Submitted:
27 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
Introduction
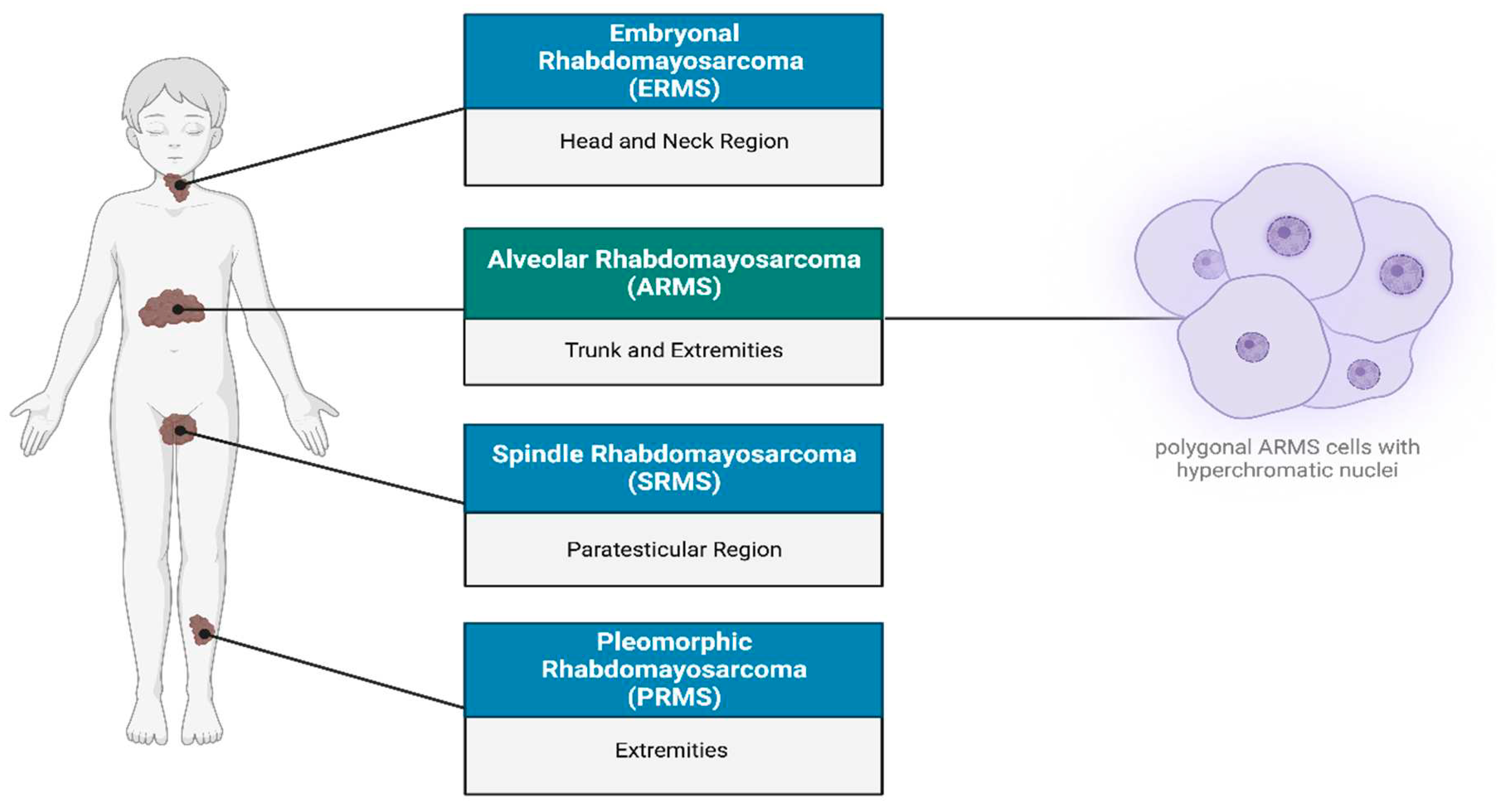
Rhabdomyosarcoma overview
Tumour Growth and Differentiation in ARMS
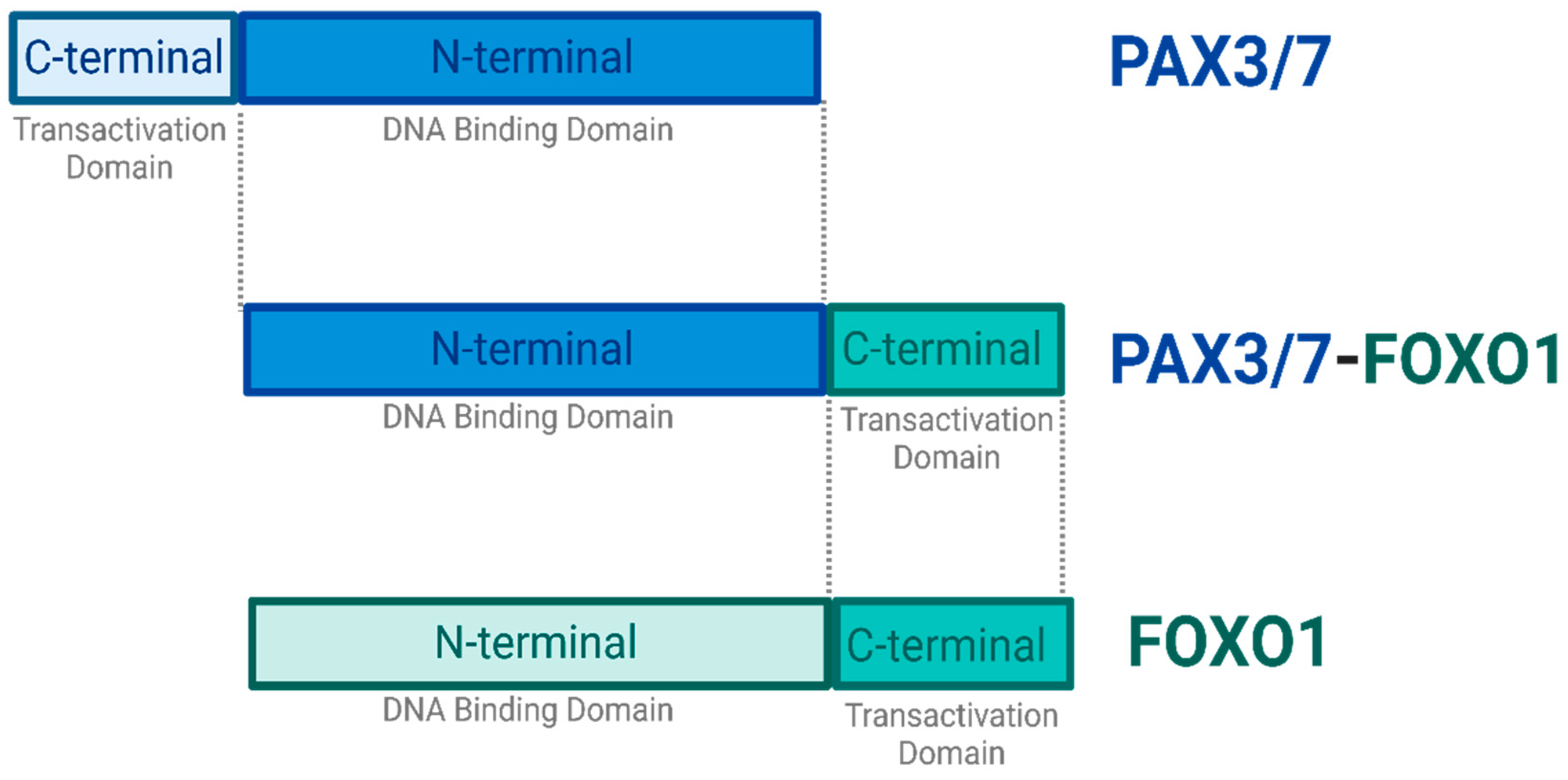
PAX3/7 and FOXO1
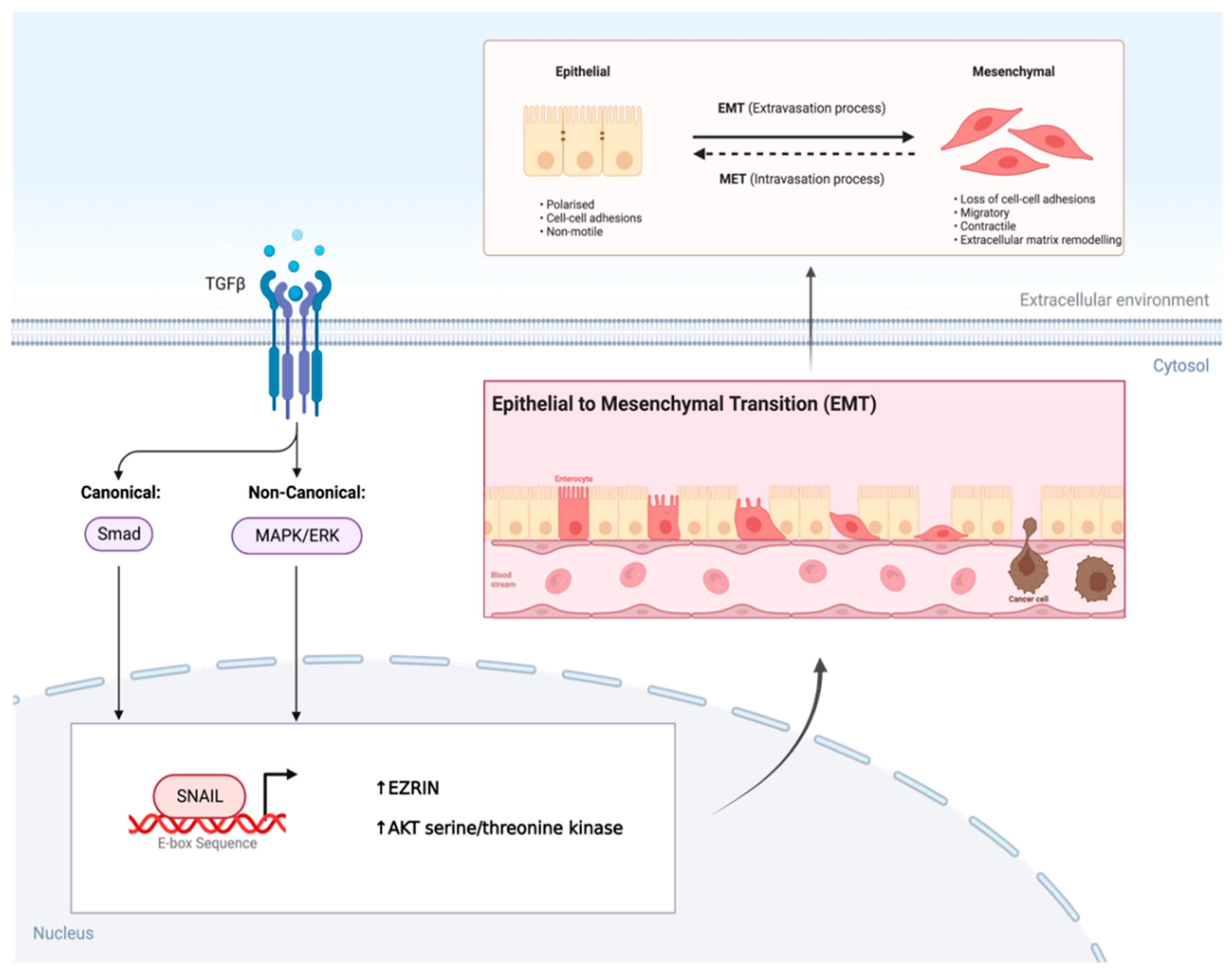
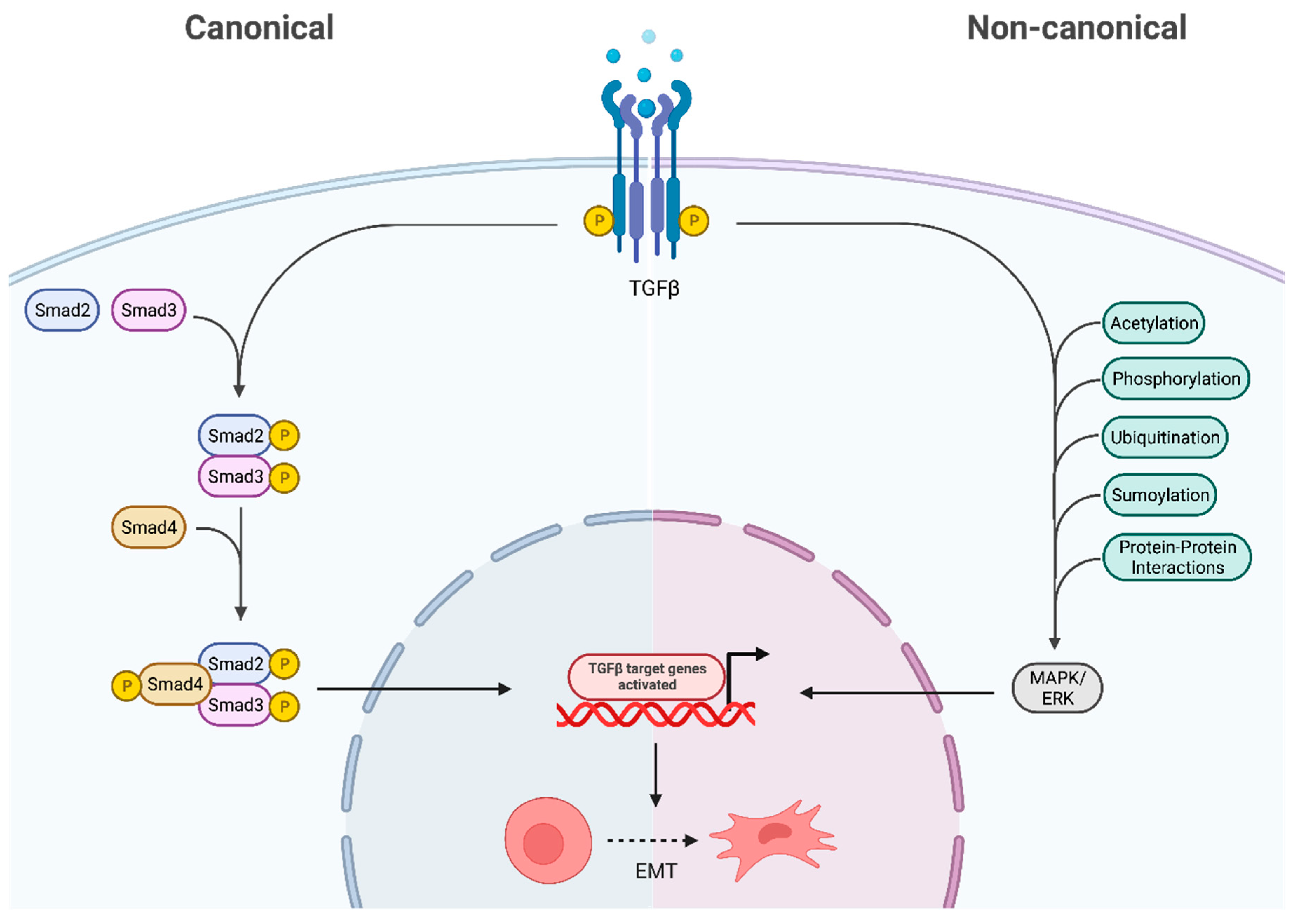
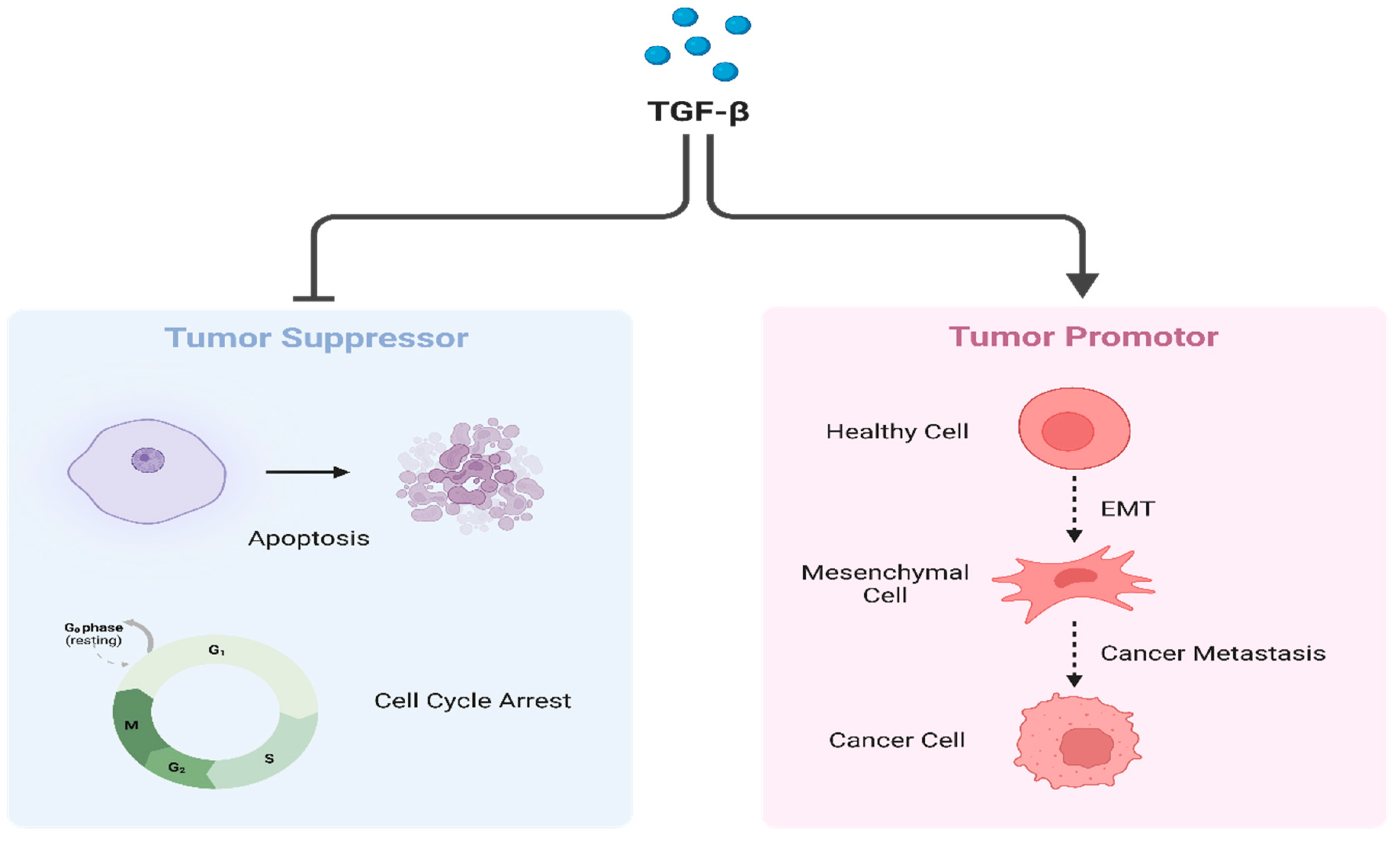
Transforming Growth Factor-β
ARMS Chemotherapy Drugs and Their Impact in the Tumor Cells Differentiation
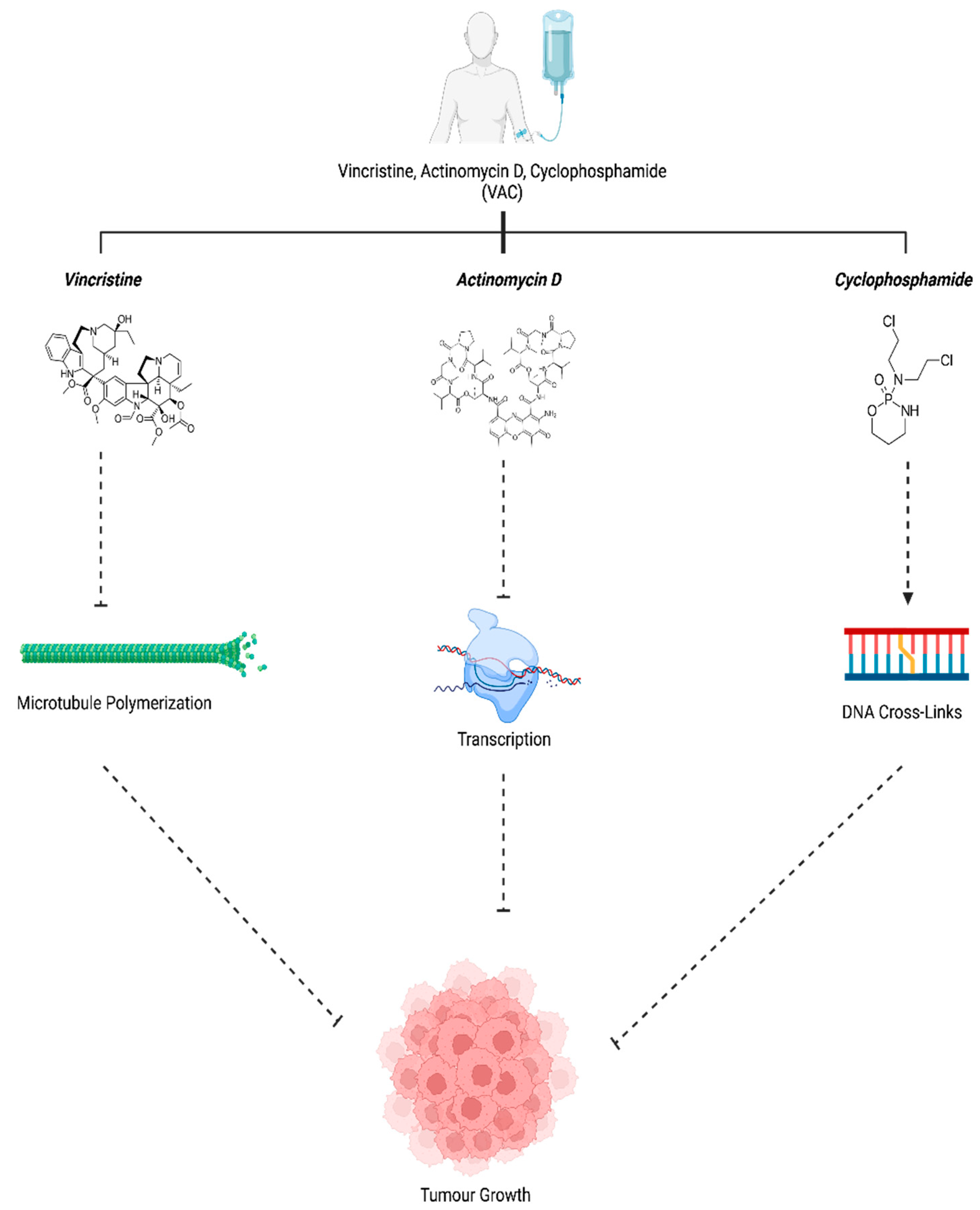
Vincristine, Actinomysin D and Cyclophosphamide (VAC)
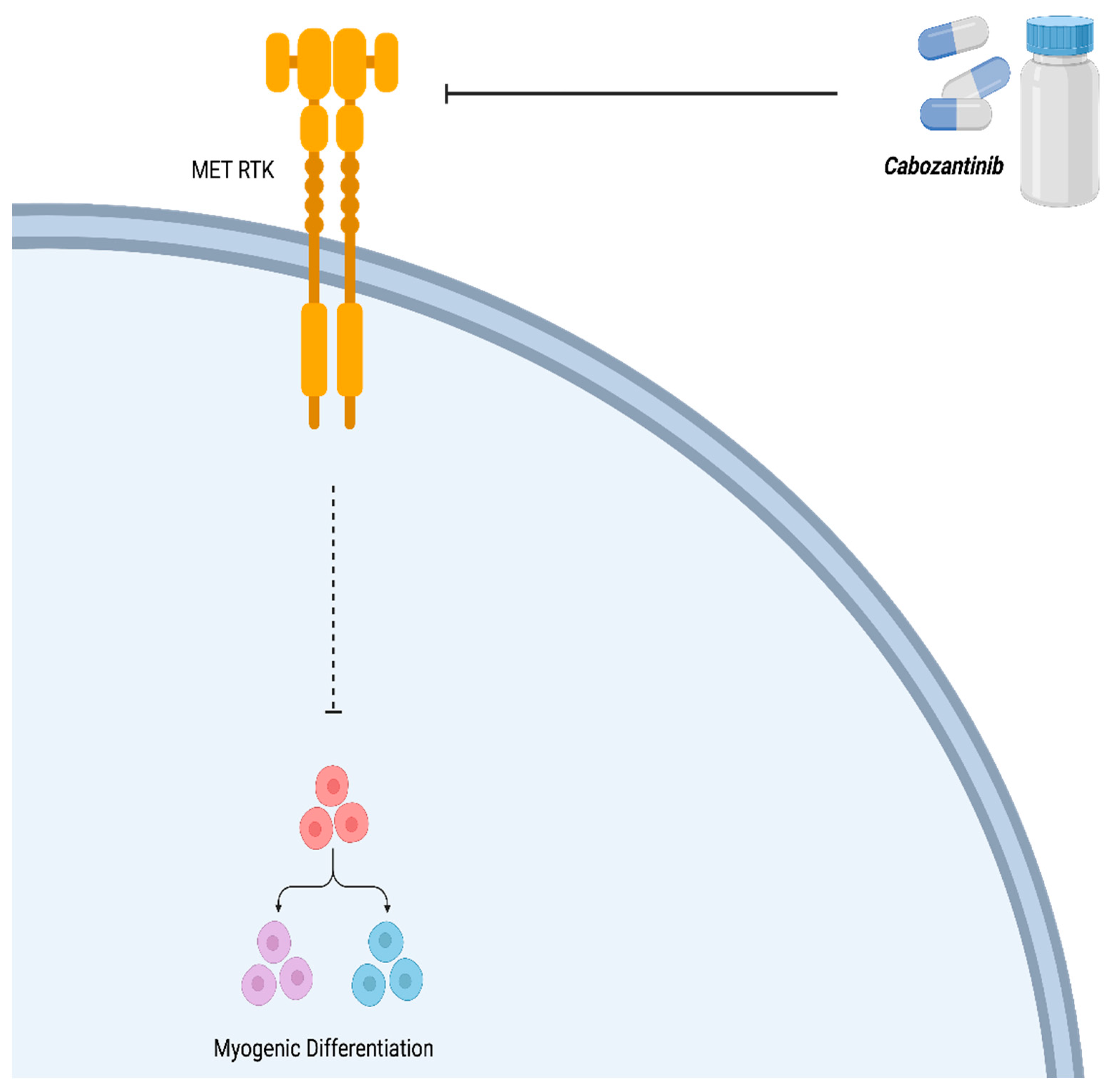
Cabozantinib (XL184)
Bortezomib
Vinorelbine
AZD1775
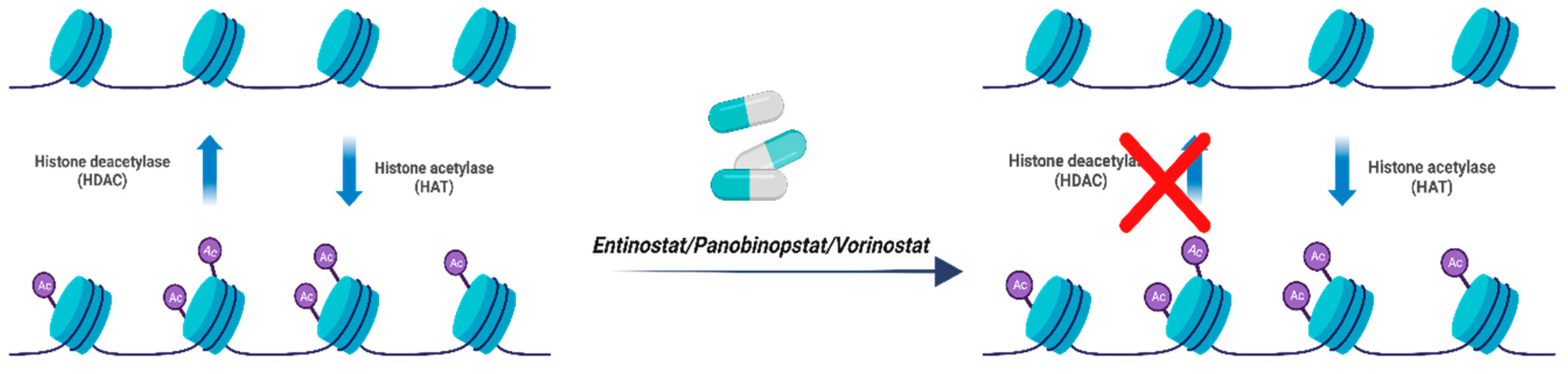
Entinostat, Panobinopstat and Vorinostat
Critotinib, Bevacizumab (mAb) and Regorafenib
All Trans Retinoic Acid (ATRA)
Cisplatin
5-Azacytidine
| Drug/Compound | Molecular Target | Reference |
|---|---|---|
| Vincristine, Actinomycin D, and Cyclophosphamide (VAC) | Microtubule Polymerization, Guanine nucleotide in DNA, cross-linkages with guanine N-7 respectively | (40, 42) |
| Cabozantinib (XL184) | Tyrosine Kinase (MET) | (35) |
| Bortezomib | 26s proteosome | (35) |
| Vinorelbin | Microtubular Proteins | (9) |
| AZD1775 | Wee 1 | (35) |
| Entinostat, Panobinopstat and Vorinostat | Histone Deacetylase (HDAC) | (34) |
| Critotinib, Bevacizumab (mAb) and Regorafenib | Receptor Tyrosine Kinae (RTK) | (34) |
