Submitted:
27 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and methods
2.1. Study design
2.2. Recruitment
2.3. Dietary intake measurements and adherence to the Mediterranean Diet
2.4. Anthropometric, body composition, physical activity and resting metabolic rate analysis.
2.5. Biochemical analysis and blood pressure measurement.
2.6. Statistical Analysis
3. Results
3.1. Subjects sociodemographic characteristics
3.2. Dietary intake.
3.3. Biochemical measurements
3.4. Anthropometric, body composition, resting metabolic rate and physical activity measurements
3.5. Principal component analysis (PCA)
4. Discussion.
Strengths and weaknesses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luli, M.; Yeo, G.; Farrell, E.; Ogden, J.; Parretti, H.; Frew, E.; Bevan, S.; Brown, A.; Logue, J.; Menon, V.; et al. The Implications of Defining Obesity as a Disease: A Report from the Association for the Study of Obesity 2021 Annual Conference; EClinicalMedicine 2023, 58, 101962. [CrossRef]
- Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 December 2023).
- World Health Organization [WHO]. Regional Office for Europe WHO European Regional Obesity: Report 2022; WHO: Geneva, Switzerland, 2022; ISBN 978-92-890-5773-8.
- González-Muniesa, P.; Mártinez-González, M.A.; Hu, F.B.; Després, J.P.; Matsuzawa, Y.; Loos, R.J.F.; Moreno, L.A.; Bray, G.A.; Martinez, J.A. Obesity. Nat Rev Dis Primers 2017, 3, 17034. [CrossRef]
- Di Cesare, M.; Bentham, J.; Stevens, G.A.; Zhou, B.; Danaei, G.; Lu, Y.; Bixby, H.; Cowan, M.J.; Riley, L.M.; Hajifathalian, K.; et al. Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19.2 Million Participants. The Lancet 2016, 387, 1377–1396. [CrossRef]
- Ezzati, M.; Riboli, E. Behavioral and Dietary Risk Factors for Noncommunicable Diseases. N Engl J Med 2013, 369, 954–964. [CrossRef]
- WHO. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; WHO: Geneva, Switzerland, 2009; ISBN 978-92-4-156387-1.
- Reilly, S.M.; Saltiel, A.R. Adapting to Obesity with Adipose Tissue Inflammation. Nat Rev Endocrinol 2017, 13, 633–643. [CrossRef]
- Chung, W.K.; Leibel, R.L. Considerations Regarding the Genetics of Obesity. Obesity 2008, 16, S33–S39. [CrossRef]
- Losavio, J.; Keenan, M.J.; Gollub, E.A.; Silver, H.J. Factors That Predict Weight Loss Success Differ by Diet Intervention Type. Front Nutr 2023, 10, 1192747. [CrossRef]
- Tsatsoulis, A.; Paschou, S.A. Metabolically Healthy Obesity: Criteria, Epidemiology, Controversies, and Consequences. Curr Obes Rep 2020, 9, 109–120. [CrossRef]
- Chooi, Y.C.; Ding, C.; Magkos, F. The Epidemiology of Obesity. Metabolism 2019, 92, 6–10. [CrossRef]
- Dent, R.; McPherson, R.; Harper, M.E. Factors Affecting Weight Loss Variability in Obesity. Metabolism 2020, 113, 154388. [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339 (6123), 1084–1088. [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A. V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [CrossRef]
- Asarian, L.; Geary, N. Sex Differences in the Physiology of Eating. Am J Physiol Regul Integr Comp Physiol 2013, 305, 1215–1267. [CrossRef]
- Cuevas-Sierra, A.; Milagro, F.I.; Guruceaga, E.; Cuervo, M.; Goni, L.; García-Granero, M.; Martinez, J.A.; Riezu-Boj, J.I. A Weight-Loss Model Based on Baseline Microbiota and Genetic Scores for Selection of Dietary Treatments in Overweight and Obese Population. Clinical Nutrition 2022, 41, 1712–1723. [CrossRef]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gaudier, G.I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of Obesity. Curr Obes Rep 2020, 9, 272–279. [CrossRef]
- Cai, T.; Tian, L.; Wong, P.H.; Wei, L.J. Analysis of Randomized Comparative Clinical Trial Data for Personalized Treatment Selections. Biostatistics 2011, 12, 270–282. [CrossRef]
- De Toro-Martín, J.; Arsenault, B.J.; Després, J.-P.; Vohl, M.-C. Precision Nutrition: A Review of Personalized Nutritional Approaches for the Prevention and Management of Metabolic Syndrome. Nutrients 2017, 9, 913. [CrossRef]
- Gómez-Juaristi, M.; Martínez-López, S.; Sarria, B.; Bravo, L.; Mateos, R. Bioavailability of Hydroxycinnamates in an Instant Green/Roasted Coffee Blend in Humans. Identification of Novel Colonic Metabolites. Food Funct 2018, 9, 331–343. [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.C.; et al. Dietary Polyphenol Intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Eur J Nutr 2016, 55, 1359–1375. [CrossRef]
- Martínez-López, S.; Sarriá, B.; Mateos, R.; Bravo-Clemente, L. Moderate Consumption of a Soluble Green/Roasted Coffee Rich in Caffeoylquinic Acids Reduces Cardiovascular Risk Markers: Results from a Randomized, Cross-over, Controlled Trial in Healthy and Hypercholesterolemic Subjects. Eur J Nutr 2019, 58, 865–878. [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct 2014, 5, 1695–1717. [CrossRef]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo-Clemente, L. Regularly Consuming a Green/Roasted Coffee Blend Reduces the Risk of Metabolic Syndrome. Eur J Nutr 2018, 57, 269–278. [CrossRef]
- Hinojosa-Nogueira, D.; Pérez-Burillo, S.; Garciá-Rincón, I.; Rufián-Henares, J.A.; Pastoriza, S. A Useful and Simple Tool to Evaluate and Compare the Intake of Total Dietary Polyphenols in Different Populations. Public Health Nutr 2021, 24, 3818–3824. [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database (Oxford) 2010, 2010, bap024. [CrossRef]
- 28. Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018, 378, e34. [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for Energy. EFSA J 2013, 11, 3005. [CrossRef]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412 - 41. [CrossRef]
- Meier, J.J.; Hü, K.; Holst, J.J.; Deacon, C.F.; Schmiegel, W.H.; Nauck, M.A. Reduced Insulinotropic Effect of Gastric Inhibitory Polypeptide in First-Degree Relatives of Patients With Type 2 Diabetes. Diabetes 2001, 50, 2497 - 2504. [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J Clin Endocrinol Metab 2000; 85, 2402-2410. [CrossRef]
- Vargas Franco, V. Estadística Descriptiva Para Ingeniería Ambiental Con SPSS; Vargas Franco, V., Eds.; Universidad Nacional de Colombia Editorial UN: Cali, Colombia, 2007; p. 49, ISBN 978-958-33-9319-3.
