Submitted:
29 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
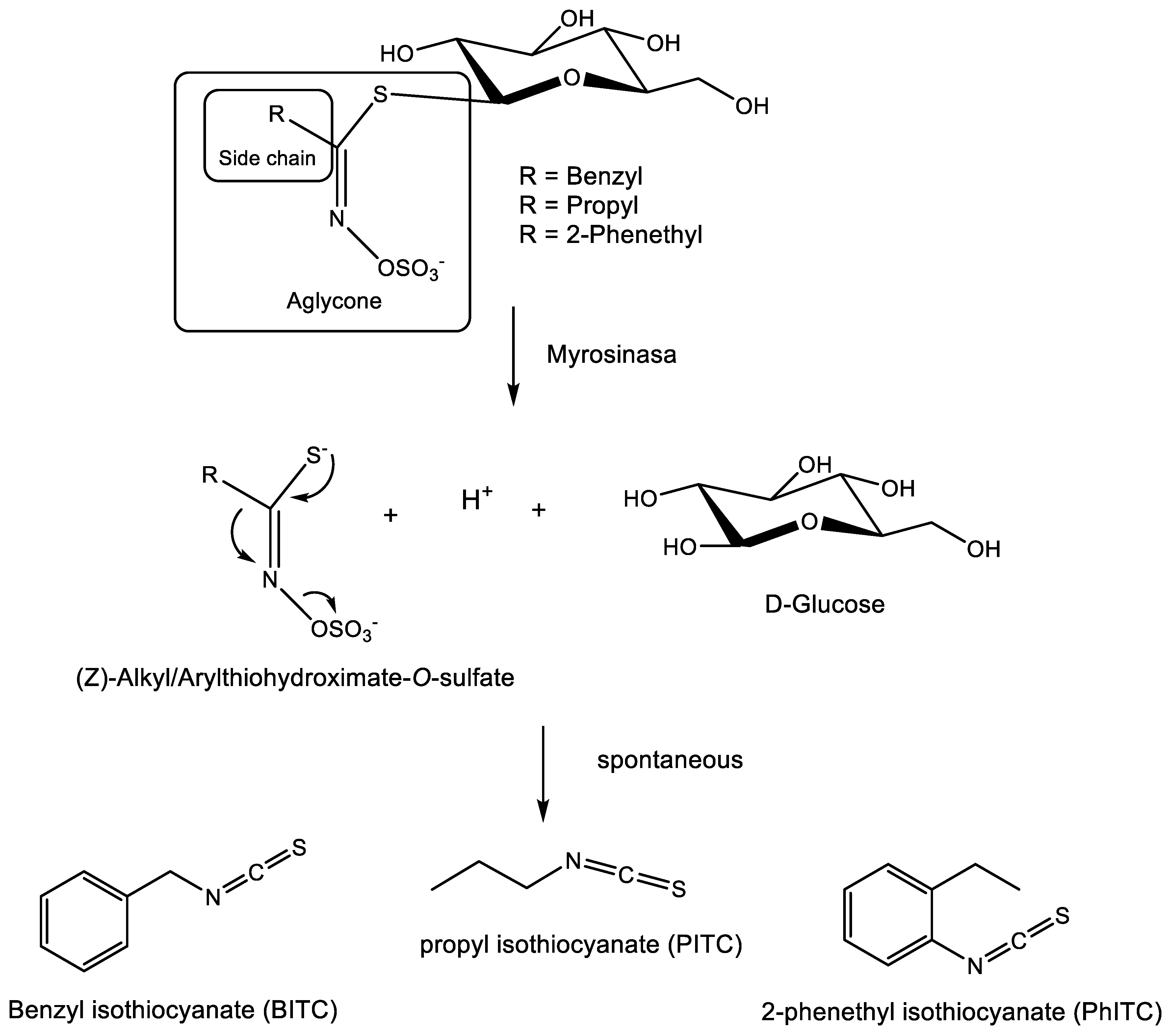
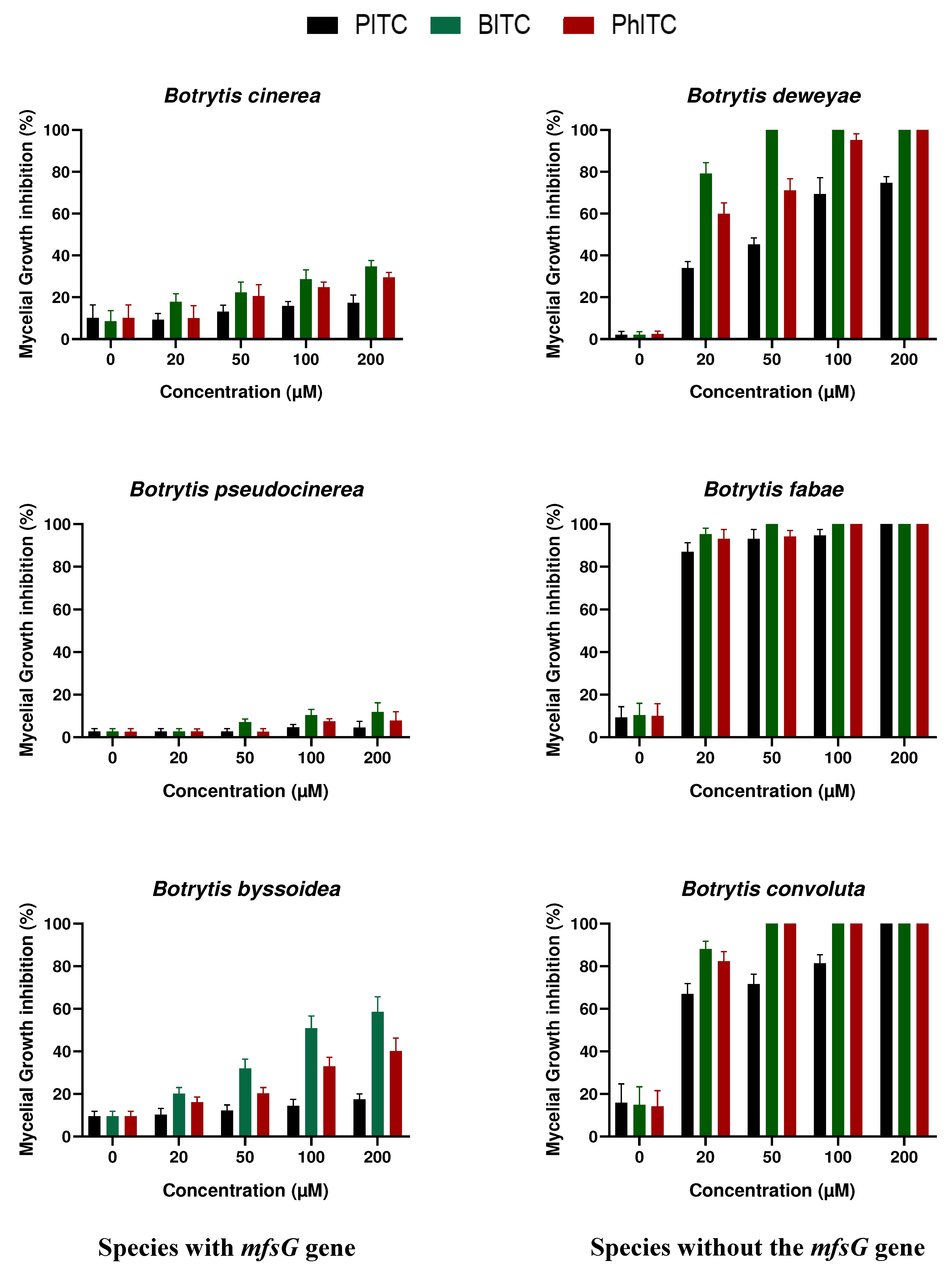
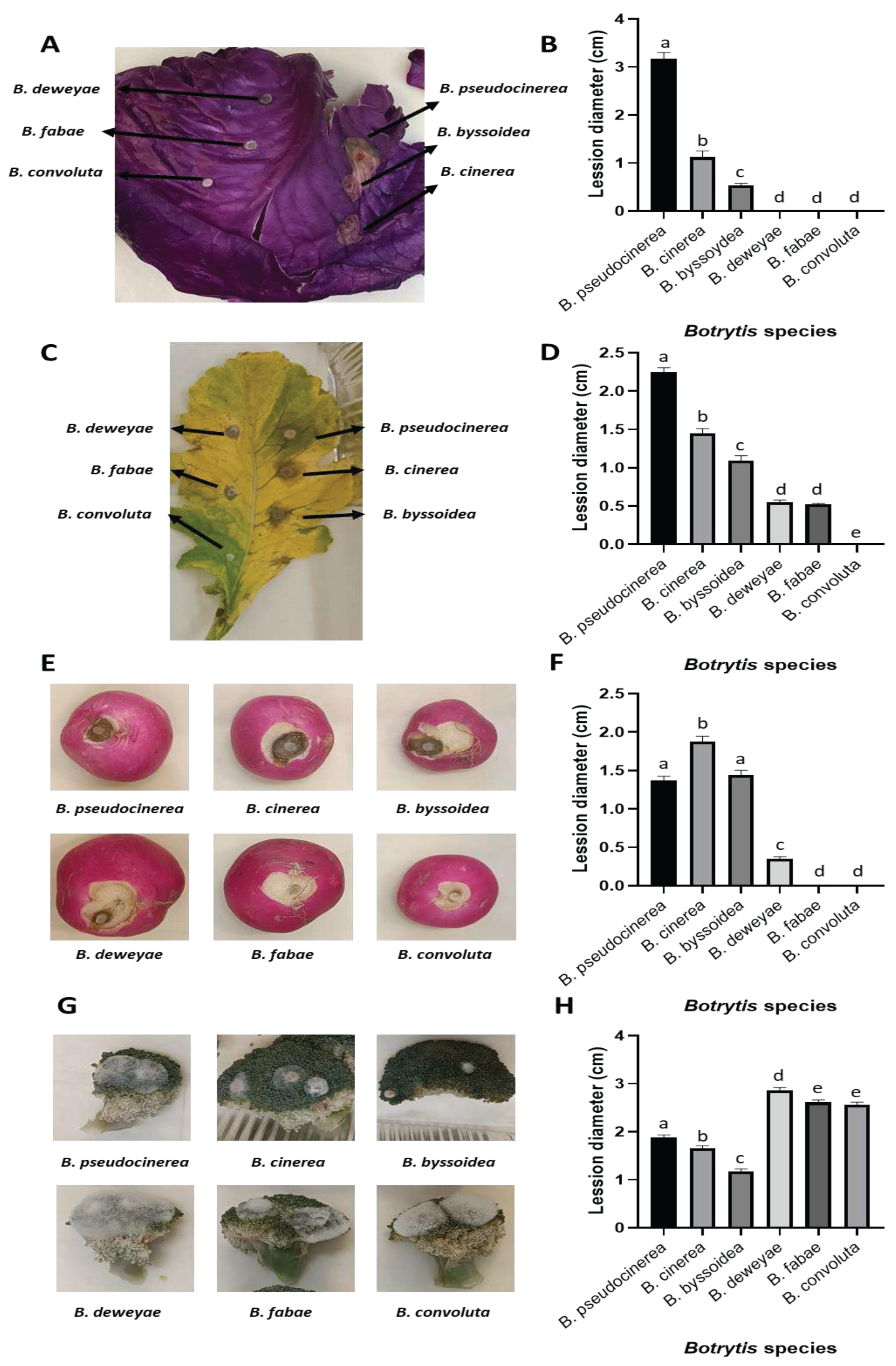
2.1. Evaluation of the Tolerance of Botrytis Species to Glucosinolate Breakdown Products
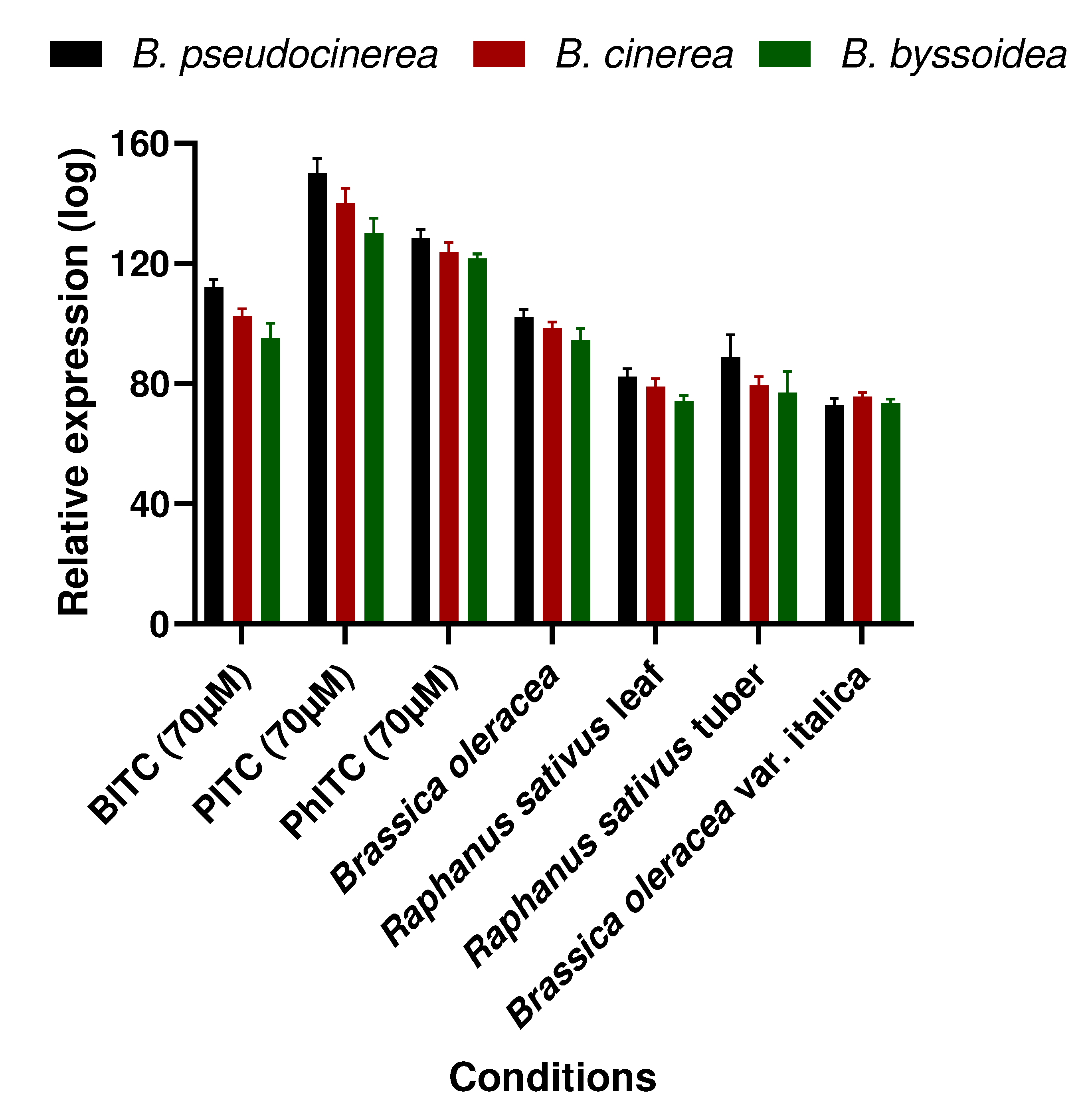
2.2. Glucosinolate Breakdown Products and Infection Processes Induce the mfsG Gene
3. Discussion
4. Materials and Methods
4.1. Organisms, Media and Culture Conditions
4.2. Vegetative Growth
4.3. Virulence Assay
4.4. Quantitative Real-Time PCR (qRT-PCR)
4.5. Bioinformatic Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Smith, D.A.; Banks, S.W. Formation and biological properties of isoflavonoid phytoalexins. Prog. Clin. Biol. Res. 1986, 213, 113–24. [Google Scholar]
- Arbona, V.; Gómez-Cadenas, A. Omics in Plant Disease Resistance; Caister Academic Press, 2016; ISBN 9781910190357. [Google Scholar]
- VanEtten, H.D.; Mansfield, J.W.; Bailey, J.A.; Farmer, E.E. Two classes of plant antibiotics: phytoalexins versus “Phytoanticipins” . Plant Cell 1994, 6, 1191–1192. [Google Scholar] [CrossRef]
- JPMorrissey, A.O. Fungal resistance to plant antibiotics as a mechanism of pathogenesis. Microbiol. Mol. Biol. Rev. 1999, 63, 708–724. [Google Scholar] [CrossRef]
- Kliebenstein, D.J. Secondery metabolites and plant/environment interactions: a view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- VanEtten, H.D.; Matthews, D.E.; Matthews, P.S. Phytoalexin detoxification: importance for pathogenicity and practical implications. Annu. Rev. Phytopathol. 