| All Trans Retinoic Acid (ATRA) | retinoic acid receptors (RARs) | (20) |
| Cisplatin | DNA | (53) |
| 5-Azacytidine | DNA methyltransferase | (56) |
Conclusion and Future Direction
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Aghaei M, Nasimian A, Rahmati M, Kawalec P, Machaj F, Rosik J, et al. The Role of BiP and the IRE1alpha-XBP1 Axis in Rhabdomyosarcoma Pathology. Cancers (Basel). 2021, 13.
- Eguia-Aguilar P, Lopez-Martinez B, Retana-Contreras C, Perezpena-Diazconti M. Alveolar rhabdomyosarcoma: Origin and prognostic implications of molecular findings. Bol Med Hosp Infant Mex. 2016, 73, 405–410.
- Stefanek E, Samiei E, Kavoosi M, Esmaeillou M, Roustai Geraylow K, Emami A, et al. A bioengineering method for modeling alveolar Rhabdomyosarcoma and assessing chemotherapy responses. MethodsX. 2021, 8, 101473. [Google Scholar] [CrossRef] [PubMed]
- Nhung TH, Minh VL, Tuyet TT, Cuong TM, Lam NL, Trang HT, et al. Orbital rhabdomyosarcoma in a 19-year-old male patient: A case report and literature review. Radiol Case Rep. 2023, 18, 2744–2749. [Google Scholar] [CrossRef]
- Skapek SX, Ferrari A, Gupta AA, Lupo PJ, Butler E, Shipley J, et al. Rhabdomyosarcoma. Nat Rev Dis Primers. 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Emami A, Shojaei S, da Silva Rosa SC, Aghaei M, Samiei E, Vosoughi AR, et al. Mechanisms of simvastatin myotoxicity: The role of autophagy flux inhibition. Eur J Pharmacol. 2019, 862, 172616. [Google Scholar] [CrossRef] [PubMed]
