Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
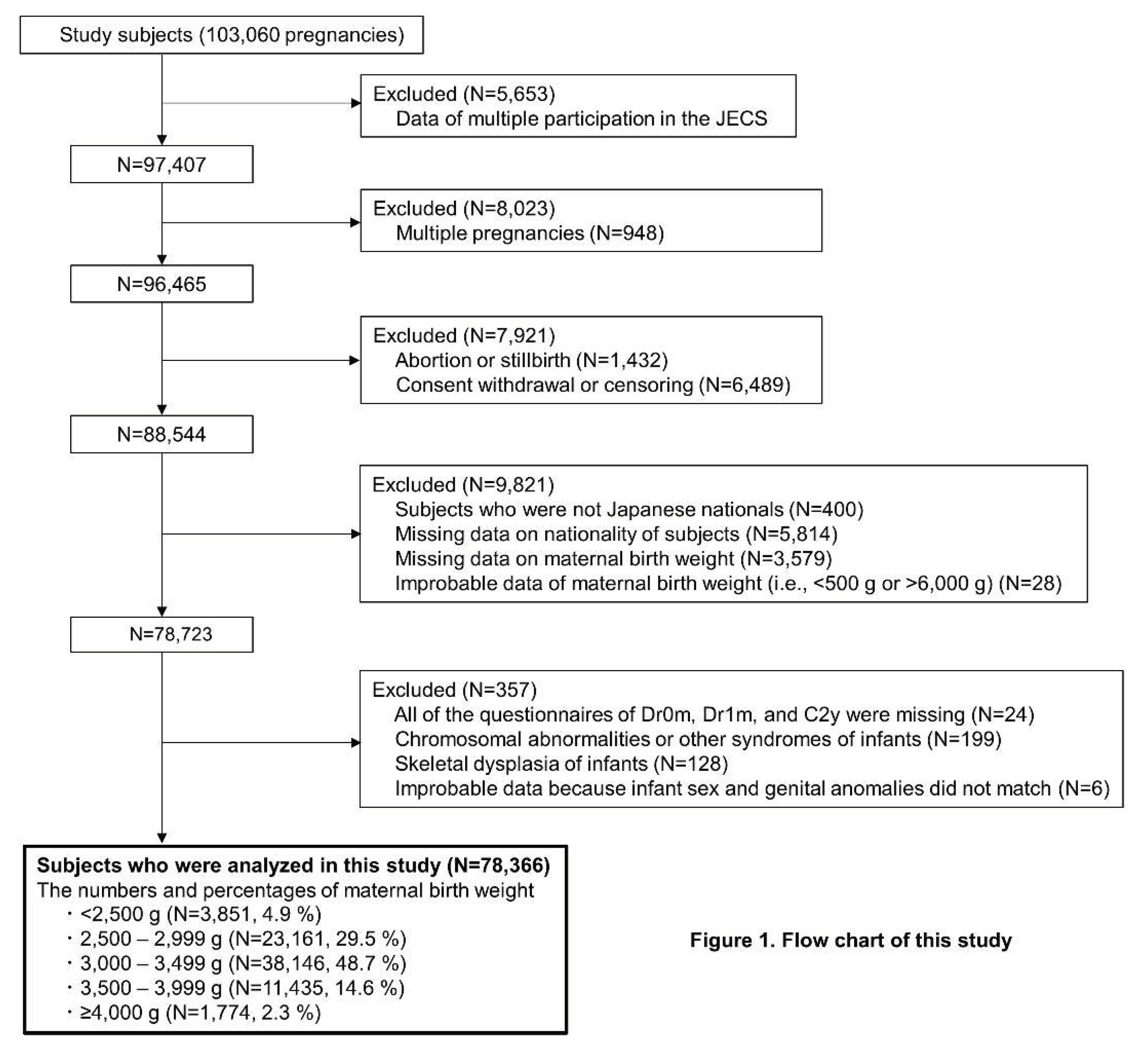
2.1. Study Design
2.2. Maternal Birth Weight (MBW)
2.3. Infant Congenital Malformations Selection
2.4. Data Collection and Classification of Other Variables
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
3.2. Prevalence of Infant Congenital Malformations
3.3. Association between MBW and Prevalence of Infant Congenital Malformations
3.4. Association between MBW and Prevalence of Infant Congenital Malformations Stratified by Infant Sex
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Oliveira, C.; Fett-Conte, A. Birth Defects: Risk Factors and Consequences. J Pediatr Genet 2015, 02, 085–090. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Fukuoka, H. Developmental Origins of Health and Disease Theory in Cardiology. Journal of Cardiology 2020, 76, 14–17. [Google Scholar] [CrossRef]
- Hanson, M. The Birth and Future Health of DOHaD. J Dev Orig Health Dis 2015, 6, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Egeland, G.M.; Skjærven, R.; Irgens, L.M. Birth Characteristics of Women Who Develop Gestational Diabetes: Population Based Study. BMJ 2000, 321, 546–547. [Google Scholar] [CrossRef] [PubMed]
- Innes, K.E. Association of a Woman’s Own Birth Weight With Subsequent Risk for Gestational Diabetes. JAMA 2002, 287, 2534. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.C.; Williams, M.A.; Luthy, D.A.; Emanuel, I.; Shy, K. Weight at Birth and Subsequent Risk of Preeclampsia as an Adult. Am J Obstet Gynecol 2003, 189, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Andraweera, P.H.; Dekker, G.; Leemaqz, S.; McCowan, L.; Myers, J.; Kenny, L.; Walker, J.; Poston, L.; Roberts, C.T. ; the SCOPE Consortium Effect of Birth Weight and Early Pregnancy BMI on Risk for Pregnancy Complications. Obesity 2019, 27, 237–244. [Google Scholar] [CrossRef]
- Bay, B.; Ingerslev, H.J.; Lemmen, J.G.; Degn, B.; Rasmussen, I.A.; Kesmodel, U.S. Preimplantation Genetic Diagnosis: A National Multicenter Obstetric and Neonatal Follow-up Study. Fertility and Sterility 2016, 106, 1363–1369. [Google Scholar] [CrossRef]
- Á Rogvi, R.; Forman, J.L.; Damm, P.; Greisen, G. Women Born Preterm or with Inappropriate Weight for Gestational Age Are at Risk of Subsequent Gestational Diabetes and Pre-Eclampsia. PLoS ONE 2012, 7, e34001. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and Study Design of the Japan Environment and Children’s Study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). Journal of Epidemiology 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Nishigori, H.; Obara, T.; Nishigori, T.; Metoki, H.; Ishikuro, M.; Mizuno, S.; Sakurai, K.; Tatsuta, N.; Nishijima, I.; Fujiwara, I.; et al. Drug Use before and during Pregnancy in Japan: The Japan Environment and Children’s Study. Pharmacy 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.; Ogawa, K.; Kanazawa, S.; Kawasaki, M.; Morisaki, N.; Mito, A.; Sago, H.; Horikawa, R.; Arata, N. Association of Maternal Birth Weight with the Risk of Low Birth Weight and Small-for-Gestational-Age in Offspring: A Prospective Single-Center Cohort Study. PLoS ONE 2021, 16, e0251734. [Google Scholar] [CrossRef]
- Low Birthweight: Country, Regional and Global Estimates; Wardlaw, T., Wardlaw, T.M., Weltgesundheitsorganisation, *!!! REPLACE !!!*, Eds.; World Health Organization: Geneva, 2004; ISBN 978-92-806-3832-5. [Google Scholar]
- Wagata, M.; Ishikuro, M.; Obara, T.; Nagai, M.; Mizuno, S.; Nakaya, N.; Nakamura, T.; Hirata, T.; Tsuchiya, N.; Metoki, H.; et al. Low Birth Weight and Abnormal Pre-Pregnancy Body Mass Index Were at Higher Risk for Hypertensive Disorders of Pregnancy. Pregnancy Hypertension 2020, 22, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Mezawa, H.; Tomotaki, A.; Yamamoto-Hanada, K.; Ishitsuka, K.; Ayabe, T.; Konishi, M.; Saito, M.; Yang, L.; Suganuma, N.; Hirahara, F.; et al. Prevalence of Congenital Anomalies in the Japan Environment and Children’s Study. Journal of Epidemiology 2019, 29, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, Y.; Tatsuta, N.; Iwai-Shimada, M.; Nakai, K.; Arima, T.; Fujiwara, I.; Yaegashi, N.; Takeuchi, A.; Nakayama, S.F. The Association between Gestational Use of Personal Care Products and Neonatal Urological Abnormality at Birth: The Japan Environment and Children’s Study. Reproductive Toxicology 2020, 93, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Iwama, N.; Sugiyama, T.; Metoki, H.; Saito, M.; Hoshiai, T.; Watanabe, Z.; Tanaka, K.; Sasaki, S.; Sakurai, K.; Ishikuro, M.; et al. Associations between Glycosylated Hemoglobin Level at Less than 24 Weeks of Gestation and Adverse Pregnancy Outcomes in Japan: The Japan Environment and Children’s Study (JECS). Diabetes Research and Clinical Practice 2020, 169, 108377. [Google Scholar] [CrossRef] [PubMed]
- Heinze, G.; Schemper, M. A Solution to the Problem of Separation in Logistic Regression. Statistics in Medicine 2002, 21, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 29 January 2024).
