Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Experimental
2.1. Catalyst synthesis
2.2. Physicochemical characterization
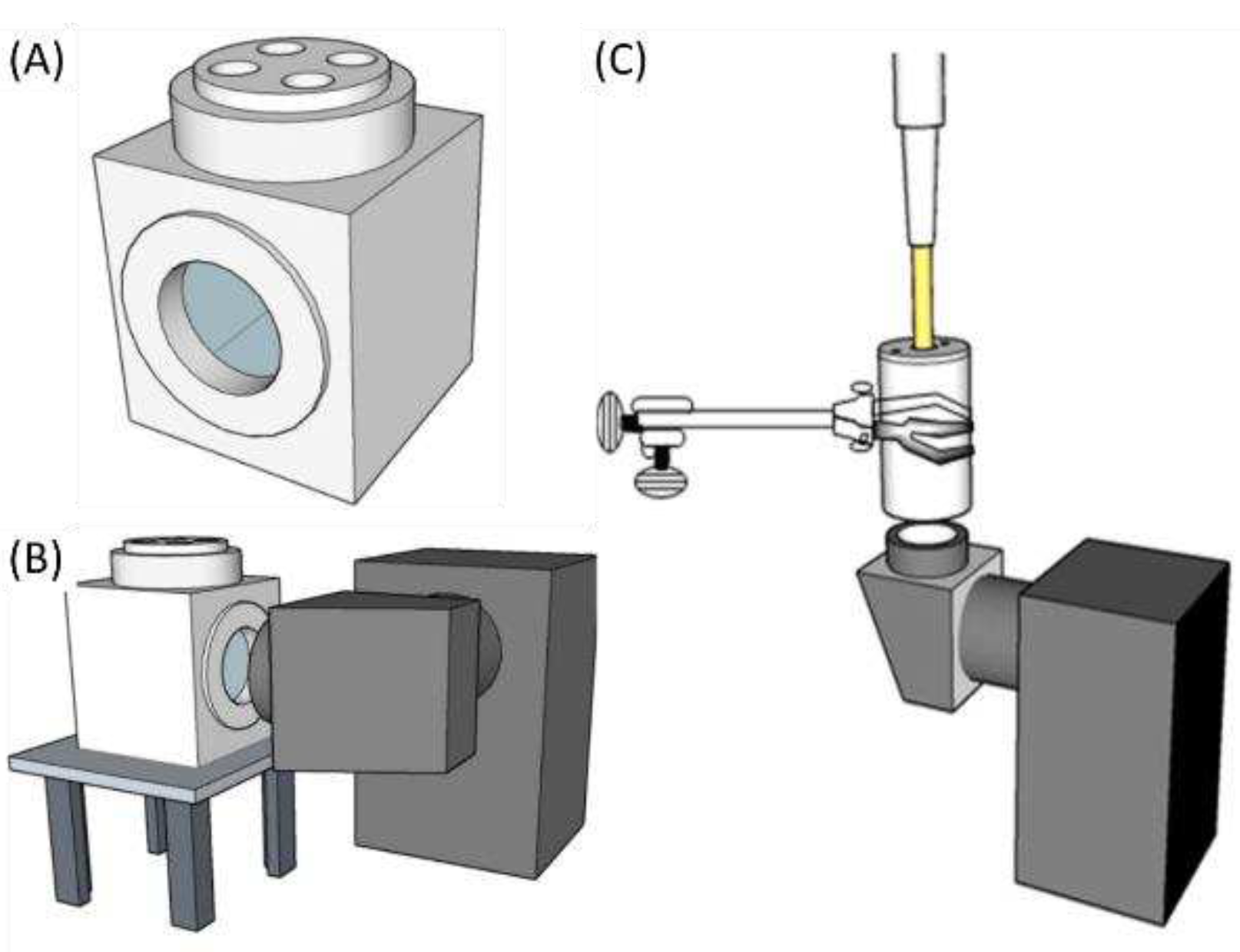
2.3. Photochemical Properties
2.4. Electrochemical characterization
2.5. Photoelectrochemical characterization
3. Results and discussion
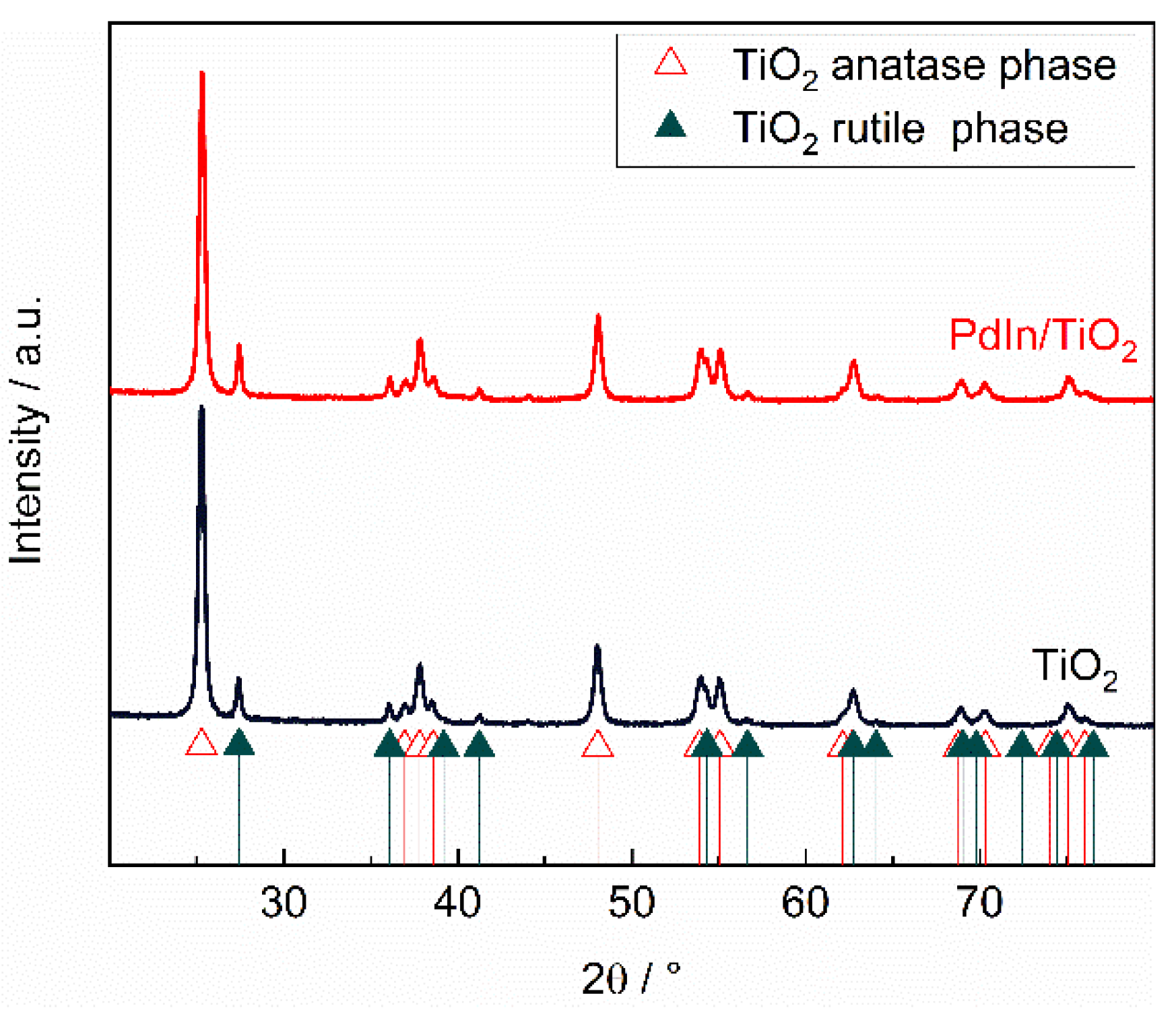
3.1. Characterization
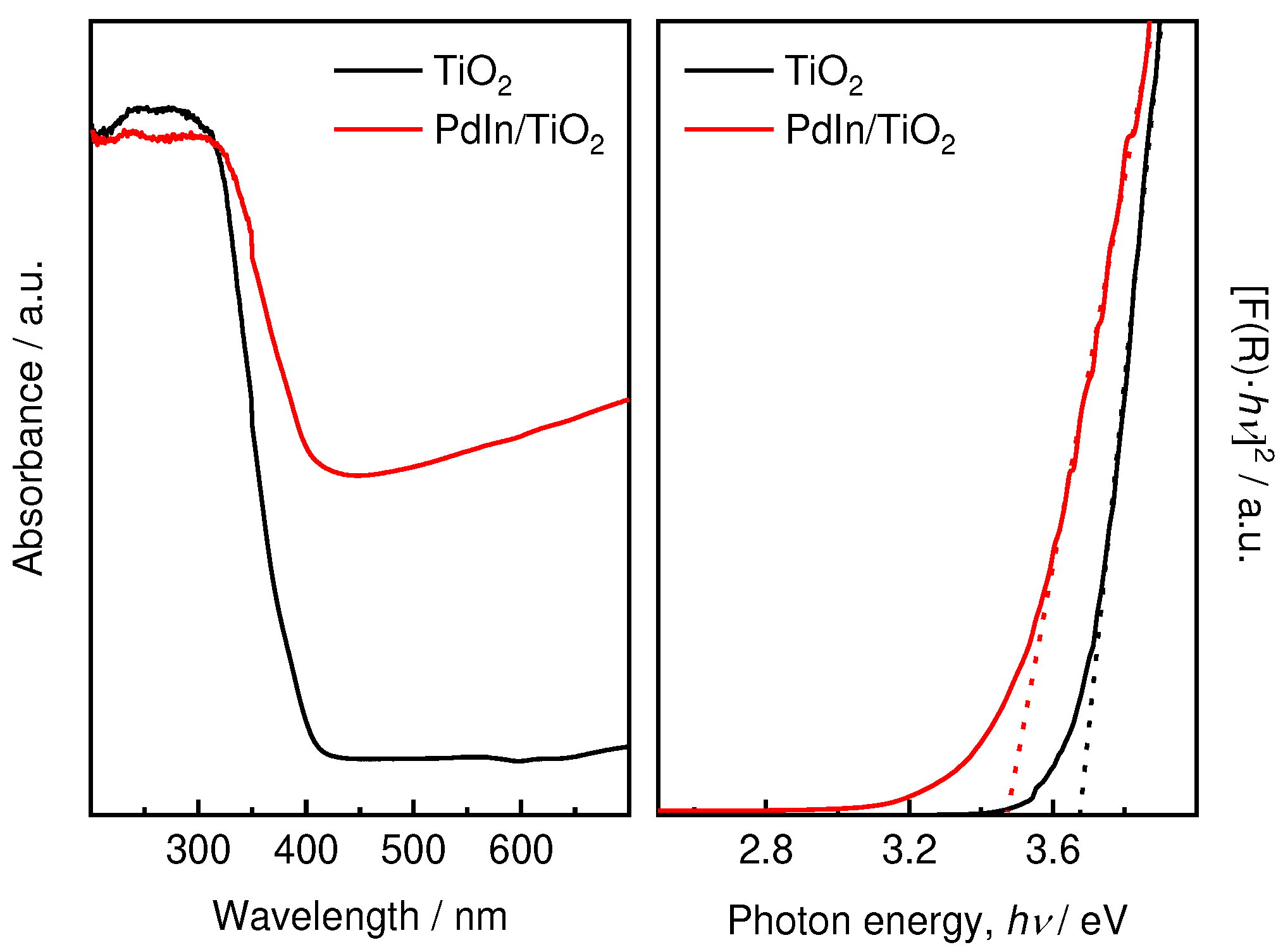
UV-DRS analysis
Physicochemical properties
3.2. Hydrogen evolution reaction (HER)
3.3. Paracetamol oxidation reaction
4. Conclusions
Supplementary Materials
CRediT authorship contribution statement
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feng, C.; Chen, Z.; Jing, J.; Sun, M.; Tian, J.; Lu, G.; Ma, L.; Li, X.; Hou, J. Significantly Enhanced Photocatalytic Hydrogen Production Performance of G-C3N4/CNTs/CdZnS with Carbon Nanotubes as the Electron Mediators. J Mater Sci Technol 2021, 80, 75–83. [Google Scholar] [CrossRef]
- Kim, D.; Yong, K. Boron Doping Induced Charge Transfer Switching of a C3N4/ZnO Photocatalyst from Z-Scheme to Type II to Enhance Photocatalytic Hydrogen Production. Appl Catal B 2021, 282. [Google Scholar] [CrossRef]
- Fernandez-Ibanez, P.; McMichael, S.; Rioja Cabanillas, A.; Alkharabsheh, S.; Tolosana Moranchel, A.; Byrne, J.A. New Trends on Photoelectrocatalysis (PEC): Nanomaterials, Wastewater Treatment and Hydrogen Generation. Curr Opin Chem Eng 2021, 34, 100725. [Google Scholar] [CrossRef]
