Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction*- The Good, the Bad and the Incredible about the Immune Reactions of Vector Insects against Parasites and Pathogens
2. Diseases Vectored by Insects
| Vectors | Diseases | Pathogens | Distribution | At Risk |
|---|---|---|---|---|
| Aedes, Anopheles , Culex, Mansonia | Lymphatic filariasis | Nematode worms Brugia spp., and Wucheria bancrofti | Tropical and subtropical regions of SE Asia, Central and South America, Africa, West Pacific | 882 million |
| Aedes | Dengue | Flavivirus | Tropical, subtropical and spreading to Europe | 3.9 billion in 129 countries |
| Aedes | Yellow fever | Flavivirus | Endemic in tropical regions of Africa and Latin America | 900 million |
| Aedes | Chikungunya | Alphavirus | Tropical, subtropical and temperate regions. | ¾ of the World population at risk. |
| Aedes | Zika | Flavivirus | The Americas, Europe, India and 89 countries | Over 2 billion at risk |
| Anopheles complex with 484 recognised species but An.gambiae carries the deadliest form. | Malaria | Protozoan parasite with 5 Plasmodium species. | In 2021, the African region carried 95% of cases | Nearly half the World was at risk of malaria in 2021 |
| Culex spp. | Arboviruses | West Nile Virus, (Both Flaviviruses) Japanese encephalitis |
USA, Canada, Caribbean, Central and South America. South East Asia and West Pacific |
These and other arboviruses (Zika etc) risk emerging pandemics |
| Blackflies Simulium spp |
Onchocerciasis River blindness | Vector-borne nematode worms eg. Onchocercas volvulus | 99% in Africa but also foci in Brazil, Venezuela and Yemen | 123 million |
| Sandflies Phlebotomus spp and Lutzomyia spp. | Leishmaniasis | Protozoan parasite more than 20 species | Africa, Americas (Brazil), Middle East, South Asia, Mediterranean | 99 countries |
| Tsetse flies Glossina spp. |
African trypanosomiasis | Protozoan parasites, Trypanosoma brucei | Sub-Saharan Africa | 55 million people but control now and less than 1000 cases in 2022 |
| Lice Pediculus humanus and Pthirus pubis |
Louse-borne typhus and severe allergic reactions | Rickettsiae Rickettsia prowazekii |
Epidemics in wars, prisons and refugee camps in colder regions | Global diseases of poverty |
| Fleas Xenopsylla cheopis |
Plague | Gram-negative bacterium Yersinia pestis | Associated with close living in deprived areas with rat infestations | Global distribution |
| Triatomine bugs Rhodnius and Triatoma spp. | Chagas disease | Protozoan Trypanosoma cruzi | Mainly South, Central America but also North America now | 70 million in the Americas |
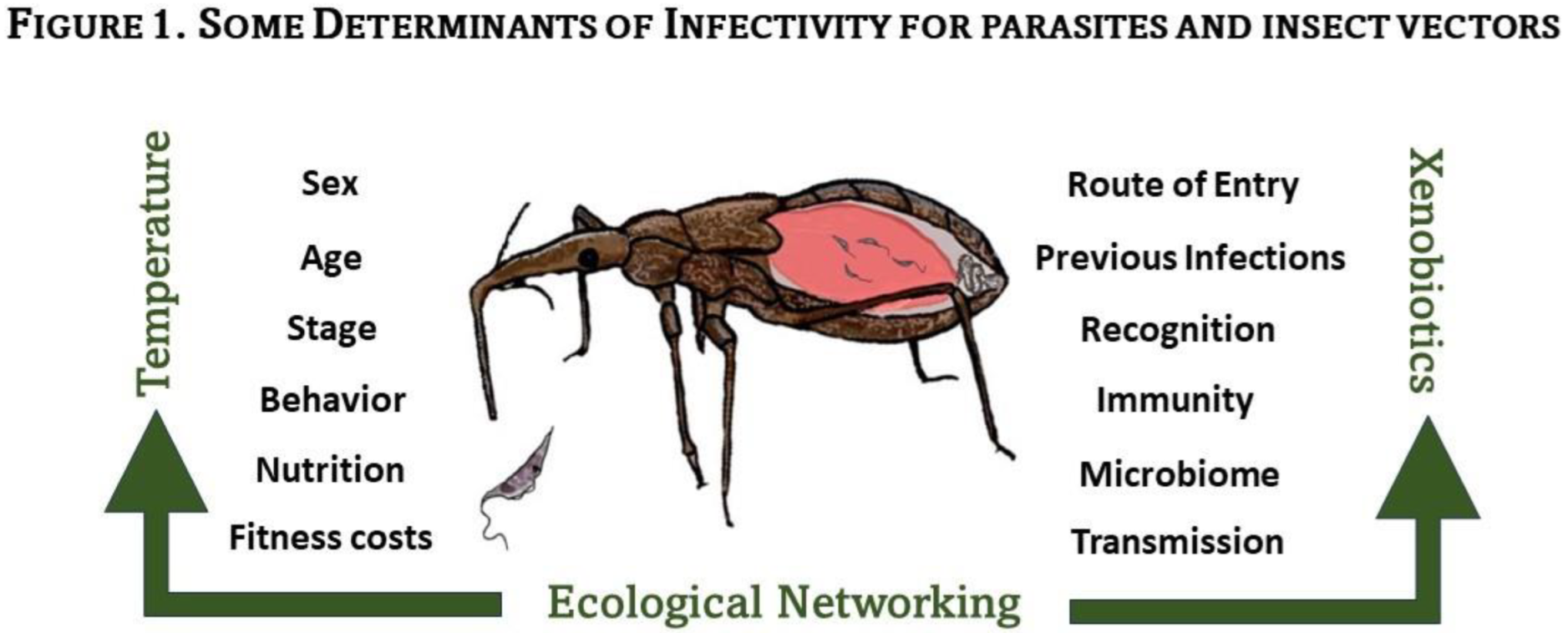
3. Typical Insect Immune Scenario
4. Vector Cellular Immunity
4.1. Haemocyte Types
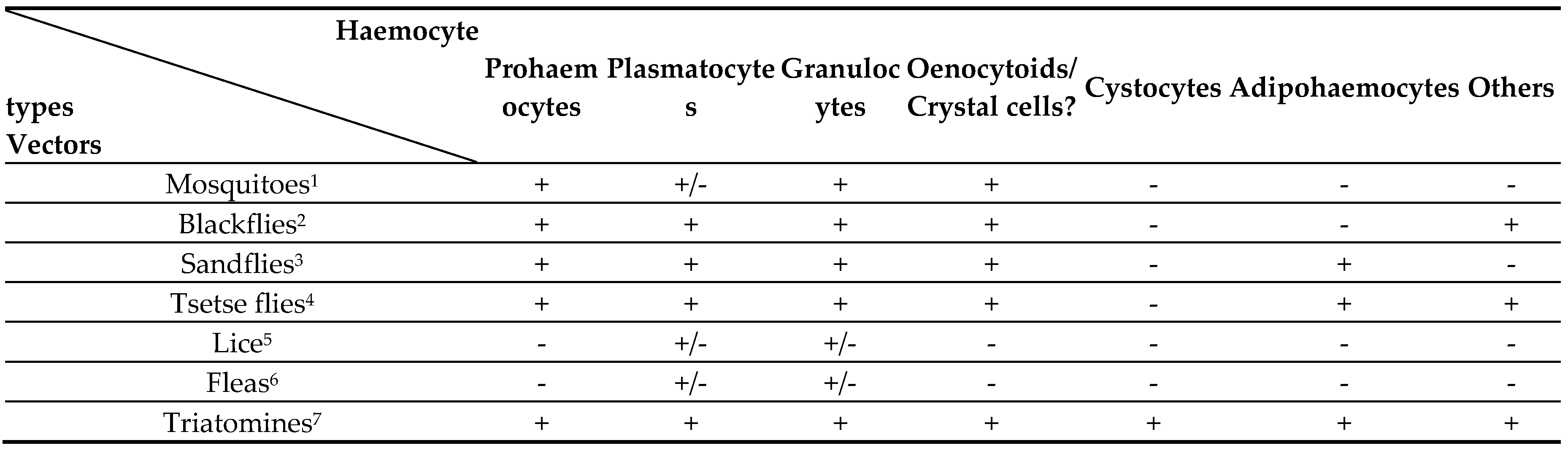 |
4.2. Cellular Defence Mechanisms
4.2.1. Coagulation
4.2.2. Phagocytosis
- In blackflies, phagocytosis of erythrocytes and bacteria is mediated by plasmatocytes and granulocytes with evidence of these cells also being involved in remodelling tissues [104].
- With sandflies, Leishmania development occurs exclusively in the gut with research mainly confined to this organ and interaction with the microbiota [8], so that consideration of the possible role of the haemocytes has been neglected. Lutzomyia longipalpis embryonic cell lines used for studying innate immunity in sandflies, however, have active Toll and Imd pathways and internalisation of Leishmania parasites was reported [127], so that the haemocytes may well be activated following infection. In lice and fleas more information on phagocytosis has been published recently.
- In head and body lice, the relative phagocytic activities of the haemocytes have been compared following injections of Escherichia coli or Staphylococcus aureus and showed that the body lice had a reduced immune response compared to the head lice [115]. In addition, haemocytes have been identified engulfing endosymbionts during their migrations around the body of the lice [128]. The reduced phagocytic competence of the body lice may be related to the increased pathogen vectoring capacity of these insects [128]. The presence in lice of the genes for the main signalling pathways, except Imd, may indicate the potential for activation of the haemocytes [129].
- The phagocytic activity of fleas has been the subject to similar research to that in lice [111], so that following inoculations of E. coli the phagocytic activity of the haemocytes increased significantly. This was accompanied by a general enhancement of antimicrobial resistance of the haemolymph, probably also involving humoral immune factors induced via signalling pathways [130] (see section 5. Vector Humoral Immunity, below).
- ∙ Two other major vectors in which phagocytosis has been recorded are the tsetse flies, Glossina spp. and the triatomines, Rhodnius and Triatoma. The main parasites involved in these insects are the African trypanosomes and T. cruzi, respectively. These parasites are mainly confined to the vector gut, although African trypanosomes do migrate in the vector during maturation [12], and Rhodnius also hosts T. rangeli which invades the haemocoel [131].
4.2.3. Nodules and Capsules
5. Vector Humoral Immunity
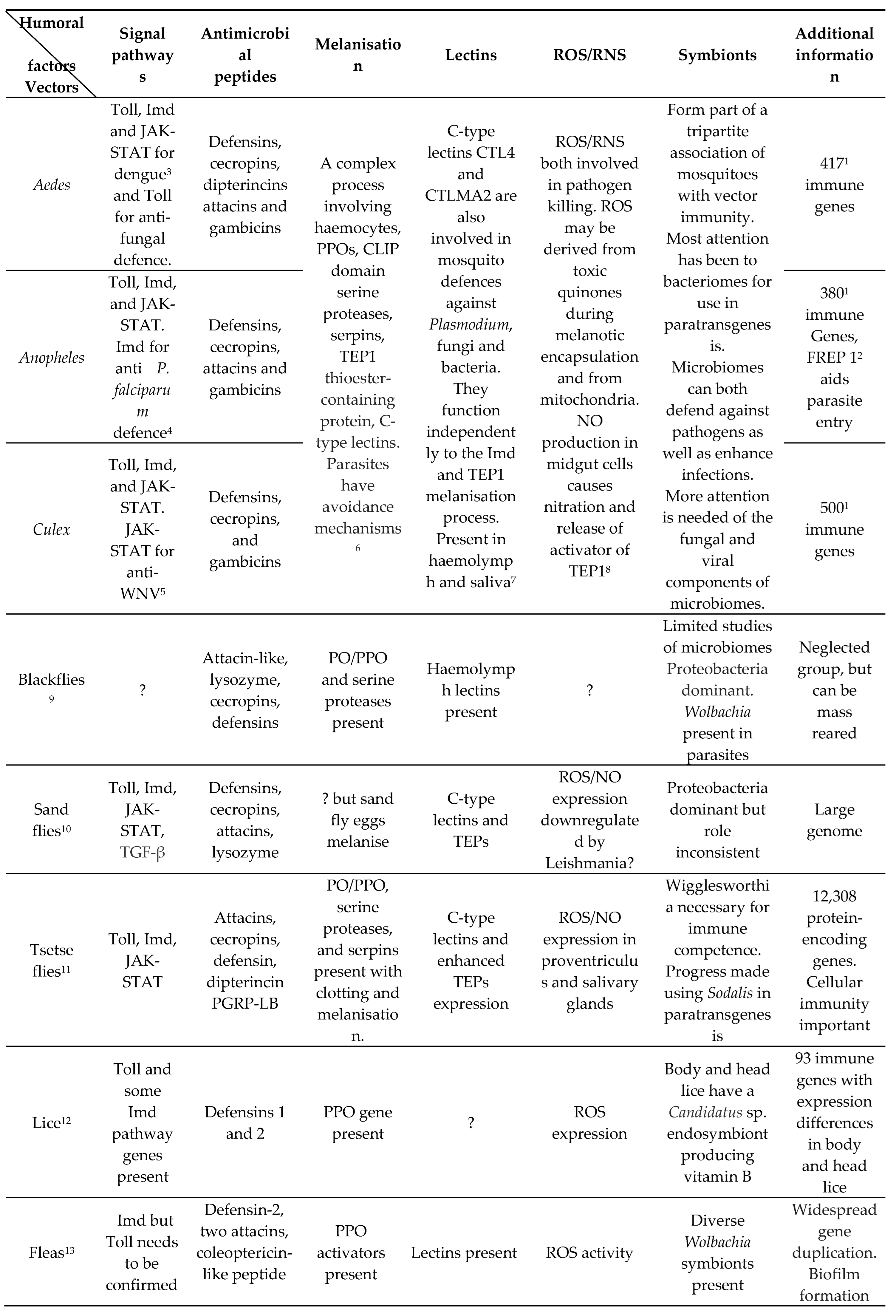 
|
5.1. Mosquitoes (Table 1 and Table 3)
- i.
