Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. General aspects of visceral leishmaniasis
1.2. Development of Vaccines Against Canine Visceral Leishmaniasis
1.2.1. Commercially available vaccines for Canine Visceral Leishmaniasis
1.2.2. Chimeric proteins used as potential vaccine candidates for visceral leishmaniasis
2. Patents of Chimeric Proteins (2010-2023)
2.1. Protein targets
3. Discussion and future perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ready, P. D. Biology of Phlebotomine Sand Flies as Vectors of Disease Agents. Annu. Rev. Entomol., 2013, 58, 227–250. [Google Scholar] [CrossRef]
- Maurício, I. L.; Stothard, J. R.; Miles, M. A. The Strange Case of Leishmania Chagasi. Parasitol. Today Pers. Ed, 2000, 16, 188–189. [Google Scholar] [CrossRef]
- Lainson, R.; Shaw, J. J. Parasitology, 10th ed.; Hodder Arnold: London, 2005. [Google Scholar]
- Desjeux, P. Leishmaniasis: Current Situation and New Perspectives. Comp. Immunol. Microbiol. Infect. Dis., 2004, 27, 305–318. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I. D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PloS One, 2012, 7, e35671. [Google Scholar] [CrossRef]
- Leishmaniasis https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed Jan 8, 2024).
- Dantas-Torres, F. The Role of Dogs as Reservoirs of Leishmania Parasites, with Emphasis on Leishmania (Leishmania) Infantum and Leishmania (Viannia) Braziliensis. Vet. Parasitol., 2007, 149, 139–146. [Google Scholar] [CrossRef]
- Verçosa, B. L. A.; Melo, M. N.; Puerto, H. L. D.; Mendonça, I. L.; Vasconcelos, A. C. Apoptosis, Inflammatory Response and Parasite Load in Skin of Leishmania (Leishmania) Chagasi Naturally Infected Dogs: A Histomorphometric Analysis. Vet. Parasitol., 2012, 189, 162–170. [Google Scholar] [CrossRef]
- Prado, P. F. do; Rocha, M. F.; Sousa, J. F. de; Caldeira, D. I.; Paz, G. F.; Dias, E. S. Epidemiological Aspects of Human and Canine Visceral Leishmaniasis in Montes Claros, State of Minas Gerais, Brazil, between 2007 and 2009. Rev. Soc. Bras. Med. Trop., 2011, 44, 561–566. [Google Scholar] [CrossRef]
- Courtenay, O.; Quinnell, R. J.; Garcez, L. M.; Shaw, J. J.; Dye, C. Infectiousness in a Cohort of Brazilian Dogs: Why Culling Fails to Control Visceral Leishmaniasis in Areas of High Transmission. J. Infect. Dis., 2002, 186, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Costa, C. H. N.; Tapety, C. M. M.; Werneck, G. L. Controle da leishmaniose visceral em meio urbano: estudo de intervenção randomizado fatorial. Rev. Soc. Bras. Med. Trop., 2007, 40, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C. M.; Pires, M. M.; da Silva, K. M.; Assis, F. D.; Gonçalves Filho, J.; Perri, S. H. V. Relationship between Dog Culling and Incidence of Human Visceral Leishmaniasis in an Endemic Area. Vet. Parasitol., 2010, 170, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Teva, A.; dos-Santos, C. B.; Santos, F. N.; Pinto, I. de-Souza; Fux, B.; Leite, G. R.; Falqueto, A. Field Trial of Efficacy of the Leish-Tec® Vaccine against Canine Leishmaniasis Caused by Leishmania Infantum in an Endemic Area with High Transmission Rates. PLoS ONE, 2017, 12 (9), e0185438. [CrossRef]
- Coura-Vital, W.; Ker, H. G.; Roatt, B. M.; Aguiar-Soares, R. D. O.; Leal, G. G. de A.; Moreira, N. das D.; Oliveira, L. A. M.; de Menezes Machado, E. M.; Morais, M. H. F.; Corrêa-Oliveira, R.; et al. Evaluation of Change in Canine Diagnosis Protocol Adopted by the Visceral Leishmaniasis Control Program in Brazil and a New Proposal for Diagnosis. PloS One, 2014, 9 (3), e91009. [CrossRef]
- França-Silva, J. C.; Giunchetti, R. C.; Mariano, R. M. da S.; Machado-Coelho, G. L. L.; Teixeira, L. de A. S.; Barata, R. A.; Michalsky, É. M.; Rocha, M. F.; Fortes-Dias, C. L.; Dias, E. S. The Program for the Control of Visceral Leishmaniasis in Brazil: The Effect of the Systematic Euthanasia of Seropositive Dogs as a Single Control Action in Porteirinha, a Brazilian City with an Intense Transmission of Visceral Leishmaniasis. Pathog. Basel Switz., 2023, 12 (8), 1060. [CrossRef]
- Costa, C. H. N. How Effective Is Dog Culling in Controlling Zoonotic Visceral Leishmaniasis? A Critical Evaluation of the Science, Politics and Ethics behind This Public Health Policy. Rev. Soc. Bras. Med. Trop., 2011, 44, 232–242. [Google Scholar] [CrossRef]
- Otranto, D.; Dantas-Torres, F. The Prevention of Canine Leishmaniasis and Its Impact on Public Health. Trends Parasitol., 2013, 29, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Maia-Elkhoury, A. N. S.; Alves, W. A.; Sousa-Gomes, M. L. de; Sena, J. M. de; Luna, E. A. Visceral Leishmaniasis in Brazil: Trends and Challenges. Cad. Saúde Pública, 2008, 24, 2941–2947. [Google Scholar] [CrossRef] [PubMed]
- Gramiccia, M.; Gradoni, L. The Current Status of Zoonotic Leishmaniases and Approaches to Disease Control. Int. J. Parasitol., 2005, 35, 1169–1180. [Google Scholar] [CrossRef]
- Reis, A. B.; Giunchetti, R. C.; Carrillo, E.; Martins-Filho, O. A.; Moreno, J. Immunity to Leishmania and the Rational Search for Vaccines against Canine Leishmaniasis. Trends Parasitol., 2010, 26, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, M.; Khamesipour, A.; Mobedi, I.; Zarei, Z.; Hashemi-Fesharki, R. Double-Blind Randomized Efficacy Field Trial of Alum Precipitated Autoclaved Leishmania Major Vaccine Mixed with BCG against Canine Visceral Leishmaniasis in Meshkin-Shahr District, I.R. Iran. Vaccine, 2004, 22, 4097–4100. [Google Scholar] [CrossRef]
- Leite, J. C.; Gonçalves, A. A. M.; de Oliveira, D. S.; Resende, L. A.; Boas, D. F. V.; Ribeiro, H. S.; Pereira, D. F. S.; da Silva, A. V.; Mariano, R. M. da S.; Reis, P. C. C.; et al. Transmission-Blocking Vaccines for Canine Visceral Leishmaniasis: New Progress and Yet New Challenges. Vaccines, 2023, 11 (10), 1565. [CrossRef]
