Submitted:
29 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
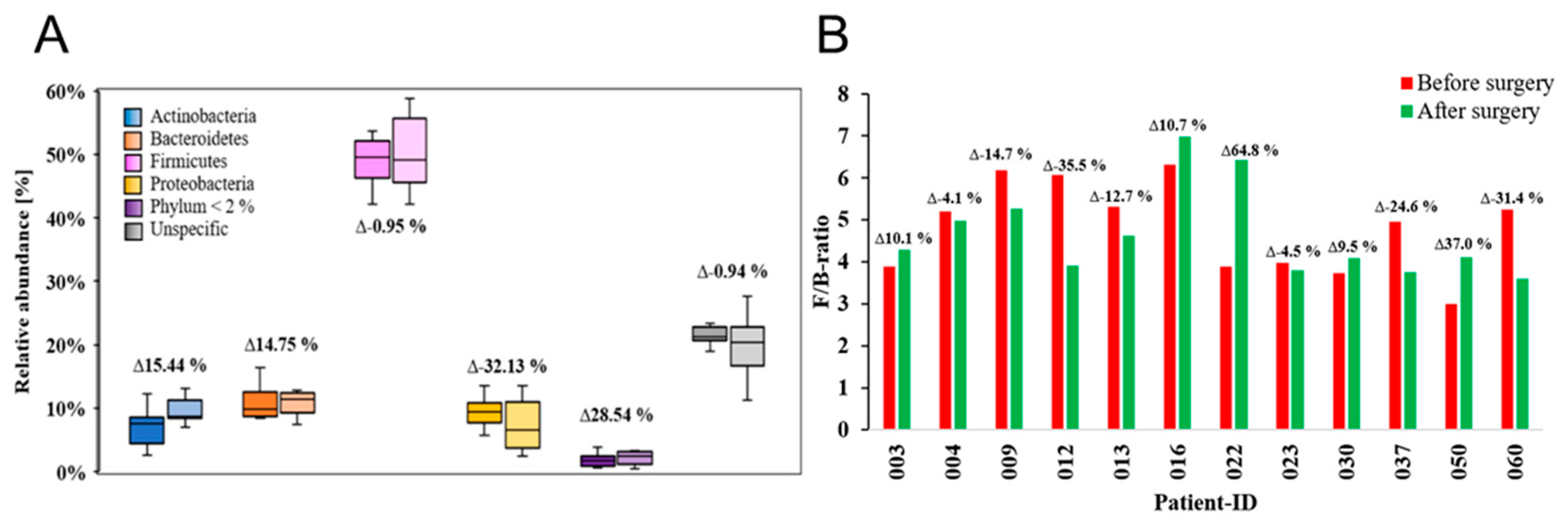
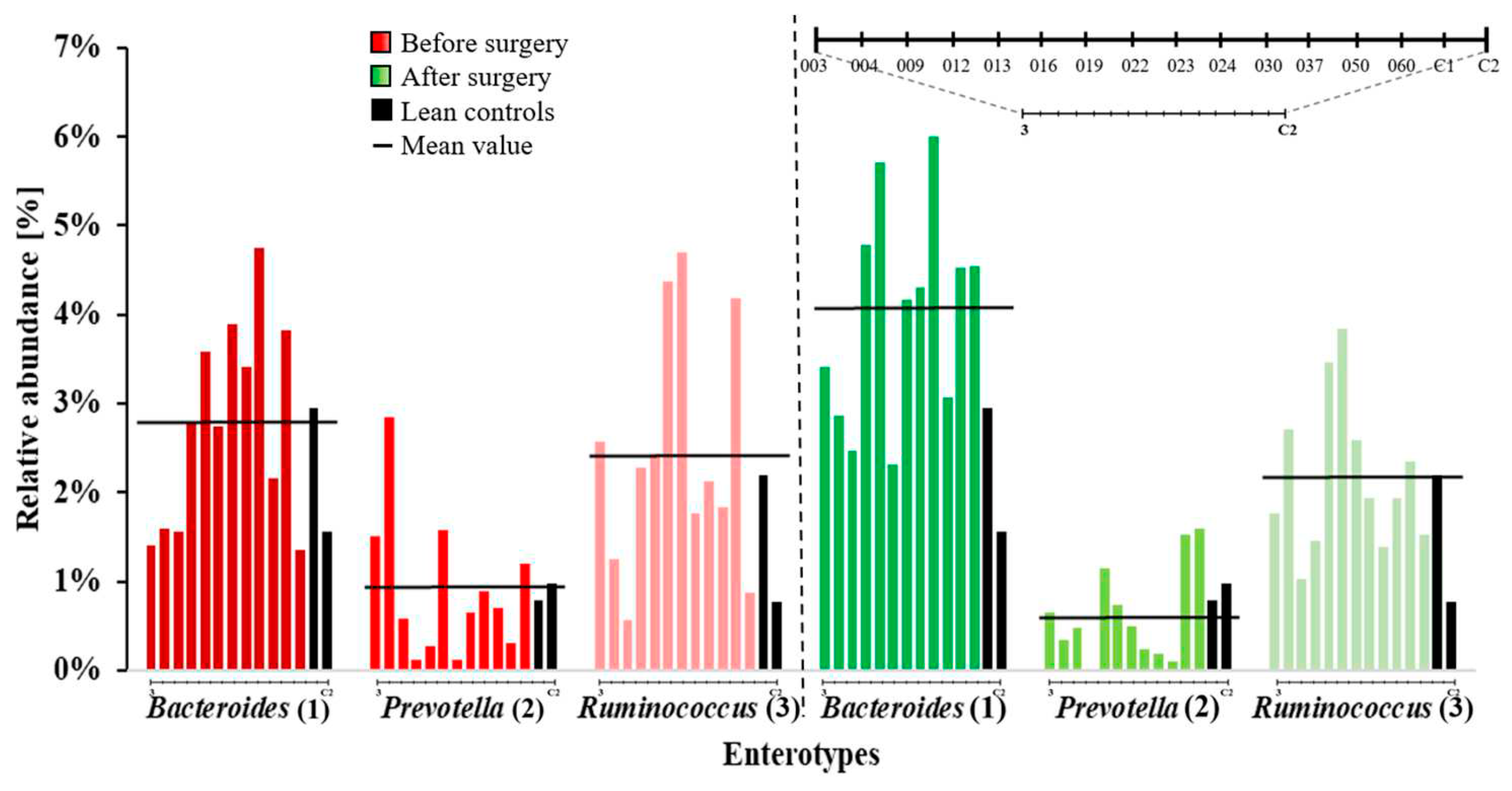
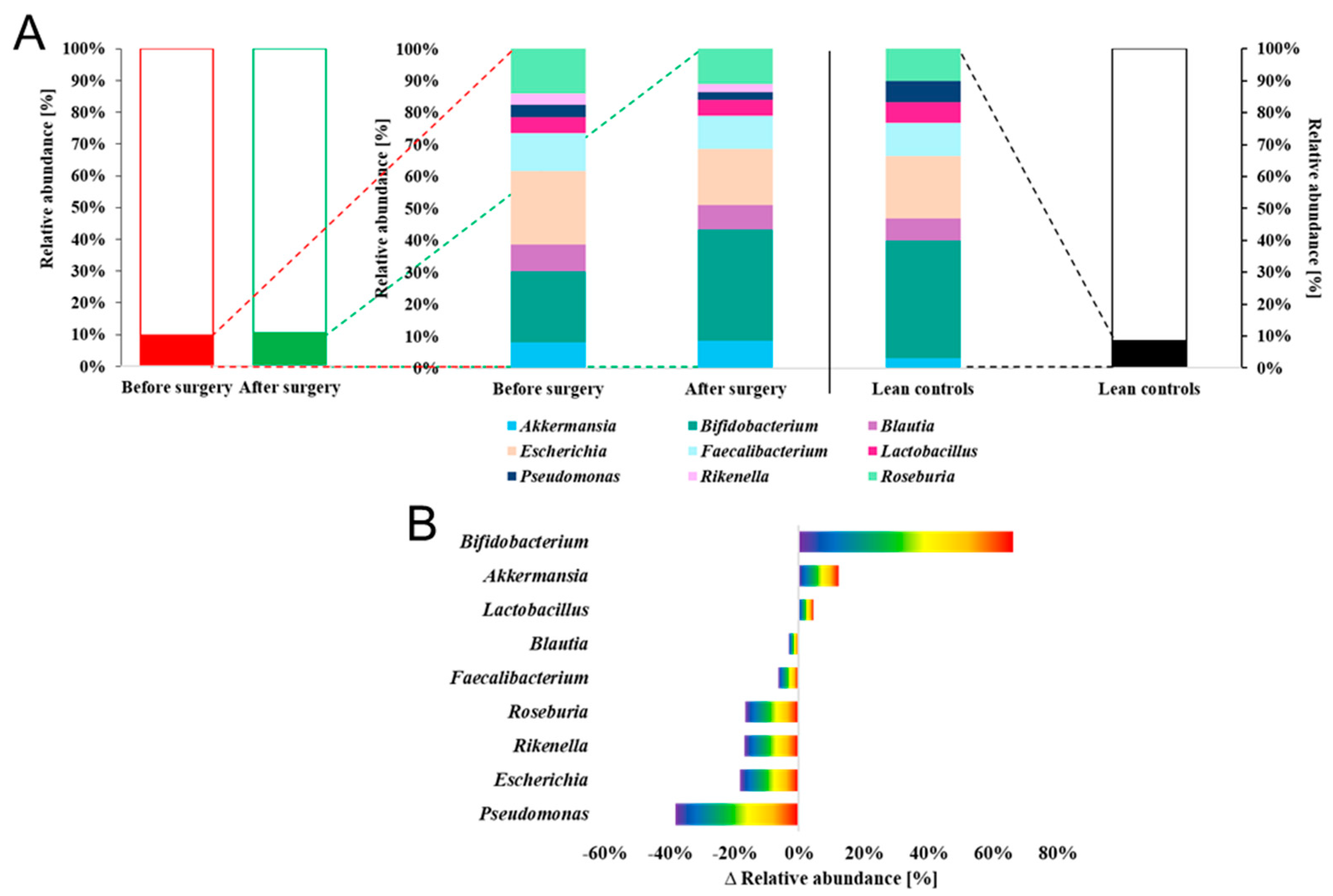
2. Results
3. Discussion
4. Materials and Methods
4.1 Study Cohort
4.2 Anthropometric Measurements and Clinical Data
4.3 16S rDNA Next-Generation Sequencing
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harnack, L.J.; Jeffery, R.W.; Boutelle, K.N. Temporal Trends in Energy Intake in the United States: An Ecologic Perspective. Am J Clin Nutr 2000, 71, 1478–1484. [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proceedings of the National Academy of Sciences 2009, 106, 2365–2370. [CrossRef]
- Stein, C.J.; Colditz, G.A. The Epidemic of Obesity. J Clin Endocrinol Metab 2004, 89, 2522–2525. [CrossRef]
- Fernandez, M.L. The Metabolic Syndrome. Nutr Rev 2008, 65, S30–S34. [CrossRef]
- Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organ Tech Rep Ser 2000, 894, i–xii, 1–253.
- Green, M.; Arora, K.; Prakash, S. Microbial Medicine: Prebiotic and Probiotic Functional Foods to Target Obesity and Metabolic Syndrome. Int J Mol Sci 2020, 21, 2890. [CrossRef]
