Submitted:
27 January 2024
Posted:
29 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patients
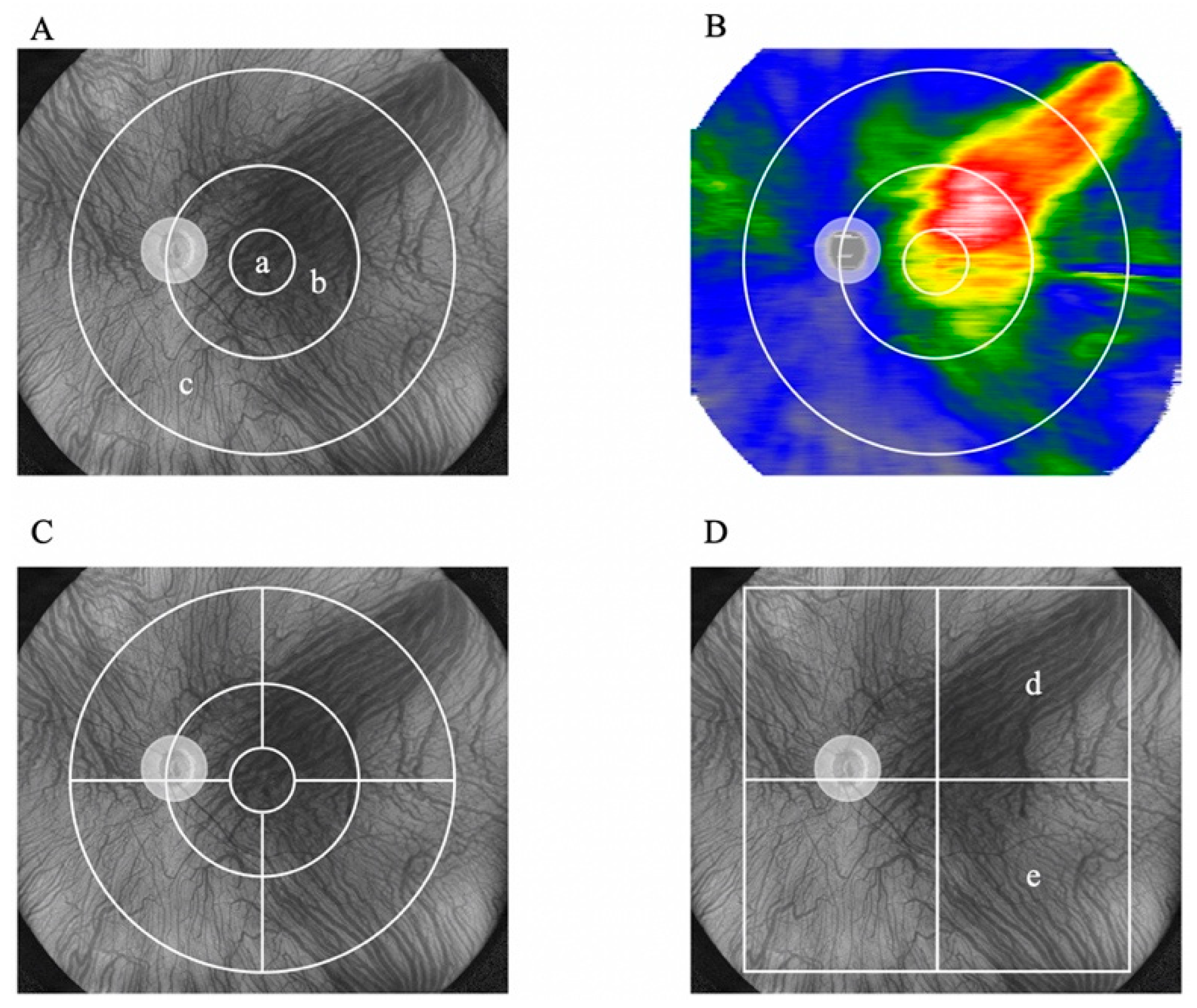
2.2. Evaluation of Choroidal Thickness via UWF Swept-Source OCT
2.3. Statistical Analyses
3. Results
3.1. Characteristics of the Enrolled Patients
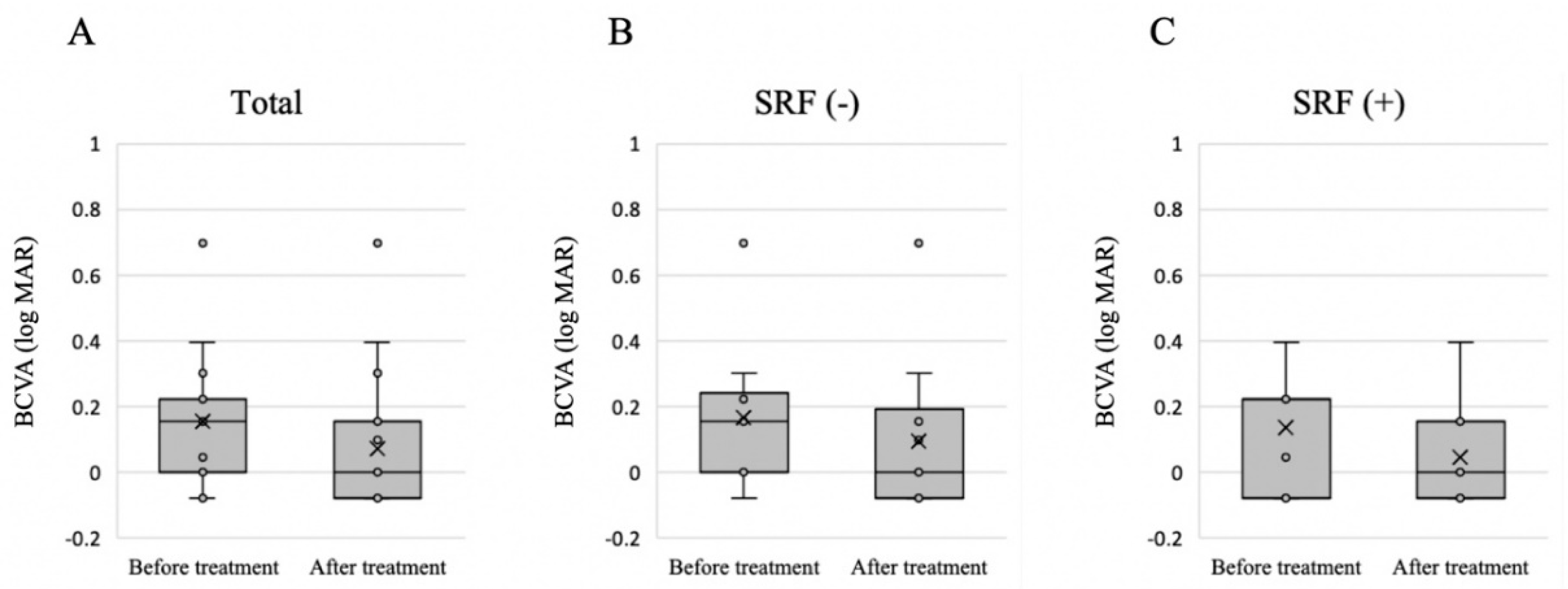
3.2. Treatment Outcome and Factors Related to the Presence of SRF after Treatment
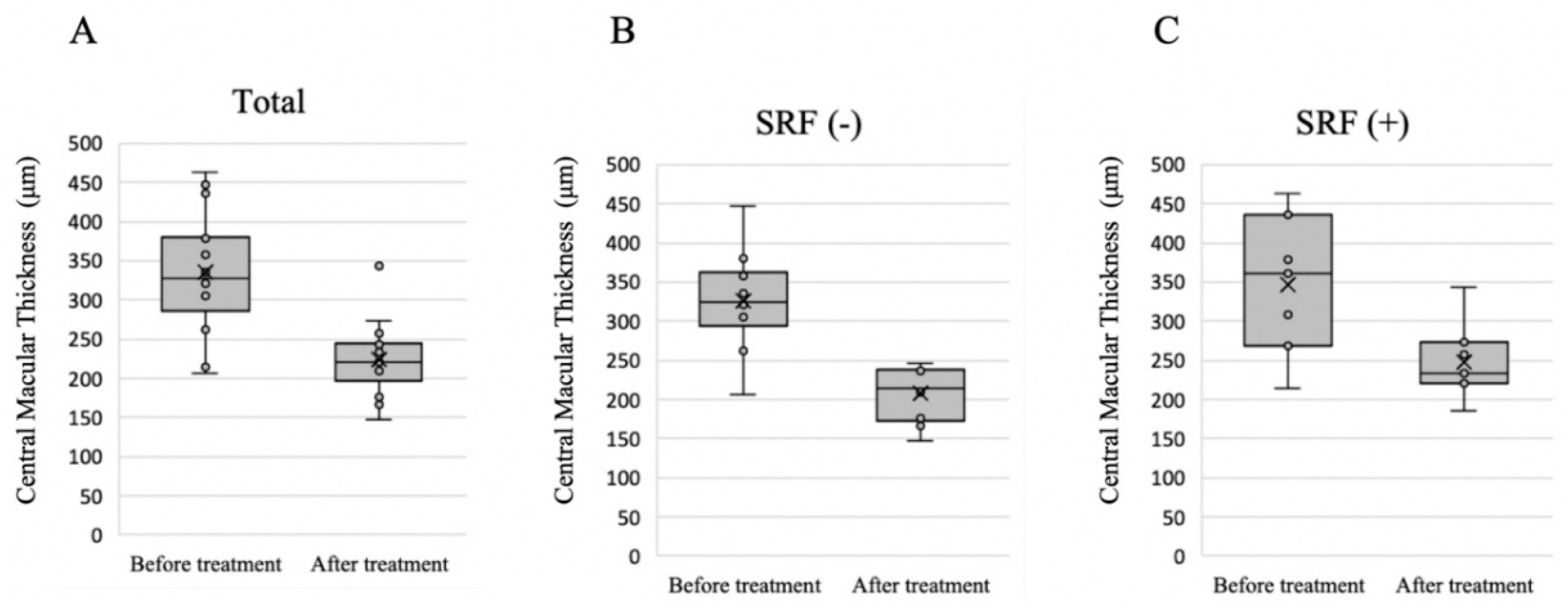
3.3. Choroidal Thickness before and after Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pang, C.E.; Freund, K.B. Pachychoroid neovasculopathy. Retina 2015, 35, 1–9. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y. Pachychoroid disease: a new perspective on exudative maculopathy. Jpn. J. Ophthalmol. 2020, 64, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Notomi, S.; Shiose, S.; Ishikawa, K.; Fukuda, Y.; Kano, K.; Mori, K.; Wada, I.; Kaizu, Y.; Matsumoto, H.; Akiyama, M.; et al. Drusen and pigment abnormality predict the development of neovascular age-related macular degeneration in Japanese patients. PLOS ONE 2021, 16, e0255213. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Notomi, S.; Shiose, S.; Kano, K.; Hashimoto, S.; Fujiwara, K.; Akiyama, M.; Ishikawa, K.; Hisatomi, T.; Sonoda, K.-H. Differences in Central and Peripheral Choroidal Thickness among the Subtypes of Age-Related Macular Degeneration in an Asian Population. J. Clin. Med. 2023, 12, 5364. [Google Scholar] [CrossRef]
- Ishikura, M.; Muraoka, Y.; Nishigori, N.; Takahashi, A.; Miyake, M.; Ueda-Arakawa, N.; Miyata, M.; Ooto, S.; Tsujikawa, A. Widefield Choroidal Thickness of Eyes with Central Serous Chorioretinopathy Examined by Swept-Source OCT. Ophthalmol. Retin. 2022, 6, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Funatsu, R.; Sonoda, S.; Terasaki, H.; Shiihara, H.; Mihara, N.; Horie, J.; Sakamoto, T. Choroidal morphologic features in central serous chorioretinopathy using ultra-widefield optical coherence tomography. Graefe's Arch. Clin. Exp. Ophthalmol. 2023, 261, 971–979. [Google Scholar] [CrossRef]
