Introduction
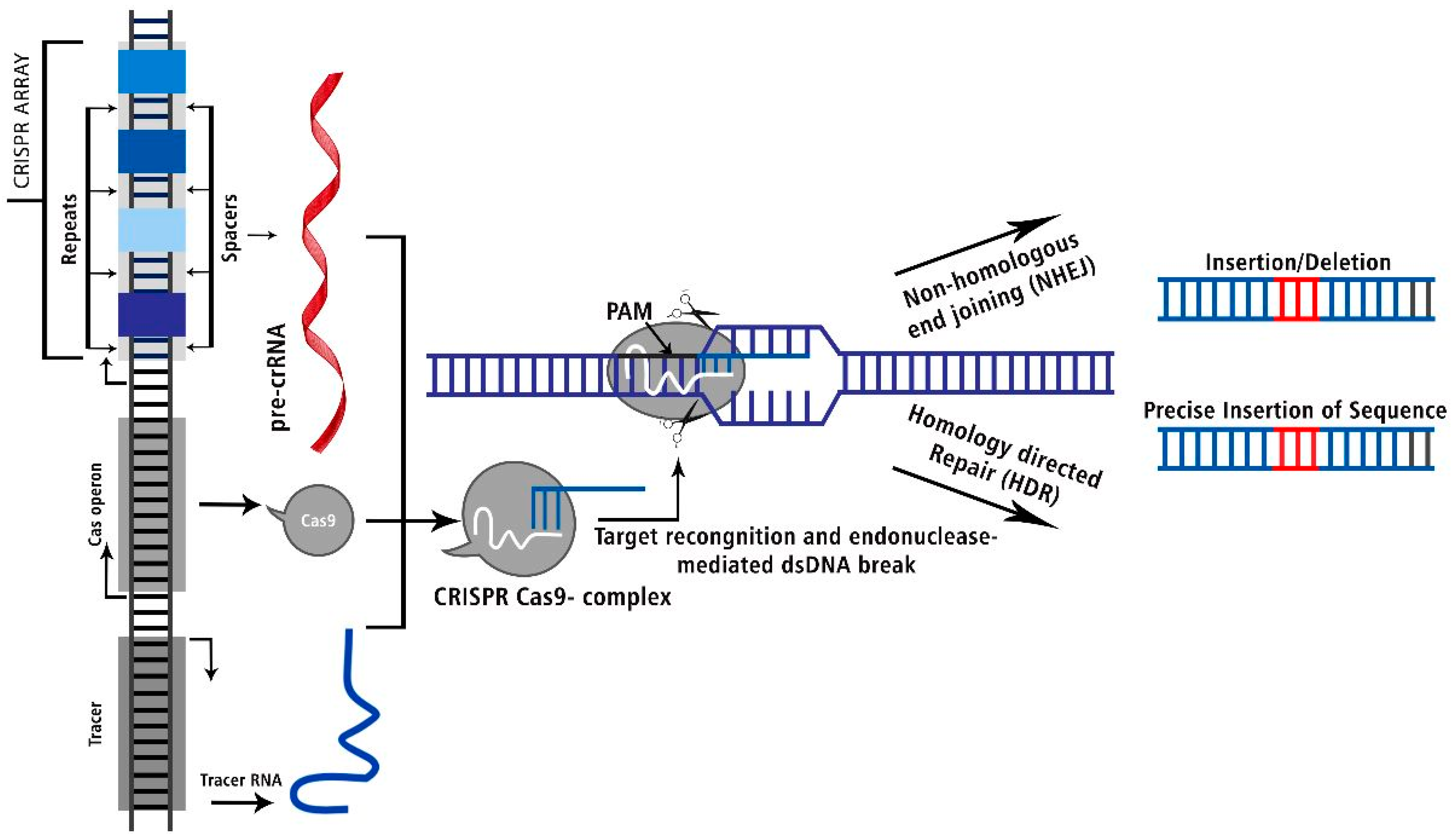
Researchers have made a lot of progress in the area of gene mapping since high-throughput sequencing methods and statistics have improved. Academics are now very interested in how genes affect traits at the genetic level. It used to be hard and take a long time to turn off or overexpress a gene in a living organism to find out what it did. In the last few decades, a new method called "genome editing" has been widely used and has helped a lot with the study of functional genomics, the creation of engineered species, and gene therapy. Genome editing is based on nucleases with a sequence-specific DNA-binding domain and a non-specific DNA-cutting domain. These nucleases are versatile, adaptable, and made in a way that makes them easy to use. The cell's DNA repair machinery makes the appropriate changes, such as insertions, deletions, and exchanges, once it finds the damaged spots. The brief method of insertion and/ or deletion of Non-Homologous End Joining (NHEJ) and Homology Direct Repair (HDR) sequences at targeted dsDNA is give in
Figure 1.
