1. Introduction
Exposure to jet fuels can occur through a variety of routes, with the most likely routes being dermal (skin) and inhalation (respiratory). Generally speaking, ingestion as a route of exposure for jet fuel has not been widely studied or considered a priority for toxicological studies, given that the more occupational routes have taken precedent due to their greater likelihood of occurrence. However, oral exposure can occur in situations where food or drinking water comes into contact with the fuel, such as may occur with spills or accidental leaks. Both types of these incidents have occurred in recent years, including the contamination of drinking water with Jet Propellant (JP)-5 aboard a U.S. aircraft carrier, the USS Nimitz, in September of 2022 (LaGrone 2023, Ziezulewicz 2023) and a series of unfortunate leaks that culminated in the accidental release of as much as 14,000 gallons of JP-5 from the Navy’s Red Hill Fuel Storage Facility at Joint Base Pearl Harbor-Hickam (JBPHH) in late 2021 (Miko et al 2023). During the JBPHH incident, the residential water for ~93,000 Service Members, military families, employees, and the surrounding communities were impacted, with many residents voicing concerns over the potential short- and long-term health impacts of fuel consumption (Miko et al 2023). Without the appropriate studies to consult, there has been little scientific basis for addressing concerns regarding oral exposure or answering the health-related questions raised by these events, including whether jet fuels such as JP-5 can potentially impact hormone levels, reproduction and/or development.
Several studies have detailed cases of self-reported symptoms which included dizziness, attention-deficits, headache, and various cognitive impairments with exposure to JP-5 vapors (Porter 1990, Proctor et al 2011, Smith et al 1997, Tu et al 2004). In contrast, there are significantly fewer published studies reporting effects of exposure to jet fuel on other aspects of physiology, including endocrine function. A 2002 study reported that “low” concentration occupational exposures of fuel and solvents (primarily JP-8) among female U.S. Air Force personnel resulted in adverse effects on endocrine markers associated with nonconceptive menstrual cycles, suggesting that jet fuel, or components thereof, can act as endocrine disruptors (Reutman et al 2002). In support of this hypothesis, a 2015 publication suggested that benzene, toluene, ethylbenzene and xylene, all four of which are constituents in jet fuels, may have endocrine disrupting properties at lower levels than expected (Bolden et al 2015). Likewise, a 2018 focusing on groundwater contamination with conventional oil and gas suggested potential impacts on endocrine bioactivities (Kassotis et al 2018), while another previous study in male rats indicated that jet fuel can alter protein expression in rodent testes (Witzmann et al 2003).
The current study used male and female Sprague Dawley rats and oral exposure to JP-5 to establish potential endocrine disruption and changes to reproductive health resulting from JP-5 ingestion. By using exposure periods that encompass a full spermatogenesis cycle for male rats, and approximately four ovulatory cycles for females, the study was designed to detect impacts to reproductive health in adult rats. Further, observing fetal development in rats exposed to JP-5 prior to mating and throughout pregnancy allowed for insight into the developmental effects following JP-5 exposure. Additionally, an in vitro assay involving human estrogen, androgen and glucocorticoid receptors in human cells provided insight into possible adverse effects due to ingestion of jet fuel.
2. Materials and Methods
Jet Fuels
Jet fuels were obtained through the U.S. Air Force Fuels and Energy Branch at Wright-Patterson Air Force Base. U.S. Navy jet fuel JP-5 (El Paso Corporation, Houston, TX, USA) was used for animal studies. U.S. Air Force jet fuel JP-8 (AGE Refining, San Antonio, TX, USA) and camelina plant derived jet fuel (Bio-oil Derived Synthetic Paraffinic Kerosene, UPO LLC, Des Plaines, IL, USA) were used in the in vitro assays, as well as JP-5.
Animal Study Design
Sprague Dawley rats aged ~80 days were used in this study. Rats were maintained with a 12 hour:12 hour electronically controlled light:dark cycle and provided dry chow and water ad libitum in a temperature (20-26°C) and humidity (30-70%) controlled vivarium. A blood sample was collected from all rats pre-exposure to use as a baseline for reproductive hormone levels. Food consumption and body weights were recorded daily during exposure for all animals and daily during gestation for bred females, and at a minimum of twice per week otherwise.
Exposures were stagger-started in accordance with the reproductive physiology for each sex so that both sexes completed exposures at the same time to begin breeding. The rat spermatogenesis cycle is approximately 52 days in Sprague Dawley rats, with an additional 8.5 days required for epididymal transit; accordingly, male rats were exposed for 5 days/week for 9 weeks to encompass a complete spermatogenesis cycle with maturation of sperm in the epididymis. The rat ovulatory cycle is 4-5 days; accordingly, females were exposed for 5 days/week for 3 weeks to encompass approximately 4 ovulatory cycles prior to either breeding or euthanasia and necropsy, with exposures to jet fuel continuing through pregnancy.
Exposure Groups
This study incorporated two sets of female rats, with one group exposed for the collection of ovulatory cycle data during the last week of exposure before being euthanized and ovaries collected at necropsy for histopathological examination. The second set of female rats was used for breeding and gestation, with euthanasia at gestation day 20 in order to examine physical characteristics of the fetuses. Male rats were euthanized after mating to collect the testes for sperm analysis and histopathological examination (Table 1).
Oral Administration
Animals were exposed via gavage with a 20-gauge, 3-inch curved stainless steel cannula ball-tipped gavage needle affixed to a glass syringe. Appropriate dose volumes were drawn into the syringe and the excess liquid was wiped from the stainless-steel gavage needle prior to administration. Control rats were administered 2.0 ml/kg body weight with distilled water. JP-5 “low” dose rats were administered 750 mg/kg of neat JP-5 at a volume of 0.86 ml/kg body weight. JP-5 “high” dose rats were administered 2,000 mg/kg of neat JP-5 at a volume of 2.31 ml/kg body weight. Dose volumes were based on calculated fuel density of JP-5 at 868 mg/ml. Male rats were exposed for 5 days/week for 9 weeks and through mating. Female rats were exposed for 5 days/week for 3 weeks and, for animals that were bred, throughout pregnancy to gestational day 20.
Vaginal Cytology
During the final 7 days of exposure, including 5 days of gavage, vaginal lavage and cytology were used to assess ovulatory cycles. Using a pipette, approximately 20-25 µl of sterile 0.9% physiological saline was used to perform the lavage. The pipette tip was inserted approximately 3-5 mm in the vaginal opening, the fluid was expelled into the vagina and then flushed back and forth into the vagina 2-3 times, finally to be re-collected using the pipette. The lavage liquid was placed into an appropriate square on a labeled microscope slide and allowed to air dry for at least 24 hours prior to staining. Slides were stained with 0.1% methyl blue for approximately 1 minute and de-stained in distilled water for approximately 1-2 minutes. The slides were viewed under a microscope at 10x-40x magnification to assess the estrous cycle stage. The predominance of any one specific type of cell was considered to be representative of a stage of the estrous cycle. Proestrus was indicated by a predominance of nucleated round epithelial cells. Estrus was indicated by a predominance of irregularly shaped cornified epithelial cells in which the nucleus is not well-defined or absent. Metestrus was indicated by an approximately equal distribution of leukocytes, cornified epithelial cells and nucleated round epithelial cells. Diestrus was indicated by a predominance of leukocytes. A cycle was considered abnormal if there were three or more days in a row in the estrus phase; a cycle was also considered abnormal with four or more days of diestrus in a row.
Hormone analyses
Blood samples were collected from each rat prior to the onset of exposure and at the time of necropsy. Reproductive hormone levels were assessed using a multi-array immunoassay kit (Meso Scale Discovery, Rockville, MD, USA) that quantified the levels of estradiol, progesterone, testosterone and dehydroepiandrosterone (DHEA) simultaneously in one sample. These measurements were conducted in triplicate and data with a coefficient of variation (CV) greater than 30 were omitted.
Mating Procedure
Males and females were paired (25 pairs per exposure group) for cohabitation until visible evidence of mating in the form of a vaginal plug was observed. Exposures to jet fuel continued until mating. Following successful mating, males from each pair were euthanized for sperm analysis.