- Fung, A.T.M.; Yannuzzi, L.A.; Freund, K. Type 1 (sub-retinal pigment epithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012, 32, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hiroe, T.; Morimoto, M.; Mimura, K.; Ito, A.; Akiyama, H. Efficacy of treat-and-extend regimen with aflibercept for pachychoroid neovasculopathy and Type 1 neovascular age-related macular degeneration. Jpn. J. Ophthalmol. 2018, 62, 144–150. [Google Scholar] [CrossRef]
- Schworm, B.; Luft, N.; Keidel, L.F.; Kreutzer, T.C.; Herold, T.R.; Priglinger, S.G.; Siedlecki, J. Vanishing pachy-choroid in pachychoroid neovasculopathy under long-term anti-vascular endothelial growth factor therapy. BMC Ophthalmol. 2021, 21, 269. [Google Scholar] [CrossRef]
- Sartini, F.; Figus, M.; Casini, G.; Nardi, M.; Posarelli, C. Pachychoroid neovasculopathy: a type-1 choroidal neovascularization belonging to the pachychoroid spectrum-pathogenesis, imaging and available treatment options. Int Ophthalmol. 2020, 40, 3577–3589. [Google Scholar] [CrossRef] [PubMed]
- Maruko, I.; Iida, T.; Sugano, Y.; Ojima, A.; Ogasawara, M.; Spaide, R.F. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010, 117, 1792–1799. [Google Scholar] [CrossRef]
- Yamada-Okahara, N.; Kyo, A.; Hirayama, K.; Yamamoto, M.; Kohno, T.; Honda, S. Practical treatment options for persistent central serous chorioretinopathy and early visual and anatomical outcomes. Jpn. J. Ophthalmol. 