1989, 27, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Ahiahonu, P.W.K. Metabolism and detoxification of phytoalexins and analogs by phytopathogenic fungi. Phytochemistry 2005, 66, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Ahiahonu, P.W.K. Probing the phytopathogenic stem rot fungus with phytoalexins and analogues: unprecedented glucosylation of camalexin and 6-methoxycamalexin. Bioorg. Med. Chem. 2002, 10, 3307–3312. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, H.; Del Sorbo, G.; De Waard, M.A. The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol. Plant Microbe Interact. 2001, 14, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, H.J.; Raaijmakers, J.M.; De Waard, M.A. Fungal ABC transporters and microbial interactions in natural environments. Mol. Plant Microbe Interact. 2002, 15, 1165–1172. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef]
- Burow, M.; Wittstock, U. Regulation and function of specifier proteins in plants. Phytochem. Rev. 2009, 8, 87–99. [Google Scholar] [CrossRef]
- Borek, V.; Morra, M.J.; Brown, P.D.; McCaffrey, J.P. Allelochemicals produced during sinigrin decomposition in Soil. J. Agric. Food Chem. 1994, 42, 1030–1034. [Google Scholar] [CrossRef]
- Andréasson, E.; J#xD8;rgensen, L.B.; Höglund, A.-S.; Rask, L.; Meijer, J. Different myrosinase and idioblast distribution in Arabidopsis and Brassica napus. Plant Physiol. 2001, 127, 1750–1763. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, G.; Schoonbeek, H.J.; De Waard, M.A. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Rogers, B. The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 2001, 3, 207–214. [Google Scholar] [PubMed]
- Urban, M.; Bhargava, T.; Hamer, J.E. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 1999, 18, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.B.; Suresh, A.; Deng, Y.Z.; Naqvi, N.I. A multidrug resistance transporter in Magnaporthe is required for host penetration and for survival during oxidative stress. Plant Cell 2006, 18, 3686–3705. [Google Scholar] [CrossRef]
