Submitted:
19 January 2024
Posted:
30 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Patient enrollment and stratification
2.2. Surgical details
2.3. Patient assessments and Measured outcomes
2.4. Statistics
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sooriakumaran P, Pavan N, Wiklund PN, Roach 3rd M. Surgery Versus Radiation for High-risk Prostate Cancer: The Fight Continues. But Is It Time To Call a Draw and Reach Consensus? Eur Urol [Internet]. 2019 Apr 1;75(4):556–7. [CrossRef]
- Mossanen M, Nepple KG, Grubb RL 3rd, Androile GL, Kallogjeri D, Klein EA, et al. Heterogeneity in Definitions of High-risk Prostate Cancer and Varying Impact on Mortality Rates after Radical Prostatectomy. Eur Urol Oncol. 2018 Jun;1(2):143–8. [CrossRef]
- Kishan AU, Karnes RJ, Romero T, Wong JK, Motterle G, Tosoian JJ, et al. Comparison of Multimodal Therapies and Outcomes Among Patients With High-Risk Prostate Cancer With Adverse Clinicopathologic Features. JAMA Netw open. 2021 Jul;4(7):e2115312. [CrossRef]
- Abdollah F, Sood A, Sammon JD, Hsu L, Beyer B, Moschini M, et al. Long-term cancer control outcomes in patients with clinically high-risk prostate cancer treated with robot-assisted radical prostatectomy: results from a multi-institutional study of 1100 patients. Eur Urol. 2015 Sep;68(3):497–505. [CrossRef]
- Deng W, Chen R, Zhu K, Cheng X, Xiong Y, Liu W, et al. Functional Preservation and Oncologic Control following Robot-Assisted versus Laparoscopic Radical Prostatectomy for Intermediate- and High-Risk Localized Prostate Cancer: A Propensity Score Matched Analysis. J Oncol. 2021;2021:4375722. [CrossRef]
- Tavukçu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol [Internet]. 2016/12/08. 2016 Dec;57(Suppl 2):S172–84. Available from: https://pubmed.ncbi.nlm.nih.gov/27995221. [CrossRef]
- Association, WM. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA [Internet]. 2013 Nov 27;310(20):2191–4. [CrossRef]
- Cornford P, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur Urol. 2021 Feb;79(2):263–82. [CrossRef]
- Choo R, Danjoux C, Gardner S, Morton G, Szumacher E, Loblaw DA, et al. Prospective study evaluating postoperative radiotherapy plus 2-year androgen suppression for post-radical prostatectomy patients with pathologic T3 disease and/or positive surgical margins. Int J Radiat Oncol Biol Phys. 2009 Oct;75(2):407–12. [CrossRef]
- Kumar A, Patel VR, Panaiyadiyan S, Seetharam Bhat KR, Moschovas MC, Nayak B. Nerve-sparing robot-assisted radical prostatectomy: Current perspectives. Asian J Urol. 2021 Jan;8(1):2–13. [CrossRef]
- Menon M, Shrivastava A, Kaul S, Badani KK, Fumo M, Bhandari M, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol. 2007 Mar;51(3):648. [CrossRef]
- Shin TY, Lee YS. Robot-assisted radical prostatectomy with clipless intrafascial neurovascular bundle-sparing approach: surgical technique and one-year functional and oncologic outcomes. Sci Rep. 2020 Oct;10(1):17595. [CrossRef]
- Dell’Oglio P, Tappero S, Longoni M, Buratto C, Scilipoti P, Secco S, et al. Retzius-sparing Robot-assisted Radical Prostatectomy in High-risk Prostate Cancer Patients: Results from a Large Single-institution Series. Eur Urol open Sci. 2022 Apr;38:69–78. [CrossRef]
- Greco F, Hoda MR, Wagner S, Reichelt O, Inferrera A, Magno C, et al. Bilateral vs unilateral laparoscopic intrafascial nerve-sparing radical prostatectomy: evaluation of surgical and functional outcomes in 457 patients. BJU Int. 2011 Aug;108(4):583–7. [CrossRef]
- Asimakopoulos AD, Miano R, Di Lorenzo N, Spera E, Vespasiani G, Mugnier C. Laparoscopic versus robot-assisted bilateral nerve-sparing radical prostatectomy: comparison of pentafecta rates for a single surgeon. Surg Endosc. 2013 Nov;27(11):4297–304. [CrossRef]
- Ou Y-C, Yang C-K, Kang H-M, Chang K-S, Wang J, Hung S-W, et al. Pentafecta Outcomes of 230 Cases of Robotic-assisted Radical Prostatectomy with Bilateral Neurovascular Bundle Preservation. Anticancer Res. 2015 Sep;35(9):5007–13.