Scientists have made a number of fake nuclease systems so they can change DNA. The zinc-finger nuclease (ZFN) is a type of nuclease that is made in a lab and is used a lot. ZFNs have two parts in common: the DNA-binding Cys2-His2 domain and the DNA-cutting FokI restriction endonuclease domain. Use of transcription activator-like effector nucleases (TALENs), which are made from a protein made by the plant-pathogenic bacteria Xanthomonas, is another popular way to change the genome. In the part of TALENs that bind to DNA, there are 33–35 patterns of amino acids that are the same. All of these patterns work with different nucleotides. By moving around recognition patterns made up of a small number of amino acids, TALENs can be made to find a certain DNA sequence. The clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) protein 9 system is a new tool that can be used in place of ZFNs and TALENs. CRISPR/Cas9 uses short RNA to cut particular sequences of DNA. ZFNs and TALENs, on the other hand, use proteins to do the same thing. CRISPR/Cas9 is easy to use and has made a lot of progress in the last year because it only needs flexible RNA to make sequence-specificity. This article gives an overview of CRISPR/Cas9 at the molecular level and talks about its uses, limits, and possible uses in therapy.
CRISPR-Cas Genome Editing Technology
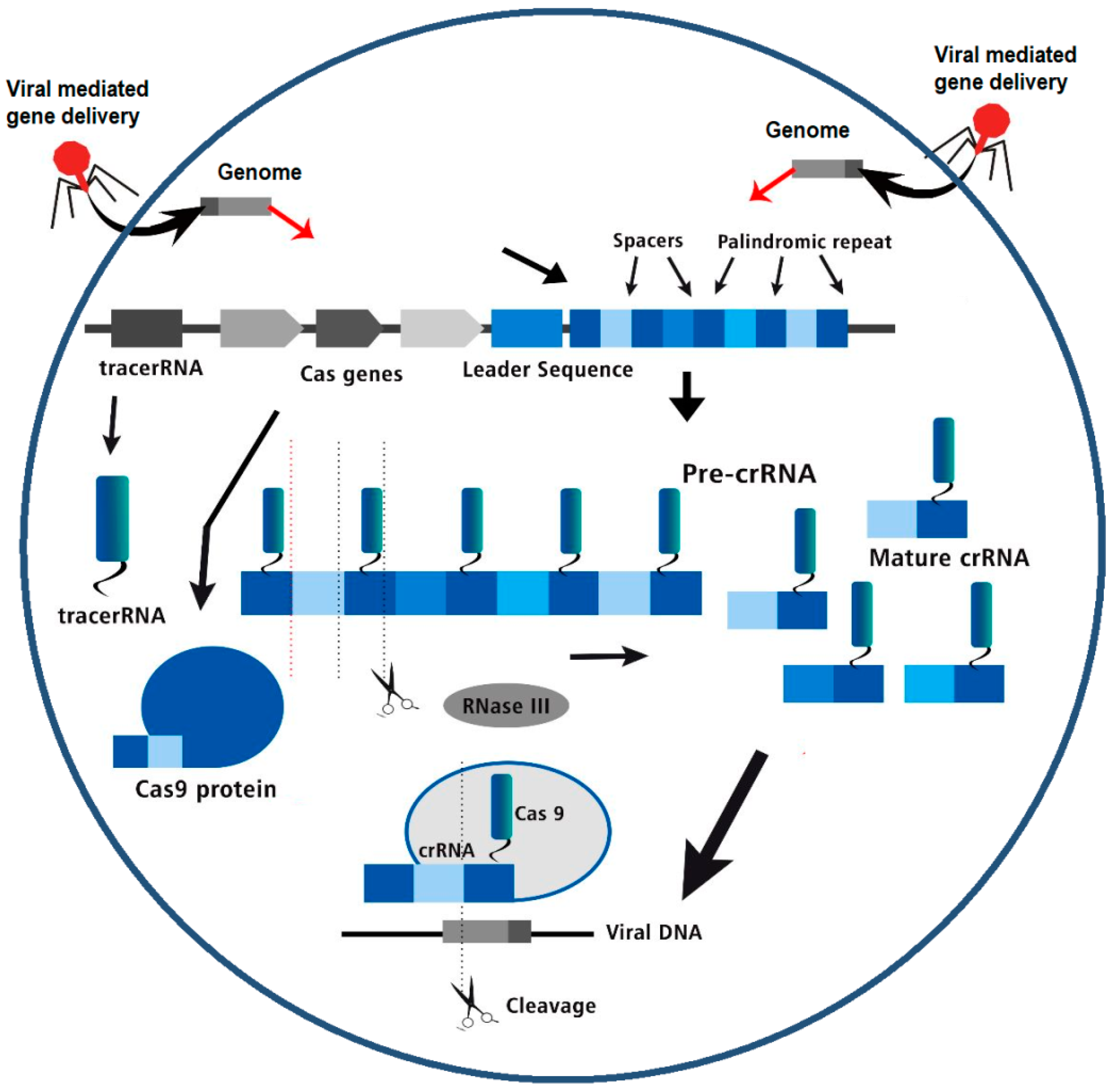
CRISPR/Cas is a learned defence system in bacteria and archaea that protects them from viruses and phages. It uses CRISPR RNA (crRNA) to find foreign DNA and Cas nucleases to cut it out. Almost 40% of bacterial genomes have CRISPR/Cas, and almost 90% of archaeal genomes do as well. The CRISPR locus has sequences that don't repeat, called spacers, that split the conserved repetitions (CRs). This event is shown in
Figure 1. Cas nuclease is an enzyme in the CRISPR/Cas system. It cuts up foreign DNA into pieces that can then be put into the CRISPR locus of the host. Cas uses these gaps as templates for transcription to make crRNA, which tells it to cut the DNA of the invading virus at the right places (
Figure 2 and
Figure 3). For crRNA production, gap closing, and DNA invasion, more than 40 different families of Cas proteins are needed. Most people divide CRISPR/Cas systems into Type I, Type II, and Type III based on how the Cas proteins are coded and how they look. For the type II CRISPR/Cas system, all you need is Cas9, which is a single Cas protein. Cas9 has both an HNH nuclease domain and a nuclease domain like the one in RuvC. The CRISPR/Cas9 method has been shown to be an easy way to change genomes that works well.