- Le Rutte, E. A.; Coffeng, L. E.; Malvolti, S.; Kaye, P. M.; de Vlas, S. J. The Potential Impact of Human Visceral Leishmaniasis Vaccines on Population Incidence. PLoS Negl. Trop. Dis., 2020, 14, e0008468. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Frasca, K.; Scherrer, S.; Henao-Martínez, A. F.; Newman, S.; Ramanan, P.; Suarez, J. A. A Review of Leishmaniasis: Current Knowledge and Future Directions. Curr. Trop. Med. Rep., 2021, 8, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Abdian, N.; Gholami, E.; Zahedifard, F.; Safaee, N.; Rafati, S. Evaluation of DNA/DNA and Prime-Boost Vaccination Using LPG3 against Leishmania Major Infection in Susceptible BALB/c Mice and Its Antigenic Properties in Human Leishmaniasis. Exp. Parasitol., 2011, 127, 627–636. [Google Scholar] [CrossRef]
- Araújo, M. S. S.; de Andrade, R. A.; Sathler-Avelar, R.; Magalhães, C. P.; Carvalho, A. T.; Andrade, M. C.; Campolina, S. S.; Mello, M. N.; Vianna, L. R.; Mayrink, W.; et al. Immunological Changes in Canine Peripheral Blood Leukocytes Triggered by Immunization with First or Second Generation Vaccines against Canine Visceral Leishmaniasis. Vet. Immunol. Immunopathol., 2011, 141, 64–75. [Google Scholar] [CrossRef]
- Nagill, R.; Kaur, S. Vaccine Candidates for Leishmaniasis: A Review. Int. Immunopharmacol., 2011, 11, 1464–1488. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sundar, S. Leishmaniasis: Vaccine Candidates and Perspectives. Vaccine, 2012, 30, 3834–3842. [Google Scholar] [CrossRef] [PubMed]
- Thomaz-Soccol, V.; Ferreira da Costa, E. S.; Karp, S. G.; Junior Letti, L. A.; Soccol, F. T.; Soccol, C. R. Recent Advances in Vaccines Against Leishmania Based on Patent Applications. Recent Pat. Biotechnol., 2018, 12, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Giunchetti, R. C.; Silveira, P. C.; Resende, L. A.; Leite, J. C.; Melo-Júnior, O.; Rodrigues-Alves, M.; Costa, L. M.; Lair, D.; Chaves, V.; Soares, I.; et al. Canine visceral leishmaniasis biomarkers and their employment in vaccines - ScienceDirect https://www.sciencedirect.com/science/article/pii/S0304401719301025?via%3Dihub (accessed Jan 8, 2024).
- Graciano, R. C. D.; Ribeiro, J. A. T.; Macêdo, A. K. S.; de S Lavareda, J. P.; de Oliveira, P. R.; Netto, J. B.; Nogueira, L. M.; Machado, J. M.; Camposda-Paz, M.; Giunchetti, R. C.; et al. Recent Patents Applications in Red Biotechnology: A Mini-Review. Recent Pat. Biotechnol., 2019, 13, 170–186. [Google Scholar] [CrossRef] [PubMed]
- de Lana, M.; Giunchetti, R. C. Dogs as a Model for Chemotherapy of Chagas Disease and Leishmaniasis. Curr. Pharm. Des., 2021, 27, 1741–1756. [Google Scholar] [CrossRef] [PubMed]
- Murray, H. W.; Cervia, J. S.; Hariprashad, J.; Taylor, A. P.; Stoeckle, M. Y.; Hockman, H. Effect of Granulocyte-Macrophage Colony-Stimulating Factor in Experimental Visceral Leishmaniasis. J. Clin. Invest., 1995, 95, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Smirlis, D.; Soteriadou, K. P.; Karagouni, E. Vaccination with Leishmania Histone H1-Pulsed Dendritic Cells Confers Protection in Murine Visceral Leishmaniasis. Vaccine, 2012, 30, 5086–5093. [Google Scholar] [CrossRef]
- Das, A.; Ali, N. Vaccine Prospects of Killed but Metabolically Active Leishmania against Visceral Leishmaniasis. Expert Rev. Vaccines, 2012, 11, 783–785. [Google Scholar] [CrossRef]
- Dumas, C.; Muyombwe, A.; Roy, G.; Matte, C.; Ouellette, M.; Olivier, M.; Papadopoulou, B. Recombinant Leishmania Major Secreting Biologically Active Granulocyte-Macrophage Colony-Stimulating Factor Survives Poorly in Macrophages in Vitro and Delays Disease Development in Mice. Infect. Immun., 2003, 71, 6499–6509. [Google Scholar] [CrossRef]
- Lage, D. P.; Martins, V. T.; Duarte, M. C.; Garde, E.; Chávez-Fumagalli, M. A.; Menezes-Souza, D.; Roatt, B. M.; Tavares, C. a. P.; Soto, M.; Coelho, E. a. F. Prophylactic Properties of a Leishmania-Specific Hypothetical Protein in a Murine Model of Visceral Leishmaniasis. Parasite Immunol., 2015, 37, 646–656. [Google Scholar] [CrossRef]
- Martins, V. T.; Lage, D. P.; Duarte, M. C.; Costa, L. E.; Garde, E.; Rodrigues, M. R.; Chávez-Fumagalli, M. A.; Menezes-Souza, D.; Roatt, B. M.; Tavares, C. A. P.; et al. A New Leishmania-Specific Hypothetical Protein, LiHyT, Used as a Vaccine Antigen against Visceral Leishmaniasis. Acta Trop., 2016, 154, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Green, S. J.; Mellouk, S.; Hoffman, S. L.; Meltzer, M. S.; Nacy, C. A. Cellular Mechanisms of Nonspecific Immunity to Intracellular Infection: Cytokine-Induced Synthesis of Toxic Nitrogen Oxides from L-Arginine by Macrophages and Hepatocytes. Immunol. Lett., 1990, 25, 15–19. [Google Scholar] [CrossRef]
- Blackwell, J. M.; Fakiola, M.; Ibrahim, M. E.; Jamieson, S. E.; Jeronimo, S. B.; Miller, E. N.; Mishra, A.; Mohamed, H. S.; Peacock, C. S.; Raju, M.; et al. Genetics and Visceral Leishmaniasis: Of Mice and Man. Parasite Immunol., 2009, 31, 254–266. [Google Scholar] [CrossRef]
- Kaye, P. M.; Aebischer, T. Visceral Leishmaniasis: Immunology and Prospects for a Vaccine. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis., 2011, 17, 1462–1470. [Google Scholar] [CrossRef]
- Moreno, J.; Vouldoukis, I.; Martin, V.; McGahie, D.; Cuisinier, A.-M.; Gueguen, S. Use of a LiESP/QA-21 Vaccine (CaniLeish) Stimulates an Appropriate Th1-Dominated Cell-Mediated Immune Response in Dogs. PLoS Negl. Trop. Dis., 2012, 6, e1683. [Google Scholar] [CrossRef] [PubMed]
- Velez, R.; Gállego, M. Commercially Approved Vaccines for Canine Leishmaniosis: A Review of Available Data on Their Safety and Efficacy. Trop. Med. Int. Health TM IH, 2020, 25, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Baxarias, M.; Homedes, J.; Mateu, C.; Attipa, C.; Solano-Gallego, L. Use of Preventive Measures and Serological Screening Tools for Leishmania Infantum Infection in Dogs from Europe. Parasit. Vectors, 2022, 15, 134. [Google Scholar] [CrossRef] [PubMed]
- CaniLeish | Agência Europeia de Medicamentos https://www.ema.europa.eu/en/medicines/veterinary/EPAR/canileish#ema-inpage-item-product-info (accessed Jan 14, 2024).
