenic ability among other MSCs, suggesting superiority in cartilage repair [
19,
20,
21,
22,
23,
24,
25,
26,
27]. These cells have a close anatomical contact with cartilage suggesting a close bias towards the production of cartilage, becoming a good candidate to cartilage tissue-engineering [
23].
Several researches have shown encouraging results in clinical trials, justifying the use of UC-MSCs in treatments for cartilage regeneration [
24,69]. The UC-MSC derived from the Wharton jelly (WJ) have the highest proliferation potential, differentiation and immunogenic abilities[
12]. They also have the ability to release trophic factors that make them an excellent candidate for use in the clinical setting to provide cell-based restoration of hyaline-like cartilage. In allogeneic administrations, these cells stimulate little or no host immune response and can be stored for long periods while maintaining viability [
28]. UC-MSCs also have shown the ability of
in vitro induction of the production of glycosaminoglycans and collagen type II [
29].
Recent studies support the use of MSCs conditioned medium (CM) as a new therapeutic method for articular cartilage repair. CM results from the modification of the culture conditions of MSCs, through which components of the medium naturally secreted under standard conditions (secretome) can be produced and secreted in an adapted way. The CM is made up of extracellular vesicles and exosomes and also of a soluble portion rich in proteins, cytokines, growth factors, lipids and chemokines[
30]. These factors with both anti-inflammatory and pro-inflammatory effects related to chondrocyte catabolic and anabolic processes interplay allowing remodulation and improving cartilage's structure and biomechanical properties [
31,
32,
33,
34]. The simple modification of the local inflammatory environment is partially the key to the deceleration of OA progression by attenuating activated macrophages and sinoviocytes of secreting the massive amount of metabolites, harmful for articular cartilage ECM and chondrocyte [
32,
35]. When compared with PBS in in vivo studies with rats, it has been highlighted that CM has a remarkable articular-protective effect, maintaining subchondral bone structure, producing a significantly more abundant cartilage matrix and inhibiting chondrocyte apoptosis with enhanced autophagy [
36]. It was also demonstrated that CM might intensify the positive effects of cell-based therapies[
30].
The main objective of this research is to understand and describe the regenerative ability of a novel therapeutic product that combines equine SM- MSCs (eSM-MSCs) and equine UC-MSCs CM (eUC-MSCs CM) when applied in partial and full thickness cartilage defects of an equine patient.
2. Materials and Methods
2.1. Ethics and Regulation
This study was carried out in accordance with Organismo Responsável pelo Bem Estar Animal (ORBEA) from ICBAS-UP, project number: P289/ORBEA/2018 recommendations and authorization. Treatments were performed after the signature of a consent from the patient’s legal tutor, after a thorough explanation on the procedure and possible risks and associated effects, in accordance with national regulations and approval from the competent authorities.
2.2. Patient identification and clinical evaluation
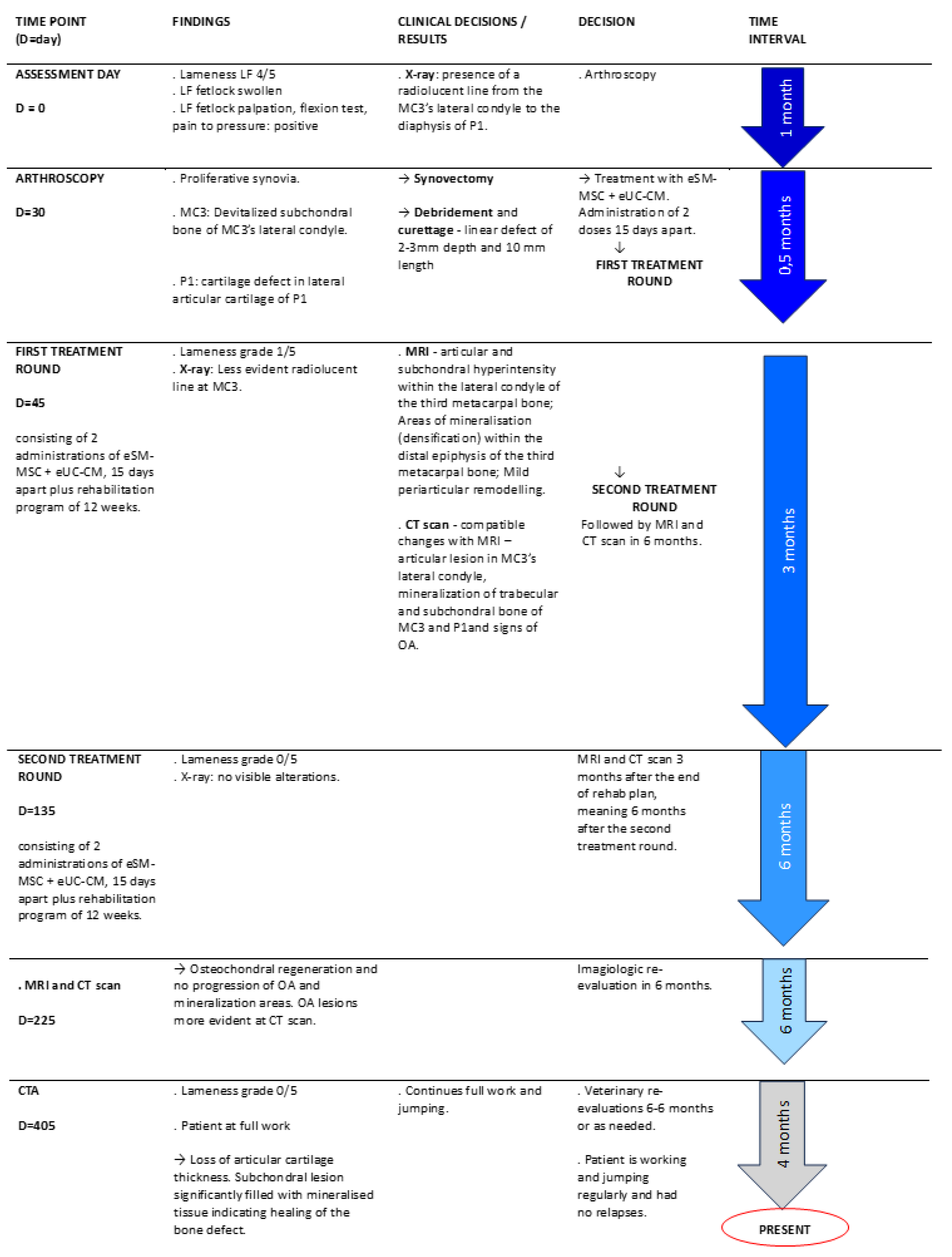
A five-year-old showjumper stallion was evaluated due to an acute lesion of the left forelimb (LF) metacarpophalangeal joint (MCj) with associated lameness. Patient undergone identification, anamnesis, physical and orthopaedic examination. Lameness was evaluated at walk and trot on a hard surface and scored in a scale of 0 to 5, according to American Association of Equine Practitioners (AAEP) parameters [
37]. As complementary diagnostic exams, regional nerve blocks, as well as radiographs (X-rays), arthroscopy, magnetic resonance image (MRI), computer tomography (CT) and computed tomography arthrography (CTA) were performed at different times of the therapeutical intervention,
The patient was treated with a combination of eSM-MSCs and eUC-MSC CM, followed by a rehabilitation program and medical follow-up. The same combination was previously used in an equine orthopaedic injury[
38].
As this was an acute case, the equine has not been under any other medical treatments (including nonsteroidal anti-inflammatory drugs, intra-articular corticosteroids, hyaluronan, glycosaminoglycans, hemoderivative treatments and other MSCs preparations) neither received any additional medical treatment (except for the ones described in the treatment plan) for at least 2 months before and after the cell-based treatment.
2.4. Diagnostic complementary exams
2.4.1. Regional Nerve Blocks.
Three regional nerve blocks were performed on the LF: digital palmar, abaxial and a low four-point nerve block. It was used lidocaine 2% (Anestesin® 20mg/ml, Orion Corporation, Finland), 2ml medially and laterally in each assessment, using 25G needles. Regional nerve blocks were performed from distal to proximal until the painful region was determined.
2.4.2. Radiographs
Radiological examination (X-ray) of the left metacarpophalangeal joint was performed with a digital system—CareRay Cw series® (CareRay, Suzhou, China), radiological constants: 72 Kv, 0.8 mA. The distance between the X-ray generator (Orange 1060 HF, EcoRay, Seoul, Republic of Korea) and the flat panel was approximately 66 cm. Seven standard views – lateromedial (LM), dorsopalmar (DP), Latero-medial flexed meta-carpophalangeal joint (LM Flex), oblique dorsolateral-plantaromedial (DLPMO), oblique dorsomedial-plantarolateral (DMPLO), oblique palmarolateral-dorsomedial oblique (PLDMO) and Palmaromedial-Dorsolateral oblique (PMDLO)– were obtained. Radiological examination was performed on assessment day and those views who presented alterations were repeated each 30 days until the end of the rehabilitation program.
2.4.3. Arthroscopy
The patient was premedicated with penicillin procaine (Depocilina 300mg/mL®, 12mg/Kg, IM, MSD Portugal), phenylbutazone (Phenylarthrite®, 2,2 mg/kg, IV, Vetoquinol, France) and detomidine (Domosedan®, 0.02 mg/kg, IV, Orion Corporation, Finland). Arthroscopic examination was performed under general anaesthesia being the patient in dorsal recumbency. A dorsal approach was performed to the LF MCj. Briefly, a stab incision on the dorsal surface of the MCj was performed as well as debridement of the tissues to access the dorsal pouch of the MCj. A needle arthroscope 18 cm length x 4,5 mm diameter and a needle visualization suite (KARL STORZ® SE & Co. KG, Tuttlingen, Germany) were introduced into the joint allowing a good evaluation, access and management of the surgical findings of the dorsal aspect of the joint.
2.4.4. Magnetic Resonance Image
MRI was performed under general anaesthesia. LF metacarpophalangeal joint was the component explored. The protocol included sagittal -Time weighted image (TW) 2*W and Short Tau Inversion Recovery (STIR), dorsal – T1W GRE and STIR, transverse T1W multiecho gradient recalled echo (GRE), T2W fast spin echo (FSE) and STIR. Two MRI exams were performed, in the end of each treatment round, approximately 6 months apart.
