Submitted:
29 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Origin, Principle, and Advantages of mowDH
3. Research Progress of the mowDH Technology
3.1. Genotype Effects
3.2. Environmental Factors
3.3. Treatment of Wheat Spikes and Timing of Pollination
3.4. Hormone Treatment
3.5. Embryo Rescue
3.6. Doubling Treatment
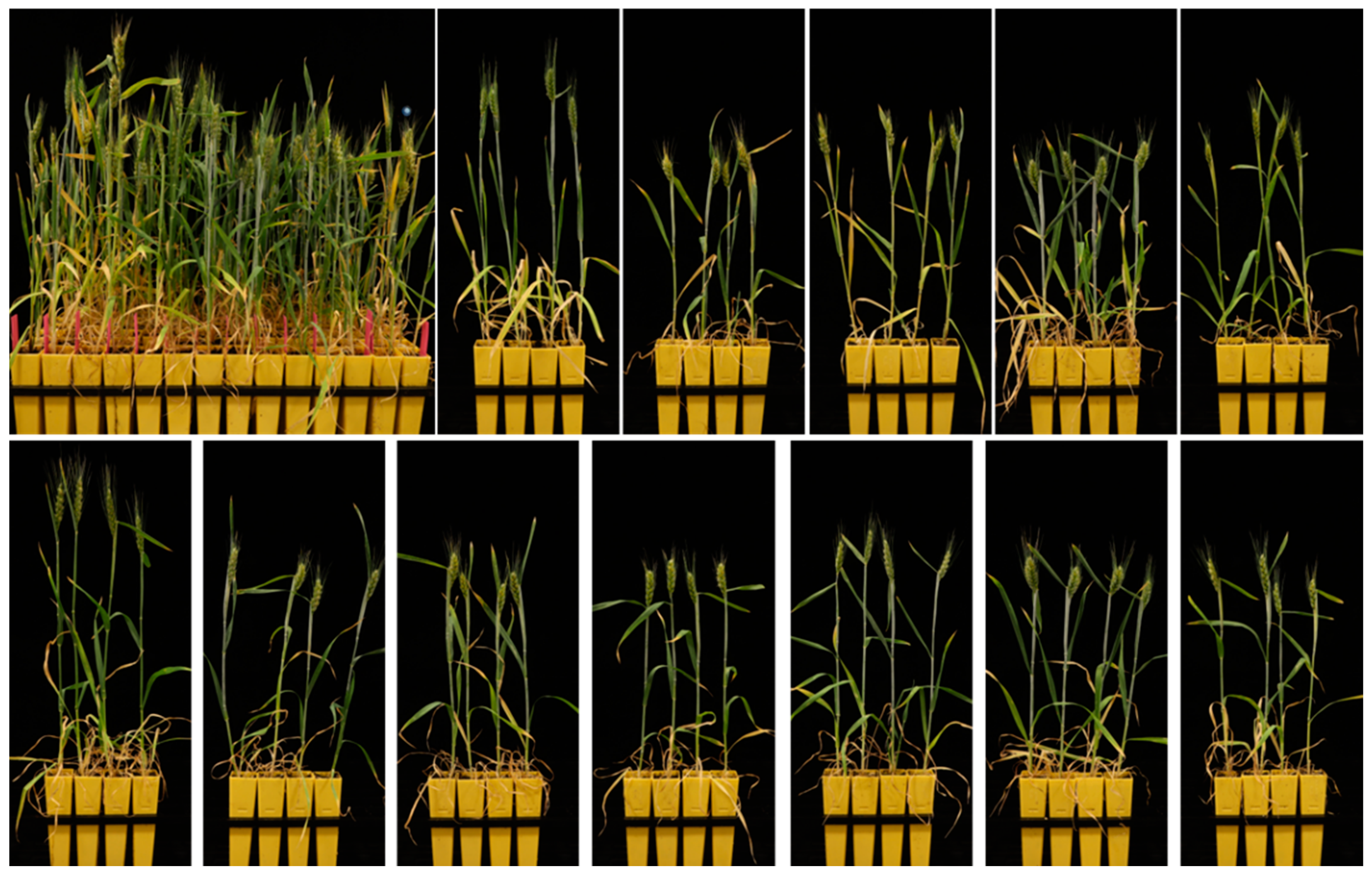
4. Stability of Doubled Haploids
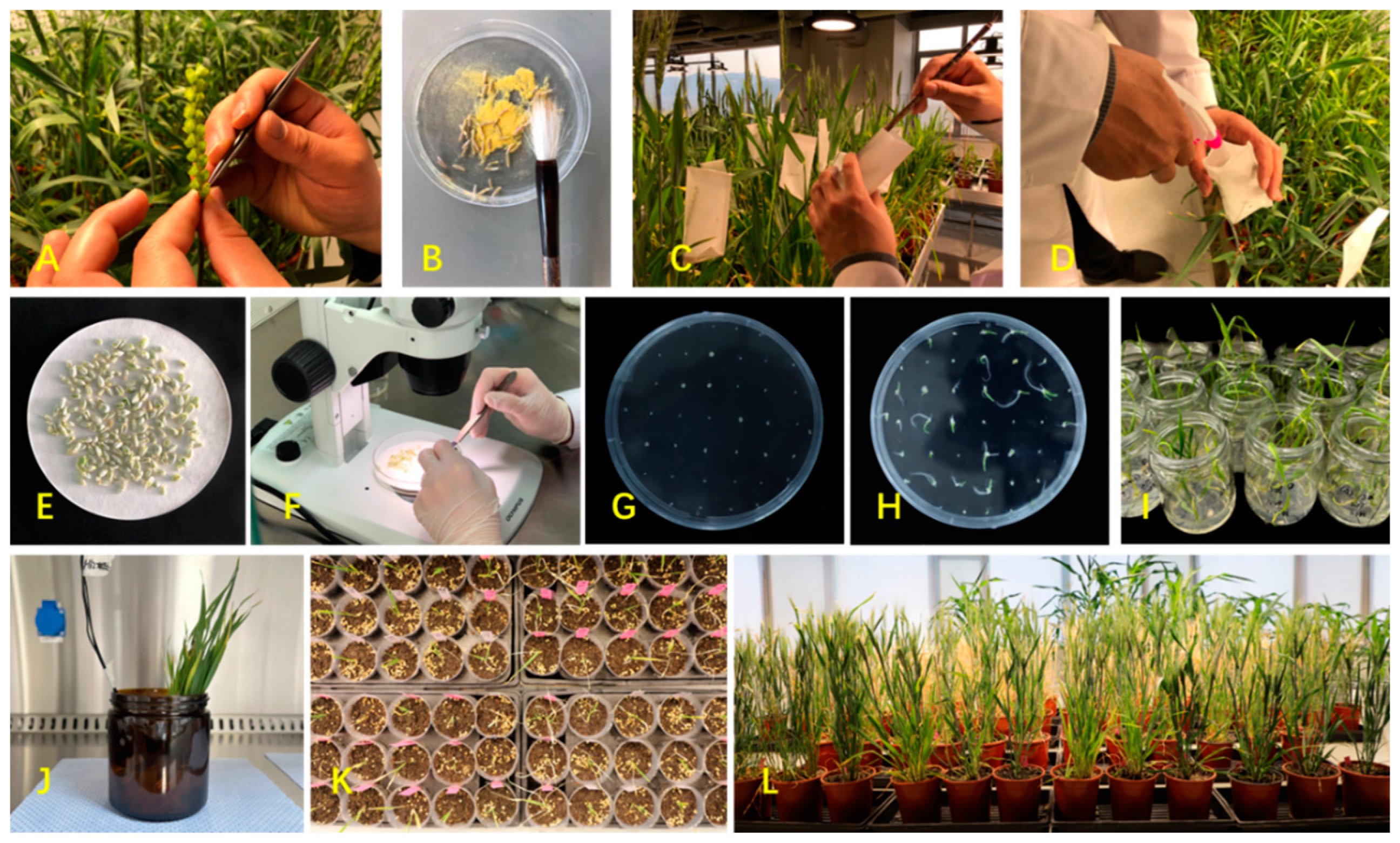
5. An Optimized mowDH Procedure for Winter Wheat
- (1)
- Wheat breeding lines (≥ F3 generation) and hybrid maize varieties are maintained in environmentally controlled facilities: wheat under 20-24 ℃, day/night of 20h/4h, light intensity > 30000 lux, and humidity 60-65%; maize under 22-24 ℃, day/night of 12h/12h, light intensity > 10000 lux, and humidity 55-60%. A set of ten maize seeds are sown once a week, to continuously supply pollen grains. Winter or semi-winter wheat is vernalized under 4 ℃ for 40 days before shifting to regular growth.
- (2)
- When heading, wheat spikes are manually emasculated.
- (3)
- Mature pollen grains are collected from maize tassel around 10 am, and are applied with a brush to the emasculated wheat florets at the feathery pistil stage. The pollination time is recorded.
- (4)
- At 24 hours post pollination, 2,4-D (100 ppm) is sprayed on top of the pollinated wheat florets.
- (5)
- At 15 days post pollination, manually pollinated spikes and any immature seeds born are harvested.
- (6)
- Immature seeds are sterilized in 70% alcohol for 1 minute, and in 15% sodium hypochlorite for 20 minutes, and then rinsed with sterile water five times.
- (7)
- Within a clean hood, haploid embryos are isolated from the sterilized immature seeds using a dissection microscope, and isolated haploid embryos are then planted on ½ MS medium (½ MS + 20 g/L sucrose + 2.4 g/L plant agar, pH 5.8).
