1. Introduction
Acute skin infections, regardless of etiology (bacterial, viral, or fungal), are very frequent in dermatological practice. They range from superficial clinical forms with moderate local inflammatory phenomena, to deep infections that may involve structures adjacent to the skin, such as in necrotizing fasciitis. Whether mild or severe, any can aggravate further and lead to sepsis by triggering a complex cascade of dysfunction and even failure across multiple organs and body systems. Therefore, addressing the first clinical signs of skin infections promptly and effectively is the most appropriate therapeutic approach [
1].
Currently, sepsis of any cause is considered a serious threat to public health globally, and the international research community is engaged in discussions of sepsis definitions, treatments, preventive measures [
2]. The understanding of sepsis has undergone reconceptualization over time. In 1991, for instance, the American College of Chest Physicians described
sepsis as a
systemic inflammatory response syndrome (SIRS) caused by infection, with possible further aggravation to
severesepsis in case of organ dysfunction with hypoperfusion or hypotension, and to
septic shock in case of hypotension despite adequate volemic resuscitation. In 2016, the Third International Consensus Definitions for Sepsis and Septic Shock defined
sepsis-3 as life-threatening organ dysfunction, acknowledging the severe, lethal threat of a pathogen invading the body [
3,
4]. The diagnosis of organ dysfunction is established when the Sequential Organ Failure Assessment (SOFA) score changes by at least two points as a result of the acute infection [
5].
To illustrate the scale of the problem, sepsis-related mortality rate was 41% in Europe and 28.3% in the United States according to public health data from 2012 [
6]. Then, in 2017, 48.9 million cases of sepsis were reported worldwide, resulting to 11 million deaths, which amounted to 19.7% of all deaths that year [
7]. According to our review of the literature, these are still the most cited data in newer studies in the absence of updated epidemiological reports.
The starting point of infection in sepsis patients has been described, but few studies highlight the importance of skin semiology in the early recognition of sepsis. One such study conducted in Colombia on a group of patients diagnosed with sepsis showed that skin and soft tissue infections were the fourth leading cause of sepsis (9.4%), after urinary tract infections (27.6%), lower respiratory tract infections (27.4%), and intra-abdominal infections (10.8%). Almost a quarter of sepsis episodes originating in skin infections progressed to septic shock, and 28% of these patients had to be transferred to intensive care units. Of them, 8% succumbed to their conditions [
8].
The skin is home to an incredibly diverse microbiome, and it is normally well equipped to maintain a healthy balance. When the skin’s defense mechanisms are overrun by proliferating pathogens, the ensuing acute skin infections are cellulitis, erysipelas, trophic ulcers in advanced stages of chronic venous insufficiency, bullous dermatoses (pemphigus, pemphigoid, erythema multiforme) with extensive denudation, erythroderma. The bacterial agents most often involved are Methicillin-resistant
Staphylococcus aureus (MRSA),
Escherichia coli,
Pseudomonas aeruginosa, species of the genus
Streptococcus,
Klebsiella pneumoniae,
Proteus mirabilis,
Enterococcus [
9]. The onset of infectious dermatoses is abrupt, with soft tissue erythema, induration, local heat, pain, as well as general symptoms such as fever, followed by the deterioration of vital functions and single/multiple organ dysfunction in the case of sepsis complications.
The appropriate management of acute skin infections depends on their severity. Mild forms can be effectively resolved through local antibiotic therapy, while severe infections require systemic antibiotic therapy. Initiation should be prompt and with agents empirically proven to be sensitive to gram-positive microorganisms, such as penicillinase-resistant penicillin, cephalosporins, macrolides or fluoroquinolones. In case of severe sepsis, adequate treatment is multifactorial and multidisciplinary, as it may also include the administration of vasopressor agents, steroids, anticoagulants, anti-inflammatories, as well as glycemic control, ventilation support, and even (early) resuscitation[
10,
11]. In making such therapeutic decisions, medical teams must consider not only the gateway of infection and the pathogenspotentially involved, but also the patient's age and comorbidities. In most cases, a broad-spectrum empiric antibiotic with good tissue penetration is administered first, with the possibility of later switching to a narrow-spectrum antibiotic therapy based on the antibiogram results [
12].
1.1. Study aims
In this study,we aimed to evaluate the incidence of skin lesions with loss of tissue that can become infected and complicated further by cutaneous-onset sepsis, in the context of tertiary care provided by the dermatology unit of the largest emergency hospital in NE Romania. The specific objectives included: (1) surveying hospital records over a period of three years to identify cases of skin lesions with loss of tissue, (2) compiling a comprehensive database on all admitted patients – generalcharacteristics, types of lesions, etiological agents, clinical signs and symptoms, comorbidities, (3) analyzing the data to identify statistically significant associations between variables, and (4) reporting on results internationallyto update awareness and understanding of relevant issues.
The study is relevant considering the scarcity of incidence data on cutaneous-onset sepsis in the literature, and the implications for multidisciplinary clinical practice and further research. Skin lesions are not typically regarded as emergencies, but lack of timely diagnosis and treatment can lead to infections and then on to sepsis, delaying recovery, increasing treatment complexity and financial costs, delaying recovery, undermining quality of life, and even threatening the patient’s life.
2. Materials and Methods
3.1. Study coordinates, inclusion and exclusion criteria
This was a retrospective observational study of patients admitted to the Clinic of Dermatology-Venereology of the Emergency Clinical County Hospital "Sf. Spiridon" Iași, in NE Romania, between January 2020 and December 2022. The study was conducted with the formal approval of the institutional Research Ethics Committees of the hospital and the medical university.