2.4.5. Computed Tomography scanning
CT was performed in order to compare the results with the images from MRI exams. Taking advantage of the fact that the patient was under general anaesthesia, a Cone-beam CT examination was performed at the time of each MRI exam.
2.4.6. Computed Tomography Arthrography
A CTA was performed six months after the patient returned to full work in order to monitor the healing of the osteochondral lesion on the dorsal aspect of the articular cartilage of the lateral condyle of MC3. The exam was performed with the patient under standing sedation with detomidine (Domosedan®) and iohexol (Omnipaque™ 300, GE Healthcare, UK) was used as contrast.
2.5. Donor selection and SM collection
eSM-MSCs donor was a young and healthy six months old foal who’s cause of death was a field accident. Collection preparation and procedures were previously described [
39].
2.6. eSM-MSCs: isolation, culture, characterization and CM analysis
After collection, equine synovial membrane, was prepared at the Laboratory of Veterinary Cell-based Therapies from ICBAS-UP. The isolation protocol of eSM-MSCs had been developed by Regenera
®[
39]. eSM-MSCs were characterized through trilineage differentiation, immunohistochemistry and karyotype analysis. eSM-MSCs CM preparation and analysis were also performed as previously described [
39].
2.7. UC-MSCs: isolation, culture, characterization and CM preparation and analysis
eUC-MSCs were isolated from equine UC - Wharton's jelly - and the connective tissue surrounding UC. It was expanded to form culture of adherent cells with fibroblastic morphology. Trilineage differentiation, immunophenotype and bacteriological control were previously performed and described [
38]. CM preparation and analysis were previously described [
38]. This process is a patented technology owned by Regenera
® [
38].
2.8. eSM-MSCs + eUC-MSC CM solution preparation
The therapeutic solution for intra-articular clinical application was a combination of allogenic eSM-MSCs suspended in eUC-MSC CM. Prior to preparation of the final therapeutic combination, eSM-MSC and UC-MSC CM were produced and preserved as described above. Cryopreserved P3 eSM-MSCs batches were suspended in treated animal’s autologous serum. UC-MSCs was thawed and added to the suspension to a final 1:1 concentration. 2 ml of eSM-MSCs (1x10
7 cells) solution was suspended in UC-MSC CM and transferred to a perforable capped vial and preserved on ice until the moment of administration. This therapeutic combination is currently a patented technology owned by REGENERA® (PCT/IB2019/052006, WO2019175773). This preparation was previously described [
38].
2.9. Treatment Protocol
Treatment protocol consisted of two administrations, fifteen days apart, of the prepared therapeutical combination at the LF MCj through a dorsal access. After treatment, the limb was bandaged for 24 hours and the horse received a single dose of phenylbutazone (Phenylarthrite®). The horse was monitored during the 48 hours after treatment and a rehabilitation program was initiated (
Table 1). Periodical veterinary and radiological assessments were performed during the rehabilitation program as presented at
Table 1. Six months after the first treatment administration, the patient has undergone MRI and CT exams. This protocol is from now one called first treatment round. A second treatment round was performed after the evaluation of MRI and CT exams and was accomplished under the same protocol.
2.9.1. Intra-articular eSM-MSCs + eUC-MSC CM administration
Patient was sedated with detomidine (Domosedan®), the LF MCj field was clipped and skin was aseptically prepared with chlorohexidine soap and then chlorohexidine solution and alcohol. The prepared therapeutical combination was loaded to a 2ml syringe, homogenized and injected in the LF MCj.
2.9.2. Rehabilitation program and clinical evaluations
Clinical improvement was assessed through lameness evaluation, pain to pressure and limb tumefaction/inflammation. Radiological evaluations were made every 30 days after first dose treatment, until the end of the rehabilitation program. Rehabilitation program consisted of an exercise-controlled program including stall confinement with increasing time of exercise [
9,
40,
41,
42,
43]. After intra-articular treatment, the patient underwent a rehabilitation program which consisted of two days of box rest followed by 13 days of 10 minutes hand-walk. At day 15 the second treatment was performed followed by the same 15 days rehabilitation program, up to day 30. Between day 30 and day 45 the work consisted of 20 min hand-walking, between day 45 and day 60 the work was 30 minutes of hand-walking, between day 60 and day 75, 30 minutes of hand walking plus 5 minutes trot and finally between day 75 and day 90, the patient was submitted to 30 minutes of hand-walking plus 10 minutes of trot. After 12 weeks of the established program (
Table 1) and of a veterinary revaluation, it was decided if the patient could return to full work. Three months after the return to full work, an MRI and a CT scan were performed to validate the healing of the defects.
3. Results
3.1. Horse evaluation
On assessment day, the LF MCj was swollen and the horse presented a lameness grade 4/5 scored accordingly with AAEP lameness grading scale [
37]. Palpation, manipulation, flexion test, and pain to pressure were performed and all had a positive reaction.
After the completion of the rehabilitation program, the horse still presented some lameness (1/5). Three months later, an MRI and CT scan examinations of the LF MCj was performed and some evidences of articular defects were still present so it was decided to implement another round of treatments, which were performed 6 months after the first one. After the second treatment round, lameness grade was 0/5 and there was no pain at palpation, pressure or flexion. Three months after the completion of the second treatment round, the horse was back to normal workout and a CTA was to evaluate the joint and healing of osteochondral tissues (
Figure 1).
3.2. Diagnostic Complementary Exams
3.2.1. Regional Nerve blocks.
Low four-point nerve block of LF eliminated pain and lameness, validating the injured anatomical area.
3.2.2. Radiographs
Metacarpophalangeal joint radiographs revealed the presence of a radiolucent region at the lateral condyle of the third metacarpus. Lateral and Dorsopalmar projections did not present considerable abnormalities (
Figure 2a,b). A radiolucent line was perceptible from the MC3’s lateral condyle to the diaphysis of the first phalanx (P1) (
Figure 2c). Evidence of a radiolucent area in MC3’s lateral condyle, compatible with cartilage or bony lesion (
Figure 2d), was identified.
3.2.3. Arthroscopic examination
The patient was proposed to arthroscopy to accurately determine the character and extent of the lesion and to manage any abnormal finding. A synovectomy of the proliferative synovia, as well as a debridement of the devitalized subchondral of MC3’s lateral condyle, were performed. A full thickness articular defect of 2-3mm depth and 10 mm length was identified in the lateral condyle of MC3. A partial thickness articular defect in the lateral articular surface of P1 was also diagnosed (
Figure 3).
3.2.4. MRI exam
An MRI exam was performed after the first and second treatment rounds to achieve accurate images of articular cartilage and subchondral bone of the LF MCj.
The first MRI exam identified the following MCj alterations: the patient presented articular and subchondral hyperintensity within the lateral condyle of the third metacarpal bone (
Figure 4a,c,d). Areas of mineralisation (densification) within the distal epiphysis of the third metacarpal bone (bigger laterally than medially) (
Figure 4b). Mild periarticular remodelling was seen at the abaxial margins of the proximal epiphysis of the proximal phalanx, as well as at the proximal articular margins of the proximal sesamoid bones, compatible with mild osteoarthritis of the MCj (
Figure 4).
After a second round of treatment and rehabilitation program another MRI was performed demonstrating osteochondral regeneration and absence of OA’s progression. MRI exam images evidenced: articular and subchondral hyperintensity within the lateral condyle of MC3 but less defined, areas of mineralization within the distal epiphysis of MC3 (lateral bigger than medial) are static as well as the mild MCj OA (
Figure 5).
3.2.5. Computed Tomography (CT) exam
CT exams exhibited compatible changes with MRI images – articular lesion in MC3’s lateral condyle, mineralization of trabecular and subchondral bone of MC3 and P1and signs of OA (
Figure 6). Nevertheless, OA changes were more evident in the second CT scan than in the second MRI - the articular surface of lateral condyle was irregular and a focal hypoattenuating region was seen further palmarly. There was mild periarticular remodelling in both the distal aspect of MC3 and the proximal aspect of P1. These findings were static between the two exams performed six-months apart (
Figure 7).
3.2.6. Computed Tomography Arthrography
CTA was performed with the standing patient and evidenced a total filling of the lesion with resumption of normal bone structure. There is a thinning of the cartilage line indicating loss of thickness of the articular cartilage. The subchondral lesion showed significant filling with mineralised tissue indicating healing of the bone defect (
Figure 8).
3.3. eSM-MSCs and eUC-MSCs isolation, culture, characterization and conditioned medium analysis
eSM-MSCs and eUC-MSCs were successfully isolated and expanded. Cells observed radiating from the explants and those identified in culture showed clear plastic adhesion and fibroblast-like morphology, an essential feature to characterize cells as MSCs [
39]. Characterization of eSM-MSCs and eUC-MSCs was previously addressed and discussed as well as their CM analysis [
38,
39].
3.4. Treatment Results
The horse did not present any adverse reaction such as pain, inflammation or any other clinical concern that required treatment interruption, neither during the treatment itself nor during the physical rehabilitation program.
After the first treatment round, the patient revealed a clinical improvement, lameness score grade reduced significantly from 4/5 to 1/5. Joint swelling and pain on palpation were no longer noticed and the horse did not present any signs of pain or discomfort during the rehabilitation program. Through advanced diagnostic imaging, P1’s partial thickness lesion was no longer observed but the presence osteochondral lesion at MC3’s lateral condyle remained. As the goal of this treatment was a complete clinical and imagological recovery of articular cartilage and subchondral bone, a second round of treatments was performed.
After the second treatment round, the patient presented no lameness (grade 0/5), no swelling or pain to pressure and was shod with a thinner lateral branch to reduce impact in the lateral side of the joint. The patient has resumed normal and full work, including jumping exercises, remaining sound. Diagnostic imaging (MRI and CT) demonstrated the absence of joint effusion/synovitis signs, as well as the lesion’s filling. The horse was maintained in full work and three months later a CTA was performed and evidenced fulfilment of the osteochondral lesion and presence of cartilage at MC3’s condyle (
Figure 9).