- Truong, H.B.; Bae, S.; Cho, J.; Hur, J. Advances in Application of g–C3N4–Based Materials for Treatment of Polluted Water and Wastewater via Activation of Oxidants and Photoelectrocatalysis: A Comprehensive Review. Chemosphere 2022, 286, 131737. [Google Scholar] [CrossRef]
- Xie, L.; Wang, L.; Zhao, W.; Liu, S.; Huang, W.; Zhao, Q. WS2 Moiré Superlattices Derived from Mechanical Flexibility for Hydrogen Evolution Reaction. Nat Commun 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. zhu; Zhang, C.; Zeng, G. ming; Tan, X. fei; Huang, D. lian; Zhou, J. wu; Fang, Q. zhen; Yang, K. hua; Wang, H.; Wei, J.; et al. State-of-the-Art Progress in the Rational Design of Layered Double Hydroxide Based Photocatalysts for Photocatalytic and Photoelectrochemical H2/O2 Production. Coord Chem Rev 2021, 446. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Zhao, W.; Liu, S.; Zhao, Q. Oxygen-Facilitated Dynamic Active-Site Generation on Strained MoS2 during Photo-Catalytic Hydrogen Evolution. Chemical Engineering Journal 2021, 405. [Google Scholar] [CrossRef]
- Chen, Z.; Li, S.; Peng, Y.; Hu, C. Tailoring Aromatic Ring-Terminated Edges of g-C3N4nanosheets for Efficient Photocatalytic Hydrogen Evolution with Simultaneous Antibiotic Removal. Catal Sci Technol 2020, 10, 5470–5479. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Kumari, A.; Guo, C.; Naushad, M.; Vo, D.V.N.; Iqbal, J.; Stadler, F.J. Construction of Dual Z-Scheme g-C3N4/Bi4Ti3O12/Bi4O5I2 Heterojunction for Visible and Solar Powered Coupled Photocatalytic Antibiotic Degradation and Hydrogen Production: Boosting via I−/I3− and Bi3+/Bi5+ Redox Mediators. Appl Catal B 2021, 284. [Google Scholar] [CrossRef]
- Sharma, G.; Dionysiou, D.D.; Sharma, S.; Kumar, A.; Al-Muhtaseb, A.H.; Naushad, M.; Stadler, F.J. Highly Efficient Sr/Ce/Activated Carbon Bimetallic Nanocomposite for Photoinduced Degradation of Rhodamine B. Catal Today 2019, 335, 437–451. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic Wastewater Purification with Simultaneous Hydrogen Production Using MoS2 QD-Decorated Hierarchical Assembly of ZnIn2S4 on Reduced Graphene Oxide Photocatalyst. Water Res 2017, 121, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, M.; Sathe, S.M.; Ghangrekar, M.M. Hybrid Treatment Solutions for Removal of Micropollutant From Wastewaters. In Microconstituents in the Environment; Wiley, 2023; pp. 491–512.
- Babuji, P.; Thirumalaisamy, S.; Duraisamy, K.; Periyasamy, G. Human Health Risks Due to Exposure to Water Pollution: A Review. Water (Basel) 2023, 15, 2532. [Google Scholar] [CrossRef]