- Payne, J.H.; DeWind, L.T.; Commons, R.R. Metabolic Observations in Patients with Jejunocolic Shunts. The American Journal of Surgery 1963, 106, 273–289. [CrossRef]
- Siezen, R.J.; Kleerebezem, M. The Human Gut Microbiome: Are We Our Enterotypes? Microb Biotechnol 2011, 4, 550–553. [CrossRef]
- Wijngaarden, L.H.; Taselaar, A.E.; Nuijten, F.; Harst, E. van der; Klaassen, R.A.; Kuijper, T.M.; Jongbloed, F.; Ambagtsheer, G.; Klepper, M.; IJzermans, J.N.M.; et al. T and B Cell Composition and Cytokine Producing Capacity Before and After Bariatric Surgery. Front Immunol 2022, 13. [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [CrossRef]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms Linking Obesity with Cardiovascular Disease. Nature 2006, 444, 875–880. [CrossRef]
- Calle, E.E.; Thun, M.J. Obesity and Cancer. Oncogene 2004, 23, 6365–6378. [CrossRef]
- Buchwald, H.; Avidor, Y.; Braunwald, E.; Jensen, M.D.; Pories, W.; Fahrbach, K.; Schoelles, K. Bariatric Surgery. JAMA 2004, 292, 1724. [CrossRef]
- NIH Conference. Gastrointestinal Surgery for Severe Obesity. Consensus Development Conference Panel. Ann Intern Med 1991, 115, 956–961.
