1. Introduction
Pharmaceutical companies are aware of the importance of the crystalline form (or absence of crystallinity in amorphous solids) of a drug candidate or active pharmaceutical ingredient (API) in connection with its solubility and stability and, consequently, the impact on the pharmacokinetic profile of such a product [
1]. Polymorphism, defined as the ability of a molecule to form different crystal forms depending on the intermolecular associations or different conformations of the molecules in the crystal lattice, is a source of possible problems during the development of a drug (unexpected appearance of a new polymorph, disappearing polymorphs [
2], or difficulties in the synthesis of the selected one) but also opportunities from the physicochemical or patentability points of view [
3].
Although the identification of the most stable polymorph is highly recommended during the early phases of drug discovery [
4], it is true in our opinion that organic and medicinal chemists involved in the discovery phase usually do not consider the influence of the crystalline form of the molecules synthesized because the main objective is to have a sample for biological testing.
As a part of our work in the field of tyrosine kinase inhibitors, we developed years ago compound
1 (internally named
IQS016,
Figure 1) as a candidate for the treatment of leukemia [
5].
Such compound was later licensed to the company Pangea Oncology (
https://panoncology.com) and started its possible development as a DDR2 inhibitor for the treatment of squamous cell carcinoma and KRAS-mutated adenocarcinoma of the lung [
6,
7]. Although
1 presented very good
in vitro activity, the development was stopped by Pangea Oncology due to poor pharmacokinetic properties. Nevertheless, a preliminary GMP batch of
1 (named