The study enrolled consecutive patients over the age of 18 who were admitted for the diagnosis and treatment of skin lesions (ulcerations, erosions, fissures, excoriations) and symptoms indicative of associated inflammatory processes (edema, tumefaction, erythema, fever, pain, etc.). Completing a bacteriological examination on admission was a key criterion for inclusion. Patients younger than 18, with concomitant SARS-CoV-2 infection, in sepsis of non-cutaneous cause, and/or with Glasgow Coma Scores<13) were excluded from the study.
A detailed clinical analysis of skin manifestations was performed, and the patient data were organized in three study groups:
group A – infectedskin lesions and sepsis,
group B – infected lesions without sepsis,
group C – non-infected skin lesions (negative bacteriological examination results).
The three data sets were compared to identify significant associations between clinical manifestations (cutaneous, systemic) and patient characteristics (demographics, comorbidities, mental status), as well as to assess risk factors.
3.2. Definitions
The types of skin lesions with loss of tissue considered in this study were: erosions (loss of tissue down to the skin’s basal membrane), ulcerations (deep loss of tissue), fissures (linear lesions e.g., due to skin dehydration,thickening, compromised elasticity), excoriations (superficial loss of skin tissue e.g., due to scratching). Whenever bullae and vesicles were present, this was also noted and included in the analysis.
Sepsis was defined as infection-related systemic inflammatory response syndrome (SIRS) with two or more of the following criteria being met: body temperature >38oC or <3638oC, tachycardia, tachypnea, leukocytosis/leukocytopenia. The differential diagnosis of cutaneous-onset sepsis was based on the identification of primary skin lesions and the confirmation of bacterial agents in cultured samples from the lesions, distinguishing it from sepsis of other causes e.g., neoplasms, respiratory, urogenital, etc.
A milddeterioration of mental status on the Glasgow Coma Scale (scores of 13 and 14) was also taken into consideration in relation to sepsis.This scale is commonly used to assess brain injury, and assigning a GCS score is standard procedure in emergency admissions. Fully awake, responsive, cognitively agile patients receive the maximum score 15, while scores 9-12 describe moderate impairment, and 8 or less indicate severe, coma states of unconsciousness. Severely altered mental status occurs in septic shock, which was not the object of our study, and patientswould not have been referred to the dermatology unit of the hospital in the first place; they would have been admitted and treated in the ICU of the emergency department.
3.2. Statistical analysis
The statistical analysis was performed using the SPSS version 29.0 software package (SPSS Inc., Chicago, IL, USA). Categorical variables were presented as frequencies and percentages, and continuous variables as means ± standard deviation. Categorical variables were compared between groups using the Pearson Chi-squared test, and the associated risks (OR) were also calculated. Continuous variables were compared between groups using the Mann-Whitney test (because the pre-condition of normal repartition of values was not verified). To investigate the combined action of the statistically significant risk factors for infection and sepsis, the multivariate analysis was performed using a model for binary logistic regression. Statistical significance was assessed relative to the threshold of p< 0.05, and the confidence interval (CI) was set at 95%.
3. Results
3.1. General demographic characteristics
During the 3-year researched period, 509 of all admitted patients were diagnosed with infectious dermatoses and met the criteria for inclusion in the study. The patients were between 18 and 92 years old (mean age 64.22 ± 14.699). In 441 cases, lesions were infected, and sepsis was confirmed in 78 of these cases (group A).It is worth pointing out that patients with sepsis were older (mean age 65.23 ± 14.056), but not substantially (weak statistical significant(p=0.046). These age characteristics are summarized in Table 1.
Table 1.
Patient age data in the three study groups.
Table 1.
Patient age data in the three study groups.
| Study group |
N |
Mean |
St. dev. |
St. error |
Min |
Max |
Median |
| A – confirmed sepsis |
78
|
61.33
|
15.022
|
1.701
|
18
|
88
|
62.0
|
| B – infection without sepsis |
363
|
65.23
|
14.056
|
0.738
|
18
|
92
|
65.0
|
| C – non-infected lesions |
68
|
62.13
|
17.075
|
2.071
|
20
|
90
|
65.5
|
| Total |
509
|
64.22
|
14.699
|
0.652
|
18
|
92
|
65.0
|
The 441 patients with infected lesions and sepsis were mostly men (56.2%) and mostly rural residents (57.4%), and statistically non-significant differences were noted relative to the presence or absence of sepsis (see Table 2). However, sex and background differences were significant within each group. Namely, acute skin infections were more common in men than in women (57.0% vs. 43%), and in patients from rural rather than urban areas (58.1% vs. 41.9%). Similarly, sepsis was more common in men (52.6%) and rural residents (53.8%). Male patients therefore appeared to be 2.282 times more at risk of skin infections than women, while urban residence seemed to provide some protection given the 0.474 higher risk associated with rural background.
Table 2.
Demographic characteristics –group A vs. group B.
Table 2.
Demographic characteristics –group A vs. group B.