- Inostroza, P.A.; Carmona, E.; Arrhenius, Å.; Krauss, M.; Brack, W.; Backhaus, T. Target Screening of Chemicals of Emerging Concern (CECs) in Surface Waters of the Swedish West Coast. Data (Basel) 2023, 8, 93. [Google Scholar] [CrossRef]
- Ameliorative Effects of Frankincense Oil on Rats Treated with a Minimum Toxic Dose of Paracetamol. Journal of Medical and Life Science 2023, 0, 0–0. [CrossRef]
- Sacco, N.A.; Marchesini, F.A.; Gamba, I.; García, G. Photoelectrochemical Degradation of Contaminants of Emerging Concern with Special Attention on the Removal of Acetaminophen in Water-Based Solutions. 2023, 13, 524. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Japanese Journal of Applied Physics, Part 1: Regular Papers and Short Notes and Review Papers 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind Eng Chem Res 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A Review and Recent Developments in Photocatalytic Water-Splitting Using TiO2 for Hydrogen Production. Renewable and Sustainable Energy Reviews 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Yilmaz, P.; Lacerda, A.M.; Larrosa, I.; Dunn, S. Photoelectrocatalysis of Rhodamine B and Solar Hydrogen Production by TiO2 and Pd/TiO2 Catalyst Systems. Electrochim Acta 2017, 231, 641–649. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Mendow, G.; Picard, N.P.; Zoppas, F.M.; Aghemo, V.S.; Gutierrez, L.B.; Querini, C.A.; Miró, E.E. PdIn Catalysts in a Continuous Fixed Bed Reactor for the Nitrate Removal from Groundwater. International Journal of Chemical Reactor Engineering 2019, 17, 1–17. [Google Scholar] [CrossRef]
- Beltrame, T.F.; Zoppas, F.M.; Ferreira, J.Z.; Marchesini, F.A.; Bernardes, A.M. Nitrate Reduction by Electrochemical Processes Using Copper Electrode: Evaluating Operational Parameters Aiming Low Nitrite Formation. Water Science and Technology 2021, 84, 200–215. [Google Scholar] [CrossRef]
- Beltrame, T.F.; Zoppas, F.M.; Gomes, M.C.; Ferreira, J.Z.; Marchesini, F.A.; Bernardes, A.M. Electrochemical Nitrate Reduction of Brines: Improving Selectivity to N2 by the Use of Pd/Activated Carbon Fiber Catalyst. Chemosphere 2021, 279, 130832. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, F.A.; Irusta, S.; Querini, C.; Miró, E. Spectroscopic and Catalytic Characterization of Pd-In and Pt-In Supported on Al2O3 and SiO2, Active Catalysts for Nitrate Hydrogenation. Appl Catal A Gen 2008, 348, 60–70. [Google Scholar] [CrossRef]
