Submitted:
26 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
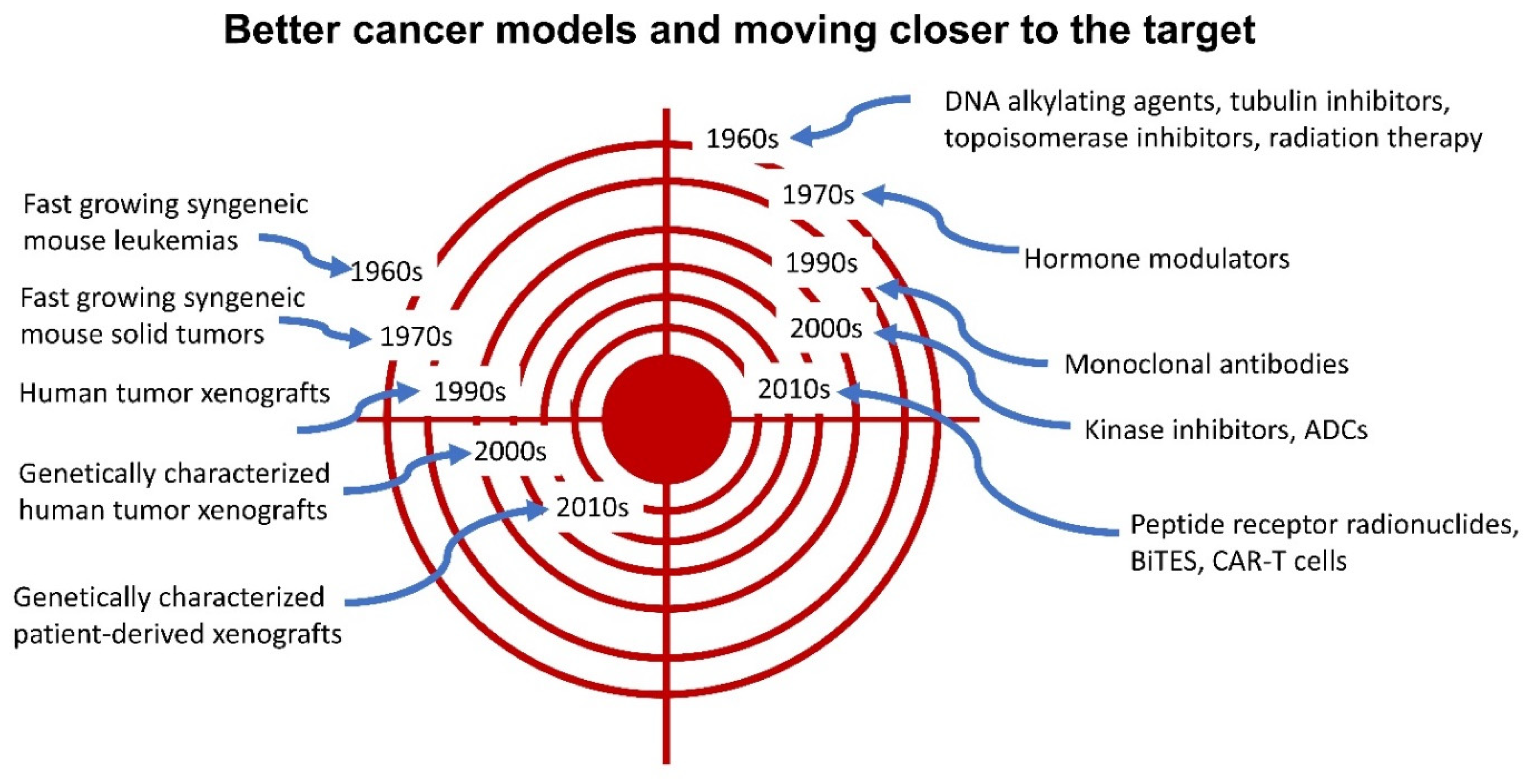
Introduction
Cancer Treatments Introduced Prior to 1970
Cancer Treatments Introduced between 1970 and 2023
Future of Cancer Treatments
Supplementary Materials
References
- Cole, M. P., Jones, C. T. & Todd, I. D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer 25, 270–275 (1971). [CrossRef]
- Wyld, L., Audisio, R. A. & Poston, G. J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 12, 115–124 (2015). [CrossRef]
- Crosby, D. et al. Early detection of cancer. Science 375, eaay9040 (2022). [CrossRef]
- Lederman, M. The early history of radiotherapy: 1895–1939. Int. J. Radiat. Oncol. 7, 639–648 (1981). [CrossRef]
- Lim, Y. K. & Kim, D. Brachytherapy: A Comprehensive Review. Prog. Med. Phys. 32, 25–39 (2021). [CrossRef]
- DeVita, V. T. & Chu, E. A History of Cancer Chemotherapy. Cancer Res. 68, 8643–8653 (2008). [CrossRef]
- Goodman, L. S. & Wintrobe, M. M. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J. Am. Med. Assoc. 132, 126–132 (1946). [CrossRef]
- Farber, S. & Diamond, L. K. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N. Engl. J. Med. 238, 787–793 (1948). [CrossRef]
- Elion, G. B., Singer, S. & Hitchings, G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J. Biol. Chem. 208, 477–488 (1954).