- upregulation of immune effector genes to antimicrobial peptides (AMPs) via signalling pathways
- ii.
- the vector PpO system, melanisation and serine proteases
- iii.
- cytotoxic/stimulatory lectin molecules
- iv.
- nitric oxide and ROS killing of parasites
- v.
- specific peptides stimulating parasite differentiation
- vi.
- glycoprotein receptors on the surface of the midgut for parasite attachment
- vii.
- role of bacterial symbionts
5.2. Blackflies (Table 1 and Table 3).
5.3. Sandflies (Table 1 and Table 3).
5.4. Tsetse flies (Table 1 and Table 3)
5.5. Lice (Table 1 and Table 3)
5.6. Fleas (Table 1 and Table 3)
5.7. Triatomines (Table 1 and Table 3).
6. Recognition, Signalling, and Priming in Vector Innate Immunity
6.1. Pathogen Recognition
6.2. Pattern Recognition Receptors (PPRs).
6.2.1. Peptidoglycan Recognition Proteins (PGRPs)
6.2.2. Immunolectins
6.2.3. Thioester-Containing Proteins (TEPs)
6.3. Signal Transduction Pathways
6.3.1. The Toll Signalling Pathway
6.3.2. The Imd Signalling Pathway
6.3.3. The JAK/STAT Pathway
6.3.4. JNK Signalling
6.3.5. Eicosanoid Signalling
6.3.6. Antiviral Immunity
6.3.7. Immune Priming.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patramool, S.; Choumet, V.; Surasombatpattana, P.; Sabatier, L.; Thomas, F.; Thongrungkiat, S.; Rabilloud, T.; Boulanger, N.; Biron, D.G.; Missé, D. Update on the proteomics of major arthropod vectors of human and animal pathogens. Proteomics 2012, 12, 3510–3523. [Google Scholar] [CrossRef]
- Soumana, I.H.; Simo, G.; Njiokou, F.; B. ; Abd-Alla, A.M.; Cuny, G.; Geiger, A. The bacterial flora of tsetse fly midgut and its effect on trypanosome transmission. J. Invertebr. Pathol. 2013, 112. Suppl, S89-93. [Google Scholar]
- Gulley, M.M.; Zhang, X.; Michel. K. The roles of serpins in mosquito immunology and physiology. J. Insect Physiol. 2013, 59, 138–147. [Google Scholar] [CrossRef]
- Flores-Villegas, A.L.; Salazar-Schettino, P.M.; Córdoba-Aguilar, A.; Gutiérrez-Cabrera, A.E.; Rojas-Wastavino, G.E.; Bucio-Torres, M.I. , Cabrera-Bravo, M. Immune defence mechanisms of triatomines against bacteria, viruses, fungi and parasites. Bull. Entomol. Res. 2015, 105, 523-532. 105.
- Garcia, G.R.; Maruyama, S.R.; Malardo, T.; Zangirolamo, A.F.; Gardinassi, L.G. The biology of hematophagous arthropods addressed by molecular high-throughput approaches. Austin J. Trop. Med. & Hyg. 2015; 1, 1004. [Google Scholar]
- Saraiva, R.G.; Kang, S.; Simões, M.L.; Angleró-Rodríguez, Y.I.; Dimopoulos, G. Mosquito gut antiparasitic and antiviral immunity. Dev. Comp. Immunol. 2016, 64, 53–64. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, M. Peptidoglycan recognition proteins in hematophagous arthropods. Dev. Comp. Immunol. 2018, 83, 89–95. [Google Scholar] [CrossRef]
- Telleria, E.L.; Martins-da-Silva, A.; Tempone, A.J.; Traub-Csekö, Y.M.M. Leishmania, microbiota and sand fly immunity. Parasitology 2018, 145, 1336–1353. [Google Scholar] [CrossRef]
- Brown, L.D. Immunity of fleas (Order Siphonaptera). Dev. Comp. Immunol. 2019, 98, 76–79. [Google Scholar] [CrossRef]
- Koenraadt, C.J.M. Infectious Diseases and Arthropods. The Lancet, Infectious Diseases 2019, 19, 1298. [Google Scholar] [CrossRef]
- Guarneri, A.A. , Schaub, G.A. Interaction of Triatomines with Their Bacterial Microbiota and Trypanosomes. In: Guarneri, A., Lorenzo, M. eds; Triatominae - The Biology of Chagas Disease Vectors. Entomology in Focus, vol 5. Springer, Cham. 2019, pp. 345-386.
- Matetovici, I.; De Vooght, L.; Van Den Abbeele, J. Innate immunity in the tsetse fly (Glossina), vector of African trypanosomes. Dev. Comp. Immunol. 2019, 98, 181–188. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Lowenberger, C. The Immune System of Triatomines. In: Guarneri, A., Lorenzo, M. eds; Triatominae - The Biology of Chagas Disease Vectors. Entomology in Focus, vol 5. Springer, Cham, 2019; pp.307-344.
- Tawidian, P.; Rhodes, V.L.; Michel, K. Mosquito-fungus interactions and antifungal immunity. Insect Biochem. Mol. Biol. 2019, 111, 103182. [Google Scholar] [CrossRef]
- King, J.G. Developmental and comparative perspectives on mosquito immunity. Dev. Comp. Immunol. 2020, 103, 103458. [Google Scholar] [CrossRef]
- Raddi, G. , Barletta, A.B.F., Efremova, M., Ramirez, J.L., Cantera, R., Teichmann, S.A., et al.; Mosquito cellular immunity at single-cell resolution. Science 2020, 369, 1128–1132. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated immunity in insects: Cells, processes and associated components in the fight against pathogens and parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Gabrieli, P.; Caccia, S.; Varotto-Boccazzi, I.; Arnoldi, I.; Barbieri, G.; Comandatore, F.; Epis, S. Mosquito trilogy: microbiota, immunity and pathogens, and their implications for the control of disease transmission. Front. Microbiol. 2021, 12, 630438. [Google Scholar] [CrossRef]
- Rosendo Machado, S.; van der Most, T.; Miesen, P. Genetic determinants of antiviral immunity in dipteran insects - compiling the experimental evidence. Dev. Comp. Immunol. 2021, 119, 104010. [Google Scholar] [CrossRef]
- Bahia, A.C.; Barletta, A.B.F.; Lopes, A.H.; De Niz, M. Editorial: Parasite interactions with insect hosts in tropical diseases. Front. Trop. Dis. 2022, 3, 992277. [Google Scholar] [CrossRef]
- Cardoso, M.A.; Brito, T.F.; Brito, I.A.dA.; Berni, M.A.; Coelho, V.L.; Pane, A. The neglected virome of triatomine Insects. Front. Trop. Dis. 2022, 3, 828712. [Google Scholar] [CrossRef]
- Cardoso-Jaime, V.; Tikhe, C.V.; Dong, S.; Dimopoulos, G. The role of mosquito hemocytes in viral infections. Viruses 2022, 14, 2088. [Google Scholar] [CrossRef]
- Cecílio, P. , Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef]
- Ouali, R.; Vieira, L.R.; Salmon, D.; Bousbata, S. Rhodnius prolixus hemolymph immuno-physiology: deciphering the systemic immune response triggered by Trypanosoma cruzi establishment in the vector using quantitative proteomics. Cells, 2022; 11, 1449. [Google Scholar]
- Ramirez, J.L.; Hampton, K.J.; Rosales, A.M.; Muturi, E.J. Multiple mosquito AMPs are needed to potentiate their antifungal effect against entomopathogenic fungi. Front. Microbiol. 2022, 13, 1062383. [Google Scholar] [CrossRef]
- Ratcliffe, N.A.; Furtado Pacheco, J.P. ; Dyson, P,; Castro, H.C.; Gonzalez, M.S.; Azambuja, P.; Mello.; C.B. Overview of paratransgenesis as a strategy to control pathogen transmission by insect vectors. Parasit. Vectors, 2022; 15, 112. [Google Scholar]
- Mwangi, V.I.; Martinez, E.G.; Leda, R.L.; Catunda, M.E.S.L.A.; Dias, A.S.; Padron Antonio, Y.; Guerra, M.D.G.V.B. Resisting an invasion: A review of the triatomine vector (Kissing bug) defense strategies against a Trypanosoma sp infection. Acta Trop. 2023, 238, 106745. [Google Scholar] [CrossRef]
- Parres-Mercader, M.; Pance, A.; Gomez-Dıaz, E. Novel systems to study vector-pathogen interactions in malaria. Front. Cell. Infect. Microbiol. 2023, 13, 1146030. [Google Scholar] [CrossRef]
- Vinayagam, S.; Rajendran, D.; Sekar, K.; Renu, K.; Sattu, K. The microbiota, the malarial parasite, and the mosquito [MMM] - A three-sided relationship. Mol. Biochem. Parasitol. 2023, 253, 111543. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L.; Aksoy, S. Microbiota in disease-transmitting vectors. Nat. Rev. Microbiol. 2023, 21, 604–618. [Google Scholar] [CrossRef]
- Zheng, R. , Wang, Q., Wu, R.; Paradkar, P.N.; Hoffmann, A.A.; Wang G-H. Holobiont perspectives on tripartite interactions among microbiota, mosquitoes, and pathogens. ISME J. 2023; 17, 1143–1152. [Google Scholar]
- Wilkerson, R.C.; Linton, Y.-M.; Strickman, D. Mosquitoes of the World; Johns Hopkins University Press, Baltimore; 2021, 1-2, 1332 pages; ISBN 978-1-421438-14-6.
- Brühl, C.A.; Després, L.; Frör, O. ; Patil. C.D.; Poulin, B.; Tetreau, G.; Allgeier S. Environmental and socioeconomic effects of mosquito control in Europe using the biocide Bacillus thuringiensis subsp. israelensis (Bti). Science of the Total Environment, 2020; 724, 137800. [Google Scholar]
- Lahondère, C.; Vinauger, C.; Okubo, R.P.; Wolff, G.H.; Chan, J.K.; Akbari, O.S.; Riffell, J.A. The olfactory basis of orchid pollination by mosquitoes. Proc. Natl Acad. Sci. USA. 2020, 117, 708–716. [Google Scholar] [CrossRef]
- Agten, S. M, Watson, E.E.; Ripoll-Rozada, J.; Dowman, L.J.; Wu, M.C.L.; Alwis, I.; Jackson, S.P.; Pereira, P.J.B.; Payne, R.J. Potent trivalent inhibitors of thrombin through hybridization of salivary sulfopeptides from hematophagous arthropods. Angew Chem. Int. Ed. Engl. 2021, 60, 5348–5356. [Google Scholar]
- Bellekom, B.; Hackett, T.D.; Lewis, O.T. A network perspective on the vectoring of human disease. Trends Parasitol. 2021, 37, 391–400. [Google Scholar] [CrossRef]
- Stork, N.E. How many species of insects and other terrestrial arthropods are there on Earth? Annu. Rev. Entomol. 2018, 63, 31–45. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Vector Borne Diseases. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases. Accessed 1st June, 2023.
- Shaw, W.R.; Catteruccia, F. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat. Microbiol. 2019, 4, 20–34. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Ethics and vector-borne diseases: WHO guidance. 2020. https://apps.who.int/iris/handle/10665/336075. Accessed 1st June 2023.
- U.S. Center for Disease Control and Prevention (CDC). Malaria‘s impact worldwide. 25 Feb 2020. https://www.cdc.gov›malaria›impact. Accessed 1st June 2023.
- World Health Organization (WHO). Lymphatic filariasis. 2023. https://www.who.int/news-room/fact-sheets/detail/lymphatic-filariasis. Accessed 2nd June 2023.
- Frallonardo, L.; Di Gennaro, F.; Panico, G.G.; Novara, R.; Pellara, E.; Cotugno, S. , Guido, G.; De Vita, E.; Ricciardi A.; Totaro, V.; et al. Onchocerciasis: current knowledge and future goals. Front. Trop. Dis, 2022, 3, 986884. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Onchocerciasis. 2022. https://www.who.int/news-room/fact-sheets/detail/onchocerciasis. Accessed 4th June 2023.
- Bern, C. Chagas’ disease. N. Engl. J. Med. 2015, 373, 456–466. [Google Scholar] [CrossRef]
- Howard, J. Plague (Black Death) bacterial infection information and facts. https://www.nationalgeographic.com/science/article/the-plague. 2020.
- Demeure, C.E.; Dussurget, O.; Mas Fiol, G.; Le Guern, A.S.; Savin, C.; Pizarro-Cerda, J. Yersinia pestis and plague: an updated view on evolution, virulence determinants, immune subversion, vaccination and diagnostics . Genes Immun. 2019, 20, 357–370. [Google Scholar]
- Angelakis, E.; Bechah, Y.; Raoult, D. The History of Epidemic Typhus. Microbiol. Spectr. 2016 Aug;4(4). doi: 10.1128/microbiolspec.PoH-0010-2015. PMID: 27726780. [CrossRef] [PubMed]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: a European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Pan American Health Organization/World Health Organization (PAHO/WHO). Response to the epidemic of Zika virus in the Americas, December 2015–2016. https://www.paho.org/en/topics/zika. Accessed 5th December 2020.