PB1 by Pangea Oncology) was prepared by the Applus
+ Laboratories (
https://www.appluslaboratories.com), and a sample of which was transferred to our laboratory. Very recently and in connection with a project in the field of pancreatic cancer, we sent two solid samples of compound
1 for the determination of the
in vitro inhibitory capability of the most relevant tyrosine kinases involved in such a disease to Reaction Biology (
https://www.reactionbiology.com). One of them came from a batch of compound
1 obtained at our laboratories (named
IQS016) and the other from the same compound prepared by Applus
+ Laboratories (named
PB1). Although both samples seemed indistinguishable regarding their appearance as powdery solids and were prepared by using the same synthetic route [
5], the contradictory results obtained (
IQS016 sample being active in front of several of the kinases studied but the
PB1 sample being totally inactive) forced us to complementary research that is described in this paper.
2. Results and Discussion
To discard possible structural differences between samples
IQS016 and
PB1 or the presence of an impurity in any of the samples that could justify the differences observed in the
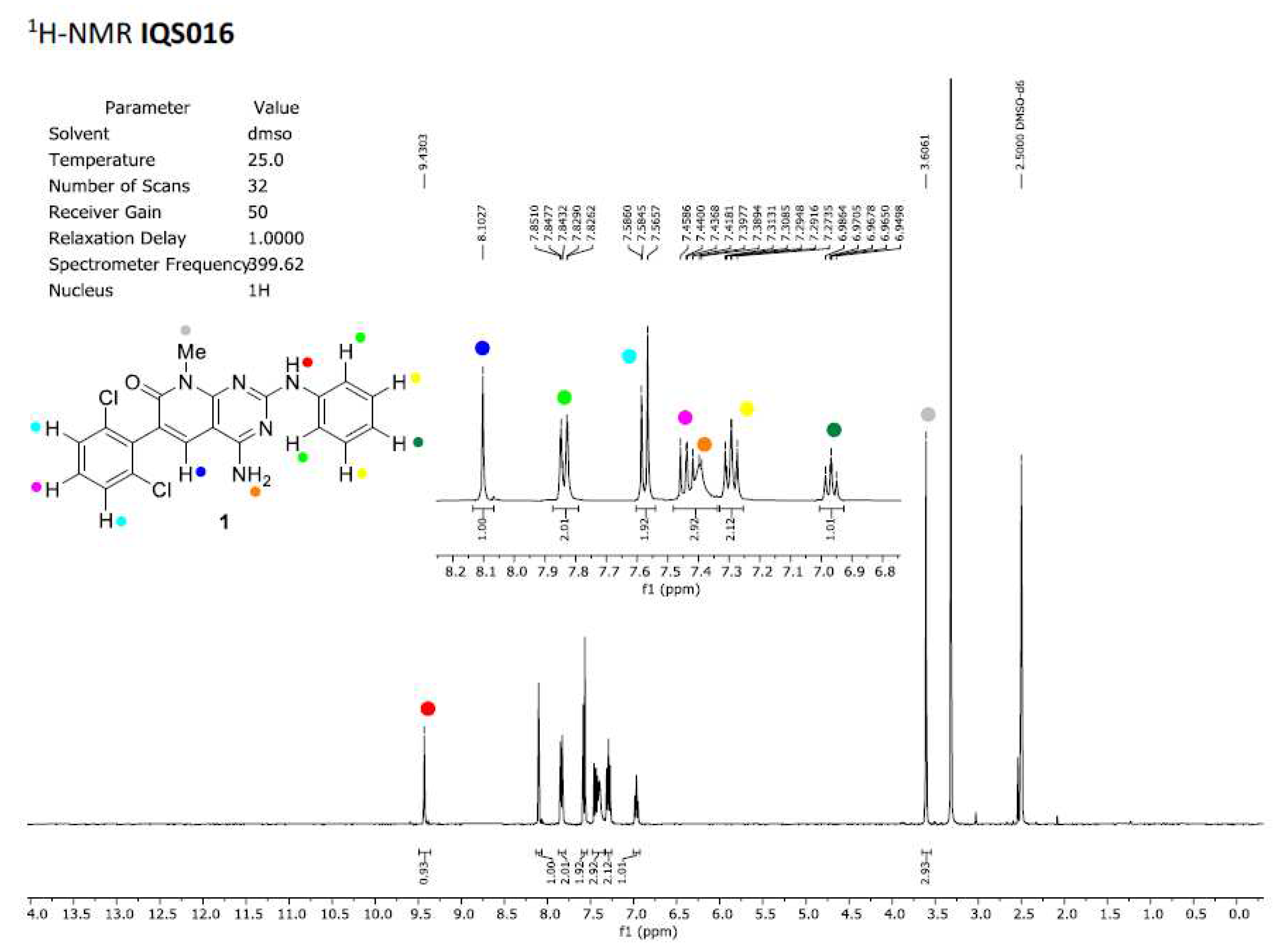
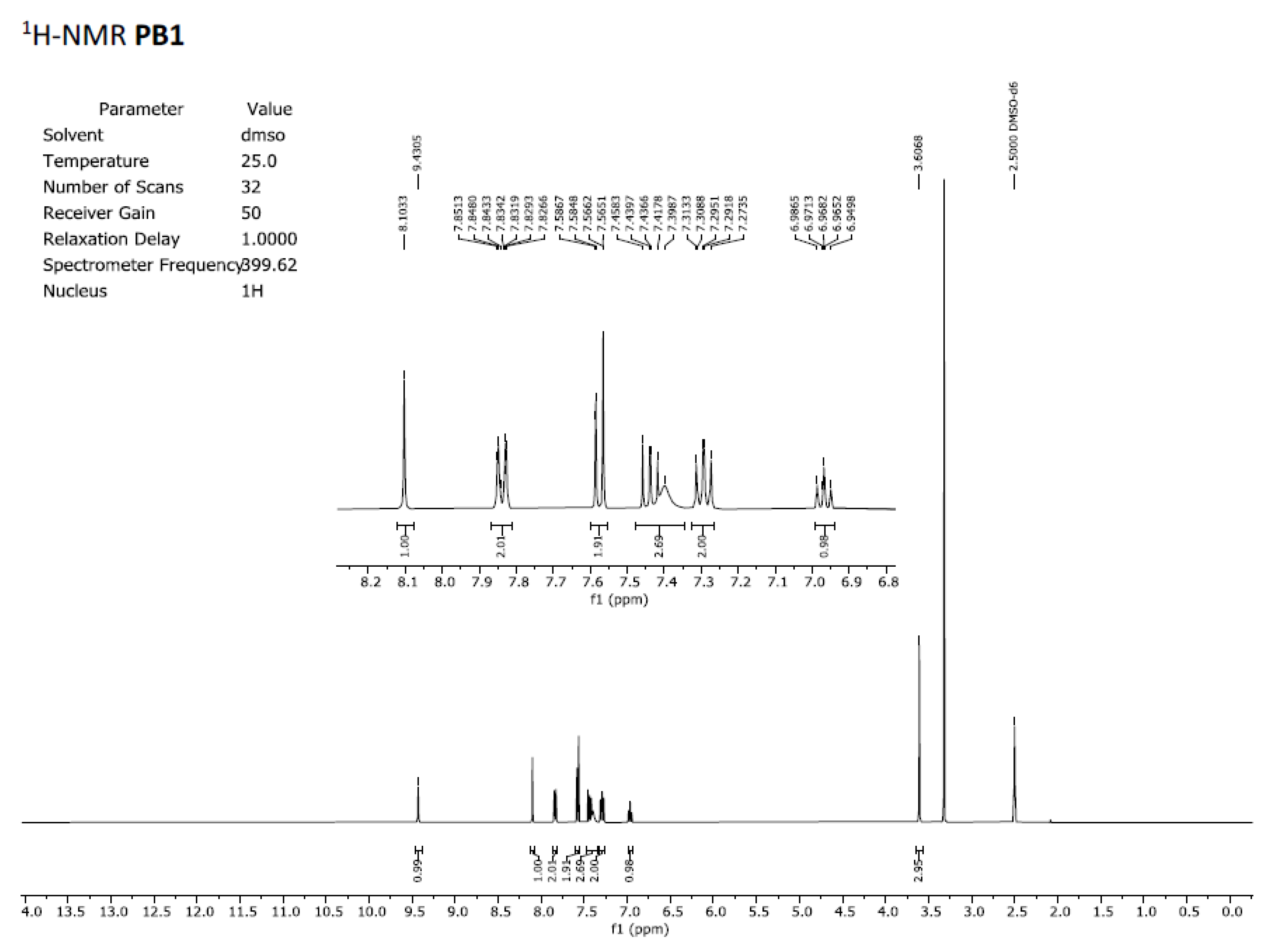
in vitro tests with isolated receptors, we first registered the
1H-NMR spectrum of both samples. Those spectra registered in DMSO-
d6 are included in
Figure 2 and
Figure 3 and clearly show that the spectra are superimposable confirming that the structure of the molecule present in both samples is the same.
Additionally, we carried out the HPLC-MS analysis of both samples. Such analysis was performed using an HPLC-MS, Agilent technologies 1200 series LC/LC MSD iQ, column X-bridge C18 (100 × 4.6 x 3.5 µm, waters) oven temperature 40 °C and a combined isocratic and linear gradient elution at a flow rate of 0.5 mL min