- Runkel, N.; Colombo-Benkmann, M.; Hüttl, T.P.; Tigges, H.; Mann, O.; Sauerland, S. Bariatric Surgery. Dtsch Arztebl Int 2011. [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proceedings of the National Academy of Sciences 2005, 102, 11070–11075. [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. <p>Saturated Fatty Acids in Obesity-Associated Inflammation</P>. J Inflamm Res 2020, Volume 13, 1–14. [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [CrossRef]
- Quigley, E.M.M. Gut Bacteria in Health and Disease. Gastroenterol Hepatol (N Y) 2013, 9, 560–569.
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The Unseen Majority. Proceedings of the National Academy of Sciences 1998, 95, 6578–6583. [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [CrossRef]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill–Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716-1724.e2. [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [CrossRef]
- Liu, B.-N.; Liu, X.-T.; Liang, Z.-H.; Wang, J.-H. Gut Microbiota in Obesity. World J Gastroenterol 2021, 27, 3837–3850. [CrossRef]
- Waters, D.L.; Ward, A.L.; Villareal, D.T. Weight Loss in Obese Adults 65years and Older: A Review of the Controversy. Exp Gerontol 2013, 48, 1054–1061. [CrossRef]
- Kong, F.; Deng, F.; Li, Y.; Zhao, J. Identification of Gut Microbiome Signatures Associated with Longevity Provides a Promising Modulation Target for Healthy Aging. Gut Microbes 2019, 10, 210–215. [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A Human Gut Microbial Gene Catalogue Established by Metagenomic Sequencing. Nature 2010, 464, 59–65. [CrossRef]
- Wu, G.D.; Bushmanc, F.D.; Lewis, J.D. Diet, the Human Gut Microbiota, and IBD. Anaerobe 2013, 24, 117–120. [CrossRef]
- Ghosh, S.; Pramanik, S. Structural Diversity, Functional Aspects and Future Therapeutic Applications of Human Gut Microbiome. Arch Microbiol 2021, 203, 5281–5308. [CrossRef]
- Ciobârcă, D.; Cătoi, A.F.; Copăescu, C.; Miere, D.; Crișan, G. Bariatric Surgery in Obesity: Effects on Gut Microbiota and Micronutrient Status. Nutrients 2020, 12, 235. [CrossRef]
- Coimbra, V.O.R.; Crovesy, L.; Ribeiro-Alves, M.; Faller, A.L.K.; Mattos, F.; Rosado, E.L. Gut Microbiota Profile in Adults Undergoing Bariatric Surgery: A Systematic Review. Nutrients 2022, 14, 4979. [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [CrossRef]
- de Wit, N.; Derrien, M.; Bosch-Vermeulen, H.; Oosterink, E.; Keshtkar, S.; Duval, C.; de Vogel-van den Bosch, J.; Kleerebezem, M.; Müller, M.; van der Meer, R. Saturated Fat Stimulates Obesity and Hepatic Steatosis and Affects Gut Microbiota Composition by an Enhanced Overflow of Dietary Fat to the Distal Intestine. American Journal of Physiology-Gastrointestinal and Liver Physiology 2012, 303, G589–G599. [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Gerardi, V.; Lopetuso, L.; Gasbarrini, A. The Gut Barrier. J Clin Gastroenterol 2012, 46, S12–S17. [CrossRef]
- Purchiaroni, F.; Tortora, A.; Gabrielli, M.; Bertucci, F.; Gigante, G.; Ianiro, G.; Ojetti, V.; Scarpellini, E.; Gasbarrini, A. The Role of Intestinal Microbiota and the Immune System. Eur Rev Med Pharmacol Sci 2013, 17, 323–333.
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Digestive and Liver Disease 2018, 50, 421–428. [CrossRef]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol 2015, 33, 496–503. [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. The Bell system technical journal 1948, 27, 379–423.