- Su, R.; Zhu, W.; Wei, Y.; Wang, C.; Feng, H.; Lin, L.; Hod, M.; Hadar, E.; Yang, H. Relationship of Maternal Birth Weight on Maternal and Neonatal Outcomes: A Multicenter Study in Beijing. J Perinatol 2016, 36, 1061–1066. [Google Scholar] [CrossRef]
- Lisi, A.; Botto, L.D.; Rittler, M.; Castilla, E.; Bianchi, F.; Botting, B.; De Walle, H.; Erickson, J.D.; Gatt, M.; De Vigan, C.; et al. Sex and Congenital Malformations: An International Perspective. Am. J. Med. Genet. 2005, 134A, 49–57. [Google Scholar] [CrossRef]
- Diab, N.S.; Barish, S.; Dong, W.; Zhao, S.; Allington, G.; Yu, X.; Kahle, K.T.; Brueckner, M.; Jin, S.C. Molecular Genetics and Complex Inheritance of Congenital Heart Disease. Genes 2021, 12, 1020. [Google Scholar] [CrossRef]
- Lee, K.-S.; Choi, Y.-J.; Cho, J.; Lee, H.; Lee, H.; Park, S.J.; Park, J.S.; Hong, Y.-C. Environmental and Genetic Risk Factors of Congenital Anomalies: An Umbrella Review of Systematic Reviews and Meta-Analyses. J Korean Med Sci 2021, 36, e183. [Google Scholar] [CrossRef] [PubMed]
- Mires, S.; Caputo, M.; Overton, T.; Skerritt, C. Maternal Micronutrient Deficiency and Congenital Heart Disease Risk: A Systematic Review of Observational Studies. Birth Defects Research 2022, 114, 1079–1091. [Google Scholar] [CrossRef]
- Choudhury, T.Z.; Majumdar, U.; Basu, M.; Garg, V. Impact of Maternal Hyperglycemia on Cardiac Development: Insights from Animal Models. Genesis 2021, 59, e23449. [Google Scholar] [CrossRef] [PubMed]
- Negrato, C.A.; Marques, P.R.; Leite, H.B.; Torigoe, C.N.; Silva, B.F.; Costa, K.; Kamei, J.M.; Zampa, C.L.; Toni, A.C.R.G.; Pereira, I.C.G.S.; et al. Glycemic and Nonglycemic Mechanisms of Congenital Malformations in Hyperglycemic Pregnancies: A Narrative Review. Archives of Endocrinology and Metabolism 2022. [Google Scholar] [CrossRef]
- Nicolaou, N.; Renkema, K.Y.; Bongers, E.M.H.F.; Giles, R.H.; Knoers, N.V.A.M. Genetic, Environmental, and Epigenetic Factors Involved in CAKUT. Nat Rev Nephrol 2015, 11, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Postoev, V.A.; Grjibovski, A.M.; Kovalenko, A.A.; Anda, E.E.; Nieboer, E.; Odland, J.Ø. Congenital Anomalies of the Kidney and the Urinary Tract: A Murmansk County Birth Registry Study. Birth Defects Research 2016, 106, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.-L.; Luh, H.; Lin, C.-Y.; Hsu, C.-N. Incidence and Risks of Congenital Anomalies of Kidney and Urinary Tract in Newborns: A Population-Based Case–Control Study in Taiwan. Medicine 2016, 95, e2659. [Google Scholar] [CrossRef]
- Macumber, I.; Schwartz, S.; Leca, N. Maternal Obesity Is Associated with Congenital Anomalies of the Kidney and Urinary Tract in Offspring. Pediatr Nephrol 2017, 32, 635–642. [Google Scholar] [CrossRef]
- Groen In ’T Woud, S.; Renkema, K.Y.; Schreuder, M.F.; Wijers, C.H.W.; Van Der Zanden, L.F.M.; Knoers, N.V.A.M.; Feitz, W.F.J.; Bongers, E.M.H.F.; Roeleveld, N.; Van Rooij, I.A.L.M. Maternal Risk Factors Involved in Specific Congenital Anomalies of the Kidney and Urinary Tract: A Case–Control Study. Birth Defects Research 2016, 106, 596–603. [Google Scholar] [CrossRef]
- Boato, R.T.; Aguiar, M.B.; Mak, R.H.; Colosimo, E.A.; Simões E Silva, A.C.; Oliveira, E.A. Maternal Risk Factors for Congenital Anomalies of the Kidney and Urinary Tract: A Case-Control Study. Journal of Pediatric Urology 2023, 19, 199–e1. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Reece, E.A.; Pavlinkova, G.; Kappen, C.; Miller, R.K. Effect of Maternal Diabetes on the Embryo, Fetus, and Children: Congenital Anomalies, Genetic and Epigenetic Changes and Developmental Outcomes. Birth Defects Research Pt C 2015, 105, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, V.; Edwards, C.F.; Bichianu, D.C. Inguinal Hernia in Premature Infants.