In order to change the genome with CRISPR/Cas9, a double-strand break (DSB) must be made and then fixed by the cell. Cas9 is guided to its target site by a tracrRNA:crRNA complex, which is made when mature crRNA joins with transactivating crRNA (tracrRNA) in the native CRISPR/Cas9 system. tracrRNA helps crRNA grow up because it is similar to crRNA in some ways. At the target site, there must be a short protospacer neighbouring motif (PAM) and a DNA sequence protospacer that matches the crRNA for sequence-specific cleavage to happen with CRISPR/Cas9. Cas9 binds to the target site, and then its HNH nuclease domain and RuvC-like nuclease domain cut the single-stranded DNA that fits the crRNA and the opposite strand, making a DSB. Researchers made a sensitive guide RNA (gRNA) that can be used to change the genome. This hybrid RNA had both crRNA and tracrRNA. There are many different kinds of CRISPR/Cas9, and each of them can find target sites with 2-4 nt PAM sequences and 20 or 24 nt gRNA sequences that match designed gRNA. Since most DNA patterns are between 22 and 29 bases long, CRISPR/Cas9 should only be able to target these. CRISPR/Cas9 is very forgiving of base pair mismatches between the guide RNA (gRNA) and its matching target sequence. However, the number, location, and spread of these mismatches may affect how well it works. It was shown that Streptococcus pyogenes' CRISPR/Cas9 could skip up to six base pair errors at target sites, for example.
DNA double-strand breaks (DSBs) caused by CRISPR/Cas9 drive cells to start DNA repair pathways like the wrong nonhomologous end-joining (NHEJ) and the right homology-directed repair (HDR). Non-homologous end-joining (NHEJ) is a fast way to fix DSBs, but it can cause changes like insertions and deletions that aren't what you want. With these changes, it is possible to turn off certain genes or parts of the genome. Gratz et al. used CRISPR/Cas9 to break DNA in the yellow part of the Drosophila genome. They then used NHEJ-mediated DNA repair to make frame-shifting indels. The HDR-mediated mechanism is more complicated than the easier NHEJ-mediated mechanism. It is activated when DNA is damaged. A identical source DNA sequence is used as a repair template for HDR-mediated error-free DNA repair. Gratz et al. replaced the yellow locus in the Drosophila genome with a 50 nt attP recombination site. They did this by injecting Cas9 with two gRNAs that target the 5′ and 3′ parts of the yellow locus and a single-strand oligodeoxynucleotide template.