- Jazayeri SB, Weissman B, Samadi DB. Outcomes following robotic-assisted laparoscopic prostatectomy: Pentafecta and Trifecta achievements. Minerva Urol Nefrol. 2018 Feb;70(1):66–73. [CrossRef]
| Variable | Overall |
| Age (yrs) Mean (± SD) Median (IQR) |
63 (±6.4) 64 (59-68) |
| PSA (ng/mL) Mean (± SD) Median (IQR) |
14 (± 14) 8.90 (5-19) |
| Nerve Sparing. n (%) Yes No |
429 (54.8%) 350 (45.2%) |
| Variable Pathologic stage, n (%) pT2 pT3a pT3b pT4 Lymph node status, n (%) pNx pN0 pN1 |
Overall | Group 1 (N=350) No NS |
Group 2 (N= 429) NS |
P Value |
|
pT2: 230 (29.56%) pT3a: 393 (50.51%) pT3b: 145 (19.02%) pT4: 7 (0.9%) |
pT2: 101 (28.85%) pT3a: 149 (42.57%) pT3b: 96 (27.42%) pT4: 4 (1.14%) |
pT2: 129 (30.14%) pT3a: 244 (57%)pT3b: 49 (12.15%) pT4: 3 (0.7%) |
0.08 | |
|
pNx: 360 (46.43%) pN0: 349 (44.91%) pN1: 66 (8.65%) |
pNx: 149 (42.69%) pN0: 164 (47%)pN1: 36 (10.31%) |
pNx: 211 (49.42%) pN0: 185 (43.24%) pN1: 30 (7.32%) |
0.09 | |
| Pathologic Gleason Score, n (%) 3+3 3+4 4+3 4+4 >4+4 Positive surgical margins. n (%) |
3+3: 81 (10.51%) 3+4: 218 (27.66%) 4+3:203 (26.36%) 4+4: 137 (17.79%) >4+4: 136 (17.66%) |
3+3: 23 (6.68%)3+4: 75 (21.80%) 4+3:88 (25.58%)4+4: 65 (18.89%) 4+4: 93 (27.03%) |
3+3: 58 (13.61%)3+4: 143 (32.39%) 4+3:115(26.99%) 4+4: 72(16.90%) 4+4: 43(10.09%) |
0.02 |
|
254 (32%) |
111 (31%) |
143 (33%) |
0.5 |
|
| Negative surgical margins, n (%) Monofocal PSM Multifocal PSM Positive surgical margins length, n (%) <= 3 mm >3 mm Localization of the PSM, n (%) Base Posterolateral Anterior Apex Follow up (months) Mean (± SD) Median (IQR) |
524 162 93 |
239 65 46 |
282 96 47 |