- Gupta, A.; Chattoo, B.B. Functional analysis of a novel ABC transporter ABC4 from Magnaporthe grisea. FEMS Microbiol. Lett. 2008, 278, 22–28. [Google Scholar] [CrossRef]
- Stefanato, F.L.; Abou-Mansour, E.; Buchala, A.; Kretschmer, M.; Mosbach, A.; Hahn, M.; Bochet, C.G.; Métraux, J.P.; Schoonbeek, H.J. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J. 2009, 58, 499–510. [Google Scholar] [CrossRef]
- Fleißner, A.; Sopalla, C.; Weltring, K.M. An ATP-binding cassette multidrug-resistance transporter is necessary for tolerance of Gibberella pulicaris to phytoalexins and virulence on potato tubers. Mol. Plant Microbe Interact. 2002, 15, 102–108. [Google Scholar] [CrossRef]
- Stergiopoulos, I.; Van Nistelrooy, J.G.M.; Kema, G.H.J.; De Waard, M.A. Multiple mechanisms account for variation in base-line sensitivity to azole fungicides in field isolates of Mycosphaerella graminicola. Pest Manag Sci. 2003, 59, 1333–1343. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, pathology and control. Botrytis Biol. Pathol. Control 2007, 1–403. [Google Scholar] [CrossRef]
- Fillinger, S.; Elad, Y. Botrytis – the Fungus, the Pathogen and its management in agricultural systems. Botrytis - Fungus, Pathog. its Manag. Agric. Syst. 2016, 1–486. [Google Scholar] [CrossRef]
- Leroux, P.; Fritz, R.; Debieu, D.; Albertini, C.; Lanen, C.; Bach, J.; Gredt, M.; Chapeland, F. Mechanisms of resistance to fungicides in field strains of Botrytis cinerea. Pest Manag. Sci. 2002, 58, 876–888. [Google Scholar] [CrossRef]
