Submitted:
26 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
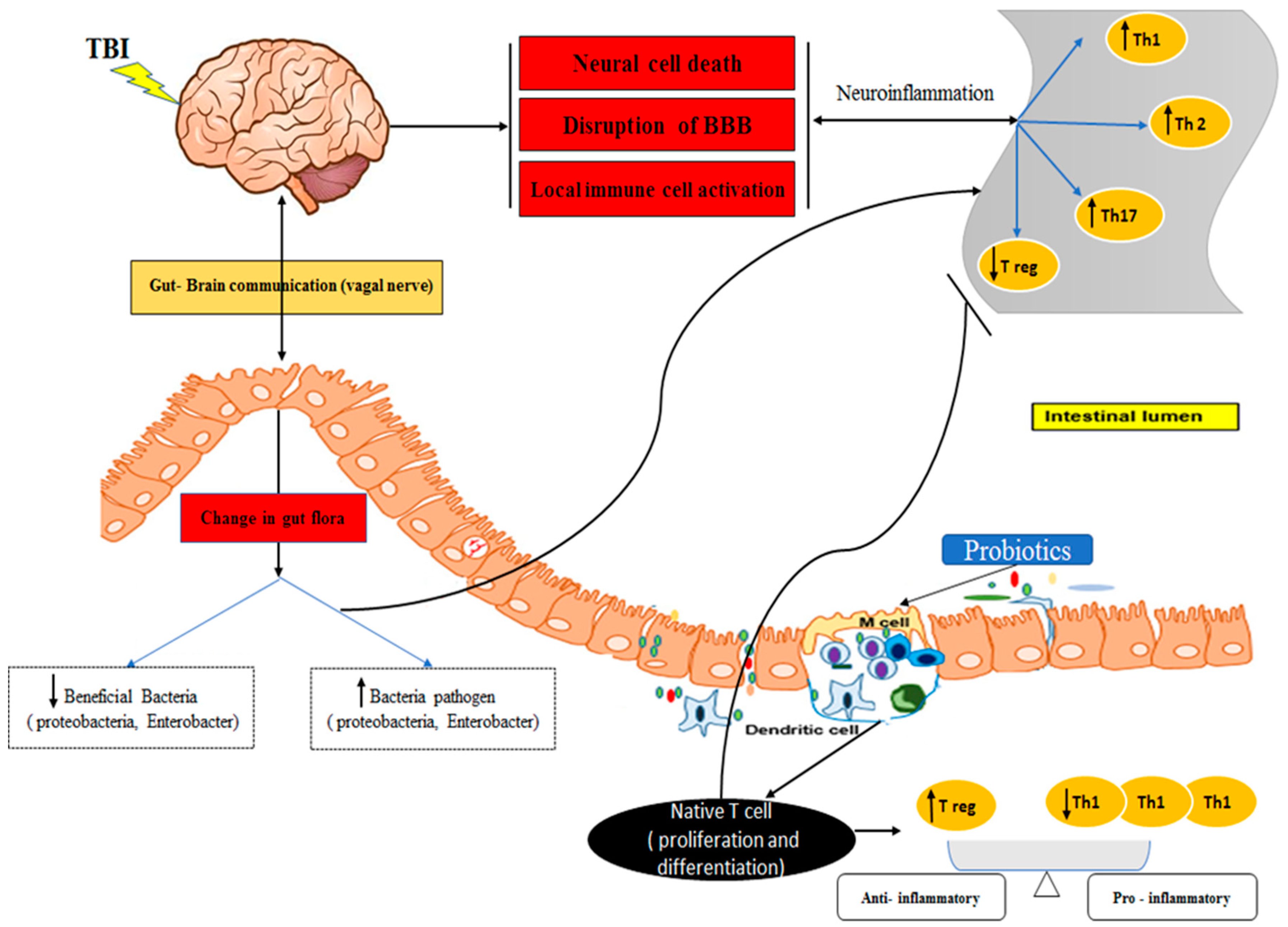
1. Introduction
2. Material and Methods
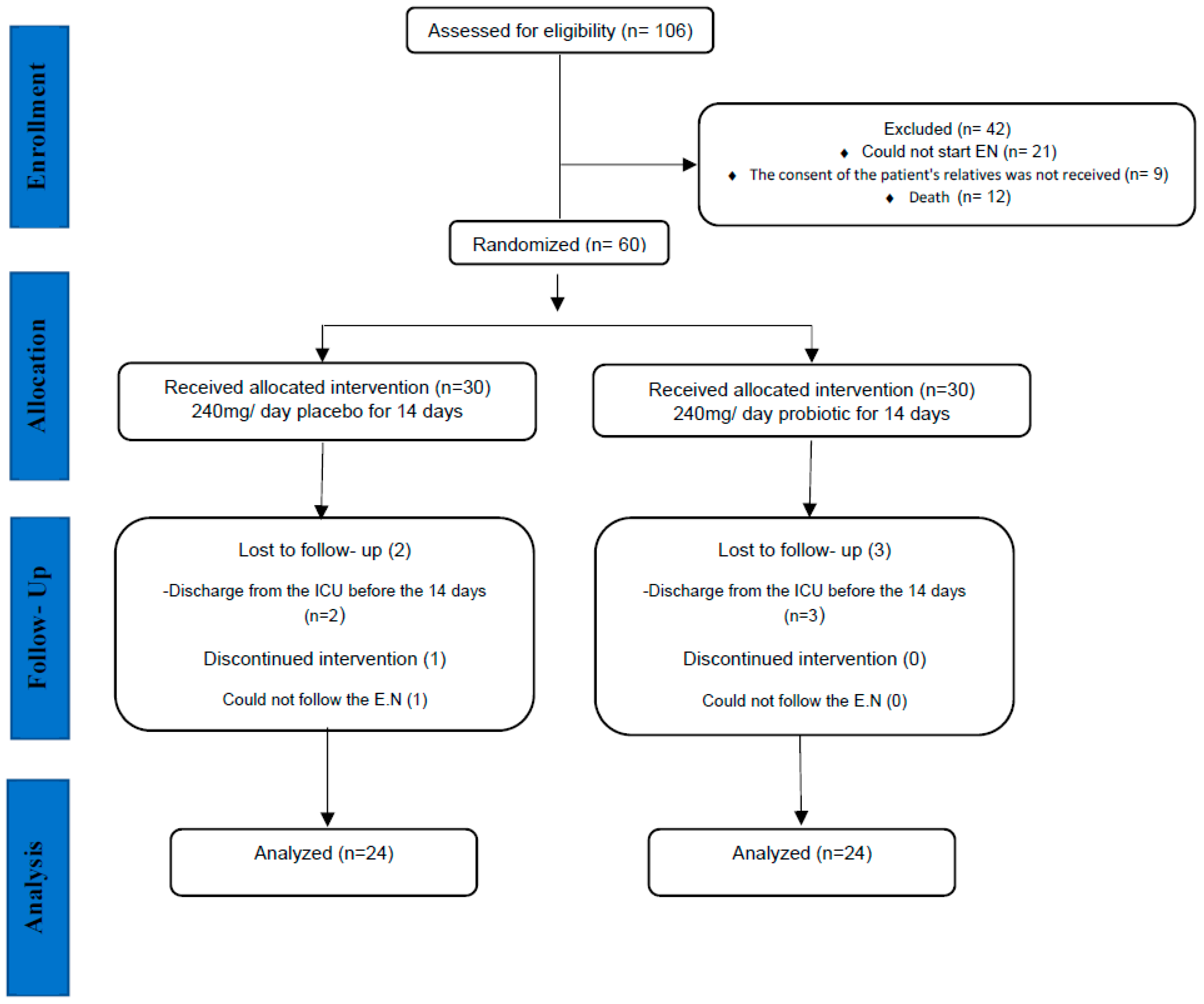
2.1. Study design
2.2. Sample size
2.3. Randomization, Blinding, and Study Protocol
2.4. Blood sampling and cell culture
2.5. Flow cytometry analysis
2.6. RNA extraction and the synthesis of complementary DNA (cDNA)
2.7. Statistical analysis
3. Results
3.1. Clinical and anthropometric information
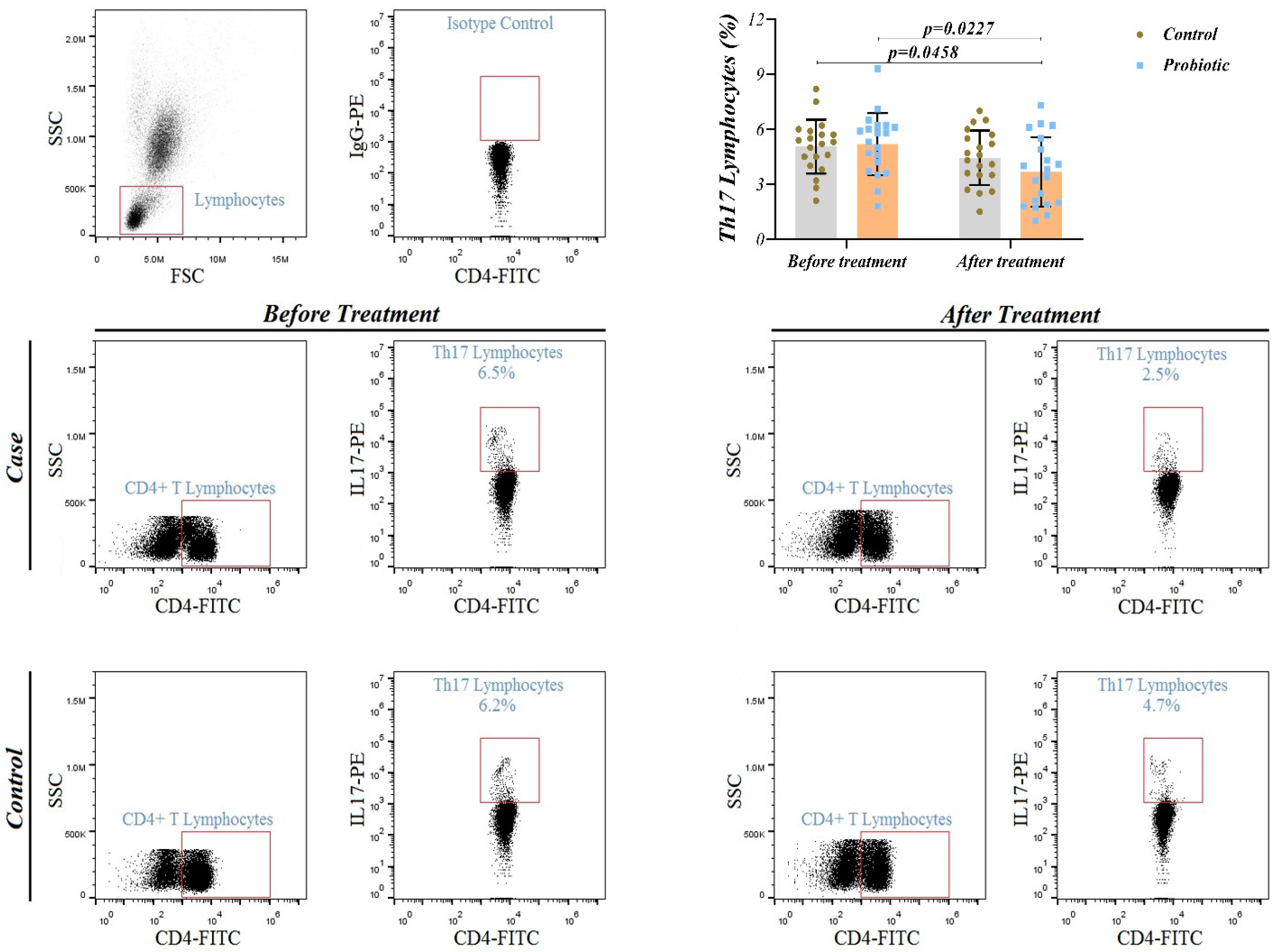
3.2. Th17 cell frequency
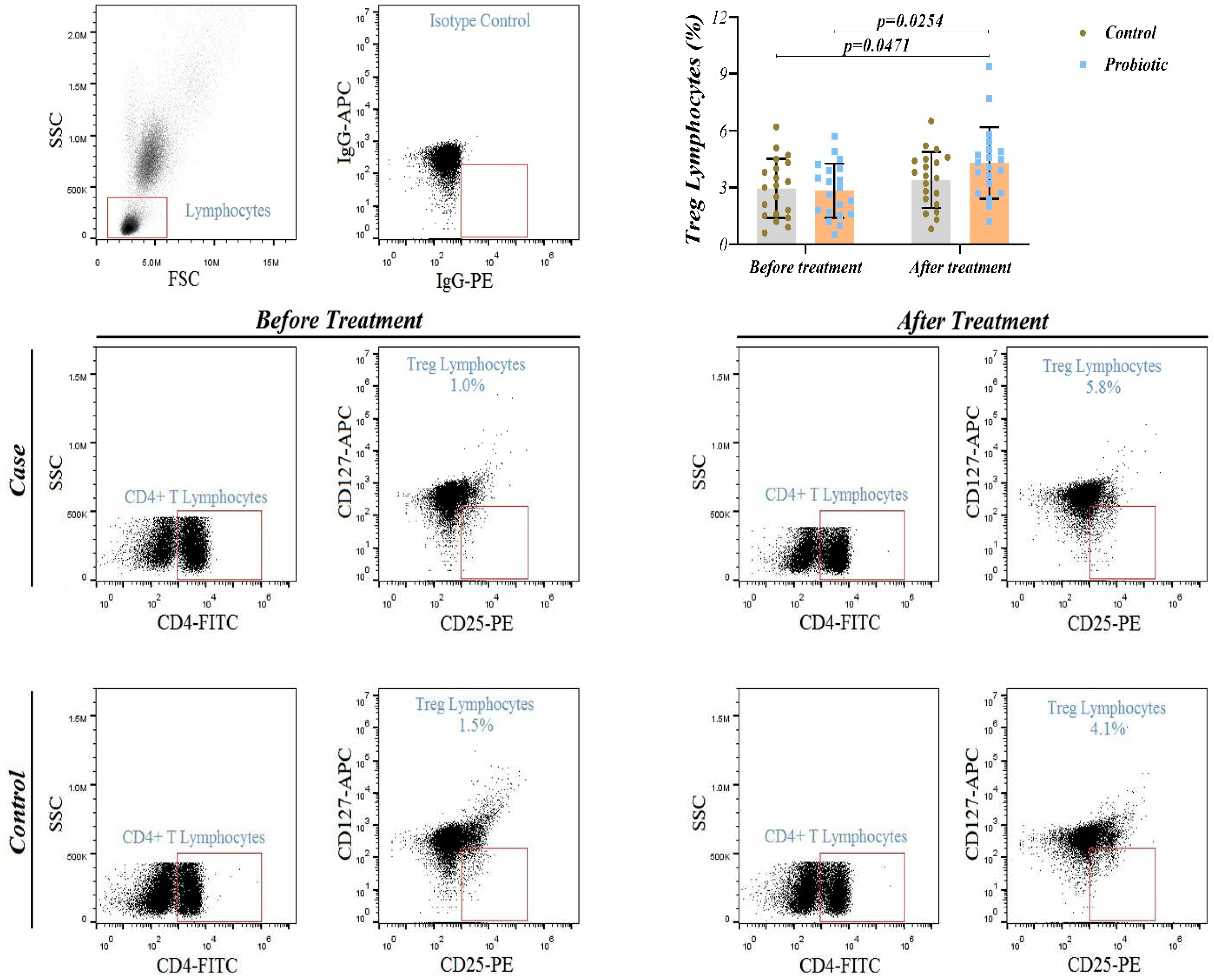
3.3. T-reg cell frequency
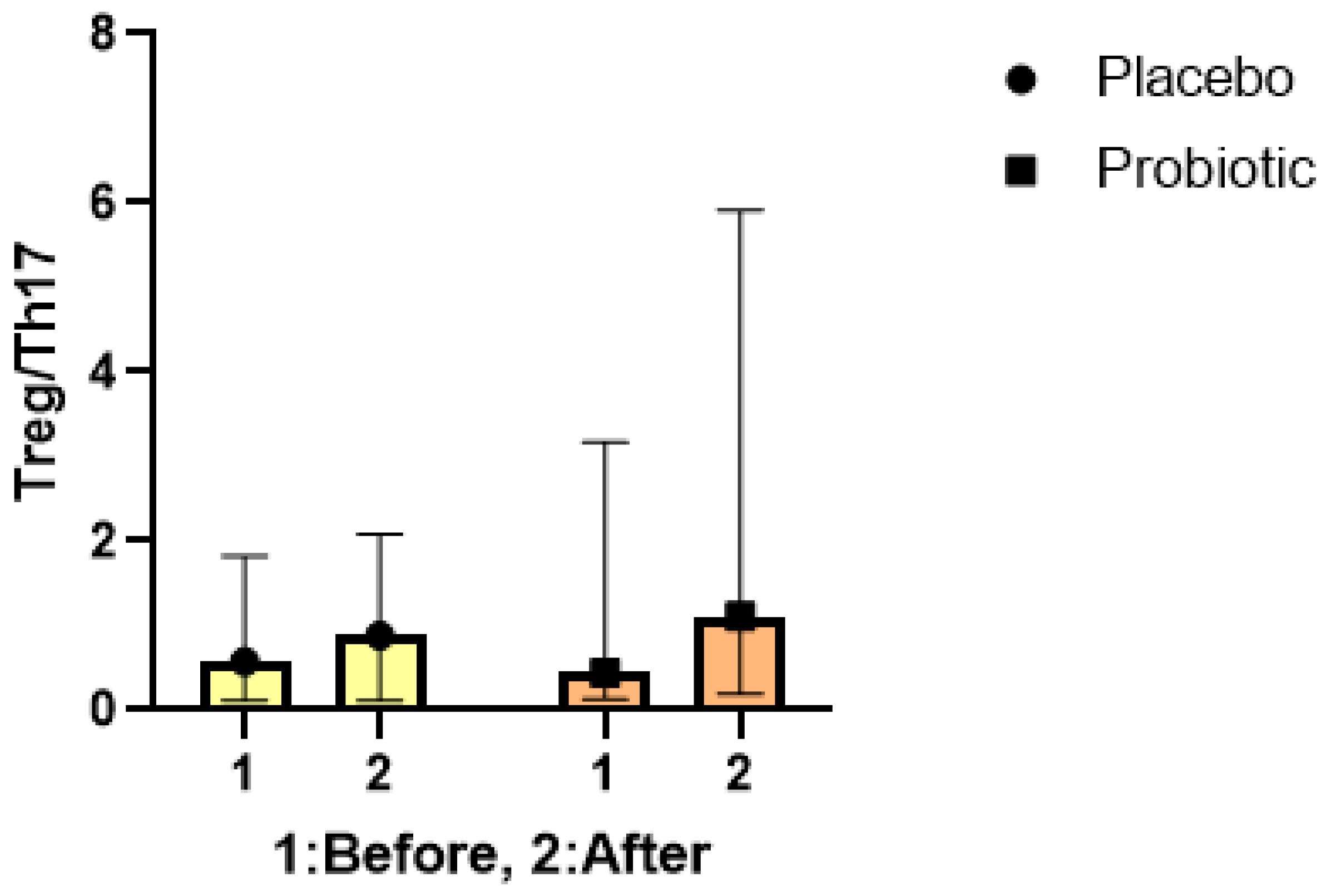
3.4. The Treg/ Th17 ratio
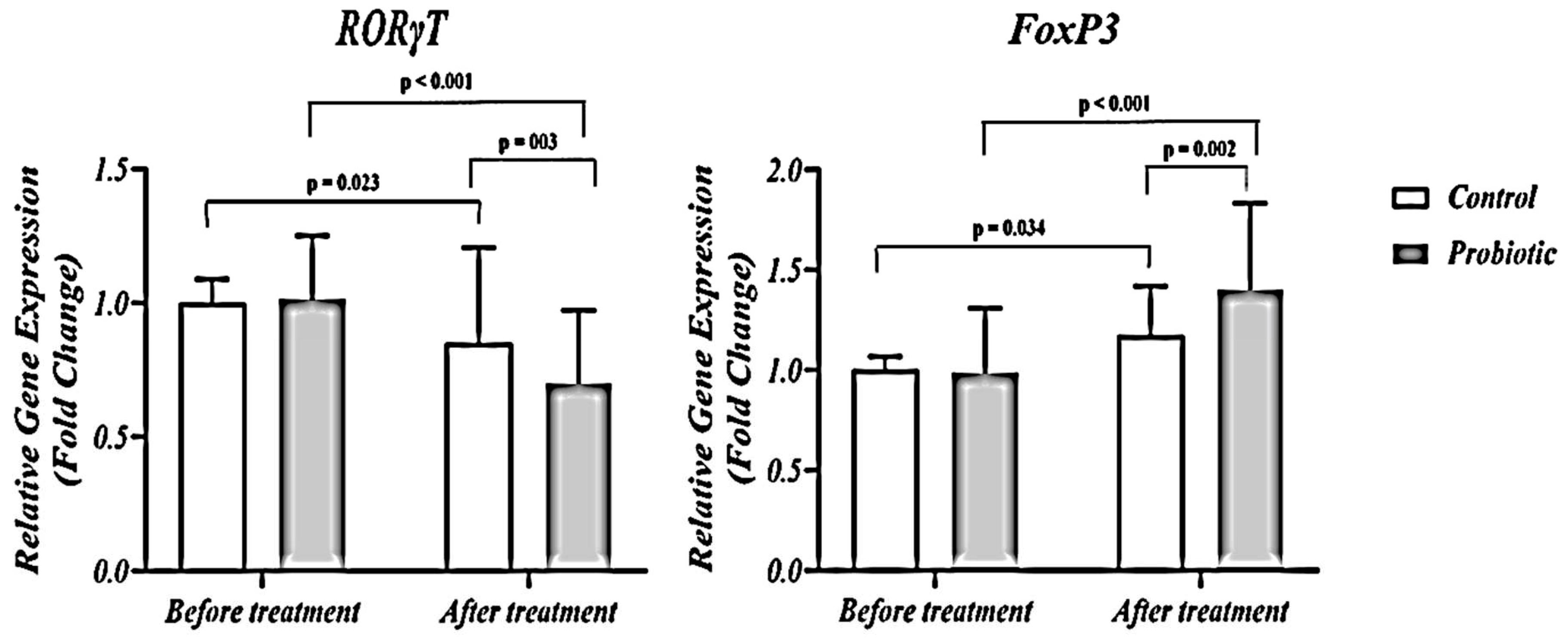
3.5. FoxP3 and RORγt expression
4. Discussion
5. Conclusion
References
- Katzenberger, R.J., B. Ganetzky, and D.A. Wassarman, The gut reaction to traumatic brain injury. Fly (Austin), 2015. 9(2): p. 68-74. [CrossRef] [PubMed]