- (8)
- Immature embryos are cultured under 20-24 °C, 16h light / 8h dark, and a light intensity of 4800 Lux. Any plantlets with shoots and roots are transferred into culture bottles (150 ml) and are cultured on ½ MS medium under 20-24 °C, 16h light / 8h dark, and a light intensity of 4800 Lux.
- (9)
- At 3-leaf stage, the culture bottle is left open for 24 hours. Haploid plantlets are then transplanted into small pots with Pindstrup substrate (PH 5.5, Pindstrup Mosebrug A/S, Denmark) for cultivation.
- (10)
- Haploid plantlets with 2-3 tillers are taken out of the small pots. Extra roots are trimmed to keep only 2-3 cm on plantlets. Haploid plantlets with trimmed roots are then soaked in colchicine solution (0.05%) for 16 hours.
- (11)
- After colchicine treatment, plantlets are rinsed with running water for 30 minutes, and then transplanted into small pots with Pindstrup substrate (PH 5.5).
- (12)
- When new tillers emerge, plantlets are then vernalized at 4 ℃ for 40 days, and then transplanted into growth pots with the seedling substrate (Jinan Fengyuan Agricultural Technology Co., China). Plants are maintained until mature.
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Zheng, Y.; Duan, L.; Wang, M.; Wang, H.; Li, H.; Li, R.; Zhang, H. Artificial Selection Trend of Wheat Varieties Released in Huang-Huai-Hai Region in China Evaluated Using DUS Testing Characteristics. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- State Council of the People’s Republic of China, Regulations on Protection of New Varieties of Plants. Beijing: China Agricultural Publishing House 1997, 4-5.
- Standing Committee of the National People’s Congress, People’s Republic of China Seed Law. Beijing: Law press 2015, 4-7.
- Wang, L.; Chang, L.; Li, H.; Ge, L.; Xin, A.; Gao, S.; Ji, W.; Sun, H.; Zhao, C. Method of Wheat Seeds Purity Testing by Molecular Markers. Journal of Triticeae Crops 2009, 29, 1–8. [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China, Standardization Administration of the People’s Republic of China. GB 4404.1-2008. Seed of food crops-Part 1: Cereals. Beijing: Standards Press of China 2008.
- Prigge, V.; Xu, X.; Li, L.; Babu, R.; Chen, S.; Atlin, G.N.; Melchinger, A.E. New Insights into the Genetics of in Vivo Induction of Maternal Haploids, the Backbone of Doubled Haploid Technology in Maize. Genetics 2012, 190, 781–793. [Google Scholar] [CrossRef] [PubMed]
- Dunwell, J.M. Haploids in flowering plants: origins and exploitation. Plant biotechnology journal 2010, 8, 377–424. [Google Scholar] [CrossRef]
- Tadesse, W.; Inagaki, M.; Tawkaz, S.; Baum, M.; Ginkel, M.V. Recent advances and application of doubled haploids in wheat breeding. African Journal of Biotechnology 2012, 11, 1. [Google Scholar] [CrossRef]
- Blakeslee, A.F.; Belling, J.; Farnham, M.E.; Bergner, A.D. A HAPLOID MUTANT IN THE JIMSON WEED, "DATURA STRAMONIUM". Science (New York, N.Y.) 1922, 55, 646–647. [Google Scholar] [CrossRef]
- Guha, S.; Maheshwari, S.C. In vitro Production of Embryos from Anthers of Datura. Nature 1964, 204, 497–497. [Google Scholar] [CrossRef]
- Niizeki, H.; Oono, K. Induction of Haploid Rice Plant from Anther Culture. Proceedings of the Japan Academy 1968, 44, 554–557. [Google Scholar] [CrossRef]
- Ho, K.M.; Jones, G.E. Mingo Barley. Canadian Journal of Plant Science 1980, 60, 279–280. [Google Scholar] [CrossRef]
- Kalinowska, K.; Chamas, S.; Unkel, K.; Demidov, D.; Lermontova, I.; Dresselhaus, T.; Kumlehn, J.; Dunemann, F.; Houben, A. State-of-the-art and novel developments of in vivo haploid technologies. Theoretical and Applied Genetics 2019. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.L.; Britt, A.B.; Tripathi, L.; Sharma, S.; Ortiz, R. Haploids: contraints and opportunities in plant breeding. Biotechnology Advances 2015. [Google Scholar] [CrossRef]
- Zargar, M.; Zavarykina, T.; Voronov, S.; Pronina, I.; Bayat, M. The Recent Development in Technologies for Attaining Doubled Haploid Plants In Vivo. Agriculture 2022, 12. [Google Scholar] [CrossRef]
- Liu, W.; Zheng, M.Y.; Polle, E.A.; Konzak, C.F. Highly Efficient Doubled-Haploid Production in Wheat (Triticum aestivum L.) via Induced Microspore Embryogenesis. Crop Science 2002, 42. [Google Scholar] [CrossRef]
- Tawkaz, S. Response of some wheat genotypes to anther culture technique for doubled haploid production. M. Sc Thesis. Sudan Academy of Science, Kartum, 2011.