Sperm Analysis and Testicular Histology
One testis and one epididymis from each male were used for assessment of sperm motility, density, morphology, and calculated average spermatid head count. The remaining testis and epididymis were used for histological examination of the seminiferous tubules.
Sperm Count: Testes were removed and weighed. The capsule was removed, and the remaining testicle was placed into a round-bottom tube. A total of 3 volumes of homogenizing solution (0.9% saline, 0.5% Triton X100) per weight of testicle was added. The testicle was homogenized using a tissue-tearor. Following homogenization, 100 µl of distilled water and 100 µl of homogenate was added to a microtube containing IDENT stain (Hamilton Thorne, Beverly, MA, USA). Samples were loaded into 2XCEL slides, cover slipped, and analyzed on the Hamilton Thorne TOX IVOS computer assisted sperm analyzer system (Hamilton Thorne, Beverly, MA).
Sperm Motility: The epididymis was removed and weighed. Immediately, the epididymis was added to 10 ml of freshly prepared M-199 media plus 0.5% bovine serum albumin (BSA) in a petri dish. Using a #11 scalpel, the distal cauda of the epididymis was punctured approximately 10-15 times (avoiding blood vessels) to allow spermatozoa to enter the media with care taken to ensure the spermatozoa avoided air contact. The dish was covered and placed on a 37°C hotplate for approximately 1 minute. Following warming, the tissue was removed, and the media was gently swirled. 12 µl of media was pipetted from the dish onto a slide and the slide was analyzed on a Hamilton Thorne TOX IVOS computer assisted sperm analyzer system (Hamilton Thorne, Beverly, MA, USA).
Sperm Morphology: The epididymis was removed and weighed. Immediately, the epididymis was added to 10 ml of freshly prepared M-199 media plus 0.5% BSA in a petri dish. Using a #11 scalpel, the distal cauda of the epididymis was punctured approximately 10-15 times (avoiding blood vessels) to allow spermatozoa to enter the media. Care was taken to ensure the spermatozoa avoided air contact. The dish was covered and placed on a 37°C hotplate for approximately 1 minute. Following warming, the tissue was removed, and the media was gently swirled. 1 ml of media was pipetted into a tube containing 100 µl of formalin. Samples were refrigerated at 4°C until analyzed. At time of analysis, 100 µl of the media sample and formalin mixture was added to a microtube containing IDENT stain (Hamilton Thorne, Beverly, MA, USA), vortexed, and allowed to rest for 2 minutes. 15 µl of sample was pipetted onto a microscope slide and cover slipped and the slide was analyzed on a Hamilton Thorne TOX IVOS computer assisted sperm analyzer system (Hamilton Thorne, Beverly, MA).
Pregnancy and Offspring Assessments
Bred females were maintained until gestation day 20, at which time they were humanely euthanized with carbon dioxide inhalation in conjunction with pneumothorax. Females then underwent necropsy and laparohysterectomy. The number of corpora lutea in each ovary was determined. The uterus was examined to determine the number of implantations, number of viable/non-viable fetuses, and number of implantation scars. Fetal position in the uterus and fetal body weights were recorded, and examinations were made for obvious external malformations or variations. External examination of all fetuses (890 total) was performed, with anogenital distance measured to assess external sexual characteristics. Fetuses were euthanized by intraperitoneal (i.p.) injection of pentobarbital. Fetuses were necropsied to locate the gonads to determine internal sex of the fetus for comparison with external sexual characteristics for concordance.
Mammalian Cell Co-transfection Assays
JP-5 was used in an in vitro assay to evaluate its ability to activate the human estrogen, androgen and/or glucocorticoid receptor. Another conventional jet fuel, JP-8, was used for comparison, as was an alternative jet fuel, camelina plant-derived Bio-oil Derived Synthetic Paraffinic Kerosene (HydroRenewable Jet; HRJ). Mammalian cell co-transfection assays were performed as previously described (Henry et al 1995, Howard et al 2000, Noonan et al 2004). Briefly, a eukaryotic expression plasmid for expression of the appropriate nuclear receptor (pRShER for expression of human estrogen receptor-, pRShAR for expression of human androgen receptor, or pRShGR for expression of human glucocorticoid receptor) along with a luciferase reporter plasmid containing the appropriate receptor response element for the particular receptor being tested (Forman et al., 1995; O’Brien et al., 1996) were co-transfected into human embryonic kidney 293 (HEK293) cells by calcium phosphate co-precipitation. A constitutively expressed pRSV--galactosidase plasmid was included in all transfections for normalization of luciferase data. Transfected cells were grown in DMEM/F12 medium containing 10% charcoal stripped fetal calf serum and specific test sterols. To test the ability of jet fuels to modulate human receptor activity, JP-8, JP-5 or HRJ (1:1 by volume) were tested at concentrations of 0, 0.001, 0.05, 0.1, 0.5, 1, 5 and 10 mg/ml. After 48 h, cells were lysed and assayed (following the addition of luciferin) for luciferase activity on a luminometer and for -galactosidase (following the addition of ONPG) at 415 nm on a microplate reader. Luciferase response was normalized to -galactosidase rate. Controls included assays without the co-transfected steroid receptor plasmid for all compounds tested. All results shown were dependent on the presence of the nuclear receptor and test compounds as well as on the presence of plasmid with the appropriate response element for the nuclear receptor being tested. All experiments were performed in triplicate, and the readings for each separate experiment were made in triplicate. Test steroids were obtained from commercially available sources. Stock solutions of fuels were made based on the density of the particular jet fuel (845 mg/ml for JP-8, 868 mg/ml for JP-5 and 794 mg/ml for HRJ) with serial dilutions in ethanol of 10, 100, and 1,000 times to facilitate reaching the concentrations listed above.
Statistical Analyses
All data analyses were performed in Microsoft Excel and Sigmaplot v.13.0. Data that passed normality and contained more than two groups were analyzed using one-way analysis of variance (ANOVA) in Sigmaplot v.13.0. Multiple comparison analyses following significant one-way ANOVA were conducted using the Holm-Sidak method. Data that did not pass normality and contained more than two groups were analyzed using the non-parametric Kruskal Wallis one-way ANOVA in Sigmaplot v.13.0. Multiple comparison analyses following significant Kruskal Wallis one-way ANOVA were conducted using the Dunn’s method. Data containing only two groups were analyzed in Microsoft Excel, using the two-tailed unpaired or paired t-test if normality is passed or the non-parametric Mann Whitney if normality was not passed. Binomial distribution analysis was used to evaluate the male to female ratios of offspring, with the assumption that the distribution should be 50% based on the fact that spermatogenesis is a meiotic process that will produce 50% Y and 50% X chromosome-bearing spermatids..
3. Results and Discussion
Effects of JP-5 exposure on body weight and food intake
Rats were randomly assigned to exposure groups and were monitored for body weight changes and food intake during jet fuel exposure. No rats lost weight over the course of the study, suggesting the jet fuel exposures were not associated with significant digestive problems that would have otherwise led to weight loss. Final weight for male rats appeared to be slightly, but significantly, dose-dependently affected by JP-5 exposure (Figure 1a). Analysis of male final weights using one-way ANOVA revealed significant difference across groups with F(2, 64) = 15.136, p < 0.001 (n = 19-24) and multiple comparison using Hold-Sidak method yielded significant reduction in weight of rats in the JP-5 2,000 mg/kg group compared to control (t = 5.479, p < 0.001) and also in the JP-5 750 mg/kg group (compared to control (t = 3.019, p = 0.007). Rats in the JP-5 2,000 mg/kg group had reduced weight compared to rats in the JP-5 750 mg/kg group with t = 2.641, p = 0.010. However, further analysis of pre-exposure weights also revealed a significant difference across groups (F(2, 64) = 15.019, p < 0.001) with rats in the JP-5 2,000 mg/kg group having significantly smaller body weight compared to control animals (t = 5.298, p < 0.001) or JP-5 Low (t = 4.121, p < 0.001).