−1 consisting of a mobile phase of water and acetonitrile, each containing 0.1% formic acid (v/v), over a 20 min run time. Detection was performed at 254 nm and by MS, ionization method with cone voltage 110 V and MS scan 100-1000.
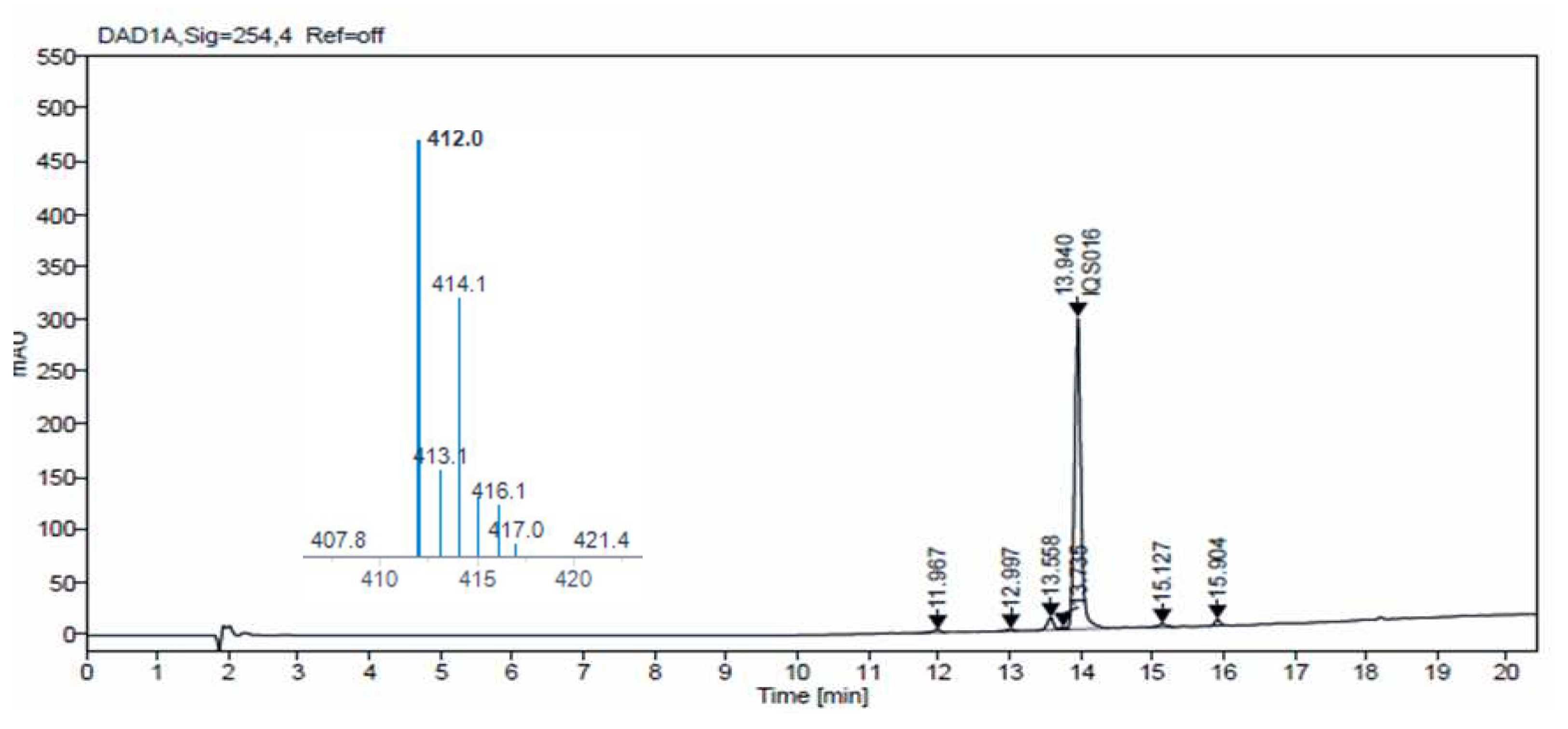
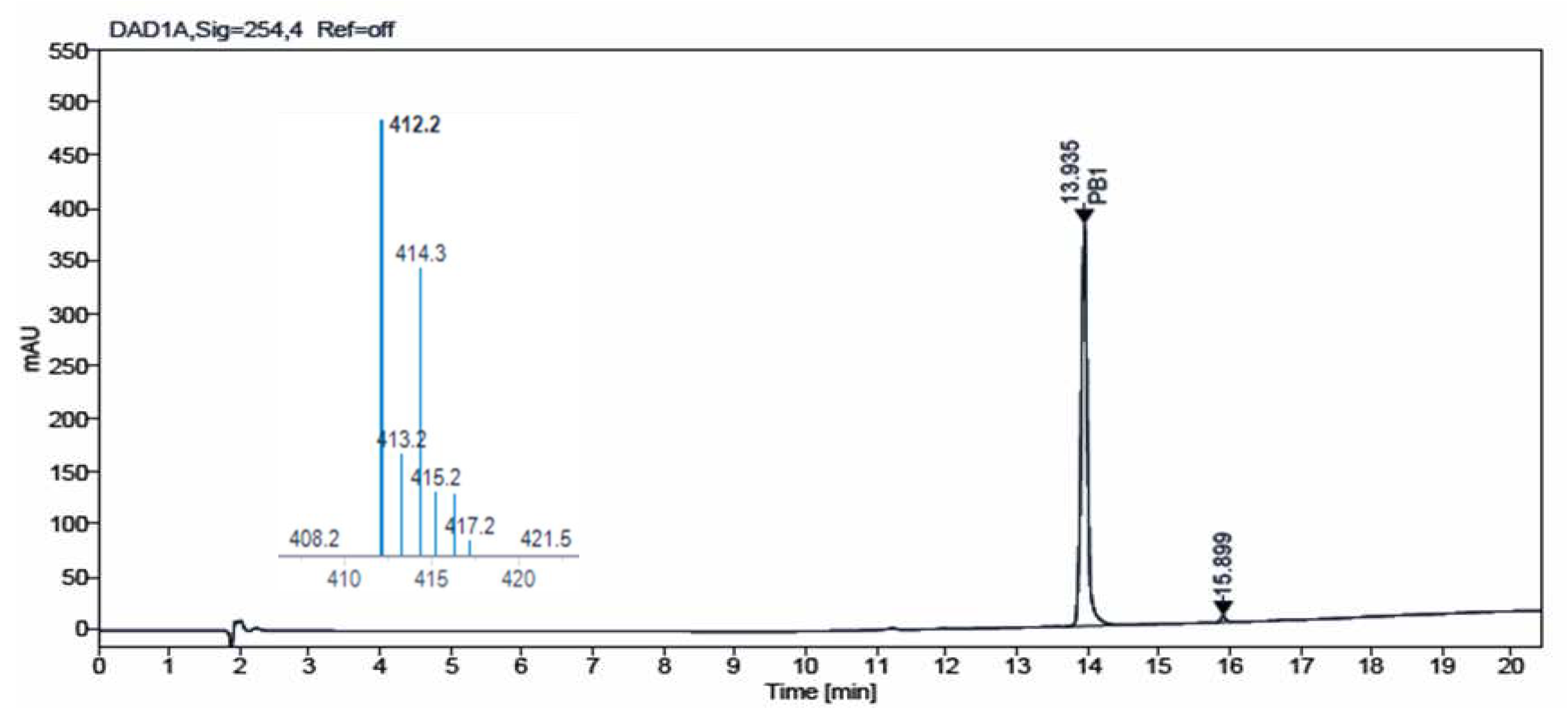
Figure 4 and
Figure 5 show the chromatograms obtained for
IQS016 and
PB1 at 254 nm. Integration by normalized areas reveals a purity of 91.5% for
IQS016 and 99.8% for
PB1. In both cases, the retention time of the main peak is 13.94 min. Each one of the chromatograms shows the mass spectrum of the main peak registered using electrospray ionization (ESI) in the positive mode, (m/z = 412, M
++1), revealing the presence of two chlorine atoms in the molecule.
Once demonstrated that both samples, IQS016 and PB1, contain the same molecular structure and that the presence of unknown impurities does not justify the difference observed in the biological testing, although the appearance of both solid samples was almost the same (a slightly colored powder), we decided to evaluate possible differences in the crystallinity of both solids.
Differential Scanning Calorimetry (DSC) is a good method to discern the presence in a solid of different crystalline forms of the same chemical substance [
8,
9,
10]. As is already known, different crystalline forms exhibit different melting points, and if the solid is amorphous, it does not have a well-defined melting point. However, its glass transition and subsequent crystallization can be measured. If the concentration of one of the crystalline forms in the sample is very low (less than 5%), two melting peaks are not detected. Instead, a broader single peak is observed.