- Martin-Giacalone BA, Weinstein PA, Plon SE, Lupo PJ. Pediatric Rhabdomyosarcoma: Epidemiology and Genetic Susceptibility. J Clin Med. 2021, 10. [Google Scholar]
- Moghadam AR, da Silva Rosa SC, Samiei E, Alizadeh J, Field J, Kawalec P, et al. Autophagy modulates temozolomide-induced cell death in alveolar Rhabdomyosarcoma cells. Cell Death Discov. 2018, 4, 52. [Google Scholar] [CrossRef]
- Allen-Rhoades W, Lupo PJ, Scheurer ME, Chi YY, Kuttesch JF, Venkatramani R, et al. Alveolar rhabdomyosarcoma has superior response rates to vinorelbine compared to embryonal rhabdomyosarcoma in patients with relapsed/refractory disease: A meta-analysis. Cancer Med. 2023, 12, 10222–10229. [Google Scholar] [CrossRef]
- Lewandowski D, Szewczyk A, Radzka J, Dubinska-Magiera M, Kazimierczak W, Daczewska M, et al. The natural origins of cytostatic compounds used in rhabdomyosarcoma therapy. Adv Clin Exp Med. 2023, 32, 1179–1191. [Google Scholar] [CrossRef]
- Nelson AC, Singh C, Pambuccian SE. Cytological diagnosis of metastatic alveolar rhabdomyosarcoma in the ascitic fluid: Report of a case highlighting the diagnostic difficulties. Cytojournal. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Sannino G, Marchetto A, Kirchner T, Grunewald TGP. Epithelial-to-Mesenchymal and Mesenchymal-to-Epithelial Transition in Mesenchymal Tumors: A Paradox in Sarcomas? Cancer Res. 2017, 77, 4556–4561. [Google Scholar] [CrossRef] [PubMed]