4. Discussion
Cartilage is a challenging tissue to regenerate. Nevertheless, in the presented case, diagnostic imaging revealed images compatible with cartilage formation in P1’s lesion and MC3’s lateral condyle, 15 months after treatment institution.
The therapy outcome was evaluated according with the clinical findings during the recovery process, advanced diagnostic imaging and the return to athletic performance. After the first treatment, a significant improvement in lameness score was noticed, suggestively due to decrease in acute bone inflammation, but the osteochondral lesion still exhibited poor filling, reflecting the slow healing of the tissues. This reinforces the importance of a complete and comprehensive monitoring approach during healing phase, as a decrease in pain could lead to a too early return to exercise during the initial stages of recovery [
44,
45,
46,
47,
48], while tissue integrity is still suboptimal, predisposing to incomplete healing and lesion recurrence. After the first treatment round, P1’s lesion was no longer evident and only increased mineralization on subchondral bone was noted, being these images compatible with osteochondral regeneration.
Still, MRI and CT scan images revealed the presence of MC3´s lateral condyle lesion – approximately 7 mm depth.
At the end of the second treatment round, the horse was clinically sound – no lameness, no radiological abnormalities were found and MRI and CT scan revealed no lesion at P1’s cartilage, MC3’s osteochondral defect was significantly less evident and no OA progression was identified. This improvement and fulfilment of cartilage and subchondral bone were interpretated as regeneration indicators, which were considered a strong advantage of this treatment. It has been demonstrated that MSCs can delay the progression of cartilage degeneration in osteoarthritis, relieving pain and improving joint function [
57], as it was observed in this case. MSCs derived from different sources (SM and UC) have varying chondrogenic capacity, proliferation and immunogenic potential [
49], and, in horses, SM-MSCs and UC-MSCs types have equal potency in modulating inflammatory processes related to musculoskeletal injuries [
11,
41,
56,
58]. This might have been the reason for the clinical improvement in pain and function also achieved, as the patient returned to athletic performance.
Six months later, the CTA confirmed the presence of a thin layer of articular cartilage in MC3´s condyle, feature also considered as a successful attainment of this protocol treatment. In this case, the use of the combination of eSM-MSCs and eUC-MCS CM with great chondrogenic and proliferative potential respectively, revealed a good clinical and imagiologic outcome [
50]. Literature lacks equine clinical studies in which MSCs are used in the treatment of full thickness cartilage defects, making it difficult to compare the results obtained in this work with the descriptions present in the scientific literature. Recently, a clinical study aimed to treat osteochondral defects with eSM-MSCs and a 3D scaffold in ponies. [85]. Tissue regeneration was documented after 3 to 6 months, aligning with the observations herein presented, highlighting the potential of eSM-MSCs and this technique to address these injuries.
The rehabilitation program carried out remained an essential step of the protocol treatment as it was guaranteed that the horse was never out of activity and had a permanent and progressive stimulus on cartilage, joints, ligaments and muscles. In an equine radius articular defect treated with autologous chondrocyte implantation (ACI), the horse stood in rest for 4 weeks (
versus 2 days in our protocol) and recovered from joint distension and lameness after 24 months (
versus 12 months with our protocol). This indicates that the stoppage time was reduced in 93% and that joint distension reduction and lameness time recovery was reduced in 50% [83]. In another report describing MSCs joint capsule rupture treatment, the horse reestablished its athletic performance one year later. Remarkably, this simpler lesion healed in one year while a more complex joint injury with an articular full thickness impairment of a high motion joint, such as the one herein described, also completely recovered after 12 months and restored the athletic performance after 13 months [84]. Early mobilizations of patients with articular lesions is advisable if protocols are taken carefully [
42,
51,
52]. These types of mobilizations include weight-bearing activities, walking in straight line, strengthening and flexibility activities. The importance of physical loading in chondrocyte maturation and phenotype maintenance is well documented and reduced biomechanical loading usually leads to atrophy [
53,
54,
55]. Similarly, an in-depth understanding of the effect of mechanical stimulation on MSC chondrogenic differentiation may facilitate the success of MSC-based cartilage regenerative therapies in joints, which have a mechanically demanding environment [
56].
MSCs have been successfully used as active biologics in the creation of advanced therapeutic medicinal products [
57]. These cell populations secrete a variety of bioactive molecules, including extracellular matrix components, that play a pivotal role in tissue regeneration. These factors are released into the culture media producing CM with therapeutic potential[
30].
The therapeutic formulation herein used combined eSM-MSCs, the cellular pool that naturally promotes the regeneration of joint tissues [
26,
32], with a CM enriched in immunomodulatory and pro-regenerative factors, produced by eUC-MSCs. This combination relies on the assumption that eUC-MSCs are the “youngest” and most adaptable kind of MSCs that naturally contribute to suppress the immune rejection in the maternal-foetal frontier medium, presenting unique immunosuppressive properties, evading host rejection and making them valuable tools for cell therapy [
33,
34,
35]. While conscious that the presented data is not sufficient to discern the real contribution of the various interveners present in this formulation to osteochondral healing and regeneration, this regenerative approach appears as a very promising option for treating osteochondral lesions and preventing osteoarthritis progression in equines.
The therapeutic protocol administered here was based on the intra-articular application of four allogeneic doses of 1x10
7 cells, deriving from the same donor, and none of them caused any local or systemic adverse effects. Low dosage injections ( 1x10
7 cells) display better clinical improvements than a higher dose [
58]. Clinical intra-articular application of MSCs is an easy, low invasive and reasonably safe procedure since no serious adverse events were reported [
58,
59,
60].
Since there are no well-defined protocols for the application of MSC-based therapies in horses, the administration scheme herein applied was decided based solely on the patient’s clinical outcome and consisted of two rounds of two administrations, three months apart. Studies concerning humans enhance the benefits of the increased number of MSCs intra-articular therapeutic applications (2 administrations and 3 administrations per year) [
61,
62]. Therefore, repeated intra-articular administrations are advisable, and in this case contributed to treatment effectiveness as observed along the follow up examinations.
In summary, intra-articular administrations of a novel product that combined eSM-MSCs and eUC-MCS CM was successfully applied in the treatment of partial and full-thickness cartilage defects in a horse. Follow-up evaluations evidenced clinical and imagiological significant improvements. In fact, the horse presented a complete recovery with return to athletic performance. Nevertheless, studies using a larger number of equine patients diagnosed with identical or comparable lesions from the clinical point of view and subjected to careful follow-ups under the same evaluating conditions, are necessary to conclude about the effectiveness of this innovative and promising therapeutic protocol.
5. Conclusions
Articular cartilage defects in equine joints, namely the metacarpophalangeal joint, can cause severe lameness, but with an accurate diagnosis and a suitable treatment, a favourable outcome is possible. This animal was monitored throughout the therapeutic protocol and was monitored for 13 months after completion of the second round of treatment. It returned and progressed in his sportive life with no lesion or clinical relapse. More studies need to be performed to validate the truly success of this novel therapeutic combination in the regeneration of osteochondral defects. The achievement of good clinical outcomes and good regenerative patterns will be a step forward on the complex and defying path of equine regenerative medicine.
Author Contributions
Conceptualization, A.C.M, J.M.S., I.L.R. and A.R.C.; methodology, A.C.M, R.D.A., J.M.S., I.L.R., B.L., A.M.R., P.S., and A.R.C.; software, A.C.S., I.L.R., B.L., and P.S.; validation, A.C.M, R.D.A., J.M.S., I.L.R., L.L., J.P.P., I.B. and A.R.C.; formal analysis, A.C.M, R.D.A., J.M.S., I.L.R., L.L., J.P.P., I.B. and A.R.C.; investigation, I.L.R., B.L., P.S., I.B., A.M.R., J.P.P., L.L., R.D.A., and A.C.M.; resources, A.C.M., J.M.S., C.M.M., R.D.A., and L.M.A.; data curation, A.C.M., L.L., J.M.S., C.M.M., R.D.A., and L.M.A.; writing—original draft preparation, I.L.R., R.D.A., A.C.M., J.P.P., and L.M.A.; writing—review and editing, I.L.R.; R.D.A.; P.S.; A.C.M.; L.L., J.P.P., J.M.S., and L.M.A.; visualization, A.C.S., I.L.R., B.L., and P.S.; supervision, A.C.M., R.D.A., L.M.A., C.M.M., L.L., and J.M.S.; project administration, A.C.M., R.D.A., L.M.A., C.M.M., and J.M.S.; funding acquisition, A.C.M., R.D.A., L.M.A., C.M.M., and J.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Prémios Santa Casa Neurociências–Prize Melo e Castro for Spinal Cord Injury Research (MC-04/17; MC-18-2021). The author Rui D. Alvites acknowledges the Centro de Estudos de Ciência Animal (CECA), Instituto de Ciências, Tecnologias e Agroambiente (ICETA), Porto University (UP), and Fundação para a Ciência e Tecnologia (FCT) for the funding and availability of all technical, structural, and human resources necessary for the development of this work. The work was supported through the project UIDB/00211/2020 funded by FCT/MCTES through national funds. The authors acknowledge FCT for funding the project 2022.04501.PTDC (Olfabionerve-Olfactory Mucosa Mesenchymal Stem Cells and Biomaterials Promoting Peripheral Nerve Regeneration) and the PhD Scholarships Ana Catarina Sousa (SFRH/BD/146689/2019), Bruna Lopes (2021.05265.BD), and Patrícia Sousa (2023.00246.BD).