- Ding, M.; Zhao, H.; Gu, J.; Li, H.; Liu, K.; Yang, M.; Li, S. Research and Breeding Application Progress of the Technique of Producing Double Haploid of Wheat by Wide Hybridization between Wheat and Maize. Agricultural Science & Technology 2017, 18, 2202–2208. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Jia, Z.; Gong, Q.; Lin, Z.; Du, L.; Pei, X.; Ye, X. Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. Journal of Experimental Botany 2019, 71, 1337–1349. [Google Scholar] [CrossRef]
- Tang, H.; Wang, K.; Zhang, S.; Han, Z.; Chang, Y.; Qiu, Y.; Yu, M.; Du, L.; Ye, X. A fast technique for visual screening of wheat haploids generated from TaMTL-edited mutants carrying anthocyanin markers. Plant Communications 2023, 4. [Google Scholar] [CrossRef]
- Kumar Niroula, R.; Prasad Bimb, H. Overview of Wheat × Maize System of Crosses for Dihaploid Induction in Wheat. World Applied Sciences Journal 2009, 7, 1037–1045. [Google Scholar]
- Ding, M.; Yang, Z.; Cui, Y.; Li, H.; Liu, K.; Zhao, H.; Yang, M.; Gu, J.; Li, S. A high-yield and multi-resistant new wheat variety bred using diploid technology - Yunmai 110. Journal of Triticeae Crops 2022, 42, 1589. [Google Scholar]
- Zhao, G. Study on Chinese Wheat Planting Regionalization (I). Journal of Triticeae Crops 2010, 30, 886–895. [Google Scholar]
- National Bureau of Statistics of China, China Statistical Yearbook 2023. China Statistics Press 2023. https://www.stats.gov.cn/sj/ndsj/2023/indexch.htm.
- Clausen, R.E.; Mann, M.C. Inheritance in Nicotiana Tabacum: V. The Occurrence of Haploid Plants in Interspecific Progenies. Proceedings of the National Academy of Sciences 1924, 10, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Gaines, E.F.; Aase, H.C. A haploid wheat plant. American Journal of Botany 1926, 13, 373–385. [Google Scholar] [CrossRef]
- BARCLAY, I.R. High frequencies of haploid production in wheat (Triticum aestivum) by chromosome elimination. Nature 1975, 256, 410–411. [Google Scholar] [CrossRef]
- Laurie, D.A.; Bennett, M.D. Wheat × maize hybridization. Canadian Journal of Genetics Cytology 1986, 28, 313–316. [Google Scholar] [CrossRef]
- Laurie, D.A. Factors Affecting Fertilization Frequency in Crosses of Triticum aestivum cv. ‘Highbury’ × Zea mays cv. ‘Seneca 60’. Plant Breeding 1989. [CrossRef]
- Laurie, D.A.; Snape, J.W. The agronomic performance of wheat doubled haploid lines derived from wheat × maize crosses. Theoretical & Applied Genetics 1990, 79, 813–816. [Google Scholar] [CrossRef]
- Laurie, D.A.; Reymondie, S. High Frequencies of Fertilization and Haploid Seedling Production in Crosses Between Commercial Hexaploid Wheat Varieties and Maize. Plant Breeding 1991, 106, 182–189. [Google Scholar] [CrossRef]
- Campbell, A.W.; Griffin, W.B.; Burritt, D.J.; Conner, A.J. Production of wheat doubled haploids via wide crosses in New Zealand wheat. New Zealand Journal of Crop and Horticultural Science 2000, 28, 185–194. [Google Scholar] [CrossRef]
- Jauhar, P.P.; Xu, S.S.; Baenziger, P.S. Haploidy in Cultivated Wheats: Induction and Utility in Basic and Applied Research. Crop Science 2009, 49, 737–755. [Google Scholar] [CrossRef]
- Niroula, R.K.; Bimb, H.P.; Thapa, D.B.; Sah, B.P.; Nayak, S. Production of haploid wheat plants from wheat (Triticum aestivum L.) × maize (Zea mays L.) cross system. Himalayan Journal of Sciences 2007, 4, 65–69. [Google Scholar] [CrossRef]
- Moradi, P.; Haghnazari, A.; Bozorgipour, R.; Sharma, B. Development of yellow rust resistant doubled haploid lines of wheat through wheat × maize crosses. International Journal of Plant Production 2009, 3, 77–88. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Abdelkhalik, S.; Shahzad, A.; Gu, J.; Yang, Z.; Ding, M.; Liu, K.; Zhao, H.; Yang, M. Development of thermo-photo sensitive genic male sterile lines in wheat using doubled haploid breeding. BMC Plant Biology 2020, 20, 246. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.D.; Finch, R.A.; Barclay, I.R. The time rate and mechanism of chromosome elimination in Hordeum hybrids. Chromosoma 1976, 54, 175–200. [Google Scholar] [CrossRef]