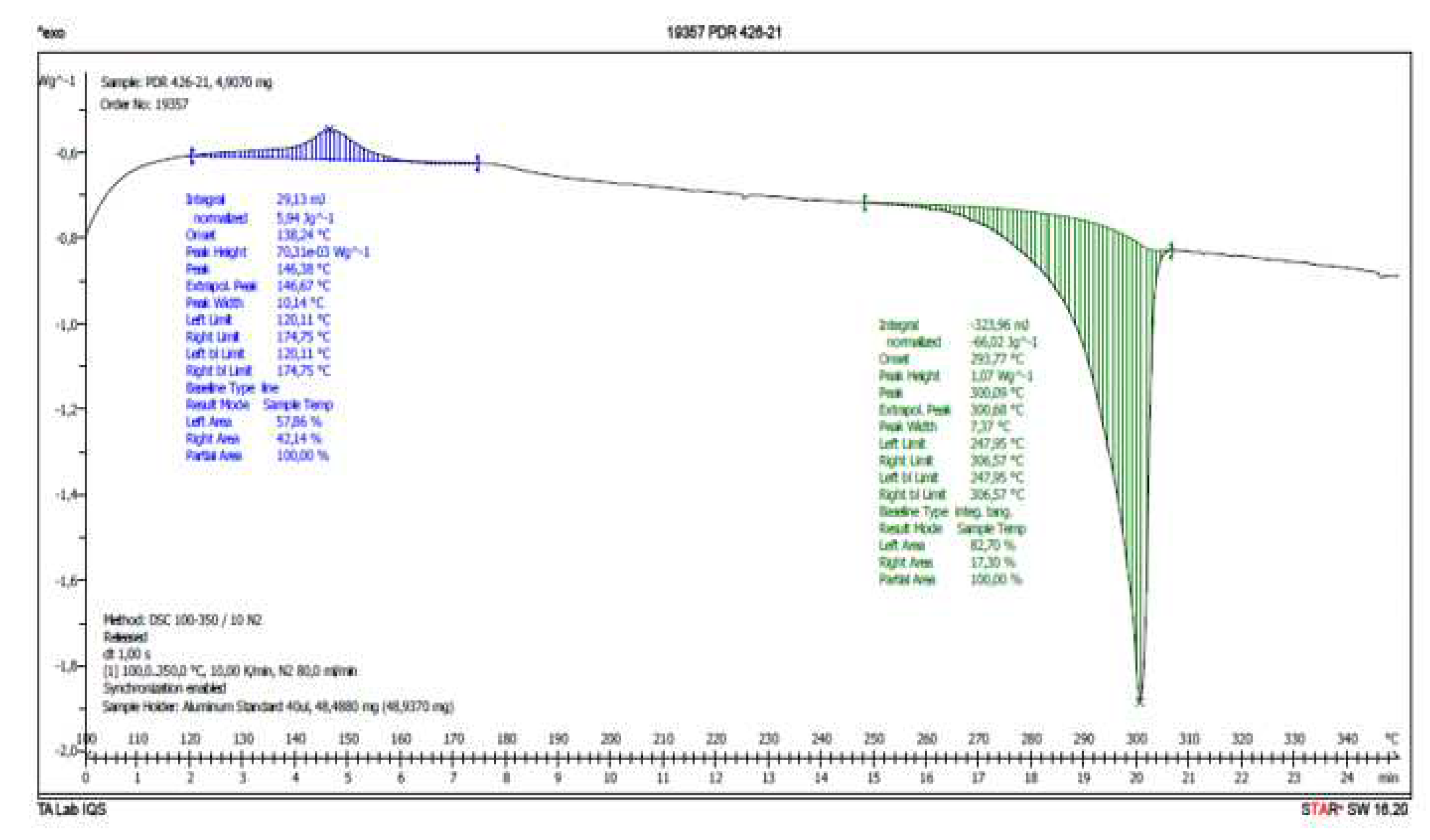
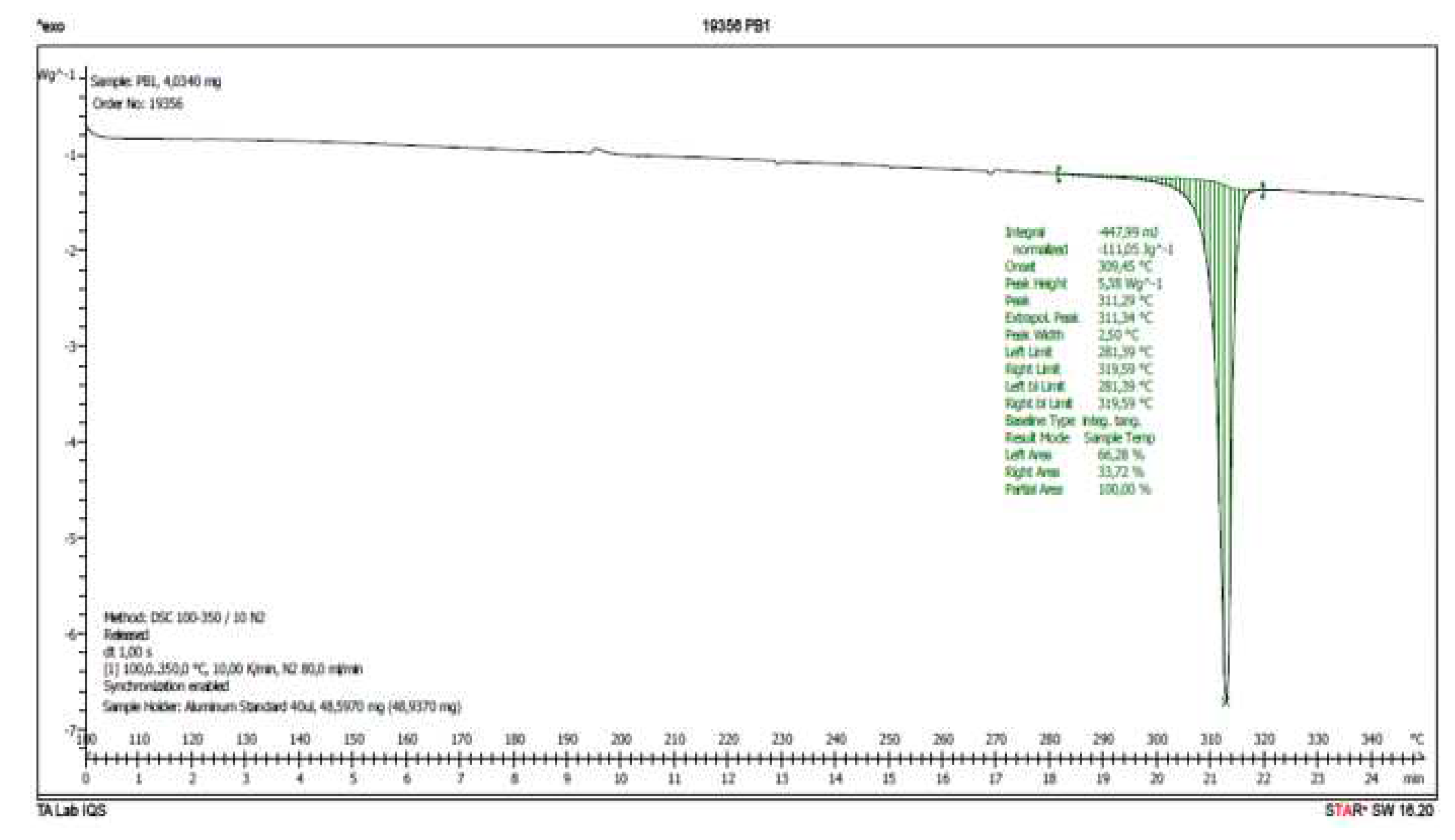
Therefore, a DSC analysis of samples
IQS016 and
PB1 was carried out.