- Moretti, R., et al., Blood-brain barrier dysfunction in disorders of the developing brain. Frontiers in neuroscience, 2015. 9: p. 40. [CrossRef] [PubMed]
- Witcher, K.G., D.S. Eiferman, and J.P. Godbout, Priming the inflammatory pump of the CNS after traumatic brain injury. Trends in neurosciences, 2015. 38(10): p. 609-620. [CrossRef] [PubMed]
- Corps, K.N., T.L. Roth, and D.B. McGavern, Inflammation and neuroprotection in traumatic brain injury. JAMA neurology, 2015. 72(3): p. 355-362. [CrossRef] [PubMed]
- Hofstetter, H.H., et al., Autoreactive T cells promote post-traumatic healing in the central nervous system. Journal of neuroimmunology, 2003. 134(1-2): p. 25-34. [CrossRef] [PubMed]
- Jones, T., E. McDaniel, and P. Popovich, Inflammatory-mediated injury and repair in the traumatically injured spinal cord. Current pharmaceutical design, 2005. 11(10): p. 1223-1236. [CrossRef]
- Popovich, P.G. and T.B. Jones, Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends in pharmacological sciences, 2003. 24(1): p. 13-17. [CrossRef] [PubMed]
- Morgan, M.E., et al., Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis & Rheumatism, 2005. 52(7): p. 2212-2221. [CrossRef] [PubMed]
- Paulos, C.M., et al., Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. The Journal of clinical investigation, 2007. 117(8): p. 2197-2204. [CrossRef]
- Mickael, M.E., S. Bhaumik, and R. Basu, Retinoid-related orphan receptor RORγt in CD4+ T-Cell–mediated intestinal homeostasis and inflammation. The American Journal of Pathology, 2020. 190(10): p. 1984-1999. [CrossRef]
- Xu, X., et al., Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. Journal of neuroinflammation, 2017. 14(1): p. 1-15. [CrossRef] [PubMed]
- Gupta, D.L., et al., Coexistence of Th1/Th2 and Th17/Treg imbalances in patients with post traumatic sepsis. Cytokine, 2016. 88: p. 214-221. [CrossRef] [PubMed]
- Luo, T., et al., Th17/Treg imbalance induced by dietary salt variation indicates inflammation of target organs in humans. Scientific reports, 2016. 6(1): p. 26767. [CrossRef] [PubMed]