- Maskarinec, G.; Novotny, R.; Tasaki, K. Nutritional Epidemiology Dietary Patterns Are Associated with Body Mass Index in Multiethnic Women. J Nutr 2000, 130, 3068-3072. [CrossRef]
- Kerver, J.M.; Yang, E.J.; Bianchi, L.; Song, W.O. Dietary Patterns Associated with Risk Factors for Cardiovascular Disease in Healthy US Adults. Am J Clin Nutr 2003, 78, 1103–1110. [CrossRef]
- Stea, T.H.; Nordheim, O.; Bere, E.; Stornes, P.; Eikemo, T.A. Fruit and Vegetable Consumption in Europe According to Gender, Educational Attainment and Regional Affiliation—A Cross-Sectional Study in 21 European Countries. PLoS One 2020, 15, e0232521. [CrossRef]
- Pour-Abbasi, M.S.; Nikrad, N.; Farhangi, M.A.; Vahdat, S.; Jafarzadeh, F. Dietary Energy Density, Metabolic Parameters, and Blood Pressure in a Sample of Adults with Obesity. BMC Endocr Disord 2023, 23, 3. [CrossRef]
- Bartrina, J. A., & Majem, L. S. Objetivos nutricionales para la población española: Consenso de la Sociedad Española de Nutrición Comunitaria 2011. RENC 2011, 17, 178–199.
- Lichnovská, R.; Gwozdziewiczová, S.; Hrebícek, J. Gender Differences in Factors Influencing Insulin Resistance in Elderly Hyperlipemic Non-Diabetic Subjects. Cardiovasc Diabetol 2002, 1, 4. [CrossRef]
- Acosta B, A.M.; Escalona O, M.; Maiz G, A.; Pollak C, F.; Leighton P, F. Determinación Del Índice de Resistencia Insulínica Mediante HOMA En Una Población de La Región Metropolitana de Chile. Rev Med Chil 2002, 130, 1227–1231. http://dx.doi.org/10.4067/S0034-98872002001100004.
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consulation; WHO: Geneva, Switzerland, 2000; ISBN 92-4-120894-5.
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic Syndrome - A New World-Wide Definition. A Consensus Statement from the International Diabetes Federation. Diabetic Med 2006, 23, 469–480. [CrossRef]
- Raiman, L.; Amarnani, R.; Abdur-Rahman, M.; Marshall, A.; Mani-Babu, S. The Role of Physical Activity in Obesity: Let’s Actively Manage Obesity. Clin Med (Lond) 2023, 23, 311–317. [CrossRef]
- Silveira, E.A.; Mendonça, C.R.; Delpino, F.M.; Elias Souza, G.V.; Pereira de Souza Rosa, L.; de Oliveira, C.; Noll, M. Sedentary Behavior, Physical Inactivity, Abdominal Obesity and Obesity in Adults and Older Adults: A Systematic Review and Meta-Analysis. Clin Nutr ESPEN 2022, 50, 63–73. [CrossRef]
- Black, A.E. Critical Evaluation of Energy Intake Using the Goldberg Cut-off for Energy Intake:Basal Metabolic Rate. A Practical Guide to Its Calculation, Use and Limitations. Int J Obes Relat Metab Disord 2000, 24, 1119-1130. [CrossRef]
- European Food Safety Authority (EFSA). Example of a Protocol for Identification of Misreporting (Under-and Over-Reporting of Energy Intake) Based on the PILOT-PANEU Project. EFSA J 2013, 11, 1–17. https://www.efsa.europa.eu/sites/default/files/efsa_rep/blobserver_assets/3944A-8-2-1.pdf.