CRISPR/Cas9 is better than ZFNs and TALENs in a number of ways. Creating, choosing, and testing proteins is a time-consuming and labor-intensive process that is necessary for ZFNs and TALENs because they use protein-guided DNA breaks. But CRISPR/Cas9 can change many parts of the genome at once by mixing Cas9 with multiple gRNAs that each have a different target site. These gRNAs are useful because they are easy to change and don't cost much to make. If we can quickly make transgenic mice with several changed genes, we might learn more about how genes work and how they talk to each other.
Applications
Genome Editing
Researchers can use CRISPR/Cas9 to make exact changes to certain parts of the genome. This helps them figure out what role target genes play in biology and disease. By delivering plasmids with Cas9 and crRNA together, CRISPR/Cas9 has been used to make exact changes to the human genome. By putting together several different gRNA and Cas9 in a CRISPR array, CRISPR/Cas9 can cause several mutations in the DNA of mammals at the same time. CRISPR/Cas9 is useful for changing human genomes, but it also works in zebrafish, mice, flies, worms Bombyx mori, and bacteria. For example, Bassett et al. showed a modified RNA injection-based CRISPR/Cas9 system that was very successful at causing targeted mutations in the Drosophila genome. By introducing Cas9 mRNA and gRNA directly into the embryo of flies, they were able to cause mutations at the target sites in as many as 88% of the flies. In 33% of the population, the changes that were made were passed on in a stable way through the germline.
CRISPR/Cas9 can be used to change the genomes of plants to give them desired traits or make them resistant to diseases. Jiang et al. put the green glow protein gene into the Arabidopsis and tobacco genomes and the bacterial blight resistance gene into the rice genome to prove that CRISPR/Cas9 can be used in plants. Miao et al. (56) showed that CRISPR/Cas9 works and can be used to change the rice genome. In the future, CRISPR/Cas9 could be used as a new way to improve food quality by changing the genomes of crops.
Transcription Regulation
Gene function and transcriptional network studies can learn a lot from knowing how gene transcription is controlled in living cells. By removing functional regions that are linked to transcription, CRISPR/Cas9 can be used to control the transcription of certain genes. Changes to the DNA make this process last forever and make it impossible to stop. CRISPR inference, or CRISPRi, is an updated version of the CRISPR/Cas9 system for RNA-guided control of transcription. Qi et al. made dCas9, which can't cut DNA because it doesn't have the power to act as a catalyst. To stop transcriptional elongation, RNA polymerase, and transcription factor binding, dCas9 was co-expressed with gRNA to make a recognition complex. Qi et al. found that when two different gRNAs were used to target the RFP and GFP genes, CRISPRi could stop the production of both RFP and GFP without any crosstalk in Escherichia coli. But CRISPRi was not very effective at stopping gene production in mammalian cells. By adding repressing or activating effector domains to dCas9, Gilbert et al. were able to use it with gRNA to control the transcription of target genes in an exact and stable way. In their study, Chen et al. showed how well CRISPRi works to control the activation of many genes, either on their own or together. CRISPRi is a unique and very specific way to change gene expression without making any lasting changes to the target DNA sequence.
Gene Therapy
Genome editing could cure diseases for good by getting rid of or changing bad genes or by adding new safe genes. Urnov et al. were able to fix a gene defect that caused disease in human cells by using HDR made by ZFNs. After that, ZFNs were used to fix gene defects that caused sickle-cell disease and haemophilia B ZFNs have been used to make immunotherapies work better and to make human cells more resistant to virus attack by turning off genes that make viruses dangerous or adding genes that make them less dangerous. CRISPR/Cas9 is a new way to edit the genome that is very effective and can be used in studies on gene therapy. It is the latest generation of designed nucleases. Ebina et al. used CRISPR/Cas9 to damage the long-terminal repeat promoter of the HIV-1 genome. This made HIV-1 less likely to be expressed in human cells that were infected with HIV. CRISPR/Cas9 can also get rid of viral proviral genes that have become part of the DNA of host cells.