- Marchesini, F.A.; Gutierrez, L.B.; Querini, C.A.; Miró, E.E. Pt,In and Pd,In Catalysts for the Hydrogenation of Nitrates and Nitrites in Water. FTIR Characterization and Reaction Studies. Chemical Engineering Journal 2010, 159, 203–211. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An Overview on Pd-Based Electrocatalysts for the Hydrogen Evolution Reaction. Inorg Chem Front 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Sarkar, S.; Peter, S.C. An Overview on Pd-Based Electrocatalysts for the Hydrogen Evolution Reaction. Inorg Chem Front 2018, 5, 2060–2080. [Google Scholar] [CrossRef]
- Sacco, N.A.; Zoppas, F.M.; Aghemo, V.S.; Beltrame, T.F.; Marchesini, F.A. Pd / In-Based Catalysts for Nitrate Catalytic Removal from Water: Synthesis Designs Aiming for Better N2 Selectivity. Water Supply 2023, 00, 1–20. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; García, G.; Hernández, I.; Albo, J. An Optofluidic Planar Microreactor with Photoactive Cu2O/Mo2C/TiO2 heterostructures for Enhanced Visible Light-Driven CO2conversion to Methanol. Journal of CO2 Utilization 2023, 67. [Google Scholar] [CrossRef]
- Yang, Y.-K.; Jiao, C.-Q.; Meng, Y.-S.; Yao, N.-T.; Jiang, W.-J.; Liu, T. Substituent Effect on Metal-to-Metal Charge Transfer Behavior of Cyanide-Bridged {Fe2Co2} Square. Inorg Chem Commun 2021, 130, 108712. [Google Scholar] [CrossRef]
- Chavez Zavaleta, R.; Fomichev, S.; Khaliullin, G.; Berciu, M. Effects of Reduced Dimensionality, Crystal Field, Electron-Lattice Coupling, and Strain on the Ground State of a Rare-Earth Nickelate Monolayer. Phys Rev B 2021, 104, 205111. [Google Scholar] [CrossRef]