- Hitchings, G. H. & Elion, G. B. The chemistry and biochemistry of purine analogs. Ann. N. Y. Acad. Sci. 60, 195–199 (1954). [CrossRef]
- Heidelberger, C. et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179, 663–666 (1957). [CrossRef]
- Pittillo, R. F., Schabel, F. M., Wilcox, W. S. & Skipper, H. E. Experimental evaluation of potential anticancer agents. XVI. Basic study of effects of certain anticancer agents on kinetic behavior of model bacterial cell populations. Cancer Chemother. Rep. 47, 1–26 (1965).
- Schabel FM. Animal models as predictive systems. in Cancer chemotherapy-fundamental concepts and recent advances. 323–355 (Year Book Medical Publisher, 1975).
- Teicher, B. A. Tumor models for efficacy determination. Mol. Cancer Ther. 5, 2435–2443 (2006). [CrossRef]
- Johnson, I. S., Armstrong, J. G., Gorman, M. & Burnett, J. P. THE VINCA ALKALOIDS: A NEW CLASS OF ONCOLYTIC AGENTS. Cancer Res. 23, 1390–1427 (1963).
- Brunner, K. W. & Young, C. W. A METHYLHYDRAZINE DERIVATIVE IN HODGKIN’S DISEASE AND OTHER MALIGNANT NEOPLASMS. THERAPEUTIC AND TOXIC EFFECTS STUDIED IN 51 PATIENTS. Ann. Intern. Med. 63, 69–86 (1965). [CrossRef]
- DeVita, V. T., Serpick, A. & Carbone, P. P. Preliminary clinical studies with ibenzmethyzin. Clin. Pharmacol. Ther. 7, 542–546 (1966). [CrossRef]
- Tan, C., Tasaka, H., Yu, K. P., Murphy, M. L. & Karnofsky, D. A. Daunomycin, an antitumor antibiotic, in the treatment of neoplastic disease. Clinical evaluation with special reference to childhood leukemia. Cancer 20, 333–353 (1967). [CrossRef]
- Freireich EJ, Karon M & Frei E III. Quadruple combination therapy (VAMP) for acute lymphocytic leukemia of childhood. Proc Am Assoc Cancer Res 5, (1964).
- Frei E III. Potential for eliminating leukemic cells in childhood acute leukemia. Proc Am Assoc Cancer Res 5, (1963).
- George, P. et al. A study of ‘total therapy’ of acute lymphocytic leukemia in children. J. Pediatr. 72, 399–408 (1968).
- Devita, V. T., Serpick, A. A. & Carbone, P. P. Combination chemotherapy in the treatment of advanced Hodgkin’s disease. Ann. Intern. Med. 73, 881–895 (1970). [CrossRef]
- Moxley, J. H., De Vita, V. T., Brace, K. & Frei, E. Intensive combination chemotherapy and X-irradiation in Hodgkin’s disease. Cancer Res. 27, 1258–1263 (1967).
- Nurgali, K., Jagoe, R. T. & Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 9, 245 (2018). [CrossRef]
- Thomas, E. D., Lochte, H. L., Lu, W. C. & Ferrebee, J. W. Intravenous Infusion of Bone Marrow in Patients Receiving Radiation and Chemotherapy. N. Engl. J. Med. 257, 491–496 (1957). [CrossRef]
- Henig, I. & Zuckerman, T. Hematopoietic stem cell transplantation-50 years of evolution and future perspectives. Rambam Maimonides Med. J. 5, e0028 (2014). [CrossRef]
- van Rood, J. J. The detection of transplantation antigens in leukocytes. Semin. Hematol. 5, 187–214 (1968).