- Smith, C.J.F.; Friedlander, S.F.; Guma, M.; Kavanaugh, A.; Chambers, C.D. Infantile Hemangiomas: An Updated Review on Risk Factors, Pathogenesis, and Treatment. Birth Defects Research 2017, 109, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Auger, N.; Del Giorgio, F.; Le-Nguyen, A.; Bilodeau-Bertrand, M.; Piché, N. Maternal Risk Factors for Paediatric Inguinal Hernia. British Journal of Surgery 2021, 109, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Murai-Takeda, A.; Kawabe, H.; Itoh, H. Low Birth Weight Trends: Possible Impacts on the Prevalences of Hypertension and Chronic Kidney Disease. Hypertens Res 2020, 43, 859–868. [Google Scholar] [CrossRef]
- Little, R.E. Birthweight and Gestational Age: Mothers’ Estimates Compared with State and Hospital Records. Am J Public Health 1986, 76, 1350–1351. [Google Scholar] [CrossRef]
| Variables | All participants (N=78,366) | Participants according to maternal birth weight | ||||
|---|---|---|---|---|---|---|
| <2,500 g (N=3,850) | 2,500–2,999 g (N=23,161) | 3,000–3,499 g (N=38,146) | 3,500–3,999 g (N=11,435) | ≥4,000 g (N=1,774) | ||
| Maternal age at the MT1 questionnaire, years | 30.9 (5.0) | 30.6 (5.2) | 30.7 (5.0) | 31.0 (4.9) | 31.2 (4.9) | 31.5 (4.8) |
| Category of maternal age at the MT1 questionnaire, N (%) | ||||||
| <25 years | 7,897 (10.1) | 498 (12.9) | 2,631 (11.4) | 3,653 (9.6) | 976 (8.5) | 139 (7.8) |
| 25–29.9 years | 22,832 (29.1) | 1,118 (29.0) | 6,971 (30.1) | 11,005 (28.8) | 3,270 (28.6) | 468 (26.4) |
| 30–34.9 years | 27,679 (35.3) | 1,298 (33.7) | 7,949 (34.3) | 13,727 (36.0) | 4,054 (35.5) | 651 (36.7) |
| ≥35 years | 19,480 (24.9) | 905 (23.5) | 5,478 (23.7) | 9,530 (25.0) | 3,061 (26.8) | 506 (28.5) |
| Missing | 478 (0.6) | 31 (0.8) | 132 (0.6) | 231 (0.6) | 74 (0.6) | 10 (0.6) |
| Pre-pregnancy BMI, kg/m2 | 21.2 (3.2) | 20.9 (3.3) | 20.9 (3.2) | 21.2 (3.2) | 21.5 (3.3) | 22.0 (3.6) |
| Category of pre-pregnancy BMI, N (%) | ||||||
| Underweight (<18.5 kg/m2) | 12,733 (16.2) | 790 (20.5) | 4,370 (18.9) | 5,973 (15.7) | 1,443 (12.6) | 157 (8.9) |
| Normal range (18.5–24.9 kg/m2) | 57,638 (73.5) | 2,682 (69.7) | 16,664 (71.9) | 28,251 (74.1) | 8,686 (76.0) | 1,355 (76.4) |
| Obese (≥25.0 kg/m2) | 7,936 (10.1) | 373 (9.7) | 2,105 (9.1) | 3,901 (10.2) | 1,297 (11.3) | 260 (14.7) |
| Missing | 59 (0.1) | 5 (0.1) | 22 (0.1) | 21 (0.1) | 9 (0.1) | 2 (0.1) |
| Parity, N (%) | ||||||
| Primipara | 33,188 (42.3) | 1,742 (45.2) | 9,997 (43.2) | 15,854 (41.6) | 4,888 (42.7) | 707 (39.9) |
| Multipara | 43,210 (55.1) | 2,015 (52.3) | 12,539 (54.1) | 21,364 (56.0) | 6,263 (54.8) | 1,029 (58.0) |
| Missing | 1,968 (2.5) | 93 (2.4) | 625 (2.7) | 928 (2.4) | 284 (2.5) | 38 (2.1) |
| Conception method, N (%) | ||||||
| Spontaneous pregnancy | 72,566 (92.6) | 3,567 (92.6) | 21,492 (92.8) | 35,385 (92.8) | 10,487 (91.7) | 1,635 (92.2) |
| Non-ART | 2,984 (3.8) | 147 (3.8) | 860 (3.7) | 1,423 (3.7) | 490 (4.3) | 64 (3.6) |
| ART | 2,446 (3.1) | 111 (2.9) | 701 (3.0) | 1,159 (3.0) | 408 (3.6) | 67 (3.8) |
| Missing | 370 (0.5) | 25 (0.6) | 108 (0.5) | 179 (0.5) | 50 (0.4) | 8 (0.5) |
| History of hypertension, N (%) | ||||||
| Yes | 355 (0.5) | 29 (0.8) | 119 (0.5) | 153 (0.4) | 46 (0.4) | 8 (0.5) |
| No | 77,536 (98.9) | 3,790 (98.4) | 22,910 (98.9) | 37,763 (99.0) | 11,317 (99.0) | 1,756 (99.0) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| History of type 1 diabetes, N (%) | ||||||
| Yes | 61 (0.1) | 3 (0.1) | 19 (0.1) | 30 (0.1) | 7 (0.1) | 2 (0.1) |
| No | 77,830 (99.3) | 3,816 (99.1) | 23,010 (99.3) | 37,886 (99.3) | 11,356 (99.3) | 1,762 (99.3) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| History of type 2 diabetes, N (%) | ||||||
| Yes | 96 (0.1) | 8 (0.2) | 31 (0.1) | 40 (0.1) | 12 (0.1) | 5 (0.3) |
| No | 77,795 (99.3) | 3,811 (99.0) | 22,998 (99.3) | 37,876 (99.3) | 11,351 (99.3) | 1,759 (99.2) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| HbA1c (NGSP) level at <24 weeks of gestation, % | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) | 5.2 (0.3) |
| History of kidney disorder, N (%) | ||||||
| Yes | 341 (0.4) | 23 (0.6) | 102 (0.4) | 150 (0.4) | 55 (0.5) | 11 (0.6) |
| No | 77,550 (99.0) | 3,796 (98.6) | 22,927 (99.0) | 37,766 (99.0) | 11,308 (98.9) | 1,753 (98.8) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| History of mental diseases, N (%) | ||||||
| Yes | 5,961 (7.6) | 323 (8.4) | 1,762 (7.6) | 2,849 (7.5) | 887 (7.8) | 140 (7.9) |
| No | 71,930 (91.8) | 3,496 (90.8) | 21,267 (91.8) | 35,067 (91.9) | 10,476 (91.6) | 1,624 (91.5) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| History of congenital heart diseases, N (%) | ||||||
| Yes | 259 (0.3) | 22 (0.6) | 79 (0.3) | 109 (0.3) | 44 (0.4) | 5 (0.3) |
| No | 77,632 (99.1) | 3,797 (98.6) | 22,950 (99.1) | 37,807 (99.1) | 11,319 (99.0) | 1,759 (99.2) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| Uterine malformation, N (%) | ||||||
| Yes | 223 (0.3) | 8 (0.2) | 74 (0.3) | 110 (0.3) | 27 (0.2) | 4 (0.2) |
| No | 77,668 (99.1) | 3,811 (99.0) | 22,955 (99.1) | 37,806 (99.1) | 11,336 (99.1) | 1,760 (99.2) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| Malformation of urinary tract or genital organs, N (%) | ||||||
| Yes | 30 (0.0) | 0 (0.0) | 11 (0.0) | 15 (0.0) | 4 (0.0) | 0 (0.0) |
| No | 77,861 (99.4) | 3,819 (99.2) | 23,018 (99.4) | 37,901 (99.4) | 11,359 (99.3) | 1,764 (99.4) |