- Goodrich, J.K.; Waters, J.L.; Poole, A.C.; Sutter, J.L.; Koren, O.; Blekhman, R.; Beaumont, M.; Van Treuren, W.; Knight, R.; Bell, J.T.; et al. Human Genetics Shape the Gut Microbiome. Cell 2014, 159, 789–799. [CrossRef]
- Beaumont, M.; Goodrich, J.K.; Jackson, M.A.; Yet, I.; Davenport, E.R.; Vieira-Silva, S.; Debelius, J.; Pallister, T.; Mangino, M.; Raes, J.; et al. Heritable Components of the Human Fecal Microbiome Are Associated with Visceral Fat. Genome Biol 2016, 17, 189. [CrossRef]
- Guo, Y.; Liu, C.; Shan, C.; Chen, Y.; Li, H.; Huang, Z.; Zou, D. Gut Microbiota after Roux-en-Y Gastric Bypass and Sleeve Gastrectomy in a Diabetic Rat Model: Increased Diversity and Associations of Discriminant Genera with Metabolic Changes. Diabetes Metab Res Rev 2017, 33. [CrossRef]
- Guo, Y.; Huang, Z.-P.; Liu, C.-Q.; Qi, L.; Sheng, Y.; Zou, D.-J. Modulation of the Gut Microbiome: A Systematic Review of the Effect of Bariatric Surgery. Eur J Endocrinol 2018, 178, 43–56. [CrossRef]
- Shao, Y.; Ding, R.; Xu, B.; Hua, R.; Shen, Q.; He, K.; Yao, Q. Alterations of Gut Microbiota After Roux-En-Y Gastric Bypass and Sleeve Gastrectomy in Sprague-Dawley Rats. Obes Surg 2017, 27, 295–302. [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.-M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science (1979) 2011, 334, 105–108. [CrossRef]
- Vandeputte, D.; De Commer, L.; Tito, R.Y.; Kathagen, G.; Sabino, J.; Vermeire, S.; Faust, K.; Raes, J. Temporal Variability in Quantitative Human Gut Microbiome Profiles and Implications for Clinical Research. Nat Commun 2021, 12, 6740. [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat Rev Gastroenterol Hepatol 2014, 11, 506–514. [CrossRef]
- Douillard, F.P.; de Vos, W.M. Functional Genomics of Lactic Acid Bacteria: From Food to Health. Microb Cell Fact 2014, 13, S8. [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut 2016, 65, 426–436. [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat Med 2017, 23, 107–113. [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat Med 2019, 25, 1096–1103. [CrossRef]
- Dao, M.C.; Belda, E.; Prifti, E.; Everard, A.; Kayser, B.D.; Bouillot, J.-L.; Chevallier, J.-M.; Pons, N.; Le Chatelier, E.; Ehrlich, S.D.; et al. Akkermansia Muciniphila Abundance Is Lower in Severe Obesity, but Its Increased Level after Bariatric Surgery Is Not Associated with Metabolic Health Improvement. American Journal of Physiology-Endocrinology and Metabolism 2019, 317, E446–E459. [CrossRef]
- Damms-Machado, A.; Mitra, S.; Schollenberger, A.E.; Kramer, K.M.; Meile, T.; Königsrainer, A.; Huson, D.H.; Bischoff, S.C. Effects of Surgical and Dietary Weight Loss Therapy for Obesity on Gut Microbiota Composition and Nutrient Absorption. Biomed Res Int 2015, 2015, 1–12. [CrossRef]