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| AAEP |
American Association of Equine Practitioners |
| ACI |
Autologous chondrocyte implantation |
| AT-MSCs |
Adipose Tissue mesenchymal stromal cells |
| BM-MSCs |
Bone Marrow mesenchymal stromal cells |
| CM |
Conditioned Medium |
| CT |
Computed Tomography |
| CTA |
Computed Tomography arthrography |
| D |
Day |
| DLPMO |
Oblique Dorsolateral-plantaromedial |
| DMPLO |
Oblique dorsomedial-plantarolateral |
| DP |
Dorso plantar |
| ECM |
Extracellular matrix |
| eSM-MSC |
Equine synovial membrane mesenchymal stem/stroma cell |
| eUC-MSC CM |
Equine Umbilical cord mesenchymal stem/stromal cell conditioned medium |
| Flex |
flexed |
| FSE |
Fast scan echo |
| G |
Gauge |
| ILGRE |
Gradient recalled echo |
| IA |
Intra-articular |
| ICBAS-UP |
Instituto de Ciências Biomédicas Abel Salazar – Universidade do Porto |
| IL |
Interleukins |
| IV |
Endovenous |
| Kg |
Kilogram |
| Kv |
Kilovolt |
| LF |
Left forelimb |
| LM |
Lateromedial |
| mA |
milliampere |
| MC3 |
Third Metacarpus |
| MCj |
Metacarpophalangeal joint |
| mg |
milligrams |
| min |
minutes |
| mL |
millilitre |
| MRI |
Magnetic Resonance Image |
| MSCs |
Mesenchymal Stem/Stromal Cell |
| OA |
Osteoarthritis |
| ORBEA |
Organismo Responsável pelo Bem-estar Animal |
| P1 |
First phalanx |
| PLDMO |
Oblique palmarolateral-dorsalmedial |
| PMDLO |
Oblique palmaromedialdorsolateral |
| SID |
Once a day |
| SM |
Synovial membrane |
| SM-MSC |
Synovial Membrane Mesenchymal Stem/Stromal Cell |
| STIR |
Short Tau Inversion Recovery |
| TW |
Time weighted image |
| UC |
Umbilical cord |
| UC-MSC MC |
Umbilical cord mesenchymal stem/stromal cell conditioned medium |
| Vet-check |
Veterinary check-up |
| W |
weight |
| WJ |
Wharton Jelly |
| X-ray |
Radiograph |
| yo |
Years-old |
References
- Linardi, R.L.; Dodson, M.E.; Moss, K.L.; King, W.J.; Ortved, K.F. The effect of autologous protein solution on the inflammatory cascade in stimulated equine chondrocytes. Frontiers in veterinary science 2019, 6, 64. [CrossRef]
- Khan, I.M.; Gilbert, S.J.; Singhrao, S.; Duance, V.C.; Archer, C.W. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater 2008, 16, 26-39.
- Crawford, D.C.; Miller, L.E.; Block, J.E. Conservative management of symptomatic knee osteoarthritis: a flawed strategy? Orthopedic reviews 2013, 5.
- Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis (20901984) 2011. [CrossRef]
- Tan, S.S.H.; Tjio, C.K.E.; Wong, J.R.Y.; Wong, K.L.; Chew, J.R.J.; Hui, J.H.P.; Toh, W.S. Mesenchymal stem cell exosomes for cartilage regeneration: a systematic review of preclinical in vivo studies. Tissue Engineering Part B: Reviews 2021, 27, 1-13. [CrossRef]
- Nam, Y.; Rim, Y.A.; Lee, J.; Ju, J.H. Current therapeutic strategies for stem cell-based cartilage regeneration. Stem cells international 2018, 2018. [CrossRef]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nature Reviews Rheumatology 2015, 11, 21-34. [CrossRef]
- Liu, T.P.; Ha, P.; Xiao, C.Y.; Kim, S.Y.; Jensen, A.R.; Easley, J.; Yao, Q.; Zhang, X. Updates on mesenchymal stem cell therapies for articular cartilage regeneration in large animal models. Frontiers in Cell and Developmental Biology 2022, 10, 982199. [CrossRef]
- Davidson, E.J. Controlled exercise in equine rehabilitation. Veterinary Clinics: Equine Practice 2016, 32, 159-165. [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: the critical role of the cell secretome. Frontiers in bioengineering and biotechnology 2019, 7, 9. [CrossRef]
- Wang, J.; Zhou, L.; Zhang, Y.; Huang, L.; Shi, Q. Mesenchymal stem cells-a promising strategy for treating knee osteoarthritis: a meta-analysis. Bone & Joint Research 2020, 9, 719-728.
- Zha, K.; Li, X.; Yang, Z.; Tian, G.; Sun, Z.; Sui, X.; Dai, Y.; Liu, S.; Guo, Q. Heterogeneity of mesenchymal stem cells in cartilage regeneration: from characterization to application. NPJ Regenerative Medicine 2021, 6, 14. [CrossRef]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: review on isolation, expansion, and constructs. Stem Cell Research 2020, 44, 101738. [CrossRef]
- Richter, W. Mesenchymal stem cells and cartilage in situ regeneration. Journal of internal medicine 2009, 266, 390-405. [CrossRef]
- Grande, D.A.; Southerland, S.S.; Manji, R.; Pate, D.W.; Schwartz, R.E.; Lucas, P.A. Repair of articular cartilage defects using mesenchymal stem cells. Tissue engineering 1995, 1, 345-353. [CrossRef]
- Otto, W.; Rao, J. Tomorrow's skeleton staff: mesenchymal stem cells and the repair of bone and cartilage. Cell proliferation 2004, 37, 97-110.
- Granero-Molto, F.; Weis, J.A.; Longobardi, L.; Spagnoli, A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert opinion on biological therapy 2008, 8, 255-268. [CrossRef]
- Ahmed, N.; Stanford, W.L.; Kandel, R.A. Mesenchymal stem and progenitor cells for cartilage repair. Skeletal Radiology 2007, 36, 909-912. [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology 2005, 52, 2521-2529. [CrossRef]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher chondrogenic potential of fibrous synovium–and adipose synovium–derived cells compared with subcutaneous fat–derived cells: Distinguishing properties of mesenchymal stem cells in humans. Arthritis & Rheumatism 2006, 54, 843-853. [CrossRef]
- Yoshimura, H.; Muneta, T.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and tissue research 2007, 327, 449-462. [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. The FASEB Journal 2004, 18, 980-982. [CrossRef]
- Jones, B.A.; Pei, M. Synovium-derived stem cells: a tissue-specific stem cell for cartilage engineering and regeneration. Tissue Engineering Part B: Reviews 2012, 18, 301-311. [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Comparison of mesenchymal tissues-derived stem cells for in vivo chondrogenesis: suitable conditions for cell therapy of cartilage defects in rabbit. Cell and tissue research 2008, 333, 207-215. [CrossRef]
- Hassanzadeh, A.; Vousooghi, N.; Rahimnia, R.; Razeghian, E.; Rajaeian, S.; Seyhoun, I.; Sharif, S.; Verdi, J. Recent advances in mesenchymal stem/stromal cells (MSCs)-based approaches for osteoarthritis (OA) therapy. Cell Biology International 2023. [CrossRef]
- De Bari, C.; Dell'Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis & Rheumatism 2001, 44, 1928-1942.
- Kubosch, E.J.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.P.; Schmal, H. The potential for synovium-derived stem cells in cartilage repair. Current stem cell research & therapy 2018, 13, 174-184. [CrossRef]
- Sadlik, B.; Jaroslawski, G.; Puszkarz, M.; Blasiak, A.; Oldak, T.; Gladysz, D.; Whyte, G.P. Cartilage Repair in the Knee Using Umbilical Cord Wharton's Jelly–Derived Mesenchymal Stem Cells Embedded Onto Collagen Scaffolding and Implanted Under Dry Arthroscopy. Arthroscopy techniques 2018, 7, e57-e63.
- Liu, S.; Hou, K.D.; Yuan, M.; Peng, J.; Zhang, L.; Sui, X.; Zhao, B.; Xu, W.; Wang, A.; Lu, S. Characteristics of mesenchymal stem cells derived from Wharton's jelly of human umbilical cord and for fabrication of non-scaffold tissue-engineered cartilage. Journal of bioscience and bioengineering 2014, 117, 229-235.
- Sriramulu, S.; Banerjee, A.; Di Liddo, R.; Jothimani, G.; Gopinath, M.; Murugesan, R.; Marotta, F.; Pathak, S. Concise review on clinical applications of conditioned medium derived from human umbilical cord-mesenchymal stem cells (UC-MSCs). International journal of hematology-oncology and stem cell research 2018, 12, 230.
- Solursh, M.; Meier, S. A conditioned medium (CM) factor produced by chondrocytes that promotes their own differentiation. Developmental Biology 1973, 30, 279-289. [CrossRef]
- Rosochowicz, M.A.; Lach, M.S.; Richter, M.; Suchorska, W.M.; Trzeciak, T. Conditioned Medium–Is it an Undervalued Lab Waste with the Potential for Osteoarthritis Management? Stem Cell Reviews and Reports 2023, 1-29.
- MIRZAEI, M.; ESHAGHI-GORJI, R.; MALEKI, E.; MALEKSHAH, A.K.; AMIRI, F.T. Investigating the comparative effect of conditioned medium from mesenchymal stem cells and fibroblast cells on articular cartilage defects. Journal of Research in Pharmacy 2023, 27.
- Huang, C.-Y.; Vesvoranan, O.; Yin, X.; Montoya, A.; Londono, V.; Sawatari, Y.; Garcia-Godoy, F. Anti-inflammatory effects of conditioned medium of periodontal ligament-derived stem cells on chondrocytes, synoviocytes, and meniscus cells. Stem cells and development 2021, 30, 537-547. [CrossRef]
- Alves da Silva, M.; Costa-Pinto, A.; Martins, A.; Correlo, V.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.; Neves, N.M. Conditioned medium as a strategy for human stem cells chondrogenic differentiation. Journal of tissue engineering and regenerative medicine 2015, 9, 714-723. [CrossRef]
- Chen, Y.-C.; Chang, Y.-W.; Tan, K.P.; Shen, Y.-S.; Wang, Y.-H.; Chang, C.-H. Can mesenchymal stem cells and their conditioned medium assist inflammatory chondrocytes recovery? PloS one 2018, 13, e0205563.
- Practitioners, A.A.o.E. Guide for veterinary service and judging of equestrian events. 1991.
- Reis, I.L.; Lopes, B.; Sousa, P.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Rêma, A.; Briote, I.; Mendonça, C.; Santos, J.M. Treatment of Equine Tarsus Long Medial Collateral Desmitis with Allogenic Synovial Membrane Mesenchymal Stem Cells Enhanced by Umbilical Cord Mesenchymal Stem Cell-Derived Conditioned Medium: Case Report. 2023.