| Missing | 475 (0.6) | 31 (0.8) | 132 (0.6) | 230 (0.6) | 72 (0.6) | 10 (0.6) |
| Currently smoking, N (%) | ||||||
| Yes | 3,104 (4.0) | 209 (5.4) | 999 (4.3) | 1,456 (3.8) | 362 (3.2) | 78 (4.4) |
| No | 74,288 (94.8) | 3,584 (93.1) | 21,877 (94.5) | 36,217 (94.9) | 10,934 (95.6) | 1,676 (94.5) |
| Missing | 974 (1.2) | 57 (1.5) | 285 (1.2) | 473 (1.2) | 139 (1.2) | 20 (1.1) |
| Continue drinking, N (%) | ||||||
| Yes | 7,943 (10.1) | 359 (9.3) | 2,304 (9.9) | 3,906 (10.2) | 1,175 (10.3) | 199 (11.2) |
| No | 69,624 (88.8) | 3,440 (89.4) | 20,623 (89.0) | 33,854 (88.7) | 10,150 (88.8) | 1,557 (87.8) |
| Missing | 799 (1.0) | 51 (1.3) | 234 (1.0) | 386 (1.0) | 110 (1.0) | 18 (1.0) |
| Use of any drug before 12 weeks of gestation (methimazole, SSRI, antidepressant drug except for SSRI, antianxiety, sleeping pill, antipsychotic, valproic acid, antiepileptic except for valproic acid, lithium carbonate, and other psychoactive drug), N (%) | 2,154 (2.7) | 133 (3.5) | 672 (2.9) | 1,011 (2.7) | 298 (2.6) | 40 (2.3) |
| Use of folic acid supplement before 12 weeks of gestation, N (%) | 25,604 (32.7) | 1,267 (32.9) | 7,493 (32.4) | 12,543 (32.9) | 3,749 (32.8) | 552 (31.1) |
| New-onset HDP, N (%) | ||||||
| Yes | 779 (1.0) | 48 (1.2) | 238 (1.0) | 386 (1.0) | 90 (0.8) | 17 (1.0) |
| No | 77,587 (99.0) | 3,802 (98.8) | 22,923 (99.0) | 37,760 (99.0) | 11,345 (99.2) | 1,757 (99.0) |
| GDM, N (%) | ||||||
| Yes | 2,132 (2.7) | 153 (4.0) | 682 (2.9) | 976 (2.6) | 272 (2.4) | 49 (2.8) |
| No | 76,092 (97.1) | 3,689 (95.8) | 22,435 (96.9) | 37,105 (97.3) | 11,143 (97.4) | 1,720 (97.0) |
| Missing | 142 (0.2) | 8 (0.2) | 44 (0.2) | 65 (0.2) | 20 (0.2) | 5 (0.3) |
| Maternal highest level of education, N (%) | ||||||
| <13 years | 26,252 (33.5) | 1,518 (39.4) | 7,994 (34.5) | 12,581 (33.0) | 3,536 (30.9) | 623 (35.1) |
| ≥13 years | 51,185 (65.3) | 2,280 (59.2) | 14,894 (64.3) | 25,107 (65.8) | 7,779 (68.0) | 1,125 (63.4) |
| Missing | 929 (1.2) | 52 (1.4) | 273 (1.2) | 458 (1.2) | 120 (1.0) | 26 (1.5) |
| Marital status, N (%) | ||||||
| Unmarried or divorced or widowed | 3,185 (4.1) | 211 (5.5) | 1,020 (4.4) | 1,466 (3.8) | 429 (3.8) | 59 (3.3) |
| Married | 74,426 (95.0) | 3,588 (93.2) | 21,923 (94.7) | 36,320 (95.2) | 10,896 (95.3) | 1,699 (95.8) |
| Missing | 755 (1.0) | 51 (1.3) | 218 (0.9) | 360 (0.9) | 110 (1.0) | 16 (0.9) |
| Annual household income (million, Japanese Yen), N (%) | ||||||
| <4 | 28,310 (36.1) | 1,551 (40.3) | 8,592 (37.1) | 13,631 (35.7) | 3,899 (34.1) | 637 (35.9) |
| 4–5.99 | 36,476 (46.5) | 1,656 (43.0) | 10,544 (45.5) | 17,902 (46.9) | 5,552 (48.6) | 822 (46.3) |
| ≥6 | 8,204 (10.5) | 353 (9.2) | 2,371 (10.2) | 4,088 (10.7) | 1,211 (10.6) | 181 (10.2) |
| Missing | 5,376 (6.9) | 290 (7.5) | 1,654 (7.1) | 2,525 (6.6) | 773 (6.8) | 134 (7.6) |
| Delivery week, weeks | 39.3 (1.5) | 39.0 (1.7) | 39.2 (1.6) | 39.3 (1.5) | 39.4 (1.5) | 39.4 (1.5) |
| Preterm delivery at <37 weeks of gestation, N (%) | ||||||
| Yes | 3,476 (4.4) | 242 (6.3) | 1,174 (5.1) | 1,571 (4.1) | 420 (3.7) | 69 (3.9) |
| No | 74,748 (95.4) | 3,600 (93.5) | 21,943 (94.7) | 36,510 (95.7) | 10,995 (96.2) | 1,700 (95.8) |
| Missing | 142 (0.2) | 8 (0.2) | 44 (0.2) | 65 (0.2) | 20 (0.2) | 5 (0.3) |
| Infant sex, N (%) | ||||||
| Male | 40,117 (51.2) | 1,963 (51.0) | 11,820 (51.0) | 19,535 (51.2) | 5,884 (51.5) | 915 (51.6) |
| Female | 38,249 (48.8) | 1,887 (49.0) | 11,341 (49.0) | 18,611 (48.8) | 5,551 (48.5) | 859 (48.4) |
| Infant birth weight, g | 3,026 (411) | 2,877 (425) | 2,933 (400) | 3,044 (395) | 3,171 (415) | 3,258 (425) |
| Category of infant birth weight in percentiles, N (%) | ||||||
| SGA (<10th percentile) | 5,751 (7.3) | 542 (14.1) | 2,418 (10.4) | 2,348 (6.2) | 401 (3.5) | 42 (2.4) |
| AGA (≥10th percentile and <90th percentile) | 62,580 (79.9) | 2,975 (77.3) | 18,625 (80.4) | 31,036 (81.4) | 8,716 (76.2) | 1,228 (69.2) |
| LGA (≥90th percentile) | 7,708 (9.8) | 221 (5.7) | 1,403 (6.1) | 3,652 (9.6) | 1,974 (17.3) | 458 (25.8) |
| Missing | 2,327 (3.0) | 112 (2.9) | 715 (3.1) | 1,110 (2.9) | 344 (3.0) | 46 (2.6) |
| Category of infant birth weight in grams, N (%) | ||||||
| Low birth weight (<2,500 g) | 6,176 (7.9) | 570 (14.8) | 2,520 (10.9) | 2,526 (6.6) | 497 (4.3) | 63 (3.6) |
| Normal birth weight (≥2,500 g and <4,000 g) | 71,333 (91.0) | 3,247 (84.3) | 20,497 (88.5) | 35,252 (92.4) | 10,684 (93.4) | 1,653 (93.2) |
| Macrosomia (≥4,000 g) | 673 (0.9) | 20 (0.5) | 91 (0.4) | 283 (0.7) | 226 (2.0) | 53 (3.0) |
| Missing | 184 (0.2) | 13 (0.3) | 53 (0.2) | 85 (0.2) | 28 (0.2) | 5 (0.3) |
| Data are expressed as mean (SD) or the number (percentage). ART, assisted reproductive technology; AGA, appropriate gestational age; BMI, body mass index; GDM, gestational diabetes mellitus; HDP, hypertensive disorders of pregnancy; LGA, large for gestational age; NGSP, National Glycohemoglobin Standardization Program; SD, standard deviation; SGA, small gestational age; SSRI, selective serotonin reuptake inhibitor. | ||||||
| Infant congenital malformations | All participants (N=78,366) | Participants according to maternal birth weight | ||||
|---|---|---|---|---|---|---|
| <2,500 g (N=3,850) | 2,500–2,999 g (N=23,161) | 3,000–3,499 g (N=38,146) | 3,500–3,999 g (N=11,435) | ≥4,000 g (N=1,774) | ||