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spaetzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Rock, F.; Hardiman, G.; Timans, J.; Kastelein, R.; Bazan, J. A family of human receptors structurally related to Drosophila Toll. Proc. Natl Acad. Sci. USA 1998, 95, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Baldwin, J.; Brar, D.; Salunke, D.B.; Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 2022, 70, 102172. [Google Scholar] [CrossRef]
- Van de Leemput, J. , Han, Z. Drosophila, a powerful model to study virus-host interactions and pathogenicity in the fight against SARS-CoV-2. Cell Biosci. 2021, 11, 110. [Google Scholar]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria mellonella: the versatile host for drug discovery, in vivo toxicity testing and characterising host-pathogen interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef]
- Lehane, M.J. The Biology of Blood-Sucking in Insects. Cambridge University Press: Second Ed. Cambridge, UK, 2009. ISBN: 9780521543958.
- Van Nouhuys, S.; Niemikapee, S.; Hanski, I. Variation in a host–parasitoid Interaction across independent populations. Insects 2012, 3, 1236–1256. [Google Scholar] [CrossRef]
- Black, J.L.; Clark, M.K.; Sword, G.A. Physiological and transcriptional immune responses of a non-model arthropod to infection with different entomopathogenic groups. PLoS ONE 2022, 17, e0263620. [Google Scholar] [CrossRef]
- Antonelli, P. , Duval, P., Luis, P.; Minard, G.; Moro, C.V. Reciprocal interactions between anthropogenic stressors and insect microbiota. Environ. Sci. Pollut. Res. 2022; 29, 64469–64488. [Google Scholar]
- Ferreira, A.B.B.; Bahia, A.C.; Pitaluga, A.N.; Barros, E.; dos Santos, D.G.; Bottino-Rojas, V.; Kubota, M.S.; de Oliveira, P.L.; Pimenta, P.F.P.; Traub-Csekö, Y.M.; Sorgine, M.H.F. Sexual dimorphism in immune responses and infection resistance in Aedes aegypti and other hematophagous insect vectors. Front. Trop. Dis. 2022, 3 Article –847109, 1–17. [Google Scholar]
- Booth, K.; Cambron, L.; Fisher, N.; Greenlee, K.J. Immune defense varies within an instar in the tobacco hornworm, Manduca sexta. Physiol. Biochem. Zool. 2015, 88, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Candian, V.; Tedeschi, R. Impact of the diet on the mortality and on gene expression of the antimicrobial peptide Tenecin 3 in Tenebrio molitor larvae infected by Beauveria bassiana. Insects 2023, 14, 359. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A. Evolutionary plasticity of insect immunity. J. Insect Physiol. 2013, 59, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Tafesh-Edwards, G.; Mohamed, A. Pathogen infection routes and host innate immunity: lessons from insects. Immunol. Lett. 2022, 247, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: the secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.H.G. , Contet, A., Krueger, K. Arthropod innate immune systems and vector-borne diseases. Biochemistry 2017, 56, 907–918. 56,.
- Dyer, N.A.; Rose, C.; Ejeh, N.O.; Acosta-Serrano, A. Flying tryps: survival and maturation of trypanosomes in tsetse flies. Trends Parasitol. 2013, 29, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Moure, U.A.E; Tan, T.; Sha, L.; Lu, X.; Shao, Z.; Yang, G.; Wang, Y.; Cui, H. Advances in the immune regulatory role of non-coding RNAs (miRNAs and lncRNAs) in insect-pathogen interactions. Front. Immunol. 2022, 13, 856457. [Google Scholar] [CrossRef] [PubMed]
- Rosetto, M.; Engstrom, Y.; Baldari, C.T.; Telford, J.L.; Hultmark, D. Signals from the IL-1 receptor homolog, Toll, can activate an immune response in a Drosophila hemocyte cell line. Biochem. Biophys. Res. Commun. 1995, 209, 111–116. [Google Scholar] [CrossRef]
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426(6962), 33–38. [Google Scholar] [CrossRef]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila innate immunity involves multiple signaling pathways and coordinated communication between different tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef]
- Collins, F.H.; Sakai, R.K.; Vernick, K.D.; Paskewitz, S.; Seeley, D.C.; Miller, L.H.; Collins, W.E.; Campbell, C.C.; Gwadz, R.W. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science 1986, 234(4776), 607–610. [Google Scholar] [CrossRef]
- Dimopoulos, G. , Richman, A., Muller, H.-M., Kafatos, F. C. Molecular immune responses of the mosquito Anopheles gambiae to bacteria and malaria parasites. Proc. Natl Acad. Sci. USA 1997, 94, 11508–11513. [Google Scholar] [CrossRef] [PubMed]
- Stower, H. Anti-mosquito immunity. Nat. Med. 2020, 26, 1009. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Dunn, P.E.; Dai, W.; Kanost, M.R.; Geng, C.X. Soluble peptidoglycan fragments stimulate antibacterial protein synthesis by fat body from larvae of Manduca sexta. Dev. Comp. Immunol. 1985, 9, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Wang, Y.; Dittmer, N.T.; Kanost, M.R.; Jiang, H. Serine protease networks mediate immune responses in extra-embryonic tissues of eggs in the tobacco hornworm, Manduca sexta. J. Innate Immun. 2022, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Verno, P.J.; Chadwick, J.S.; Aston, W.P.; Dunphy, G.B. The in vitro generation of an antibacterial activity from the fat body and hemolymph of non-immunized larvae of Galleria mellonella. Dev. Comp. Immunol. 1984, 8, 537–546. [Google Scholar] [CrossRef]
- Sheehan, G.; Margalit, A.; Sheehan, D.; Kavanagh, K. Proteomic profiling of bacterial and fungal induced immune priming in Galleria mellonella larvae. J. Insect Physiol. 2021, 131, 104213. [Google Scholar] [CrossRef]
- Kordaczuk, J.; Sułek, M.; Mak, P.; Zdybicka-Barabas, A.; Śmiałek, J.; Wojda, I. Cationic protein 8 plays multiple roles in Galleria mellonella immunity. Sci. Rep. 2022, 12, 11737. [Google Scholar] [CrossRef]
- Ashida, M.; Ohnishi, E. Activation of pre-phenol oxidase in hemolymph of the silkworm, Bombyx mori. Arch. Biochem. Biophys. 1967, 122, 411–416. [Google Scholar] [CrossRef]
- Wang, R.J.; Chen, K.; Xing, L.S.; Lin, Z.; Zou, Z.; Lu, Z. Reactive oxygen species and antimicrobial peptides are sequentially produced in silkworm midgut in response to bacterial infection. Dev. Comp. Immunol. 2020, 110, 103720. [Google Scholar] [CrossRef] [PubMed]
- Komano, H.; Mizuno, D.; Natori, S. Purification of lectin induced in the hemolymph of Sarcophaga peregrina larvae on injury. J Biol Chem. 1980. 255, 2919–29124. [CrossRef]
- Masova, A.; Sanda, M.; Jiracek, J.; Selicharova, I. Changes in the proteomes of the hemocytes and fat bodies of the flesh fly Sarcophaga bullata larvae after infection by Escherichia coli. Proteome Sci. 2010, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Freitas, C.C.; Garcia, E.S. Evidence and partial characterization of an inducible antibacterial factor in the haemolymph of Rhodnius prolixus. J. Insect Physiol. 1986, 32, 807–812. [Google Scholar] [CrossRef]
- Salcedo-Porras, N.; Oliveira, PL.; Guarneri, A.A.; Lowenberger, C. A fat body transcriptome analysis of the immune responses of Rhodnius prolixus to artificial infections with bacteria. Parasit. Vectors 2022, 15, 269. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C. Current concepts concerning insect hemocytes. American Zoologist, 1962; 2, 209–246. [Google Scholar]
- Price, C,D. ; Ratcliffe, N.A. A reappraisal of insect haemocyte classification by the examination of blood from fifteen insect orders. Z. Zellforsch. mikrosk. Anat. 1974, 147, 537–549.
- Ribeiro, C.; Brehélin, M. Insect haemocytes: what type of cell is that? J. Insect Physiol. 2006, 52, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.P.; Ratcliffe, N.A.; Renwrantz, L.R. The separation of insect haemocyte types on Percoll gradients: methodology and problems. J. Insect Physiol. 1986, 32, 167–177. [Google Scholar] [CrossRef]
- Anggraeni, T.; Ratcliffe, N.A. Studies on cell-cell co-operation during phagocytosis by purified haemocyte populations of the wax moth, Galleria mellonella. J. Insect Physiol. 1991, 37, 453–460. [Google Scholar] [CrossRef]
- Mullett, H.; Ratcliffe, N.A.; Rowley, A.F. Analysis of immune defences of the wax moth, Galleria mellonella, with anti-haemocytic monoclonal antibodies. J. Insect Physiol. 1993, 39, 897–902. [Google Scholar] [CrossRef]
- Tattikota, S.G.; Cho, B.; Liu, Y.; Hu, Y.; Barrera, V.; Steinbaugh, M.J.; Yoon, S.H.; Comjean, A.; Li, F.; Dervis, F.; et al. A single-cell survey of Drosophila blood. eLife 2020, 9, e54818. [Google Scholar] [CrossRef]
- Hultmark, D.; Andó, I. Hematopoietic plasticity mapped in Drosophila and other insects. Elife, 2022, 11, e78906. [Google Scholar] [CrossRef]
- Severo, M.S.; Landry, J.J.M.; Lindquist, R.L.; Goosmann, C.; Brinkmann, V.; Collier, P.; Hauser, A.E.; Benes, V.; Henriksson, J.; Teichmann, S.A.; Levashina, E.A. Unbiased classification of mosquito blood cells by single-cell genomics and high-content imaging. Proc. Natl Acad. Sci. USA. 2018, 115, E7568–E7577. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Mohammed, M.; Franzén, O.; Ankarklev, J.; Smith, R. Correction: Single-cell analysis of mosquito hemocytes identifies signatures of immune cell subtypes and cell differentiation. Elife 2022, 11, e85158. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, H.I.; John, A.B.; Ichwan, S.J.A.; Kamaruzzaaman, B.Y. Effect of prolonged captivity on the hemolymph profile of Tachypleus gigas using the various anticoagulant formulations. Aquac. Rep. 2021, 20, 1–9. [Google Scholar] [CrossRef]
- Hall, D.W. Mosquito hemocytes: a review. Dev. Comp. Immunol. 1983, 7, 1–12. [Google Scholar] [CrossRef]
- Castillo, J.C.; Robertson, A.E.; Strand, M.R. Characterization of hemocytes from the mosquitoes Anopheles gambiae and Aedes aegypti. Insect Biochem. Mol. Biol. 2006, 36, 891–903. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Strand, M.R. Mosquito hemocyte-mediated immune responses. Curr. Opin. Insect Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- 103. Silva, J.C.; Pessoa, F.A.C., Ríos-Velásquez, C.M., Araújo, H.R.C., Feitosa, A.P.S., Alves, L.C., Brayner, F.A., Eds.; Medeiros, J.F. 2015. Morphological characterization of hemocytes in Ectemnaspis rorotaense (Floch & Abonnenc) and Ectemnaspis trombetense (Hamada, Py-Daniel & Adler) (Diptera: Simuliidae). EntomoBrasilis, 2015; Volume 8, pp. 209–213. [Google Scholar]
- Luckhart, S.; Cupp, M.S.; Cupp, E.W. Morphological and functional classification of the hemocytes of adult female Simulium vittatum (Diptera: Simuliidae), J. Med. Entomol. 1992, 29, 457–466. [Google Scholar] [CrossRef]
- Cupp, M.S.; Chen, Y.; Cupp, E.W. Cellular hemolymph response of Simulium vittatum (Diptera: Simuliidae) to intrathoracic injection of Onchocerca lienalis (Filarioidea: Onchocercidae) microfilariae. J. Med. Entomol. 1997, 34, 56–63. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque, J.R.F. Identificação das Espécies de Flebotomíneos no Município de Timbaúba/pe e Caracterização dos Hemócitos de Lutzomyia migonei (França, 1920) (Diptera: Psychodidae) Vetor de Leishmania spp. 1918. Fundação Oswaldo Cruz Instituto Aggeu Magalhães, Mestrado Acadêmico em Biociências e Biotecnologia em Saúde, Recife, 68 pages.