2023, 67, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, Y.; Maruyama-Inoue, M.; Ito, A.; Sato, S.; Inoue, T.; Yamane, S.; Kadonosono, K. One-year outcome of combination therapy with intravitreal anti-vascular endothelial growth factor and photodynamic therapy in patients with pachychoroid neovasculopathy. Graefe's Arch. Clin. Exp. Ophthalmol. 2020, 258, 1279–1285. [Google Scholar] [CrossRef]
- Roy, R.; Saurabh, K.; Shah, D.; Goel, S. Treatment outcomes of pachychoroid neovasculopathy with photodynamic therapy and anti-vascular endothelial growth factor. Indian J. Ophthalmol. 2019, 67, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Hiroe, T.; Kishi, S. Dilatation of Asymmetric Vortex Vein in Central Serous Chorioretinopathy. Ophthalmol. Retin. 2018, 2, 152–161. [Google Scholar] [CrossRef]
- Funatsu, R.; Sonoda, S.; Terasaki, H.; Shiihara, H.; Mihara, N.; Horie, J.; Sakamoto, T. Effect of photodynamic therapy on choroid of the medial area from optic disc in patients with central serous chorioretinopathy. PLOS ONE 2023, 18, e0282057. [Google Scholar] [CrossRef]
- Nishigori, N.; Muraoka, Y.; Ishikura, M.; Kogo, T.; Ueda-Arakawa, N.; Miyata, M.; Tamura, H.; Hata, M.; Takahashi, A.; Miyake, M.; et al. Extensive reduction in choroidal thickness after photodynamic therapy in eyes with central serous chorioretinopathy. Sci. Rep. 2023, 13, 10890. [Google Scholar] [CrossRef]
- Matsumoto, H.; Hoshino, J.; Nakamura, K.; Kishi, S.; Akiyama, H. Attenuation of irradiated choroid and its regional vortex veins in central serous chorioretinopathy after photodynamic therapy. Sci. Rep. 2023, 13, 19903. [Google Scholar] [CrossRef]
- Matsumoto, H.; Mukai, R.; Hoshino, J.; Oda, M.; Matsuzaki, T.; Ishizaki, Y.; Shibasaki, K.; Akiyama, H. Choroidal congestion mouse model: Could it serve as a pachychoroid model? PLoS ONE 2021, 16, e0246115. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Mukai, R.; Saito, K.; Hoshino, J.; Kishi, S.; Akiyama, H. Vortex vein congestion in the monkey eye: A possible animal model of pachychoroid. PLOS ONE 2022, 17, e0274137. [Google Scholar] [CrossRef] [PubMed]