- Fernandes, C. B.; Junior, J. T. M.; de Jesus, C.; Souza, B. M. P. da S.; Larangeira, D. F.; Fraga, D. B. M.; Tavares Veras, P. S.; Barrouin-Melo, S. M. Comparison of Two Commercial Vaccines against Visceral Leishmaniasis in Dogs from Endemic Areas: IgG, and Subclasses, Parasitism, and Parasite Transmission by Xenodiagnosis. Vaccine, 2014, 32 (11), 1287–1295. [CrossRef]
- Coelho, E. A. F.; Tavares, C. A. P.; Carvalho, F. A. A.; Chaves, K. F.; Teixeira, K. N.; Rodrigues, R. C.; Charest, H.; Matlashewski, G.; Gazzinelli, R. T.; Fernandes, A. P. Immune Responses Induced by the Leishmania (Leishmania) Donovani A2 Antigen, but Not by the LACK Antigen, Are Protective against Experimental Leishmania (Leishmania) Amazonensis Infection. Infect. Immun., 2003, 71, 3988–3994. [Google Scholar] [CrossRef] [PubMed]
- Zanin, F. H. C.; Coelho, E. A. F.; Tavares, C. A. P.; Marques-da-Silva, E. A.; Silva Costa, M. M.; Rezende, S. A.; Gazzinelli, R. T.; Fernandes, A. P. Evaluation of Immune Responses and Protection Induced by A2 and Nucleoside Hydrolase (NH) DNA Vaccines against Leishmania Chagasi and Leishmania Amazonensis Experimental Infections. Microbes Infect., 2007, 9, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Resende, D. M.; Caetano, B. C.; Dutra, M. S.; Penido, M. L. O.; Abrantes, C. F.; Verly, R. M.; Resende, J. M.; Piló-Veloso, D.; Rezende, S. A.; Bruna-Romero, O.; et al. Epitope Mapping and Protective Immunity Elicited by Adenovirus Expressing the Leishmania Amastigote Specific A2 Antigen: Correlation with IFN-Gamma and Cytolytic Activity by CD8+ T Cells. Vaccine, 2008, 26, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- de Mendonça, L. Z.; Resende, L. A.; Lanna, M. F.; Aguiar-Soares, R. D. de O.; Roatt, B. M.; Castro, R. A. de O. E.; Batista, M. A.; Silveira-Lemos, D.; Gomes, J. de A. S.; Fujiwara, R. T.; et al. Multicomponent LBSap Vaccine Displays Immunological and Parasitological Profiles Similar to Those of Leish-Tec® and Leishmune® Vaccines against Visceral Leishmaniasis. Parasit. Vectors, 2016, 9 (1), 472. [CrossRef]
- Fernandes, A. P.; Costa, M. M. S.; Coelho, E. A. F.; Michalick, M. S. M.; de Freitas, E.; Melo, M. N.; Luiz Tafuri, W.; Resende, D. de M.; Hermont, V.; Abrantes, C. de F.; et al. Protective Immunity against Challenge with Leishmania (Leishmania) Chagasi in Beagle Dogs Vaccinated with Recombinant A2 Protein. Vaccine, 2008, 26 (46), 5888–5895. [CrossRef]
- Regina-Silva, S.; Feres, A. M. L. T.; França-Silva, J. C.; Dias, E. S.; Michalsky, É. M.; de Andrade, H. M.; Coelho, E. A. F.; Ribeiro, G. M.; Fernandes, A. P.; Machado-Coelho, G. L. L. Field Randomized Trial to Evaluate the Efficacy of the Leish-Tec® Vaccine against Canine Visceral Leishmaniasis in an Endemic Area of Brazil. Vaccine, 2016, 34, 2233–2239. [Google Scholar] [CrossRef] [PubMed]
- Wylie, C. E.; Carbonell-Antoñanzas, M.; Aiassa, E.; Dhollander, S.; Zagmutt, F. J.; Brodbelt, D. C.; Solano-Gallego, L. A Systematic Review of the Efficacy of Prophylactic Control Measures for Naturally-Occurring Canine Leishmaniosis, Part I: Vaccinations. Prev. Vet. Med., 2014, 117, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Mapa suspende fabricação e venda e determina o recolhimento de lotes de vacina contra Leishmaniose https://www.gov.br/agricultura/pt-br/assuntos/noticias/mapa-suspende-fabricacao-e-venda-e-determina-o-recolhimento-de-lotes-de-vacina-contra-leishmaniose-apos-fiscalizacao (accessed Jan 14, 2024).
- Goto, Y.; Bhatia, A.; Raman, V. S.; Liang, H.; Mohamath, R.; Picone, A. F.; Vidal, S. E. Z.; Vedvick, T. S.; Howard, R. F.; Reed, S. G. KSAC, the First Defined Polyprotein Vaccine Candidate for Visceral Leishmaniasis▿. Clin. Vaccine Immunol. CVI, 2011, 18, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Margaroni, M.; Kotsakis, S. D.; Karagouni, E. A Canine-Directed Chimeric Multi-Epitope Vaccine Induced Protective Immune Responses in BALB/c Mice Infected with Leishmania Infantum. Vaccines, 2020, 8, 350. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.; Jain, N. K. Vaccines for Visceral Leishmaniasis: A Review. J. Immunol. Methods, 2015, 422, 1–12. [Google Scholar] [CrossRef]
- Bonde, J. S.; Bülow, L. Chimeric Genes, Proteins. In Brenner’s Encyclopedia of Genetics (Second Edition); Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, 2013; pp. 519–520. [Google Scholar] [CrossRef]
- Strohl, W. Chimeric Genes, Proteins☆. In Reference Module in Life Sciences; Elsevier, 2017. [CrossRef]
- Ghosh, A.; Zhang, W. W.; Matlashewski, G. Immunization with A2 Protein Results in a Mixed Th1/Th2 and a Humoral Response Which Protects Mice against Leishmania Donovani Infections. Vaccine, 2001, 20, 59–66. [Google Scholar] [CrossRef]
- Basu, R.; Bhaumik, S.; Basu, J. M.; Naskar, K.; De, T.; Roy, S. Kinetoplastid Membrane Protein-11 DNA Vaccination Induces Complete Protection against Both Pentavalent Antimonial-Sensitive and -Resistant Strains of Leishmania Donovani That Correlates with Inducible Nitric Oxide Synthase Activity and IL-4 Generation: Evidence for Mixed Th1- and Th2-Like Responses in Visceral Leishmaniasis1. J. Immunol., 2005, 174, 7160–7171. [Google Scholar] [CrossRef]
- Rafati, S.; Zahedifard, F.; Nazgouee, F. Prime-Boost Vaccination Using Cysteine Proteinases Type I and II of Leishmania Infantum Confers Protective Immunity in Murine Visceral Leishmaniasis. Vaccine, 2006, 24, 2169–2175. [Google Scholar] [CrossRef]
- Goto, Y.; Bogatzki, L. Y.; Bertholet, S.; Coler, R. N.; Reed, S. G. Protective Immunization against Visceral Leishmaniasis Using Leishmania Sterol 24-c-Methyltransferase Formulated in Adjuvant. Vaccine, 2007, 25, 7450–7458. [Google Scholar] [CrossRef]
- Brito, R. C. F. D.; Ruiz, J. C.; Cardoso, J. M. de O.; Ostolin, T. L. V. D. P.; Reis, L. E. S.; Mathias, F. A. S.; Aguiar-Soares, R. D. de O.; Roatt, B. M.; Corrêa-Oliveira, R.; Resende, D. de M.; et al. Chimeric Vaccines Designed by Immunoinformatics-Activated Polyfunctional and Memory T Cells That Trigger Protection against Experimental Visceral Leishmaniasis. Vaccines, 2020, 8 (2), 252. [CrossRef]
- Chang, P.-L.; Wu, C.-C.; Leu, H.-J. Using Patent Analyses to Monitor the Technological Trends in an Emerging Field of Technology: A Case of Carbon Nanotube Field Emission Display. Scientometrics, 2009, 82, 5–19. [Google Scholar] [CrossRef]
- Mucke, H. A. M. What Patents Tell Us about Drug Repurposing for Cancer: A Landscape Analysis. Semin. Cancer Biol., 2021, 68, 3–7. [Google Scholar] [CrossRef]
- INPI https://www.gov.br/inpi/pt-br/copy2_of_nova-home-page (accessed Jan 8, 2024).