- Michel, B. Replication fork arrest and DNA recombination. Trends in Biochemical Sciences 2000, 25, 173–178. [Google Scholar] [CrossRef]
- Mochida, K.; Tsujimoto, H.; Sasakuma, T. Confocal analysis of chromosome behavior in wheat × maize zygotes. Genome 2004, 47, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Chan, S.W.L. Haploid plants produced by centromere-mediated genome elimination. Nature 2010, 464, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Sanei, M.; Pickering, R.; Kumke, K.; Nasuda, S.; Houben, A. Loss of centromeric histone H3 (CENH3) from centromeres precedes uniparental chromosome elimination in interspecific barley hybrids. Proceedings of the National Academy of Sciences 2011, 108, E498–E505. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhu, Q.; Wang, H.; Xiao, J.; Xing, L.; Chen, P.; Jin, W.; Wang, X.-E. Competitive Expression of Endogenous Wheat CENH3 May Lead to Suppression of Alien ZmCENH3 in Transgenic Wheat × Maize Hybrids. Journal of Genetics and Genomics 2015, 42, 639–649. [Google Scholar] [CrossRef]
- Kapoor, C.; Chaudhary, H.; Relan, A.; Manoj, N.; Singh, K.; Sharma, P. Haploid induction efficiency of diverse Himalayan maize (Zea mays) and cogon grass (Imperata cylindrica) gene pools in hexaploid and tetraploid wheats and triticale following chromosome elimination-mediated approach of doubled haploidy breeding. Cereal Research Communications 2020, 48, 539–545. [Google Scholar] [CrossRef]
- Gurtay, G.; Kutlu, I.; Avci, S. Production of haploids in ancient, local and modern wheat by anther culture and maize pollination. Acta Biologica Cracoviensia. Series Botanica 2021, 63. [Google Scholar] [CrossRef]
- Laurie, D.; Bennett, M. Cytological evidence for fertilization in hexaploid wheat × sorghum crosses. Plant Breeding 1988, 100, 73–82. [Google Scholar] [CrossRef]
- Laurie, D.A. The frequency of fertilization in wheat × pearl millet crosses. Genome 1989, 32, 1063–1067. [Google Scholar] [CrossRef]
- USHIYAMA, T.; SHIMIZU, T.; KUWABARA, T. High frequency of haploid production of wheat through intergeneric cross with teosinte. Japanese Journal of Breeding 1991, 41, 353–357. [Google Scholar] [CrossRef]
- Riera-Lizarazu, O.; Mujeeb-Kazi, A. Polyhaploid production in the Triticeae: wheat × Tripsacum crosses. Crop science 1993, 33, 973–976. [Google Scholar] [CrossRef]
- Mochida, K.; Tsujimoto, H. Production of Wheat Doubled Haploids by Pollination With Job’s Tears (Coix lachryma-jobi L.). Journal of Heredity 2001, 92, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, H.K.; Sethi, G.S.; Singh, S.; Pratap, A.; Sharma, S. Efficient haploid induction in wheat by using pollen of Imperata cylindrica. Plant Breeding 2005, 124, 96–98. [Google Scholar] [CrossRef]
- Kishii, M.; Singh, S. Haploid Production Technology: Fasten Wheat Breeding to Meet Future Food Security. In Accelerated Plant Breeding, Volume 1: Cereal Crops; Gosal, S.S., Wani, S.H., Eds.; Springer International Publishing: Cham, 2020; pp. 139–165. [Google Scholar]
- OHKAWA, Y.; SUENAGA, K.; OGAWA, T. Production of haploid wheat plants through pollination of sorghum pollen. Japanese Journal of Breeding 1992, 42, 891–894. [Google Scholar] [CrossRef]
- Inagaki, M.; Mujeeb-Kazi, A. Comparison of polyhaploid production frequencies in crosses of hexaploid wheat with maize, pearl millet and sorghum. Japanese Journal of Breeding 1995, 45, 157–161. [Google Scholar] [CrossRef]
- Inagaki, M.N.; Hash, C.T. Production of haploids in bread wheat, durum wheat and hexaploid triticale crossed with pearl millet. Plant Breeding 1998, 117, 485–487. [Google Scholar] [CrossRef]
- Ishii, T.; Ueda, T.; Tanaka, H.; Tsujimoto, H. Chromosome elimination by wide hybridization between Triticeae or oat plant and pearl millet: pearl millet chromosome dynamics in hybrid embryo cells. Chromosome Research 2010, 18, 821–831. [Google Scholar] [CrossRef]
- Suenaga, K.; Morshedi, A.R.; Darvey, N.L. Evaluation of teosinte lines as pollen parents for wheat haploid production. Cereal Research Communications 1998, 26, 119–125. [Google Scholar] [CrossRef]