- Hirooka, K.; Saito, M.; Yamashita, Y.; Hashimoto, Y.; Terao, N.; Koizumi, H.; Noda, K.; Ishida, S. Imbalanced choroidal circulation in eyes with asymmetric dilated vortex vein. Jpn. J. Ophthalmol. 2021, 66, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Kishi, S.; Matsumoto, H. A new insight into pachychoroid diseases: Remodeling of choroidal vasculature. Graefe's Arch. Clin. Exp. Ophthalmol. 2022, 260, 3405–3417. [Google Scholar] [CrossRef]
- Schlötzer-Schrehardt, U.; Viestenz, A.; Naumann, G.O.; Laqua, H.; Michels, S.; Schmidt-Erfurth, U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe's Arch. Clin. Exp. Ophthalmol. 2002, 240, 748–757. [Google Scholar] [CrossRef]
- Demircan, A.; Yesilkaya, C.; Alkin, Z. Early choriocapillaris changes after half-fluence photodynamic therapy in chronic central serous chorioretinopathy evaluated by optical coherence tomography angiography: Preliminary results. Photodiagnosis Photodyn. Ther. 2018, 21, 375–378. [Google Scholar] [CrossRef]
- Hunt, D.W.; Chan, A.H. Influence of photodynamic therapy on immunological aspects of disease - an update. Expert Opin. Investig. Drugs 2000, 9, 807–817. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Schlötzer-Schrehard, U.; Cursiefen, C.; Michels, S.; Beckendorf, A.; Naumann, G.O.H. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Investig. Opthalmology Vis. Sci. 2003, 44, 4473–4480. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Woo, S.J.; Yu, H.G.; Park, K.H. Comparison of efficacy and safety between half-fluence and full-fluence photodynamic therapy for chronic central serous chorioretinopathy. Retina 2011, 31, 119–126. [Google Scholar] [CrossRef]