- Gihring, A.; Gärtner, F.; Mayer, L.; Roth, A.; Abdelrasoul, H.; Kornmann, M.; Elad, L.; Knippschild, U. Influence of Bariatric Surgery on the Peripheral Blood Immune System of Female Patients with Morbid Obesity Revealed by High-Dimensional Mass Cytometry. Front Immunol 2023, 14. [CrossRef]
- Badal, V.D.; Vaccariello, E.D.; Murray, E.R.; Yu, K.E.; Knight, R.; Jeste, D. V.; Nguyen, T.T. The Gut Microbiome, Aging, and Longevity: A Systematic Review. Nutrients 2020, 12, 3759. [CrossRef]
- Tseng, C.-H.; Wu, C.-Y. The Gut Microbiome in Obesity. Journal of the Formosan Medical Association 2019, 118, S3–S9. [CrossRef]
- Min, Y.; Ma, X.; Sankaran, K.; Ru, Y.; Chen, L.; Baiocchi, M.; Zhu, S. Sex-Specific Association between Gut Microbiome and Fat Distribution. Nat Commun 2019, 10, 2408. [CrossRef]
- Robles-Alonso, V.; Guarner, F. [Progress in the Knowledge of the Intestinal Human Microbiota]. Nutr Hosp 2013, 28, 553–557. [CrossRef]
- Jethwani, P.; Grover, K. Gut Microbiota in Health and Diseases – A Review. Int J Curr Microbiol Appl Sci 2019, 8, 1586–1599. [CrossRef]
- Huse, S.M.; Dethlefsen, L.; Huber, J.A.; Welch, D.M.; Relman, D.A.; Sogin, M.L. Exploring Microbial Diversity and Taxonomy Using SSU RRNA Hypervariable Tag Sequencing. PLoS Genet 2008, 4, e1000255. [CrossRef]
- Lilja, S.; Stoll, C.; Krammer, U.; Hippe, B.; Duszka, K.; Debebe, T.; Höfinger, I.; König, J.; Pointner, A.; Haslberger, A. Five Days Periodic Fasting Elevates Levels of Longevity Related Christensenella and Sirtuin Expression in Humans. Int J Mol Sci 2021, 22, 2331. [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The Healthy Human Microbiome. Genome Med 2016, 8, 51. [CrossRef]
- Konopiński, M.K. Shannon Diversity Index: A Call to Replace the Original Shannon’s Formula with Unbiased Estimator in the Population Genetics Studies. PeerJ 2020, 8, e9391. [CrossRef]
- Ghaffari, S.; Abbasi, A.; Somi, M.H.; Moaddab, S.Y.; Nikniaz, L.; Kafil, H.S.; Ebrahimzadeh Leylabadlo, H. Akkermansia Muciniphila: From Its Critical Role in Human Health to Strategies for Promoting Its Abundance in Human Gut Microbiome. Crit Rev Food Sci Nutr 2023, 63, 7357–7377. [CrossRef]
- Nie, K.; Ma, K.; Luo, W.; Shen, Z.; Yang, Z.; Xiao, M.; Tong, T.; Yang, Y.; Wang, X. Roseburia Intestinalis: A Beneficial Gut Organism From the Discoveries in Genus and Species. Front Cell Infect Microbiol 2021, 11. [CrossRef]
- Elder, K.A.; Wolfe, B.M. Bariatric Surgery: A Review of Procedures and Outcomes. Gastroenterology 2007, 132, 2253–2271. [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of Diet in Shaping Gut Microbiota Revealed by a Comparative Study in Children from Europe and Rural Africa. Proceedings of the National Academy of Sciences 2010, 107, 14691–14696. [CrossRef]