- Sofianos, G.; Samaras, A.; Karaoglanidis, G. Multiple and multidrug resistance in Botrytis cinerea: molecular mechanisms of MLR/MDR strains in Greece and effects of co-existence of different resistance mechanisms on fungicide sensitivity. Front. Plant Sci. 2023, 14. [Google Scholar] [CrossRef]
- Fillinger, S.; Walker, A.-S. Chemical control and resistance management of Botrytis diseases. In Botrytis – the Fungus, the Pathogen and its Management in Agricultural Systems; Springer International Publishing: Cham, 2016; pp. 189–216. [Google Scholar]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS One 2013, 8, e70771. [Google Scholar] [CrossRef]
- Vela-Corcía, D.; Aditya Srivastava, D.; Dafa-Berger, A.; Rotem, N.; Barda, O.; Levy, M. MFS transporter from Botrytis cinerea provides tolerance to glucosinolate-breakdown products and is required for pathogenicity. Nat. Commun. 2019, 10, 2886. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liang, Z.; Zang, Y.; Zhu, Z.; Yang, J. Diversity of glucosinolates among common Brassicaceae vegetables in China. Hortic. Plant J. 2023, 9, 365–380. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed]
- Van Kan, J.A.L.L.; Stassen, J.H.M.M.; Mosbach, A.; Van Der Lee, T.A.J.J.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.; Seidl, M.F.; Cottam, E.; et al. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Saski, C.; Schnabel, G.; Xiao, S.; Hu, M. A high-quality genome resource of Botrytis fragariae, a new and rapidly spreading fungal pathogen causing strawberry gray mold in the United States. Phytopathology 2021, 111, 496–499. [Google Scholar] [CrossRef]