- East, J.; Molyneux, D.H.; Hillen, N. Haemocytes of Glossina. Ann. Trop. Med. Parasitol. 1980, 74, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Kaaya, G.P.; Ratcliffe, N.A. Comparative study of hemocytes and associated cells of some medically important dipterans. J. Morphol. 1982, 173, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Coulaud, P.J.; Lepolard, C.; Bechah, Y.; Berenger, J.M.; Raoult, D.; Ghigo, E. Hemocytes from Pediculus humanus humanus are hosts for human bacterial pathogens. Front. Cell Infect. Microbiol. 2015, 4, 183. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, M.P.; Nadeina, V.P.; Chumakova, I.V. Kletki gemolimfy blokh i ikh fagotsitarnaia aktivnost' [Hemolymph cells of fleas and their phagocytic activity. Parazitologiia 1988, 22–321-8. [Google Scholar]
- Muñoz, M.; Lin, N.; Lin, R.; King, B.; Brown, L.D. Immune defense mechanisms against a systemic bacterial infection in the cat flea (Ctenocephalides felis). J. Invert. Pathol. 2022, 195, 107850. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Garcia, E.S.; Ratcliffe, N.A. Aspects of classification of Hemiptera hemocytes from six triatomine species. Mem.Inst. Oswaldo Cruz 1991, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moyetta, N.R.; Ramos, F.O.; Leyria, J.; Canavoso, L.E.; Fruttero, L.L. Morphological and ultrastructural characterization of hemocytes in an insect model, the hematophagous Dipetalogaster maxima (Hemiptera: Reduviidae). Insects 2021, 12, 640. [Google Scholar] [CrossRef]
- Agianian, B.; Lesch, C.; Loseva, O.; Dushay, M.S. Preliminary characterization of hemolymph coagulation in Anopheles gambiae larvae. Dev. Comp. Immunol. 2007, 31, 879–888. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, J.S.; Kang, J.S.; Kwon, D.H.; Yoon, K.S.; Strycharz, J.; Koh, Y.H.; Pittendrigh, B.R.; Clark, J.M.; Lee, S.H. Comparison of the humoral and cellular immune responses between body and head lice following bacterial challenge. Insect Biochem. Mol. Biol. 2011, 41, 332–339. [Google Scholar] [CrossRef]
- Dziedziech, A.; Shivankar, S.; Theopold, U. Drosophila melanogaster responses against entomopathogenic nematodes: focus on hemolymph clots. Insects 2020, 11, 62. [Google Scholar] [CrossRef]
- Arai, I.; Ohta, M.; Suzuki, A.; Tanaka, S.; Yoshizawa, Y.; Sato, R. Immunohistochemical analysis of the role of hemocytin in nodule formation in the larvae of the silkworm, Bombyx mori. J. Insect Sci. 2013, 13, 125. [Google Scholar] [CrossRef]
- Nazario-Toole, A.E. , Wu, L. P. Phagocytosis in insect immunity. Adv. Insect Physiol. 2017, 52, 35–82. [Google Scholar]
- Salcedo-Porras, N.; Noor, S.; Cai, C.; Oliveira, P.L.; Lowenberger, C. Rhodnius prolixus uses the peptidoglycan recognition receptor rpPGRP-LC/LA to detect Gram-negative bacteria and activate the IMD pathway. Curr. Res. Insect Sci. 2020; 13, 1:100006. [Google Scholar]
- Blandin, S.A.; Levashina, E.A. Phagocytosis in mosquito immune responses. Immunol. Rev. 2007, 219, 8–16. [Google Scholar] [CrossRef] [PubMed]
- League, G.P.; Hillyer, J.F. Functional integration of the circulatory, immune, and respiratory systems in mosquito larvae: pathogen killing in the hemocyte-rich tracheal tufts. BMC Biol. 2016, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Srivastava, P.; Sirisena, P.; Dubey, S.K.; Kumar, R.; Shrinet, J.; Sunil, S. Mosquito innate immunity. Insects 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Smith, R.C. Chemical depletion of phagocytic immune cells in Anopheles gambiae reveals dual roles of mosquito hemocytes in anti-Plasmodium immunity. Proc. Natl Acad. Sci. USA. 2019, 116, 14119–14128. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.H.J.F.; Ferreira, Á.G.A.; Imler, J.L.; Marques, J.T. Distinct roles of hemocytes at different stages of infection by dengue and Zika viruses in Aedes aegypti mosquitoes. Front. Immunol. 2021, 12, 660873. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Sigle, L.T.; Rinker, D.C.; Estévez-Lao, T.Y.; Capra, J.A.; Hillyer, J.F. The immune deficiency and c-Jun N-terminal kinase pathways drive the functional integration of the immune and circulatory systems of mosquitoes. Open Biol. 2022, 12, 220111. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F. Mosquito immunity. Adv. Exp. Med. Biol. 2010, 708, 218–238. [Google Scholar]
- Tinoco-Nunes, B.; Telleria, E.L.; da Silva-Neves, M.; Marques, C.; Azevedo-Brito, D.A.; Pitaluga, A.N.; Traub-Csekö. The sandfly Lutzomyia longipalpis LL5 embryonic cell line has active Toll and Imd pathways and shows immune responses to bacteria, yeast and Leishmania. Parasit Vectors 2016, 9, 222. [Google Scholar] [CrossRef]
- Perotti, M.A.; Allen, J.M.; Reed, D.L.; Braig, H.R. Host-symbiont interactions of the primary endosymbiont of human head and body lice. FASEB J. 2007, 21, 963–1284. [Google Scholar] [CrossRef]
- Previte, D.; Olds, B.P.; Yoon, K.; Sun, W.; Muir, W.; Paige, K.N.; Lee, S.H.; Clark, J.; Koehler, J.E.; Pittendrigh, B.R. Differential gene expression in laboratory strains of human head and body lice when challenged with Bartonella quintana, a pathogenic bacterium. Insect Mol. Biol. 2014, 23, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Russell, C.W.; Johnson, K.L.; Mortensen, R.D.; Erickson, D.L. Gene expression analysis of Xenopsylla cheopis (Siphonaptera: Pulicidae) suggests a role for reactive oxygen species in response to Yersinia pestis infection. J. Med. Entomol. 2012, 49, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, P.; Garcia, E.S. Trypanosoma rangeli interactions within the vector Rhodnius prolixus - A mini review. Mem. Inst. Oswaldo Cruz, 2005, 100, 567–572. [Google Scholar] [CrossRef]
- Weiss, B.L.; Maltz, M.; Aksoy, S. Obligate symbionts activate immune system development in the tsetse fly. J. Immunol. 2012, 188, 3395–3403. [Google Scholar] [CrossRef]
- Matetovici, I. , Van Den Abbeele, J. Thioester-containing proteins in the tsetse fly (Glossina) and their response to trypanosome infection. Insect Mol. Biol. 2018; 27, 414–428. [Google Scholar]
- Hao, Z.; Kasumba, I.; Lehane, M.J.; Gibson, W.C.; Kwon, J.; Aksoy, S. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc. Natl Acad; Sci. USA. 2001; 98, 12648–12653. [Google Scholar]
- Azambuja, P.; Garcia, E.S.; Waniek, P.J.; Vieira, C.S.; Figueiredo, M.B.; Gonzalez, M.S.; Mello, C.B.; Castro, D.P.; Ratcliffe, N.A. Rhodnius prolixus: from physiology by Wigglesworth to recent studies of immune system modulation by Trypanosoma cruzi and Trypanosoma rangeli. J. Insect Physiol. 2017, 97, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Porras, N,; Guarneri, A. ; Oliveira, P.L,.Lowenberger, C. Rhodnius prolixus: Identification of missing components of the IMD immune signaling pathway and functional characterization of its role in eliminating bacteria. PLoS One, 2019, 14, e0214794. [Google Scholar]
- Salcedo-Porras, N.; Lowenberger, C. The innate immune system of kissing bugs, vectors of chagas disease. Dev. Comp. Immunol. 2019, 98, 119–128. [Google Scholar] [CrossRef]
- Schaub, G.A. An update on the knowledge of parasite–vector interactions of Chagas Disease. Res. rep. trop. med. 2021, 12, 63–76. [Google Scholar] [CrossRef]
- Alejandro, A.D.; Lilia, J.P.; Jesús, M.B.; Henry, R.M. The IMD and Toll canonical immune pathways of Triatoma pallidipennis are preferentially activated by Gram-negative and Gram-positive bacteria, respectively, but cross-activation also occurs. Parasit. Vectors 2022, 15, 256. [Google Scholar] [CrossRef]
- Borges, A.; Santos, P.N.; Furtado, A.F.; Figueiredo, R.C. Phagocytosis of latex beads and bacteria by hemocytes of the triatomine bug Rhodnius prolixus (Hemiptera: Reduvidae). Micron 2008, 39, 486–494. [Google Scholar] [CrossRef]
- Dubovskiy, I.M.; Kryukova, N.A.; Glupov, V.V.; Ratcliffe, N.A. Encapsulation and nodulation in insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar]
- Sato, R. Mechanisms and roles of the first stage of nodule formation in lepidopteran insects. J. Insect Sci. 2023, 23, 3–1-14. [Google Scholar] [CrossRef]
- Ni, W.; Bao, J.; Mo, B.; Liu, L.; Li, T.; Pan, G.; Chen, J.; Zhou, Z. Hemocytin facilitates host immune responses against Nosema bombycis. Dev. Comp. Immunol. 2020, 103, 103495. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S-H. ; Whitten, M.M.A.; Zachary, D.; Hoffmann, J.A.; Levashina, E.A. Fz2 and Cdc42 mediate melanization and actin polymerization but are dispensable for Plasmodium killing in the mosquito midgut. PLoS Pathog. 2006, 2, e133. [Google Scholar]
- Camacho, E.; Dong, Y.; Anglero-Rodriguez, Y.; Smith, D.; Jacomini, R.S.; Dimopoulos, G.; Casadevall, A. Analysis of melanotic Plasmodium spp. capsules in mosquitoes reveal eumelanin-pheomelanin composition and identify AgMesh as a modulator of parasite infection. bioRxiv, 2021.05.07.443077.
- Edgerton, E.B.; McCrea, A.R.; Berry, C.T.; Kwok, J.Y.; Thompson, L.K.; Watson, B.; Povelones, M. Activation of mosquito immunity blocks the development of transmission-stage filarial nematodes. Proc. Natl Acad; Sci. USA. 2020, 117, 3711-3717.