Figure 6 and
Figure 7 present the evaluated records corresponding to these different samples.
The main results from the evaluated DSC plots are presented in
Table 1.
There is a difference of almost 16 degrees between the onset values and 11 degrees between the peak temperature values of IQS016 and PB1 samples. Consequently, considering that they are chemically identical substances, the two records can only correspond to two different solid forms of the same substance.
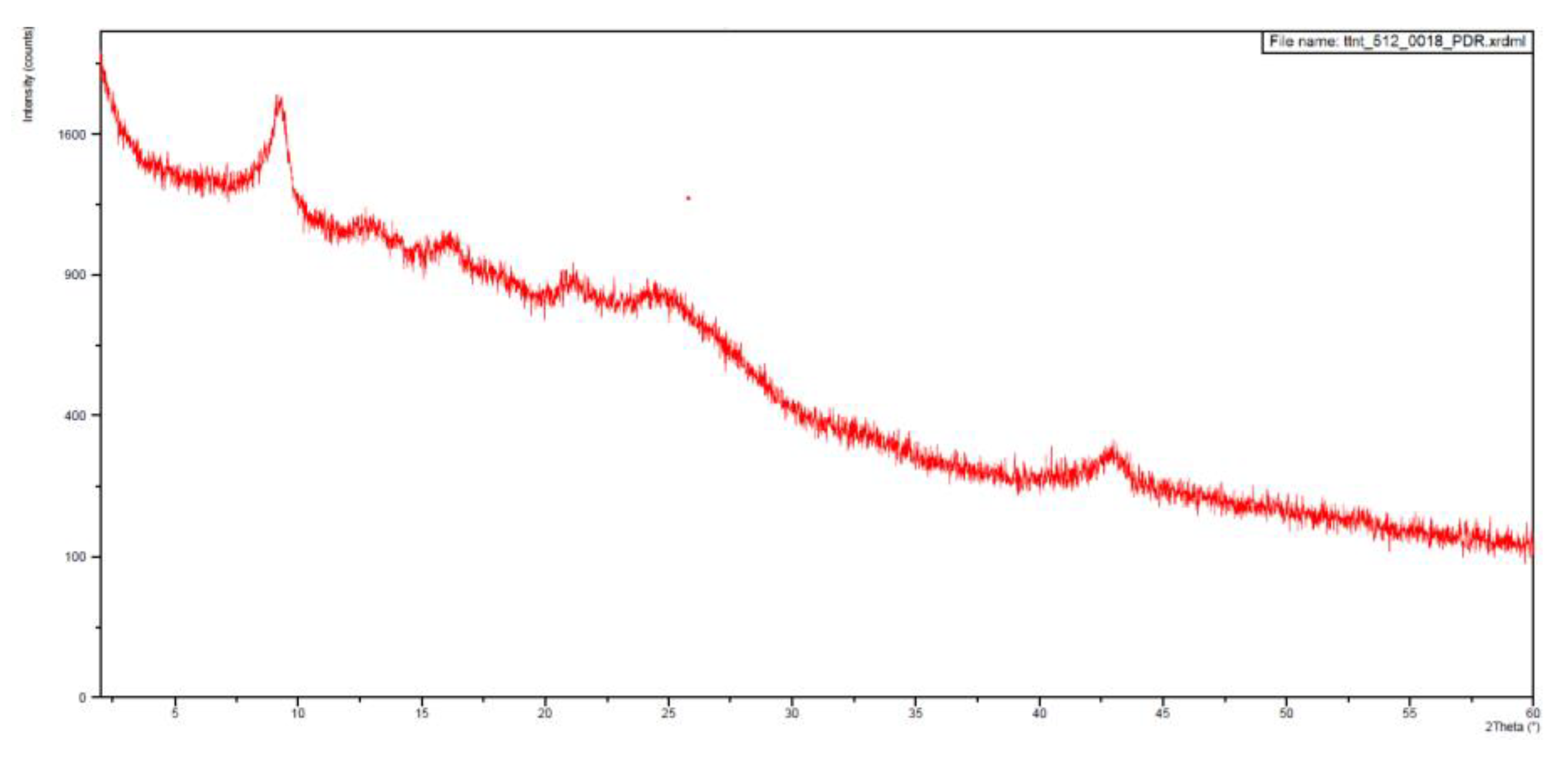
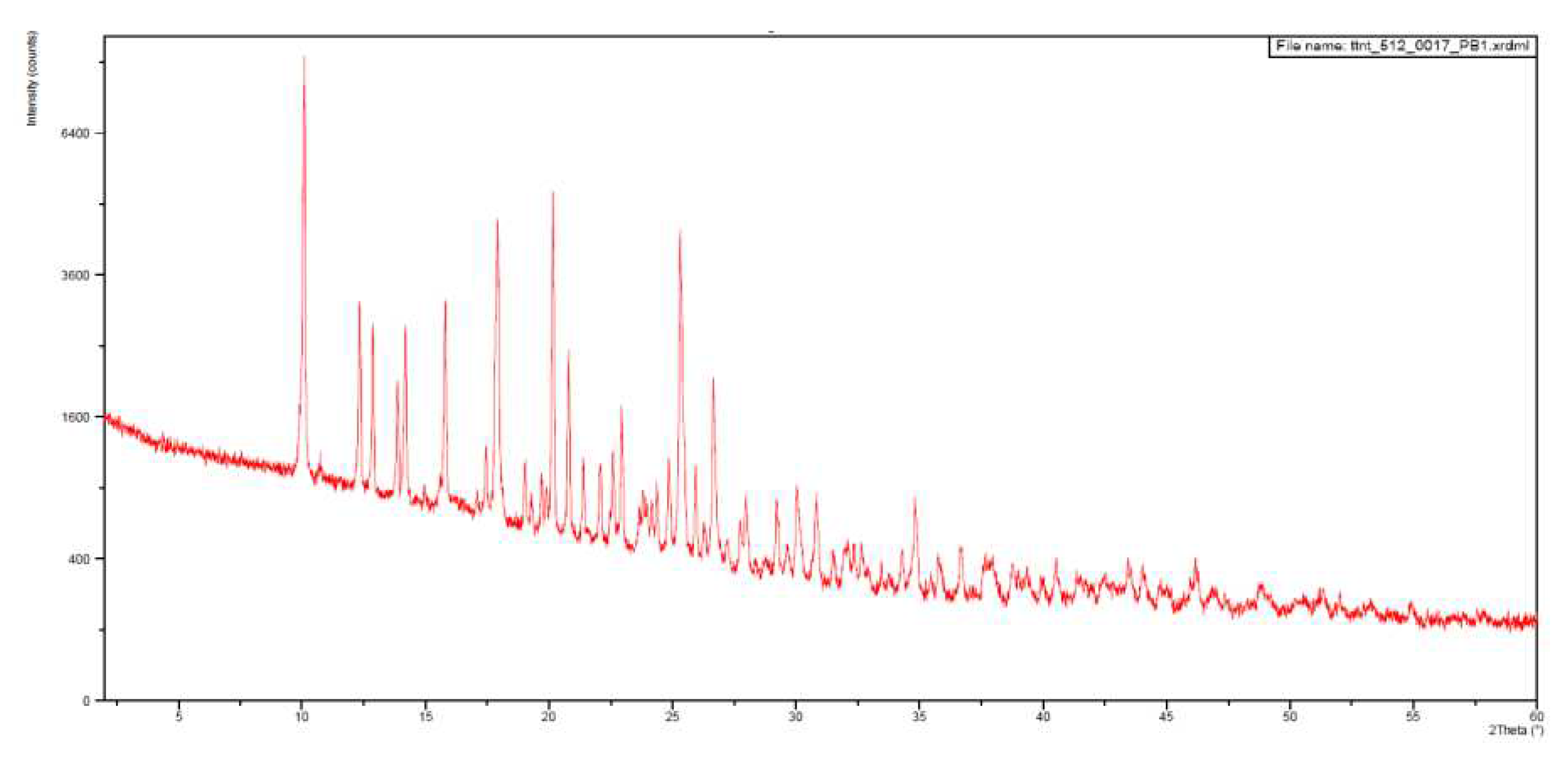
With this, somehow surprising, result in hand we decided to register the X-ray powder diffraction diagrams of both samples that are depicted in
Figure 8 and
Figure 9. The
IQS016 sample (
Figure 8) is mainly not microcrystalline, containing amorphous/partially crystalline/nanocrystalline phases. On the contrary, the
PB1 sample (
Figure 9) is microcrystalline as its X-ray powder diffractogram shows. That is to say, although both powders look quite similar,
PB1 presents a crystalline structure that could justify a lower solubility in organic solvents and water than
IQS016.
Subsequently, we decided to try to carry out the determination of the structure present in
PB1 from the X-ray powder diffraction data [
11] to confirm that such crystals contain compound
1. The powder X-ray diffractogram of
PB1 was perfectly indexed to an orthorhombic unit cell with unit cell parameters a = 18.63 Å, b = 17.49 Å and c = 12.46 Å and a volume of 4062.6 Å