- Lagutina IV, Valentine V, Picchione F, Harwood F, Valentine MB, Villarejo-Balcells B, et al. Modeling of the human alveolar rhabdomyosarcoma Pax3-Foxo1 chromosome translocation in mouse myoblasts using CRISPR-Cas9 nuclease. PLoS Genet. 2015, 11, e1004951. [Google Scholar]
- Azorsa DO, Bode PK, Wachtel M, Cheuk ATC, Meltzer PS, Vokuhl C, et al. Immunohistochemical detection of PAX-FOXO1 fusion proteins in alveolar rhabdomyosarcoma using breakpoint specific monoclonal antibodies. Mod Pathol. 2021, 34, 748–757. [Google Scholar] [CrossRef]
- Skrzypek K, Kusienicka A, Trzyna E, Szewczyk B, Ulman A, Konieczny P, et al. SNAIL is a key regulator of alveolar rhabdomyosarcoma tumor growth and differentiation through repression of MYF5 and MYOD function. Cell Death Dis. 2018, 9, 643. [Google Scholar] [CrossRef]
- Skrzypek K, Nieszporek A, Badyra B, Lasota M, Majka M. Enhancement of myogenic differentiation and inhibition of rhabdomyosarcoma progression by miR-28-3p and miR-193a-5p regulated by SNAIL. Mol Ther Nucleic Acids. 2021, 24, 888–904. [Google Scholar] [CrossRef]
- Charytonowicz E, Cordon-Cardo C, Matushansky I, Ziman M. Alveolar rhabdomyosarcoma: Is the cell of origin a mesenchymal stem cell? Cancer Lett. 2009, 279, 126–136. [Google Scholar] [CrossRef]