- Liu, C.T.; Hou, R.F.; Chen, C.C. Formation of basement membrane-like structure terminates the cellular encapsulation of microfilariae in the haemocoel of Anopheles quadrimaculatus. Parasitology 1998, 116, 511–518. [Google Scholar] [CrossRef]
- Infanger, L.-C.; Rocheleau, T.A.; Bartholomay, L.C.; Johnson, J.K.; Fuchs, J.; Higgs, S.; Christensen, B.M. (2004). The role of phenylalanine hydroxylase in melanotic encapsulation of filarial worms in two species of mosquitoes. Insect Biochem. Mol. Biol. 2004, 34, 1329–1338. [Google Scholar] [PubMed]
- Binggeli, O.; Neyen, C.; Poidevin, M.; Lemaitre, B. Prophenoloxidase activation Is required for survival to microbial infections in Drosophila. PLoS Pathog. 2014, 10, e1004067. [Google Scholar] [CrossRef]
- Zou, Z.; Shin, S.W.; Alvarez, K.S.; Bian, G.; Kokoza, V.; Raikhel, A.S. Mosquito RUNX4 in the immune regulation of PPO gene expression and its effect on avian malaria parasite infection. Proc. Natl Acad; Sci. USA. 2008; 105, 18454–18459. [Google Scholar]
- Eleftherianos, I.; Heryanto, C. Transcriptomic insights into the insect immune response to nematode infection. Genes 2021, 12, 202. [Google Scholar] [CrossRef]
- Loghry, H.J.; Kwon, H.; Smith, R.C.; Sondjaja, N.A.; Minkler, S.J.; Young, S.; Wheeler, N.J.; Zamanian, M.; Bartholomay, L.C.; Kimber, M.J. ; Extracellular vesicles secreted by Brugia malayi microfilariae modulate the melanization pathway in the mosquito host. Sci. Rep. 2023, 13, 8778. [Google Scholar] [CrossRef]
- Castillo, J.C.; Reynolds, S.E.; Eleftherianos, I. Insect immune responses to nematode parasites. Trends Parasitol. 2011, 27, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Beerntsen, B.T.; Bartholomay, L.C.; Lowery, R.J. Penetration of the mosquito midgut is not required for Brugia pahangi microfilariae to avoid the melanotic encapsulation response of Armigeres subalbatus. Vet. Parasitol. 2007, 144, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.B. , Azambuja, P., Garcia, E.S., Ratcliffe, N.A. Differential in vitro and in vivo behavior of three strains of Trypanosoma cruzi in the gut and hemolymph of Rhodnius prolixus. Exp. Parasitol. 1996, 82, 112–121. [Google Scholar] [CrossRef]
- Mello, C.B. , Nigam, Y., Garcia, E.S., Azambuja, P., Newton, R.P., Ratcliffe, N.A. Studies on a haemolymph lectin isolated from Rhodnius prolixus and its interaction with Trypanosoma rangeli. Exp. Parasitol. 1999, 91, 289–296. [Google Scholar] [CrossRef]
- Araújo, C.A.C.; Pacheco, J.P.F.; Waniek, P.J.; Geraldo, R.B.; Sibajev, A.; Dos Santos, A.L.; Evangelho, V.G.O.; Dyson, P.J.; Azambuja, P.; Ratcliffe, N.A.; Castro, H.C.; Mello, C.B. A rhamnose-binding lectin from Rhodnius prolixus and the impact of its silencing on gut bacterial microbiota and Trypanosoma cruzi. Dev. Comp. Immunol, 2021; 114, 103823. [Google Scholar]
- Kaaya, G.P. , Ratcliffe, N.A., Alemu, P. Cellular and humoral defenses of Glossina (Diptera: Glossinidae): reactions against bacteria, trypanosomes, and experimental implants. J. Med. Entomol. 1986; 23, 30–43. [Google Scholar]
- Lee, W.S.; Webster, J.A.; Madzokere, E.T.; Stephenson, E.B.; Herrero, L.J. Mosquito antiviral defense mechanisms: a delicate balance between innate immunity and persistent viral infection. Parasites Vectors 2019, 12, 165. [Google Scholar] [CrossRef]
- Coste Grahl, M.V.; Perin, A.P.A.; Lopes, F.C.; Porto, B.N.; Uberti, A.F.; Canavoso, L.E.; Stanisçuaski, F.; Fruttero, L.L. The role of extracellular nucleic acids in the immune system modulation of Rhodnius prolixus (Hemiptera: Reduviidae). Pestic. Biochem. Physiol. 2020, 167, 104591. [Google Scholar] [CrossRef]
- Holt, R.A.; Subramanian, G.M.; Halpern, A.; Sutton, G.G.; Charlab, R.; Nusskern, D.R.; Wincker, P.; Clark, A.G.; Ribeiro, J.M.; Wides, R.; et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science 2002, 298(5591), 129–149. [Google Scholar] [CrossRef] [PubMed]
- Nene, V.; Wortman, J.R.; Lawson, D.; Haas, B.; Kodira, C.; Tu, Z.J.; Loftus, B.; Xi, Z.; Megy, K.; Grabherr, M.; et al. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 2007, 316(5832), 1718–1723. [Google Scholar] [CrossRef]
- Arensburger, P.; Megy, K.; Waterhouse, R.M.; Abrudan, J.; Amedeo, P.; Antelo, B.; Bartholomay, L.; Bidwell, S.; Caler, E.; Camara, F.; et al. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science, 2010; 330(6000), 86–88. [Google Scholar]
- Boissière, A. , Tchioffo, M.T., Bachar, D., Abate, L., Marie, A., Nsango, S.E., Shahbazkia, H.R.; Awono-Ambene, P.H.; Levashina, E.A.; Christen, R.; Morlais, I. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 2012, 8, e1002742. [Google Scholar]
- Bartholomay, L.C.; Waterhouse, R.M.; Mayhew, G.F.; Campbell, C.L.; Michel, K.; Zou, Z.; Ramirez, J.L.; Das, S.; Alvarez, K.; Peter Arensburge, P.; et al. Pathogenomics of Culex quinquefasciatus and meta-analysis of infection responses to diverse pathogens. Science 2010, 330(6000), 88–90. [Google Scholar] [CrossRef]
- Zhang, G.; Niu, G.; Franca, C.M.; Dong, Y.; Wang, X.; Butler, N.S.; Dimopoulos, G.; Li, J. Anopheles midgut FREP1 mediates Plasmodium invasion. J. Biol. Chem. 2015, 290, 16490–16501. [Google Scholar] [CrossRef]
- Erlandson, M.A.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J, Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef] [PubMed]
- Kato, N. , Mueller, C. R., Fuchs, J. F., McElroy, K., Wessely, V., Higgs, S., Christensen, B.M. Evaluation of the function of a type I peritrophic matrix as a physical barrier for midgut epithelium invasion by mosquito-borne pathogens in Aedes aegypti. Vector-Borne Zoonotic Dis. 2008; 8, 701–712. [Google Scholar]
- Bai, L. , Wang, L., Vega-Rodríguez, J., Wang, G., and Wang, S. A gut symbiotic bacterium Serratia marcescens renders mosquito resistance to Plasmodium infection through activation of mosquito immune responses. Front. Microbiol. 2019, 10, 1580. [Google Scholar] [CrossRef]
- Harsh, S.; Eleftherianos, I. Flavivirus infection and regulation of host immune and tissue homeostasis in insects. Front. Immunol. 2020, 11, 618801. [Google Scholar] [CrossRef]
- Rodgers, F.H.; Gendrin, M.; Christophides, G.K. Chapter 6 - the mosquito immune system and its interactions with the microbiota: implications for disease transmission. In: Wikel, S.K.; Aksoy, S.; Dimopoulos, G, editors. Arthropod Vector: Controller of Disease Transmission, Volume 1 [Internet]: Academic; 2017, pp. 101–22. [cited 2020 Oct 29]. Available from: http://www.sciencedirect.com/science/article/pii/B9780128053508000064.
- Lowenberger, C.A. Ferdig, M.T., Bulet, P., Khalili, S., Hoffmann, J.A., Christensen, B.M. Aedes aegypti-Induced antibacterial proteins reduce the establishment and development of Brugia malayi. Exp. Parasitol. 1996, 83, 191–201.
- Ratcliffe, N.A.; Whitten, M.M.A. Vector immunity in microbe–vector interactions. Microbe–Vector Interactions in Vector-borne Diseases (ed. by Gillespie, S.H.; Smith, G.L.; Osbourn, A.), 2004, pp. 199–262, SGM Symposium 63. Cambridge University Press, Cambridge, U.K.
- Sousa, G.L.; Bishnoi, R.; Baxter, R.H.G.; Povelones, M. The CLIP-domain serine protease CLIPC9 regulates melanization downstream of SPCLIP1, CLIPA8, and CLIPA28 in the malaria vector Anopheles gambiae. PLoS Pathog. 2020, 16, e1008985. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cheng, J.; Mu, X.; Kuang, X.; Li, Z.; Wu, J.A. C-type lectin in saliva of Aedes albopictus (Diptera: Culicidae) bind and agglutinate microorganisms with broad spectrum. J. Insect Sci. 2023, 23, 1. [Google Scholar] [CrossRef]
- Chen, C.; Durrant, H.J.; Newton, R.P.; Ratcliffe, N.A. A study of novel lectins and their involvement in the activation of the prophenoloxidase system in Blaberus discoidalis. Biochem J. 1995, 310, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.L.; Dong, Y.; Mlambo, G.; Dimopoulos, G. C-type lectin 4 regulates broad-spectrum melanization-based refractoriness to malaria parasites. PLoS Biol, 2022, 20, e3001515. [Google Scholar] [CrossRef]
- Bukhari, T.; Aimanianda, V.; Bischoff, E.; Brito-Fravallo, E.; Eiglmeier, K.; Riehle, M.M.; Vernick, K.D.; Mitri, C. Genetics and immunity of Anopheles response to the entomopathogenic fungus Metarhizium anisopliae overlap with immunity to Plasmodium. Sci. Rep. 2022, 12, 6315. [Google Scholar] [CrossRef] [PubMed]
- Molina-Cruz, A.; DeJong, R.J.; Charles, B.; Gupta, L.; Kumar, S.; Jaramillo-Gutierrez, G.; Barillas-Mury, C. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 2008, 283, 3217–3223. [Google Scholar] [CrossRef]
- Barillas-Mury, C. CLIP proteases and Plasmodium melanization in Anopheles gambiae. Trends Parasitol. 2007, 23, 297–299. [Google Scholar] [CrossRef]
- Gonçalves, R.L.; Oliveira, J.H.; Oliveira, G.A.; Andersen, J.F.; Oliveira, M.F.; Oliveira, P.L.; Barillas-Mury, C. Mitochondrial reactive oxygen species modulate mosquito susceptibility to Plasmodium infection. PLoS One 2012, 7, e41083. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, G. , Seeley, D., Wolf, A.; Kafatos, F.C. Malaria infection of the mosquito Anopheles gambiae activates immune-responsive genes during critical transition stages of the parasite life cycle. EMBO J. 1998, 17, 6115–6123. [Google Scholar] [CrossRef] [PubMed]
- Luckhart, S.; Vodovotz, Y.; Cui, L.; Rosenberg, R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc. Natl Acad. Sci. USA 1998, 95, 5700–5705. [Google Scholar] [CrossRef]
- 184. Oliveira, Gde. A.; Lieberman, J.; Barillas-Mury, C. Epithelial nitration by a peroxidase/NOX5 system mediates mosquito antiplasmodial immunity. Science, 2012; 335(6070), 856–859.
- Castillo, J.C.; Ferreira, A.B.B.; Trisnadi, N.; Barillas-Mury, C. Activation of mosquito complement antiplasmodial response requires cellular immunity. Sci. Immunol. 2017, 2, eaal1505. [Google Scholar] [CrossRef]
- Keleta, Y. , Ramelow, J., Cui, L.; Li, J. Molecular interactions between parasite and mosquito during midgut invasion as targets to block malaria transmission. npj Vaccines 2021, 6, 140. [Google Scholar] [CrossRef]
- McCoy, K.D. , Weldon, C.T., Ansumana, R.; Lamin, J.M.; Stenger, D.A.; Ryan, S.J.; Bardosh, K.; Jacobsen, K.H.; Dinglasan, R.R. Are malaria transmission-blocking vaccines acceptable to high burden communities? Results from a mixed methods study in Bo, Sierra Leone. Malar. J. 2021; 20, 183. [Google Scholar]
- Molina-Cruz, A.; Canepa, G.E.; Alves, E.; Silva, T.L.; Williams, A.E.; Nagyal, S.; Yenkoidiok-Douti, L.; Nagata, B.M.; Calvo, E.; Andersen, J.; Boulanger, M.J.; Barillas-Mury, C. Plasmodium falciparum evades immunity of anopheline mosquitoes by interacting with a Pfs47 midgut receptor. Proc. Natl Acad. Sci. USA. 2020, 117, 2597–2605. [Google Scholar] [CrossRef]
- Vogel, K.J.; Coon, K.L. Chapter Seven - Functions and Mechanisms of Symbionts of Insect Disease Vectors, Editor(s): Oliver, K.M.; Russell, J.A. Advances in Insect Physiology, Academic Press, 2020, 58, 233–275. [Google Scholar]
- Ramirez, J.L. , Souza-Neto, J., Torres Cosme, R., Rovira, J., Ortiz, A., Pascale J.M., Dimopoulos, G. Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl. Trop. Dis. 2012, 6, e1561. [Google Scholar] [CrossRef]
- Gómez, M.; Martinez, D.; Muñoz, M.; Ramírez, J.D. Aedes aegypti and Ae. albopictus microbiome/virome: new strategies for controlling arboviral transmission? Parasit. Vectors 2022, 15, 287. [Google Scholar] [CrossRef]
- Scolari, F.; Casiraghi, M.; Bonizzoni, M. Aedes spp. and their microbiota: a review. Front. Microbiol. 2019, 10, 2036. [Google Scholar] [CrossRef]
- Gendrin, M.; Christophides, G.K. The Anopheles Mosquito Microbiota and Their Impact on Pathogen Transmission. In: Manguin, S., editor. Anopheles mosquitoes-new insights into malaria vectors. London: IntechOpen; 2013. pp. 525–48.
- Guégan, M.; Zouache, K.; Démichel, C.; Minard, G.; Van, T.V.; Potier, P.; Mavingui, P.; Moro, C.V. The mosquito holobiont: fresh insight into mosquito–microbiota interactions. Microbiome 2018, 6, 49. [Google Scholar] [CrossRef]
- Nilsson, L.K.J.; de Oliveira, M.R.; Marinotti, O.; Rocha, E.M.; Håkansson, S.; Tadei, W.P.; de Souza, A.Q.L.; Terenius, O. Characterization of bacterial communities in breeding waters of Anopheles darlingi in Manaus in the Amazon Basin malaria endemic area. Microb. Ecol. 2019, 78, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Bongio, N.J.; Lampe, D.J. Inhibition of Plasmodium berghei development in mosquitoes by effector proteins secreted from Asaia sp. bacteria using a novel native secretion signal. PLoS ONE 2015, 10, e0143541. [Google Scholar] [CrossRef]
- Cappelli, A.; Damiani, C.; Mancini, M.V.; Valzano, M.; Rossi, P. ; Serrao, A, Ricci, I.; Favia, G. Asaia activates immune genes in mosquito eliciting an anti-Plasmodium response: implications in malaria control. Front. Genet, 2019; 10, 836. [Google Scholar]
- Rossi, P.; Ricci, I.; Cappelli, A.; Damiani, C.; Ulissi, U.; Mancini, M.V.; Capone, A.; Epis, S.; Crotti, E.; Chouaia, B.; et al. Mutual exclusion of Asaia and Wolbachia in the reproductive organs of mosquito vectors. Parasit. Vectors 2015, 8, 278. [Google Scholar] [CrossRef]
- Wang, S.; Jacobs-Lorena, M. Genetic approaches to interfere with malaria transmission by vector mosquitoes. Trends Biotechnol. 2013, 31, 185–193. [Google Scholar] [CrossRef]
- Adler, P.H. , McCreadie, J.W. Black Flies (Simuliidae). Med. Vet. Entomol. 2019, 237–259. https://api.semanticscholar.org/CorpusID:91385523.