| |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Sex |
0.519 |
0.471 |
- |
| male |
248 |
56.2% |
41 |
52.6% |
207 |
57.0% |
|
|
|
| female |
193 |
43.8% |
37 |
47.4% |
156 |
43.0% |
|
|
|
| Background |
0.481 |
0.488 |
- |
| urban |
188 |
42.6% |
36 |
46.2% |
152 |
41.9% |
|
|
|
| rural |
253 |
57.4% |
42 |
53.8% |
211 |
58.1% |
|
|
|
| Total |
441 |
100% |
78 |
100% |
363 |
100% |
|
|
|
3.2. Clinical manifestations – cutaneous and systemic, including conscious state
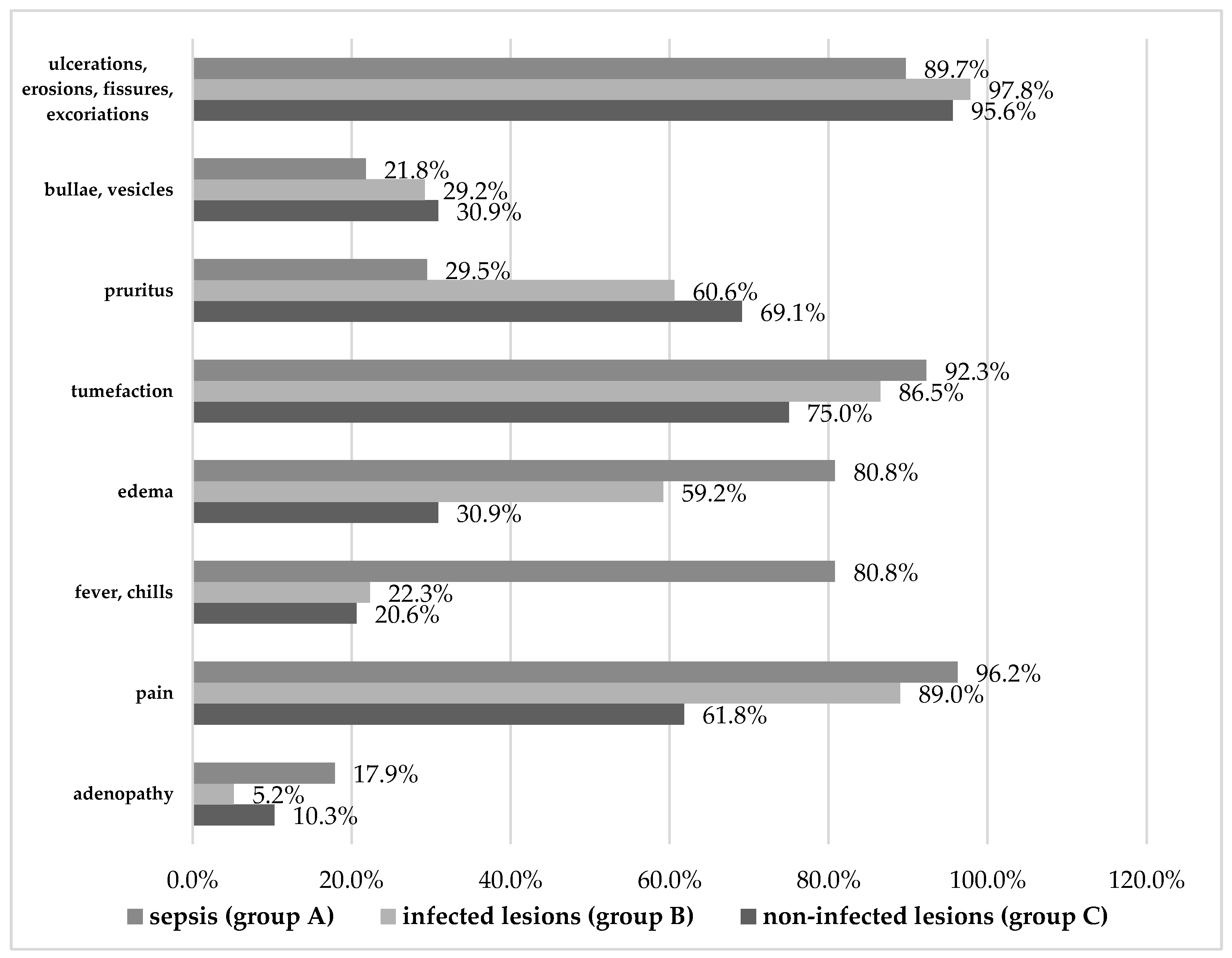
Overall, themain clinical manifestations recorded were skin ulcerations, erosions, fissures, excoriations, bullae, vesicles, pruritus, tumefaction, as well as fever, chills, pain, edema, and adenopathy(see Figure 1, Table 3, and Table 4).There were several noteworthy similarities and significant differences between the three study groups, as follows.
The topography of skin lesions was consistent across groups, with non-significant differences between patients with sepsis versus those with uncomplicated infections. Most skin manifestations occurred in the lower limbs (97.4% in group A, 93.9% in group B), and a minority of patients also had lesions on their torsos (23.1% in both groups), upper limbs (17.9% in group A, 18.5% in group B), and on their face (10.3% in group A, 8.8% in group B). It seems, therefore, that the location of the studied lesions was not a significant factor in the patients’ aggravation towards sepsis. The same can be said about the number of lesions, in the sense that patients with multiple lesions were not more likely to progress to sepsis compared to those presenting a single lesion.
While most patients across groups had skin ulcerations, erosions, fissures, and excoriations, significantly fewer patients with sepsis had such lesions (89.7%) compared to patients without complicated infections (97.8%, p=0.003) or infections altogether (95.6%).Similarly, bullae or vesicles were least present in patients with sepsis (only 21.8% compared to 29.2% in group B, p<0.001). Likewise, pruritus occurred in only 29.5% of patients with sepsis compared to more than 60% of patients in groups B and C (p<0.001). Tumefaction was the only exception, occurring in a majority ofcases in all three groups, but mostly in sepsis (p=0.016).
The opposite pattern could be seen in manifestations reaching beyond the level of the skin, such as edema. Relative to the 80.8% of patients with sepsis who manifested edema, significantly fewer patients with uncomplicated infections had edema (59.2%), and only 30.9% of uninfected patients did (p<0.001). This confirms edema as a risk factor for both infection (3.251) and sepsis (2.891).
In addition, patients with sepsis experienced systemic signs and symptoms significantly more than the other patients. Fever and chills were, by far, most common in patients with sepsis (80.8%) and much rarer in the other groups (22.3% and 20.6%, respectively, p<0.001). This translates to a 14.622-fold increased risk of sepsis in the presence of fever, which is not surprising considering what sepsis entails.