- Leal Reis, I.; Lopes, B.; Sousa, P.; Sousa, A.C.; Branquinho, M.; Caseiro, A.R.; Pedrosa, S.S.; Rêma, A.; Oliveira, C.; Porto, B. Allogenic Synovia-Derived Mesenchymal Stem Cells for Treatment of Equine Tendinopathies and Desmopathies—Proof of Concept. Animals 2023, 13, 1312. [CrossRef]
- Thomopoulos, S.; Parks, W.C.; Rifkin, D.B.; Derwin, K.A. Mechanisms of tendon injury and repair. Journal of Orthopaedic Research 2015, 33, 832-839. [CrossRef]
- Schils, S.; Turner, T. Review of early mobilization of muscle, tendon, and ligament after injury in equine rehabilitation. In Proceedings of the Proceedings of the 56th Annual Convention of the American Association of Equine Practitioners, Baltimore, Maryland, USA, 4-8 December 2010, 2010; pp. 374-380.
- Kaneps, A.J. Practical rehabilitation and physical therapy for the general equine practitioner. Veterinary Clinics: Equine Practice 2016, 32, 167-180. [CrossRef]
- Ortved, K.F. Regenerative medicine and rehabilitation for tendinous and ligamentous injuries in sport horses. Veterinary Clinics: Equine Practice 2018, 34, 359-373. [CrossRef]
- Link, T.M.; Stahl, R.; Woertler, K. Cartilage imaging: motivation, techniques, current and future significance. European radiology 2007, 17, 1135-1146. [CrossRef]
- Oei, E.H.; Wick, M.C.; Müller-Lutz, A.; Schleich, C.; Miese, F.R. Cartilage imaging: techniques and developments. In Proceedings of the Seminars in musculoskeletal radiology, 2018; pp. 245-260.
- Chu, C.R.; Williams, A.A.; Coyle, C.H.; Bowers, M.E. Early diagnosis to enable early treatment of pre-osteoarthritis. Arthritis research & therapy 2012, 14, 1-10. [CrossRef]
- Hughes, R.J.; Houlihan-Burne, D.G. Clinical and MRI considerations in sports-related knee joint cartilage injury and cartilage repair. In Proceedings of the Seminars in musculoskeletal radiology, 2011; pp. 069-088. [CrossRef]
- Li, Q.; Amano, K.; Link, T.M.; Ma, C.B. Advanced imaging in osteoarthritis. Sports Health 2016, 8, 418-428. [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomedicine & pharmacotherapy 2019, 109, 2318-2326. [CrossRef]
- Colbath, A.C.; Dow, S.W.; McIlwraith, C.W.; Goodrich, L.R. Mesenchymal stem cells for treatment of musculoskeletal disease in horses: Relative merits of allogeneic versus autologous stem cells. Equine veterinary journal 2020, 52, 654-663. [CrossRef]
- Mithoefer, K.; Hambly, K.; Logerstedt, D.; Ricci, M.; Silvers, H.; Villa, S.D. Current concepts for rehabilitation and return to sport after knee articular cartilage repair in the athlete. journal of orthopaedic & sports physical therapy 2012, 42, 254-273. [CrossRef]
- Uludag, S.; Ataker, Y.; Seyahi, A.; Tetik, O.; Gudemez, E. Early rehabilitation after stable osteosynthesis of intra-articular fractures of the metacarpal base of the thumb. Journal of Hand Surgery (European Volume) 2015, 40, 370-373. [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. Journal of Orthopaedic Research® 2018, 36, 52-63. [CrossRef]
- Chen, F.H.; Rousche, K.T.; Tuan, R.S. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nature clinical practice rheumatology 2006, 2, 373-382. [CrossRef]
- Hardmeier, R.; Redl, H.; Marlovits, S. Effects of mechanical loading on collagen propeptides processing in cartilage repair. Journal of tissue engineering and regenerative medicine 2010, 4, 1-11. [CrossRef]
- Zha, K.; Sun, Z.; Yang, Y.; Chen, M.; Gao, C.; Fu, L.; Li, H.; Sui, X.; Guo, Q.; Liu, S. Recent developed strategies for enhancing chondrogenic differentiation of MSC: impact on MSC-based therapy for cartilage regeneration. Stem Cells International 2021, 2021. [CrossRef]
- Mebarki, M.; Abadie, C.; Larghero, J.; Cras, A. Human umbilical cord-derived mesenchymal stem/stromal cells: a promising candidate for the development of advanced therapy medicinal products. Stem Cell Research & Therapy 2021, 12, 1-10. [CrossRef]
- Wei, P.; Bao, R. Intra-articular mesenchymal stem cell injection for knee osteoarthritis: Mechanisms and clinical evidence. International Journal of Molecular Sciences 2022, 24, 59. [CrossRef]
- Ma, W.; Liu, C.; Wang, S.; Xu, H.; Sun, H.; Fan, X. Efficacy and safety of intra-articular injection of mesenchymal stem cells in the treatment of knee osteoarthritis: A systematic review and meta-analysis. Medicine 2020, 99.
- Abbaszadeh, H.; Ghorbani, F.; Derakhshani, M.; Movassaghpour, A.A.; Yousefi, M.; Talebi, M.; Shamsasenjan, K. Regenerative potential of Wharton's jelly-derived mesenchymal stem cells: A new horizon of stem cell therapy. Journal of Cellular Physiology 2020, 235, 9230-9240.
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem cells translational medicine 2019, 8, 215-224. [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regenerative medicine 2018, 13, 295-307. [CrossRef]
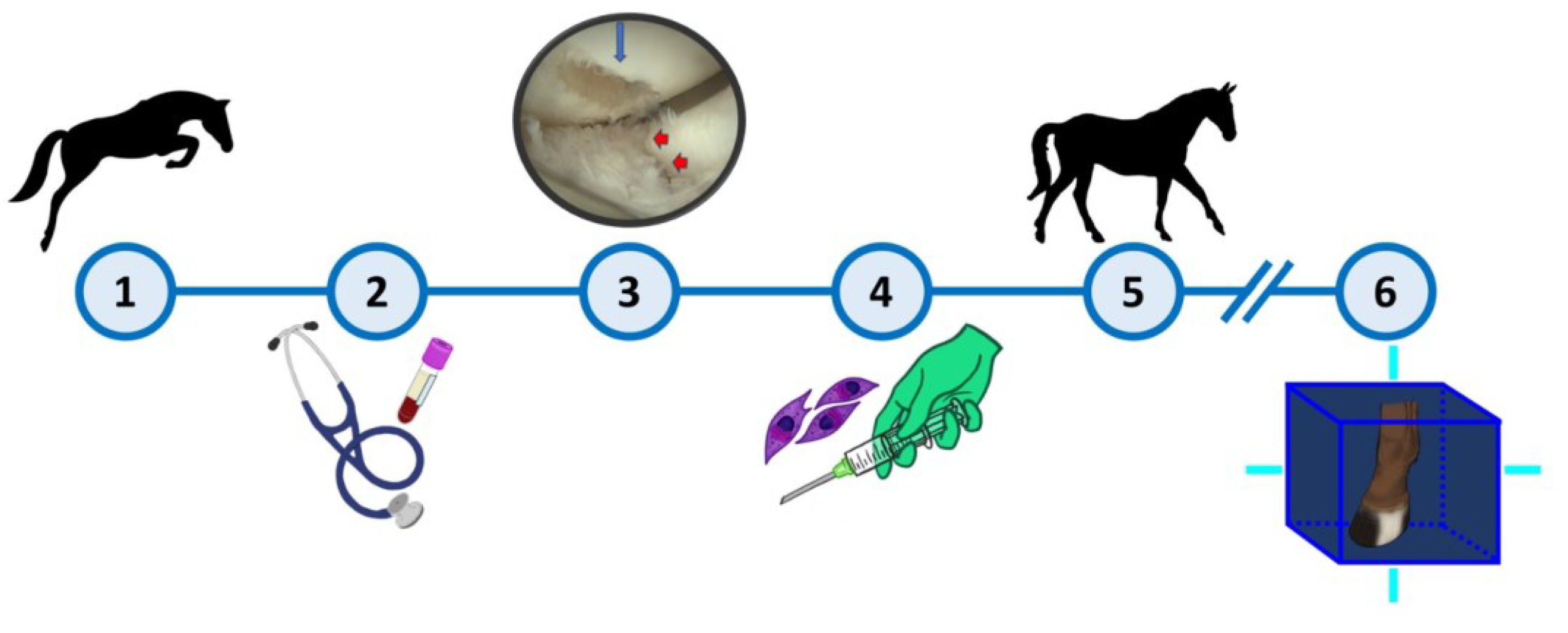
Figure 1.
Treatment timeline. 1) Horse injury. 2) A complete physical and orthopaedic examinations as well as complementary diagnostic exams were performed. 3) Arthroscopy revealed the presence of a full thickness defect at articular surface of MC3’s lateral condyle (blue arrow) and P1’s lateral articular surface (red arrow) of LF MCj. 4) Treatment protocol: 2 intra-articular administrations, 15 days apart, of 2ml of the combination eSM-MSCs (1x107 cells) + eUC-MSC CM. 5) Rehabilitation program – progressive time on walking and trotting during 12 weeks. 6) Complementary diagnostic exams: MRI + CT scan. After these exams, a second round of treatments was performed, following the same protocol. Three months after the end of the second treatment round, a CTA was performed.
Figure 1.
Treatment timeline. 1) Horse injury. 2) A complete physical and orthopaedic examinations as well as complementary diagnostic exams were performed. 3) Arthroscopy revealed the presence of a full thickness defect at articular surface of MC3’s lateral condyle (blue arrow) and P1’s lateral articular surface (red arrow) of LF MCj. 4) Treatment protocol: 2 intra-articular administrations, 15 days apart, of 2ml of the combination eSM-MSCs (1x107 cells) + eUC-MSC CM. 5) Rehabilitation program – progressive time on walking and trotting during 12 weeks. 6) Complementary diagnostic exams: MRI + CT scan. After these exams, a second round of treatments was performed, following the same protocol. Three months after the end of the second treatment round, a CTA was performed.
