Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cells and viruses
2.2. Coinfection of ZIKV and PIUV
2.3. TCID50 (Tissue Culture Infectious Dose 50%)
2.4. Intracellular replication
2.5. ZIKV and PIUV RNA Quantification
2.6. Statistics
3. Results
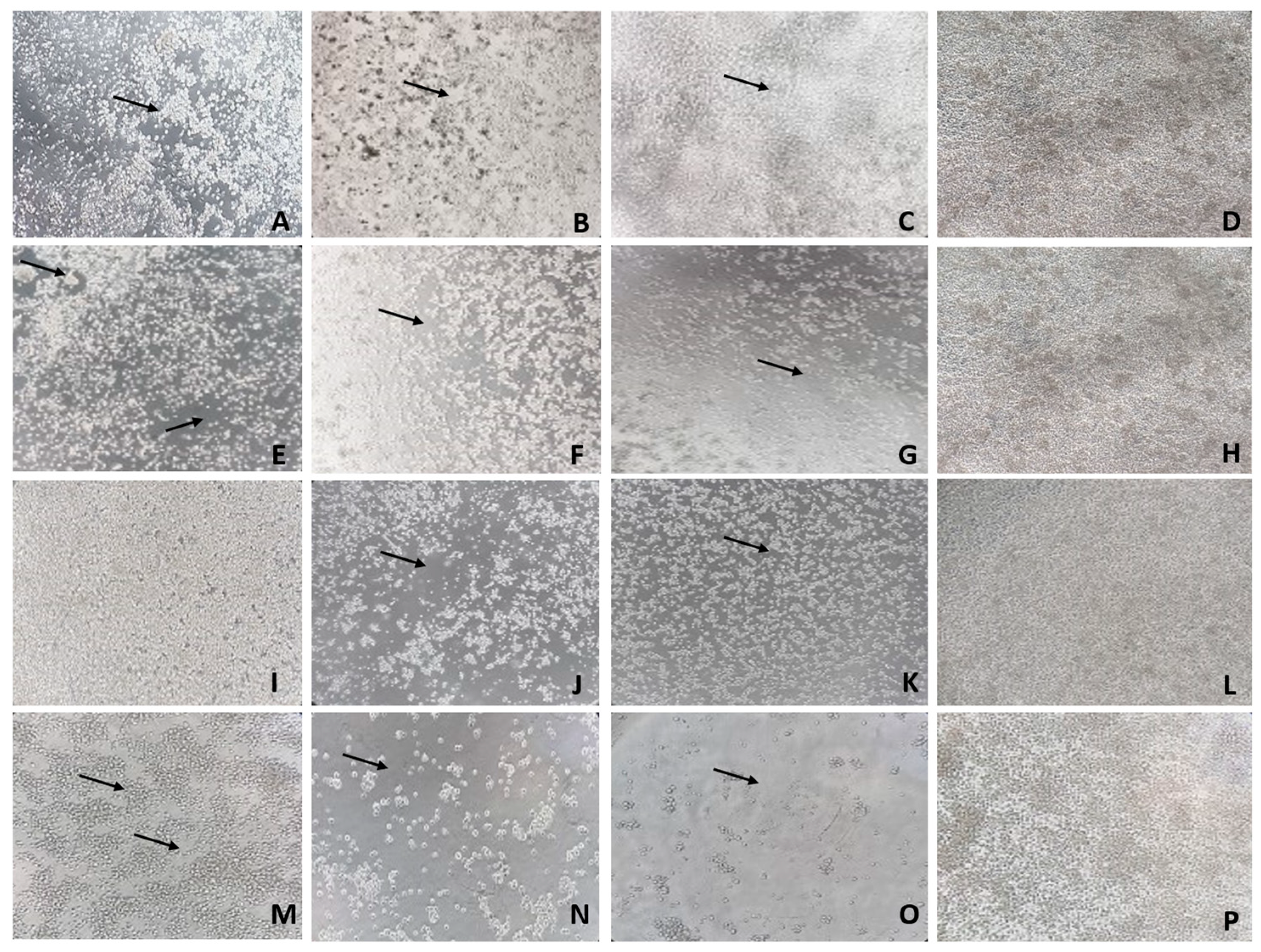
3.1. PIUV causes intense CPE in C6/36 cells
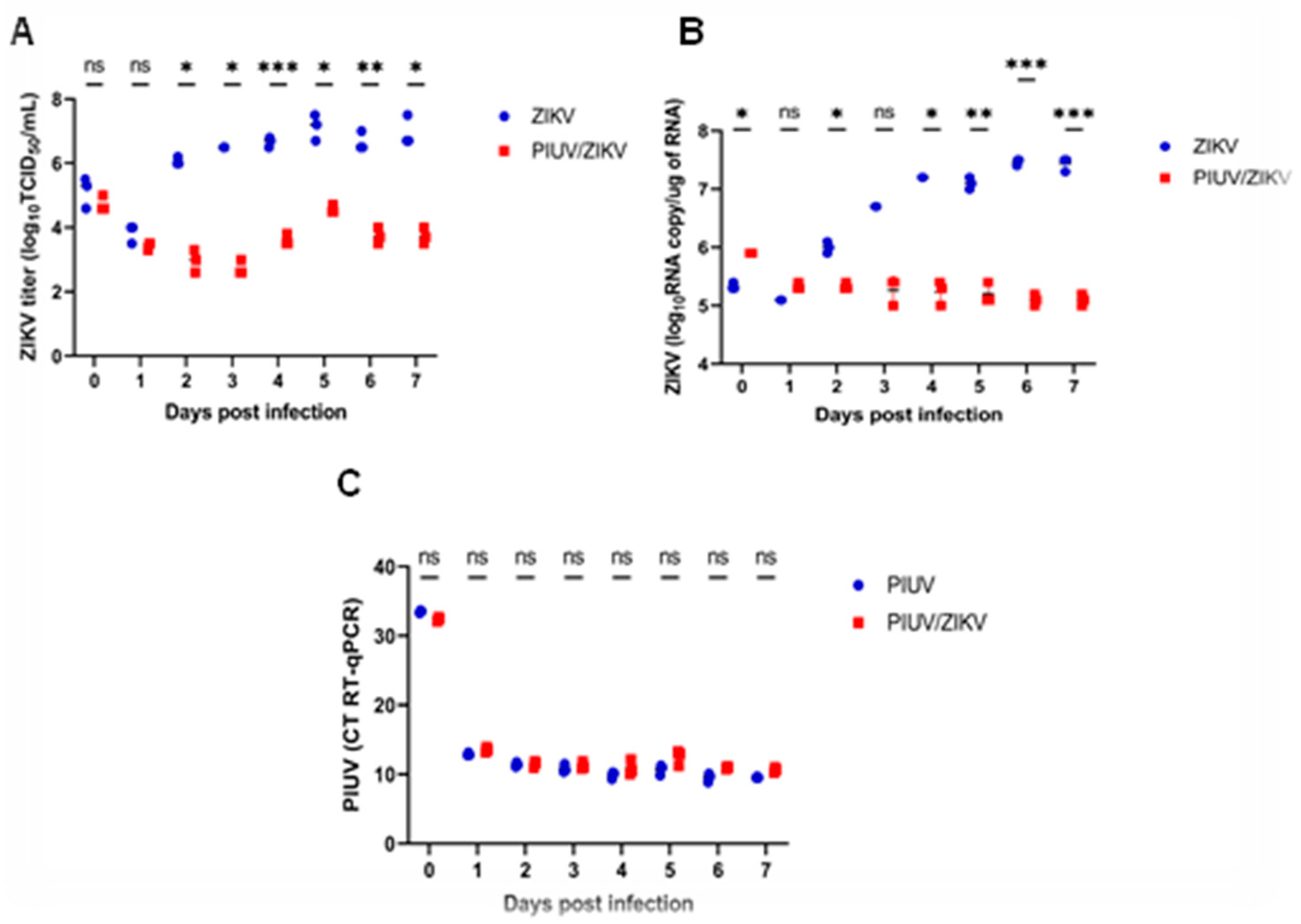