Similarly, pain was reported by almost all the patients with sepsis and infection (96.2% and 89.0%, respectively), compared to 61.8% of uninfected patients (p<0.001).Our risk analysis pointed to pain as the second highest risk factor for infection (4.999), suggesting that painful symptomscan betray infective aggravation even more than edema,a telltale sign of inflammation.
Regarding adenopathy, even if it occurred in only 17.9% of sepsis cases, it was significantly rarer in the group with uncomplicated infections (5.2%, p<0.001), so it is worth mentioning. However, the fact that it did also occur in uninfected patients with a non-significantdifference (10.3%, p=0.158) makes the result of risk analysis less useful (we calculated a 3.961-fold increased risk of developing sepsis in the presence of adenopathy).
Figure 1.
Clinical manifestations (skin-related and systemic) in the three study groups.
Figure 1.
Clinical manifestations (skin-related and systemic) in the three study groups.
Table 3.
Clinical manifestations in patients with infected lesions (with vs. without sepsis).
Table 3.
Clinical manifestations in patients with infected lesions (with vs. without sepsis).
| |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Ulcerations, erosions, fissures, excoriations |
11.907
|
0.003**
|
0.197 (0.072
÷
0.543)
|
| present |
425
|
96.4%
|
70
|
89.7%
|
355
|
97.8%
|
|
|
|
| absent |
16
|
3.6%
|
8
|
10.3%
|
8
|
2.2%
|
|
|
|
| Bullae, vesicles |
1.751
|
0.186
|
- |
| present |
123
|
27.9%
|
17
|
21.8%
|
106
|
29.2%
|
|
|
|
| absent |
318
|
72.1%
|
61
|
78.2%
|
257
|
70.8%
|
|
|
|
| Pruritus |
25.131
|
<0.001**
|
0.272 (0.160
÷
0.462)
|
| present |
243
|
55.1%
|
23
|
29.5%
|
220
|
60.6%
|
|
|
|
| absent |
198
|
44.9%
|
55
|
70.5%
|
143
|
39.4%
|
|
|
|
| Tumefaction |
1.983
|
0.159
|
- |
| present |
386
|
87.5%
|
72
|
92.3%
|
314
|
86.5%
|
|
|
|
| absent |
55
|
12.5%
|
6
|
7.7%
|
49
|
13.5%
|
|
|
|
| Edema |
12.786
|
<0.001**
|
2.891 (1.586
÷
5.272)
|
| present |
278
|
63.0%
|
63
|
80.8%
|
215
|
59.2%
|
|
|
|
| absent |
163
|
37.0%
|
15
|
19.2%
|
148
|
40.8%
|
|
|
|
| Fever, chills |
99.762
|
<0.001**
|
14.622 (7.906
÷
27.044)
|
| present |
144
|
32.7%
|
63
|
80.8%
|
81
|
22.3%
|
|
|
|
| absent |
297
|
67.3%
|
15
|
19.2%
|
282
|
77.7%
|
|
|
|
| Pain |
3.754
|
0.053
|
- |
| present |
398
|
90.2%
|
75
|
96.2%
|
323
|
89.0%
|
|
|
|
| absent |
43
|
9.8%
|
3
|
3.8%
|
40
|
11.0%
|
|
|
|
| Adenopathy |
14.992
|
<0.001**
|
3.961 (1.889
÷
8.302)
|
| present |
33
|
7.5%
|
14
|
17.9%
|
19
|
5.2%
|
|
|
|
| absent |
408
|
92.5%
|
64
|
82.1%
|
344
|
94.8%
|
|
|
|
| Total |
441
|
100%
|
78
|
100%
|
363
|
100%
|
|
|
|
Table 4.
Clinical manifestations in patients with infected vs. non-infected lesions.
Table 4.
Clinical manifestations in patients with infected vs. non-infected lesions.
| |
Total |
Infection |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group B) |
No (group C) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Ulcerations, erosions, fissures, excoriations |
1.123
|
0.391
|
-
|
| present |
420
|
97.4%
|
355
|
97.8%
|
65
|
95.6%
|
|
|
|
| absent |
11
|
2.6%
|
8
|
2.2%
|
3
|
4.4%
|
|
|
|
| Bullae, vesicles |
0.078
|
0.780
|
-
|
| present |
127
|
29.5%
|
106
|
29.2%
|
21
|
30.9%
|
|
|
|
| absent |
304
|
70.5%
|
257
|
70.8%
|
47
|
69.1%
|
|
|
|
| Pruritus |
1.760
|
0.185
|
-
|
| present |
267
|
61.9%
|
220
|
60.6%
|
47
|
69.1%
|
|
|
|
| absent |
164
|
38.1%
|
143
|
39.4%
|
21
|
30.9%
|
|
|
|
| Tumefaction |
5.842
|
0.016*
|
2.136 (1.142
÷
3.995)
|
| present |
365
|
84.7%
|
314
|
86.5%
|
51
|
75%
|
|
|
|
| absent |
66
|
15.3%
|
49
|
13.5%
|
17
|
25%
|
|
|
|
| Edema |
18.575
|
<0.001**
|
3.251 (1.866
÷
5.666)
|
| present |
236
|
54.8%
|
215
|
59.2%
|
21
|
30.9%
|
|
|
|
| absent |
195
|
45.2%
|
148
|
40.8%
|
47
|
69.1%
|
|
|
|
| Fever, chills |
0.099
|
0.753
|
-
|
| present |
95
|
22%
|
81
|
22.3%
|
14
|
20.6%
|
|
|
|
| absent |
336
|
78%
|
282
|
77.7%
|
54
|
79.4%
|
|
|
|
| Pain |
32.712
|
<0.001**
|
4.999 (2.773
÷
9.010)
|
| present |
365
|
84.7%
|
323
|
89%
|
42
|
61.8%
|
|
|
|
| absent |
66
|
15.3%
|
40
|
11%
|
26
|
38.2%
|
|
|
|
| Adenopathy |
2.587
|
0.158
|
-
|
| present |
26
|
6%
|
19
|
5.2%
|
7
|
10.3%
|
|
|
|
| absent |
405
|
94%
|
344
|
94.8%
|
61
|
89.7%
|
|
|
|
| Total |
431
|
100%
|
363
|
100%
|
68
|
100%
|
|
|
|
Last but not least, a mildly altered state of consciousness(Glasgow scores of 13 or 14)was significantly associated with the presence of sepsis. Of the 13 patients with such scores, most had confirmed sepsis (9% relative to the size of group A vs. 1.7% in group B, p=0.003, see Table 5). The risk assessment showed that, based on these data, a Glasgow score of 13 or 14pointed to a 5.866-fold higher risk of sepsis.This relationship between sepsis and impaired brain function highlights the importance of addressing and preventing sepsis not just generally, but in the context of skin lesions that can be subject to infective complications and aggravation.