- Ismail, N.A.; Ragab, S.H.; ElBaky, A.A.; Shoeib, A.R.S.; Alhosary, Y.; Fekry, D. Frequency of Firmicutes and Bacteroidetes in Gut Microbiota in Obese and Normal Weight Egyptian Children and Adults. Archives of Medical Science 2011, 3, 501–507. [CrossRef]
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring Bacterial Community of Human Gut Microbiota Reveals an Increase in Lactobacillus in Obese Patients and Methanogens in Anorexic Patients. PLoS One 2009, 4, e7125. [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L. V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The Gut Microbiota as an Environmental Factor That Regulates Fat Storage. Proceedings of the National Academy of Sciences 2004, 101, 15718–15723. [CrossRef]
- Bervoets, L.; Van Hoorenbeeck, K.; Kortleven, I.; Van Noten, C.; Hens, N.; Vael, C.; Goossens, H.; Desager, K.N.; Vankerckhoven, V. Differences in Gut Microbiota Composition between Obese and Lean Children: A Cross-Sectional Study. Gut Pathog 2013, 5, 10. [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.; Kang, D.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutrition in Clinical Practice 2012, 27, 201–214. [CrossRef]
- Xu, P.; Li, M.; Zhang, J.; Zhang, T. Correlation of Intestinal Microbiota with Overweight and Obesity in Kazakh School Children. BMC Microbiol 2012, 12, 283. [CrossRef]
- Fei, N.; Zhao, L. An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice. ISME J 2013, 7, 880–884. [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J Lipid Res 2013, 54, 2325–2340. [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [CrossRef]
- Säemann, M.D.; Böhmig, G.A.; Österreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory Effects of Sodium Butyrate on Human Monocytes: Potent Inhibition of IL-12 and Up-regulation of IL-10 Production. The FASEB Journal 2000, 14, 2380–2382. [CrossRef]
- Soliman, M.M.; Ahmed, M.M.; Salah-eldin, A.; Abdel-Aal, A.A.-A. Butyrate Regulates Leptin Expression through Different Signaling Pathways in Adipocytes. J Vet Sci 2011, 12, 319. [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [CrossRef]
- Hardy, H.; Harris, J.; Lyon, E.; Beal, J.; Foey, A. Probiotics, Prebiotics and Immunomodulation of Gut Mucosal Defences: Homeostasis and Immunopathology. Nutrients 2013, 5, 1869–1912. [CrossRef]
- Fukuda, S.; Toh, H.; Taylor, T.D.; Ohno, H.; Hattori, M. Acetate-Producing Bifidobacteria Protect the Host from Enteropathogenic Infection via Carbohydrate Transporters. Gut Microbes 2012, 3, 449–454. [CrossRef]
- Furet, J.-P.; Kong, L.-C.; Tap, J.; Poitou, C.; Basdevant, A.; Bouillot, J.-L.; Mariat, D.; Corthier, G.; Doré, J.; Henegar, C.; et al. Differential Adaptation of Human Gut Microbiota to Bariatric Surgery–Induced Weight Loss. Diabetes 2010, 59, 3049–3057. [CrossRef]