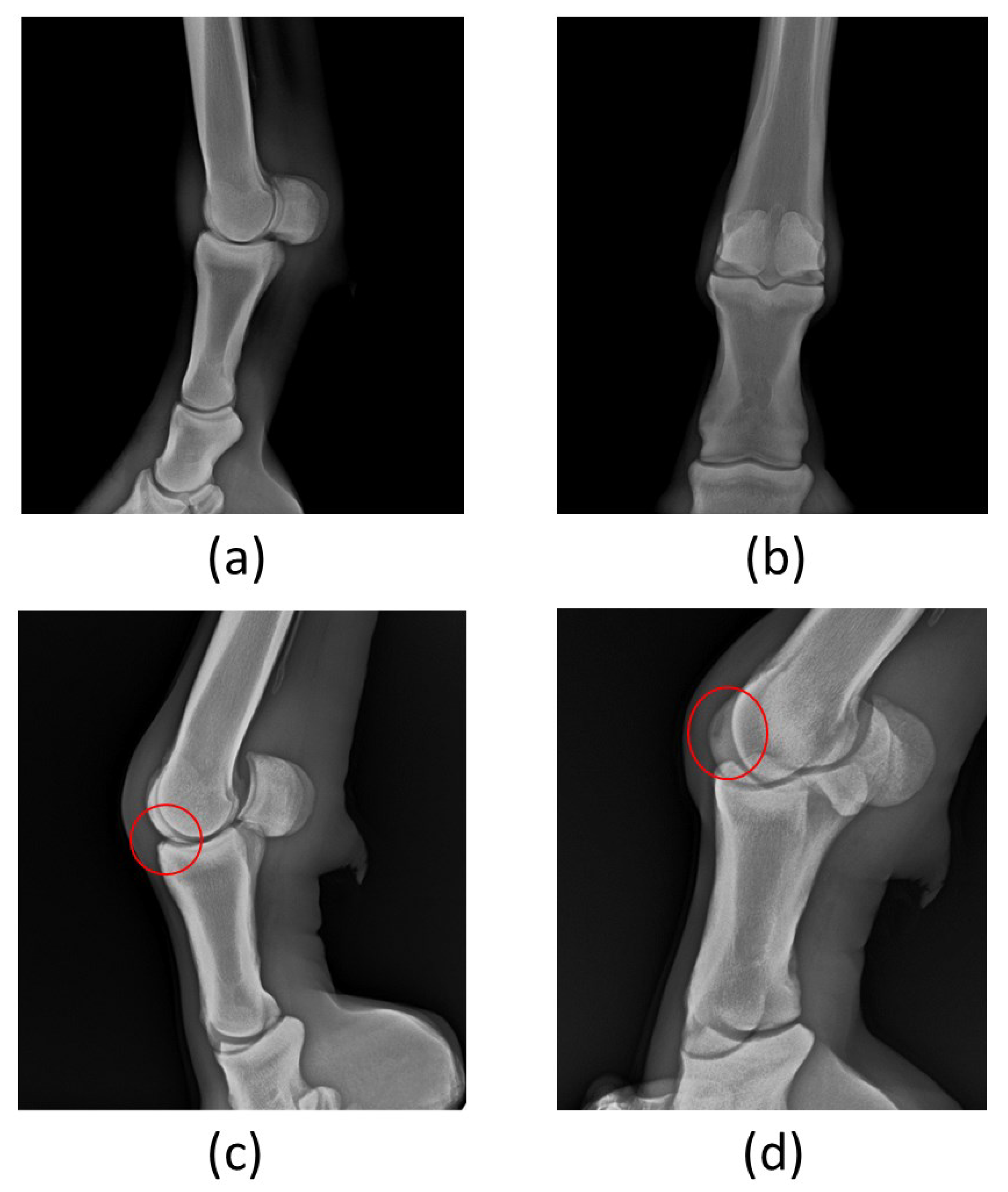
Figure 2.
Patient LF metacarpophalangeal joint radiographs before treatment. Four radiographic projections (a) Lateromedial (LM), (b) Dorsopalmar (DP), (c) LM flexed d) PLDMO slightly flexed (palmarolateral-dorsomedial Oblique) are presented. Radiological alterations are highlighted with a red circle and are seen in LM flex (c) and PLDMO flex (d).
Figure 2.
Patient LF metacarpophalangeal joint radiographs before treatment. Four radiographic projections (a) Lateromedial (LM), (b) Dorsopalmar (DP), (c) LM flexed d) PLDMO slightly flexed (palmarolateral-dorsomedial Oblique) are presented. Radiological alterations are highlighted with a red circle and are seen in LM flex (c) and PLDMO flex (d).
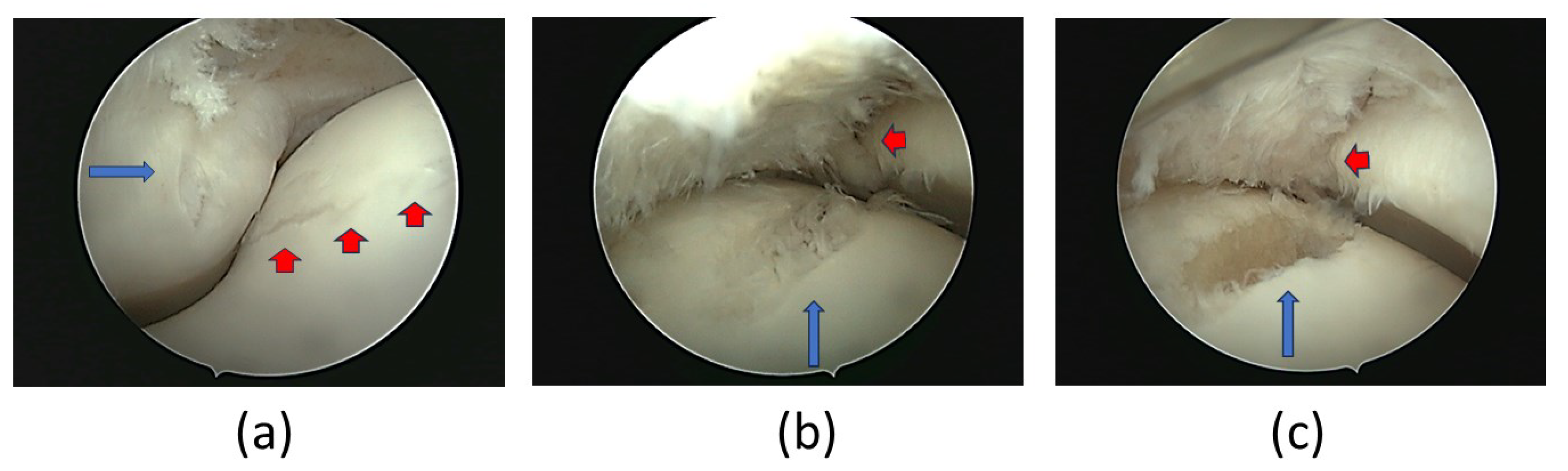
Figure 3.
Arthroscopic images of the LF metacarpophalangeal joint. (a) Lateral cartilage defect on the sagittal ridge of P1 (blue arrow) and on the lateral condyle of MC3 (red arrow). Cartilage defect in MC3´s lateral condyle with devitalized subchondral bone during (b) and after debridement (c). Cartilage defect at P1 remains visible (blue arrow) in (b) and (c) images.
Figure 3.
Arthroscopic images of the LF metacarpophalangeal joint. (a) Lateral cartilage defect on the sagittal ridge of P1 (blue arrow) and on the lateral condyle of MC3 (red arrow). Cartilage defect in MC3´s lateral condyle with devitalized subchondral bone during (b) and after debridement (c). Cartilage defect at P1 remains visible (blue arrow) in (b) and (c) images.
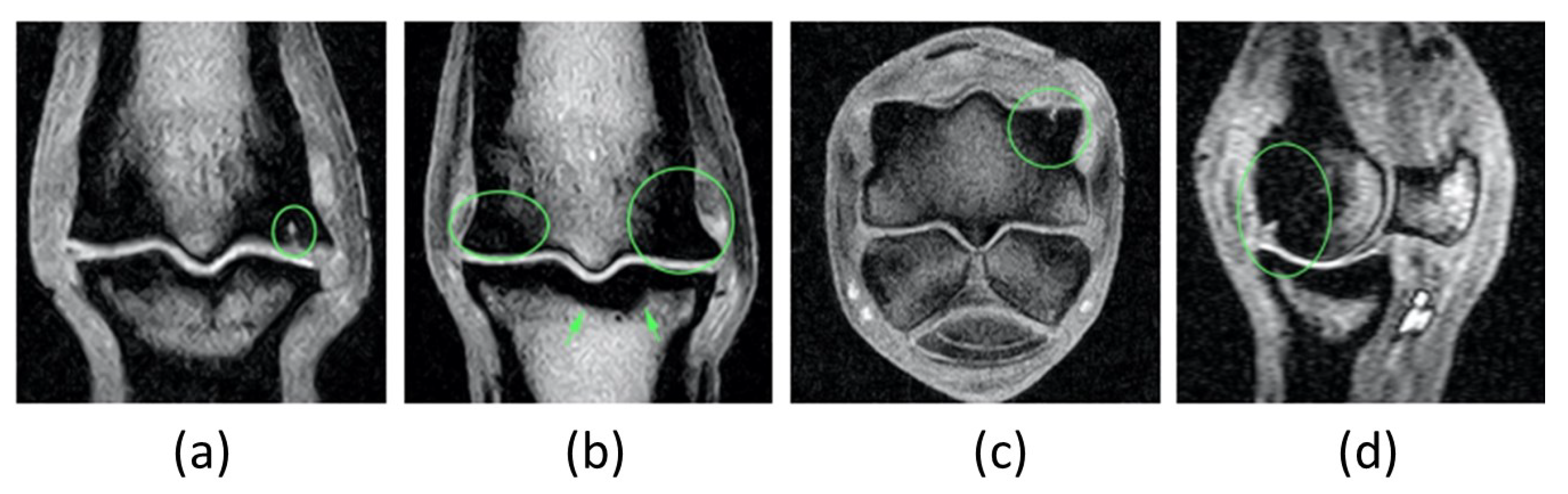
Figure 4.