Table 5.
Glasgow scores – patient group A vs. group B.
Table 5.
Glasgow scores – patient group A vs. group B.
| Glasgow Score 13-14 |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| present |
13 |
2.9% |
7 |
9% |
6 |
1.7% |
12.030 |
.003** |
5.866
(1.914÷17.975) |
| absent |
428 |
97.1% |
71 |
91% |
357 |
98.3% |
|
|
|
| Total |
441 |
100% |
78 |
100% |
363 |
100% |
|
|
|
3.3. Main acute skin conditions and the etiological agents responsible for infections
As summarized in Table 6, the types of skin infections diagnosed in our patients were venous ulcers (68.3%), microbial eczema (61.9%), cellulitis (21.1%), superinfected bullous dermatosis such as pemphigus, pemphigoid, Stevens-Johnson syndrome (6.6%)erysipelas (5%), and erythroderma (2%).Other dermatoses featuring loss of tissue or the disruption of the skin barrier, such as atopic dermatitis, psoriasis, vasculitis, and ulcerated skin neoplasms amounted to 27%.
While venous ulcers were almost equally present in groups A and B (66.7% and 68.6%), they were by far the least common in uninfected patients (13.2%, p<0.001). At the same time, microbial eczema was significantly more frequent among patients with uncomplicated infections (68.9%) than both sepsis patients (29.5%) and uninfected patients (27.9%) (p<0.001). On the other hand,48.7% of patients with sepsis had cellulitis, while only 15.2% of patients with uncomplicated infections and just one uninfected patient did (p<0.001). Erysipelas was infrequent in all groups, but rarest among patients with uncomplicated infections (3.9% in group B vs. 10.3% in group A, p=0.038, and 17.6% in group C, p<0.001, respectively).Overall, cellulitis presented the highest risk for sepsis (5.320), followed by erythroderma (3.870) and erysipelas (2.849).Other differences in the incidence of venous ulcers, erythroderma, superinfected bullous dermatosis, and other dermatoses disruptive of the skin barrier were not significant.
Table 6.
Acute skin conditions in patients with vs. without sepsis.
Table 6.
Acute skin conditions in patients with vs. without sepsis.
| |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Venous ulcers |
0.110
|
0.740
|
-
|
| present |
301
|
68.3%
|
52
|
66.7%
|
249
|
68.6%
|
|
|
|
| absent |
140
|
31.7%
|
26
|
33.3%
|
114
|
31.4%
|
|
|
|
| Microbial eczema |
42.227
|
<0.001**
|
0.189 (0.111
÷
0.323)
|
| present |
273
|
61.9%
|
23
|
29.5%
|
250
|
68.9%
|
|
|
|
| absent |
168
|
38.1%
|
55
|
70.5%
|
113
|
31.1%
|
|
|
|
| Cellulitis |
43.470
|
<0.001**
|
5.320 (3.136
÷
9.026)
|
| present |
93
|
21.1%
|
38
|
48.7%
|
55
|
15.2%
|
|
|
|
| absent |
348
|
78.9%
|
40
|
51.3%
|
308
|
84.8%
|
|
|
|
| Superinfected bullous dermatoses |
1.149
|
0.284
|
-
|
| present |
29
|
6.6%
|
3
|
3.8%
|
26
|
7.2%
|
|
|
|
| absent |
412
|
93.4%
|
75
|
96.2%
|
337
|
92.8%
|
|
|
|
| Erysipelas |
5.548
|
0.038*
|
2.849 (1.152
÷
7.048)
|
| present |
22
|
5%
|
8
|
10.3%
|
14
|
3.9%
|
|
|
|
| absent |
419
|
95%
|
70
|
89.7%
|
349
|
96.1%
|
|
|
|
| Erythroderma |
4.518
|
0.056
|
3.870 (1.015
÷
14.757)
|
| present |
9
|
2%
|
4
|
5.1%
|
5
|
1.4%
|
|
|
|
| absent |
432
|
98%
|
74
|
94.9%
|
358
|
98.6%
|
|
|
|
| Other dermatosesfeaturing loss of tissue or the disruption of the skin barrier |
2.801
|
0.094
|
-
|
| present |
119
|
27%
|
27
|
34.6%
|
92
|
25.3%
|
|
|
|
| absent |
322
|
73%
|
51
|
65.4%
|
271
|
74.7%
|
|
|
|
| Total |
441
|
100%
|
78
|
100%
|
363
|
100%
|
|
|
|
Table 7.
Acute skin conditions in patients with uncomplicated infections vs. without infection.
Table 7.