- Thomas, E. D. et al. Marrow Transplantation for Acute Nonlymphoblastic Leukemia in First Remission. N. Engl. J. Med. 301, 597–599 (1979). [CrossRef]
- Penack, O. et al. How much has allogeneic stem cell transplant–related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 4, 6283–6290 (2020). [CrossRef]
- Norton, L. Cancer log-kill revisited. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 3–7 (2014). [CrossRef]
- Rygaard, J. & Poulsen, C. O. HETEROTRANSPLANTATION OF A HUMAN MALIGNANT TUMOUR TO “NUDE” MICE. Acta Pathol. Microbiol. Scand. 77, 758–760 (1969). [CrossRef]
- Bibby, M. C. Orthotopic models of cancer for preclinical drug evaluation. Eur. J. Cancer 40, 852–857 (2004). [CrossRef]
- Tentler, J. J. et al. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 9, 338–350 (2012). [CrossRef]
- Meisel, J. L., Venur, V. A., Gnant, M. & Carey, L. Evolution of Targeted Therapy in Breast Cancer: Where Precision Medicine Began. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 38, 78–86 (2018). [CrossRef]
- Arya, M., Shergill, I. S., Grange, P. & Emberton, M. Hormone therapy: a revolution in understanding prostate cancer. Lancet Oncol. 9, 1112 (2008). [CrossRef]
- Schechter, A. L. et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312, 513–516 (1984). [CrossRef]
- Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001). [CrossRef]
- Enewold, L. & Thomas, A. Real-World Patterns of EGFR Testing and Treatment with Erlotinib for Non-Small Cell Lung Cancer in the United States. PLOS ONE 11, e0156728 (2016). [CrossRef]
- Horak, P. et al. Standards for the classification of pathogenicity of somatic variants in cancer (oncogenicity): Joint recommendations of Clinical Genome Resource (ClinGen), Cancer Genomics Consortium (CGC), and Variant Interpretation for Cancer Consortium (VICC). Genet. Med. Off. J. Am. Coll. Med. Genet. 24, 986–998 (2022). [CrossRef]
- Li, M. M. et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. JMD 19, 4–23 (2017). [CrossRef]
- Senapati, J. et al. Management of chronic myeloid leukemia in 2023 – common ground and common sense. Blood Cancer J. 13, 58 (2023). [CrossRef]
- Sonkin, D. & Simon, R. Early Detection is as Important as Imatinib in CML Treatment Success. http://www.preprints.org/manuscript/201910.0207/v1 (2019). [CrossRef]
- Correia, J. H., Rodrigues, J. A., Pimenta, S., Dong, T. & Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 13, 1332 (2021). [CrossRef]
- Shastry, M. et al. Rise of Antibody-Drug Conjugates: The Present and Future. Am. Soc. Clin. Oncol. Educ. Book e390094 (2023. [CrossRef]
- Bagchi, S., Yuan, R. & Engleman, E. G. Immune Checkpoint Inhibitors for the Treatment of Cancer: Clinical Impact and Mechanisms of Response and Resistance. Annu. Rev. Pathol. Mech. Dis. 16, 223–249 (2021). [CrossRef]
- Tawbi, H. A. et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 386, 24–34 (2022). [CrossRef]
- Johnson, D. B., Nebhan, C. A., Moslehi, J. J. & Balko, J. M. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 19, 254–267 (2022). [CrossRef]
- Tian, Z., Liu, M., Zhang, Y. & Wang, X. Bispecific T cell engagers: an emerging therapy for management of hematologic malignancies. J. Hematol. Oncol.J Hematol Oncol 14, 75 (2021). [CrossRef]
- Dmitriy Sonkin, Yue Liu, Raymond Pagliarini, Bill Tschantz, & Michael Morrissey. Identifying SILV as ADC target in melanoma. NVS-DFCI Jt. RETREAT (2010).
- Chen, Y. et al. The melanosomal protein PMEL17 as a target for antibody drug conjugate therapy in melanoma. J. Biol. Chem. 287, 24082–24091 (2012). [CrossRef]
- Franke, V. et al. High response rates for T-VEC in early metastatic melanoma (stage IIIB/C-IVM1a). Int. J. Cancer 145, 974–978 (2019). [CrossRef]
- Hofland, J., Brabander, T., Verburg, F. A., Feelders, R. A. & De Herder, W. W. Peptide Receptor Radionuclide Therapy. J. Clin. Endocrinol. Metab. 107, 3199–3208 (2022). [CrossRef]
- Sartor, O. et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 385, 1091–1103 (2021). [CrossRef]
- Sterner, R. C. & Sterner, R. M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). [CrossRef]
- Medina, J. E. et al. Cell-free DNA approaches for cancer early detection and interception. J. Immunother. Cancer 11, e006013 (2023). [CrossRef]
- Vozenin, M.-C., Bourhis, J. & Durante, M. Towards clinical translation of FLASH radiotherapy. Nat. Rev. Clin. Oncol. 19, 791–803 (2022). [CrossRef]
- Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022). [CrossRef]