- Li, D.W.; Qio, J.W.; Ouyang, P.; Yao, Q.X.; Dawei, L.D.; Jiwen, Q.; Ping, O.; Qingxiao, Y. High frequncies of fertilization and embryo formation in hexaploid wheat × Tripsacum dactyloides crosses. Theoretical and Applied Genetics 1996, 92, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.E. Cogongrass (Imperata cylindrica)—Biology, Ecology, and Management. Critical Reviews in Plant Sciences 2004, 23, 367–380. [Google Scholar] [CrossRef]
- Mayel, A.; Chaudhary, H.K.; Badiyal, A.; Jamwal, N.S. Comparative Pollination Efficiency of Freshly Harvested Pollen of Imperata cylindrica and Zea mays for Haploid Induction in Bread Wheat. Cereal Research Communications 2016, 44, 162–171. [Google Scholar] [CrossRef]
- Kapoor, C.; Chaudhary, H.K.; Sharma, P.; Relan, A.; Manoj, N.V.; Singh, K.; Sood, V.K. In vivo haploid induction potential of Himalayan maize (Zea mays) and cogon grass (Imperata cylindrica) gene pools in different segregational cycles of intra and inter-generic crosses of wheat. Plant Genetic Resources: Characterization and Utilization 2021, 19, 522–529. [Google Scholar] [CrossRef]
- Dhiman, R.; Rana, V.; Chaudhary, H. Himalayan maize - potential pollen source for maize mediated system of chromosome elimination approach in DH breeding of bread wheat. Cereal Research Communications 2012, 40, 246–255. [Google Scholar] [CrossRef]
- Khokhar, M.I.; Razaq, A.; Iqbal, J.; Anwar, M.J.; Iqbal, M.Z.; Sajid, u.R. Choice of maize genotype affects wheat haploid seed and success of embryo rescue. RADS Journal of Biological Research & Applied Sciences 2019, 10, 1–5. [Google Scholar] [CrossRef]
- Rather, S.A.; Chaudhary, H.K.; Kaila, V. Influence of different wheat and Imperata cylindrica genetic backgrounds on haploid induction efficiency in wheat doubled haploid breeding. Czech Journal of Genetics Plant Breeding 2014, 50, 195–200. [Google Scholar] [CrossRef]
- Verma, V.; Bains, N.S.; Mangat, G.S.; Nanda, G.S.; Gosal, S.S.; Singh, K. Maize genotypes show striking differences for induction and regeneration of haploid wheat embryos in the wheat × maize system. Crop science 1999, 39, 1722–1727. [Google Scholar] [CrossRef]
- Brazauskas, G.; Pašakinskienė, I.; Ruzgas, V. Improved approaches in wheat × maize crossing for wheat doubled haploid production. Biologija 2005, 51. [Google Scholar]
- Samuel Jeberson, M.; Kumar Chaudhary, H.; Kumar Chahota, R.; Hussain Wani, S. Doubled haploid production in advanced back cross generations and molecular cytogenetic characterization of rye chromatin in triticale wheat derived doubled haploid lines. Biocell 2021, 45, 1651–1659. [Google Scholar] [CrossRef]
- Sharma, S.; Sethi, G.S.; Chaudhary, H.K. Influence of winter and spring wheat genetic backgrounds on haploid induction parameters and trait correlations in the wheat × maize system. Euphytica 2005, 144, 199–205. [Google Scholar] [CrossRef]
- Laurie, D.A.; Bennett, M.D. The production of haploid wheat plants from wheat × maize crosses. Theoretical and Applied Genetics 1988, 76, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, K.; Tamaki, M.; Nakajima, K. Influence of wheat (Triticum aestivum) and maize (Zea mays) genotypes on haploid wheat production in crosses between wheat and maize. Nogyo Seibutsu Shigen Kenkyujo kenkyu hokoku 1991, 131–142. [Google Scholar]
- Suenaga, K.; Nakajima, K. Efficient production of haploid wheat (Triticum aestivum) through crosses between Japanese wheat and maize (Zea mays). Plant Cell Reports 1989, 8, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sethi, G.S.; Chaudhary, H.K. Differential responsiveness of winter and spring wheat genotypes to maize-mediated production of haploids. Cereal Research Communications 2004, 32, 201–207. [Google Scholar] [CrossRef]
- Avcı, S.; Kutlu, İ. Comparison of orchard-grass and sweet maize for doubled haploid plant production via wide hybridization in bread wheat. Turkish Journal of Agriculture-Food Science Technology 2020, 8, 1548–1552. [Google Scholar] [CrossRef]