- Valero-Jiménez, C.A.; Steentjes, M.B.F.; Slot, J.C.; Shi-Kunne, X.; Scholten, O.E.; van Kan, J.A.L. Dynamics in secondary metabolite gene clusters in otherwise highly syntenic and stable genomes in the fungal genus Botrytis. Genome Biol. Evol. 2020, 12, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, M.C.; Harper, L.A.; Lopez-Ruiz, F.J. Positive Selection of Transcription Factors Is a Prominent Feature of the Evolution of a Plant Pathogenic Genus Originating in the Miocene. Genome Biol. Evol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Piślewska-Bednarek, M.; Sánchez-Vallet, A.; Molina, A.; Glawischnig, E.; Gigolashvili, T.; Bednarek, P. Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol. Plant 2016, 9, 682–695. [Google Scholar] [CrossRef]
- Yun, H.S.; Kang, B.G.; Kwon, C. Arabidopsis immune secretory pathways to powdery mildew fungi. Plant Signal. Behav. 2016, 11, e1226456. [Google Scholar] [CrossRef]
- He, Y.; Xu, J.; Wang, X.; He, X.; Wang, Y.; Zhou, J.; Zhang, S.; Meng, X. The Arabidopsis Pleiotropic drug resistance transporters PEN3 and PDR12 mediate camalexin secretion for resistance to Botrytis cinerea. Plant Cell 2019, 31, 2206–2222. [Google Scholar] [CrossRef] [PubMed]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Chen, J.; Ullah, C.; Reichelt, M.; Beran, F.; Yang, Z.L.; Gershenzon, J.; Hammerbacher, A.; Vassão, D.G. The phytopathogenic fungus Sclerotinia sclerotiorum detoxifies plant glucosinolate hydrolysis products via an isothiocyanate hydrolase. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- de Waard, M.A.; Andrade, A.C.; Hayashi, K.; Schoonbeek, H.; Stergiopoulos, I.; Zwiers, L. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 2006, 62, 195–207. [Google Scholar] [CrossRef]