- Espacenet – patent search https://worldwide.espacenet.com/ (accessed Jan 8, 2024).
- WIPO - Search International and National Patent Collections https://patentscope.wipo.int/search/en/search.jsf (accessed Jan 8, 2024).
- Siqueira-Neto, J. L.; Debnath, A.; McCall, L.-I.; Bernatchez, J. A.; Ndao, M.; Reed, S. L.; Rosenthal, P. J. Cysteine Proteases in Protozoan Parasites. PLoS Negl. Trop. Dis., 2018, 12, e0006512. [Google Scholar] [CrossRef] [PubMed]
- Agallou, M.; Athanasiou, E.; Koutsoni, O.; Dotsika, E.; Karagouni, E. Experimental Validation of Multi-Epitope Peptides Including Promising MHC Class I- and II-Restricted Epitopes of Four Known Leishmania Infantum Proteins. Front. Immunol., 2014, 5, 268. [Google Scholar] [CrossRef]
- Seyed, N.; Taheri, T.; Vauchy, C.; Dosset, M.; Godet, Y.; Eslamifar, A.; Sharifi, I.; Adotevi, O.; Borg, C.; Rohrlich, P. S.; et al. Immunogenicity Evaluation of a Rationally Designed Polytope Construct Encoding HLA-A*0201 Restricted Epitopes Derived from Leishmania Major Related Proteins in HLA-A2/DR1 Transgenic Mice: Steps toward Polytope Vaccine. PLoS ONE, 2014, 9, e108848. [Google Scholar] [CrossRef] [PubMed]
- Noormehr, H.; Zavaran Hosseini, A.; Soudi, S.; Beyzay, F. Enhancement of Th1 Immune Response against Leishmania Cysteine Peptidase A, B by PLGA Nanoparticle. Int. Immunopharmacol., 2018, 59, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.; Zahedifard, F.; Saljoughian, N.; Doroud, D.; Jamshidi, S.; Mahdavi, N.; Shirian, S.; Daneshbod, Y.; Hamid Zarkesh-Esfahani, S.; Papadopoulou, B.; et al. Immunological Comparison of DNA Vaccination Using Two Delivery Systems against Canine Leishmaniasis. Vet. Parasitol., 2015, 212, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, R.; Nillegoda, N. B.; Mayer, M. P.; Bukau, B. The Hsp70 Chaperone Network. Nat. Rev. Mol. Cell Biol., 2019, 20, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Clare, D. K.; Saibil, H. R. ATP-Driven Molecular Chaperone Machines. Biopolymers, 2013, 99, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Shonhai, A.; Maier, A. G.; Przyborski, J. M.; Blatch, G. L. Intracellular Protozoan Parasites of Humans: The Role of Molecular Chaperones in Development and Pathogenesis. Protein Pept. Lett., 2011, 18, 143–157. [Google Scholar] [CrossRef]
- Kaur, T.; Sobti, R. C.; Kaur, S. Cocktail of Gp63 and Hsp70 Induces Protection against Leishmania Donovani in BALB/c Mice. Parasite Immunol., 2011, 33, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, M.; Karimi, M. H.; Kalani, M.; Ebrahimnezhad, S.; Namayandeh, M.; Moravej, A. Immunostimulatory Effects of Leishmania Infantum HSP70 Recombinant Protein on Dendritic Cells in Vitro and in Vivo. Immunotherapy, 2014, 6, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Burns, J. M.; Shreffler, W. G.; Benson, D. R.; Ghalib, H. W.; Badaro, R.; Reed, S. G. Molecular Characterization of a Kinesin-Related Antigen of Leishmania Chagasi That Detects Specific Antibody in African and American Visceral Leishmaniasis. Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 775–779. [Google Scholar] [CrossRef] [PubMed]
- de Souza, L. M. B.; Carvalho, J.; Bates, M. D.; Petterle, R. R.; Thomaz-Soccol, V.; Bates, P. A. Production of a Kinesin-Related Recombinant Protein (Lbk39) from Leishmania Braziliensis by Leishmania Tarentolae Promastigotes and Its Application in the Serodiagnosis of Leishmaniasis. One Health, 2019, 8, 100111. [Google Scholar] [CrossRef] [PubMed]
- Freire, M. L.; Assis, T. S. M. de; Avelar, D. M. de; Rabello, A.; Cota, G. Evaluation of a New Brand of Immunochromatographic Test for Visceral Leishmaniasis in Brazil Made Available from 2018. Rev. Inst. Med. Trop. Sao Paulo, 2018, 60, e49. [Google Scholar] [CrossRef]
- Garcia, V. S.; Guerrero, S. A.; Gugliotta, L. M.; Gonzalez, V. D. G. A Lateral Flow Immunoassay Based on Colored Latex Particles for Detection of Canine Visceral Leishmaniasis. Acta Trop., 2020, 212, 105643. [Google Scholar] [CrossRef]
- Assis, T. S. M. de; Braga, A. S. da C.; Pedras, M. J.; Barral, A. M. P.; Siqueira, I. C. de; Costa, C. H. N.; Costa, D. L.; Holanda, T. A.; Soares, V. Y. R.; Biá, M.; et al. Validação Do Teste Imunocromatográfico Rápido IT-LEISH® Para o Diagnóstico Da Leishmaniose Visceral Humana. Epidemiol. E Serviços Saúde, 2008, 17 (2), 107–116. [CrossRef]
- Pedras, M. J.; de Gouvêa Viana, L.; de Oliveira, E. J.; Rabello, A. Comparative Evaluation of Direct Agglutination Test, rK39 and Soluble Antigen ELISA and IFAT for the Diagnosis of Visceral Leishmaniasis. Trans. R. Soc. Trop. Med. Hyg., 2008, 102, 172–178. [Google Scholar] [CrossRef]
- Romero, H. D.; Silva, L. de A.; Silva-Vergara, M. L.; Rodrigues, V.; Costa, R. T.; Guimarães, S. F.; Alecrim, W.; Moraes-Souza, H.; Prata, A. Comparative Study of Serologic Tests for the Diagnosis of Asymptomatic Visceral Leishmaniasis in an Endemic Area. Am. J. Trop. Med. Hyg., 2009, 81 (1), 27–33.