- Niroula, R.K.; Thapa, D.B. Response of wheat genotypes to maize mediated polyhaploid production. American-Eurasian Journal of Agronomy 2009, 2, 156–161. [Google Scholar]
- Gu, J.; Liu, K.; Li, S.; Tian, Y.; Yang, H.; Yang, M. Study on the in vitro culture of cut plants in wheat haploid embryo induction by a wheat × maize cross. Frontiers of Agriculture in China 2008, 2, 391–395. [Google Scholar] [CrossRef]
- Khan, M.A.; Ahmad, J. In vitro wheat haploid embryo production by wheat × maize cross system under different environmental conditions. Pak J Agric Sci 2011, 48, 49–53. [Google Scholar] [CrossRef]
- Khan, H.; Bhardwaj, S.C.; Gangwar, O.P.; Prasad, P.; Rathore, R. Efficiency of double haploid production in wheat through wide hybridization and embryo rescue. Indian Journal of Genetics and Plant Breeding 2017, 77, 428–430. [Google Scholar] [CrossRef]
- Hussain, M.; Niaz, M.; Iqbal, M.; Iftikhar, T.; Ahmad, J. Emasculation techniques and detached tiller culture in wheat × maize crosses. J. Agric. Res 2012, 50, 1–19. [Google Scholar]
- Martins-Lopes, P.F.; Guedes-Pinto, H.; Pinto-Carnide, O.; Snape, J. The effect of spikelet position on the success frequencies of wheat haploid production using the maize cross system. Euphytica 2001, 121, 265–271. [Google Scholar] [CrossRef]
- Mahato, A.; Chaudhary, H.K. Auxin induced haploid induction in wide crosses of durum wheat. Cereal Research Communications 2019, 47, 552–565. [Google Scholar] [CrossRef]
- Shubham; Sandal, S.S.; Walia, P.; Upadhyay, V. Application of Auxins in Haploid Embryo Induction in Hexaploidy Wheat. International Journal of Environment and Climate Change 2023, 13, 251–255. [Google Scholar] [CrossRef]
- Juzoń, K.; Warchoł, M.; Dziurka, K.; Czyczyło-Mysza, I.M.; Marcińska, I.; Skrzypek, E. The effect of 2,4-dichlorophenoxyacetic acid on the production of oat (Avena sativa L.) doubled haploid lines through wide hybridization. PeerJ 2022, 10. [Google Scholar] [CrossRef]
- Kaushik, N.; Sirohi, M.; Khanna, V.K. Influence of age of the embryo and method of hormone application on haploid embryo formation in wheat × maize crosses. In Proceedings of the Proceedings of the 4th International Crop Science Congress. Brisbane, Australia, 2004; p. 771.
- Usha, P.; Khanna, V.K. Effect of hormonal treatments on haploid formation and in vitro haploid regeneration in wheat × maize system. International Journal of Plant Sciences (Muzaffarnagar) 2017, 12, 234–239. [Google Scholar]
- Kumlehn, J.; Stein, N. Biotechnological approaches to barley improvement; Springer: 2014; Volume 69.
- Slama-Ayed, O.; Bouhaouel, I.; Ayed, S.; De Buyser, J.; Picard, E.; Amara, H.S. Efficiency of three haplomethods in durum wheat (Triticum turgidum subsp. durum Desf.): Isolated microspore culture, gynogenesis and wheat × maize crosses. Czech Journal of Genetics and Plant Breeding 2019, 55, 101–109. [Google Scholar] [CrossRef]
- Cherkaoui, S.; Lamsaouri, O.; Chlyah, A.; Chlyah, H. Durum wheat × maize crosses for haploid wheat production: influence of parental genotypes and various experimental factors. Plant Breeding 2000, 119, 31–36. [Google Scholar] [CrossRef]
- Goyal, P. Improving the efficiency of detached tiller culture and plant regeneration in wheat × maize system of doubled haploid production in wheat. PhD Dissertation, Punjab Agricultural University, Ludhiana, 2016. [Google Scholar]
- Chen, X. A Study on the Increasing Frequences of Plant Production During Embryo Culture in Crosses Between Wheat and Maize. SCIENTIA AGRICUTURA SINICA 1996. [Google Scholar]
- Hooghvorst, I.; Nogués, S. Chromosome doubling methods in doubled haploid and haploid inducer-mediated genome-editing systems in major crops. Plant Cell Reports 2021, 40, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, W.; Tawkaz, S.; Inagaki, M.; Picard, E.; Baum, M. Methods and applications of doubled haploid technology in wheat breeding. ICARDA, Aleppo, Syria. 36 pp. International Center for Agricultural Research in the Dry Areas PO Box 2013, 114, 5055. [Google Scholar]