- Chagas, A.C.; Calvo, E.; Pimenta, P.F.; Ribeiro, J.M. An insight into the sialome of Simulium guianense (DIPTERA: SIMUlIIDAE), the main vector of River Blindness Disease in Brazil. BMC Genomics 2011, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mu, L.; Zhuang, L.; Han, Y.; Liu, T.; Li, J.; Yang, Y.; Yang, H.; Wei, L. A cecropin-like antimicrobial peptide with anti-inflammatory activity from the black fly salivary glands. Parasit. Vectors 2015, 8, 561. [Google Scholar] [CrossRef]
- Ham, P.J.; Albuquerque, C.; Baxter, A.J.; Chalk, R.; Hagen, H.E. Approaches to vector control: new and trusted. 1. Humoral immune responses in blackfly and mosquito vectors of filariae. Trans. R. Soc. Trop. Med. Hyg. 1994, 88, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Kläger, S.L.; Watson, A.; Achukwi, D.; Hultmark, D.; Hagen, H.-E. Humoral immune response of Simulium damnosum s.l. following filarial and bacterial infections. Parasitology 2002, 125, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Ham, P.J. Vector immunity: new potential for disease control? Annales de la Societe Beige de Medecine Tropicale 1991, 71, 179–l87. [Google Scholar]
- Efon Ekangouo, A.; Nana Djeunga, H.C.; Sempere, G.; Kamgno, J.; Njiokou, F.; Moundipa Fewou, P.; Geiger, A. Bacteriome diversity of blackflies' gut and association with Onchocerca volvulus, the causative agent of onchocerciasis in Mbam Valley (Center Region, Cameroon). Pathogens 2021, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Leishmaniasis. 2023. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed 15 Nov 2023.
- Dillon, R.J.; Ivens, A.C.; Churcher, C.; Holroyd, N.; Quail, M.A.; Rogers, M.E.; Soares, M.B.; Bonaldo, M.F.; Casavant, T.L.; Lehane, M.J.; Bates, P.A. Analysis of ESTs from Lutzomyia longipalpis sand flies and their contribution toward understanding the insect-parasite relationship. Genomics 2006, 88, 831–840. [Google Scholar] [CrossRef]
- Boulanger, N.; Lowenberger, C.; Volf, P.; Ursic, R.; Sigutova, L.; Sabatier, L.; Svobodova, M.; Beverley, S.M.; Späth, G.; Reto Brun, R.; et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 7140–7146. [Google Scholar] [CrossRef] [PubMed]
- Coutinho-Abreu, I.V. , Serafim, T.D., Meneses, C.; Kamhawi, S.; Oliveira, F.; Valenzuela, J.G. Leishmania infection induces a limited differential gene expression in the sand fly midgut. BMC Genomics, 2020; 21, 608. [Google Scholar]
- Kykalová, B.; Tichá, L.; Volf, P.; Loza Telleria, E. Phlebotomus papatasi antimicrobial peptides in larvae and females and a gut-specific defensin upregulated by Leishmania major Infection. Microorganisms 2021, 9, 2307. [Google Scholar] [CrossRef]
- Omondi, Z.N.; Arserim, S.K.; Töz, S.; Özbel, Y. Host-parasite interactions: Regulation of Leishmania infection in sand fly. Acta Parasitol. 2022, 67, 606–618. [Google Scholar] [CrossRef]
- Campolina, T.B.; Villegas, L.E.M.; Monteiro, C.C.; Pimenta, P.F.P.; Secundino, N.F. C Tripartite interactions: Leishmania, microbiota and Lutzomyia longipalpis. PLoS Negl. Trop. Dis. 2020, 14, e0008666. [Google Scholar] [CrossRef]
- Dostálová, A. , Volf, P. Leishmania development in sand flies: parasite-vector interactions overview. Parasites Vectors, 2012; 5, 276. [Google Scholar]
- Wijerathna, T.0; Gunathunga, S.; Gunathilaka, H.N. Recent developments and future directions in the paratransgenesis based control of Leishmania transmission. Biol. Control 2020, 145, 104260. [Google Scholar] [CrossRef]
- Kelly, P.H.; Bahr, S.M.; Serafim, T.D.; Ajami, N.J.; Petrosino, J.F.; Meneses, C.; Kirby, J.R.; Valenzuela, J.G.; Kamhawi, S.; Wilson, M.E. The gut microbiome of the vector Lutzomyia longipalpis is essential for survival of Leishmania infantum. mBio, 2017; 38, e01121-16. [Google Scholar]
- Jeffries, C.L.; Rogers, M.E.; Walker, T. Establishment of a method for Lutzomyia longipalpis sand fly embryo microinjection: The first step towards potential novel control strategies for leishmaniasis. Wellcome Open Res. 2018, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.D.; Coutinho-Abreu, I.V.; Dey, R.; Kissinger, R.; Valenzuela, J.G.; Oliveira, F.; Kamhawi, S. Leishmaniasis: the act of transmission. Trends Parasitol. 2021, 37, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Aoki, V.; Abdeladhim, M.; Li, N.; Cecilio, P.; Prisayanh, P.; Diaz, L.A.; Valenzuela, J.G. Some good and some bad: Sand fly salivary proteins in the control of leishmaniasis and in autoimmunity. Front. Cell. Infect. Microbiol. 2022, 12, 839932. [Google Scholar] [CrossRef] [PubMed]
- Krafsur, E.S. Tsetse fies: genetics, evolution, and role as vectors. Infect. Genet. Evol. 2009, 9, 124–141. [Google Scholar] [CrossRef] [PubMed]
- International Glossina Genome Initiative. Genome sequence of the tsetse fly (Glossina morsitans): vector of African trypanosomiasis. Science. 2014 Apr 25; 344(6182): 380-386. PMID: 24763584; PMCID: PMC4077534. [CrossRef]
- Attardo, G.M. , Abd-Alla, A.M.M., Acosta-Serrano, A.; Allen, J.E.; Bateta, R.; Benoit, J.B.; Bourtzis, K.; Caers, J.; Caljon, G.; Christensen, M.B.; et al. Comparative genomic analysis of six Glossina genomes, vectors of African trypanosomes. Genome Biol. 2019, 20, 187. [Google Scholar] [CrossRef]
- Weiss, B.L.; Wang, J.; Aksoy, S. Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 2011, 9, e1000619. [Google Scholar] [CrossRef] [PubMed]
- Benoit, J.B.; Vigneron, A.; Broderick, N. A, Wu, Y.; Sun, J.S.; Carlson, J.R.; Aksoy, S.; Weiss, B.L. Symbiont-induced odorant binding proteins mediate insect host hematopoiesis. Elife 2017, 6, e19535. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Yang, G.; Aksoy, S. Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc. Natl Acad. Sci. USA. 2009, 106, 2133–2138. [Google Scholar] [CrossRef]
- Wang, J.; Aksoy, S. PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring. Proc. Natl Acad. Sci. USA. 2012, 109, 10552–10557. [Google Scholar] [CrossRef]
- Amanzougaghene, N. , Mediannikov, O., Ly, T.D.A.; Gautret, P.; Davoust, B.; Fenollar, F.; Izri, A. Molecular investigation and genetic diversity of Pediculus and Pthirus lice in France. Parasites Vectors 2020, 13, 177. [Google Scholar] [CrossRef]
- Kirkness, E.F.; Haas, B.J.; Sun, W.; Braig, H.R.; Perotti, M.; Clark, J.M.; Lee, S.H.; Robertson, H.M.; Kennedy, R.C.; Elhaik, E. et al. Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl Acad. Sci. USA. 2010, 107, 12168-12173. doi:10.1073/pnas.1003379107. Epub 2010 Jun 21. Erratum in: Proc Natl Acad Sci USA. 2011 Apr 12, 108(15), 6335. Krause, E. [corrected to Kraus, E. PMID: 20566863; PMCID: PMC2901460. [CrossRef] [PubMed]
- Amanzougaghene, N.; Fenollar, F.; Raoult, D.; Mediannikov, O. Where are we with human lice? A review of the current state of knowledge. Front. Cell, Infect. Microbiol, 2020; 9, 474. [Google Scholar]
- Kim, J.H. , Previte, D.J., Yoon, K.S., Murenzi, E., Koehler, J.E., Pittendrigh, B.R., Lee, S.H.; Clarke, J.M. Comparison of the proliferation and excretion of Bartonella quintana between body and head lice following oral challenge. Insect Mol. Biol. 2017, 26, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, C.N.; Burkhart, C.G. Bacterial symbiotes, their presence in head lice, and potential treatment avenues. J. Cutan. Med. Surg. 2006, 10, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.E. Resume of the Siphonaptera (Insecta) of the World. J. Med. Entomol. 1999, 35, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Bitam, I.; Dittmar, K.; Parola, P.; Whiting, M.F.; Raoult, D. Fleas and flea-borne diseases. Int. J. Infect. Dis. 2010, 14, e667–76. [Google Scholar] [CrossRef]
- Miarinjara, A.; Bland, D.M.; Belthoff, J.R.; Hinnebusch, B.J. Poor vector competence of the human flea, Pulex irritans, to transmit Yersinia pestis. Parasites Vectors 2021, 14, 317. [Google Scholar] [CrossRef]
- Danchenko, M.; Laukaitis, H.J.; Macaluso, K.R. Dynamic gene expression in salivary glands of the cat flea during Rickettsia felis infection. Pathog. Dis. 2021, 79, ftab020. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Verhoeve, V.I.; Gillespie, J.J.; Johnston, J.S.; Guillotte, M.L.; Rennoll-Bankert, K.E.; Rahman, S.; Hagen, D.; Elsik, C.G.; Macaluso, K.R.; Azad, A.F. A chromosome-level assembly of the cat flea genome uncovers rampant gene duplication and genome size plasticity. BMC Biology 2020, 18. [Google Scholar] [CrossRef]
- Bland, D.M.; Martens, C.A.; Virtaneva, K.; Kanakabandi, K.; Long, D.; Rosenke, R.; Saturday, G.A.; Hoyt, F.H.; Bruno, D.P.; Ribeiro, J.M.; et al. Transcriptomic profiling of the digestive tract of the rat flea, Xenopsylla cheopis, following blood feeding and infection with Yersinia pestis. PLoS Negl. Trop. Dis. 2020, 14, e0008688. [Google Scholar] [CrossRef]
- Vadyvaloo, V.; Viall, A.K.; Jarrett, C.O.; Hinz, A.K.; Sturdevant, D.E.; Joseph Hinnebusch, B. Role of the PhoP-PhoQ gene regulatory system in adaptation of Yersinia pestis to environmental stress in the flea digestive tract. Microbiology (Reading, England), 2015; 161, 1198–1210. [Google Scholar]
- Dreher-Lesnick, S.M.; Ceraul, S.M.; Lesnick, S.C.; Gillespie, J.J.; Anderson, J.M.; Jochim, R.C.; Valenzuela, J.G.; A. F. Azad, A.F. Analysis of Rickettsia typhi-infected and uninfected cat flea (Ctenocephalides felis) midgut cDNA libraries: deciphering molecular pathways involved in host response to R. typhi infection. Insect Mol. Biol. 2010, 19, 229–241. [Google Scholar] [CrossRef]
- Laukaitis, H.J.; Macaluso, K. Unpacking the intricacies of Rickettsia–vector interactions. Trends Parasitol. 2021, 37, 734–746. [Google Scholar] [CrossRef]
- Beliavskaia, A.; Tan, K-K. ; Sinha, A.; Husin, N.A.; Lim, F.S.; Loong, S.K.; Bell-Sakyi, L.; Carlow, C.K.S.; AbuBakar, S.; Darby, A.C.; Makepeace, B.L.; Khoo, J.J. Metagenomics of culture isolates and insect tissue illuminate the evolution of Wolbachia, Rickettsia and Bartonella symbionts in Ctenocephalides spp. Fleas. Microbial Genomics 2023, 9, 001045. [Google Scholar]
- Coura, J.R.; Viñas, P.A. Chagas Disease: A New Worldwide Challenge. Nature, 2010; 465(7301), S6–7. [Google Scholar] [CrossRef]
- Vieira, C.B.; Praça, Y.R.; Bentes, K.L.; da Santos Santiago, P.B.; Silva, S.M.M.; Silva, G.D.S.; Motta, F.N.; Bastos, I.M.D.; Santana, J.M.; de Araújo, C.N.; et al. Triatomines: trypanosomatids, bacteria, and viruses potential vectors? Front. Cell. Infect. Microbiol. 2018, 8, 405. [Google Scholar] [CrossRef]
- Dario, M.A.; Pavan, M.G.; Rodrigues, M.S.; Lisboa, C.V.; Kluyber, D.; Desbiez, A.L.J.; Herrera, H.M.; Roque, A.L.R.; Lima, L.; Teixeira, M.M.G.; Jansen, A.M. Trypanosoma rangeli genetics, mammalian hosts, and geographical diversity from five Brazilian biomes. Pathogens 2021, 10, 736. [Google Scholar] [CrossRef]
- Tobie, E.J. Observation on the development of Trypanosoma rangeli in the haemocoel of Rhodnius prolixus. J. Invertebr. Pathol, 1970, 15, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.C.P. , Schofield, C.J. Controle da transmissão transfusional da doença de Chagas na Iniciativa do Cone Sul. Rev. Soc. Bras. Med. Trop. 1998, 31, 373–383. [Google Scholar] [CrossRef]
- Wigglesworth, V.B. The Principles of Insect Physiology, (seventh ed.), Chapman and Hall, London (1974).