- Medeiros, F. A. C.; Souza Filho, J. A. de; Barbosa, J. R.; Donato, L. E.; Figueiredo, F. B.; Werneck, G. L.; Paz, G. F.; Thompson, M.; Marcelino, A. P. Phase II Validation Study of the rK39 ELISA Prototype for the Diagnosis of Canine Visceral Leishmaniasis in Brazil. Cad. Saúde Pública, 2021, 37, e00041320. [Google Scholar] [CrossRef]
- Salari, S.; Sharifi, I.; Keyhani, A. R.; Ghasemi Nejad Almani, P. Evaluation of a New Live Recombinant Vaccine against Cutaneous Leishmaniasis in BALB/c Mice. Parasit. Vectors, 2020, 13, 415. [Google Scholar] [CrossRef]
- Jardim, A.; Hanson, S.; Ullman, B.; McCubbin, W. D.; Kay, C. M.; Olafson, R. W. Cloning and Structure-Function Analysis of the Leishmania Donovani Kinetoplastid Membrane Protein-11. Biochem. J., 1995, 305 Pt 1, 315–320. [Google Scholar] [CrossRef]
- Lacerda, D. I.; Cysne-Finkelstein, L.; Nunes, M. P.; De-Luca, P. M.; Genestra, M. da S.; Leon, L. L. P.; Berrêdo-Pinho, M.; Mendonça-Lima, L.; Matos, D. C. de S.; Medeiros, M. A.; et al. Kinetoplastid Membrane Protein-11 Exacerbates Infection with Leishmania Amazonensis in Murine Macrophages. Mem. Inst. Oswaldo Cruz, 2012, 107, 238–245. [CrossRef]
- Mortazavidehkordi, N.; Fallah, A.; Abdollahi, A.; Kia, V.; Khanahmad, H.; Najafabadi, Z. G.; Hashemi, N.; Estiri, B.; Roudbari, Z.; Najafi, A.; et al. A Lentiviral Vaccine Expressing KMP11-HASPB Fusion Protein Increases Immune Response to Leishmania Major in BALB/C. Parasitol. Res., 2018, 117, 2265–2273. [Google Scholar] [CrossRef]
- Bourreau, E.; Collet, M.; Prévot, G.; Milon, G.; Ashimoff, D.; Hasagewa, H.; Parra-Lopez, C.; Launois, P. IFN-γ-Producing CD45RA+ CD8+ and IL-10-Producing CD45RA– CD4+ T Cells Generated in Response to LACK in Naive Subjects Never Exposed to Leishmania. Eur. J. Immunol., 2002, 32, 510–520. [Google Scholar] [CrossRef]
- Pérez-Jiménez, E.; Kochan, G.; Gherardi, M. M.; Esteban, M. MVA-LACK as a Safe and Efficient Vector for Vaccination against Leishmaniasis. Microbes Infect., 2006, 8, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Sacks, D. L.; Brown, D. R.; Reiner, S. L.; Charest, H.; Glaichenhaus, N.; Seder, R. A. Vaccination with DNA Encoding the Immunodominant LACK Parasite Antigen Confers Protective Immunity to Mice Infected with Leishmania Major. J. Exp. Med., 1997, 186, 1137–1147. [Google Scholar] [CrossRef]
- Maasho, K.; Satti, I.; Nylén, S.; Guzman, G.; Koning, F.; Akuffo, H. A Leishmania Homologue of Receptors for Activated C-Kinase (LACK) Induces Both Interferon-Gamma and Interleukin-10 in Natural Killer Cells of Healthy Blood Donors. J. Infect. Dis., 2000, 182, 570–578. [Google Scholar] [CrossRef]
- Bourreau, E.; Pascalis, H.; Prévot, G.; Kariminia, A.; Jolly, N.; Milon, G.; Buffet, P.; Michel, R.; Meynard, J.-B.; Boutin, J.-P.; et al. Increased Production of Interferon-Gamma by Leishmania Homologue of the Mammalian Receptor for Activated C Kinase-Reactive CD4+ T Cells among Human Blood Mononuclear Cells: An Early Marker of Exposure to Leishmania? Scand. J. Immunol., 2003, 58, 201–210. [Google Scholar] [CrossRef]
- Carvalho, L. P.; Passos, S.; Dutra, W. O.; Soto, M.; Alonso, C.; Gollob, K. J.; Carvalho, E. M.; Ribeiro de Jesus, A. Effect of LACK and KMP11 on IFN-Gamma Production by Peripheral Blood Mononuclear Cells from Cutaneous and Mucosal Leishmaniasis Patients. Scand. J. Immunol., 2005, 61, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Azeredo-Coutinho, R. B. G.; Matos, D. C. S.; Armôa, G. G. R.; Maia, R. M.; Schubach, A.; Mayrink, W.; Mendonça, S. C. F. Contrasting Human Cytokine Responses to Promastigote Whole-Cell Extract and the Leishmania Analogue Receptor for Activated C Kinase Antigen of L. Amazonensis in Natural Infection versus Immunization. Clin. Exp. Immunol., 2008, 153, 369–375. [Google Scholar] [CrossRef]
- Stober, C. B.; Jeronimo, S. M. B.; Pontes, N. N.; Miller, E. N.; Blackwell, J. M. Cytokine Responses to Novel Antigens in a Peri-Urban Population in Brazil Exposed to Leishmania Infantum Chagasi. Am. J. Trop. Med. Hyg., 2012, 87, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Julia, V.; Glaichenhaus, N. CD4(+) T Cells Which React to the Leishmania Major LACK Antigen Rapidly Secrete Interleukin-4 and Are Detrimental to the Host in Resistant B10.D2 Mice. Infect. Immun., 1999, 67, 3641–3644. [Google Scholar] [CrossRef]
- Afonso, L. C. C.; Scharton, T. M.; Vieira, L. Q.; Wysocka, M.; Trinchieri, G.; Scott, P. The Adjuvant Effect of Interleukin-12 in a Vaccine Against Leishmania Major. Science, 1994, 263, 235–237. [Google Scholar] [CrossRef]
- Mougneau, E.; Altare, F.; Wakil, A. E.; Zheng, S.; Coppola, T.; Wang, Z. E.; Waldmann, R.; Locksley, R. M.; Glaichenhaus, N. Expression Cloning of a Protective Leishmania Antigen. Science, 1995, 268, 563–566. [Google Scholar] [CrossRef]
- Melby, P. C.; Yang, J.; Zhao, W.; Perez, L. E.; Cheng, J. Leishmania Donovani P36(LACK) DNA Vaccine Is Highly Immunogenic but Not Protective against Experimental Visceral Leishmaniasis. Infect. Immun., 2001, 69, 4719–4725. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, E. A.; Coelho, E. A. F.; Gomes, D. C. O.; Vilela, M. C.; Masioli, C. Z.; Tavares, C. A. P.; Fernandes, A. P.; Afonso, L. C. C.; Rezende, S. A. Intramuscular Immunization with P36(LACK) DNA Vaccine Induces IFN-γ Production but Does Not Protect BALB/c Mice against Leishmania Chagasi Intravenous Challenge. Parasitol. Res., 2005, 98, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Fernández Cotrina, J.; Iniesta, V.; Monroy, I.; Baz, V.; Hugnet, C.; Marañon, F.; Fabra, M.; Gómez-Nieto, L. C.; Alonso, C. A Large-Scale Field Randomized Trial Demonstrates Safety and Efficacy of the Vaccine LetiFend® against Canine Leishmaniosis. Vaccine, 2018, 36, 1972–1982. [Google Scholar] [CrossRef] [PubMed]