- Wehling, H.; Lusher, J. People with a Body Mass Index ⩾30 Under-Report Their Dietary Intake: A Systematic Review. J Health Psychol 2019, 24, 2042–2059. [CrossRef]
- Malinowska, A.M.; Mlodzik-Czyzewska, M.A.; Chmurzynska, A. Dietary Patterns Associated with Obesity and Overweight: When Should Misreporters Be Included in Analysis? Nutrition 2020, 70, 110605. [CrossRef]
- Hannon, B.A.; Thompson, S. V.; An, R.; Teran-Garcia, M. Clinical Outcomes of Dietary Replacement of Saturated Fatty Acids with Unsaturated Fat Sources in Adults with Overweight and Obesity: A Systematic Review and Meta-Analysis of Randomized Control Trials. Ann Nutr Metab 2017, 71, 107–117. [CrossRef]
- Vernarelli, J.A.; Mitchell, D.C.; Rolls, B.J.; Hartman, T.J. Dietary Energy Density Is Associated with Obesity and Other Biomarkers of Chronic Disease in US Adults. Eur J Nutr 2015, 54, 59–65. [CrossRef]
- Seifu, C.N.; Fahey, P.P.; Hailemariam, T.G.; Frost, S.A.; Atlantis, E. Dietary Patterns Associated with Obesity Outcomes in Adults: An Umbrella Review of Systematic Reviews. Public Health Nutr 2021, 24, 6390–6414. [CrossRef]
- Morenga, L. Te; Mallard, S.; Mann, J. Dietary Sugars and Body Weight: Systematic Review and Meta-Analyses of Randomised Controlled Trials and Cohort Studies. BMJ 2012, 346, e7492. [CrossRef]
- Khan, T.A.; Sievenpiper, J.L. Controversies about Sugars: Results from Systematic Reviews and Meta-Analyses on Obesity, Cardiometabolic Disease and Diabetes. Eur J Nutr 2016, 55, 25–43. [CrossRef]
- Simopoulos, A.P. Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Diseases. Food Rev Int 2004, 20, 77–90. [CrossRef]
- Clemente-Suárez, V.J.; Beltrán-Velasco, A.I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients 2023, 15, 2749. [CrossRef]
- Muscogiuri, G.; Verde, L.; Vetrani, C.; Barrea, L.; Savastano, S.; Colao, A. Obesity: A Gender-View. J Endocrinol Invest 2023, 1-8. [CrossRef]
- Ruiz, E.; Rodriguez, P.; Valero, T.; Ávila, J.M.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Dietary Intake of Individual (Free and Intrinsic) Sugars and Food Sources in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 275. [CrossRef]
- Kastorini, C.M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The Effect of Mediterranean Diet on Metabolic Syndrome and Its Components: A Meta-Analysis of 50 Studies and 534,906 Individuals. J Am Coll Cardiol 2011, 57, 1299–1313. [CrossRef]
- Rice Bradley, B.H. Dietary Fat and Risk for Type 2 Diabetes: A Review of Recent Research. Curr Nutr Rep 2018, 7, 214–226. [CrossRef]
- Cerrillo, I.; Saralegui-Díez, P.; Morilla-Romero-de-la-Osa, R.; González de Molina, M.; Guzmán, G.I. Nutritional Analysis of the Spanish Population: A New Approach Using Public Data on Consumption. Int J Environ Res Public Health 2023, 20, 1642. [CrossRef]
- Varraso, R.; Garcia-Aymerich, J.; Monier, F.; Le Moual, N.; De Batlle, J.; Miranda, G.; Pison, C.; Romieu, I.; Kauffmann, F.; Maccario, J. Assessment of Dietary Patterns in Nutritional Epidemiology: Principal Component Analysis Compared with Confirmatory Factor Analysis. Am J Clin Nutr 2012, 96, 1079–1092. [CrossRef]
- Newby, P.K.; Tucker, K.L. Empirically Derived Eating Patterns Using Factor or Cluster Analysis: A Review. Nutr Rev 2004, 62, 177–203. [CrossRef]
- Newby, P.K.; Muller, D.; Hallfrisch, J.; Andres, R.; Tucker, K.L. Food Patterns Measured by Factor Analysis and Anthropometric Changes in Adults. Am J Clin Nutr 2004, 80, 504-513. [CrossRef]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr Opin Lipidol 2002, 13, 3–9. [CrossRef]