3. The number of molecules in the unit cell was calculated to be Z = 8. The space group
Pbca was assigned based on the systematic absences and the subsequent Pawley pattern matching [
12] fitted very well with the experimental X-ray powder diffractogram, being the agreement factor of 2.63%. Its crystal structure was solved by using the Global Optimization Simulated Annealing approach integrated in Topas [
13,
14]. Some constraints were introduced, considering the molecule as a rigid body using the Z-matrix notation, which was allowed to rotate and translate in the three directions within the unit cell. Planar restrictions were applied to the aromatic rings and the phenyl and dichlorophenyl rings were allowed to rotate about two fixed points. A chemical sense solution with an agreement factor of 13.4% was obtained. The crystal structure so obtained was subsequently refined by the Rietveld method also using TOPAS v6 software, giving a satisfying result with a low Rwp value of 6.59 %. The final Pattern matching and Rietveld plots for the crystal structure refinement are shown in the supporting information.
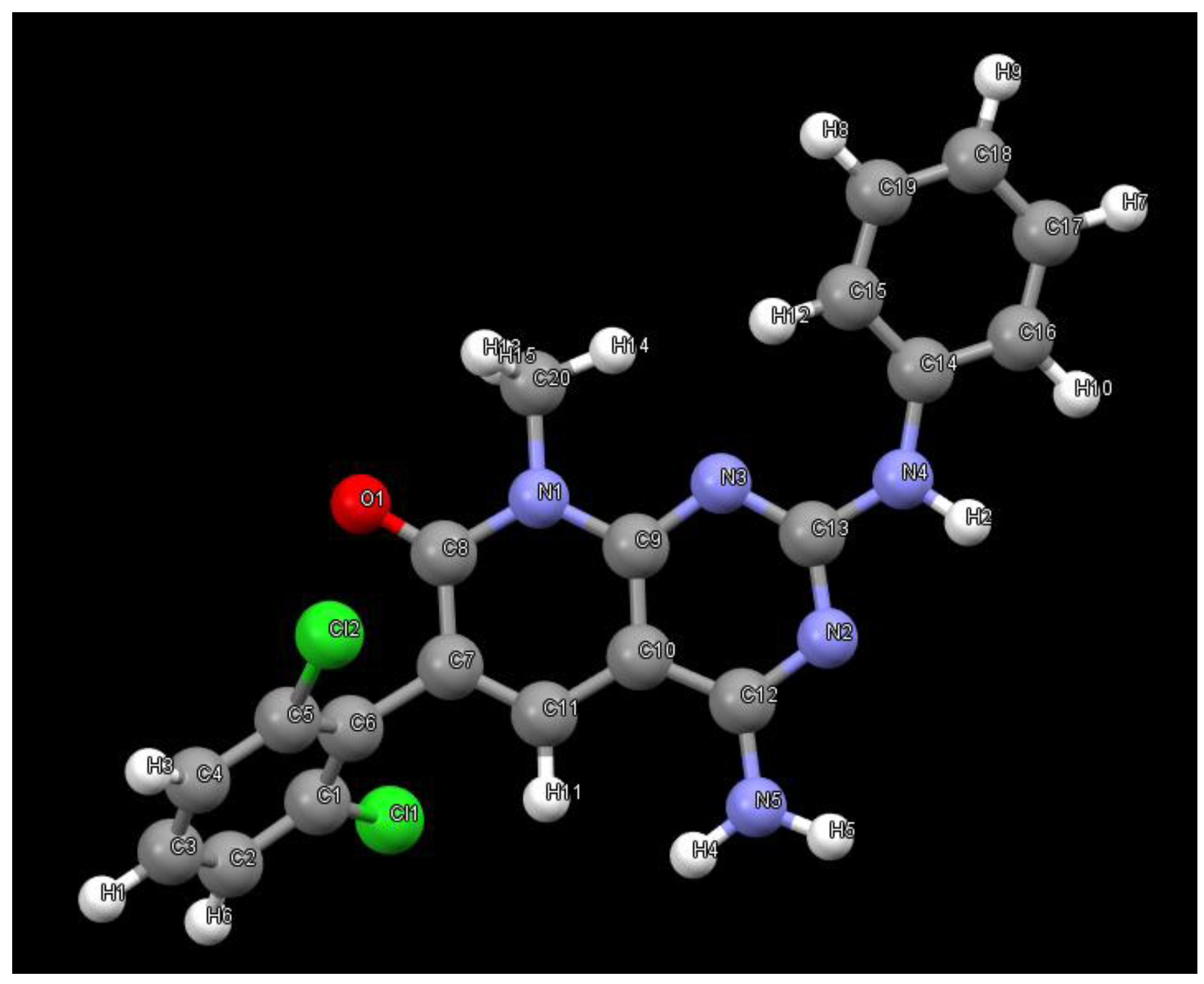
Figure 10 shows the structure present in the
PB1 sample determined from the powder X-ray diffraction data. As it can be seen the structure corresponds to the tyrosine kinase inhibitor
1 (
Figure 1).