Acute skin conditions in patients with uncomplicated infections vs. without infection.
| |
Total |
Infection |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group B) |
No (group C) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Venous ulcers |
73.049 |
<0.001** |
14.319 (6.862÷29.878) |
| present |
258 |
59.9% |
249 |
68.6% |
9 |
13.2% |
|
|
|
| absent |
173 |
40.1% |
114 |
31.4% |
59 |
86.8% |
|
|
|
| Microbial eczema |
40.897 |
<0.001** |
5.706 (3.213÷10.134) |
| present |
269 |
62.4% |
250 |
68.9% |
19 |
27.9% |
|
|
|
| absent |
162 |
37.6% |
113 |
31.1% |
49 |
72.1% |
|
|
|
| Cellulitis |
9.482 |
0.002** |
11.964 (1.627÷87.988) |
| present |
56 |
13.0% |
55 |
15.2% |
1 |
1.5% |
|
|
|
| absent |
375 |
87.0% |
308 |
84.8% |
67 |
98.5% |
|
|
|
| Superinfected bullous dermatoses |
0.230 |
0.632 |
- |
| present |
32 |
7.4% |
26 |
7.2% |
6 |
8.8% |
|
|
|
| absent |
399 |
92.6% |
337 |
92.8% |
62 |
91.2% |
|
|
|
| Erysipelas |
19.214 |
<0.001** |
0.187 (0.082÷0.425) |
| present |
26 |
6.0% |
14 |
3.9% |
12 |
17.6% |
|
|
|
| absent |
405 |
94.0% |
349 |
96.1% |
56 |
82.4% |
|
|
|
| Erythroderma |
0.877 |
0.305 |
- |
| present |
7 |
1.6% |
5 |
1.4% |
2 |
2.9% |
|
|
|
| absent |
424 |
98.4% |
358 |
98.6% |
66 |
97.1% |
|
|
|
| Other dermatoses featuring loss of tissue or the disruption of the skin barrier |
27.735 |
<0.001** |
0.252 (0.148÷0.431) |
| present |
131 |
30.4% |
92 |
25.3% |
39 |
57.4% |
|
|
|
| absent |
300 |
69.6% |
271 |
74.7% |
29 |
42.6% |
|
|
|
| Total |
431 |
100% |
363 |
100% |
68 |
100% |
|
|
|
The main bacterial agents evidencedin the cultured samples of skin lesionsfrom the 441 patients with uncomplicated infections and with sepsis were Staphylococcus aureus (43.3%), Pseudomonas aeruginosa (33.8%), Escherichia coli (12.9%), Klebsiella pneumoniae (9.8%), and Streptococcus β-hemolytic (5.9%).As can be seen in Table 8, only K. pneumoniaeandS. β-hemolyticwere significantly more common in the sepsis group (17.9% vs. 8%, p=0.007, and 11.5% vs. 4.7%, p=0.031, respectively). The noted differences can also be expressed as a 2.655 times higher risk of sepsis in streptococcal infections and a 2.519-fold increase in sepsis risk in the presence of K. pneumoniae.
In addition, various other species of bacteria were detected (30.4%) without significant variationsrelative to the presence or absence of sepsis (Proteus mirabilis, Serratia marcescens, Citrobacter freundii, Providenciastuartii, Stenotrophomonas maltophilia, Morganella morganii, and Enterobacter genus). The same can be said about the viral agents found in 9.5% of cases. The hepatitis B and C viruses identified were inactive and did not play an active role in the studied pathologies.
Table 8.
Etiological agents in the infected lesions with vs. without sepsis.
Table 8.
Etiological agents in the infected lesions with vs. without sepsis.
| |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| S. aureus |
1.451
|
0.228
|
-
|
| present |
191
|
43.3%
|
29
|
37.2%
|
162
|
44.6%
|
|
|
|
| absent |
250
|
56.7%
|
49
|
62.8%
|
201
|
55.4%
|
|
|
|
| P. aeruginosa |
0.029
|
0.865
|
-
|
| present |
149
|
33.8%
|
27
|
34.6%
|
122
|
33.6%
|
|
|
|
| absent |
292
|
66.2%
|
51
|
65.4%
|
241
|
66.4%
|
|
|
|
| E. coli |
2.125
|
0.145
|
-
|
| present |
57
|
12.9%
|
14
|
17.9%
|
43
|
11.8%
|
|
|
|
| absent |
384
|
87.1%
|
64
|
82.1%
|
320
|
88.2%
|
|
|
|
| K. pneumoniae |
7.237
|
0.007**
|
2.519 (1.262