- Campbell, S.D.I.; Brosnan, B.J.; Chu, A.K.Y.; Skeaff, C.M.; Rehrer, N.J.; Perry, T.L.; Peddie, M.C. Sedentary Behavior and Body Weight and Composition in Adults: A Systematic Review and Meta-Analysis of Prospective Studies. Sports Med 2018, 48, 585–595. [CrossRef]
- Edwardson, C.L.; Gorely, T.; Davies, M.J.; Gray, L.J.; Khunti, K.; Wilmot, E.G.; Yates, T.; Biddle, S.J.H. Association of Sedentary Behaviour with Metabolic Syndrome: A Meta-Analysis. PLoS One 2012, 7, e34916. [CrossRef]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J Fam Med 2020, 41, 365–373. [CrossRef]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse Changes in Body Composition During the Menopausal Transition and Relation to Cardiovascular Risk: A Contemporary Review. Womens Health Rep (New Rochelle) 2022, 3, 573–581. [CrossRef]
- Holven, K.B.; Roeters van Lennep, J. Sex Differences in Lipids: A Life Course Approach. Atherosclerosis 2023, 384. [CrossRef]
- Buday, B.; Pach, P.F.; Literati-Nagy, B.; Vitai, M.; Kovacs, G.; Vecsei, Z.; Koranyi, L.; Lengyel, C. Sex Influenced Association of Directly Measured Insulin Sensitivity and Serum Transaminase Levels: Why Alanine Aminotransferase Only Predicts Cardiovascular Risk in Men? Cardiovasc Diabetol 2015, 14. [CrossRef]
- Caballería, L.; Pera, G.; Auladell, M.A.; Torán, P.; Muñoz, L.; Miranda, D.; Alumà, A.; Casas, J.D.; Sánchez, C.; Gil, D.; et al. Prevalence and Factors Associated with the Presence of Nonalcoholic Fatty Liver Disease in an Adult Population in Spain. Eur J Gastroenterol Hepatol 2010, 22, 24–32. [CrossRef]
- Du, T.; Sun, X.; Yuan, G.; Zhou, X.; Lu, H.; Lin, X.; Yu, X. Sex Differences in the Impact of Nonalcoholic Fatty Liver Disease on Cardiovascular Risk Factors. Nutr Metab Cardiovasc Dis 2017, 27, 63–69. [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Baldelli, E.; Marrazzo, A.; Romagnoli, D.; Lonardo, A. NAFLD as a Sexual Dimorphic Disease: Role of Gender and Reproductive Status in the Development and Progression of Nonalcoholic Fatty Liver Disease and Inherent Cardiovascular Risk. Adv Ther 2017, 34, 1291–1326. [CrossRef]
- Maranon, R.; Reckelhoff, J.F. Sex and Gender Differences in Control of Blood Pressure. Clin Sci (Lond) 2013, 125, 311–318. [CrossRef]
- Connelly, P.J.; Currie, G.; Delles, C. Sex Differences in the Prevalence, Outcomes and Management of Hypertension. Curr Hypertens Rep 2022, 24, 185-192. [CrossRef]
- Garaulet, M.; Pérez-Llamas, F.; Canteras, M.; Tebar, F.J.; Zamora, S. Endocrine, Metabolic and Nutritional Factors in Obesity and Their Relative Significance as Studied by Factor Analysis. Int J Obes Relat Metab Disord 2001, 25, 243-251. [CrossRef]
- Sweeney, J. P; Weihrauch, J. L. Summary of Available Data for Cholesterol in Foods and Methods for Its Determination. CRC Crit Rev Food Sci Nutr 1976, 8, 131-159. [CrossRef]
- Neuenschwander, M.; Stadelmaier, J.; Eble, J.; Grummich, K.; Szczerba, E.; Kiesswetter, E.; Schlesinger, S.; Schwingshackl, L. Substitution of Animal-Based with Plant-Based Foods on Cardiometabolic Health and All-Cause Mortality: A Systematic Review and Meta-Analysis of Prospective Studies. BMC Med 2023, 21. [CrossRef]
- Ferrari, L.; Panaite, S.-A.; Bertazzo, A.; Visioli, F. Animal- and Plant-Based Protein Sources: A Scoping Review of Human Health Outcomes and Environmental Impact. Nutrients 2022, 14, 5115. [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients 2020, 12, 3704. [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of Dietary Polyphenols on Metabolic Syndrome Features in Humans: A Systematic Review. Obes Rev 2016, 17, 573–586. [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants (Basel) 2023, 12, 416. [CrossRef]