- Hayashi, K.; Schoonbeek, H. jan; De Waard, M.A. Bcmfs1, a novel major facilitator superfamily transporter from Botrytis cinerea, provides tolerance towards the natural toxic compounds camptothecin and cercosporin and towards fungicides. Appl Environ. Microbiol. 2002, 68, 4996–5004. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Schoonbeek, H. jan; De Waard, M.A. Modulators of membrane drug transporters potentiate the activity of the DMI fungicide oxpoconazole against Botrytis cinerea. Pest Manag. Sci. 2003, 59, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, I.; Gielkens, M.M.C.; Goodall, S.D.; Venema, K.; De Waard, M.A. Molecular cloning and characterisation of three new ATP-binding cassette transporter genes from the wheat pathogen Mycosphaerella graminicola. Gene 2002, 289, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Shafran, H.; Miyara, I.; Eshed, R.; Prusky, D.; Sherman, A. Development of new tools for studying gene function in fungi based on the Gateway system. Fungal Genet. Biol. 2008, 45, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Acero, F.J.; Cantoral, J.M.; Javier Fernández-Acero, F.; Carbú, M.; Garrido, C.; Vallejo, I.; Cantoral, J.M. Proteomic advances in phytopathogenic fungi. Current Proteomics 2007, 4(2), 79–88. [Google Scholar] [CrossRef]
- Fernández Acero, F.J.; Carbú, M.; El-Akhal, M.R.; Garrido, C.; González-Rodríguez, V.E.; Cantoral, J.M. Development of proteomics-based fungicides: New strategies for environmentally friendly control of fungal plant diseases. Int. J. Mol. Sci. 2011, 12, 795–816. [Google Scholar] [CrossRef]
- Hahn, M.; Scalliet, G. One cut to change them all: CRISPR/Cas, a groundbreaking tool for genome editing in Botrytis cinerea and other fungal plant pathogens. Phytopathology® 2021, 111, 474–477. [Google Scholar] [CrossRef]
- Chen, J.; Lai, Y.; Wang, L.; Zhai, S.; Zou, G.; Zhou, Z.; Cui, C.; Wang, S. CRISPR/Cas9-mediated efficient genome editing via blastospore-based transformation in entomopathogenic fungus Beauveria bassiana. Sci. Rep. 2017, 7, 45763. [Google Scholar] [CrossRef]