3.2. PIUV inhibits ZIKV replication in C6/36, but ZIKV does not interfere with PIUV replication
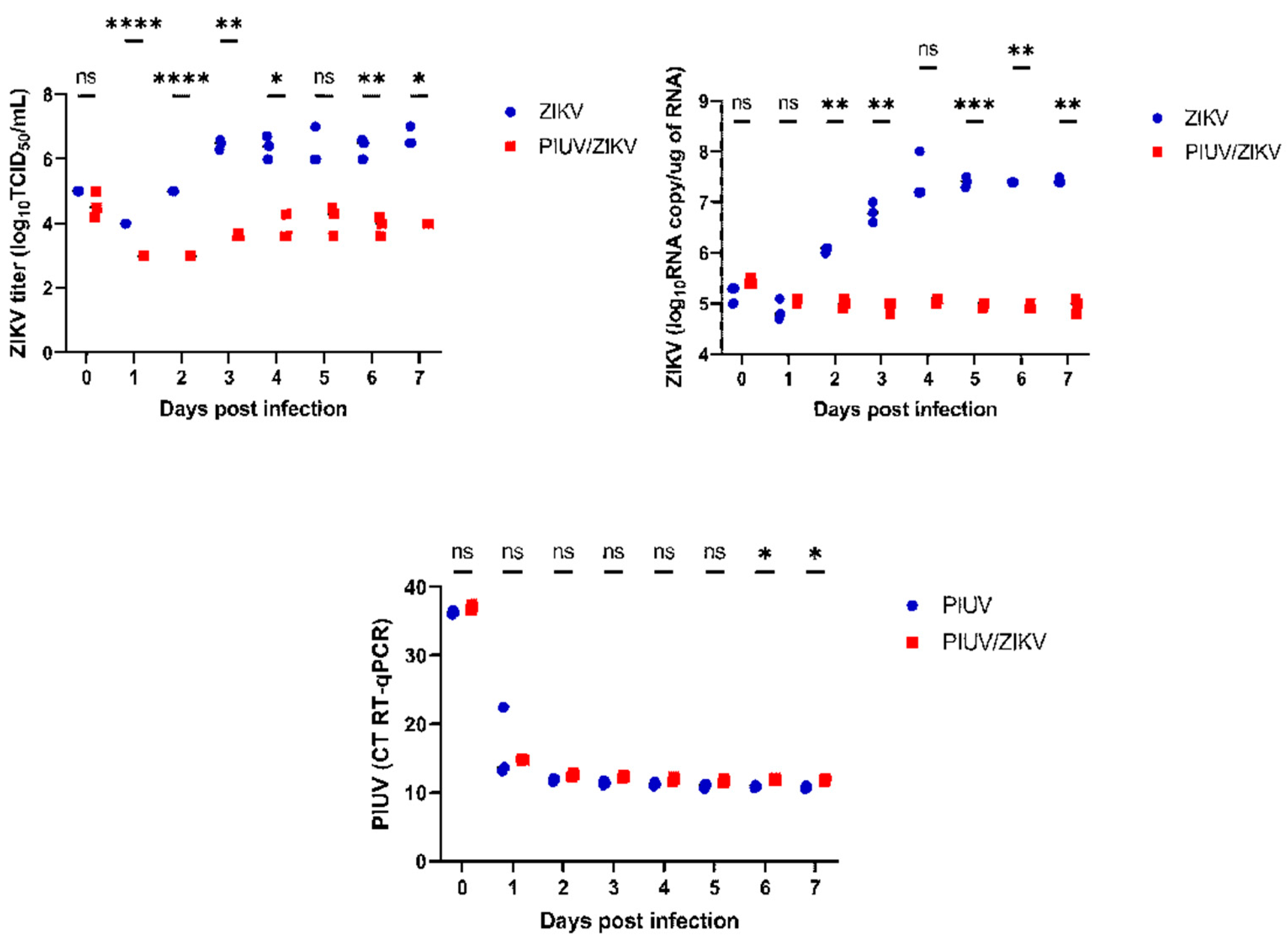
3.3. PIUV inhibition of ZIKV replication is dose-dependent
3.4. PIUV CPE was predominant in PIUV/ZIKV coinfection
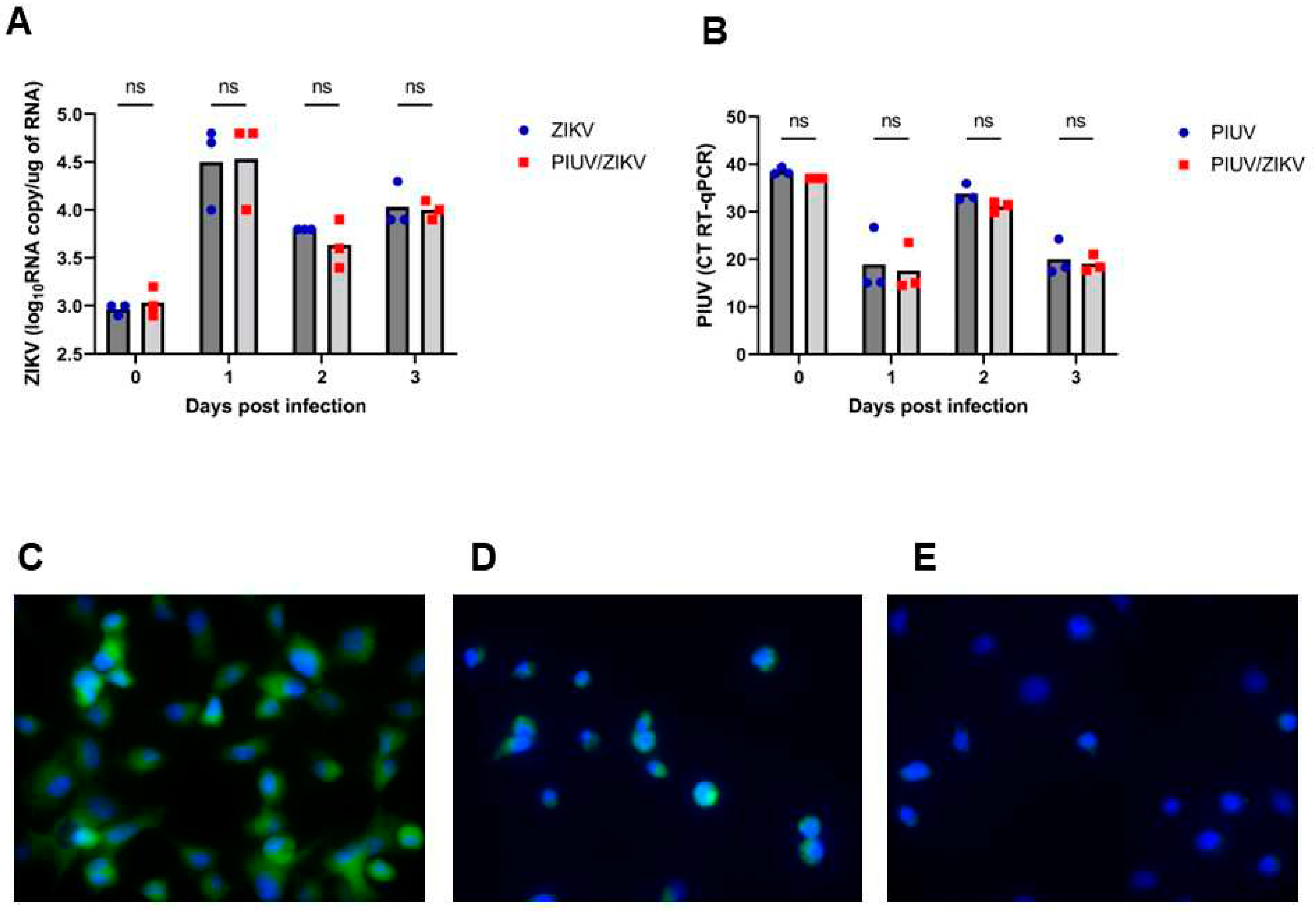
3.5. PIUV does not inhibit ZIKV cellular entry and likely inhibits intracellular replication in C6/36 cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vasconcelos, P.F.C.; Calisher, C.H. Emergence of Human Arboviral Diseases in the Americas, 2000-2016. Vector-Borne Zoonotic Dis. 2016, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Soni S, Gill VJS, Anusheel, Singh J, Chhabra J, Gill GJS, Bakshi R. Dengue, Chikungunya, and Zika: The Causes and Threats of Emerging and Re-emerging Arboviral Diseases. Cureus. 2023 Jul 11;15(7):e41717. [CrossRef]
- Masmejan, S.; Musso, D.; Vouga, M.; Pomar, L.; Dashraath, P.; Stojanov, M.; Panchaud, A.; Baud, D. Zika Virus. Pathogens 2020, 9, 898. [Google Scholar] [CrossRef] [PubMed]
- CDC. Centers for Disease Control and Prevention. 2022. Available at: https://www.cdc.gov/campylobacter/guillain-barre.html.