- Dehury, T.; Kumar, S.; Rath, C. Structural Transformation and Bandgap Engineering by Doping Pr in HfO2 Nanoparticles. Mater Lett 2021, 302, 130413. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Zhang, G.; Xu, T.; Wan, P.; Sun, X. A Metallic CoS2 Nanopyramid Array Grown on 3D Carbon Fiber Paper as an Excellent Electrocatalyst for Hydrogen Evolution. J Mater Chem A Mater 2015, 3, 6306–6310. [Google Scholar] [CrossRef]
- Bazan-Aguilar, A.; García, G.; Pastor, E.; Rodríguez, J.L.; Baena-Moncada, A.M. In-Situ Spectroelectrochemical Study of Highly Active Ni-Based Foam Electrocatalysts for Hydrogen Evolution Reaction. Appl Catal B 2023, 336, 122930. [Google Scholar] [CrossRef]
- López, M.; Exner, K.S.; Viñes, F.; Illas, F. Theoretical Study of the Mechanism of the Hydrogen Evolution Reaction on the V2C MXene: Thermodynamic and Kinetic Aspects. J Catal 2023, 421, 252–263. [Google Scholar] [CrossRef]
- Nematollahi, D.; Shayani-Jam, H.; Alimoradi, M.; Niroomand, S. Electrochemical Oxidation of Acetaminophen in Aqueous Solutions: Kinetic Evaluation of Hydrolysis, Hydroxylation and Dimerization Processes. Electrochim Acta 2009, 54, 7407–7415. [Google Scholar] [CrossRef]