- Lucas, S.M., N.J. Rothwell, and R.M. Gibson, The role of inflammation in CNS injury and disease. British journal of pharmacology, 2006. 147(S1): p. S232-S240. [CrossRef] [PubMed]
- Yang, W., et al., Translocation and Dissemination of Gut Bacteria after Severe Traumatic Brain Injury. Microorganisms, 2022. 10(10): p. 2082. [CrossRef] [PubMed]
- Caputi, V. and M.C. Giron, Microbiome-gut-brain axis and toll-like receptors in Parkinson’s disease. International journal of molecular sciences, 2018. 19(6): p. 1689. [CrossRef] [PubMed]
- Cui, Y., et al., Orally administered brain protein combined with probiotics increases Treg differentiation to reduce secondary inflammatory damage following craniocerebral trauma. Frontiers in Immunology, 2022. 13: p. 928343. [CrossRef] [PubMed]
- Ng, S., et al., Mechanisms of action of probiotics: recent advances. Inflammatory bowel diseases, 2009. 15(2): p. 300-310. [CrossRef]
- Celorrio, M., et al., Innate and Peripheral Immune Alterations after Traumatic Brain Injury Are Regulated in a Gut Microbiota-Dependent Manner in Mice. Journal of neurotrauma, 2023. 40(7-8): p. 772-787. [CrossRef]