- INAGAKI, M. Chromosome doubling of the wheat haploids obtained from crosses with Hordeum bulbosum L. Japanese Journal of Breeding 1985, 35, 193–195. [Google Scholar] [CrossRef]
- Khan, M.A.; Shaukat, S.; Ahmad, J.; Kashif, M.; Khan, A.S.; Iqbal, M.Z. Use of intergeneric cross for production of doubled haploid wheat (Triticum aestivum L.). Journal of Science, Technology and Development 2012, 31, 295–300. [Google Scholar]
- Niu, Z.; Jiang, A.; Abu Hammad, W.; Oladzadabbasabadi, A.; Xu, S.S.; Mergoum, M.; Elias, E.M. Review of doubled haploid production in durum and common wheat through wheat × maize hybridization. Plant breeding 2014, 133, 313–320. [Google Scholar] [CrossRef]
- Sharma, P.; Chaudhary, H.K.; Manoj, N.V.; Kumar, P. New protocol for colchicine induced efficient doubled haploidy in haploid regenerants of tetraploid and hexaploid wheats at in vitro level. Cereal research communications 2019, 47, 356–368. [Google Scholar] [CrossRef]
- Comeau, A.; Nadeau, P.; Plourde, A.; Simard, R.; Maës, O.; Kelly, S.; Harper, L.; Lettre, J.; Landry, B.; St-Pierre, C.A. Media for the in ovulo culture of proembryos of wheat and wheat-derived interspecific hybrids or haploids. Plant Science 1992, 81, 117–125. [Google Scholar] [CrossRef]
- Kammholz, S.J.; Grams, R.A.; Banks, P.M.; Sutherland, M.W. Segregation of glutenins in wheat × maize-derived doubled haploid wheat populations. Australian journal of agricultural research 1998, 49, 1253–1260. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, L.; Sun, J. Molecular evidence on maize specific DNA fragment transferred into wheat through sexual hybridization. Science in China Series C: Life Sciences 1998, 41, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Brazauskas, G.; Pasakinskiene, I.; Jahoor, A. AFLP analysis indicates no introgression of maize DNA in wheat × maize crosses. Plant Breeding 2004, 123, 117–121. [Google Scholar] [CrossRef]
- Schmid, T.E.; Xu, W.; Adler, I.D. Detection of aneuploidy by multicolor FISH in mouse sperm after in vivo treatment with acrylamide, colchicine, diazepam or thiabendazole. Mutagenesis 1999, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.B.S.R. Chemically induced aneuploidy in higher plants. Mutagenesis 1990, 5, 105–126. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, S.S.; Dhesi, J.S.; Gill, B.S.; Svendsgaard, D. Evaluation of 10 chemicals for aneuploidy induction in the hexaploid wheat assay. Mutagenesis 1991, 6, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, K.; Nakajima, K. Variation in doubled haploid plants of wheat obtained through wheat (Triticum aestivum) × maize (Zea mays) crosses. Plant breeding 1993, 111, 120–124. [Google Scholar] [CrossRef]
- Shrestha, S.; Koo, D.H.; Evers, B.; Wu, S.; Walkowiak, S.; Hucl, P.; Pozniak, C.; Fritz, A.; Poland, J. Wheat doubled haploids have a marked prevalence of chromosomal aberrations. The Plant Genome 2023, 16. [Google Scholar] [CrossRef]
- Lv, J.; Yu, K.; Wei, J.; Gui, H.; Liu, C.; Liang, D.; Wang, Y.; Zhou, H.; Carlin, R.; Rich, R.; et al. Generation of paternal haploids in wheat by genome editing of the centromeric histone CENH3. Nature Biotechnology 2020, 38, 1397–1401. [Google Scholar] [CrossRef]


| Pollen source | First reported | Rates of haploid embryos (%) | Average rate of haploid embryos (%) | Rates of haploid plantlets (%) | Average rate of haploid plantlets (%) | Wheat genotype dependence | References |
|---|---|---|---|---|---|---|---|
| Maize | [28] | 1.6-60.7 | 17.0 | 16.3-86.6 | 58.0 | weak | [35,36,60,61,62] |
| Sorghum | [45] | 0-42.1 | 18.0 | 56.4-63.3 | 59.9 | strong | [52,53] |
| Pearl millet | [46] | 0.3-39.4 | 18.1 | 44.6-72.2 | 53.1 | weak | [53,54,55] |
| Teosinte | [47] | 12.5-57.5 | 40.5 | 34.6-90.3 | 72.2 | weak | [47,56] |
| Tripsacum | [48] | 5.0-59.0 | 22.9 | 69.3-83.3 | 78.5 | weak | [48,57] |
| Job’s tears | [49] | 10.6 | 10.6 | 26.1 | 26.1 | unknown | [49] |
| Imperata cylindrica | [50] | 0-64.7 | 27.4 | 18.1-84.9 | 48.9 | weak | [50,59,60,63] |
| Ae. caudata | [51] | No data | No data | No data | No data | unknown | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