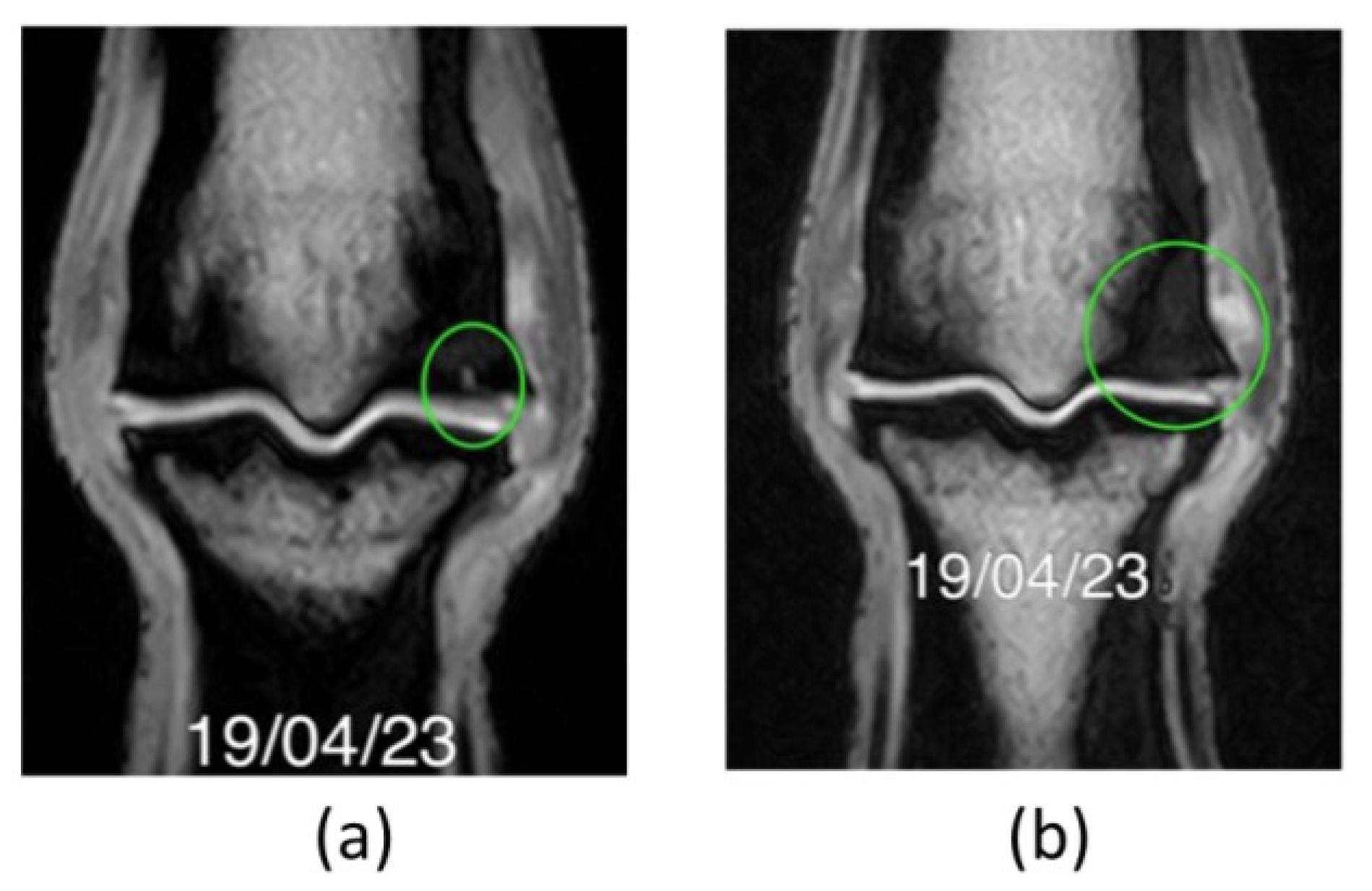
MRI examination: first exam. A well-defined T1W linear (7 mm length approximately) hyperintensity seen extending from the articular surface of the dorso-disto-abaxial aspect of the lateral condyle of the third metacarpal bone into the subchondral bone (green circle in (a), (c) and (d). The adjacent subchondral and trabecular bone show extensive and ill-defined low signal intensity on all sequences (green circle in (b)). The opposing subchondral bone of the proximal phalanx is mildly thickened, especially axially (green arrow in (b)). An area of ill-defined low signal intensity on all sequences is also seen at the dorso-disto-abaxial aspect of the medial condyle of the third metacarpal bone, its extension is smaller than the one described in the lateral one (left green circle is smaller than right green circle in (b)). .
Figure 4.
MRI examination: first exam. A well-defined T1W linear (7 mm length approximately) hyperintensity seen extending from the articular surface of the dorso-disto-abaxial aspect of the lateral condyle of the third metacarpal bone into the subchondral bone (green circle in (a), (c) and (d). The adjacent subchondral and trabecular bone show extensive and ill-defined low signal intensity on all sequences (green circle in (b)). The opposing subchondral bone of the proximal phalanx is mildly thickened, especially axially (green arrow in (b)). An area of ill-defined low signal intensity on all sequences is also seen at the dorso-disto-abaxial aspect of the medial condyle of the third metacarpal bone, its extension is smaller than the one described in the lateral one (left green circle is smaller than right green circle in (b)). .
Figure 5.
MRI examination: second exam (a) Previously described linear T1W hyperintensity/hypoattenuating within the lateral condyle of the third metacarpal bone is still visible (green circle) in the current examination, although it has less defined margination. (b) Area of mineralization in the distal epiphysis of the third metacarpal bone - the degree of hypointensity/hyperattenuation of the subchondral and trabecular bone adjacent to the previously described subchondral lesion within the lateral condyle of the third metacarpal bone (green circle), as well as the opposing subchondral bone of the proximal phalanx, is similar in the current examination compared with the previous one.
Figure 5.
MRI examination: second exam (a) Previously described linear T1W hyperintensity/hypoattenuating within the lateral condyle of the third metacarpal bone is still visible (green circle) in the current examination, although it has less defined margination. (b) Area of mineralization in the distal epiphysis of the third metacarpal bone - the degree of hypointensity/hyperattenuation of the subchondral and trabecular bone adjacent to the previously described subchondral lesion within the lateral condyle of the third metacarpal bone (green circle), as well as the opposing subchondral bone of the proximal phalanx, is similar in the current examination compared with the previous one.
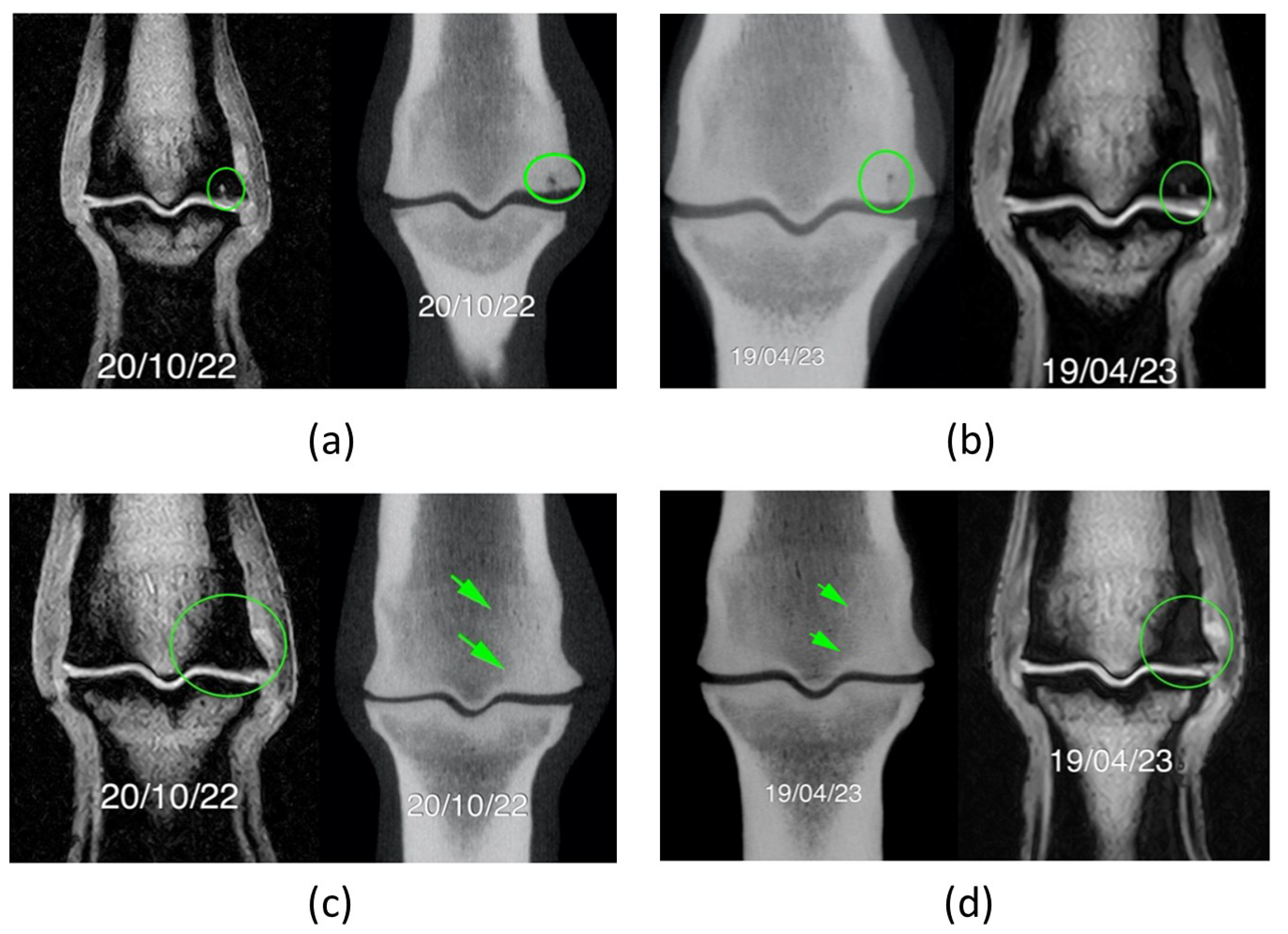