- Mayor-Ruiz, C. et al. Rational discovery of molecular glue degraders via scalable chemical profiling. Nat. Chem. Biol. 16, 1199–1207 (2020). [CrossRef]
- Munir, T. et al. Chronic Lymphocytic Leukemia Therapy Guided by Measurable Residual Disease. N. Engl. J. Med. NEJMoa2310063 (2023). [CrossRef]
- Harjunpää, H. & Guillerey, C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 200, 108–119 (2020). [CrossRef]
- Rudin, C. M. et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J. Hematol. Oncol.J Hematol Oncol 16, 66 (2023). [CrossRef]
- Zhou, S., Liu, M., Ren, F., Meng, X. & Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 9, 38 (2021). [CrossRef]
- Frampton, J. E. Teserpaturev/G47Δ: First Approval. BioDrugs 36, 667–672 (2022). [CrossRef]
- Tian, Y., Xie, D. & Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Signal Transduct. Target. Ther. 7, 117 (2022). [CrossRef]
- Malfitano, A. M., Di Somma, S., Iannuzzi, C. A., Pentimalli, F. & Portella, G. Virotherapy: From single agents to combinatorial treatments. Biochem. Pharmacol. 177, 113986 (2020). [CrossRef]
- Hawkins, E. R., D’Souza, R. R. & Klampatsa, A. Armored CAR T-Cells: The Next Chapter in T-Cell Cancer Immunotherapy. Biol. Targets Ther. 15, 95–105 (2021). [CrossRef]
- Tousley, A. M. et al. Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 615, 507–516 (2023). [CrossRef]
- Wellhausen, N. et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 15, eadi1145 (2023). [CrossRef]
- Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022). [CrossRef]
- Maia, A. et al. 641 Strong immune response to therapeutic vaccination with EO2401 microbiome derived therapeutic vaccine + nivolumab: interim report of the EOGBM1–18/ROSALIE study. in Regular and Young Investigator Award Abstracts A671–A671 (BMJ Publishing Group Ltd, 2022). [CrossRef]
- Rupaimoole, R. & Slack, F. J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 16, 203–222 (2017). [CrossRef]
- Padda, I. S., Mahtani, A. U. & Parmar, M. Small Interfering RNA (siRNA) Therapy. in StatPearls (StatPearls Publishing, 2023).
- Cuciniello, R., Filosa, S. & Crispi, S. Novel approaches in cancer treatment: preclinical and clinical development of small non-coding RNA therapeutics. J. Exp. Clin. Cancer Res. 40, 383 (2021). [CrossRef]
- Garland, D. E., Moses, B. & Salyer, W. Long-term follow-up of fracture nonunions treated with PEMFs. Contemp. Orthop. 22, 295–302 (1991).
- Vadalà, M. et al. Mechanisms and therapeutic effectiveness of pulsed electromagnetic field therapy in oncology. Cancer Med. 5, 3128–3139 (2016). [CrossRef]
- Niess, H. et al. Genetic engineering of mesenchymal stromal cells for cancer therapy: turning partners in crime into Trojan horses. Innov. Surg. Sci. 1, 19–32 (2016). [CrossRef]
- Vincent, R. L. et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science 382, 211–218 (2023). [CrossRef]
- Raman, V. et al. Intracellular Salmonella delivery of an exogenous immunization antigen refocuses CD8 T cells against cancer cells, eliminates pancreatic tumors and forms antitumor immunity. Front. Immunol. 14, 1228532 (2023). [CrossRef]
- Baraldi, J. H., Martyn, G. V., Shurin, G. V. & Shurin, M. R. Tumor Innervation: History, Methodologies, and Significance. Cancers 14, 1979 (2022). [CrossRef]
- Venkatesh, H. S. Targeting electrochemical communication between neurons and cancer. Sci. Transl. Med. 15, eadi5170 (2023). [CrossRef]
- Tan, I.-L. et al. Targeting the non-coding genome and temozolomide signature enables CRISPR-mediated glioma oncolysis. Cell Rep. 42, 113339 (2023). [CrossRef]
- Yang, H. et al. KLIPP - a precision CRISPR approach to target structural variant junctions in cancer. http://biorxiv.org/lookup/doi/10.1101/2023.05.10.540176 (2023). [CrossRef]
| Cancer treatment modality | Prior to 1970 | 1970 to 2023 | Future |
|---|---|---|---|
| Surgery | ✓ | ✓ | ✓ |
| Radiation therapy | ✓ | ✓ | ✓ |
| Chemotherapy | ✓ | ✓ | ✓ |
| Allogeneic hematopoietic stem cell transplantation | ✓ | ✓ | ✓ |
| Pharmacological hormone therapy | ✓ | ✓ | |
| Treatments targeting genes with oncogenic alterations and related signaling pathways | ✓ | ✓ | |
| Photodynamic therapy | ✓ | ✓ | |
| Antibody drug conjugates | ✓ | ✓ | |
| Immune check point inhibitors | ✓ | ✓ | |
| Bispecific T-cell engagers | ✓ | ✓ | |
| Oncolytic virus therapy | ✓ | ✓ | |
| Chimeric antigen receptor T cell therapy | ✓ | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