- Sjölander, A.; Baldwin, T. M.; Curtis, J. M.; Bengtsson, K. L.; Handman, E. Vaccination with Recombinant Parasite Surface Antigen 2 from Leishmania Major Induces a Th1 Type of Immune Response but Does Not Protect against Infection. Vaccine, 1998, 16, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Sparwasser, T.; Lipford, G. B. Consequences of Bacterial CpG DNA-Driven Activation of Antigen-Presenting Cells. Curr. Top. Microbiol. Immunol., 2000, 247, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Iborra, S.; Carrión, J.; Anderson, C.; Alonso, C.; Sacks, D.; Soto, M. Vaccination with the Leishmania Infantum Acidic Ribosomal P0 Protein plus CpG Oligodeoxynucleotides Induces Protection against Cutaneous Leishmaniasis in C57BL/6 Mice but Does Not Prevent Progressive Disease in BALB/c Mice. Infect. Immun., 2005, 73, 5842–5852. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.; Alonso, C.; Requena, J. M. The Leishmania Infantum Acidic Ribosomal Protein LiP2a Induces a Prominent Humoral Response in Vivo and Stimulates Cell Proliferation in Vitro and Interferon-Gamma (IFN-Gamma) Production by Murine Splenocytes. Clin. Exp. Immunol., 2000, 122, 212–218. [Google Scholar] [CrossRef]
- Fernandes, A. P.; Coelho, E. A. F.; Machado-Coelho, G. L. L.; Grimaldi, G.; Gazzinelli, R. T. Making an Anti-Amastigote Vaccine for Visceral Leishmaniasis: Rational, Update and Perspectives. Curr. Opin. Microbiol., 2012, 15, 476–485. [Google Scholar] [CrossRef]
- Lage, D. P.; Ludolf, F.; Silveira, P. C.; Machado, A. S.; Ramos, F. F.; Dias, D. S.; Ribeiro, P. A. F.; Costa, L. E.; Vale, D. L.; Tavares, G. S. V.; et al. Screening Diagnostic Candidates from Leishmania Infantum Proteins for Human Visceral Leishmaniasis Using an Immunoproteomics Approach. Parasitology, 2019, 146, 1467–1476. [Google Scholar] [CrossRef]
- Ribeiro, P. A. F.; Dias, D. S.; Lage, D. P.; Martins, V. T.; Costa, L. E.; Santos, T. T. O.; Ramos, F. F.; Tavares, G. S. V.; Mendonça, D. V. C.; Ludolf, F.; et al. Immunogenicity and Protective Efficacy of a New Leishmania Hypothetical Protein Applied as a DNA Vaccine or in a Recombinant Form against Leishmania Infantum Infection. Mol. Immunol., 2019, 106, 108–118. [Google Scholar] [CrossRef]
- Oliveira-da-Silva, J. A.; Machado, A. S.; Tavares, G. S. V.; Ramos, F. F.; Lage, D. P.; Ludolf, F.; Steiner, B. T.; Reis, T. A. R.; Santos, T. T. O.; Costa, L. E.; et al. Biotechnological Applications from a Leishmania Amastigote-Specific Hypothetical Protein in the Canine and Human Visceral Leishmaniasis. Microb. Pathog., 2020, 147, 104283. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C. B. Nucleoside Hydrolase NH 36: A Vital Enzyme for the Leishmania Genus in the Development of T-Cell Epitope Cross-Protective Vaccines. Front. Immunol., 2019, 10, 813. [Google Scholar] [CrossRef]
- Alves-Silva, M. V.; Nico, D.; Morrot, A.; Palatnik, M.; Palatnik-de-Sousa, C. B. A Chimera Containing CD4+ and CD8+ T-Cell Epitopes of the Leishmania Donovani Nucleoside Hydrolase (NH36) Optimizes Cross-Protection against Leishmania Amazonesis Infection. Front. Immunol., 2017, 8, 100. [Google Scholar] [CrossRef]
- Iborra, S.; Soto, M.; Carrión, J.; Nieto, A.; Fernández, E.; Alonso, C.; Requena, J. M. The Leishmania Infantum Acidic Ribosomal Protein P0 Administered as a DNA Vaccine Confers Protective Immunity to Leishmania Major Infection in BALB/c Mice. Infect. Immun., 2003, 71, 6562–6572. [Google Scholar] [CrossRef]
- Chenik, M.; Louzir, H.; Ksontini, H.; Dilou, A.; Abdmouleh, I.; Dellagi, K. Vaccination with the Divergent Portion of the Protein Histone H2B of Leishmania Protects Susceptible BALB/c Mice against a Virulent Challenge with Leishmania Major. Vaccine, 2006, 24, 2521–2529. [Google Scholar] [CrossRef]
- Carrión, J. Mechanisms of Immunity to Leishmania Major Infection in Mice: The Contribution of DNA Vaccines Coding for Two Novel Sets of Histones (H2A–H2B or H3–H4). Comp. Immunol. Microbiol. Infect. Dis., 2011, 34, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Baharia, R. K.; Tandon, R.; Sahasrabuddhe, A. A.; Sundar, S.; Dube, A. Nucleosomal Histone Proteins of L. Donovani: A Combination of Recombinant H2A, H2B, H3 and H4 Proteins Were Highly Immunogenic and Offered Optimum Prophylactic Efficacy against Leishmania Challenge in Hamsters. PLOS ONE, 2014, 9, e97911. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Ghoshal, A.; Mandal, C.; Shaha, C. Leishmania Cell Surface Prohibitin: Role in Host-Parasite Interaction. Cell. Microbiol., 2010, 12, 432–452. [Google Scholar] [CrossRef] [PubMed]
- Joshi, B.; Ko, D.; Ordonez-Ercan, D.; Chellappan, S. P. A Putative Coiled-Coil Domain of Prohibitin Is Sufficient to Repress E2F1-Mediated Transcription and Induce Apoptosis. Biochem. Biophys. Res. Commun., 2003, 312, 459–466. [Google Scholar] [CrossRef]