Convergently, we decided to try to obtain single crystals from a sample of IQS016, which is more soluble than PB1, by using the evaporative crystallization technique. Approximately 4 mg of the IQS016 sample were dissolved in MeOH, acetone, and DMSO. Two replicates of each sample, one in a closed vial and the other open to air, were prepared. After one day, crystals appeared in the open samples in MeOH and acetone, and after one week in the open sample in DMSO. In no case, crystals were formed in the closed vials.
The crystal structure present in the crystals grown in MeOH and DMSO from the
IQS016 sample was determined by single-crystal X-ray diffraction.
1 crystallizes from MeOH in the orthorhombic space group
Pbca, with Z = 8 for the formula unit C
20H
15Cl
2N
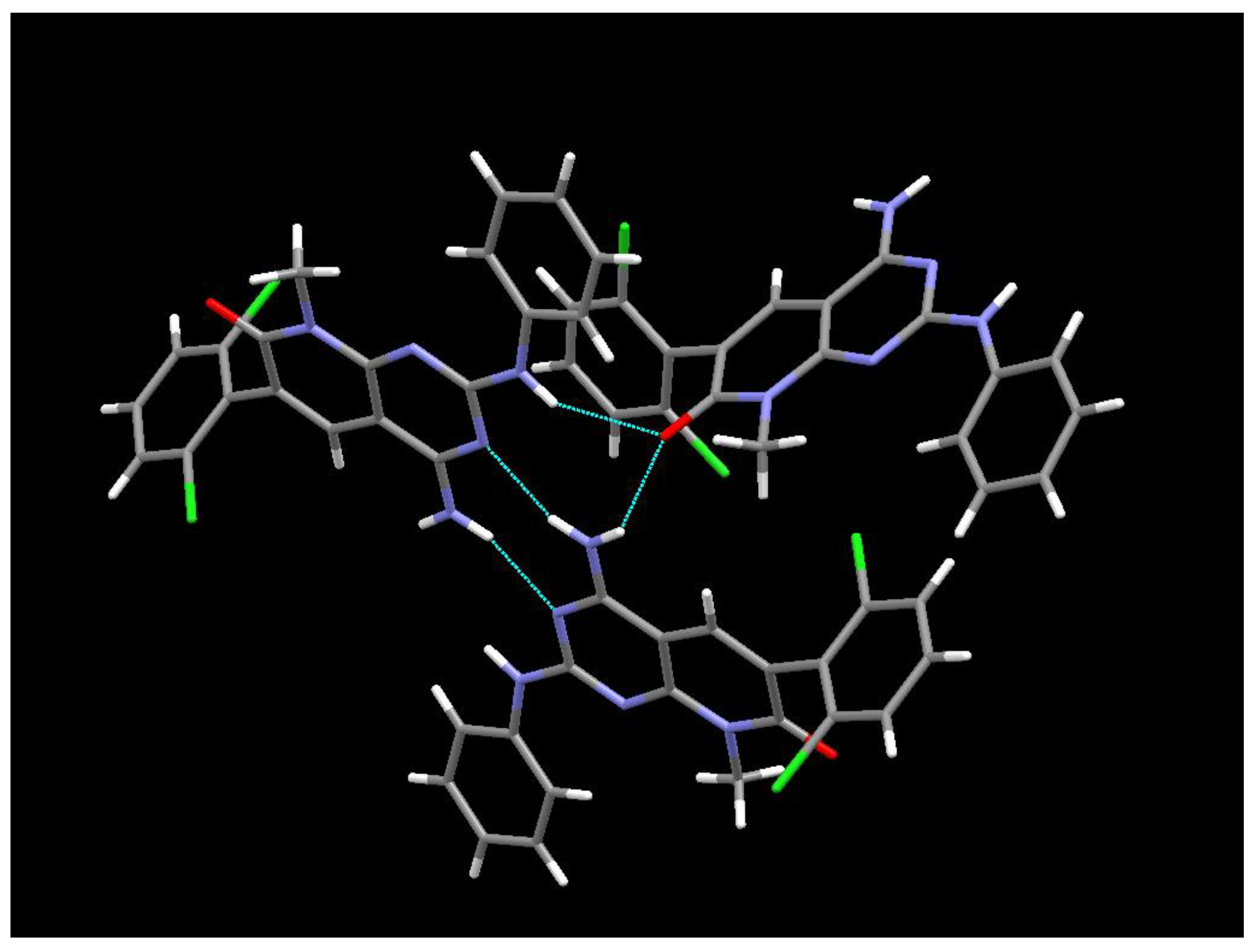
5O. The ORTEP diagram and atomic numbering together with the crystallographic data are summarized in supporting information. This structure is the same anhydrous form as the one obtained from powder X-ray diffraction data, however in this case it has been determined at 100K. The structure presents an arrangement of three hydrogen bonds between three molecules of
1 (
Figure 11) forming a R
23(8) supramolecular heterosynthon [
15]: between the C7 carbonyl group and N-H at C2 (2.27 Å), between the same carbonyl group and one of the hydrogens of the NH
2 group at C4 (2.14 Å), and between the second hydrogen of such NH
2 group at C4 and the pyrimidine ring nitrogen N3 (2.07 Å).