- Mesquita, R.D.; Vionette-Amaral, R.J.; Lowenberger, C.; Rivera-Pomar, R.; Monteiro, F.A.; Minx, P.; Spieth, J.; Carvalho, A.B.; Panzera, F.; Lawson, D. et al. Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc. Natl Acad. Sci. USA, 2015; 112(48), 14936–14941. [Google Scholar]
- Zumaya-Estrada, F.A.; Martínez-Barnetche, J.; Lavore, A.; Rivera-Pomar, R.; Rodríguez, M.H. Comparative genomics analysis of triatomines reveals common first line and inducible immunity-related genes and the absence of Imd canonical components among hemimetabolous arthropods. Parasit. Vectors 2018, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Stanley, D. Eicosanoid signaling in insect immunology: new genes and unresolved Issues. Genes (Basel) 2021, 12, 211. [Google Scholar] [CrossRef]
- Carmona-Peña, S.P.; Contreras-Garduño, J.; Castro, D.P.; Manjarrez, J.; Vázquez-Chagoyán, J.C. The innate immune response of triatomines against Trypanosoma cruzi and Trypanosoma rangeli with an unresolved question: Do triatomines have immune memory? Acta Trop. 2021, 224, 106108. [Google Scholar] [CrossRef]
- Eberhard, F.E. , Klimpel, S., Guarneri, A.A.; Tobias, N.J. Exposure to Trypanosoma parasites induces changes in the microbiome of the Chagas disease vector Rhodnius prolixus. Microbiome 2022, 10, 45. [Google Scholar] [CrossRef]
- Barbosa, H.J.; Quevedo, Y.S.; Torres, A. M, Veloza, G.A.G.; Martínez, J.C.C.; Urrea-Montes, D.A.; Robello-Porto, C.; Vallejo, G.A. Comparative proteomic analysis of the hemolymph and salivary glands of Rhodnius prolixus and R. colombiensis reveals candidates associated with differential lytic activity against Trypanosoma cruzi I and T. cruzi II. bioRxiv, 2023. [Google Scholar] [CrossRef]
- Whitten, M.M.A. , Mello, C.B., Gomes, S.A.O., Nigam, Y., Azambuja, P., Garcia, E.S., Ratcliffe, N.A. Role of superoxide and reactive nitrogen intermediates in Rhodnius prolixus (Reduviidae)/Trypanosoma rangeli interactions. Exp. Parasitol, 2001; 98, 44–57. [Google Scholar]
- Whitten, M.; Sun, F.; Tew, I.; Schaub, G.; Soukou, C.; Nappi, A.; Ratcliffe, N. Differential modulation of Rhodnius prolixus nitric oxide activities following challenge with Trypanosoma rangeli, T. cruzi and bacterial cell wall components. Insect Biochem. Mol. Biol. 2007, 37, 440–452. [Google Scholar] [CrossRef]
- Castro, D.P. , Moraes, C.S., Gonzalez, M.S., Ratcliffe, N.A., Azambuja, P., Garcia, E.S. Trypanosoma cruzi immune response modulation decreases microbiota in Rhodnius prolixus gut and is crucial for parasite survival and development. PLoS One 2012, 7, e36591. [Google Scholar]
- Batista, K.K.D.S.; Vieira, C.S.; Florentino, E.B.; Caruso, K.F.B.; Teixeira, P.T.P.; Moraes, C.D.S.; Genta, F.A.; Azambuja, P.; Castro, D.P. Nitric oxide effects on Rhodnius prolixus's immune responses, gut microbiota and Trypanosoma cruzi development. J. Insect Physiol. 2020, 126, 104100. [Google Scholar] [CrossRef]
- Gomes, S.A.; Feder, D.; Garcia, E.S.; Azambuja, P. Suppression of the prophenoloxidase system in Rhodnius prolixus orally infected with Trypanosoma rangeli. J. Insect Physiol. 2003, 49, 829–837. [Google Scholar] [CrossRef]
- Paranaiba, L.F.; Guarneri, A.A.; Torrecilhas, A.C.; Melo, M.N.; Soares, R.P. Extracellular vesicles isolated from Trypanosoma cruzi affect early parasite migration in the gut of Rhodnius prolixus but not in Triatoma infestans. Mem. Inst. Oswaldo Cruz, 2019; 114, e190217. [Google Scholar]
- Mantel, P.Y.; Marti, M. The role of extracellular vesicles in Plasmodium and other protozoan parasites. Cell. Microbiol. 2014, 16, 344–354. [Google Scholar] [CrossRef]
- Stanley, D.W. , Kim, Y. Eicosanoid signaling in insects: from discovery to plant protection. Crit. Rev. Plant Sci. 2014, 33, 20–63. [Google Scholar] [CrossRef]
- Stanley, D.; Kim, Y. Prostaglandins and other eicosanoids in insects: biosynthesis and biological actions. Front. Physiol. 2019, 9, 1927. [Google Scholar] [CrossRef]
- 263. . Barletta, A.B.F., Trisnadi, N., Ramirez, J.L., Barillas-Mury, C. Mosquito midgut prostaglandin release establishes systemic immune priming. iScience 2019, 19, 54–62. [Google Scholar] [CrossRef]
- Garcia, E.S.; Machado, E.M.; Azambuja, P. Effects of eicosanoid biosynthesis inhibitors on the prophenoloxidase-activating system and microaggregation reactions in the hemolymph of Rhodnius prolixus infected with Trypanosoma rangeli. J. Insect Physiol. 2004, 50, 157–165. [Google Scholar] [CrossRef]
- Tunctan, B.; Korkmaz, B.; Cuez, T.; Kemal Buharalioglu, C.; Sahan-Firat, S.; Falck, J.; Malik, K.U. Contribution of vasoactive eicosanoids and nitric oxide production to the effect of selective cyclooxygenase-2 inhibitor, NS-398, on endotoxin-induced hypotension in rats. Basic Clin, Pharmaco.l Toxicol. 2010, 107, 877–882. [Google Scholar] [CrossRef]
- Wang, P.H.; He, J.G. Nucleic acid sensing in invertebrate antiviral immunity. Int. Rev. Cell Mol. Biol. 2019, 345, 287–360. [Google Scholar]
- Zhang, W.; Tettamanti, G.; Bassal, T.; Heryanto, C.; Eleftherianos, I.; Mohamed, A. Regulators and signalling in insect antimicrobial innate immunity: Functional molecules and cellular pathways. Cell Signal. 2021, 83, 110003. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, J.; Feng, D.; Wang, X.; Zhang, R. Immune functions of pattern recognition receptors in Lepidoptera. Front. Immunol. 2023, 14, 1203061. [Google Scholar] [CrossRef]
- Michel, T. , Reichhart, J.M., Hoffmann, J.A.; Royet, J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature, 2001; 414, 756–759. [Google Scholar]
- Monahan, A.; Kleino, A.; Silverman, N. Readapting the role of PGRP-SD in bacterial sensing and Immune activation. Immunity 2016, 45, 951–953. [Google Scholar] [CrossRef]
- Iatsenko, I.; Kondo, S.; Mengin-Lecreulx, D.; Lemaitre, B. PGRP-SD, an extracellular pattern-recognition receptor, enhances peptidoglycan-mediated activation of the Drosophila Imd pathway. Immunity 2016, 45, 1013–1023. [Google Scholar] [CrossRef]
- Choe, K.M.; Werner, T.; Stöven, S.; Hultmark, D.; Anderson, K.V. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science 2002, 296(5566), 359–362. [Google Scholar] [CrossRef]
- Gottar, M.; Gobert, V.; Michel, T.; Belvin, M.; Duyk, G.; Hoffmann, J.A.; Ferrandon, D.; Royet, J. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature 2002, 416(6881), 640–644. [Google Scholar] [CrossRef]
- Rämet, M.; Manfruelli, P.; Pearson, A.; Mathey-Prevot, B.; Ezekowitz, R.A. Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 2002, 416(6881), 644–648. [Google Scholar] [CrossRef]
- Takehana, A.; Katsuyama, T. ; Yano T, Oshima, Y.; Takada, H.; Aigaki, T.; Kurata, S. Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc. Natl Acad. Sci. USA, 2002; 99, 13705–13710. [Google Scholar]
- Takehana, A.; Yano, T.; Mita, S.; Kotani A, Oshima Y, Kurata S. Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J. 2004, 23, 4690–4700. [Google Scholar] [CrossRef]
- Yano, T.; Mita, S.; Ohmori, H.; Oshima, Y.; Fujimoto, Y.; Ueda, R.; Takada, H.; Goldman, W.E.; Fukase, K.; Silverman, N.; Yoshimori, T.; Kurata, S. Autophagic control of listeria through intracellular innate immune recognition in Drosophila. Nat. Immunol. 2008, 9, 908–916. [Google Scholar] [CrossRef]
- Das, S.; Dong, Y.; Garver, L.; Dimopoulos, G. Specificity of the innate immune system: a closer look at the mosquito pattern-recognition receptor repertoire'. In Rolff J., Reynolds, S. (eds), Insect Infection and Immunity: Evolution, Ecology, and Mechanisms, 2009, 69-85 (Oxford University Press, 2009; online edn, Oxford Academic, 1 Sept. 2009, accessed 30 Dec. 2023. [CrossRef]
- Zhu, Y.; Yu, X.; Cheng, G. Insect C-type lectins in microbial infections. Adv. Exp. Med. Biol. 2020, 1204, 129–140. [Google Scholar]
- Dong, Y.; Manfredini, F.; Dimopoulos, G. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 2009, 5, e1000423. [Google Scholar] [CrossRef]
- Waterhouse, R.M.; Kriventseva, E.V.; Meister, S.; Xi, Z.; Alvarez, K.S.; Bartholomay, L.C.; Barillas-Mury, C.; Bian, G.; Blandin, S.; Christensen, B.M.; et al. Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 2007, 316(5832), 1738–1743. [Google Scholar] [CrossRef]
- Dong, Y.; Dimopoulos, G. Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J. Biol. Chem. 2009, 284, 9835–9844. [Google Scholar] [CrossRef]
- Blandin, S.; Levashina, E.A. Thioester-containing proteins and insect immunity. Mol. Immunol. 2004, 40, 903–908. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef]
- Dostálová, A.; Rommelaere, S.; Poidevin, M.; Lemaitre, B. Thioester-containing proteins regulate the Toll pathway and play a role in Drosophila defence against microbial pathogens and parasitoid wasps. BMC Biol. 2017, 15, 79. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Thioester-containing protein-4 regulates the Drosophila immune signaling and function against the pathogen Photorhabdus. J. Innate Immun. 2017, 9, 83–93. [Google Scholar] [CrossRef]
- Levashina, E.A.; Moita, L.F.; Blandin, S.; Vriend, G.; Lagueux, M.; Kafatos, F.C. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 2001, 104, 709–718. [Google Scholar] [CrossRef]
- Blandin, S.; Shiao, S.H.; Moita, L.F.; Janse, C.J.; Waters, A.P.; Kafatos, F.C.; Levashina, E.A. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 2004, 116, 661–670. [Google Scholar] [CrossRef]
- Yassine, H.; Kamareddine, L.; Osta, M.A. The mosquito melanization response Is implicated in defense against the entomopathogenic fungus Beauveria bassiana. PLoS Pathog. 2012, 8, e1003029. [Google Scholar] [CrossRef]
- Weng, S.C.; Li, H.H.; Li, J.C.; Liu, W.L.; Chen, C.H.; Shiao, S.H. A thioester-containing protein controls dengue virus infection in Aedes aegypti through modulating immune response. Front. Immunol. 2021, 12, 670122. [Google Scholar] [CrossRef]
- Imler, J.L.; Ferrandon, D.; Royet, J.; Reichhart, J.M.; Hetru, C.; Hoffmann, J.A. Toll-dependent and Toll-independent immune responses in Drosophila. J. Endotoxin Res. 2004, 10, 241–246. [Google Scholar] [CrossRef]
- Valanne, S.; Kallio, J.; Kleino, A.; Rämet, M. Large-scale RNAi screens add both clarity and complexity to Drosophila NF-κB signaling. Dev, Comp. Immunol, 2012; 37, 9–18. [Google Scholar]
- Parker, J.S.; Mizuguchi, K.; Gay, N.J. A family of proteins related to Spätzle, the toll receptor ligand, are encoded in the Drosophila genome. Proteins 2001, 45, 71–80. [Google Scholar] [CrossRef]
- Weber, A.N.; Tauszig-Delamasure, S.; Hoffmann, J.A.; Lelièvre, E.; Gascan, H.; Ray, K.P.; Morse, M.A.; Imler, J.L.; Gay, N.J. Binding of the Drosophila cytokine Spätzle to Toll is direct and establishes signaling. Nat. Immunol. 2003, 4, 794–800. [Google Scholar] [CrossRef]
- Sun, H.; Bristow, B.N.; Qu, G.; Wasserman, S.A. A heterotrimeric death domain complex in Toll signaling. Proc. Natl Acad. Sci. USA 2002, 99, 12871–12876. [Google Scholar] [CrossRef]
- Wu, L.P.; Anderson, K.V. Regulated nuclear import of Rel proteins in the Drosophila immune response. Nature 1998, 392(6671), 93–97. [Google Scholar] [CrossRef]
- Frolet, C.; Thoma, M.; Blandin, S.; Hoffmann, J.A.; Levashina, E.A. Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 2006, 25, 677–685. [Google Scholar] [CrossRef]