- Sarriá, B.; Sierra-Cinos, J.L.; García-Diz, L.; Martínez-López, S.; Mateos, R.; Bravo-Clemente, L. Green/Roasted Coffee May Reduce Cardiovascular Risk in Hypercholesterolemic Subjects by Decreasing Body Weight, Abdominal Adiposity and Blood Pressure. Foods 2020, 9, 1191. [CrossRef]
- van de Woestijne, A.P.; Monajemi, H.; Kalkhoven, E.; Visseren, F.L.J. Adipose Tissue Dysfunction and Hypertriglyceridemia: Mechanisms and Management. Obes Rev 2011, 12, 829–840. [CrossRef]
- Gómez-Hernández, A.; Beneit, N.; Díaz-Castroverde, S.; Escribano, Ó. Differential Role of Adipose Tissues in Obesity and Related Metabolic and Vascular Complications. Int J Endocrinol 2016, 2016. [CrossRef]
- Zhou, W.; Shi, Y.; Li, YQ.; Ping, Z.; Wang, C.; Liu, X.; Lu, J.; Mao, Z.X.; Zhao, J.; Yin, L.; et al. Body Mass Index, Abdominal Fatness, and Hypertension Incidence: A Dose-Response Meta-Analysis of Prospective Studies. J Hum Hypertens 2018, 32, 321–333. [CrossRef]
- Sánchez-Tapia, M.; Tovar, A.R.; Torres, N. Diet as Regulator of Gut Microbiota and Its Role in Health and Disease. Arch Med Res 2019, 50, 259–268. [CrossRef]
- Garaulet, M.; Ordovás, J.M.; Madrid, J.A. The Chronobiology, Etiology and Pathophysiology of Obesity. Int J Obes (Lond) 2010, 34, 1667–1683. [CrossRef]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.H.; Turek, F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity 2009, 17, 2100–2102. [CrossRef]
- Garaulet, M.; Corbalán, M.D.; Madrid, J.A.; Morales, E.; Baraza, J.C.; Lee, Y.C.; Ordovas, J.M. CLOCK Gene Is Implicated in Weight Reduction in Obese Patients Participating in a Dietary Programme Based on the Mediterranean Diet. Int J Obes (Lond) 2010, 34, 516–523. [CrossRef]
- Annesi, J.J. Coaction of Exercise and Eating Improvements Within a Behavioral Obesity Treatment: Directionality and Psychological Mechanisms. Res Q Exerc Sport 2023, 94, 826–838. [CrossRef]
- Elks, C.E.; Hoed, M. den; Zhao, J.H.; Sharp, S.J.; Wareham, N.J.; Loos, R.J.F.; Ong, K.K. Variability in the Heritability of Body Mass Index: A Systematic Review and Meta-Regression. Front Endocrinol (Lausanne) 2012, 3. [CrossRef]
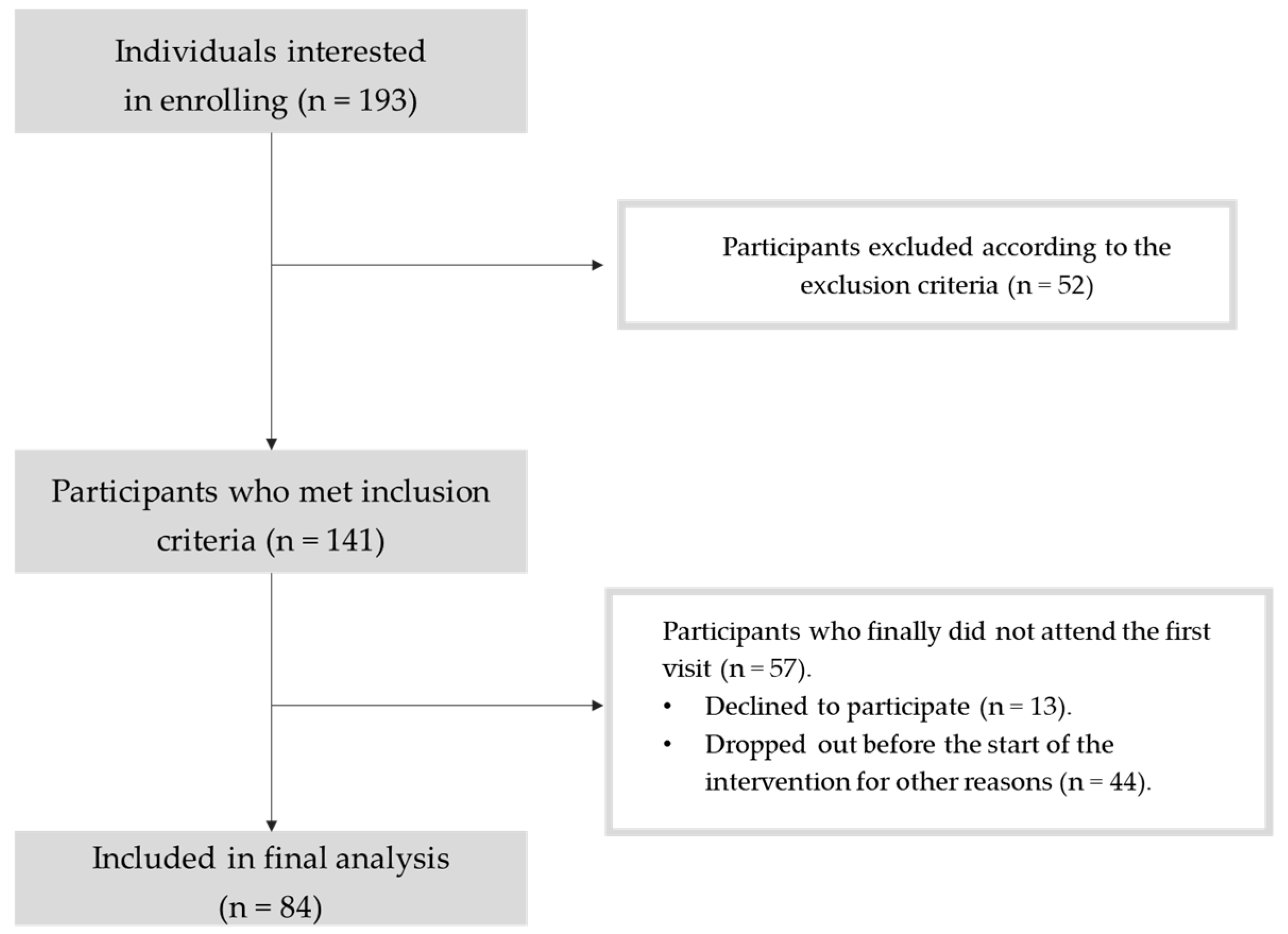
| Absolute frequency (n) | Relative frequency (%) | |
|---|---|---|
| Men | 35 | 41.7 |
| Women | 49 | 58.3 |
| European origin | 78 | 97.5 |
| Latin-American origin | 2 | 2.5 |
| Low educational level | 7 | 8.7 |
| Medium educational level | 14 | 17.5 |
| High educational level | 59 | 73.8 |
| Mean ± SD | Median (IQR) | |
| Monthly income per person (€) | 1358 ± 727 | 1167 (900) |
| Monthly income per family unit (€) | 3298 ± 1493 | 3000 (1900) |
| Age | 49.7 ± 9.9 | 51.0 (11.3) |
| Total (n = 81) | Men (n = 35) | Women (n = 46) |
P value |
||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%CV) |
Median (IQR) | Mean ± SD |
Median (IQR) | Mean ± SD |
Median (IQR) | ||
| Edible food intake (g) a | 2091 ± 572 (27.3 %) |
2038 (646) | 2103 ± 456 | 2053 (513) | 2082 ± 651 | 1967 (773) | 0.498 |
| Energy intake (kcal) | 2015 ± 521 (25.9 %) |
1960 (716) | 2095 ± 509 | 2062 (780) | 1953 ± 527 | 1907 (809) | 0.227 |
| Energy density (kcal/g)b | 1.00 ± 0.25 (24.8 %) |
1.00 (0.32) | 1.01 ± 0.20 | 1.00 (0.27) | 0.99 ± 0.28 | 0.99 (0.35) | 0.643 |
| Proteins (g) a | 88.9 ± 23.9 (26.9 %) |
88.8 (22.4) | 89.5 ± 24.3 | 88.8 (18.6) | 88.38 ± 23.8 | 87.2 (28.4) | 0.985 |
| Carbohydrates (g) | 186 ± 56 (30.1 %) |
179 (72) | 200.6 ± 57.6 | 183 (88.5) | 174.7 ± 52.6 | 177 (62.5) | 0.038* |
| Simple sugars (g) a | 75.8 ± 27.7 (36.6 %) |
70.8 (34.2) | 77.7 ± 28.0 | 73.1 (29.3) | 74.3 ± 27.8 | 68.1 (37.1) | 0.448 |
| Intrinsic sugars (g) | 47.9 ± 17.6 (36.8 %) |