| Nervous system, Cases (%) | 205 (0.26) | 14 (0.36) | 60 (0.26) | 93 (0.24) | 33 (0.29) | 5 (0.28) |
| Anencephaly, Cases (%) | 11 (0.01) | 0 (0.00) | 4 (0.02) | 5 (0.01) | 2 (0.02) | 0 (0.00) |
| Encephalocele, Cases (%) | 14 (0.02) | 0 (0.00) | 3 (0.01) | 7 (0.02) | 4 (0.03) | 0 (0.00) |
| Microcephaly, Cases (%) | 25 (0.03) | 1 (0.03) | 9 (0.04) | 12 (0.03) | 2 (0.02) | 1 (0.06) |
| Hydrocephalus, Cases (%) | 59 (0.08) | 7 (0.18) | 12 (0.05) | 32 (0.08) | 7 (0.06) | 1 (0.06) |
| Holoprosencephaly, Cases (%) | 20 (0.03) | 2 (0.05) | 6 (0.03) | 7 (0.02) | 5 (0.04) | 0 (0.00) |
| Craniotabes, Cases (%) | 58 (0.07) | 4 (0.10) | 19 (0.08) | 22 (0.06) | 12 (0.10) | 1 (0.06) |
| Agenesis of corpus callosum, Cases (%) | 17 (0.02) | 1 (0.03) | 4 (0.02) | 8 (0.02) | 4 (0.03) | 0 (0.00) |
| Spina bifida, Cases (%) | 24 (0.03) | 2 (0.05) | 7 (0.03) | 12 (0.03) | 1 (0.01) | 2 (0.11) |
| Eye, ear, and face, Cases (%) | 228 (0.29) | 7 (0.18) | 71 (0.31) | 118 (0.31) | 27 (0.24) | 5 (0.28) |
| Eyelid coloboma, Cases (%) | 12 (0.02) | 1 (0.03) | 3 (0.01) | 8 (0.02) | 0 (0.00) | 0 (0.00) |
| Microphthalmia (anophthalmia), Cases (%) | 13 (0.02) | 0 (0.00) | 2 (0.01) | 9 (0.02) | 1 (0.01) | 1 (0.06) |
| Cataract, Cases (%) | 28 (0.04) | 1 (0.03) | 8 (0.03) | 15 (0.04) | 4 (0.03) | 0 (0.00) |
| Microtia, Cases (%) | 39 (0.05) | 0 (0.00) | 13 (0.06) | 24 (0.06) | 2 (0.02) | 0 (0.00) |
| Atresia of external auditory canal, Cases (%) | 35 (0.04) | 0 (0.00) | 13 (0.06) | 14 (0.04) | 7 (0.06) | 1 (0.06) |
| Cryptotia, Cases (%) | 30 (0.04) | 0 (0.00) | 14 (0.06) | 12 (0.03) | 3 (0.03) | 1 (0.06) |
| Low-set ear, Cases (%) | 29 (0.04) | 1 (0.03) | 11 (0.05) | 14 (0.04) | 3 (0.03) | 0 (0.00) |
| Hearing loss, Cases (%) | 61 (0.08) | 5 (0.13) | 15 (0.06) | 31 (0.08) | 8 (0.07) | 2 (0.11) |
| Facial cleft, Cases (%) | 7 (0.01) | 0 (0.00) | 2 (0.01) | 4 (0.01) | 1 (0.01) | 0 (0.00) |
| Cleft lip and/or cleft palate, Cases (%) | 165 (0.21) | 12 (0.31) | 56 (0.24) | 70 (0.18) | 26 (0.23) | 1 (0.06) |
| Circulatory system, Cases (%) | 1,141 (1.46) | 72 (1.87) | 331 (1.43) | 516 (1.35) | 187 (1.64) | 35 (1.97) |
| Congenital heart disease, Cases (%) | 1,068 (1.36) | 69 (1.79) | 311 (1.34) | 485 (1.27) | 172 (1.50) | 31 (1.75) |
| Arrhythmia, Cases (%) | 84 (0.11) | 3 (0.08) | 25 (0.11) | 34 (0.09) | 17 (0.15) | 5 (0.28) |
| Respiratory system, Cases (%) | 32 (0.04) | 1 (0.03) | 7 (0.03) | 17 (0.04) | 6 (0.05) | 1 (0.06) |
| Pulmonary sequestration, Cases (%) | 8 (0.01) | 0 (0.00) | 2 (0.01) | 3 (0.01) | 3 (0.03) | 0 (0.00) |
| CCAM, Cases (%) | 14 (0.02) | 1 (0.03) | 3 (0.01) | 7 (0.02) | 3 (0.03) | 0 (0.00) |
| Pulmonary hypoplasia, Cases (%) | 14 (0.02) | 0 (0.00) | 3 (0.01) | 9 (0.02) | 1 (0.01) | 1 (0.06) |
| Digestive system, Cases (%) | 62 (0.08) | 1 (0.03) | 26 (0.11) | 25 (0.07) | 9 (0.08) | 1 (0.06) |
| Oesophageal atresia, Cases (%) | 6 (0.01) | 0 (0.00) | 4 (0.02) | 1 (0.00) | 1 (0.01) | 0 (0.00) |
| Duodenal atresia, Cases (%) | 11 (0.01) | 0 (0.00) | 3 (0.01) | 6 (0.02) | 1 (0.01) | 1 (0.06) |
| Small intestinal atresia, Cases (%) | 17 (0.02) | 0 (0.00) | 5 (0.02) | 10 (0.03) | 2 (0.02) | 0 (0.00) |
| Imperforate anus (anorectal anomaly), Cases (%) | 35 (0.04) | 1 (0.03) | 16 (0.07) | 12 (0.03) | 5 (0.04) | 1 (0.06) |
| Urinary system (CAKUT), Cases (%) | 271 (0.35) | 17 (0.44) | 66 (0.28) | 135 (0.35) | 39 (0.34) | 14 (0.79) |
| Hydronephrosis, Cases (%) | 229 (0.29) | 13 (0.34) | 61 (0.26) | 109 (0.29) | 33 (0.29) | 13 (0.73) |
| Cystic renal anomalies, Cases (%) | 31 (0.04) | 2 (0.05) | 4 (0.02) | 19 (0.05) | 5 (0.04) | 1 (0.06) |
| Renal agenesis, Cases (%) | 17 (0.02) | 2 (0.05) | 2 (0.01) | 11 (0.03) | 2 (0.02) | 0 (0.00) |
| Bladder exstrophy/cloacal exstrophy, Cases (%) | 2 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.01) | 0 (0.00) | 0 (0.00) |
| Genital organs in male infants, Cases/N (%) | 448/40,117(1.12) | 32/1,963(1.63) | 139/11,820(1.18) | 194/19,535(0.99) | 74/5,884(1.26) | 9/915(0.98) |
| Hypospadias, Cases/N (%) | 271/40,117(0.68) | 21/1,963(1.07) | 85/11,820(0.72) | 118/19,535(0.60) | 40/5,884(0.68) | 7/915(0.77) |
| Cryptorchidism/nonpalpable testis, Cases/N (%) | 266/40,117(0.66) | 18/1,963(0.92) | 82/11,820(0.69) | 115/19,535(0.59) | 46/5,884(0.78) | 5/915(0.55) |
| Micropenis, Cases/N (%) | 4/40,117(0.01) | 0/1,963(0.00) | 1/11,820(0.01) | 2/19,535(0.01) | 1/5,884(0.02) | 0/915(0.00) |
| Bifid scrotum, Cases/N (%) | 8/40,117(0.02) | 0/1,963(0.00) | 3/11,820(0.03) | 3/19,535(0.02) | 2/5,884(0.03) | 0/915(0.00) |
| Genital organs in female infants, Cases/N (%) | 11/38,249(0.03) | 0/1,887(0.00) | 4/11,341(0.04) | 6/18,611(0.03) | 1/5,551(0.02) | 0/859(0.00) |
| Clitoral hypertrophy, Cases/N (%) | 7/38,249(0.02) | 0/1,887(0.00) | 4/11,341(0.04) | 3/18,611(0.02) | 0/5,551(0.00) | 0/859(0.00) |
| Abnormal vagina opening, Cases/N (%) | 4/38,249(0.01) | 0/1,887(0.00) | 0/11,341(0.00) | 3/18,611(0.02) | 1/5,551(0.02) | 0/859(0.00) |
| Musculoskeletal system, Cases (%) | 289 (0.37) | 8 (0.21) | 94 (0.41) | 135 (0.35) | 40 (0.35) | 12 (0.68) |
| Congenital diaphragmatic hernia, Cases (%) | 29 (0.04) | 1 (0.03) | 9 (0.04) | 13 (0.03) | 6 (0.05) | 0 (0.00) |
| Umbilical hernia, Cases (%) | 32 (0.04) | 3 (0.08) | 8 (0.03) | 14 (0.04) | 4 (0.03) | 3 (0.17) |
| Gastroschisis, Cases (%) | 5 (0.01) | 0 (0.00) | 2 (0.01) | 1 (0.00) | 1 (0.01) | 1 (0.06) |