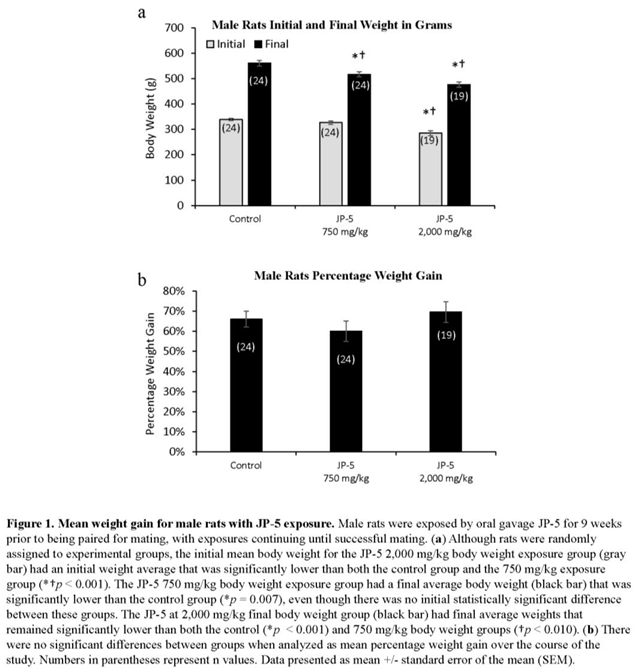
Because of the significant difference in the pre-exposure weights of rats in the JP-5 2,000 mg/kg group, we evaluated the body weight data as percentage weight gained over the course of the study. When normalized as percentage weight gained over the course of the study, there were no differences noted for weight gain in male rats in the study (Figure 1b). Percent weight gain data were analyzed using one-way ANOVA, yielding F(2, 64) = 1.023, p = 0.365 (n = 19-24). Data failed normality and thus were also analyzed using Kruskal Wallis one-way ANOVA yielding H = 5.947 (df=2), p = 0.051.
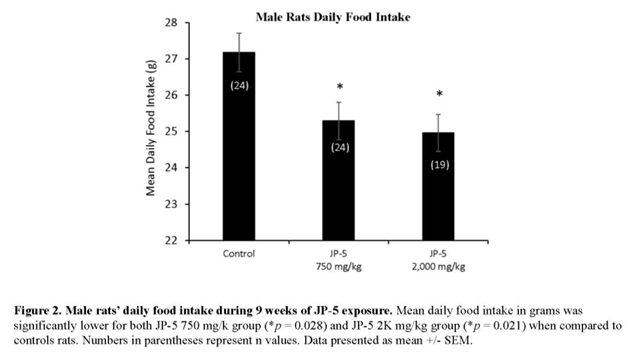
Mean daily food intake was significantly lower for male rats in both JP-5 exposure groups (Figure 2). Data passed normality and thus were analyzed using one-way ANOVA, revealing significant differences among groups with F(2, 64) = 4.832, p =0.011, n = 19-24. Multiple comparison using Holm-Sidak method yielded significantly lower food consumption by rats in the JP-5 750 mg/kg group (t = 2.527, p = 0.028) and by rats in the JP-5 2,000 mg/kg group (t = 2.786, p = 0.021) compared to food consumed by rats in the control group. While lower food intake may indicate some signs of gastric distress, the overall fact that the animals continued to gain weight during the course of exposures shows that there were no digestion problems associated with the jet fuels at these exposure levels.
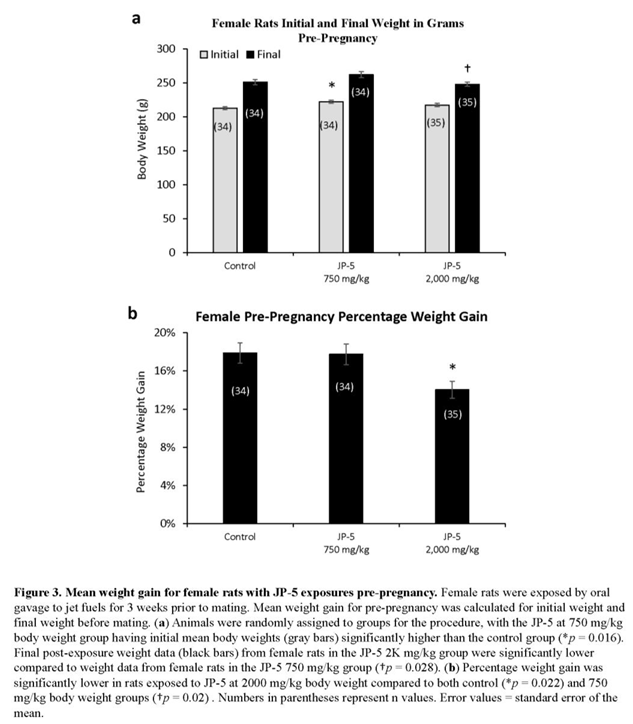
Female rats were randomly assigned to either control, JP-5 750 mg/kg or JP-5 2,000 mg/kg exposure groups with three weeks of exposure before either pre-mating or ovulatory assessments as described in Materials and Methods. The random assignment of animals to the different groups resulted in significantly higher initial weight for the female rats in the JP-5 750 mg/kg group when compared to the control group (t = 2.857, p = 0.016, n = 34-35 Holm-Sidak method, following significant one-way ANOVA with F(2, 100) = 4.083, p =0.02) (Figure 3a, gray bars). Analysis of the female post-exposure, pre-pregnancy weight data with one-way ANOVA revealed significant differences across groups with F(2,100) = 3.850, p = 0.025 and multiple comparison using Holm-Sidak yielded significantly reduced final weight of female rats in the JP-5 2,000 mg/kg group when compared to female rats in the JP-5 750 mg/kg group (t = 2.651, p = 0.028) (Figure 3b, black bars). Final, post-exposure weight data were also normalized to the initial, pre-exposure values to measure percent of weight gained. Analysis of the post-exposure, pre-pregnancy weight gain data using one-way ANOVA revealed significant difference across groups with F(2, 100) = 4.817, p = 0.01. Multiple comparison using Holm-Sidak yielded significantly less weight gain in female rats in the JP-5 2,000 mg/kg group compared to control (t = 2.730, p = 0.022) or compared to JP-5 750 mg/kg group (t = 2.630, p = 0.020) (Figure 3b). Weight gain of female rats in the JP-5 750 mg/kg group was not statistically different than the control females (t = 0.010, p = 0.921).
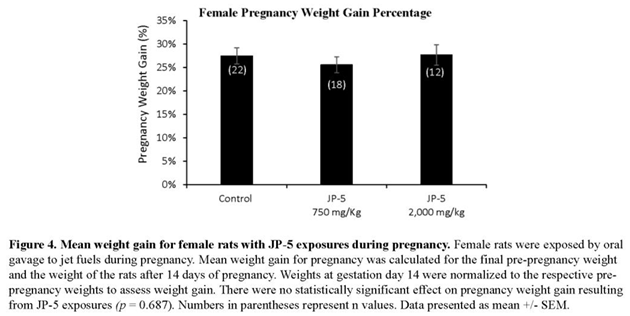
Only a subset of female rats were bred and became pregnant. Pregnancy weights were evaluated as the weight gained on gestation day (GD) 14 during pregnancy, normalized to the respective pre-pregnancy weight (Figure 4). Pregnancy weight gain data passed normality and was analyzed using one-way ANOVA yielding no statistical differences among groups with F(2, 49) = 0.378, p = 0.687, n = 12-22 (Figure 4).