- Miekus K, Lukasiewicz E, Jarocha D, Sekula M, Drabik G, Majka M. The decreased metastatic potential of rhabdomyosarcoma cells obtained through MET receptor downregulation and the induction of differentiation. Cell Death Dis. 2013, 4, e459. [Google Scholar] [CrossRef]
- Ramadan F, Saab R, Hussein N, Clezardin P, Cohen PA, Ghayad SE. Non-coding RNA in rhabdomyosarcoma progression and metastasis. Front Oncol. 2022, 12, 971174. [Google Scholar] [CrossRef]
- Skrzypek K, Kot M, Konieczny P, Nieszporek A, Kusienicka A, Lasota M, et al. SNAIL Promotes Metastatic Behavior of Rhabdomyosarcoma by Increasing EZRIN and AKT Expression and Regulating MicroRNA Networks. Cancers (Basel). 2020, 12. [Google Scholar]
- Skrzypek K, Adamek G, Kot M, Badyra B, Majka M. Progression and Differentiation of Alveolar Rhabdomyosarcoma Is Regulated by PAX7 Transcription Factor-Significance of Tumor Subclones. Cells. 2021, 10. [Google Scholar]
- Laubscher D, Gryder BE, Sunkel BD, Andresson T, Wachtel M, Das S, et al. BAF complexes drive proliferation and block myogenic differentiation in fusion-positive rhabdomyosarcoma. Nat Commun. 2021, 12, 6924. [Google Scholar] [CrossRef]
- Schmitt-Ney M, Camussi G. The PAX3-FOXO1 fusion protein present in rhabdomyosarcoma interferes with normal FOXO activity and the TGF-beta pathway. PLoS ONE. 2015, 10, e0121474. [Google Scholar]
- Petragnano F, Pietrantoni I, Camero S, Codenotti S, Milazzo L, Vulcano F, et al. Clinically relevant radioresistant rhabdomyosarcoma cell lines: Functional, molecular and immune-related characterization. J Biomed Sci. 2020, 27, 90. [Google Scholar]