- Walker, A.S.; Gautier, A.; Confais, J.; Martinho, D.; Viaud, M.; Pêcheur, P. Le; Dupont, J.; Fournier, E. Botrytis pseudocinerea, a new cryptic species causing gray mold in french vineyards in sympatry with Botrytis cinerea. Phytopathology 2011, 101, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- van Kan, J.A.L.; Shaw, M.W.; Grant-Downton, R.T. Botrytis species: Relentless necrotrophic thugs or endophytes gone rogue? Mol. Plant Pathol. 2014, 15, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C. Two undescribed species of Botrytis associated with the neck rot disease of onion bulbs. Phytopathology 1925, 15, 708–713. [Google Scholar]
- Whetzel & Drayton Botrytis convoluta. Mycologia 1932, 24(6), 475.
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. 2012. [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: architecture and applications. BMC Bioinformatics 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–17. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, W320–W324. [Google Scholar] [CrossRef]

| Species | Isolate | NCBI Gene Accession Number |
NCBI Protein Accession Number | Gene Identity with B. cinerea B05.10 (%) | Length (pb) | Reference |
|---|---|---|---|---|---|---|
| B. cinerea | B05.10 | XM_024693262.1 | XP_024549048.1 | 100% | 1379 | [32,33] |
| B. fragariae | BVB16 | XM_037342786.1 | XP_037186706.1 | 86.72% | 1296 | [34] |
| B. byssoidea | MUCL 94 | XM_038882714.1 | XP_038726455.1 | 84.47% | 1296 | [35] |
|
B. pseudocinerea |
BP362 | JAHXJK010000103.1: 18998-20539 | - | 99.33% | 1541 | [36] |
| B. medusae | B555 | JAHXJK010000103.1: 15498-16733 | - | 90.47% | 1235 | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