- Bolling, B.G.; Weaver, S.C.; Tesh, R.B.; Vasilakis, N. Insect-specific virus discovery: Significance for the arbovirus community. Viruses 2015, 7, 4911–4928. [Google Scholar] [CrossRef] [PubMed]
- Gómez M, Martinez D, Muñoz M, Ramírez JD. Aedes aegypti and Ae. albopictus microbiome/virome: new strategies for controlling arboviral transmission? Parasit Vectors. 2022 Aug 9;15(1):287. [CrossRef]
- Carvalho, V.L.; Long, M.T. Insect-Specific Viruses: An overview and their relationship to arboviruses of concern to humans and animals. Virology 2021, 557, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Charles, J.; Tangudu, C.S.; Hurt, S.L.; Tumescheit, C.; Firth, A.E.; Garcia-Rejon, J.E.; Machain-Williams, C.; Blitvich, B.J. Discovery of a novel Tymoviridae-like virus in mosquitoes from Mexico. Arch. Virol. 2019, 164, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Kallies, R.; Kopp, A.; Zirkel, F.; Estrada, A.; Gillespie, T.R.; Drosten, C.; Junglen, S. Genetic characterization of goutanap virus, a novel virus related to negeviruses, cileviruses and higreviruses. Viruses 2014, 6, 4346–4357. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.R.T.; Contreras-Gutierrezb, M.A.; Guzman, H.; Martins, L.C.; Barbiratoh, M.F.; Saviti, C.; Baltaj, V.; Uribec, S.; Viverob, R.; Suaza, J.D.; et al. Genetic characterization, molecular epidemiology, and phylogenetic relationships of insect-specific viruses in the taxon Negevirus. Virology 2017, 504, 152–167. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ye, Z.-X.; He, Y.-J.; Zhang, Y.; Wang, X.; Huang, H.-J.; Zhuo, J.-C.; Sun, Z.-T.; Yan, F.; Chen, J.-P.; et al. Discovery of Two Novel Negeviruses in a Dungfly Collected from the Arctic. Viruses 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Vasilakis N, Forrester NL, Palacios G, Nasar F, Savji N, Rossi SL, Guzman H, Wood TG, Popov V, Gorchakov R, González AV, Haddow AD, Watts DM, da Rosa AP, Weaver SC, Lipkin WI, Tesh RB. Negevirus: a proposed new taxon of insect-specific viruses with wide geographic distribution. J Virol. 2013 Mar;87(5):2475-88. [CrossRef]
- Ramos-Gonzalez, P.L.; Dos Santos, G.F.; Chabi-Jesus, C.; Harakava, R.; Kitajima, E.W.; Freitas-Astua, J. Passion Fruit Green Spot Virus Genome Harbors a New Orphan ORF and Highlights the Flexibility of the 5′-End of the RNA2 Segment Across Cileviruses. Front. Microbiol. 2020, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Lenz, O.; Pribylova, J.; Franova, J.; Koloniuk, I. Fragaria vesca-associated virus 1, a new virus related to negeviruses. Arch. Virol. 2020, 165, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Potter-Birriel JM, Pollio AR, Knott BD, Chunashvili T, Fung CK, Conte MA, Reinbold-Wasson DD, Hang J. Metagenomics analysis reveals presence of the Merida-like virus in Gorgia. Front Microbiol. 2023 Oct 12;14:1258810. [CrossRef]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 5, 427(2):90-7. [CrossRef]
- Hobson-Peters, J.; Yam, A.W.; Lu, J.W.; Setoh, Y.X.; May, F.J.; Kurucz, N.; Walsh, S.; Prow, N.A.; Davis, S.S.; Weir, R.; Melville, L.; Hunt, N.; Webb, R.I.; Blitvich, B.J.; Whelan, P.; Hall, R.A. A new insect-specific flavivirus from northern Australia suppresses replication of West Nile virus and Murray Valley encephalitis virus in co-infected mosquito cells. PLoS One 2013, (2): e56534. [CrossRef]