- Wang S, Guo L, Dong L, Guo L, Li S, Zhang J, et al. TGF-beta1 signal pathway may contribute to rhabdomyosarcoma development by inhibiting differentiation. Cancer Sci. 2010, 101, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Dalvand A, da Silva Rosa SC, Ghavami S, Marzban H. Potential role of TGFBeta and autophagy in early crebellum development. Biochem Biophys Rep. 2022, 32, 101358. [Google Scholar]
- Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGF-beta Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harb Perspect Biol. 2016, 8.
- Siapoush S, Rezaei R, Alavifard H, Hatami B, Zali MR, Vosough M, et al. Therapeutic implications of targeting autophagy and TGF-beta crosstalk for the treatment of liver fibrosis. Life Sci. 2023, 329, 121894. [Google Scholar] [CrossRef]
- Derynck R, Budi EH. Specificity, versatility, and control of TGF-beta family signaling. Sci Signal. 2019, 12.
- Alizadeh J, Glogowska A, Thliveris J, Kalantari F, Shojaei S, Hombach-Klonisch S, et al. Autophagy modulates transforming growth factor beta 1 induced epithelial to mesenchymal transition in non-small cell lung cancer cells. Biochim Biophys Acta Mol Cell Res. 2018, 1865, 749–768. [Google Scholar] [CrossRef]
- Esmaeilzadeh A, Mohammadi V, Elahi R. Transforming growth factor beta (TGF-beta) pathway in the immunopathogenesis of multiple sclerosis (MS); molecular approaches. Mol Biol Rep. 2023, 50, 6121–6131. [Google Scholar] [CrossRef] [PubMed]
- Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-beta signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Tzavlaki K, Moustakas A. TGF-beta Signaling. Biomolecules. 2020, 10.
- Chen C, Dorado Garcia H, Scheer M, Henssen AG. Current and Future Treatment Strategies for Rhabdomyosarcoma. Front Oncol. 2019, 9, 1458. [Google Scholar] [CrossRef]
- Kahen E, Yu D, Harrison DJ, Clark J, Hingorani P, Cubitt CL, et al. Identification of clinically achievable combination therapies in childhood rhabdomyosarcoma. Cancer Chemother Pharmacol. 2016, 78, 313–323. [Google Scholar] [CrossRef]
- Makimoto, A. Optimizing Rhabdomyosarcoma Treatment in Adolescents and Young Adults. Cancers (Basel). 2022, 14. [Google Scholar] [CrossRef]
- George P, Journey LJ, Goldstein MN. Effect of vincristine on the fine structure of HeLa cells during mitosis. J Natl Cancer Inst. 1965, 35, 355–375. [Google Scholar]
- Gidding CE, Kellie SJ, Kamps WA, de Graaf SS. Vincristine revisited. Crit Rev Oncol Hematol. 1999, 29, 267–287. [Google Scholar] [CrossRef]
- Awosika AO, Below J, J MD. Vincristine. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Jameshia Below declares no relevant financial relationships with ineligible companies. Disclosure: Joe M Das declares no relevant financial relationships with ineligible companies.2023.