Scientists use nucleases that they have made to change the genome of induced pluripotent stem (iPS) cells. iPS cells are very useful for disease modelling and gene therapy because they can keep dividing and changing into different types of cells forever. Horri et al. used CRISPR/Cas9 to make an iPS cell model for a disease called immunodeficiency, centromeric region instability, and facial abnormalities (ICF), which is caused by a mutation in the DNMT3B gene. In this study, plasmids that coded for Cas9 and gRNA were put into modified iPS cells to stop DNMT3B from working. Ding et al. tested how well CRISPR/Cas9 and TALENs worked to change the DNA of iPS cells by using the same hPSC lines and delivery method. Researchers found that CRISPR/Cas9 worked better than TALENs. But there is still a long way to go before CRISPR/Cas9 can be used in gene therapy. CRISPR/Cas9 must be very precise to avoid making harmful changes in places it isn't supposed to. Target site selection, gRNA design, and looking for off-target sites across the whole genome all take a lot of work.
Challenges
There are still a lot of big problems to solve before CRISPR/Cas9 can fully live up to its promise in genome editing. These problems include off-target errors, PAM dependence, making gRNAs, and figuring out how to deliver them.
Off-Target Mutations
Off-target mutations are a big worry about changing the genome with CRISPR/Cas9. When it comes to human cells, CRISPR/Cas9 is more likely to cause off-target changes than ZFNs or TALENs. There may be multiple DNA sequences in a big genome that are the same as or very similar to the DNA sequences that are being looked for. When CRISPR/Cas9 cuts complementary or very similar DNA sequences instead of the target DNA sequence, this can lead to off-target changes. A mutation that wasn't meant to happen could cause a cell to die or change. In order to make CRISPR/Cas9 less harmful to cells, researchers are putting more effort into getting rid of its off-target changes. To make sure CRISPR/Cas9 is precise, it is best to choose target sites with fewer off-target sites and mismatches between the gRNA and its matching sequence. CasOT, which was made by Xiao et al., is a flexible search engine that can find possible off-target sites in whole genomes. The amount of CRISPR/Cas9 used can also affect off-target changes, so it's important to keep an eye on that. It looked like methylation of target DNA sequences didn't change the selectivity of CRISPR/Cas9. Once Cas9 is changed into nickase, the on-target cleavage of CRISPR/Cas9 still works well, and changes that happen off-target happen less often.
PAM Dependence
In theory, CRISPR/Cas9 could be used on any DNA pattern with the right programmable gRNA. But for CRISPR/Cas9 to be specific, the gRNA and target sequences must also match, and a PAM sequence of 2–5 nucleotides must be right after the target sequence. For example, Streptococcus pyogenes has a PAM sequence called NGG, Streptococcus thermophiles has PAM sequences called NGGNG and NNAGAAW, and Neisseria meningitidis has a PAM sequence called NNNNGATT. Hsu et al. recently wrote about a NAG PAM that only guided DNA cleavage about 20% as well as NGG PAM. But the number of genetic targets is limited by the way CRISPR/Cas9 cuts DNA, which depends on PAM. In NGG PAM and NAG PAM, for example, there is a target site every 8 nt, but in NGGNG PAM and NNAGAAW PAM, the numbers are 32 and 256, respectively. On the other hand, PAM dependency makes CRISPR/Cas9 more specialised. With a longer PAM, CRISPR/Cas9 should cause fewer off-target changes than with a shorter PAM.
gRNA Production
The creation of gRNA is another important task for editing the genome with CRISPR/Cas9. At the moment, it is hard to use RNA polymerase II to make gRNA because the mRNA that is copied by RNA polymerase II goes through a lot of processing and changes after it is copied. Right now, RNA polymerase III uses the U3 and U6 snRNA regulators to make gRNA in living cells. On the other hand, the U3 and U6 snRNA genes are housekeeping genes that are expressed everywhere. This means that they can't be used to make gRNA that is special to certain tissues or cells. The lack of widely available RNA polymerase III limits how useful U3- and U6-based gRNA synthesis can be. Gao et al. made an artificial gene called RGR. The mRNA they made had the right gRNA and ribozyme sequences at both ends of the gRNA. In vitro and yeast self-cleavage were used to make mature gRNAs, and they caused sequence-specific cleavage.