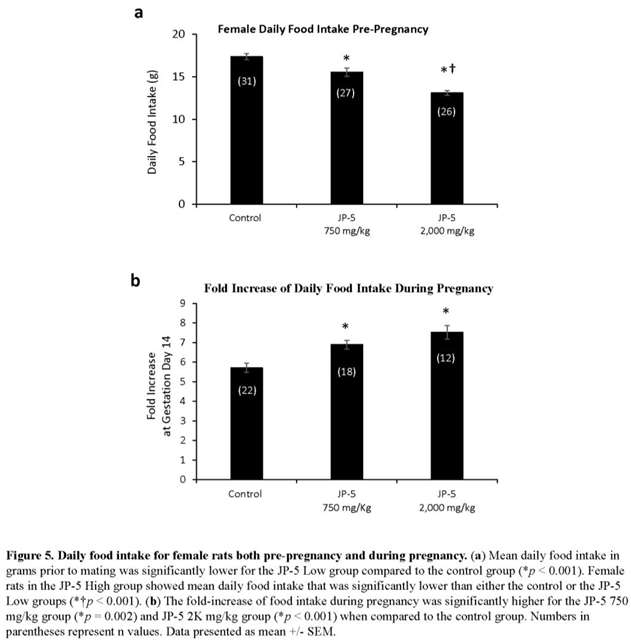
Results showed a dose-dependent increase in the average daily food intake of female rats resulting from JP-5 exposure (Figure 5a). Pre-pregnancy, post-exposure food intake data for female rats were reduced following JP-5 exposure as analyzed using one-way ANOVA revealing significant differences across groups with F(2, 81) = 30.139, p < 0.001, n = 26-31. Reduced daily food intake was observed for the JP-5 750 mg/kg group compared to control (t = 3.354, p < 0.001, Holm-Sidak comparison), and for the JP-5 2,000 mg/kg group compared to control (t = 7.760, p < 0.001, Holm-Sidak comparison). The food intake from female rats in the JP-5 2,000 mg/kg group was also significantly less than food intake from female rats in the JP-5 750 mg/kg group (t = 4.297, p < 0.001).
A subset of female rats exposed to JP-5 750 mg/kg or JP-5 2,000 mg/kg that became pregnant had increased food consumption during pregnancy relative to their food intake pre-pregnancy, as measured on GD 14 (Figure 5b). Data for the fold increase in food consumption during pregnancy passed normality and thus one-way ANOVA was used to assess statistical significance. There was statistically significant difference across groups with F(2, 49) = 12.586, p < 0.001, n = 12-22. Multiple comparison using the Holm-Sidak method yielded significantly higher food intake from pregnant rats in the JP-5 750 mg/kg group compared to control (t = 3.483, p = 0.002) and also from pregnant rats in the JP-5 2,000 mg/kg group compared to control (t = 4.700, p < 0.001).
JP-5 effects on male rat reproductive organ weights, sperm production, and sperm motility
There were no significant differences in absolute mean of left and right testis weights across the exposure groups (p > 0.05, one-way ANOVA and Kruskal Wallis, n = 19-24) (Figure 6a). However, there were dose-dependent effects of JP-5 exposure on testis weight when normalized to body weight, with both the JP-5 750 mg/kg and JP-5 2,000 mg/kg exposure groups having higher normalized testis weight than the control group (Figure 6b). One-way ANOVA of the normalized left testis weight data resulted in F(2, 63) = 19.900, p < 0.001, and multiple comparison analysis using the Holm-Sidak method yielded significant differences between control and JP-5 2,000 mg/kg group (t = 6.309, p < 0.001), control and JP-5 750 mg/kg group (t = 2.908, p = 0.005), and between JP-5 2,000 mg/kg and JP-5 750 mg/kg group (t = 3.512, p = 0.002). The normalized right testis weight data failed normality and thus Kruskal Wallis one-way ANOVA was used to assess statistical significance, resulting in H = 23.732 (df=2), p < 0.001. Multiple comparison of the normalized right testis weight data was performed using the Dunn’s method and yielded significant differences between JP-5 2,000 mg/kg and control (Q = 4.871, p < 0.001), and between JP-5 2,000 mg/kg and JP-5 750 mg/kg (Q = 2.744, p = 0.018). There was a trending increase in weight of right testis from the JP-5 750 mg/kg group compared to control, however, this was not statistically significant with Q = 2.212, p = 0.08.
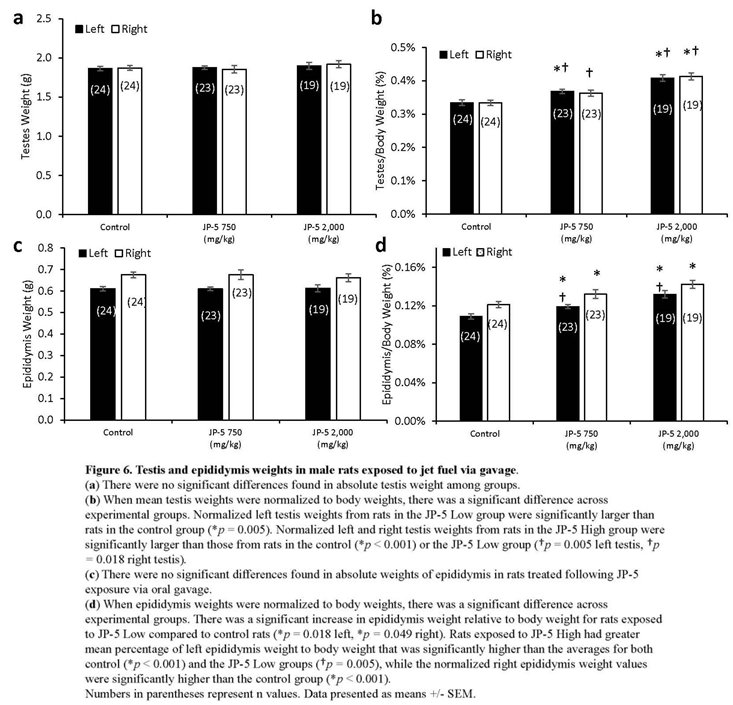
There were no significant differences in absolute mean of left and right epididymis weights across the groups (p > 0.05, one-way ANOVA and Kruskal Wallis, n = 19-24) (Figure 6c). However, there was a dose-dependent increase in epididymis weights when normalized to the respective body weights resulting from JP-5 exposure (Figure 6d). One-way ANOVA of the normalized left epididymis weight data resulted in F(2, 63) = 15.002, p <0.001, and multiple comparison analysis using the Holm-Sidak method yielded significant differences between control and JP-5 2,000 mg/kg group (t = 5.476, p < 0.001), control and JP-5 750 mg/kg group (t = 2.437, p = 0.018), and between JP-5 2,000 mg/kg and JP-5 750 mg/kg group (t = 3.131, p = 0.005). The normalized right epididymis weight data failed normality and thus Kruskal Wallis one-way ANOVA was used to assess statistical significance, resulting in H = 15.956 (df=2), p < 0.001. Multiple comparison of the normalized right epididymis weight data using the Dunn’s method yielded significant difference between JP-5 2,000 mg/kg and control (Q = 3.943, p < 0.001), and between JP-5 Low and control (Q = 2.399, p = 0.049). Comparison between JP-5 750 mg/kg and JP-5 2,000 mg/kg exposure groups yielded Q = 1.647, p = 0.299.
Taking into consideration that the rats used in this study were already mature, the fact that absolute weights of the testes and epididymis are not statistically different across the groups is an indication that the test article was not exerting a toxic influence on these already developed organs. It should be noted, as well, that the rats with the largest normalized organ to body weight ratios (Figures 6b and 6c, for testis and epididymis, respectively) were both of the JP-5 exposed groups that also showed the lowest absolute body weight (Figure 1a) and the lowest average daily food intake (Figure 2) which lends support to the supposition that these observation are most likely attributable to the JP-5 exposures upsetting digestion and potentially suppressing appetite.
There were no apparent effects of JP-5 exposures on sperm production measured per gram of testis mass (data passed normality, F(2, 65) = 0.243, p = 0.785, n = 19-25, one-way ANOVA) (Figure 7a). Sperm motility showed no adverse effects in either of the JP-5 exposure groups, with no significant differences from controls for percent rapid motility (data failed normality, H = 0.445, df = 2, p = 0.801, n=19-25, Kruskal Wallis) (Figure 7b), percent medium motility (data failed normality, H = 5.378, df = 2, p = 0.068, n=19-25, Kruskal Wallis) (Figure 7c), or percent slow motility (data failed normality, H = 0.547, df = 2, p = 0.761, n=19-25, Kruskal Wallis) (Figure 7c).