- Liu, M., J. Mao, and S. Zhang, Effect of intervention of probiotics in advance on Treg/Th17 in premature mice. BioMed Research International, 2022. 2022. [CrossRef]
- Vonder Haar, C., et al., Frontal traumatic brain injury increases impulsive decision making in rats: a potential role for the inflammatory cytokine interleukin-12. Journal of neurotrauma, 2017. 34(19): p. 2790-2800. [CrossRef] [PubMed]
- Li, M., et al., Role of regulatory T cell in clinical outcome of traumatic brain injury. Chinese medical journal, 2015. 128(08): p. 1072-1078. [CrossRef] [PubMed]
- Yu, Y., et al., Regulatory T cells exhibit neuroprotective effect in a mouse model of traumatic brain injury Retraction in/10.3892/mmr. 2017.6299. Molecular medicine reports, 2016. 14(6): p. 5556-5566. [CrossRef] [PubMed]
- Kipnis, J., et al., Dual effect of CD4+ CD25+ regulatory T cells in neurodegeneration: a dialogue with microglia. Proceedings of the National Academy of Sciences, 2004. 101(suppl_2): p. 14663-14669. [CrossRef]
- Bao, W., Y. Lin, and Z. Chen, The peripheral immune system and traumatic brain injury: Insight into the role of T-helper cells. International Journal of Medical Sciences, 2021. 18(16): p. 3644. [CrossRef]
- Bigler, E.D., Neuroimaging biomarkers in mild traumatic brain injury (mTBI). Neuropsychology review, 2013. 23: p. 169-209. [CrossRef] [PubMed]
- Muhammad, S., et al., Brain immune interactions—novel emerging options to treat acute ischemic brain injury. Cells, 2021. 10(9): p. 2429. [CrossRef] [PubMed]
- Suganya, K. and B.-S. Koo, Gut–brain axis: role of gut microbiota on neurological disorders and how probiotics/prebiotics beneficially modulate microbial and immune pathways to improve brain functions. International journal of molecular sciences, 2020. 21(20): p. 7551. [CrossRef]
- Zhu, C.S., et al., A review of traumatic brain injury and the gut microbiome: insights into novel mechanisms of secondary brain injury and promising targets for neuroprotection. Brain sciences, 2018. 8(6): p. 113. [CrossRef]