Delivery Methods
There are also questions about the best way to put CRISPR/Cas9 into organisms that haven't been solved. For example, DNA and RNA injections can be used to give CRISPR/Cas9 by injecting plasmids that make Cas9 and gRNA (30) or by injecting CRISPR components as RNA. Methods of delivery can be more or less effective based on the cells and tissues that are being targeted. CRISPR/Cas9 delivery methods that are new and strong need more time for study and development.
Conclusion
Genome editing was first used on the fruit fly Drosophila melanogaster. Since then, it has been used on many different kinds of species. The best tool for changing the genome would have a simple, fast, and cheap set of nucleases that could target any part of the genome without making any changes that weren't meant to happen. After some problems are fixed, CRISPR/Cas9 could become a reliable and easy-to-use tool for changing the genome. The versatility and ease of use of CRISPR/Cas9 make it possible to study how genes work in biology and fix genetic problems. The specificity, off-target effects, and delivery methods of CRISPR/Cas9, as well as other parts of the system, need more study. The results of recent deep genome sequencing, for example, will help choose the right target sites and make gRNA that is very particular.
Author Contributions
This work was carried out in collaboration among all authors. Taha Nazir designed the study of proposed hypothesis and compile the scientific contents. Nida Taha elaborated study to make it more credible. Whereas, Hameed A Mirza managed the literature searches and citation part of the manuscript. Thus, all authors have read and approved the final manuscript for publication in this journal.
Funding
This project is not-funded from any local and/ or international organization.
Data Availability Statement
All study information and possible research data successfully incorporated for publication.
Acknowledgments
We acknowledge the technical and scientific support of, 1. Advanced Multiple Inc., Mississauga ON, L5T2M9 Canada. 2. A.S. Chemical Laboratories Inc., Concord, ON L4K4M4 Canada.
Conflicts of Interest
The authors also declare that they are no any potential and/ or completing conflict of interest.
Ethical Approval and Consent to participate
All procedures performed in studies are not involving human participants. Therefore there is no need of the ethical approval of the institutional and/or national research committee and 1964 Helsinki declaration and its later amendments or comparable ethical standards. For type of studies no formal consent is required.
Animal Rights
Additionally, this research studies no animals involved. The authors indicate the procedures followed are in accordance with the standards set forth in the eighth edition of Guide for the Care and Use of Laboratory Animals; published by the National Academy of Sciences, The National Academies Press, Washington, D.C.).
Consent for publication
Authors agree and grant consent to publish this article in this research journal.
References
- Abudayyeh, O.O. et al. (2019) A cytosine deaminase for programmable single-base RNA editing. Science 365, 382–386. [CrossRef]
- Billon, P. et al. (2017) CRISPR-mediated base editing enables efficient disruption of eukaryotic genes through induction of STOP codons. Mol. Cell 67, 1068–1079. [CrossRef]
- Biotechnol. J. 16, 1918–1927 Okada, A. et al. (2019) CRISPR/Cas9-mediated knockout of Ms1 enables the rapid generation of male-sterile hexaploid wheat lines for use in hybrid seed production. Plant Biotechnol. 17, 1905–1913. [CrossRef]
- Braun, C.J. et al. (2016) Versatile in vivo regulation of tumor phenotypes by dCas9-mediated transcriptional perturbation. Proc. Natl. Acad. Sci. U. S. A. 113, E3892–E3900. [CrossRef]
- Cai, Y. et al. (2018) CRISPR/Cas9-mediated targeted mutagenesis of GmFT2a delays flowering time in soya bean. Plant Biotechnol. J. 16, 176–185.
- Chandrasekaran, J. et al. (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 17, 1140–1153. [CrossRef]
- Li, J. et al. (2018) Whole genome sequencing reveals rare off-target mutations and considerable inherent genetic or/and somaclonal variations in CRISPR/ Cas9-edited cotton plants. Plant Biotechnol. J. 17, 858–868. [CrossRef]
- Li, J.F. et al. (2013) Multiplex and homologous recombination–mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat. Biotechnol. 31, 688–691. [CrossRef]
- Li, X. et al. (2018) Base editing with a Cpf1-cytidine deaminase fusion. Nat. Biotechnol. 36, 324–327.