0.3 |
|
130 123 |
53 57 |
77 66 |
0.5 |
|
|
63 100 54 91 |
28 (8%) 35 (10%) 27 (8%) 41 (12%) |
30 (7%) 65 (15%) 27 (6%) 50 (12%) |
0.5 |
|
|
192 (±14) 132 (80-180) |
219 (±448) 133 (85-181) |
170 (±296) 125 (78-180) |
0.7 | |
| Biochemical recurrence rate, n (%) Adjuvant therapy. n (%) |
No: 447 (58.33%) Yes: 328 (41.67%) |
No: 178 (54.57%) Yes: 172(45.43%) |
No:273(61.39%)Yes: 156(38.61%) | 0.09 |
| Yes: 135(17.67%) No: 640(82.33%) |
Yes: 90 (25.14%) No: 260(74.86%) |
Yes: 49(15.10%)No:380(84.90%) | 0.07 |
| Univariable analysis for predictors of Biochemical recurrence rate | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variabile | OR | Lower CI | Upper CI | P Value | ||||
| Age > 70 yrs | 1 | 0.7 | 1.5 | 0.7 | ||||
|
Preoperative PSA value <= 10 ng/mL vs > 10 ng/mL |
1.6 |
1.2 |
2.0 |
<0.001 | ||||
| Positive surgical margin (PSM) | 1.5 | 1.2 | 1.9 | <0.001 | ||||
|
Surgical margin status Negative Positive Monofocal Positive Multifocal |
Reference 1.3 1.6 |
1.1 1.2 |
1.9 2.1 |
<0.001 0.005 <0.001 |
||||
|
PSM Length < 3mm vs >= 3mm |
1.4 |
1.1 |
1.9 |
0.007 | ||||
|
Pathologic Gleason Score 3+3 ≤ 3+4 vs 3+3 4+3 vs 3+3 4+4 vs ≤ 3+3 ≥ 4+5 vs ≤ 3+3 |
Reference 1.6 2.3 2.6 3.8 |
1 1.3 1.5 2.3 |
2.8 3.8 4.5 6.4 |
<0.001 0.05 0.01 0.001 0.001 |
||||
|
Pathologic stage Organ confined vs Locally advanced |
2.0 |
1.5 |
2.7 |
<0.001 |
||||
|
Lymph node status Nx N0 vs Nx N1 vs Nx |
Reference 0.9 4.3 |
0.7 3.0 |
1.1 6.0 |
<0.001 0.5 <0.001 |
||||
|
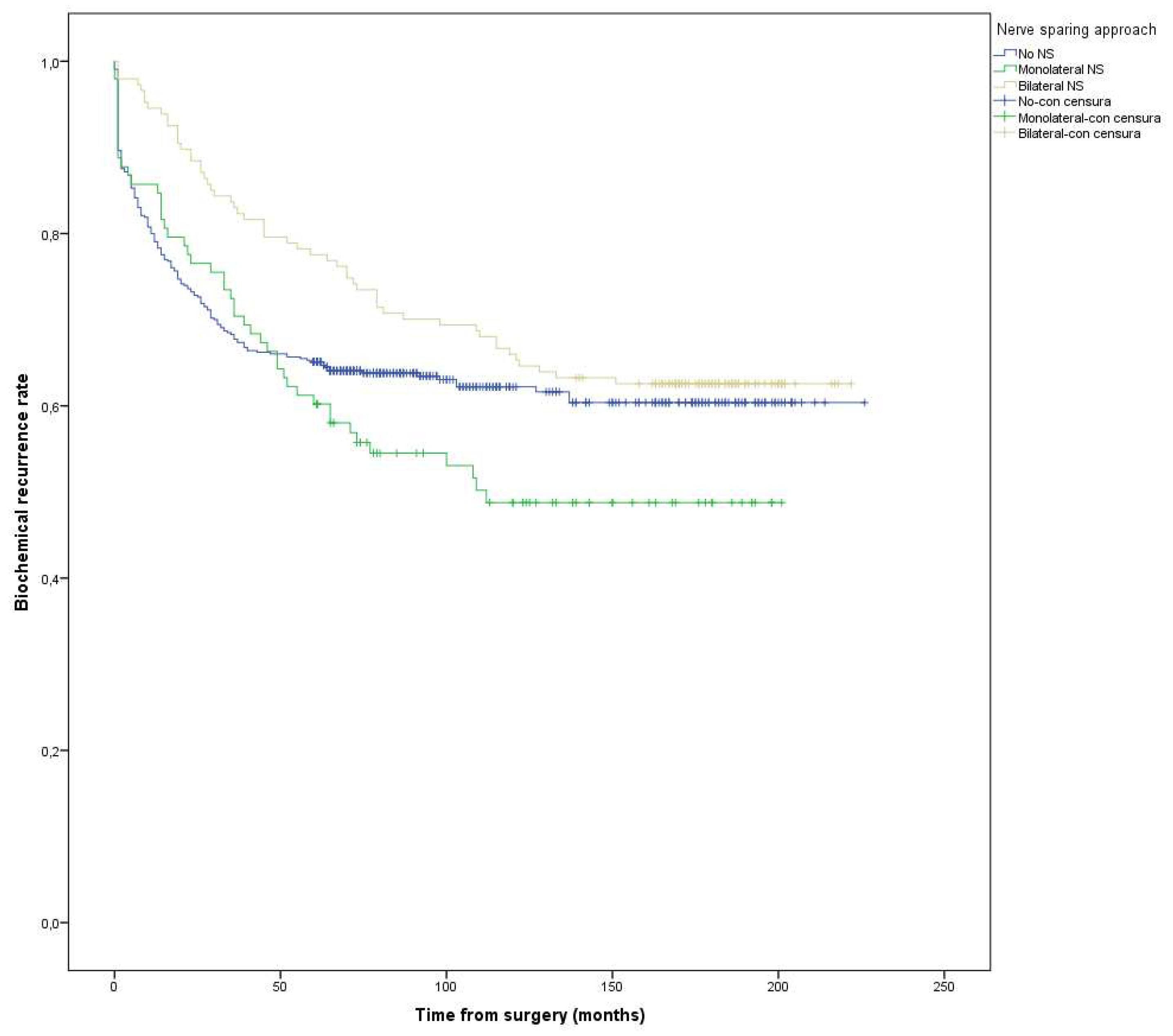
Nerve sparing (NS) approach No NS Monolateral NS vs NO-NS Bilateral NS vs NO-NS |
Reference 1.2 0.7 |