- Nicoló, M.; Eandi, C.M.; Alovisi, C.; Grignolo, F.M.; Traverso, C.E.; Musetti, D.; Piccolino, F.C. Half-fluence versus half-dose photodynamic therapy in chronic central serous chorioretinopathy. Am. J. Ophthalmol. 2014, 157, 1033–1037. [Google Scholar] [CrossRef]
- Shiragami, C.; Takasago, Y.; Osaka, R.; Kobayashi, M.; Ono, A.; Yamashita, A.; Hirooka, K. Clinical Features of Central Serous Chorioretinopathy With Type 1 Choroidal Neovascularization. Am. J. Ophthalmol. 2018, 193, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Minnella, A.M.; Centini, C.; Gambini, G.; Savastano, M.C.; Pagliei, V.; Falsini, B.; Rizzo, S.; Ciasca, G.; Maceroni, M. Choroidal Thickness Changes After Intravitreal Aflibercept Injections in Treatment-Naïve Neovascular AMD. Adv. Ther. 2022, 39, 3248–3261. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Kano, M.; Yamamoto, A.; Saito, M.; Maruko, I.; Sekiryu, T.; Okada, A.A.; Iida, T. Subfoveal Choroidal Thickness during Aflibercept Therapy for Neovascular Age-Related Macular Degeneration: Twelve-Month Results. Ophthalmology 2016, 123, 617–624. [Google Scholar] [CrossRef] [PubMed]
| 17 eyes of 16 patients, n (%) or mean ± SD | |
|---|---|
| Male | 9 (56.3 %) |
| Age (y) | 65.1 ± 12.5 |
| Axial length (mm) | 24.3 ± 1.1 |
| BCVA (logMAR) | 0.15 ± 0.20 |
| GLD (μm) | 3500 ± 905 |
| CMT (μm) | 335.1 ± 73.7 |
| Dominant side of deep choroidal veins | |
| Upper dominant | 8 (47.1%) |
| Lower dominant | 3 (17.6%) |
| Asymmetry | 6 (35.3%) |
| SRF (-) | SRF (+) | P-value | |
|---|---|---|---|
| n | 10 | 7 | |
| Male | 6 (60.0%) | 4 (56.3%) | 0.64 |
| Age (y) | 61.0 ± 11.1 | 71.0 ± 12.5 | 0.11 |
| Axial length (mm) | 24.4 ± 0.8 | 24.2 ± 1.4 | 0.64 |
| BCVA (logMAR) | 0.17 ± 0.22 | 0.14 ± 0.18 | 0.76 |