48.3 (22.5) | 47.9 ± 17.7 | 48.2 (23.6) | 47.9 ± 17.8 | 48.3 (21.3) | 0.986 |
| Added sugars (g) a | 28.1 ± 18.8 (66.9 %) |
24.9 (20.3) | 30.3 ± 20.0 | 25.8 (20.1) | 26.5 ± 17.9 | 23.5 (21.2) | 0.381 |
| Lipids (g) a | 92.1 ± 29.9 (32.4 %) |
86.8 (42.5) | 92.6 ± 27.9 | 90.4 (46.4) | 91.7 ± 31.6 | 83.7 (44.1) | 0.706 |
| SFA (g) a | 28.6 ± 10.7 (37.5 %) |
24.6 (17.0) | 20.0 ± 11.1 | 25.3 (16.2) | 28.3 ± 10.5 | 24.3 (17.2) | 0.706 |
| MUFA (g) a | 40.5 ± 14.3 (35.3 %) |
37.5 (13.7) | 40.3 ± 12.9 | 37.3 (13.1) | 40.6 ± 15.4 | 38.7 (15.3) | 0.838 |
| PUFA (g) a | 12.3 ± 5.3 (43.2 %) |
11.2 (5.3) | 12.1 ± 4.3 | 11.2 (5.4) | 12.5 ± 6.0 | 11.3 (4.6) | 0.637 |
| 6 (g) a | 10.3 ± 4.9 (47.6 %) |
9.2 (5.0) | 10.1 ± 3.9 | 9.7 (5.5) | 10.4 ± 5.6 | 8.8 (4.9) | 0.520 |
| 3 (g)a | 1.9 ± 1.0 (50.6 %) |
1.5 (1.1) | 1.81 ± 1.01 | 1.40 (0.95) | 1.93 ± 0.91 | 1.80 (1.15) | 0.359 |
| 6/3 ratio a | 6.5 ± 3.8 (59.0 %) |
6.1 (4.5) | 6.91 ± 4.52 | 6.43 (3.98) | 6.13 ± 3.19 | 5.05 (4.67) | 0.564 |
| Total dietary cholesterol (mg) a | 339 ± 132 (39.0 %) |
317 (163) | 354 ± 142 | 317 (157) | 326 ± 123 | 319 (161) | 0.577 |
| Cholesterol (mg/1000kcal) a | 172.3 ± 68.5 (39.7 %) |
166.1 (80.6) | 173.8 (78.8) | 166.1 (69.1) | 171.1 ± 60.3 | 169.1 (90.5) | 0.659 |
| Alcohol (g) a | 6.7 ± 9.6 (143.9 %) |
2.9 (10.1) | 8.6 ± 12.3 | 4.1 (13.3) | 5.3 ± 6.8 | 2.8 (7.9) | 0.503 |
| Dietary fibre (g) a | 19.8 ± 8.1 (40.7 %) |
19.7 (10.5) | 20.5 ± 9.0 | 19.8 (11.8) | 19.2 ± 7.3 | 19.2 (8.4) | 0.501 |
| Dietary fibre (g / 1000 kcal) a | 10.0 ± 3.5 (34.7 %) |
9.7 (5.0) | 9.9 ± 4.2 | 9.2 (3.9) | 10.0 ± 2.8 | 10.1 (5.0) | 0.370 |
| Total (poly)phenols (mg)a | 1278 ± 809 (63.3 %) |
1073 (1017) | 1264 ± 731 | 1073 (688) | 1288 ± 871 | 1075 (1095) | 0.802 |
|
(Poly)phenols (mg/1000 kcal) a |
642 ± 371 (57.8 %) |
535 (426) | 620 ± 364 | 509 (412) | 658 ± 383 | 594 (437) | 0.659 |
| Total (n = 81) | Men (n = 35) | Women (n = 46) |
P value |
||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%CV) |
Median (IQR) | Mean ± SD |
Median (IQR) | Mean ± SD |
Median (IQR) | ||
| % Carbohydrates | 36.9 ± 6.4 (17.4 %) |
37.1 (10.3) | 38.4 ± 6.6 | 39.6 (11.5) | 35.8 ± 6.1 | 36.5 (9.1) | 0.067 |
| % Simple sugars | 14.9 ± 4.1 (27.4 %) |
14.7 (4.9) | 15.1 ± 4.7 | 15.0 (5.7) | 14.8 ± 3.6 | 14.4 (4.4) | 0.792 |
| % Intrinsic sugars | 9.7 ± 3.4 (35.1 %) |
9.1 (4.9) | 9.3 ± 3.5 | 8.9 (5.1) | 10.0 ± 3.3 | 9.9 (5.6) | 0.352 |
| % Added sugars | 5.4 ± 3.1 (57.0 %) |
5.1 (4.2) | 5.6 ± 3.4 | 5.4 (4.4) | 5.2 ± 2.8 | 5.1 (4.1) | 0.541 |
| % Proteins | 17.9 ± 2.9 (16.3 %) |
17.8 (4.1) | 17.2 ± 2.8 | 16.8 (3.8) | 18.3 ± 2.9 | 18.5 (4.4) | 0.089 |
| % Lipids | 40.9 ± 6.0 (14.6 %) |
40.7 (9.7) | 39.5 ± 5.8 | 38.2 (8.5) | 41.9 ± 6.0 | 41.4 (8.0) | 0.072 |
| % SFA a | 12.6 ± 2.6 (20.7 %) |
12.4 (3.7) | 12.3 ± 3.1 | 12.0 (3.5) | 12.8 ± 2.2 | 13.1 (3.5) | 0.113 |
| % MUFA | 18.1 ± 3.8 (21.2 %) |
17.5 (4.5) | 17.2 ± 3.5 | 16.9 (4.0) | 18.7 ± 4.0 | 18.1 (4.3) | 0.096 |
| % PUFA a | 5.5 ± 1.8 (33.2 %) |
5.2 (2.2) | 5.3 ± 1.6 | 5.1 (2.6) | 5.7 ± 2.0 | 5.3 (1.9) | 0.396 |
| %3 a | 0.87 ± 0.50 (52.3 %) |
0.76 (0.59) | 0.79 ± 0.46 | 0.61 (0.44) | 0.92 ± 0.45 | 0.87 (0.55) | 0.088 |
| % α-Linolenic acid a | 0.5 ± 0.2 (42.3 %) |
0.44 (0.23) | 0.46 ± 0.21 | 0.40 (0.17) | 0.55 ± 0.22 | 0.49 (0.22) | 0.029* |
| %6 a | 4.6 ± 1.7 (37.0 %) |
4.1 (2.1) | 4.4 ± 1.5 | 4.3 (2.3) | 4.7 ± 1.9 | 4.1 (1.9) | 0.652 |
| % Linoleic acid a | 4.5 ± 1.7 (37.5 %) |
4.1 (2.1) | 4.3 ± 1.5 | 4.1 (2.2) | 4.6 ± 1.8 | 4.0 (1.8) | 0.744 |
| % Trans FA | 0.38 ± 0.19 (49.3 %) |
0.38 (0.27) | 0.38 ± 0.17 | 0.36 (0.23) | 0.39 ± 0.21 | 0.41 (0.29) | 0.871 |
| % Alcohol a | 2.3 ± 3.3 (145.5 %) |