÷
5.031)
|
| present |
43
|
9.8%
|
14
|
17.9%
|
29
|
8%
|
|
|
|
| absent |
398
|
90.2%
|
64
|
82.1%
|
334
|
92%
|
|
|
|
| S. β-hemolytic |
5.438
|
0.031*
|
2.655 (1.137
÷
6.200)
|
| present |
26
|
5.9%
|
9
|
11.5%
|
17
|
4.7%
|
|
|
|
| absent |
415
|
94.1%
|
69
|
88.5%
|
346
|
95.3%
|
|
|
|
| Other bacterial species |
0.124
|
0.724
|
-
|
| present |
134
|
30.4%
|
25
|
32.1%
|
109
|
30%
|
|
|
|
| absent |
307
|
69.6%
|
53
|
67.9%
|
254
|
70%
|
|
|
|
| Viral agents |
0.033
|
0.855
|
-
|
| present |
42
|
9.5%
|
7
|
9%
|
35
|
9.6%
|
|
|
|
| absent |
399
|
90.5%
|
71
|
91%
|
328
|
90.4%
|
|
|
|
| Total |
441
|
100%
|
78
|
100%
|
363
|
100%
|
|
|
|
3.4. Comorbidities
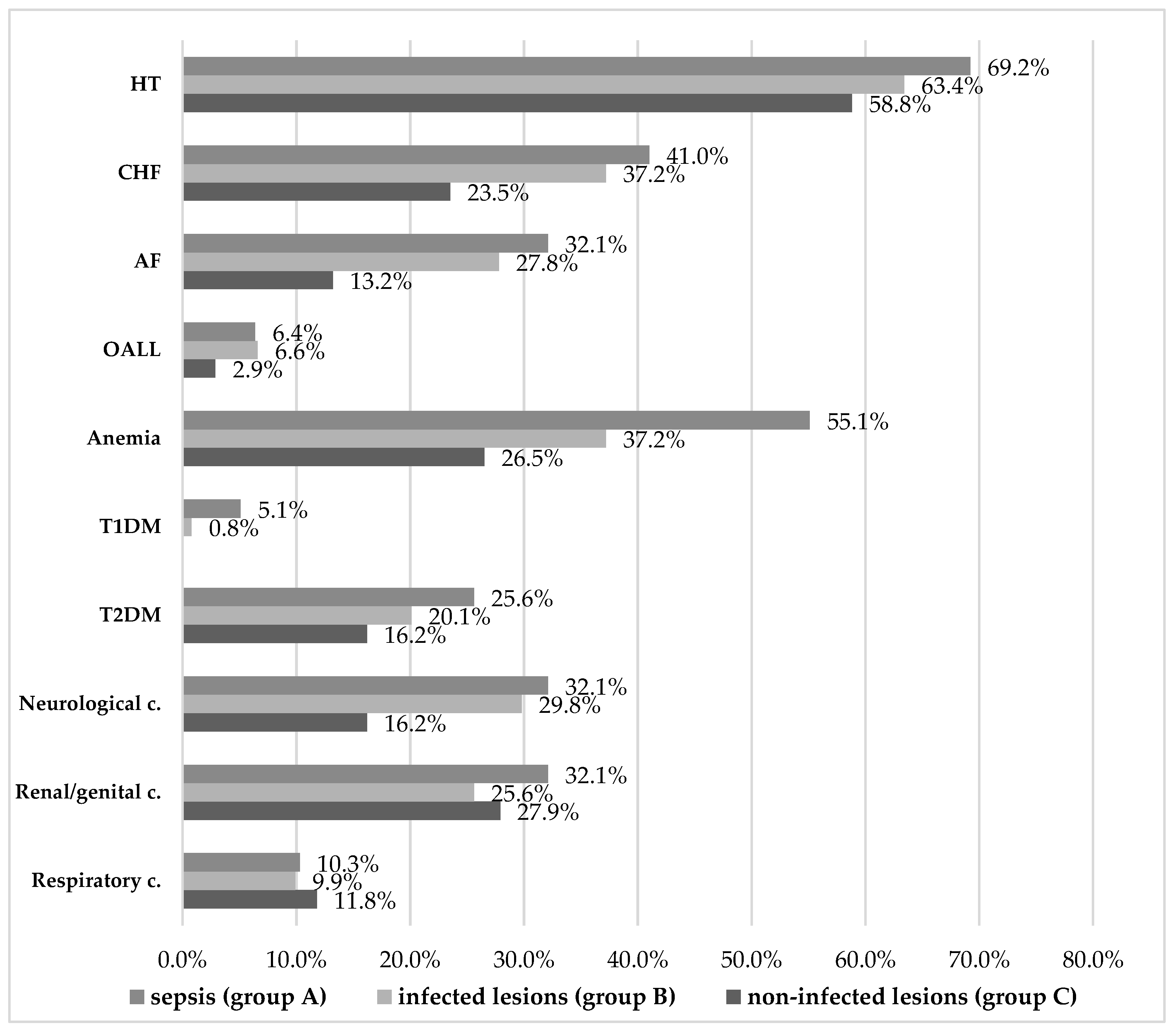
The associated pathologies of the patients enrolled in the study included cardiovascular diseases (hypertension / HT, chronic heart failure / CHF, atrial fibrillation / AF, obliterating arteriopathy of the lower limbs / OALL), microcytic hypochromic anemia, diabetesmellitus types 1 and 2 (T1DM and T2DM), neurological, renal/genital, and respiratory conditions summarized in Figure 2, and in Tables 9 and 10.
Overall, the most common underlying conditionswere cardiovascular, of which hypertension was prevalent in all study groups, with a non-significant 10% difference between patients with sepsis versus uninfected lesions. On the other hand, established chronic heart failure was significantly more frequent among patients with sepsis and those with uncomplicated infections, compared to uninfected patients. According to our data, the risk of infection was 1.924 times higher in the presence of NYHA I and II CHF. The same can be said about patients with established histories of atrial fibrillation, which increased the risk of infection 2.527-fold. Lower limb arteriopathy was the least common and differences were not conclusive.
Figure 2.
Comorbidities in the three study groups.
Figure 2.
Comorbidities in the three study groups.
Microcytic hypochromic anemia is another comorbidity that stands out as significantly more common in the sepsis group (55.1% vs. 37.2%, p=0.003). A risk of sepsis 2.075 times higher was thus calculated for patients with anemia, which suggests the importance of timely, adequate treatment of both the underlying hematological condition and of any incipient infection of skin lesions in patients with anemia.
Regarding diabetes, it appears that type 1 diabetes mellitus was significantly associated with sepsis, butpatient numbers were too small to make a reliable determination (only 7 cases). This does not mean that T1DM patients are unlikely to develop skin conditions with related infections or sepsis, but rather that it is possible such patients were prioritized for treatment in the emergency, diabetes, or another department. The fact that all T1DM patients admitted in our unit had infected lesions or even sepsis is suggestive of the risks associated with T1DM. The 6.486-fold risk of these patients invitesfurther targeted research of the role of underlying T1DM in the progression to sepsis of skin lesion infections.
At the same time, type 2 diabetes was more common (104 vs. 7 patients overall), and it was noted in all three study groups. Even if it was most frequent in patients with sepsis (25.6%) and with infected lesions (20.1%), and less so in uninfected patients (16.2%), these differences did not achieve statistical significance.
Table 9.