| GLD (μm) | 3620 ± 980 | 3329 ± 828 | 0.53 |
| CMT (μm) | 326.7 ± 64.6 | 347.1 ± 89.2 | 0.59 |
| Asymmetry of vortex vein | 0.83 | ||
| Upper dominant | 4 (40.0%) | 4 (57.1%) | |
| Lower dominant | 2 (20.0%) | 1 (14.3%) | |
| Asymmetry | 4 (40.0%) | 2 (28.6%) |
| Areas | Before treatment | After treatment | P-value |
|---|---|---|---|
| 3-mm subfield | 359.1 ± 62.0 | 359.1 ± 62.0 | <0.001 |
| 3–9-mm subfield | 287.9 ± 48.1 | 253.5 ± 50.2 | <0.001 |
| Supratemporal 3–9 mm | 348.8 ± 60.0 | 305.5 ± 58.5 | <0.001 |
| Infratemporal 3–9 mm | 320.5 ± 66.9 | 278.1 ± 63.7 | <0.001 |
| Supranasal 3–9 mm | 314.8 ± 72.6 | 287.1 ± 70.5 | <0.001 |
| Infranasal 3–9 mm | 275.8 ± 59.3 | 244.4 ± 64.6 | <0.001 |
| 9–18-mm subfield | 236.1 ± 40.5 | 215.8 ± 38.9 | <0.001 |
| Supratemporal 9–18 mm | 281.1 ± 54.9 | 255.9 ± 51.8 | <0.001 |
| Infratemporal 9–18 mm | 233.1 ± 51.7 | 210.6 ± 47.7 | <0.001 |
| Supranasal 9–18 mm | 252.1 ± 62.4 | 232.9 ± 59.7 | <0.001 |
| Infranasal 9–18 mm | 178.1 ± 38.4 | 163.8 ± 36.3 | <0.001 |
| Areas | Before treatment | After treatment | Ratio | P-value |
|---|---|---|---|---|
| 9-mm subfield | 282.3 ± 53.1 | 258.5 ± 48.1 | 0.88 ± 0.07 | 0.002 |
| 9–18-mm subfield | 222.6 ± 47.1 | 198.6 ± 49.0 | 0.91 ± 0.06 |
| Asymmetrical pattern (n=11; upper dominant, n=8, lower dominant, n=3) | ||||
| Areas | Before treatment | After treatment | Ratio | P-value |
| Dominant side | 273.5 ± 49.4 | 257.5 ± 53.0 | 0.94 ± 0.05 | 0.68 |
| Non-dominant side | 253.2 ± 36.6 | 237.8 ± 47.7 | 0.94 ± 0.05 | |
| Symmetrical pattern (n=6) | ||||
| Areas | Before treatment | After treatment | Ratio | P-value |
| Upper area | 270.5 ± 65.3 | 241.7 ± 64.4 | 0.89 ± 0.08 | 0.11 |
| Lower area | 186.5 ± 27.6 | 153.3 ± 36.0 | 0.83 ± 0.18 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