1.0 (3.2) | 2.8 ± 4.0 | 1.2 (4.3) | 1.9 ± 2.7 | 1.0 (2.5) | 0.565 |
| % Dietary fibre a | 2.0 ± 0.7 (34.7%) |
1.9 (1.0) | 2.0 ± 0.8 | 1.8 (0.8) | 2.0 ± 0.6 | 2.0 (1.0) | 0.370 |
| Total (n = 83) |
Men (n = 35) |
Women (n = 48) |
|||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%CV) |
Median (IQR) |
Mean ± SD |
Median (IQR) | Mean ± SD |
Median (IQR) | P value | |
| TC (mg/dL) | 211.0 ± 31.1 (14.7 %) |
214 (42.5) | 204.1 ± 31.7 | 205 (36.5) | 216.0 ± 30.2 | 223 (46.8) | 0.085 |
| TG (mg/dL) a | 127.7 ± 68.1 (53.3 %) |
111 (52) | 144.8 ± 80.6 | 121 (61.5) | 115.3 ± 55.0 | 104.5 (50) | 0.091 |
| HDL (mg/dL) a | 61.6 ± 17.0 (27.6 %) |
56 (26) | 54.3 ± 16.1 | 51 (14) | 66.9 ± 15.8 | 68 (23.3) | <0.001*** |
| LDL (mg/dL) | 124.0 ± 25.0 (20.2 %) |
126.8 (36.6) | 120.9 ± 25.6 | 120.6 (36.5) | 126.2 ± 24.6 | 128.6 (36.9) | 0.337 |
| VLDL (mg/dL) a | 25.5 ± 13.6 (53.2 %) |
22.0 (10.3) | 28.9 ± 16.1 | 24.0 (12.0) | 23.0 ± 10.9 | 21.0 (10.3) | 0.094 |
| HbA1c (%) | 5.75 ± 0.35 (6.1 %) |
5.70 (0.40) | 5.71 ± 0.32 | 5.70 (0.35) | 5.77 ± 0.37 | 5.80 (0.40) | 0.504 |
| FBG (mg/dL) | 93.1 ± 11.3 (12.2 %) |
92 (12.5) | 92.3 ± 12.0 | 91 (11.5) | 93.7 ± 11.0 | 93 (13.3) | 0.586 |
| Insulin (µUI/mL) a | 10.5 ± 5.7 (54.2 %) |
9.2 (6.6) | 11.1 ± 5.7 | 10.5 (5.1) | 10.0 ± 5.7 | 8.0 (7.0) | 0.173 |
| HOMA-IR a | 2.41 ± 1.32 (54.5 %) |
1.98 (1.66) | 2.51 ± 1.22 | 2.41 (1.22) | 2.34 ± 1.39 | 1.84 (2.05) | 0.243 |
| HOMA-β a | 146 ± 123 (84.2 %) |
111 (97) | 153 ± 127 | 119 (123) | 141 ± 121 | 98 (83) | 0.437 |
| QUICKI a | 0.343 ± 0.027 (7.8 %) |
0.344 (0.040) | 0.340 ± 0.027 | 0.335 (0.026) | 0.345 ± 0.026 | 0.348 (0.047) | 0.233 |
| AST (UI/L) a | 24.8 ± 12.0 (48.4 %) |
22.0 (8.0) | 27.6 ± 14.5 | 24.0 (9.3) | 22.9 ± 9.5 | 21.0 (5.5) | 0.014* |
| ALT (UI/L) a | 30.1 ± 18.9 (62.9 %) |
24.0 (16.0) | 34.6 ± 18.5 | 30.0 (20.0) | 26.8 ± 18.8 | 21.0 (10.0) | 0.006** |
| hsCRP (mg/dL) a | 0.345 ± 0.562 (162.8 %) |
0.153 (0.301) | 0.350 ± 0.662 | 0.158 (0.248) | 0.341 ± 0.484 | 0.151 (0.440) | 0.843 |
| Blood pressure | |||||||
| SBP (mmHg) a | 126.2 ± 18.6 (14.7 %) |
124.7 (25.1) | 130.5 ± 14.2 | 130.7 (18.7) | 123.2 ± 20.8 | 121.3 (25.0) | 0.01* |
| DBP (mmHg) a | 84.8 ± 10.9 (12.8 %) |
82.0 (14.8) | 86.9 ± 11.1 | 87.3 (18.6) | 83.3 ± 10.6 | 80.7 (12.7) | 0.110 |
| Total (n = 84) | Men (n = 35) | Women (n = 49) | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%CV) |
Median (IQR) |
Mean ± SD |
Median (IQR) | Mean ± SD |
Median (IQR) | P value | |
| Height (cm) | 165.4 ± 8.1 (4.9 %) |
163.6 (13) | 172.1 ± 6.7 | 173.3 (7.2) | 160.7 ± 5.0 | 160.1 (5.6) | <0.001*** |
| Weight (kg) b | 83.6 ± 11.1 (13.2 %) |
83.6 (12.0) | 91.2 ± 10.9 | 87.3 (14.9) | 78.1 ± 7.4 | 77.6 (10.2) | <0.001*** |
| BMI (kg/m2) | 30.5 ± 2.9 (9.6 %) |
30.4 (3.5) | 30.8 ± 2.8 | 30.8 (3.3) | 30.3 ± 3.0 | 30.1 (4.6) | 0.471 |
| WC (cm) | 96.0 ± 11.5 (11.9 %) |
94.7 (15.4) | 102.2 ± 11.1 | 103.9 (15.1) | 91.5 ± 9.5 | 91.6 (10.8) | <0.001*** |
| HC (cm) a | 108.5 ± 6.8 (6.3 %) |
106.8 (10.4) | 105.9 ± 6.1 | 104.5 (6.2) | 110.5 ± 6.6 | 110.2 (10) | <0.001*** |
| WC/HC b | 0.89 ± 0.11 (12.8 %) |
0.88 (0.15) | 0.97 ± 0.11 | 0.96 (0.16) | 0.83 ± 0.08 | 0.85 (0.12) | <0.001*** |
| WC / Height | 0.58 ± 0.07 (11.3 %) |
0.58 (0.09) | 0.59 ± 0.06 | 0.60 (0.10) | 0.57 ± 0.07 | 0.57 (0.08) | 0.107 |
| SUMM 6 FOLDS a | 153.6 (33.0) (21.5 %) |
162.1 (46.5) | 131.7 ± 30.1 | 130.0 (36.8) | 169.3 ± 25.3 | 170.2 (24.6) | <0.001*** |
| Body composition measured by bioimpedance | |||||||
| Fat weight (kg) | 30.2 ± 7.3 (24.0 %) |
29.8 (9.7) | 28.4 ± 7.6 | 27.3 (9.9) | 31.5 ± 6.8 | 31.3 (9.1) | 0.053 |
| % Body fat | 36.2 ± 7.8 (21.5 %) |