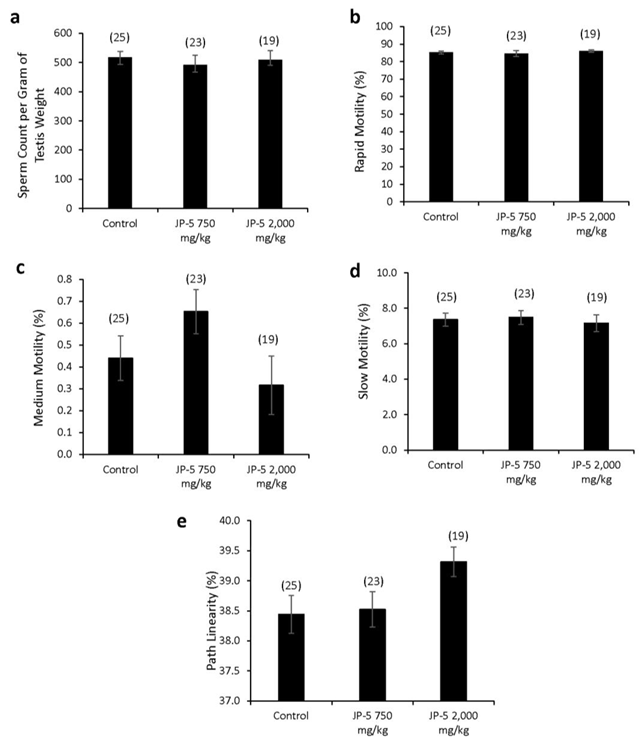
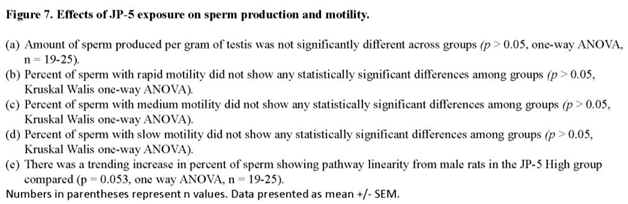
There was a trending increase in sperm path linearity from rats in the JP-5 2,000 mg/kg group compared to control (Figure 7e). Data failed normality and Kruskal Wallis one-way ANOVA yielded H = 5.862 (df=2), p = 0.053 (n = 19-25). Direct comparison between the control and JP-5 2,000 mg/kg group data using two-tailed, unpaired t-test yielded p = 0.04. However, direct comparison using the non-parametric Mann Whitney Rank Sum Test yielded p = 0.065.
Other sperm traits analyzed included: path velocity, progressive velocity, track speed, lateral amplitude, beat frequency, straightness, linearity and elongation, and percent static (data not shown), with no statistically significant differences or trends to lend or interpretation for the difference for path linearity for sperm exposed to the higher concentration of JP-5. There were also no adverse effects due to JP-5 exposures on sperm morphology (data not shown).
Hormonal effects on male rats
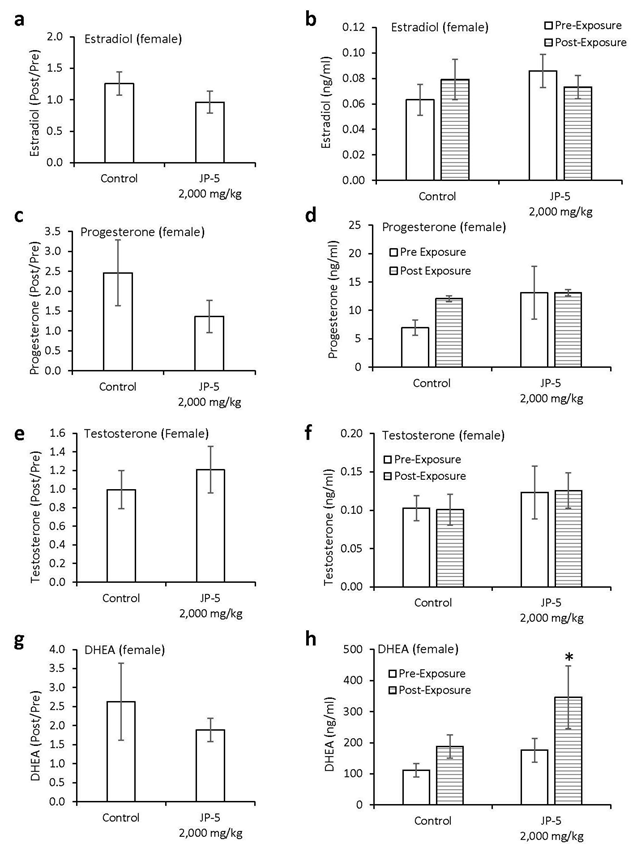
Levels of hormones were evaluated for a subset of males following experimental exposures and mating as described in Materials and Methods. Only control rats and rats exposed to 2,000 mg JP-5 /kg were included in these evaluations. There were no significant reductions in the estradiol level from male rats compared to control when data were analyzed as ratios of post- to pre-exposure values (p = 0.45, two-tailed, unpaired t-test, n = 8 control, 7 JP-5 2,000 mg/kg group) (Figure 8a). However, a comparison of pre-exposure with post-exposure values within the JP-5 2,000 mg/kg group showed a significant reduction in estradiol levels (p = 0.028, two-tailed paired t-test); a difference that was not present in the control group (p = 0.5) (Figure 8b).
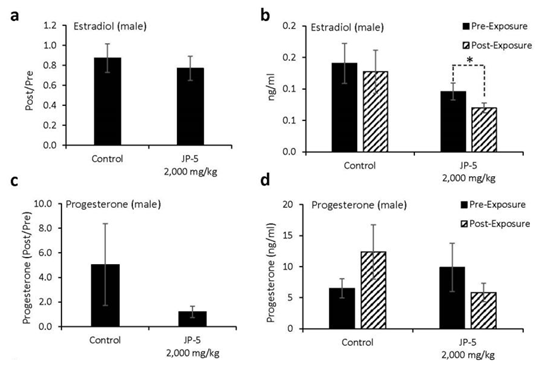
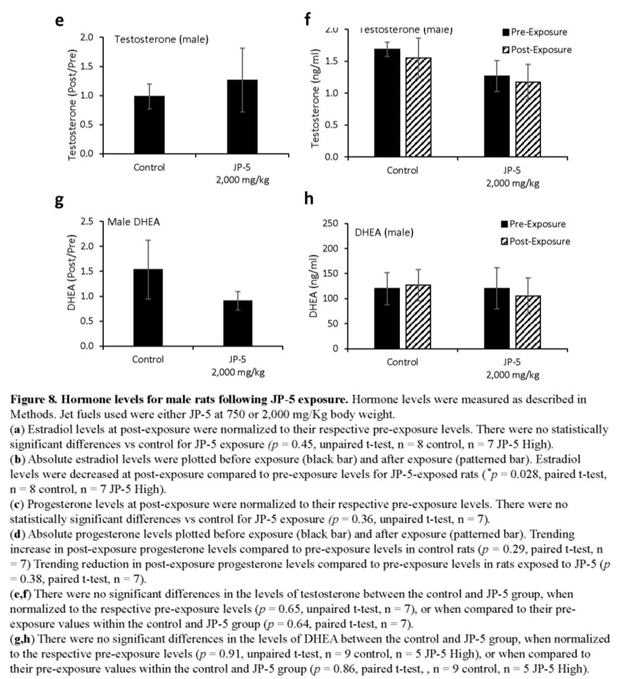
There were no statistically significant differences in progesterone post- to pre-exposure ratios noted (p = 0.36, two-tailed, unpaired t-test, n = 7) (Figure 8c), but the data did show that while control rats had a trending increase (p = 0.29) in progesterone levels following mating, the JP-5 exposed rats had a trending decrease (p = 0.38) in progesterone at the conclusion of the experimental procedures (Figure 8d).
There were no significant differences observed in testosterone levels when data were analyzed as post- to pre-exposure ratios (p = 0.65, two-tailed, unpaired t-test, n = 7) (Figure 8e) or when post-exposure values were statistically compared to pre-exposure values for control and JP-5 2,000 mg/kg groups (p = 0.64, 0.79, two-tailed, paired t-test) (Figure 8f).