0.9 0.5 |
1.7 1.0 |
0.6 0.1 0.1 |
||||
| Multivariable analysis for predictors of Biochemical recurrence rate | ||||||||
| Variabile | OR | Lower CI | Upper CI | P Value | ||||
| Age >70 yrs | 1.0 | 0.9 | 1.5 | 0.6 | ||||
|
Preoperative PSA value <= 10 ng/mL vs > 10 ng/mL |
1.4 |
1.5 |
2.8 |
<0.001 | ||||
| Positive surgical margin (PSM) | 1.5 | 1.3 | 1.8 | 0.005 | ||||
|
Surgical margin status Negative Positive Monofocal Positive Multifocal |
Reference 1.4 2.3 |
0.9 1.5 |
2.4 3.6 |
<0.001 0.1 <0.001 |
||||
|
PSM Length < 3mm vs >= 3mm |
1.5 |
0.8 |
2.8 |
0.1 |
||||
|
Pathologic Gleason Score 3+3 ≤ 3+4 vs 3+3 4+3 vs 3+3 4+4 vs ≤ 3+3 ≥ 4+5 vs ≤ 3+3 |
Reference 1.6 2.1 2.5 3.4 |
0.9 1.2 1.4 1.9 |
2 3.5 4.4 6 |
<0.001 0.07 <0.001 <0.001 <0.001 |
||||
|
Pathologic stage Organ confined vs Locally advanced |
2.1 |
1.5 |
2.1 |
<0.001 |
||||
|
Lymph node status Nx N0 vs Nx N1 vs Nx |
Reference 0.7 2.5 |
0.7 1.6 |
1 3.8 |
<0.001 0.1 <0.001 |
||||
|
Nerve sparing (NS) approach No NS Monolateral NS vs NO-NS Bilateral NS vs NO-NS |
Reference 0.7 1.3 |
0.5 1.0 |
1.5 1.8 |
0.06 0.7 0.03 |
||||
| Univariable analysis for predictors of Cancer specific survival | ||||||||
| Variabile | HR | 95% CI | Upper CI | P Value | ||||
| Age > 70 anni | 0.3 | 0.04 | 2.8 | 0.3 | ||||
|
Preoperative PSA value <= 10 ng/mL vs > 10 ng/mL |
0.5 |
0.2 |
1.6 |
0.3 |
||||
| Positive surgical margin (PSM) | 1.1 | 0.4 | 3.1 | 0.7 | ||||
|
Surgical margin status Negative Positive Monofocal Positive Multifocal |
Reference 1.4 0.8 |
0.4 0.2 |
4.5 3.2 |
0.7 0.5 0.8 |
||||
|
PSM Length < 3mm vs >= 3mm |
0.7 |
0.2 |
2.3 |