- Romo, H.; Kenney, J.L.; Blitvich, B.J.; Brault, A.C. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nasar, F.; Erasmus, J.H.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat virus induces both homologous and heterologous interference. Virology 2015, 484:51-58. [CrossRef]
- Erasmus, J.H.; Auguste, A.J.; Kaelber, J.T.; Luo, H.; Rossi, S.L.; Fenton, K.; Leal, G.; Kim, D.Y.; Chiu, W.; Wang, T.; Frolov, I.; Nasar, F.; Weaver, S.C. A chikungunya fever vaccine utilizing an insect-specific virus platform. Nat Med 2017, 23(2):192-199. [CrossRef]
- Erasmus JH, Needham J, Raychaudhuri S, Diamond MS, Beasley DW, Morkowski S, Salje H, Fernandez Salas I, Kim DY, Frolov I, Nasar F, Weaver SC. Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection. PLoS Negl Trop Dis. 2015 Oct 22;9(10):e0004119. [CrossRef]
- Erasmus JH, Weaver SC. Biotechnological Applications of an Insect-Specific Alphavirus. DNA Cell Biol. 2017 Dec;36(12):1045-1049. [CrossRef]
- Patterson, E.I.; Kautz, T.F.; Contreras-Gutierrez, M.A.; Guzman, H.; Tesh, R.B.; Hughes, G.L.; Forrester, N.L. Negeviruses Reduce Replication of Alphaviruses during Coinfection. J. Virol. 2021, 95, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Rio, D.C.; Ares, M.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5439. [Google Scholar] [CrossRef] [PubMed]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Pauvolid-Corrêa A, Solberg O, Couto-Lima D, Kenney J, Serra-Freire N, Brault A, Nogueira R, Langevin S, Komar N. Nhumirim virus, a novel flavivirus isolated from mosquitoes from the Pantanal, Brazil. Arch Virol. 2015 Jan;160(1):21-7. [CrossRef]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796–2808. [Google Scholar] [CrossRef] [PubMed]
- Hall-Mendelin, S.; McLean, B.J.; Bielefeldt-Ohmann, H.; Hobson-Peters, J.; Hall, R.A.; van den Hurk, A.F. The insect-specific Palm Creek virus modulates West Nile virus infection in and transmission by Australian mosquitoes. Parasit Vectors 2016, 25;9(1):414. [CrossRef]
- Kent, R.J.; Crabtree, M.B.; Miller, B.R. Transmission of West Nile virus by Culex quinquefasciatus say infected with Culex Flavivirus Izabal. PLoS Negl Trop Dis 2010, 4;4(5): e671. [CrossRef]
- Newman, C.M.; Cerutti, F.; Anderson, T.K.; Hamer, G.L.; Walker, E.D.; Kitron, U.D.; Ruiz, M.O.; Brawn, J.D.; Goldberg, T.L. Culex flavivirus and West Nile virus mosquito coinfection and positive ecological association in Chicago, United States. Vector Borne Zoonotic Dis 2011, 11(8):1099-105. [CrossRef]
- Newman, C.M.; Krebs, B.L.; Anderson, T.K.; Hamer, G.L.; Ruiz, M.O.; Brawn, J.D.; Brown, W.M; Kitron, U.D.; Goldberg, T.L. Culex Flavivirus During West Nile Virus Epidemic and Interepidemic Years in Chicago, United States. Vector Borne Zoonotic Dis 2017, 17(8):567-575. [CrossRef]
- Crockett, R.K.; Burkhalter, K.; Mead, D.; Kelly, R.; Brown, J.; Varnado, W.; Roy, A.; Horiuchi, K.; Biggerstaff, B.J.; Miller, B.; Nasci, R. Culex flavivirus and West Nile virus in Culex quinquefasciatus populations in the southeastern United States. J Med Entomol 2012, 49(1):165-74. [CrossRef]
- Hollingsworth BD, Grubaugh ND, Lazzaro BP, Murdock CC. Leveraging insect-specific viruses to elucidate mosquito population structure and dynamics. PLoS Pathog. 2023 Aug 31;19(8):e1011588. [CrossRef]
- Guo Z, Jing W, Liu J, Liu M. The global trends and regional differences in incidence of Zika virus infection and implications for Zika virus infection prevention. PLoS Negl Trop Dis. 2022 Oct 21;16(10):e0010812. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