| Scoliosis, Cases (%) | 2 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.01) | 0 (0.00) | 0 (0.00) |
| Limbs, N (%) | 221 (0.28) | 4 (0.10) | 75 (0.32) | 105 (0.28) | 29 (0.25) | 8 (0.45) |
| Polydactyly of upper limb, Cases (%) | 79 (0.10) | 2 (0.05) | 31 (0.13) | 38 (0.10) | 6 (0.05) | 2 (0.11) |
| Syndactyly of upper limb, Cases (%) | 37 (0.05) | 1 (0.03) | 14 (0.06) | 13 (0.03) | 8 (0.07) | 1 (0.06) |
| Brachydactyly of upper limb, Cases (%) | 10 (0.01) | 1 (0.03) | 5 (0.02) | 3 (0.01) | 1 (0.01) | 0 (0.00) |
| Cleft hand of upper limb, Cases (%) | 6 (0.01) | 0 (0.00) | 3 (0.01) | 3 (0.01) | 0 (0.00) | 0 (0.00) |
| Defect of upper limb, Cases (%) | 2 (0.00) | 0 (0.00) | 1 (0.00) | 1 (0.00) | 0 (0.00) | 0 (0.00) |
| Polydactyly of lower limb, Cases (%) | 82 (0.10) | 0 (0.00) | 22 (0.09) | 43 (0.11) | 13 (0.11) | 4 (0.23) |
| Syndactyly of lower limb, Cases (%) | 88 (0.11) | 2 (0.05) | 21 (0.09) | 45 (0.12) | 16 (0.14) | 4 (0.23) |
| Short toe, Cases (%) | 2 (0.00) | 0 (0.00) | 1 (0.00) | 1 (0.00) | 0 (0.00) | 0 (0.00) |
| Cleft foot, Cases (%) | 6 (0.01) | 0 (0.00) | 3 (0.01) | 3 (0.01) | 0 (0.00) | 0 (0.00) |
| Defect of lower limb, Cases (%) | 3 (0.00) | 0 (0.00) | 1 (0.00) | 2 (0.01) | 0 (0.00) | 0 (0.00) |
| Skin, Cases (%) | 610 (0.78) | 43 (1.12) | 174 (0.75) | 292 (0.77) | 89 (0.78) | 12 (0.68) |
| Angioma, Cases (%) | 600 (0.77) | 43 (1.12) | 172 (0.74) | 286 (0.75) | 87 (0.76) | 12 (0.68) |
| Epidermolysis bullosa hereditaria/ incontinence of pigment, Cases (%) | 10 (0.01) | 0 (0.00) | 2 (0.01) | 6 (0.02) | 2 (0.02) | 0 (0.00) |
| Inguinal Hernia, Cases (%) | 382 (0.49) | 31 (0.81) | 106 (0.46) | 175 (0.46) | 59 (0.52) | 11 (0.62) |
| Data are expressed as the number (percentage). Abbreviations: CAKUT, congenital anomalies of the kidney and urinary tract; CCAM, congenital cystic adenomatoid malformation | ||||||
| Infant congenital malformations | Maternal birth weight | ||||
|---|---|---|---|---|---|
| <2,500 g (N=3,850) | 2,500–2,999 g (N=23,161) | 3,000–3,499 g (N=38,146) | 3,500–3,999 g (N=11,435) | ≥4,000 g (N=1,774) | |
| Nervous system | |||||
| Cases (%) | 14 (0.36) | 60 (0.26) | 93 (0.24) | 33 (0.29) | 5 (0.28) |
| Model 1, Crude OR (95% CI)a | 1.538 (0.848–2.590) | 1.066 (0.768–1.469) | Reference | 1.196 (0.794–1.756) | 1.256 (0.470–2.724) |
| Model 2, Adjusted OR (95% CI)a, b | 1.242 (0.947–1.628) | 1.029 (0.878–1.206) | Reference | 1.098 (0.905–1.332) | 1.144 (0.751–1.744) |
| Eye, ear, and face | |||||
| Cases (%) | 7 (0.18) | 71 (0.31) | 118 (0.31) | 27 (0.24) | 5 (0.28) |
| Model 1, Crude OR (95% CI)a | 0.626 (0.273–1.222) | 0.994 (0.738–1.329) | Reference | 0.774 (0.501–1.153) | 0.997 (0.372–2.134) |
| Model 2, Adjusted OR (95% CI)a, b | 0.792 (0.552–1.136) | 0.998 (0.864–1.153) | Reference | 0.875 (0.714–1.073) | 0.987 (0.649–1.502) |
| Cleft lip and/or cleft palate | |||||
| Cases (%) | 12 (0.31) | 56 (0.24) | 70 (0.18) | 26 (0.23) | 1 (0.06) |
| Model 1, Crude OR (95% CI)a | 1.759 (0.918–3.096) | 1.321 (0.927–1.872) | Reference | 1.254 (0.789–1.936) | 0.457 (0.052–1.666) |
| Model 2, Adjusted OR (95% CI)a, b | 1.331 (0.994–1.784) | 1.154 (0.973–1.368) | Reference | 1.114 (0.896–1.385) | 0.668 (0.304–1.466) |
| Circulatory system | |||||
| Congenital heart diseases | |||||
| Cases (%) | 69 (1.79) | 311 (1.34) | 485 (1.27) | 172 (1.50) | 31 (1.75) |
| Model 1, Crude OR (95% CI) | 1.417 (1.099–1.828) | 1.057 (0.916–1.220) | Reference | 1.186 (0.995–1.413) | 1.381 (0.958–1.992) |
| Model 2, Adjusted OR (95% CI)b | 1.388 (1.075–1.792) | 1.050 (0.909–1.212) | Reference | 1.179 (0.989–1.405) | 1.370 (0.949–1.977) |
| Arrhythmia | |||||
| Cases (%) | 3 (0.08) | 25 (0.11) | 34 (0.09) | 17 (0.15) | 5 (0.28) |
| Model 1, Crude OR (95% CI)a | 1.005 (0.273–2.639) | 1.218 (0.723–2.024) | Reference | 1.693 (0.931–2.969) | 3.435 (1.240–7.793) |
| Model 2, Adjusted OR (95% CI)a, b | 1.029 (0.613–1.729) | 1.123 (0.881–1.432) | Reference | 1.284 (0.977–1.688) | 1.775 (1.157–2.725) |
| Urinary system (CAKUT) | |||||
| Cases (%) | 17 (0.44) | 66 (0.28) | 135 (0.35) | 39 (0.34) | 14 (0.79) |
| Model 1, Crude OR (95% CI) | 1.249 (0.753–2.070) | 0.805 (0.599–1.081) | Reference | 0.964 (0.674–1.377) | 2.240 (1.289–3.891) |
| Model 2, Adjusted OR (95% CI)b | 1.254 (0.756–2.083) | 0.809 (0.602–1.087) | Reference | 0.957 (0.669–1.368) | 2.194 (1.261–3.819) |
| Genital organs in male infants, N | N=1,963 | N=11,820 | N=19,535 | N=5,884 | N=915 |
| Cases (%) | 32 (1.63) | 139 (1.18) | 194 (0.99) | 74 (1.26) | 9 (0.98) |
| Model 1, Crude OR (95% CI) | 1.652 (1.133–2.408) | 1.186 (0.953–1.477) | Reference | 1.270 (0.970–1.662) | 0.990 (0.506–1.939) |
| Model 2, Adjusted OR (95% CI)b | 1.648 (1.130–2.405) | 1.189 (0.954–1.481) | Reference | 1.264 (0.965–1.655) | 0.974 (0.497–1.907) |
| Hypospadias in male infants | |||||
| Cases (%) | 21 (1.07) | 85 (0.72) | 118 (0.60) | 40 (0.68) | 7 (0.77) |
| Model 1, Crude OR (95% CI) | 1.779 (1.116–2.837) | 1.192 (0.901–1.577) | Reference | 1.126 (0.786–1.614) | 1.269 (0.590–2.727) |
| Model 2, Adjusted OR (95% CI)b | 1.804 (1.130–2.881) | 1.199 (0.906–1.587) | Reference | 1.115 (0.778–1.599) | 1.250 (0.581–2.691) |
| Cryptorchidism/nonpalpable testis in male infants | |||||
| Cases (%) | 18 (0.92) | 82 (0.69) | 115 (0.59) | 46 (0.78) | 5 (0.55) |
| Model 1, Crude OR (95% CI) | 1.563 (0.949–2.574) | 1.180 (0.888–1.567) | Reference | 1.331 (0.944–1.875) | 0.928 (0.378–2.277) |