- Lage, D. P.; Ribeiro, P. A. F.; Dias, D. S.; Mendonça, D. V. C.; Ramos, F. F.; Carvalho, L. M.; de Oliveira, D.; Steiner, B. T.; Martins, V. T.; Perin, L.; et al. A Candidate Vaccine for Human Visceral Leishmaniasis Based on a Specific T Cell Epitope-Containing Chimeric Protein Protects Mice against Leishmania Infantum Infection. NPJ Vaccines, 2020, 5, 75. [Google Scholar] [CrossRef]
- Dias, D. S.; Ribeiro, P. A. F.; Martins, V. T.; Lage, D. P.; Ramos, F. F.; Dias, A. L. T.; Rodrigues, M. R.; Portela, Á. S. B.; Costa, L. E.; Caligiorne, R. B.; et al. Recombinant Prohibitin Protein of Leishmania Infantum Acts as a Vaccine Candidate and Diagnostic Marker against Visceral Leishmaniasis. Cell. Immunol., 2018, 323, 59–69. [Google Scholar] [CrossRef]
- Kedzierski, L.; Montgomery, J.; Bullen, D.; Curtis, J.; Gardiner, E.; Jimenez-Ruiz, A.; Handman, E. A Leucine-Rich Repeat Motif of Leishmania Parasite Surface Antigen 2 Binds to Macrophages through the Complement Receptor 3. J. Immunol. Baltim. Md 1950, 2004, 172, 4902–4906. [Google Scholar] [CrossRef]
- Lincoln, L. M.; Ozaki, M.; Donelson, J. E.; Beetham, J. K. Genetic Complementation of Leishmania Deficient in PSA (GP46) Restores Their Resistance to Lysis by Complement. Mol. Biochem. Parasitol., 2004, 137, 185–189. [Google Scholar] [CrossRef]
- Chamakh-Ayari, R.; Bras-Gonçalves, R.; Bahi-Jaber, N.; Petitdidier, E.; Markikou-Ouni, W.; Aoun, K.; Moreno, J.; Carrillo, E.; Salotra, P.; Kaushal, H.; et al. In Vitro Evaluation of a Soluble Leishmania Promastigote Surface Antigen as a Potential Vaccine Candidate against Human Leishmaniasis. PLoS ONE, 2014, 9, e92708. [Google Scholar] [CrossRef] [PubMed]
- Handman, E.; Osborn, A. H.; Symons, F.; van Driel, R.; Cappai, R. The Leishmania Promastigote Surface Antigen 2 Complex Is Differentially Expressed during the Parasite Life Cycle. Mol. Biochem. Parasitol., 1995, 74, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kemp, M.; Handman, E.; Kemp, K.; Ismail, A.; Mustafa, M. D.; Kordofani, A. Y.; Bendtzen, K.; Kharazmi, A.; Theander, T. G. The Leishmania Promastigote Surface Antigen-2 (PSA-2) Is Specifically Recognised by Th1 Cells in Humans with Naturally Acquired Immunity to L. Major. FEMS Immunol. Med. Microbiol., 1998, 20, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Petitdidier, E.; Pagniez, J.; Papierok, G.; Vincendeau, P.; Lemesre, J.-L.; Bras-Gonçalves, R. Recombinant Forms of Leishmania Amazonensis Excreted/Secreted Promastigote Surface Antigen (PSA) Induce Protective Immune Responses in Dogs. PLoS Negl. Trop. Dis., 2016, 10, e0004614. [Google Scholar] [CrossRef] [PubMed]
- Ommen, G.; Chrobak, M.; Clos, J. The Co-Chaperone SGT of Leishmania Donovani Is Essential for the Parasite’s Viability. Cell Stress Chaperones, 2010, 15, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Dias, D. S.; Ribeiro, P. A. F.; Martins, V. T.; Lage, D. P.; Portela, Á. S. B.; Costa, L. E.; Salles, B. C. S.; Lima, M. P.; Ramos, F. F.; Santos, T. T. O.; et al. Recombinant Small Glutamine-Rich Tetratricopeptide Repeat-Containing Protein of Leishmania Infantum: Potential Vaccine and Diagnostic Application against Visceral Leishmaniasis. Mol. Immunol., 2017, 91, 272–281. [Google Scholar] [CrossRef]
- Zhang, W.-W.; Matlashewski, G. Loss of Virulence in Leishmania Donovani Deficient in an Amastigote-Specific Protein, A2. Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 8807–8811. [Google Scholar] [CrossRef]
- Almeida, A. P. M. M.; Machado, L. F. M.; Doro, D.; Nascimento, F. C.; Damasceno, L.; Gazzinelli, R. T.; Fernandes, A. P.; Junqueira, C. New Vaccine Formulations Containing a Modified Version of the Amastigote 2 Antigen and the Non-Virulent Trypanosoma Cruzi CL-14 Strain Are Highly Antigenic and Protective against Leishmania Infantum Challenge. Front. Immunol., 2018, 9, 465. [Google Scholar] [CrossRef]
- Toepp, A.; Larson, M.; Wilson, G.; Grinnage-Pulley, T.; Bennett, C.; Leal-Lima, A.; Anderson, B.; Parrish, M.; Anderson, M.; Fowler, H.; et al. Randomized, Controlled, Double-Blinded Field Trial to Assess Leishmania Vaccine Effectiveness as Immunotherapy for Canine Leishmaniosis. Vaccine, 2018, 36, 6433–6441. [Google Scholar] [CrossRef]
- Azam, S. S.; Abro, A.; Raza, S.; Saroosh, A. Structure and Dynamics Studies of Sterol 24-C-Methyltransferase with Mechanism Based Inactivators for the Disruption of Ergosterol Biosynthesis. Mol. Biol. Rep., 2014, 41, 4279–4293. [Google Scholar] [CrossRef] [PubMed]
- Palatnik-de-Sousa, C. B.; Nico, D. The Delay in the Licensing of Protozoal Vaccines: A Comparative History. Front. Immunol., 2020, 11, 204. [Google Scholar] [CrossRef] [PubMed]
- Brito, R. C. F.; Guimarães, F. G.; Velloso, J. P. L.; Corrêa-Oliveira, R.; Ruiz, J. C.; Reis, A. B.; Resende, D. M. Immunoinformatics Features Linked to Leishmania Vaccine Development: Data Integration of Experimental and In Silico Studies. Int. J. Mol. Sci., 2017, 18, 371. [Google Scholar] [CrossRef] [PubMed]
- Khan, M. A. A.; Ami, J. Q.; Faisal, K.; Chowdhury, R.; Ghosh, P.; Hossain, F.; Abd El Wahed, A.; Mondal, D. An Immunoinformatic Approach Driven by Experimental Proteomics: In Silico Design of a Subunit Candidate Vaccine Targeting Secretory Proteins of Leishmania Donovani Amastigotes. Parasit. Vectors, 2020, 13, 196. [Google Scholar] [CrossRef]
- Brasil. Manual de vigilância e controle da leishmaniose visceral - 1a Edição.