Similarly, there were no significant differences observed in DHEA levels when data were analyzed as post- to pre-exposure ratios (p = 0.91, two-tailed, unpaired t-test, n = 9 control, 5 JP-5 2,000 mg/kg) (Figure 8g) or when post-exposure values were statistically compared to pre-exposure values for control and JP-5 2,000 mg/kg groups (p = 0.86, 0.39, two-tailed, paired t-test) (Figure 8h).
Effects on female rats
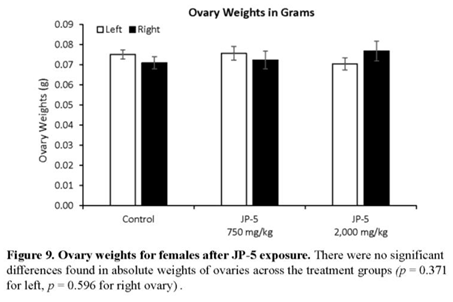
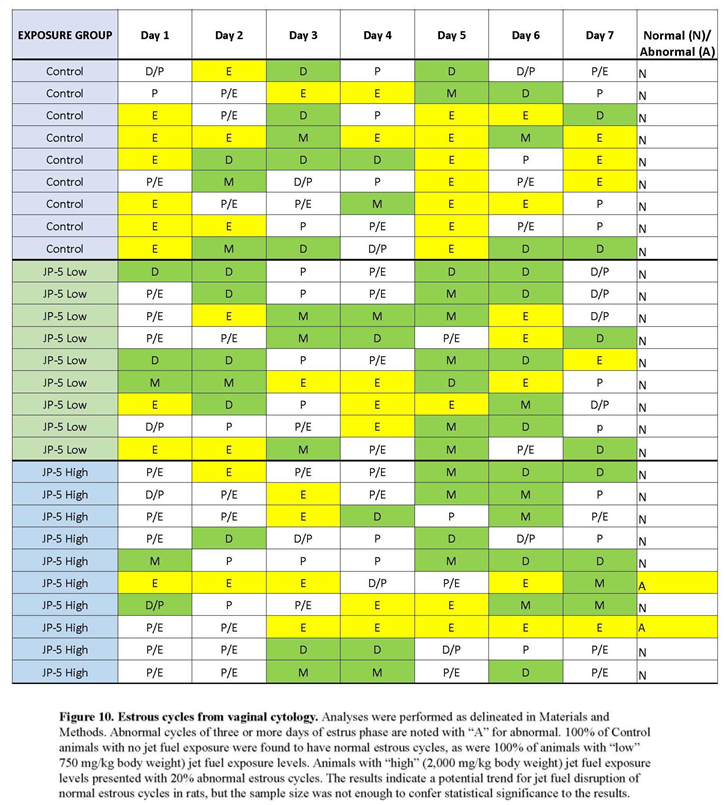
There were no effects on ovary weights for females exposed to JP-5 as shown in Figure 9. One-way ANOVA of left ovary weight data revealed F(2, 25) = 1.031, p = 0.371 (n = 9 control, JP-5 750 mg/kg, n = 10 JP-5 2,000 mg/kg). One-way ANOVA of right ovary weight data yielded F(2, 25) = 0.529, p = 0.596 (n = 9 control, JP-5 750 mg/kg, n = 10 JP-5 2,000 mg/kg). There were, however, disruptions in estrous cycles noted with JP-5 2,000 mg/kg exposure for two of the 10 females as shown in Figure 10, but the sample size did not confer statistical significance.
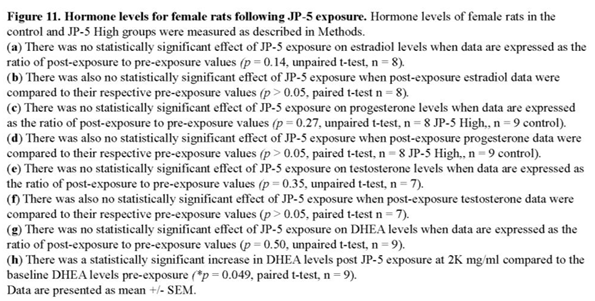
Neither estradiol nor progesterone levels were significantly affected by JP-5 exposure for both estradiol and progesterone levels (Figure 11a–d). There were trending non-significant reductions in the estradiol level from female rats compared to control when data were analyzed as ratios of post- to pre-exposure values (p = 0.14, two-tailed, unpaired t-test, n = 8) (Figure 11a). A comparison of pre-exposure with post exposure levels within exposure groups did not show statistically significant differences when analyzed using two-tailed paired t-test (p > 0.05). However, a slight trending increase was noted in the control post-exposure average compared to control pre-exposure level whereas there was a sight trending decrease in the JP-5 2,000 mg/kg post-exposure average compared to JP-5 2,000 mg/kg pre-exposure level (Figure 11b).
There were trending non-significant reductions in the progesterone level from female rats compared to control when data were analyzed as ratios of post- to pre-exposure values (p = 0.27, two-tailed, unpaired t-test, n = 8 JP-5, 9 control) (Figure 11c). A comparison of pre-exposure with post exposure levels within exposure groups did not show statistically significant differences when analyzed using two-tailed paired t-test (p > 0.05, paired t-test) (Figure 11d).
There were very slight trending increases in the testosterone level from female rats compared to control when data were analyzed as ratios of post- to pre-exposure levels (p = 0.35, two-tailed, unpaired t-test, n = 7) (Figure 11e). There was no noticeable difference in the averaged post-exposure value compared to the pre-exposure value of testosterone levels from control or JP-5 2,000 mg/kg groups (p > 0.05) (Figure 11f).
There were slight trending increases in the DHEA level from female rats compared to control when data were analyzed as ratios of post- to pre-exposure levels (p = 0.50, two-tailed, unpaired t-test, n = 9) (Figure 11g). Interestingly, there was an increase in the averaged post-exposure value of DHEA compared to the pre-exposure value in the JP-5 2,000 mg/kg group (p = 0.049); a difference that was not present in the control group (p = 0.1) (Figure 11h).
Offspring effects
Fetal evaluations were performed on GD 20 as described in Materials and Methods. Weight of offspring was significantly lower following JP-5 750 mg/kg exposure for both males (Figure 12a) and females (Figure 12b). Male and female offspring weight data failed normality and thus the non-parametric Kruskal Wallis one-way ANOVA were used to assess statistical significance. Kruskal Wallis analysis of male offspring data revealed significant across experimental groups with H = 9.990 (df=2), p = 0.007 (n = 92-167 as indicated in Figure 12a graph). Multiple comparison using the Dunn’s method yielded significantly lower male offspring weight from the JP-5 750 mg/kg group compared to the control group with Q = 2.525, p = 0.035. Kruskal Wallis analysis of female offspring data revealed a significant difference across experimental groups with H = 9.961 (df=2), p = 0.007 (n = 134-169 as indicated in Figure 12b graph). Multiple comparison using the Dunn’s method yielded significantly lower male offspring weight from the JP-5 750 mg/kg group compared to the control group with Q = 2.999, p = 0.008.