| Model 2, Adjusted OR (95% CI)b | 1.515 (0.919–2.498) | 1.170 (0.880–1.556) | Reference | 1.332 (0.945–1.879) | 0.912 (0.371–2.240) |
| Musculoskeletal system | |||||
| Limbs | |||||
| Cases (%) | 4 (0.10) | 75 (0.32) | 105 (0.28) | 29 (0.25) | 8 (0.45) |
| Model 1, Crude OR (95% CI) | 0.377 (0.139–1.023) | 1.177 (0.875–1.584) | Reference | 0.921 (0.610–1.390) | 1.641 (0.799–3.373) |
| Model 2, Adjusted OR (95% CI)b | 0.370 (0.136–1.007) | 1.168 (0.868–1.573) | Reference | 0.912 (0.604–1.378) | 1.634 (0.794–3.363) |
| Skin | |||||
| Angioma | |||||
| Cases (%) | 43 (1.12) | 174 (0.75) | 292 (0.77) | 89 (0.78) | 12 (0.68) |
| Model 1, Crude OR (95% CI) | 1.495 (1.083–2.064) | 0.990 (0.819–1.197) | Reference | 1.015 (0.798–1.291) | 0.902 (0.505–1.609) |
| Model 2, Adjusted OR (95% CI)b | 1.491 (1.079–2.059) | 0.993 (0.821–1.201) | Reference | 1.004 (0.789–1.278) | 0.882 (0.494–1.576) |
| Inguinal hernia | |||||
| Cases (%) | 31 (0.81) | 106 (0.46) | 175 (0.46) | 59 (0.52) | 11 (0.62) |
| Model 1, Crude OR (95% CI) | 1.761 (1.200–2.584) | 0.998 (0.783–1.270) | Reference | 1.125 (0.837–1.513) | 1.354 (0.735–2.494) |
| Model 2, Adjusted OR (95% CI)b | 1.746 (1.189–2.565) | 0.997 (0.783–1.271) | Reference | 1.119 (0.832–1.504) | 1.344 (0.729–2.479) |
| a Firth logistic regression model was applied. b Adjusted for maternal age in the MT1 questionnaire, pre-pregnancy BMI, conception method, parity (primipara or not), history of mental illness, history of kidney disease, history of congenital heart disease, history of uterine malformation and/or urogenital malformation, smoking status, alcohol consumption, marital status, education level, annual income, use of any drug before 12 weeks of gestation (methimazole, SSRI, antidepressant drug except for SSRI, antianxiety, sleeping pill, antipsychotic, valproic acid, antiepileptic except for valproic acid, lithium carbonate, and other psychoactive drug), use of folic acid supplement at <12 weeks of gestation, HbA1c level at <24 weeks of gestation, and infant sex. In the analysis of the association between maternal birth weight and genital organs in male infants, the infant sex was not included in the model. Abbreviations: BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CI, confidence interval; HbA1c, glycosylated haemoglobin; OR, odds ratio; SSRI, selective serotonin reuptake inhibitor. | |||||
| Infant congenital malformations | Maternal birth weight | ||||
|---|---|---|---|---|---|
| <2,500 g | 2,500–2,999 g | 3,000–3,499 g | 3,500–3,999 g | ≥4,000 g | |
| The number of male and female infants | |||||
| Male infants, N | 1,963 | 11,820 | 19,535 | 5,884 | 915 |
| Female infants, N | 1,887 | 11,341 | 18,611 | 5,551 | 859 |
| Nervous system | |||||
| Male infants | |||||
| Cases (%) | 9 (0.46) | 30 (0.25) | 55 (0.28) | 17 (0.29) | 2 (0.22) |
| Model 1, Crude OR (95% CI)a | 1.706 (0.803–3.240) | 0.908 (0.577–1.402) | Reference | 1.047 (0.594–1.755) | 0.961 (0.199–2.804) |
| Model 2, Adjusted OR (95% CI)a, b | 1.306 (0.936–1.821) | 0.952 (0.769–1.178) | Reference | 1.024 (0.790–1.328) | 0.988 (0.536–1.822) |
| Female infants | |||||
| Cases (%) | 5 (0.26) | 30 (0.26) | 38 (0.20) | 16 (0.29) | 3 (0.35) |
| Model 1, Crude OR (95% CI)a | 1.409 (0.511–3.172) | 1.301 (0.803–2.089) | Reference | 1.438 (0.786–2.516) | 1.971 (0.537–5.149) |
| Model 2, Adjusted OR (95% CI)a, b | 1.192 (0.780–1.823) | 1.138 (0.906–1.428) | Reference | 1.208 (0.916–1.592) | 1.437 (0.850–2.428) |
| Eye, ear, and face | |||||
| Male infants | |||||
| Cases (%) | 2 (0.10) | 45 (0.38) | 68 (0.35) | 12 (0.20) | 3 (0.33) |
| Model 1, Crude OR (95% CI)a | 0.363 (0.075–1.048) | 1.098 (0.750–1.593) | Reference | 0.605 (0.315–1.066) | 1.090 (0.301–2.768) |
| Model 2, Adjusted OR (95% CI)a, b | 0.599 (0.326–1.101) | 1.048 (0.874–1.257) | Reference | 0.777 (0.580–1.040) | 1.041 (0.618–1.753) |
| Female infants | |||||
| Cases (%) | 5 (0.26) | 26 (0.23) | 50 (0.27) | 15 (0.27) | 2 (0.23) |
| Model 1, Crude OR (95% CI)a | 1.074 (0.393–2.374) | 0.861 (0.530–1.365) | Reference | 1.029 (0.563–1.775) | 1.072 (0.221–3.141) |
| Model 2, Adjusted OR (95% CI)a, b | 1.047 (0.687–1.591) | 0.932 (0.744–1.168) | Reference | 1.015 (0.773–1.334) | 1.014 (0.552–1.865) |
| Cleft lip and/or cleft palate | |||||
| Male infants | |||||
| Cases (%) | 9 (0.46) | 33 (0.28) | 43 (0.22) | 14 (0.24) | 1 (0.11) |
| Model 1, Crude OR (95% CI)a | 2.179 (1.014–4.208) | 1.274 (0.806–1.995) | Reference | 1.107 (0.590–1.957) | 0.735 (0.083–2.729) |
| Model 2, Adjusted OR (95% CI)a, b | 1.473 (1.052–2.064) | 1.132 (0.912–1.406) | Reference | 1.050 (0.789–1.396) | 0.845 (0.386–1.846) |
| Female infants | |||||
| Cases (%) | 3 (0.16) | 23 (0.20) | 27 (0.15) | 12 (0.22) | 0 (0.00) |
| Model 1, Crude OR (95% CI)a | 1.255 (0.338–3.358) | 1.403 (0.803–2.433) | Reference | 1.525 (0.754–2.914) | 0.393 (0.003–2.802) |
| Model 2, Adjusted OR (95% CI)a, b | 1.137 (0.678–1.906) | 1.183 (0.913–1.533) | Reference | 1.219 (0.890–1.671) | 0.626 (0.169–2.314) |
| Circulatory system | |||||
| Congenital heart diseases | |||||
| Male infants | |||||
| Cases (%) | 34 (1.73) | 144 (1.22) | 206 (1.05) | 82 (1.39) | 17 (1.86) |
| Model 1, Crude OR (95% CI) | 1.654 (1.147–2.384) | 1.157 (0.934–1.434) | Reference | 1.326 (1.025–1.716) | 1.776 (1.078–2.926) |