- Lu DF, Wang YS, Li C, Wei GJ, Chen R, Dong DM, et al. Actinomycin D inhibits cell proliferations and promotes apoptosis in osteosarcoma cells. Int J Clin Exp Med. 2015, 8, 1904–1911. [Google Scholar]
- Marchal JA, Prados J, Melguizo C, Fernandez JE, Velez C, Alvarez L, et al. Actinomycin D treatment leads to differentiation and inhibits proliferation in rhabdomyosarcoma cells. J Lab Clin Med. 1997, 130, 42–50. [Google Scholar] [CrossRef]
- Ogino MH, Tadi P. Cyclophosphamide. StatPearls. Treasure Island (FL) ineligible companies. Disclosure: Prasanna Tadi declares no relevant financial relationships with ineligible companies.2023.
- Chuk MK, Widemann BC, Minard CG, Liu X, Kim A, Bernhardt MB, et al. A phase 1 study of cabozantinib in children and adolescents with recurrent or refractory solid tumors, including CNS tumors: Trial ADVL1211, a report from the Children's Oncology Group. Pediatr Blood Cancer. 2018, 65, e27077. [Google Scholar] [CrossRef] [PubMed]
- Casanova M, Ferrari A, Bisogno G, Merks JH, De Salvo GL, Meazza C, et al. Vinorelbine and low-dose cyclophosphamide in the treatment of pediatric sarcomas: Pilot study for the upcoming European Rhabdomyosarcoma Protocol. Cancer. 2004, 101, 1664–1671. [Google Scholar] [CrossRef] [PubMed]
- Liang C, Qiao G, Liu Y, Tian L, Hui N, Li J, et al. Overview of all-trans-retinoic acid (ATRA) and its analogues: Structures, activities, and mechanisms in acute promyelocytic leukaemia. Eur J Med Chem. 2021, 220, 113451. [Google Scholar] [CrossRef] [PubMed]
- Ni X, Hu G, Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit Rev Food Sci Nutr. 2019;59(sup1):S71-S80.
- Szymanski L, Skopek R, Palusinska M, Schenk T, Stengel S, Lewicki S, et al. Retinoic Acid and Its Derivatives in Skin. Retinoic Acid and Its Derivatives in Skin. Cells. 2020, 9. [Google Scholar]
- le Maire A, Teyssier C, Balaguer P, Bourguet W, Germain P. Regulation of RXR-RAR Heterodimers by RXR- and RAR-Specific Ligands and Their Combinations. Cells. 2019, 8.
- O'Brien E, Tse C, Tracy I, Reddin I, Selfe J, Gibson J, et al. Pharmacological EZH2 inhibition combined with retinoic acid treatment promotes differentiation and apoptosis in rhabdomyosarcoma cells. Clin Epigenetics. 2023, 15, 167. [Google Scholar] [CrossRef] [PubMed]
- Williams AP, Waters AM, Stewart JE, Atigadda VR, Mroczek-Musulman E, Muccio DD, et al. A novel retinoid X receptor agonist, UAB30, inhibits rhabdomyosarcoma cells in vitro. J Surg Res. 2018, 228, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chen J, Li Q. Implication of retinoic acid receptor selective signaling in myogenic differentiation. Sci Rep. 2016, 6, 18856. [CrossRef]
- Gudas, LJ. Retinoids induce stem cell differentiation via epigenetic changes. Semin Cell Dev Biol. 2013, 24, 701–705. [Google Scholar] [CrossRef]
- Qi L, Luo Q, Zhang Y, Jia F, Zhao Y, Wang F. Advances in Toxicological Research of the Anticancer Drug Cisplatin. Chem Res Toxicol. 2019, 32, 1469–1486. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- arrabi A, Perrin D, Kavoosi M, Sommer M, Sezen S, Mehrbod P; et al. Rhabdomyosarcoma: Current Therapy, Challenges, and Future Approaches to Treatment Strategies. Cancers (Basel). 2023, 15.
- Jin S, Cojocari D, Purkal JJ, Popovic R, Talaty NN, Xiao Y, et al. 5-Azacitidine Induces NOXA to Prime AML Cells for Venetoclax-Mediated Apoptosis. Clin Cancer Res. 2020, 26, 3371–3383. [Google Scholar] [CrossRef] [PubMed]
- Mahoney SE, Yao Z, Keyes CC, Tapscott SJ, Diede SJ. Genome-wide DNA methylation studies suggest distinct DNA methylation patterns in pediatric embryonal and alveolar rhabdomyosarcomas. Epigenetics. 2012, 7, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Zhu Q, Liang F, Cai S, Luo X, Duo T, Liang Z, et al. KDM4A regulates myogenesis by demethylating H3K9me3 of myogenic regulatory factors. Cell Death Dis. 2021, 12, 514. [Google Scholar] [CrossRef] [PubMed]
- Filip K, Lewinska A, Adamczyk-Grochala J, Marino Gammazza A, Cappello F, Lauricella M; et al. 5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma beta-TC-6 Cells. Cells. 2022, 11.
- Mirani B, Pagan E, Shojaei S, Duchscherer J, Toyota BD, Ghavami S, et al. A 3D bioprinted hydrogel mesh loaded with all-trans retinoic acid for treatment of glioblastoma. Eur J Pharmacol. 2019, 854, 201–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