- Kong, L.-C.; Tap, J.; Aron-Wisnewsky, J.; Pelloux, V.; Basdevant, A.; Bouillot, J.-L.; Zucker, J.-D.; Doré, J.; Clément, K. Gut Microbiota after Gastric Bypass in Human Obesity: Increased Richness and Associations of Bacterial Genera with Adipose Tissue Genes. Am J Clin Nutr 2013, 98, 16–24. [CrossRef]
- Li, J. V.; Ashrafian, H.; Bueter, M.; Kinross, J.; Sands, C.; le Roux, C.W.; Bloom, S.R.; Darzi, A.; Athanasiou, T.; Marchesi, J.R.; et al. Metabolic Surgery Profoundly Influences Gut Microbial-Host Metabolic Cross-Talk. Gut 2011, 60, 1214–1223. [CrossRef]
- Zhang, H.; DiBaise, J.K.; Zuccolo, A.; Kudrna, D.; Braidotti, M.; Yu, Y.; Parameswaran, P.; Crowell, M.D.; Wing, R.; Rittmann, B.E.; et al. Human Gut Microbiota in Obesity and after Gastric Bypass. Proceedings of the National Academy of Sciences 2009, 106, 2365–2370. [CrossRef]
- Depommier, C.; Everard, A.; Druart, C.; Plovier, H.; Van Hul, M.; Vieira-Silva, S.; Falony, G.; Raes, J.; Maiter, D.; Delzenne, N.M.; et al. Supplementation with Akkermansia Muciniphila in Overweight and Obese Human Volunteers: A Proof-of-Concept Exploratory Study. Nat Med 2019, 25, 1096–1103. [CrossRef]
- Raber, H.F.; Kubiczek, D.H.; Bodenberger, N.; Kissmann, A.K.; D’souza, D.; Hu, X.; Mayer, D.; Xu, P.; Knippschild, U.; Spellerberg, B.; et al. Flucell-selex Aptamers as Specific Binding Molecules for Diagnostics of the Health Relevant Gut Bacterium Akkermansia Muciniphila. Int J Mol Sci 2021, 22. [CrossRef]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted Distribution of the Butyrate Kinase Pathway among Butyrate-Producing Bacteria from the Human Colon. J Bacteriol 2004, 186, 2099–2106. [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia Spp.: A Marker of Health? Future Microbiol 2017, 12, 157–170. [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia Muciniphila Gen. Nov., Sp. Nov., a Human Intestinal Mucin-Degrading Bacterium. Int J Syst Evol Microbiol 2004, 54, 1469–1476. [CrossRef]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut Microbiota in Human Adults with Type 2 Diabetes Differs from Non-Diabetic Adults. PLoS One 2010, 5, e9085. [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E. V.; Jeraldo, P.R.; Chen, X.; et al. Multiple Sclerosis Patients Have a Distinct Gut Microbiota Compared to Healthy Controls. Sci Rep 2016, 6, 28484. [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; DeSantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut Microbiota in Multiple Sclerosis: Possible Influence of Immunomodulators. Journal of Investigative Medicine 2015, 63, 729–734. [CrossRef]