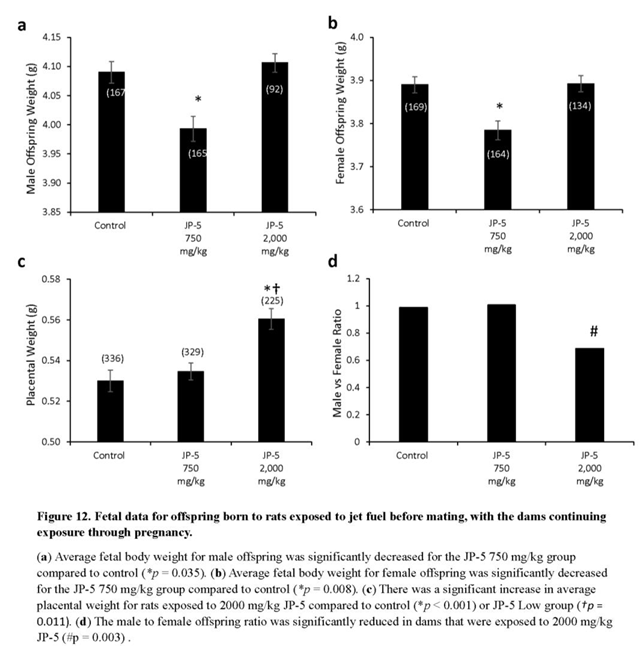
Interestingly, the fetal weights trended back to the same as controls for JP-5 2,000 mg/kg exposed rats for both males (Figure 12a) and females (Figure 12b); the reason for the seeming paradox can be discerned from the increased placental weight noted for JP-5 2,000 mg/kg exposure (Figure 12c). Combined male and female placental weight data also failed normality and statistically analyzed using Kruskal Wallis one-way ANOVA, yielding significant difference across groups with H = 18.262 (df=2), p < 0.001 (n = 225-336). Multiple comparison using the Dunn’s method yielded significantly greater placental weight from the JP-5 2,000 mg/kg group compared to control (Q = 4.247, p < 0.001) or the JP-5 750 mg/kg group (Q = 2.912, p = 0.011). Estrogen has a positive effect on vascularization and growth of both the placenta and the fetus (Albrecht and Pepe, 2010) and the increased placental weight noted could be the result of increased estrogenic compound exposure, which would point to JP-5 acting as an endocrine disruptor, specifically as an estrogen mimic. The seeming paradox of fetal weight loss with a lower exposure level of JP-5, while the weight is increased with higher exposure can be attributed to the larger placenta, which would provide more nutrients to the offspring of the JP-5 2,000 mg/kg exposed dams.
Figure 12d shows the male to female ratio of offspring for the exposure groups, with approximately the expected 1:1 value for both the control and JP-5 750 mg/kg exposure groups, with the control group having 167 male to 168 female fetuses while the JP-5 750 mg/kg group had 165 male to 164 female fetuses. The JP-5 2,000 mg/kg group, on the other hand, showed an approximately 0.69:1 ratio of males to females, which was found to be a statistically significant ratio difference (p = 0.003) using binomial distribution analysis with the assumption that the distribution should be 50% based on the fact that spermatogenesis is a meiotic process that will produce 50% Y and 50% X chromosome-bearing spermatids (Figure 12d). There were fewer dams in the final analysis for the JP-5 2,000 mg/kg group (16) compared to the control and JP-5 750 mg/kg groups (20 each), with the fetuses per dam ratio being 15.3 for the control group, 15.5 for the JP-5 750 mg/kg group and 14.1 for the JP-5 2,000 mg/kg group. There were 16 resorptions noted in the JP-5 2,000 mg/kg group, vs 11 each in the control and JP-5 750 mg/kg groups (data not shown), which means that the resorption to fetus ratio was 0.07 for the JP-5 2,000 mg/kg group, as compared to 0.04 for the control and JP-5 750 mg/kg groups. Normalizing the resorption rate for JP-5 to the control rate would mean that the expected resorptions for the JP-5 2,000 mg/kg group would be 9.01, suggesting that there were 7 resorptions that could potentially be attributed to the effects of JP-5 2,000 mg/kg exposure; if all 7 of these excess resorptions were assumed to be male, the ratio of male to female fetuses would be 0.74:1, which would still fail the binomial distribution assumption of 1:1, or 50% male, with p = 0.013. The importance of the fact that the binomial distribution would still be significantly skewed towards more females than should be expected is that this indicates a mechanism of action that leads to success of fertilization by X chromosome-bearing spermatids, rather than that that the JP-5 2,000 mg/kg exposure was preferentially toxic to male fetuses, although this preferential toxicity to male fetuses may also be true, but there is no way to know the sex associated with the resorptions.
The fetuses were further inspected for anogenital distance, abnormalities of which can be an indication of endocrine disruption, and for gross abnormalities. There were no indications that the JP-5 exposures produced any alterations in anogenital distance (data not shown).
In vitro activation of human estrogen receptors by jet fuels
Due to the potential endocrine disrupting effects noted for the study described thus far, we tested the ability of JP-5 and other jet fuels to directly activate human hormone receptors. The jet fuels selected for testing were JP-5, JP-8 and a Bio-oil Derived Synthetic Paraffinic Kerosene derived from the camelina plant, we will refer to as HydroRenewable Jet (HRJ). While the animal study provides some evidence of JP-5 being an estrogen mimic, the structural evaluation of its components provides some indication that it might have structural similarity to estradiol (Figure 13). We also tested JP-8, a similar U.S. Air Force jet fuel that contains similar constituents. The compound -sitosterol is a known estrogen receptor activator (Gutendorf & Westendorf 2001) and since it is a component of camelina plant oil, it seemed possible it could survive refinement into jet fuel which turns camelina oil into HRJ fuel (Figure 13). In addition to the human estrogen receptor assays, an assay was performed with the human androgen receptor and the human glucocorticoid receptor to test hormone receptor specificity of the jet fuels. Only the estrogen receptor evaluations showed any sign of being activated by jet fuels, with the camelina-derived synthetic HRJ showing no indication of activating the steroid receptors.
Figure 14a shows the concentration response curve that resulted from addition of increasing concentration of 17β-estradiol, which is a known agonist of the human estrogen receptor- α. 17β-estradiol was used in separate experiments at concentrations of 0, 0.1 nm, 1 nm, 10 nm, 100 nm and 1000 nm in the mammalian cell co-transfection assay using HEK293 cells as described in Materials and Methods. Luciferase activity is expressed as fold activation vs. zero concentration control. Results were normalized using the constitutively active β-galactosidase expression plasmid that shows cell viability. These results show that the test system was working as expected.
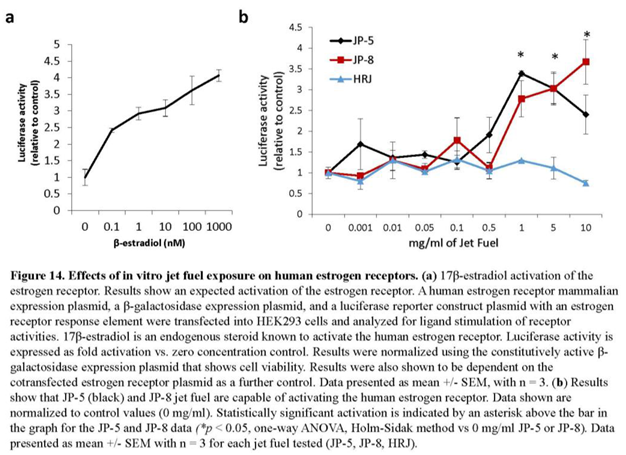
To test the ability of jet fuels to modulate human estrogen receptor-α activity, JP-8, JP-5 or HRJ were tested at concentrations of 0, 0.1, 1, 5 and 10 mg/ml. Results show that JP-5 and JP-8 were able to positively regulate the human estrogen receptor with a statistically significant level of activity reached beginning at a concentration of 1 mg/mL (Figure 14b). Data analyses were conducted using one-way ANOVA across the different concentrations for each jet fuel. Analysis of JP-5 data revealed significant difference across concentration groups with F(8, 18) = 6.403, p < 0.001, n = 3. Multiple comparison using the Holm Sidak method yielded significant activation of ER in the 1 (p < 0.001), 5 (p = 0.003) and 10 (p = 0.043) mg/ml groups when compared to 0 mg/ml. Analysis of JP-8 data revealed significant difference across concentration groups with F(8, 18) = 8.125, p < 0.001, n = 3, with significant activation of the estrogen receptor in the 1 ( p< 0.001), 5 ( p= 0.007), 10 (p = 0.016) mg/ml groups when compared to 0 mg/ml. Analysis of the HRJ data did not reveal significant activation of the estrogen receptor at the concentrations tested with F(8,18) = 1.471, p = 0.235, n = 3.