0.6 |
||||
|
Pathologic Gleason Score 3+3 ≤ 3+4 vs 3+3 4+3 vs 3+3 4+4 vs ≤ 3+3 ≥ 4+5 vs ≤ 3+3 |
Reference 0.8 0.7 1.4 16.2 |
0.1 0.1 0.1 1.7 |
5.0 11 4.0 150 |
0.03 0.8 0.7 0.7 0.01 |
||||
|
Pathologic stage Organ confined vs Locally advanced |
3.3 |
0.4 |
26 |
0.2 | ||||
|
Lymph node status Nx N0 vs Nx N1 vs Nx |
Reference 1.4 1.5 |
0.5 1.5 |
4.1 5.9 |
0.04 0.6 <0.001 |
||||
|
Nerve sparing (NS) approach No NS Monolateral NS vs NO-NS Bilateral NS vs NO-NS |
Reference 0.9 1.0 |
0.9 0.9 |
1.5 1.8 |
0.09 0.09 0.09 |
||||
| Multivariable analysis for predictors of Cancer specific survival | ||||||||
| Variabile | HR | Lower CI | Upper CI | P Value | ||||
| Age > 70 anni | 0.6 | 0.2 | 20 | 0.8 | ||||
|
Preoperative PSA value <= 10 ng/mL vs > 10 ng/mL |
0.1 |
0.01 |
1.6 |
0.1 |
||||
| Positive surgical margin (PSM) | 0.4 | 0.3 | 11 | 0.8 | ||||
|
Surgical margin status Negative Positive Monofocal Positive Multifocal |
Reference 0.9 1.0 |
0.7 0.04 |
1.8 90 |
0.9 0.8 0.9 |
||||
|
PSM Length < 3mm vs >= 3mm |
0.9 |
0.05 |
16 |
0.9 |
||||
|
Pathologic Gleason Score 3+3 ≤ 3+4 vs 3+3 4+3 vs 3+3 4+4 vs ≤ 3+3 ≥ 4+5 vs ≤ 3+3 |
Reference 0.6 16 0.7 0.2 |
0.04 0.6 0.03 0.01 |
11 456 20 4 |
0.2 0.8 0.09 0.9 0.3 |
||||
|
Pathologic stage Organ confined vs Locally advanced |
11 |
0.5 |
243 |
0.1 | ||||
|
Lymph node status Nx N0 vs Nx N1 vs Nx |
Reference 1.2 41 |
0.8 1.6 |
1.5 1000 |
0.001 0.06 <0.001 |
||||
|
Nerve sparing (NS) approach No NS Monolateral NS vs NO-NS Bilateral NS vs NO-NS |
Reference 0.9 1.0 |
0.9 0.9 |
1.5 1.8 |
0.09 0.09 0.09 |
||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