- Ou, Z.; Deng, L.; Lu, Z.; Wu, F.; Liu, W.; Huang, D.; Peng, Y. Protective Effects of Akkermansia Muciniphila on Cognitive Deficits and Amyloid Pathology in a Mouse Model of Alzheimer’s Disease. Nutr Diabetes 2020, 10, 12. [CrossRef]
- Heintz-Buschart, A.; Pandey, U.; Wicke, T.; Sixel-Döring, F.; Janzen, A.; Sittig-Wiegand, E.; Trenkwalder, C.; Oertel, W.H.; Mollenhauer, B.; Wilmes, P. The Nasal and Gut Microbiome in Parkinson’s Disease and Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Movement Disorders 2018, 33, 88–98. [CrossRef]
- Kissmann, A.K.; Rosenau, F.; Herwig, A.; Diedrich, V. Short Photoperiod-Dependent Enrichment of Akkermansia Spec. as the Major Change in the Intestinal Microbiome of Djungarian Hamsters (Phodopus Sungorus). Int J Mol Sci 2023, 24. [CrossRef]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.-Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science (1979) 2011, 334, 105–108. [CrossRef]
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res 2009, 19, 2317–2323. [CrossRef]
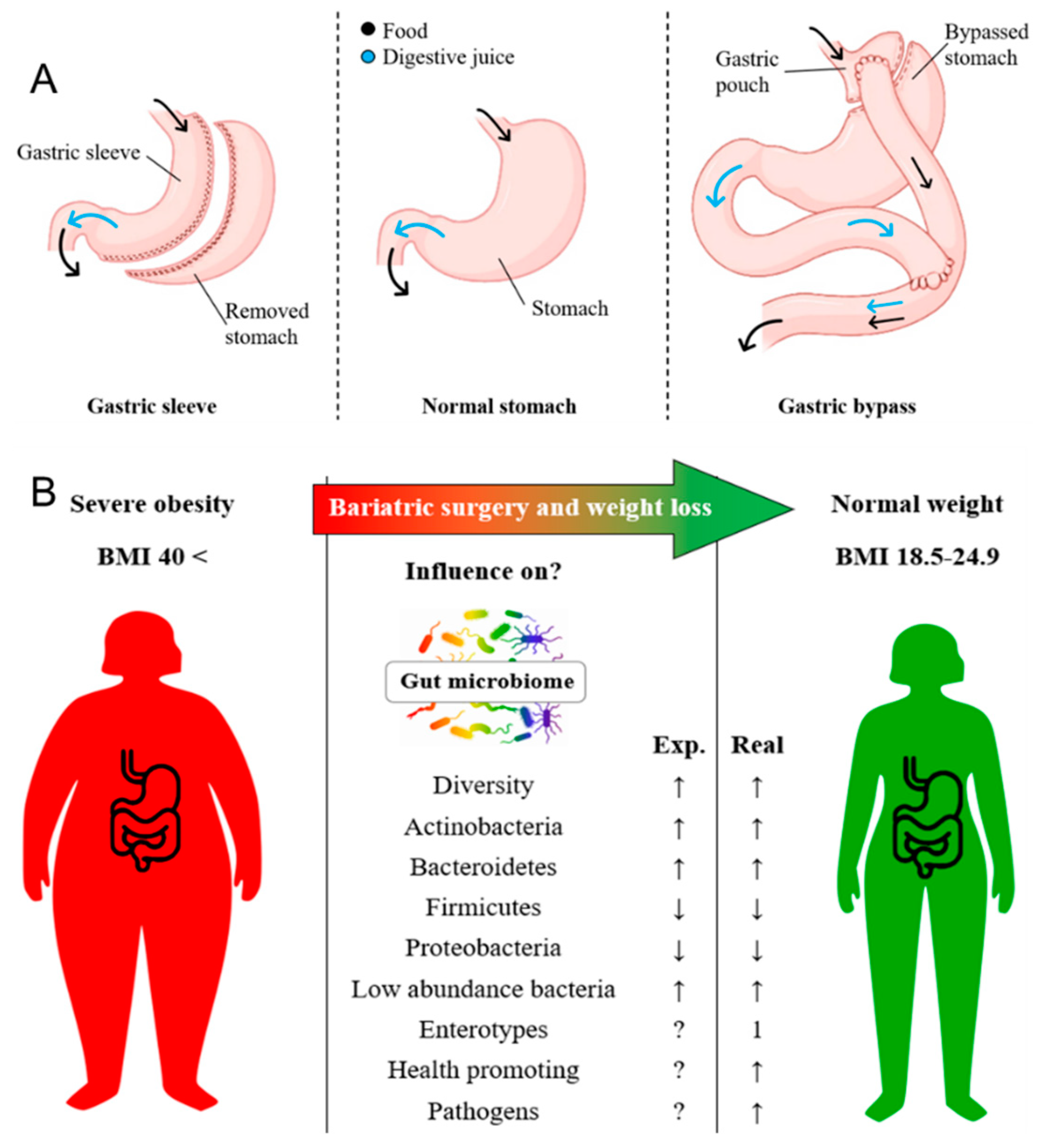
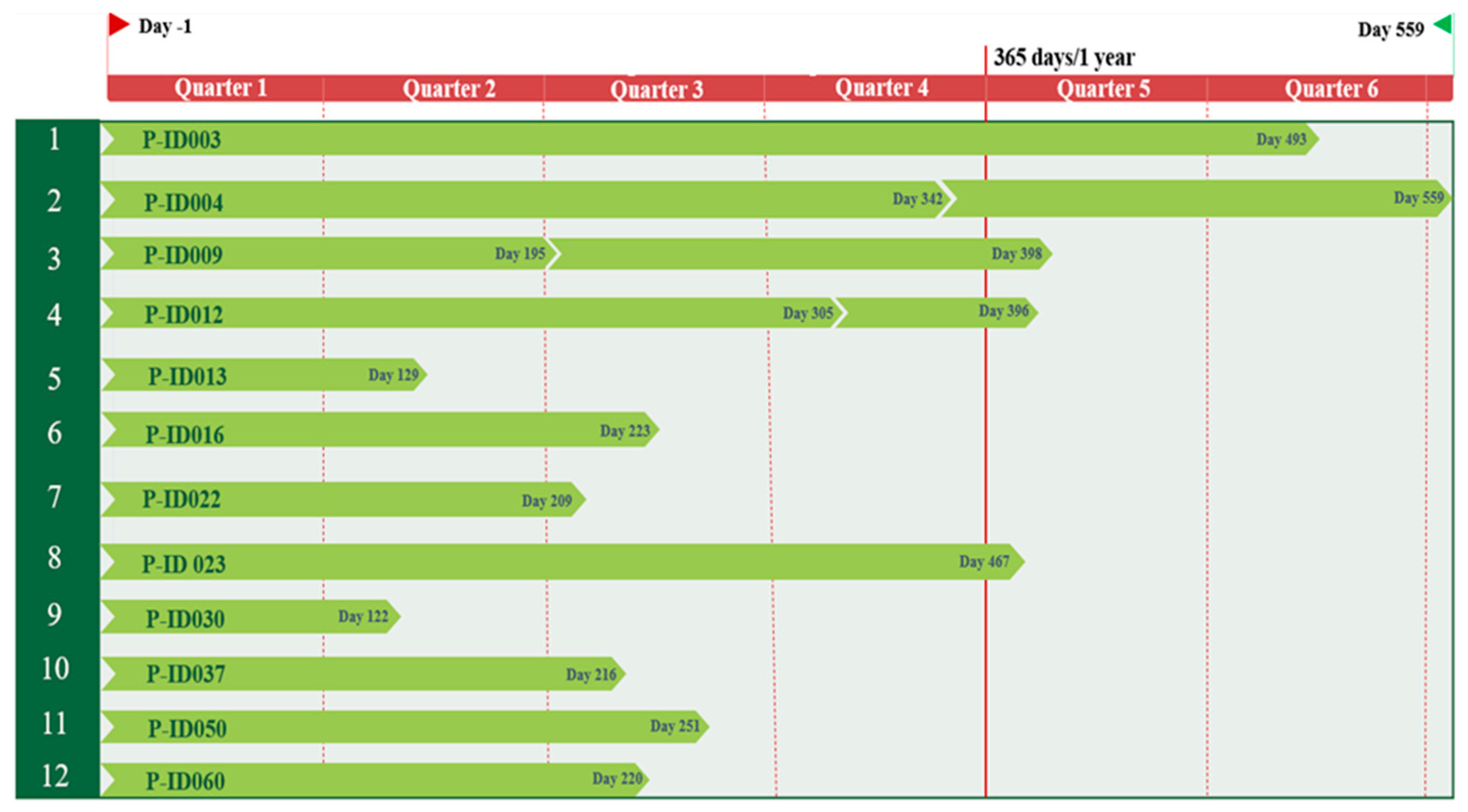
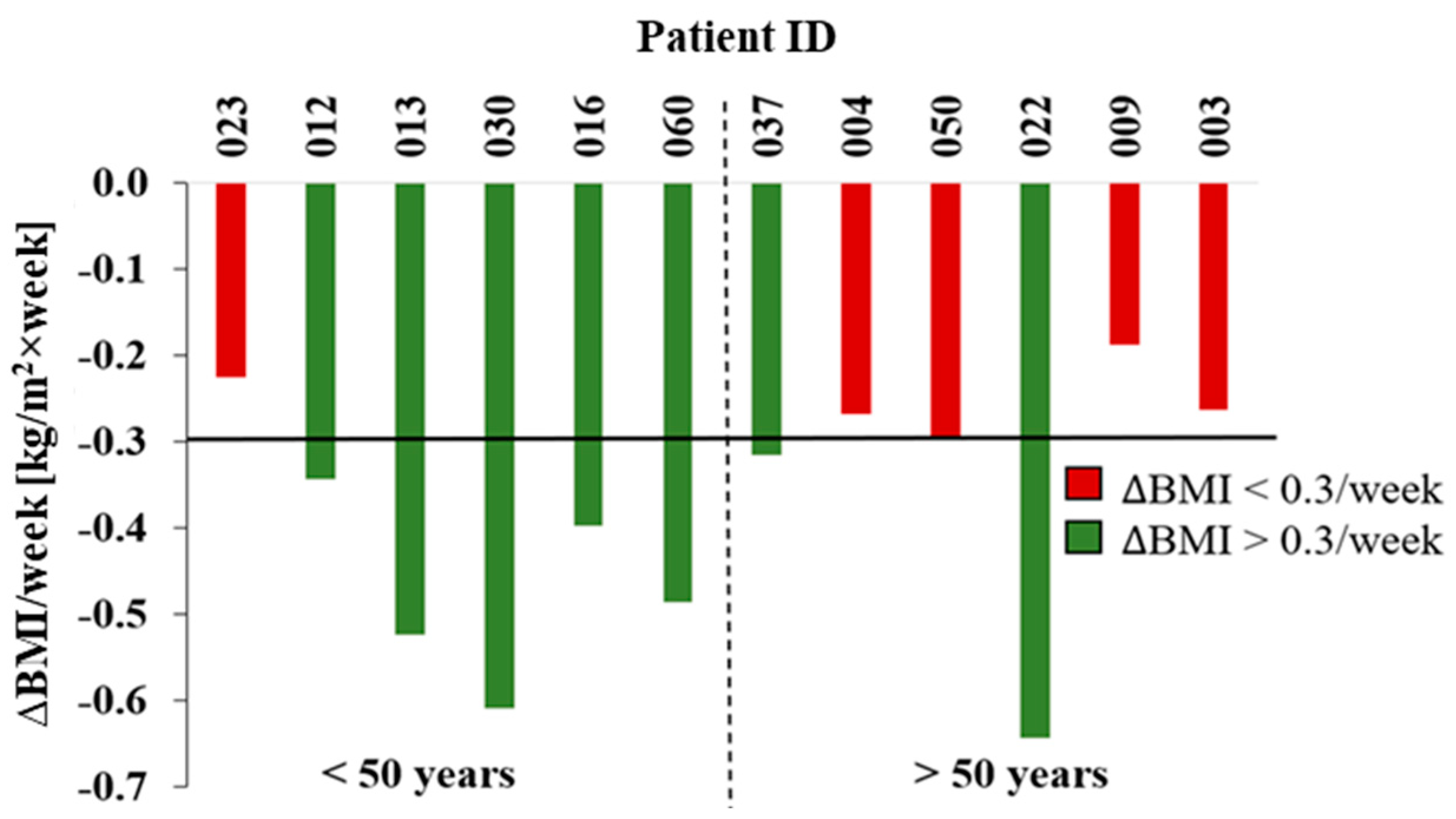
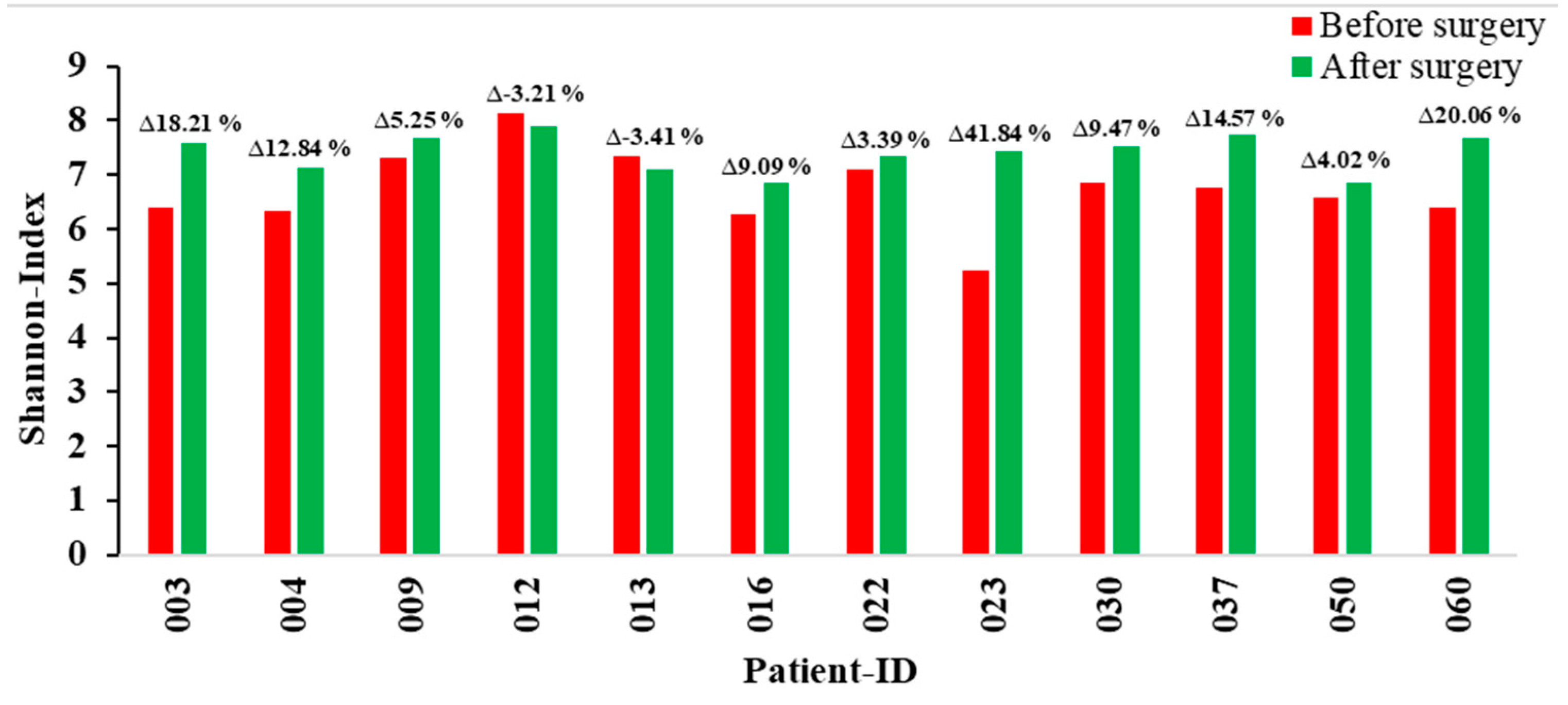
| S-ID | Sex | Surgery | Age [years] | Height [cm] | Start BMI [kg/m2] | Weight before surgery [kg] |
Weight after surgery [kg] |
End BMI [kg/m2] | Arterial hypertonia | Hyperthyroidism | Arthrosis | Prediabetes | Depressive disorder | Smoking |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 003 | F | S | 65 | 163 | 44 | 117 | 72 | 27 | ✓ | ✓ | ✓ | |||
| 004 | F | S | 54 | 162 | 61 | 159 | 103 | 39 | ✓ | ✓ | ✓ | |||
| 009 | F | S | 57 | 177 | 42 | 131 | 98 | 31 | ✓ | ✓ | ✓ | ✓ | ||
| 012 | F | S | 30 | 158 | 47 | 118 | 70 | 28 | ✓ | ✓ | ||||
| 013 | F | S | 30 | 173 | 50 | 150 | 121 | 40 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| 016 | F | S | 39 | 163 | 41 | 109 | 75 | 28 | ✓ | |||||
| 022 | F | S | 55 | 173 | 49 | 147 | 89 | 30 | ✓ | |||||
| 023 | F | S | 27 | 167 | 41 | 114 | 72 | 26 | ✓ | ✓ | ✓ | |||
| 030 | F | S | 33 | 170 | 42 | 122 | 91 | 32 | ✓ | ✓ | Ex | |||
| 037 | F | S | 45 | 165 | 43 | 117 | 91 | 33 | ✓ | |||||
| 050 | F | S | 54 | 152 | 50 | 115 | 91 | 39 | ✓ | |||||
| 060 | F | B | 40 | 170 | 50 | 144 | 100 | 35 | ✓ | |||||
| C1 | F | - | 27 | 158 | 22 | 56 | 22 | |||||||
| C2 | M | - | 32 | 179 | 22 | 70 | 22 | |||||||
| S-ID | Before Surgery | After Surgery | Change of Enterotype |
|---|---|---|---|
| 003 | III | I | ✓ |
| 004 | II | I | ✓ |
| 009 | I | I | |
| 012 | I | I | |
| 013 | I | I | |
| 016 | III | III | |
| 022 | III | I | ✓ |
| 023 | I | I | |
| 030 | I | I | |
| 037 | I | I | |
| 050 | III | I | ✓ |
| 060 | I | I | |
| C1 | I I |
||
| C2 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