- Georgel, P.; Naitza, S.; Kappler, C.; Ferrendon, D.; Zachary, D.; Swimmer, C.; Kopczynski, C.; Duyk, G.; Reichhart, J.-M.; Hoffmann, J.A.J.D.C. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev. Cell 2001, 1, 503–514. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Ertürk-Hasdemir, D.; Broemer, M.; Leulier, F.; Lane, W.S.; Paquette, N.; Hwang, D.; Kim, C.H.; Stöven, S.; Meier, P.; Silverman, N. Two roles for the Drosophila IKK complex in the activation of Relish and the induction of antimicrobial peptide genes. Proc. Natl Acad. Sci. USA. 2009, 106, 9779–9784. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Nascimento-Silva, M.C.L.; Talyuli, O.A.C.; Oliveira, J.H.; Pereira, L.O.; Oliveira, P.L.; Sorgine, M.H. Microbiota activates IMD pathway and limits Sindbis infection in Aedes aegypti. Parasites Vectors 2017, 10, 103. [Google Scholar] [CrossRef]
- Garver, L.S.; Bahia, A.C.; Das, S.; Souza-Neto, J.A. , Shiao, J.; Dong, Y.; Dimopoulos, G. Anopheles Imd pathway factors and effectors in infection intensity-dependent anti- Plasmodium action. PLoS Pathog, 2012; 8, e1002737. [Google Scholar]
- Telleria, E.L.; Sant'Anna, M.R.; Ortigão-Farias, J.R.; Pitaluga, A.N.; Dillon, V.M.; Bates, P.A.; Traub-Csekö, Y.M.; Dillon, R.J. Caspar-like gene depletion reduces Leishmania infection in sand fly host Lutzomyia longipalpis. J. Biol. Chem. 2012, 287, 12985–12993. [Google Scholar] [CrossRef]
- Vomáčková Kykalová, B.; Sassù, F.; Volf, P.; Telleria, E.L. RNAi-mediated gene silencing of Phlebotomus papatasi defensins favors Leishmania major infection. Front. Physiol. 2023, 14, 1182141. [Google Scholar] [CrossRef]
- Rennoll, S.A.; Rennoll-Bankert, K.E.; Guillotte, M.L.; Lehman, S.S.; Driscol, l T. P.; Beier-Sexton, M.; Rahman, M.S.; Gillespie, J.J.; Azad, A.F. The cat flea (Ctenocephalides felis) immune deficiency signaling pathway regulates Rickettsia typhi infection. Infect. Immun. 2017, 86, e00562–17. [Google Scholar]
- Rebeil, R.; Jarrett, C.O.; Driver, J.D.; Ernst, R.K.; Oyston, P.C.; Hinnebusch, B.J. Induction of the Yersinia pestis PhoP-PhoQ regulatory system in the flea and its role in producing a transmissible infection. J. Bacteriol. 2013, 195, 1920–1930. [Google Scholar] [CrossRef]
- Hu, C.; Aksoy, S. Innate immune responses regulate trypanosome parasite infection of the tsetse fly Glossina morsitans morsitans. Mol. Microbiol. 2006, 60, 1194–1204. [Google Scholar] [CrossRef]
- Batista, K.K.S.; Vieira, C.S.; Figueiredo, M.B.; Costa-Latgé, S.G.; Azambuja, P.; Genta, F.A.; Castro, D.P. Influence of Serratia marcescens and Rhodococcus rhodnii on the humoral immunity of Rhodnius prolixus. Int. J. Mol. Sci. 2021, 22, 10901. [Google Scholar] [CrossRef]
- Vieira, C.S.; Moreira, O.C.; Batista, K.K.S.; Ratcliffe, N.A.; Castro, D.P.; Azambuja, P. The NF-κB inhibitor, IMD-0354, affects immune gene expression, bacterial microbiota and Trypanosoma cruzi infection in Rhodnius prolixus midgut. Front. Physiol. 2018, 9, 1189. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cosentino-Gomes, D.; Vieira, L.P.; Rocco-Machado, N.; Gondim, K.C.; Meyer-Fernandes, J.R. Identification of uncoupling protein 4 from the blood-sucking insect Rhodnius prolixus and its possible role on protection against oxidative stress. Insect Biochem. Mol. Biol. 2014, 50, 24–33. [Google Scholar] [CrossRef]
- Yoon, K.A.; Lee, D.E.; Lee, S.H.; Kim, J.H. Exploring the potential role of defensins in differential vector competence of body and head lice for Bartonella quintana. Parasit. Vectors 2023, 16, 183. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Kageyama, D.; Yokoi, K.; Jouraku, A.; Tanaka, H.; Futahashi, R.; Fukatsu, T. Functional crosstalk across IMD and Toll pathways: insight into the evolution of incomplete immune cascades. Proc. R. Soc. B. 2019, 286, 20182207. [Google Scholar] [CrossRef]
- Gulia-Nuss, M. , Nuss, A., Meyer, J.; Sonenshine, D.E.; Roe, R.M.; Waterhouse, R.M.; Sattelle, D.B.; de la Fuente, J.; Ribeiro, J.M.; Megy, K.; et al. Genomic insights into the Ixodes scapularis tick vector of Lyme disease. Nat. Commun. 2016, 7, 10507. [Google Scholar] [CrossRef]
- Sidak-Lofti, L.C.; Rosche, K.L.; Pence, N.; Ujczo, J.K.; Hurtado, J.; Fisk, E.A.; Goodman, A.G.; Noh, S.M.; Peters, J.W.; Shaw, D.K. The unfolded protein response triggers the immune deficiency pathway in ticks. mBio. 2022, 13(6), e0292222. [Google Scholar]
- Cabral, S.; de Paula, A.; Samuels, R.; da Fonseca, R.; Gomes, S.; Silva, J.R.; Mury, F. Aedes aegypti (Diptera: Culicidae) immune responses with different feeding regimes following infection by the entomopathogenic fungus Metarhizium anisopliae. Insects 2020, 11, 95. [Google Scholar] [CrossRef]
- Millymaki, H.; Ramet, M. JAK/STAT pathway in Drosophila immunity. Scand. J. Immunol. 2014, 79, 377–385. [Google Scholar] [CrossRef]
- Brown, S. , Hu, N., & Hombria, J.C. Novel level of signalling control in the JAK/STAT pathway revealed by in situ visualisation of protein-protein interaction during Drosophila development. Development, 2003; 130, 3077–3084. [Google Scholar]
- Bina, S.; Zeidler, M. JAK/STAT Pathway signalling in Drosophila melanogaster. Landes Bioscience 2009. www.ncbi.nlm.nih.gov › books › NBK6034.
- Zeidler, M. , Bach, E.; Perrimon, N. The roles of the Drosophila JAK/STAT pathway. Oncogene, 2000; 19, 2598–2606. [Google Scholar]
- Gupta, L.; Molina-Cruz, A.; Kumar, S.; Rodrigues, J.; Dixit, R.; Zamora, R.E.; Barillas-Mury, C. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 2009, 5, 498–507. [Google Scholar] [CrossRef]
- Bahia, A.C.; Kubota, M.S.; Tempone, A.J.; Araujo, H.R.C.; Guedes, B.A.M.; Orfano, A.S.; Tadei, W.P.; Rıos-Velasquez, C.M.; Han, Y.S.; Nagila, F.C.; et al. The JAK-STAT pathway controls Plasmodium vivax load in early stages of Anopheles aquasalis infection. PLoS Negl. Trop. Dis. 2011, 5, e1317. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Sim, S.; Dimopoulos, G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc. Natl Acad. Sci. USA. 2009, 106, 17841–17846. [Google Scholar] [CrossRef]
- Telleria, E.L.; Azevedo-Brito, D.A.; Kykalová, B.; Tinoco-Nunes, B.; Pitaluga, A.N.; Volf, P. Traub-Csekö, Y.M. Leishmania infantum infection modulates the JAK-STAT pathway in Lutzomyia longipalpis LL5 embryonic cells and adult females, and affects parasite growth in the sand fly. Front. Trop. Dis, 2021. [Google Scholar] [CrossRef]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Genet. Dev. 2002, 12, 14–21. [Google Scholar] [CrossRef]
- Tafesh-Edwards, G. , Eleftherianos, I. JNK signaling in Drosophila immunity and homeostasis. Immunology Letters 2020, 226, 7–11. [Google Scholar] [CrossRef]
- Garver, L.S.; de Almeida, O.G.; Barillas-Mury, C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 2013, 9, e1003622. [Google Scholar] [CrossRef]
- Chowdhury, A.; Modahl, C.M.; Tan, S.T.; Wong, W.X.B; Missé, D.; Vial, T.; Kini, R.M.; Pompon, J.F. JNK pathway restricts DENV2, ZIKV and CHIKV infection by activating complement and apoptosis in mosquito salivary glands. PLoS Pathog. 2020, 16, e1008754. [Google Scholar] [CrossRef]
- Yajima, M.; Takada, M.; Takahashi, N.; Kikuchi, H.; Natori, S.; Oshima, Y.; Kurata, S. A newly established in vitro culture using transgenic Drosophila reveals functional coupling between the phospholipase A2-generated fatty acid cascade and lipopolysaccharide-dependent activation of the immune deficiency (imd) pathway in insect immunity. Biochem. J. 2003, 371 (Pt 1), 205–210. [Google Scholar] [CrossRef]
- Kwon, H.; Hall, D.R.; Smith, R.C. Prostaglandin E2 signaling mediates oenocytoid immune cell function and lysis, limiting bacteria and Plasmodium oocyst survival in Anopheles gambiae. Front. Immunol. 2021, 12, 680020. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Alves, E.S.T.L.; Talyuli, O.A.C.; Luna-Gomes, T.0; Sim, S.; Angleró-Rodríguez, Y.; Dimopoulos, G.; Bandeira-Melo, C.; Sorgine, C.M.H.F. Prostaglandins regulate humoral immune responses in Aedes aegypti. PLoS Negl.Trop Dis. 2020, 14, e0008706. [Google Scholar] [CrossRef]
- Figueiredo, M.B.; Genta, F.A.; Garcia, E.S.; Azambuja, P. Lipid mediators and vector infection: Trypanosoma rangeli inhibits Rhodnius prolixus hemocyte phagocytosis by modulation of phospholipase A2 and PAF-acetylhydrolase activities. J. Insect Physiol. 2008, 54, 1528–1537. [Google Scholar] [CrossRef]
- Mussabekova, A.; Daeffler, L.; Imler, J.L. Innate and intrinsic antiviral immunity in Drosophila. Cell. Mol. Life Sci. 2017, 74, 2039–2054. [Google Scholar] [CrossRef]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W. A novel system for the launch of Alphavirus RNA synthesis reveals a role for the Imd pathway in arthropod antiviral response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, G.; Wu, J. ;Xi, Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl Acad. Sci. USA, 2012; 109, E23-31. [Google Scholar]
- Behura, S.K.; Gomez-Machorro, C.; Harker, B.W.; DeBruyn, B.; Lovin, D.D.; Hemme, R.R.; Mori, A.; Romero-Severson, J.; Severson, D.W. Global cross-talk of genes of the mosquito Aedes aegypti in response to dengue virus infection. PLoS Negl. Trop. Dis. 2011, 5, e1385. [Google Scholar] [CrossRef] [PubMed]
- Angleró-Rodríguez, Y.I.; MacLeod, H.J.; Kang, S.; Carlson, J.S.; Jupatanakul, N.; Dimopoulos, G. Aedes aegypti molecular responses to Zika Virus: modulation of Infection by the Toll and Jak/Stat immune pathways and virus host factors. Front. Microbiol. 2017, 8, 2050. [Google Scholar] [CrossRef]
- Dong, S.; Dimopoulos, G. Aedes aegypti Argonaute 2 controls arbovirus infection and host mortality. Nat. Commun. 2023, 14, 5773. [Google Scholar] [CrossRef]
- Goto, A.; Okado, K.; Martins, N.; Cai, H.; Barbier, V.; Lamiable, O.; Troxler, L.; Santiago, E. ; Kuhn L, Paik, D.; et al. The kinase IKKβ regulates a STING- and NF-κB-dependent antiviral response pathway in Drosophila. Immunity, 2018; 49, 225-234, e4. [Google Scholar]
- Hartman, R.S.; Karp, R.D. Short-term immunologic memory in the allograft response of the American cockroach, Periplaneta americana. Transplantation 1989, 47, 920–922. [Google Scholar] [CrossRef]
- Faulhaber, L.M.; Karp, R.D. A diphasic immune response against bacteria in the American cockroach. Immunology 1992, 75, 378–381. [Google Scholar]
- Lanz-Mendoza, H. , Garduño, J.C. Insect Innate Immune Memory. In: Cooper, E. (eds), 2018, Advances in Comparative Immunology. Springer, Cham. [CrossRef]
- Sheehan, G.; Farrell, G.; Kavanagh, K. Immune priming: the secret weapon of the insect world. Virulence 2020, 11, 238–246. [Google Scholar] [CrossRef]
- 243. Vilcinskas, A. Mechanisms of transgenerational immune priming in insects. Dev. Comp. Immunol. 2021, 124, 104205. [Google Scholar] [CrossRef]
- Prakash, A.; Khan, I. Why do insects evolve immune priming? A search for crossroads. Dev. Comp. Immunol. 2022, 126, 104246. [Google Scholar] [CrossRef]
- Gomes, F.M.; Silva, M.; Molina-Cruz, A.; Barillas-Mury, C. Molecular mechanisms of insect immune memory and pathogen transmission. PLoS Pathog. 2022, 18, e1010939. [Google Scholar] [CrossRef]
- Vargas, V.; Cime-Castillo, J.; Lanz-Mendoza, H. Immune priming with inactive dengue virus during the larval stage of Aedes aegypti protects against the infection in adult mosquitoes. Sci Rep. 2020, 10, 6723. [Google Scholar] [CrossRef]
- Cabrera, K.; Hoard, D.S.; Gibson, O.; Martinez, D.I.; Wunderlich, Z. Drosophila immune priming to Enterococcus faecalis relies on immune tolerance rather than resistance. PLOS Pathogens 2023, 19, e1011567. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