- Li, Z. et al. (2015) Cas9-guide RNA directed genome editing in soybean. Plant Physiol. 169, 960–970. [CrossRef]
- Li, Z. et al. (2017) A potent Cas9-derived gene activator for plant and mammalian cells. Nat. Plants 3, 930–936. [CrossRef]
- Liang, Z. et al. (2017) Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nat. Commun. 8, 14261. [CrossRef]
- Liu, L. et al. (2017) C2c1-sgRNA complex structure reveals RNA-guided DNA cleavage mechanism. Mol. Cell 65, 310–322. [CrossRef]
- Liu, Q. et al. (2018) Hi-TOM: a platform for high[1]throughput tracking of mutations induced by CRISPR/Cas systems. Sci. China Life Sci. 62, 1–7. [CrossRef]
- Liu, X. et al. (2017) Application of CRISPR/Cas9 in plant biology. Acta Pharm. Sin. B 7, 292–302. [CrossRef]
- Odipio, J. et al. (2017) Efficient CRISPR/Cas9 genome editing of phytoene desaturase in cassava. Front. Plant Sci. 8, 1780. [CrossRef]
- Ordon, J. et al. (2017) Generation of chromosomal deletions in dicotyledonous plants employing a user-friendly genome editing toolkit. Plant J. 89,155–168. [CrossRef]
- Ortigosa, A. et al. (2019) Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol. J. 17, 665–673.
- Pan, C. et al. (2016) CRISPR/Cas9-mediated efficient and heritable targeted mutagenesis in tomato plants in the first and later generations. Sci. Rep. 6, 24765. [CrossRef]
- Peng, A. et al. (2017) Engineering canker-resistant plants through CRISPR/Cas9-targeted editing of the susceptibility gene CsLOB1 promoter in citrus. Plant Biotechnol. J. 15, 1509–1519.
- Ren, B. et al. (2019) Cas9-NG greatly expands the targeting scope of the genome-editing toolkit by recognizing NG and other atypical PAMs in rice. Mol. Plant 12, 1015–1026. [CrossRef]
- Safari, F. et al. (2019) CRISPR Cpf1 proteins: structure, function and implications for genome editing. Cell Biosci 9, 36. [CrossRef]
- Schaeffer, S.M. and Nakata, P.A. (2015) CRISPR/ Cas9-mediated genome editing and gene replacement in plants: transitioning from lab to field. Plant Sci. 240, 130–142. [CrossRef]
- Scheben, A. et al. (2017) Towards CRISPR/Cas crops–bringing together genomics and genome editing. New Phytol. 216, 682–698.
- Schiml, S. et al. (2016) Repair of adjacent single[1]strand breaks is often accompanied by the formation of tandem sequence duplications in plant genomes. Proc. Natl. Acad. Sci. U. S. A. 113, 7266– 7271. [CrossRef]
- Tian, S. et al. (2017) Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 36, 399–406. [CrossRef]
- Ueta, R. et al. (2017) Rapid breeding of parthenocarpic tomato plants using CRISPR/Cas9. Sci. Rep. 7, 507. [CrossRef]
- Veres, A. et al. (2014) Low incidence of off-target mutations in individual CRISPR-Cas9 and TALEN targeted human stem cell clones detected by whole-genome sequencing. Cell Stem Cell 15, 27–30. [CrossRef]
- Wang, C. et al. (2019) Clonal seeds from hybrid rice by simultaneous genome engineering of meiosis and fertilization genes. Nat Biotechnol. 37, 283–286. [CrossRef]
- Wang, J. et al. (2018) xCas9 expands the scope of genome editing with reduced efficiency in rice. Plant Biotechnol. J. 17, 709–711. [CrossRef]
- Charpentier, E. (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. [CrossRef]
- Yang, H. et al. (2017) CRISPR/Cas9-mediated genome editing efficiently creates specific mutations at multiple loci using one sgRNA in Brassica napus. Sci. Rep. 7, 7489. [CrossRef]
- Yin, K. et al. (2017) Progress and prospects in plant genome editing. Nat. Plants 3, 17107. [CrossRef]
- Zaidi, S.S. et al. (2017) CRISPR-Cpf1: a new tool for plant genome editing. Trends Plant Sci. 22, 550–553. [CrossRef]
- Zischewski, J. et al. (2017) Detection of on-target and off-target mutations generated by CRISPR/ Cas9 and other sequence-specific nucleases. Biotechnol. Adv. 35, 95–104. [CrossRef]
- Zong, Y. et al. (2017) Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 35, 438–440. [CrossRef]
- Zong, Y. et al. (2018) Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 36, 950–953. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).