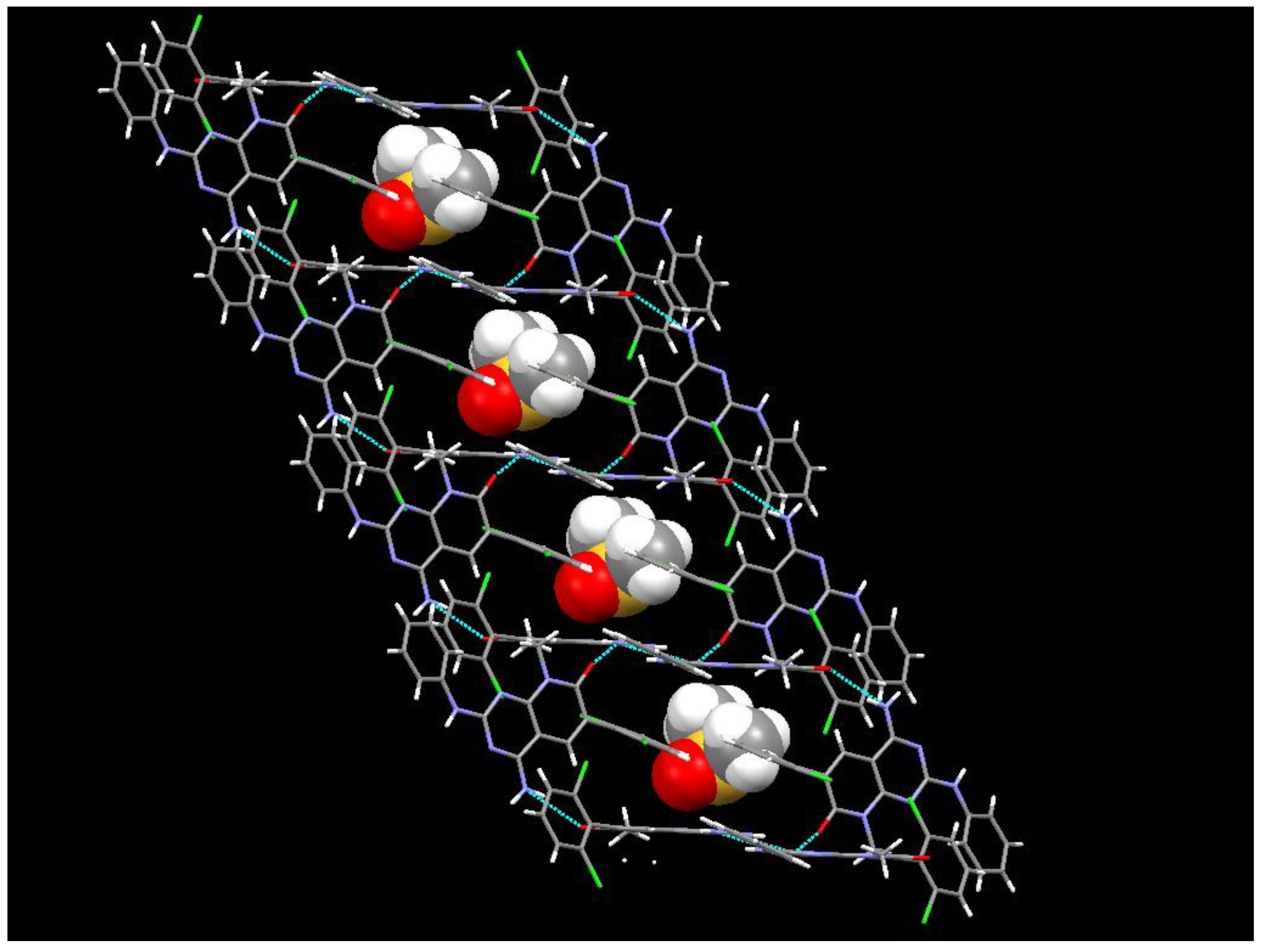
In the case of the crystals grown in DMSO, the single crystal X-ray diffraction analysis showed that compound
1 crystallizes in the monoclinic space group
C12/c1, with Z = 8 for the formula unit C
22H
21Cl
2N
5O
2S, thus confirming a 1:1 co-crystallization between
1 and DMSO. The ORTEP diagram and atomic numbering together with the crystallographic data are summarized in the supporting information. In such solvate, the DMSO, being one of the best hydrogen-bond acceptors, is not involved in any hydrogen bond with
1 and occupies the spaces between the
1 molecules (
Figure 12). In fact, the hydrogen bonds between the pyridopyrimidine molecules are the same as those present in the crystals grown from MeOH.
The different crystalline nature of the samples of the tyrosine kinase inhibitor 1 named IQS016 and PB1, the first amorphous and the second microcrystalline, can be the origin of the inconsistency observed in the results of the biological testing of both samples carried out at Reaction Biology. The test consists of a radiometric protein kinase assay (33PanQinase® Activity Assay) used for measuring the kinase inhibitory activity of the samples against the selected isolated kinases. The results are expressed as residual activity of the selected kinases after treatment with the indicated compounds at a certain µM concentration. Staurosporine is used as a positive control.
Reaction Biology offers two options for compound preparation and shipping to their facilities in Germany: as frozen DMSO stock solutions packed in dry ice or as solids. During our research in the field of tyrosine kinase inhibitors, we have routinely sent the compounds as solids but, probably, the crystalline less soluble form of PB1 has affected its results.
To prove such a hypothesis, we sent for evaluation three different samples: one solid sample of
IQS016, one solid sample of
PB1, and a sample of
PB1 dissolved in DMSO and frozen. For comparison purposes, we selected 4 tyrosine kinases of the whole set considered in our study on pancreatic cancer: EGFR (Epidermal Growth Factor Receptor), FGFR1 (Fibroblast Growth Factor Receptor 1), FGFR2 (Fibroblast Growth Factor Receptor 2), and VEGFR2 (Vascular Endothelial Growth Factor Receptor-2). The
PB1 samples were evaluated at two different concentrations, 0.5 and 10 µM, while the
IQS016 sample used as a reference was evaluated at 10 µM. The results obtained are summarized in
Table 2.
As can be seen in the table, while the solid sample of PB1 is almost inactive (only presents an intermediate activity for EGFR at 10 µM which is the kinase more sensible to compound 1), the activity of the PB1 sample sent dissolved in DMSO presents the same activity profile and similar residual activity values at 10 µM to those of IQS016 sent as a solid. So, we can conclude that the false negative obtained for the activity values of the PB1 sample observed and described at the beginning of this paper was due to the unexpected crystallinity and lower solubility of the PB1 sample.
Finally, concerning the origin of the crystallinity of the
PB1 sample, we must exclude that this is due to a different synthetic itinerary because both samples,
IQS016 and
PB1, were prepared using the same protocol described by our group [
5]. However, Applus
+ Laboratories introduced a difference in the final purification because while
IQS016 was purified using column chromatography and concentrated in vacuo to afford the corresponding solid,
PB1 was suspended in acetone at reflux and stirred for 30 min and then cooled at room temperature and filtered. Without any doubt, this disaggregation was responsible for the crystallinity of such a sample.
We consider that this example case is a good warning for organic and medicinal chemists working in the discovery phase of the possible impact of crystallinity and polymorphism during a phase of the research of a new drug in which such solid-state properties are not routinely considered. In our case, this experience has convinced us to send the compounds to Reaction Biology in a DMSO solution from now on.