- Gómez-Llorente, C., S. Munoz, and A. Gil, Role of Toll-like receptors in the development of immunotolerance mediated by probiotics. Proceedings of the Nutrition Society, 2010. 69(3): p. 381-389. [CrossRef]
- Zhou, K., et al., RETRACTED: Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3β/PTEN axis. Journal of Cerebral Blood Flow & Metabolism, 2017. 37(3): p. 967-979. [CrossRef]
- Zhang, W., et al., Transcriptional and posttranslational regulation of Th17/Treg balance in health and disease. European Journal of Immunology, 2021. 51(9): p. 2137-2150. [CrossRef] [PubMed]
- Weaver, C.T., et al., The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annual Review of Pathology: Mechanisms of Disease, 2013. 8: p. 477-512. [CrossRef] [PubMed]
- Liu, Y.-J., et al., Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics, 2020. 10(12): p. 5225. [CrossRef]
- Kwon, H.-K., et al., Generation of regulatory dendritic cells and CD4+ Foxp3+ T cells by probiotics administration suppresses immune disorders. Proceedings of the National Academy of Sciences, 2010. 107(5): p. 2159-2164. [CrossRef]
- Kreymann, K., et al., ESPEN guidelines on enteral nutrition: intensive care. Clinical nutrition, 2006. 25(2): p. 210-223. [CrossRef] [PubMed]
- McMahon, P.J., et al., Symptomatology and functional outcome in mild traumatic brain injury: results from the prospective TRACK-TBI study. Journal of neurotrauma, 2014. 31(1): p. 26-33. [CrossRef] [PubMed]
| Probiotic (n=24) | Placebo (n=24) | Pc | |
|---|---|---|---|
| Age (years) a | 36.08 (13.65) | 39.33 (16.32) | 0.059 |
| Gender, n (%) b | |||
| Males | 20 (83.30) | 17 (70.80) | 0.073 |
| Females | 4 (16.70) | 7 (29.20) | 0.054 |
| Marital status, n (%) b | 11 (45.80) | 13 (54.20) | 0.062 |
| BMI(Kg/m2) a | 24.17 (4.21) | 24.94 (4.50) | 0.648 |
| MAC (cm) a | 27.00 (2.68) | 27.16 (3.05) | 0.929 |
| TSF (cm) a | 17.70 (4.55) | 16.25 (4.96) | 0.853 |
| GCS score a | 5.21 ± 1.37 | 7.01 ± 1.94 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