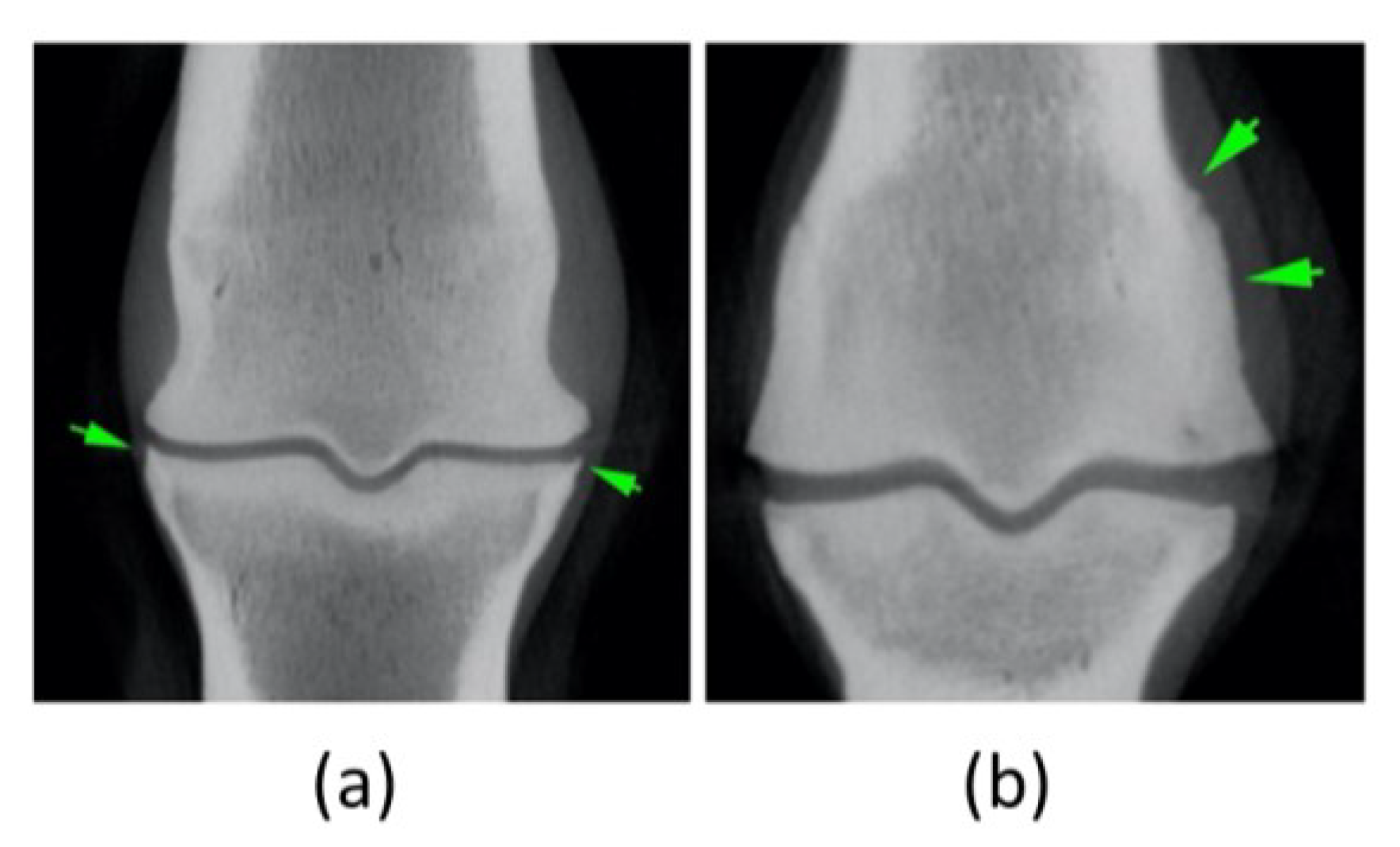
Figure 6.
Comparison of MRI and CT scan images. a) and b) refer to the same region, 6 months apart, as well as c) and d). (a) Lateral condyle presented a focal hypoattenuating region, seen further palmarly (green circle). (b) After the second treatment the degree of hypointensity/hyperattenuation within the lateral condyle of the third metacarpal bone is less defined. (c) and (d) Areas of mineralization of subchondral bone are similar meaning they are static, being lateral bigger than medial (green circle in MRI and green arrows in CT scan image).
Figure 6.
Comparison of MRI and CT scan images. a) and b) refer to the same region, 6 months apart, as well as c) and d). (a) Lateral condyle presented a focal hypoattenuating region, seen further palmarly (green circle). (b) After the second treatment the degree of hypointensity/hyperattenuation within the lateral condyle of the third metacarpal bone is less defined. (c) and (d) Areas of mineralization of subchondral bone are similar meaning they are static, being lateral bigger than medial (green circle in MRI and green arrows in CT scan image).
Figure 7.
Mild metacarpophalangeal joint osteoarthritis evidenced at the second CT scan. Evidence of mild periarticular reaction of the metacarpophalangeal joint (green arrows) better evaluated with CT scan images: (a) Proximal aspect of the proximal phalanx (b) Distal aspect of the third metacarpal bone. These findings are static - images six-months apart – and are compatible with mild OA.
Figure 7.
Mild metacarpophalangeal joint osteoarthritis evidenced at the second CT scan. Evidence of mild periarticular reaction of the metacarpophalangeal joint (green arrows) better evaluated with CT scan images: (a) Proximal aspect of the proximal phalanx (b) Distal aspect of the third metacarpal bone. These findings are static - images six-months apart – and are compatible with mild OA.
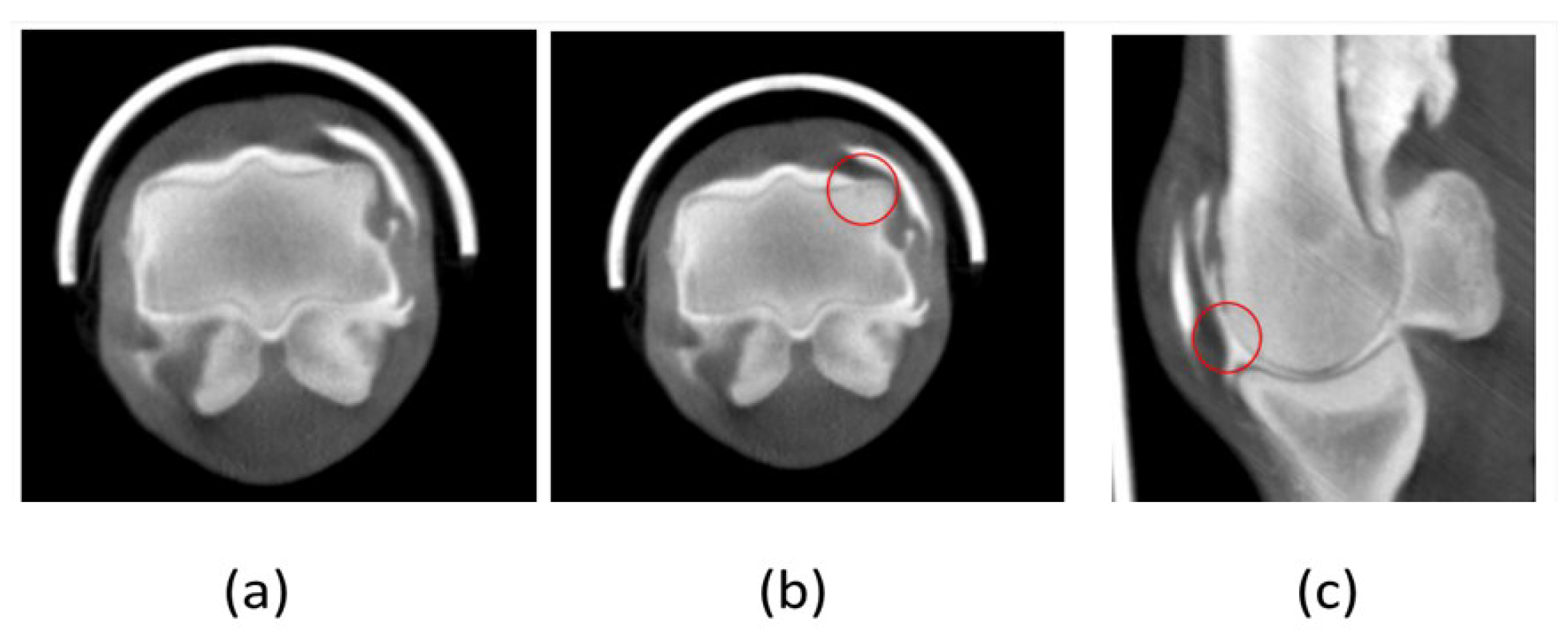
Figure 8.
Computed Tomography with arthrography. (a) Presence of articular cartilage enveloping the entire condyle, despite having a smaller thickness. (b) and (c) Subchondral bone lesion fulfilment with normal bone density and presence of cartilage was noticed (red circle).
Figure 8.
Computed Tomography with arthrography. (a) Presence of articular cartilage enveloping the entire condyle, despite having a smaller thickness. (b) and (c) Subchondral bone lesion fulfilment with normal bone density and presence of cartilage was noticed (red circle).
Figure 9.
Treatment chronogram.
Figure 9.
Treatment chronogram.
Table 1.
Physical rehabilitation program.
Table 1.
Physical rehabilitation program.
| Week |
Exercise |
| 0-2 |
2 days: stall confinement
Handwalk: 10 min
Day 15: new treatment |
| 3-4 |
2 days: stall confinement
Handwalk: 10 min
VET-CHECK + X-ray |
| 5 |
Handwalk: 15 min |
| 6 |
Handwalk: 20 min
VET-CHECK + X-ray |
| 7 |
Handwalk: 25 min |
| 8 |
Handwalk: 30 min
VET-CHECK + X-ray |
| 9-10 |
Handwalk: 30 min + 5 min trot |
| 11-12 |
Handwalk: 30 min + 10 min trot
VET-CHECK + X-ray |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).