35.6 (11.8) | 30.8 ± 6.5 | 30.5 (7.8) | 40.1 ± 6.2 | 39.7 (9.1) | <0.001*** |
| VFA (cm2) a | 141.9 ± 43.6 (30.7 %) |
137.6 (70.7) | 125.9 ± 40.8 | 115.4 (53.2) | 153.3 ± 42.2 | 155.9 (56.2) | 0.003 ** |
| SMM (kg) b | 29.9 ± 6.4 (21.5 %) |
27.7 (9.3) | 35.9 ± 5.2 | 36.0 (5.2) | 25.7 ± 2.9 | 25.8 (2.8) | <0.001*** |
| % Muscle mass | 35.7 ± 5.1 (14.3 %) |
35.8 (7.0) | 39.4 ± 4.5 | 39.2 (4.7) | 33.1 ± 3.6 | 33.0 (5.1) | <0.001*** |
| SMI (kg/m2) b | 7.94 ± 0.95 (12.0 %) |
7.70 (1.40) | 8.79 ± 0.74 | 8.60 (0.95) | 7.33 ± 0.51 | 7.30 (0.70) | <0.001*** |
| Total | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| Mean ± SD (%CV) |
Median (IQR) |
Mean ± SD |
Median (IQR) | Mean ± SD |
Median (IQR) | P value | |
|
RMR (kcal/day) (n = 56) |
1778 ± 310 (17.4 %) |
1712 (472) | 2038 ± 240 | 2077 (364) | 1596 ± 206 | 1577 (233) | <0.001*** |
|
TEE (n = 50) |
2427 ± 453 (18.7 %) |
2372 (507) | 2723 ± 419 | 2628 (325) | 2194 ± 329 | 2175 (278) | <0.001*** |
|
Average PAE (kcal/day) a (n = 60) |
463 ± 185 (39.9 %) |
447(169.1) | 483.6 ± 216.4 | 443.5 (190.7) | 447.4 ± 158.2 | 454.1 (140.6) | 0.662 |
|
METs a (n = 60) |
1.14 ± 0.09 (7.6 %) |
1.12 (0.09) | 1.14 ± 0.10 | 1.12 (0.10) | 1.13 ± 0.08 | 1.12 (0.08) | 0.676 |
|
Steps per day a (n = 60) |
7961 ± 3149 (42.0 %) |
7281 (3445) | 7521 ± 3829 | 6866 (2048) | 8297 ± 2521 | 8193 (3353) | 0.048* |
|
PAL (n = 50) |
1.36 ± 0.10 (7.0 %) |
1.36 (0.11) | 1.34 ± 0.10 | 1.33 (0.12) | 1.38 ± 0.09 | 1.37 (0.09) | 0.084 |
| Components | |||
|---|---|---|---|
| 1 | 2 | 3 | |
| Proteins a | 0.859 | ||
| Lipids | 0.832 | ||
| MUFA a | 0.821 | ||
| Energy intake | 0.761 | 0.515 | |
| Dietary cholesterol | 0.752 | ||
| SFA a | 0.731 | 0.552 | |
| PUFA a | 0.627 | ||
| Total (poly)phenol intake a | 0.883 | ||
| Dietary fibre | 0.814 | ||
| Intrinsic sugars | 0.755 | ||
| Added sugars a | 0.893 | ||
| Carbohydrates | 0.727 | ||
| Eigenvalues | 6.125 | 1.618 | 1.258 |
| Percentage of total variance | 51.040 | 13.483 | 10.487 |
| Components | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| % Body fat | 0.975 | |||
| % Muscle mass | -0.944 | |||
| Visceral fat area | 0.934 | |||
| SUMM 6 skinfolds a | 0.803 | |||
| BMI | 0.639 | 0.503 | ||
| TG a | 0.934 | |||
| VLDL a | 0.933 | |||
| HDL a | -0.808 | |||
| HOMA-IR a | 0.665 | |||
| DBP a | 0.918 | |||
| SBP | 0.905 | |||
| Waist/hip ratio | 0.551 | 0.565 | ||
| Total cholesterol | 0.986 | |||
| LDL | 0.921 | |||
| Eigenvalues | 4.268 | 3.717 | 2.260 | 1.357 |
| Percentage of total variance | 30.487 | 26.552 | 16.143 | 9.695 |
| Components | |||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Lipids | 0.942 | ||||||
| MUFA a | 0.875 | ||||||
| SFA a | 0.841 | ||||||
| Energy intake | 0.837 | ||||||
| Proteins a | 0.788 | ||||||
| PUFA a | 0.757 | ||||||
| Dietary cholesterol | 0.594 | ||||||
| % Body fat | 0.967 | ||||||
| % Muscle mass | -0.935 | ||||||
| Visceral fat area | 0.924 | ||||||
| SUMM 6 skinfolds a | 0.786 | ||||||
| BMI | 0.642 | 0.553 | |||||
| TG a | 0.884 | ||||||
| VLDL a | 0.882 | ||||||
| HDL a | -0.824 | ||||||
| HOMA-IR a | 0.674 | ||||||
| Waist/hip ratio | 0.626 | 0.534 | |||||
| CT | 0.931 | ||||||
| LDL | 0.922 | ||||||
| SBP | 0.897 | ||||||
| DBP a | 0.885 | ||||||
| Intrinsic sugars | 0.828 | ||||||
| Total (poly)phenol intake a | 0.824 | ||||||
| Dietary fibre a | 0.681 | ||||||
| Carbohydrates | 0.740 | ||||||
| Added sugars a | 0.717 | ||||||
| Eigenvalues | 6.736 | 4.394 | 3.431 | 2.439 | 1.700 | 1.310 | 1.172 |
| Percentage of total variance | 25.908 | 16.902 | 13.195 | 9.379 | 6.540 | 5.040 | 4.508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