- Chenlo, F.; Moreira, R.; Pereira, G.; Vá zquez, M.J. Viscosity of Binary and Ternary Aqueous Systems of NaH2PO4, Na2HPO4, Na3PO4, KH2PO4, K2HPO4, and K3PO4. The Kinematic Viscosities of Aqueous Solutions of NaH; 1996;
- Pournaghi-Azar, M.H.; Kheradmandi, S.; Saadatirad, A. Simultaneous Voltammetry of Paracetamol, Ascorbic Acid, and Codeine on a Palladium-Plated Aluminum Electrode: Oxidation Pathway and Kinetics. Journal of Solid State Electrochemistry 2010, 14, 1689–1695. [Google Scholar] [CrossRef]
- Zoubir, J.; Bakas, I.; Assabbane, A. A Simple Platform for the Electro-Catalytic Detection of the Dimetridazole Using an Electrochemical Sensor Fabricated by Electro-Deposition of Ag on Carbon Graphite: Application: Orange Juice, Tomato Juice and Tap Water. Heliyon 2021, 7. [Google Scholar] [CrossRef]

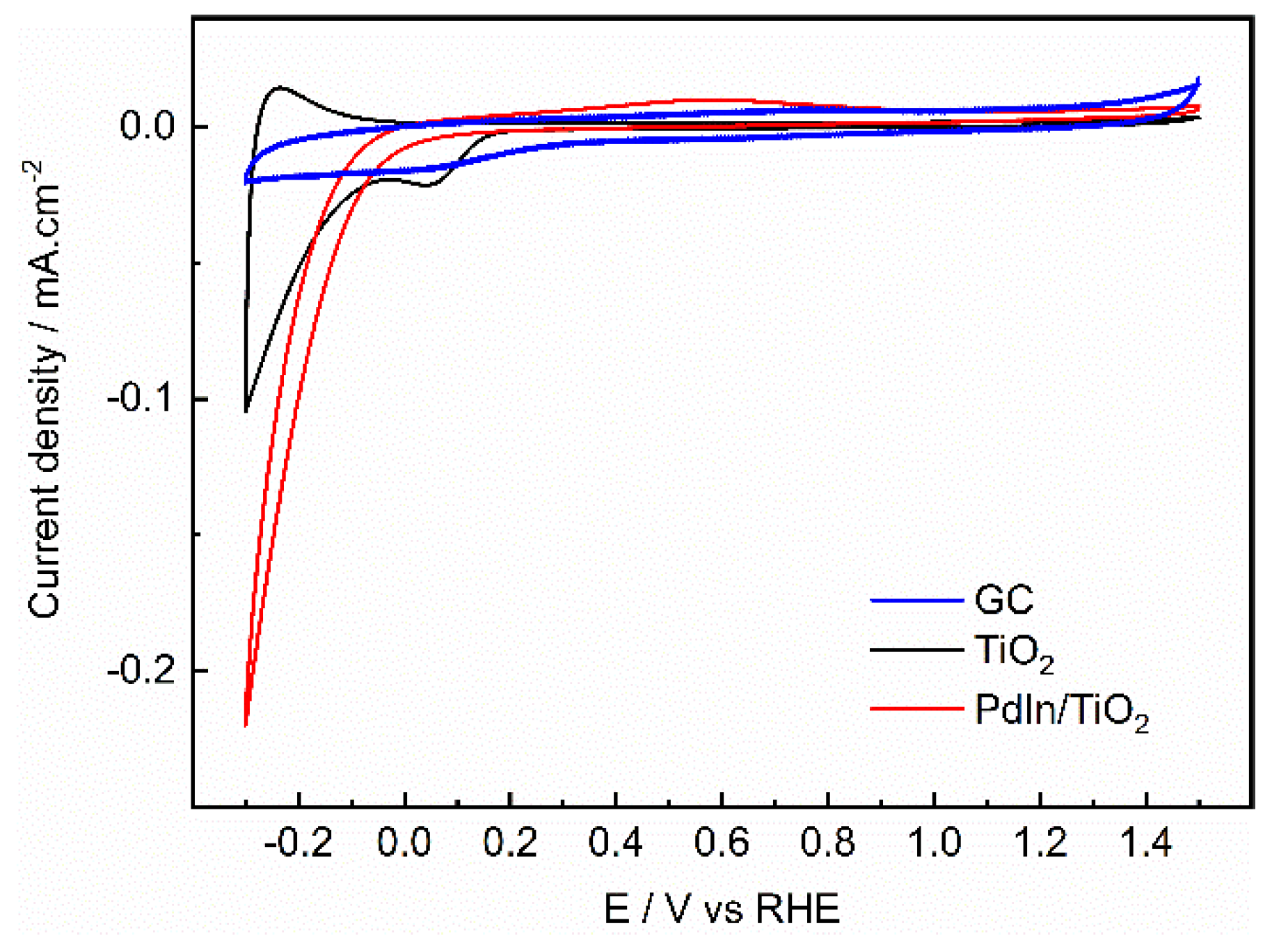
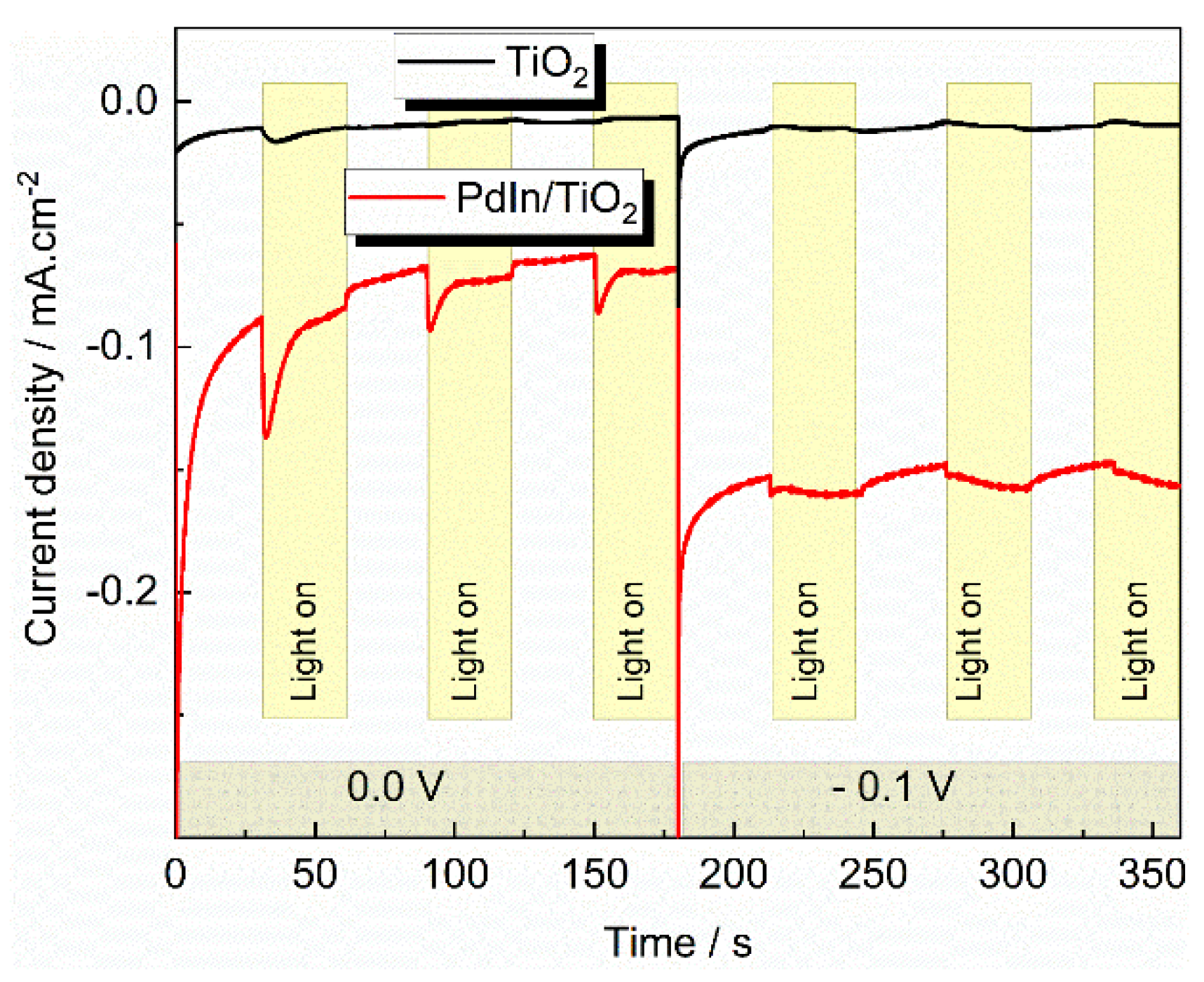
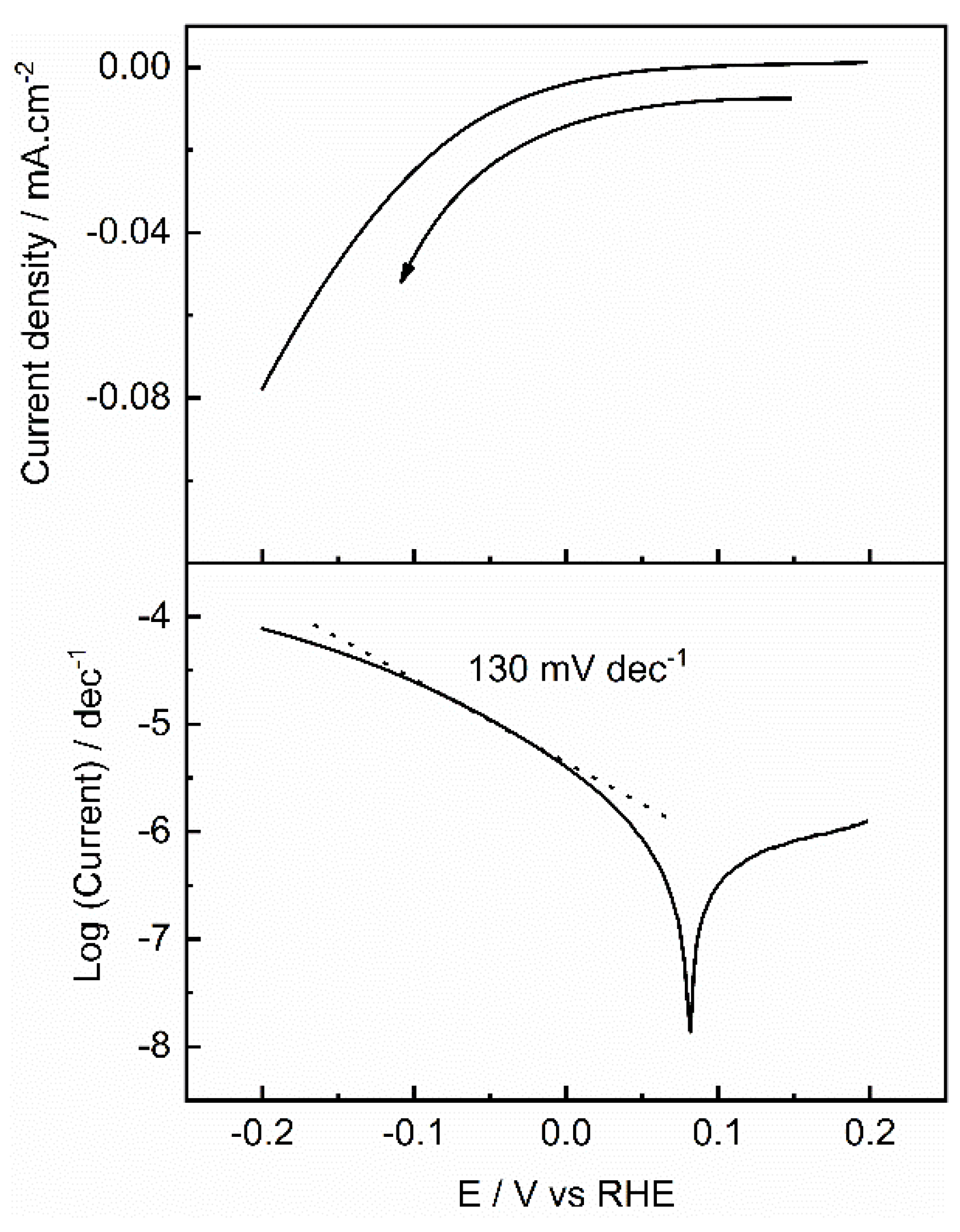
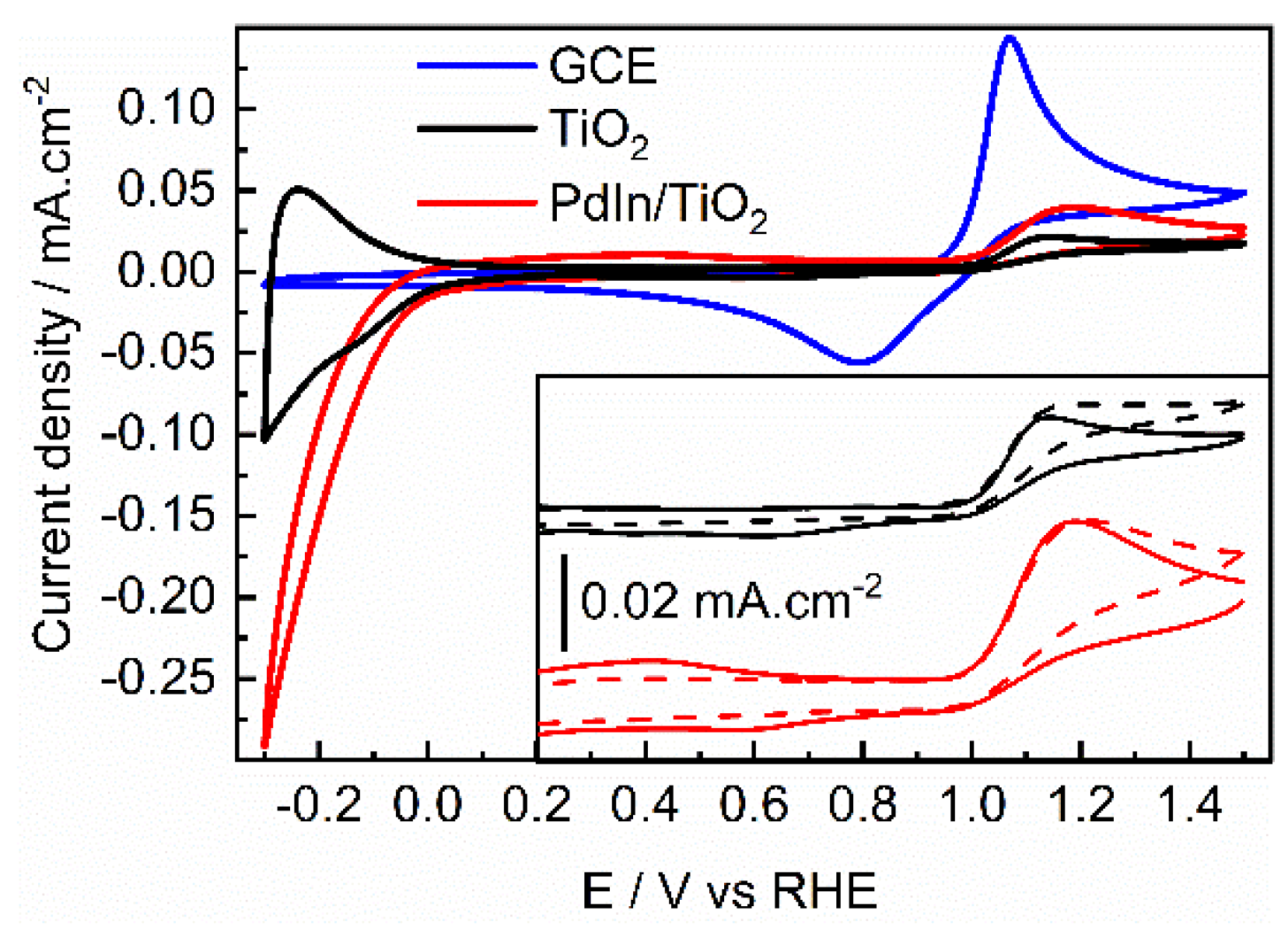
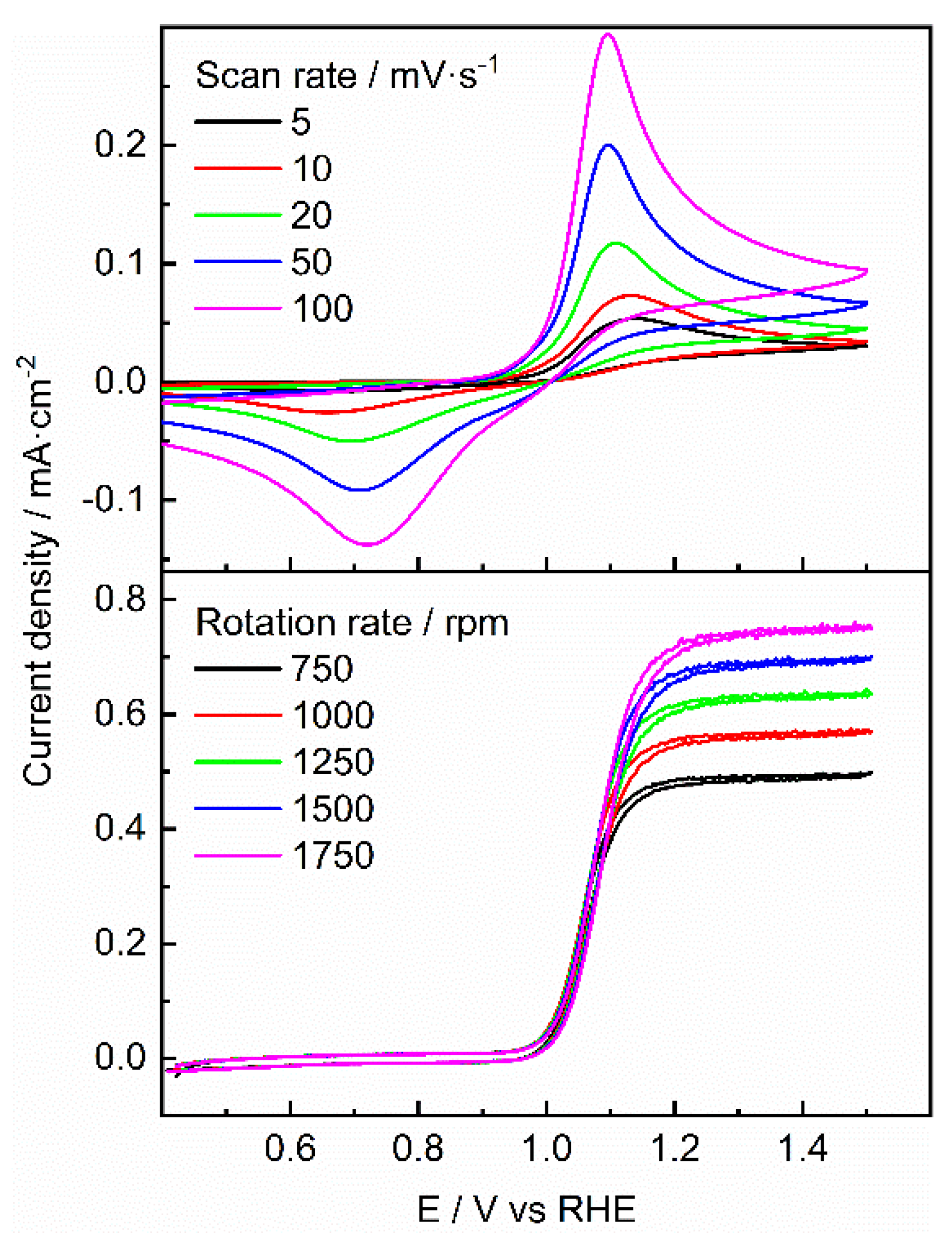
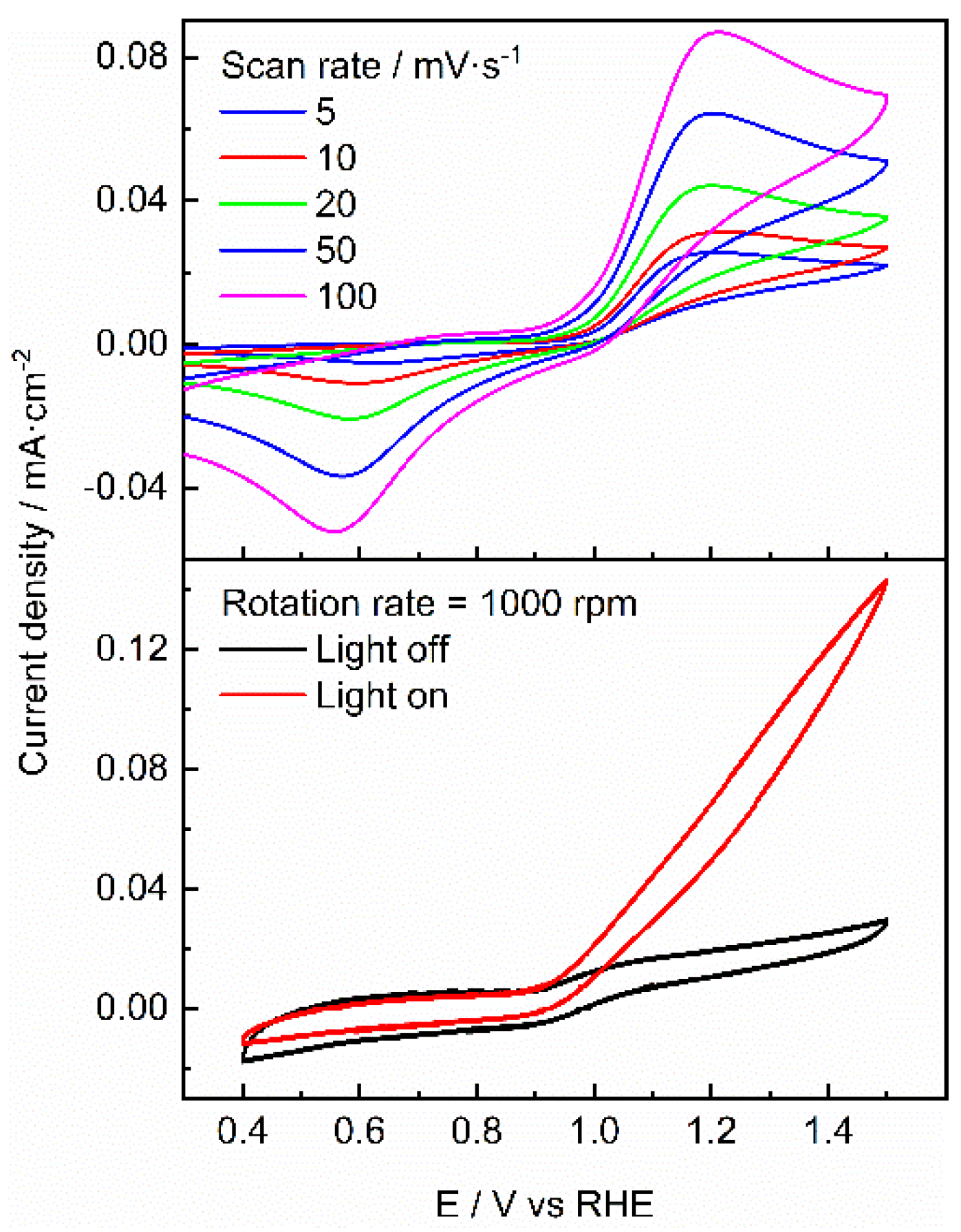
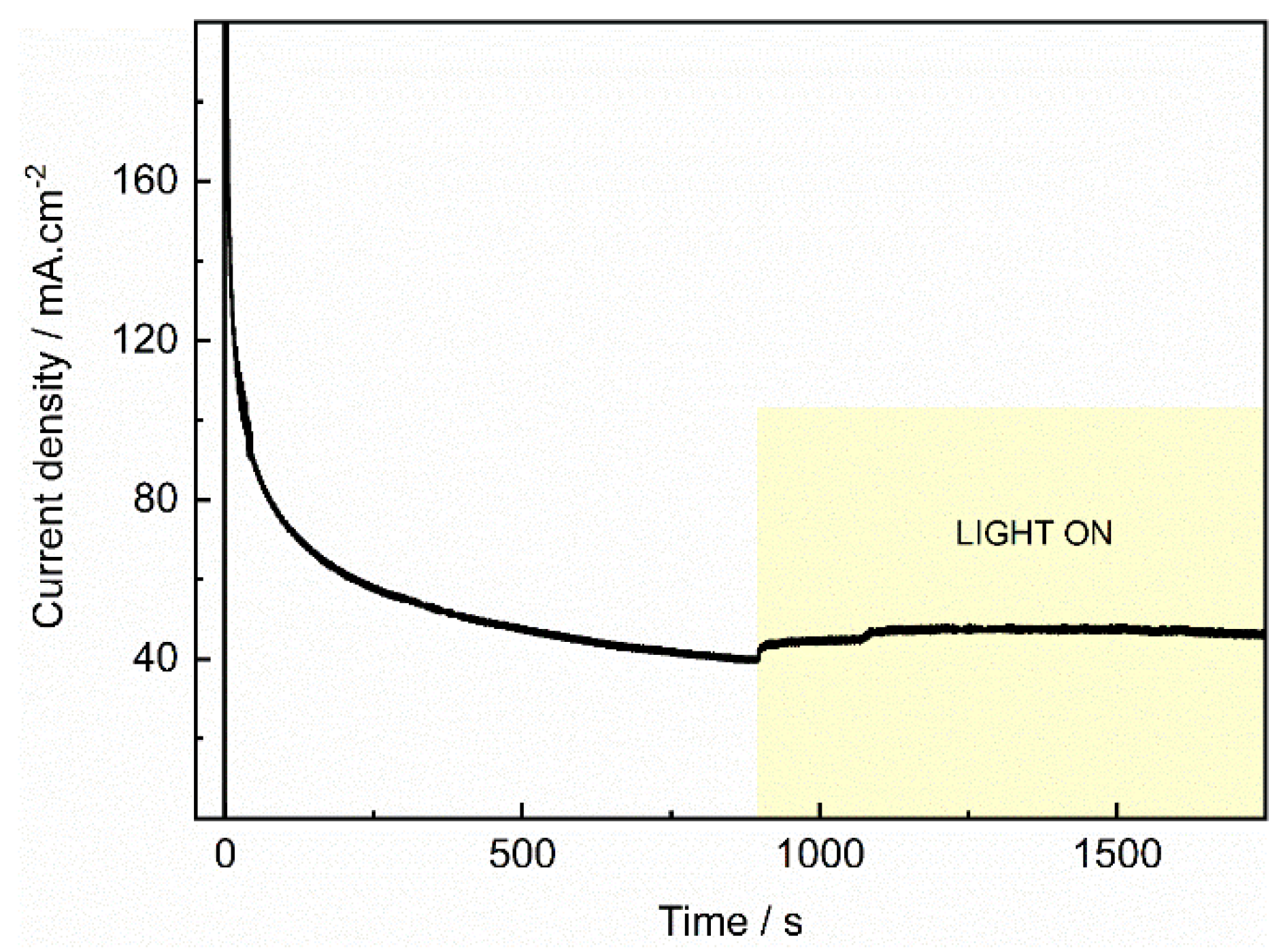
| Photocatalyst | EDLC A (mF/cm2) | EDLCC (mF/cm2) | Electroactive surface area (cm2) |
|---|---|---|---|
| GC | 0.00003 | -0.00003 | 0.6 |
| TiO2 | 0.00011 | -0.0001 | 2.5 |
| PdIn/TiO2 | 0.0004 | -0.0004 | 8.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