| Model 2, Adjusted OR (95% CI)b | 1.615 (1.119–2.332) | 1.154 (0.931–1.430) | Reference | 1.317 (1.017–1.704) | 1.745 (1.058–2.877) |
| Female infants | |||||
| Cases (%) | 35 (1.85) | 167 (1.47) | 279 (1.50) | 90 (1.62) | 14 (1.63) |
| Model 1, Crude OR (95% CI)a | 1.257 (0.869–1.762) | 0.983 (0.809–1.191) | Reference | 1.087 (0.852–1.374) | 1.125 (0.631–1.845) |
| Model 2, Adjusted OR (95% CI)a, b | 1.113 (0.934–1.327) | 0.988 (0.897–1.087) | Reference | 1.041 (0.925–1.173) | 1.062 (0.815–1.383) |
| Arrhythmia | |||||
| Male infants | |||||
| Cases (%) | 0 (0.00) | 13 (0.11) | 15 (0.08) | 9 (0.15) | 2 (0.22) |
| Model 1, Crude OR (95% CI)a | 0.321 (0.003–2.379) | 1.440 (0.684–2.994) | Reference | 2.036 (0.874–4.500) | 3.447 (0.682–11.141) |
| Model 2, Adjusted OR (95% CI)a, b | 0.558 (0.169–1.947) | 1.190 (0.855–1.656) | Reference | 1.427 (0.988–2.059) | 1.840 (0.999–3.387) |
| Female infants | |||||
| Cases (%) | 3 (0.16) | 12 (0.11) | 19 (0.10) | 8 (0.14) | 3 (0.35) |
| Model 1, Crude OR (95% CI)a | 1.771 (0.469–4.912) | 1.052 (0.504–2.117) | Reference | 1.462 (0.618–3.172) | 3.897 (1.031–10.823) |
| Model 2, Adjusted OR (95% CI)a, b | 1.394 (0.833–2.333) | 1.056 (0.763–1.462) | Reference | 1.171 (0.808–1.698) | 1.788 (1.047–3.052) |
| Urinary system (CAKUT) | |||||
| Male infants | |||||
| Cases (%) | 8 (0.41) | 51 (0.43) | 102 (0.52) | 31 (0.53) | 12 (1.31) |
| Model 1, Crude OR (95% CI) | 0.780 (0.379–1.603) | 0.826 (0.589–1.156) | Reference | 1.009 (0.674–1.510) | 2.532 (1.387–4.622) |
| Model 2, Adjusted OR (95% CI)b | 0.808 (0.392–1.664) | 0.837 (0.597–1.173) | Reference | 0.996 (0.665–1.491) | 2.470 (1.350–4.517) |
| Female infants | |||||
| Cases (%) | 9 (0.48) | 15 (0.13) | 33 (0.18) | 8 (0.14) | 2 (0.23) |
| Model 1, Crude OR (95% CI)a | 2.805 (1.287–5.547) | 0.759 (0.404–1.362) | Reference | 0.850 (0.374–1.723) | 1.617 (0.330–4.845) |
| Model 2, Adjusted OR (95% CI)a, b | 1.619 (1.154–2.273) | 0.860 (0.647–1.141) | Reference | 0.926 (0.649–1.321) | 1.267 (0.691–2.322) |
| Musculoskeletal system | |||||
| Limbs | |||||
| Male infants | |||||
| Cases (%) | 1 (0.05) | 46 (0.39) | 56 (0.29) | 16 (0.27) | 4 (0.44) |
| Model 1, Crude OR (95% CI) | 0.178 (0.025–1.281) | 1.359 (0.919–2.009) | Reference | 0.948 (0.544–1.654) | 1.527 (0.553–4.221) |
| Model 2, Adjusted OR (95% CI)b | 0.176 (0.024–1.270) | 1.354 (0.915–2.003) | Reference | 0.922 (0.528–1.609) | 1.514 (0.547–4.194) |
| Female infants | |||||
| Cases (%) | 3 (0.16) | 29 (0.26) | 49 (0.26) | 13 (0.23) | 4 (0.47) |
| Model 1, Crude OR (95% CI)a | 0.696 (0.191–1.791) | 0.978 (0.613–1.533) | Reference | 0.914 (0.481–1.621) | 1.974 (0.642–4.658) |
| Model 2, Adjusted OR (95% CI)a, b | 0.822 (0.491–1.375) | 0.981 (0.788–1.221) | Reference | 0.960 (0.719–1.281) | 1.404 (0.882–2.233) |
| Skin | |||||
| Angioma | |||||
| Male infants | |||||
| Cases (%) | 16 (0.82) | 72 (0.61) | 99 (0.51) | 35 (0.59) | 5 (0.55) |
| Model 1, Crude OR (95% CI)a | 1.655 (0.946–2.713) | 1.205 (0.887–1.630) | Reference | 1.186 (0.797–1.723) | 1.180 (0.439–2.540) |
| Model 2, Adjusted OR (95% CI)b | 1.292 (0.999–1.670) | 1.101 (0.948–1.278) | Reference | 1.082 (0.896–1.307) | 1.082 (0.709–1.651) |
| Female infants | |||||
| Cases (%) | 27 (1.43) | 100 (0.88) | 187 (1.00) | 52 (0.94) | 7 (0.81) |
| Model 1, Crude OR (95% CI)a | 1.452 (0.979–2.134) | 0.878 (0.686–1.118) | Reference | 0.938 (0.683–1.266) | 0.864 (0.379–1.675) |
| Model 2, Adjusted OR (95% CI)a, b | 1.196 (0.980–1.461) | 0.939 (0.832–1.059) | Reference | 0.963 (0.827–1.122) | 0.914 (0.636–1.314) |
| Inguinal hernia | |||||
| Male infants | |||||
| Cases (%) | 23 (1.17) | 56 (0.47) | 106 (0.54) | 30 (0.51) | 9 (0.98) |
| Model 1, Crude OR (95% CI)a | 2.209 (1.378–3.397) | 0.876 (0.630–1.205) | Reference | 0.950 (0.624–1.404) | 1.912 (0.916–3.530) |
| Model 2, Adjusted OR (95% CI)a, b | 1.484 (1.189–1.851) | 0.938 (0.800–1.099) | Reference | 0.972 (0.798–1.186) | 1.373 (0.989–1.907) |
| Female infants | |||||
| Cases (%) | 8 (0.42) | 50 (0.44) | 69 (0.37) | 29 (0.52) | 2 (0.23) |
| Model 1, Crude OR (95% CI)a | 1.207 (0.548–2.326) | 1.193 (0.826–1.711) | Reference | 1.425 (0.912–2.171) | 0.778 (0.161–2.252) |
| Model 2, Adjusted OR (95% CI)a, b | 1.092 (0.772–1.543) | 1.091 (0.914–1.302) | Reference | 1.195 (0.968–1.475) | 0.877 (0.474–1.622) |
| a Firth logistic regression model was applied. b Adjusted for maternal age at the MT1 questionnaire, pre-pregnancy BMI, conception method, parity (primipara or not), history of mental illness, history of kidney disease, history of congenital heart disease, history of uterine malformation and/or urogenital malformation, smoking status, alcohol consumption, marital status, education level, annual income, use of any drug before 12 weeks of gestation (methimazole, SSRI, antidepressant drug except for SSRI, antianxiety, sleeping pill, antipsychotic, valproic acid, antiepileptic except for valproic acid, lithium carbonate, and other psychoactive drug), use of folic acid supplement at <12 weeks of gestation, and HbA1c level at <24 weeks of gestation. Abbreviations: BMI, body mass index; CAKUT, congenital anomalies of the kidney and urinary tract; CI, confidence interval; HbA1c, glycosylated haemoglobin; OR, odds ratio; SSRI, selective serotonin reuptake inhibitor. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