The androgen receptor was shown to be responsive to testosterone as expected, but exposures to jet fuels showed no statistically significant effects in the assay (data not shown). Likewise, dexamethasone results showed the assay was working as expected for the glucocorticoid receptor, but the assay showed no indication that jet fuels modulated glucocorticoid activity (data not shown).
4. Conclusions
The importance of understanding the effects of ingested jet fuel has been brought into the spotlight by recent events where accidental spills led to the presence of jet fuel in drinking water. This study used relatively high concentrations of JP-5 jet fuel to look at potential endocrine disruption in adult rats, effects on mating and fertility, and effects on fetuses due to parental exposure. There were some indications of gastric distress, which would be expected from ingesting high concentrations of jet fuel, which manifested as significantly lower daily food intake for males (Figure 2), while females showed significantly lower daily food intake prior to pregnancy (Figure 5a), but that trend was reversed during pregnancy with the females rats then showing higher food intake when exposed to JP-5 (Figure 5b). For the males, this diminished food intake may have manifested as lower overall body weights for rats exposed to JP-5 (Figure 1a), but the percentage weight gain was similar for all rats including controls (Figure 1b). For females, only those rats exposed to JP-5 at the higher concentration used in the study were found to have significantly lower final weights pre-pregnancy in both absolute weight (Figure 3a) and percentage weight gained (Figure 3b), while the pregnant rats showed no difference in percentage weight gained (Figure 4). The fact that the pregnant females ate significantly more food with JP-5 exposure may indicate some compensatory eating during pregnancy for being malnourished before mating, but the results indicate nothing beyond the expected gastric distress that might be expected from oral exposure to jet fuel.
The study found no effects on male reproduction in terms of mating behavior. We attribute noted differences in weights of testes and epididymides when compared to body weight with JP-5 exposure (Figures 6b,d) to diminished body weight gain of the adult rats, but the observation is included since size alterations of these organs would be noteworthy if attributable to exposure. There were no observations for gross abnormalities (data not shown). There were no negative effects on sperm count (Figure 7a) or motility (Figure 7b–d), but there was a trending, non-significant, increase in path linearity for sperm (Figure 7e) which is not normally looked at as an adverse effect since linearity has a relationship to fertilization success. In context with other findings in this study that indicate JP-5 is capable of regulating estrogen receptor activity as discussed further below, it should be noted that if this increased path linearity for sperm exposed to JP-5 holds true, a possible explanation could be that estrogen receptors present in sperm have been shown to regulate motility (Skibińska et al 2023), so while attributing the observation to JP-5 exposure is only a remote possibility, it could warrant further study.
Estradiol, a hormone that is an endogenous ligand of the estrogen receptor, is associated with normal sexual behavior in both males and females. Figure 8b shows how post-exposure estradiol levels were significantly lower for male rats following more than 9 weeks of JP-5 exposure, while there were no statistical differences comparing post to pre-exposure estradiol levels in control rats. Since the rats mated successfully, there were no noted effects of these altered levels, but these diminished estradiol levels could indicate the possibility of negative feedback to estradiol production due to JP-5 exposure. While female rats did not show a significant diminishment of estradiol levels over the course of a shorter period of exposure than the males (3 weeks for females vs. more than 9 weeks for males), there was a JP-5 associated “trend” in diminishment of estradiol levels comparing post-exposure to pre-exposure animals (Figure 11b). These estradiol levels provide some evidence of endocrine disruption, with the possibility of being tied to JP-5 serving as an estrogen mimic. This study did not entertain the idea that some fuel constituents may, in fact, be hormone receptor antagonists.
Progesterone is a hormone that is normally associated with female sexual traits and pregnancy, but it is also important for normal male sexuality and increased levels of progesterone may be associated with sexual activity in males (Alvarenga et al 2010), therefore the trending increase in progesterone levels for male rats in the control group noted in this study should be looked at from the perspective that the rats had been involved in mating prior to the final blood collection for hormone analyses, which should explain the increase in progesterone levels noted in the control group (Figure 8d). The fact that the JP-5 exposed groups did not show a similar increase in progesterone levels despite the same mating circumstances prior to the hormone analyses makes the diminishment in progesterone levels with JP-5 exposure all the more worthy of note, even if it is not statistically significant. Female rats also showed a trending non-significant reduction in progesterone levels when viewed as post vs pre-exposure levels (Figure 11d). These observations provide further evidence of endocrine disruption due to JP-5 exposure.
Testosterone and DHEA are androgenic hormones strongly associated with male sexual characteristics and behavior, but they are also important in females. There were no observed differences in the androgenic hormones evaluated in this study for male rats, but the females in the study did show a statistically significant increase in DHEA levels compared post to pre-exposure with JP-5. While these changes in DHEA levels are slight, it is possible they are due endocrine disruption properties of JP-5 and warrant further study.
The rat estrous cycle is usually 4-5 days in duration and is divided into 4 phases: estrus, metestrus, diestrus, and proestrus (Stump et al 2012). Remaining in estrus phase for 3 days or longer is considered an aberrant cycle (Parker 2012). Both estrogen and progesterone are commonly associated with normal estrous cycles and perturbations of levels of these hormones could lead to altered estrous cycles. As shown in Figure 10, JP-5 at the 2,000 mg/kg body weight exposure level showed a trend towards aberrancy with 20% abnormal cycles, providing further support for the endocrine disrupting potential of JP-5.
Estrogen plays a positive role in both placental and fetal angiogenesis and is associated with the vascularization and growth of the placenta and fetus (Albrecht & Pepe 2010). JP-5 exposure at 750 mg/kg body weight exhibits a toxic effect that resulted in significantly diminished fetal weights for both males (Figure 12a) and females (Figure 12b). Paradoxically, this diminished fetal weight for both sexes was not present at the higher concentration of JP-5 used (Figures 12a,b). The significantly larger placenta sizes shown in Figure 12c for dams exposed to the higher level of JP-5 could be an explanation for this observation and would lend further support to JP-5 acting as an endocrine disruptor, particularly acting as an estrogen mimic.
The increased number of female offspring with the higher concentration of JP-5 is not explained by the number of excess fetal resorptions, since even if all these resorptions were male, a fact we cannot know, there would still be significantly more females. It is difficult to provide an explanation without further study, but one possibility to consider is that the JP-5 exposure provides an advantage in fertilization success to X chromosome-bearing spermatids in either survival or motility. There is also the possibility that JP-5 is more toxic to the developing male fetus and therefore caused more fetal resorptions for males. The fact that there were only resorptions and not non-viable fetuses present would perhaps indicate that the problems targeting male offspring are occurring in early development or prior to fertilization.
The most noteworthy finding of this study is the observation of estrogen receptor activation by the jet fuels JP-5 and JP-8 using an in vitro activation assay with human estrogen receptor and human embryonic kidney cells, with statistically significant activation compared to controls beginning at levels of 1 mg/ml (Figure 14b). The cycloparaffin and aromatic structures present in both JP-5 and JP-8 have some similarity to the shapes of estrogenic and androgenic compounds as shown in Figure 13. The alternative jet fuel (HRJ) used in the steroid receptor assay is derived from the camelina plant, which is known to produce a phytoestrogen with demonstrated ability to regulate the estrogen receptor (Gutendorf & Westendorf 2001), but the HRJ jet fuel showed no signs of estrogen receptor modulation in our study (Figure 14b).
While this study used relatively high concentrations of JP-5 to elucidate the potential of endocrine disruption, further studies would be needed with more physiologically and environmentally relevant exposure levels to gain a proper understanding of the potential dangers of JP-5 exposure to the endocrine system. The results do, however, provide strong support to the notion that JP-5 can act as an endocrine disruptor. The finding that JP-5 and JP-8 can both activate the estrogen receptor using an in vitro assay in human cells with a human estrogen receptor lends very strong support to the notion that these jet fuels can function as estrogen mimics. Further characterization is needed to help resolve potential hazards associated with ingestion of these jet fuels. We currently have ongoing studies that will further investigate the role of estrogen receptors following oral exposures to JP-5. We will also evaluate whether prolonged repeated exposures to lower doses of JP-5 can have detectable effects on neurological and endocrinological endpoints in offspring.