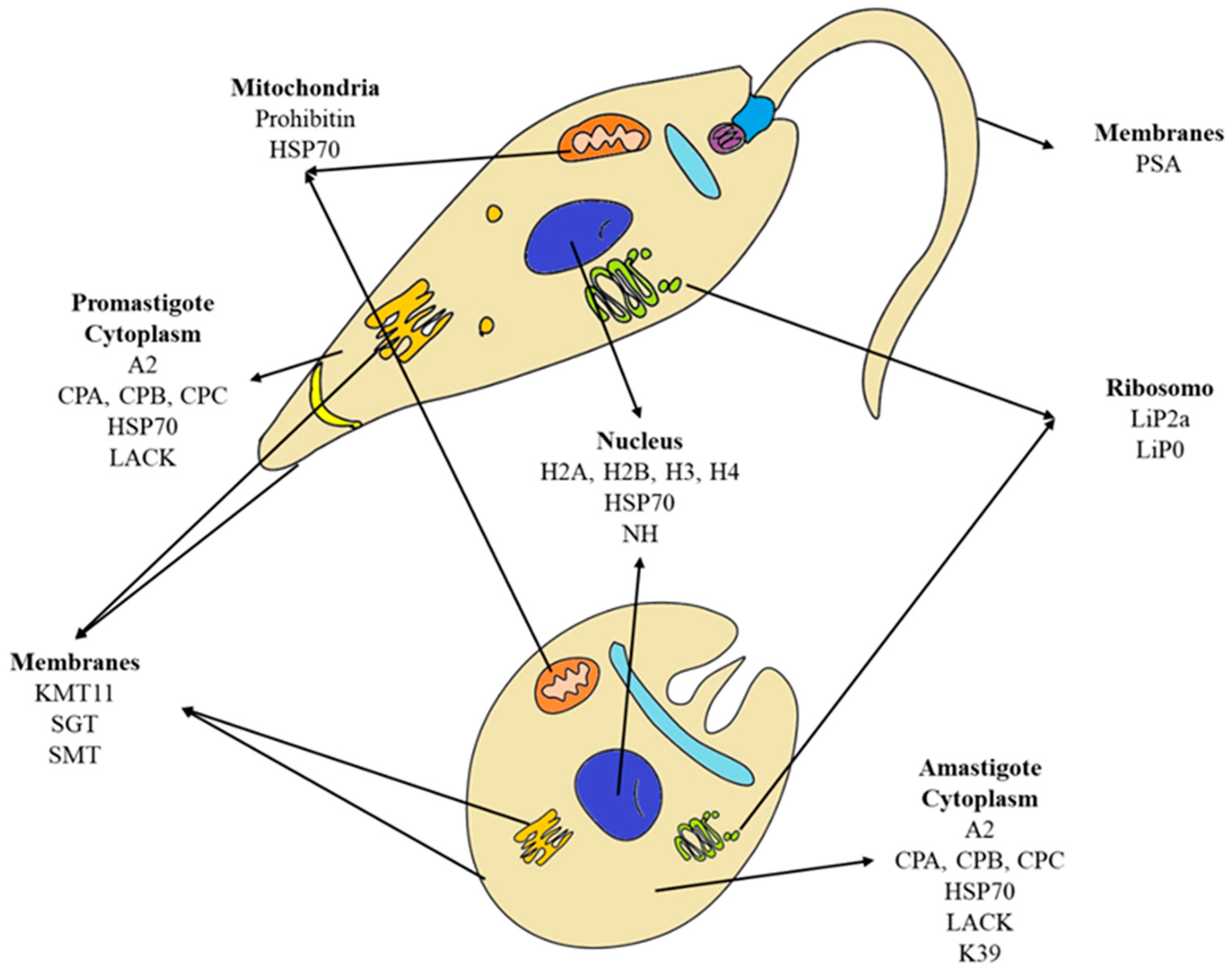
| Original patent title | Publication number | Priority Country | Deposit year | Chimera composition | In silico analysis | In vitro analysis | In vivo analysis |
|---|---|---|---|---|---|---|---|
| “Proteína quimérica, kit, método para diagnóstico de leishmaniose, uso de proteína quimérica, composição vacinal contra leishmaniose visceral, e, uso de uma composição vacinal” | BR 10 2021 00079 4 | Brazil | 2021 | A2 and K39 | - | ↑IFN-γ in spleen cell culture | ↑IgG e IgG2, in BALB/c mice. ↓spleen and liver, parasite load by limiting dilution, in BALB/c mice |
| “Quimera sintética multiepitópica como vacuna y tratamiento frente a leishmaniosis en mamíferos” | ES2795149 | Spain | 2020 | H2A, H2B, H3, and H4 | Human and mice MHC* class I and II alleles prediction | ↑IFN-γ and IL-12 after culture stimulation. ↑leishmanicidal effect in infected BMDC** |
↓spleen and liver, parasite load by limiting dilution, in BALB/c mice |
| “Vacinas compostas de proteínas quiméricas poliepítopos contra a leishmaniose visceral humana e/ou canina” | BR 10 2018 008197 7 | Brazil | 2018 | VAC-1: H2A, LACK LiP2a, LiP0, and CPC | MHC class I and II alleles prediction | ↑IFN-γ, TNF-α, CD4+ T cells, and CD8+ T cells, after culture stimulation ↑CD4+ T lymphocytes with central memory phenotype in VAC-1 and VAC-2 ↑CD8+ T lymphocytes with central memory phenotype in VAC-1 and VAC-2. Effector memory phenotype in VAC-1 |
↓spleen parasite load by Real Time PCR (qPCR), in BALB/c mice |
| VAC-2: CPA, CPB, PSA-50S, and A2 | |||||||
| “Proteína quimérica recombinante, vacina contra leishmanioses e uso” | BR 10 2017 025621 9 | Brazil | 2017 | Prohibitin; SGT; LiHyp5 | Human MHC class I and II alleles prediction | ↑ IFN-γ, IL-12, and GM-CSF in spleen cell culture ↑ PBMCs proliferation in human and dog cells |
↓spleen, liver, draining lymph nodes, and bone marrow parasite load by limiting dilution, in BALB/c mice |
| “Proteína quimérica, composição vacinal contra leishmanioses e usos” | BR 10 2016 006121 0 | Brazil | 2016 | LiHyp1, LiHyp6, LiHyV, and HRF | Human and mice MHC class I and II alleles prediction | ↑ IFN-γ, IL-12, and GM-CSF in spleen cell culture | ↓spleen, liver, draining lymph nodes, and bone marrow parasite load by limiting dilution, in BALB/c mice |
| Recombinant polyprotein vaccines for the treatment and diagnosis of leishmaniasis | US20130177584 | United States | 2013 | KSA (KM11, SMT e A2) | - | - | ↓liver by limiting dilution, in C57BL/6 (L. infantum challenge), and BALB/c mice (L. donovani challenge) |
| Vaccines comprising leishmania polypeptides for the treatment and diagnosis of leishmaniasis | WO 2014/160987 | United States | 2013 | NS and NSC | - | ↑IFN-γ in spleen cell culture | ↓liver by qPCR, in BALB/c mice |
| “Quimera multicomponente para su uso como vacuna frente a la infección por Leishmania spp. En mamíferos” | WO 2013/110824 | Spain | 2011 | HISA70 (H2A, H2B, H3, H4, A2, HSP70) | - | - | ↓spleen and liver by limiting dilution, in BALB/c mice |
| Vaccines comprising non-specific nucleoside hydrolase and sterol 24-c-methyltransferase (SMT) polypeptides for the treatment and diagnosis of Leishmaniasis | WO 2012064659 | United States | 2010 | NS | - | ↑IFN-γ by spleen cell culture, in BALB/c mice ↑IFN-γ, and ↑IgG in non-human primates |
↓liver by limiting dilution, in BALB/c mice ↑IgG1 and IgG2 titration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