Comorbidities in patients with infected lesions – with vs. without sepsis.
Table 9.
Comorbidities in patients with infected lesions – with vs. without sepsis.
| |
Total |
Sepsis |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group A) |
No (group B) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Hypertension |
0.965
|
0.326
|
-
|
| present |
284
|
64.4%
|
54
|
69.2%
|
230
|
63.4%
|
|
|
|
| absent |
157
|
35.6%
|
24
|
30.8%
|
133
|
36.6%
|
|
|
|
| Chronic heart failure |
0.401
|
0.526
|
-
|
| present |
167
|
37.9%
|
32
|
41%
|
135
|
37.2%
|
|
|
|
| absent |
274
|
62.1%
|
46
|
59%
|
228
|
62.8%
|
|
|
|
| Atrial fibrillation |
0.562
|
0.453
|
-
|
| present |
126
|
28.6%
|
25
|
32.1%
|
101
|
27.8%
|
|
|
|
| absent |
315
|
71.4%
|
53
|
67.9%
|
262
|
72.2%
|
|
|
|
| Obliterating arteriopathy of the lower limbs (OALL) |
0.004
|
0.948
|
-
|
| present |
29
|
6.6%
|
5
|
6.4%
|
24
|
6.6%
|
|
|
|
| absent |
412
|
93.4%
|
73
|
93.6%
|
339
|
93.4%
|
|
|
|
| Anemia |
8.583
|
0.003**
|
2.075 (1.266
÷
3.402)
|
| present |
178
|
40.4%
|
43
|
55.1%
|
135
|
37.2%
|
|
|
|
| absent |
263
|
59.6%
|
35
|
44.9%
|
228
|
62.8%
|
|
|
|
| Type 1 diabetes mellitus |
7.606
|
0.021*
|
6.486 (1.422
÷
29.590)
|
| present |
7
|
1.6%
|
4
|
5.1%
|
3
|
0.8%
|
|
|
|
| absent |
434
|
98.4%
|
74
|
94.9%
|
360
|
99.2%
|
|
|
|
| Type 2 diabetes mellitus |
1.180
|
0.277
|
-
|
| present |
93
|
21.1%
|
20
|
25.6%
|
73
|
20.1%
|
|
|
|
| absent |
348
|
78.9%
|
58
|
74.4%
|
290
|
79.9%
|
|
|
|
| Neurological conditions |
0.161
|
0.688
|
-
|
| present |
133
|
30.2%
|
25
|
32.1%
|
108
|
29.8%
|
|
|
|
| absent |
308
|
69.8%
|
53
|
67.9%
|
255
|
70.2%
|
|
|
|
| Renal / genital conditions |
1.355
|
0.244
|
-
|
| present |
118
|
26.8%
|
25
|
32.1%
|
93
|
25.6%
|
|
|
|
| absent |
323
|
73.2%
|
53
|
67.9%
|
270
|
74.4%
|
|
|
|
| Respiratory conditions |
0.008
|
0.928
|
-
|
| present |
44
|
10%
|
8
|
10.3%
|
36
|
9.9%
|
|
|
|
| absent |
397
|
90%
|
70
|
89.7%
|
327
|
90.1%
|
|
|
|
| Total |
441
|
100%
|
78
|
100%
|
363
|
100%
|
|
|
|
Last but not least, neurological conditions were significantly more common in patients with sepsis (32.1%) and infected lesions (29.8%) than in the uninfected group (16.2%). Neurological health plays a role in the body’s ability to heal and to fight off pathogens, as this result appears to illustrate with regard to the skin barrier. The distribution of other, respiratory, renal/genital conditions noted in our patients was not associated with our patients’ status relative to infection and sepsis.
Table 10.
Comorbidities in patients with infected lesions (without sepsis) vs. non-infected lesions.
Table 10.
Comorbidities in patients with infected lesions (without sepsis) vs. non-infected lesions.
| |
Total |
Infection |
Pearson
Chi-squared |
OR
95% CI |
| Yes (group B) |
No (group C) |
| |
N |
% |
N |
% |
N |
% |
Chi2
|
p |
|
| Hypertension |
0.504
|
0.478
|
-
|
| present |
270
|
62.6%
|
230
|
63.4%
|
40
|
58.8%
|
|
|
|
| absent |
161
|
37.4%
|
133
|
36.6%
|
28
|
41.2%
|
|
|
|
| Chronic heart failure |
4.696
|
0.030*
|
1.924 (1.057
÷
3.504)
|
| present |
151
|
35.0%
|
135
|
37.2%
|
16
|
23.5%
|
|
|
|
| absent |
280
|
65.0%
|
228
|
62.8%
|
52
|
76.5%
|
|
|
|
| Atrial fibrillation |
6.412
|
0.011*
|
2.527 (1.208
÷
5.286)
|
| present |
110
|
25.5%
|
101
|
27.8%
|
9
|
13.2%
|
|
|
|
| absent |
321
|
74.5%
|
262
|
72.2%
|
59
|
86.8%
|
|
|
|
| Obliterating arteriopathy of the lower limbs (OALL) |
1.361
|
0.402
|
-
|
| present |
26
|
6.0%
|
24
|
6.6%
|
2
|
2.9%
|
|
|
|
| absent |
405
|
94.0%
|
339
|
93.4%
|
66
|
97.1%
|
|
|
|
| Anemia |
2.874
|
0.090
|
-
|
| present |
153
|
35.5%
|
135
|
37.2%
|
18
|
26.5%
|
|
|
|
| absent |
278
|
64.5%
|
228
|
62.8%
|
50
|
73.5%
|
|
|
|
| Type 1 diabetes mellitus |
0.566
|
1.000
|
-
|
| present |
3
|
0.7%
|
3
|
0.8%
|
|
0% |
|
|
|
| absent |
428
|
99.3%
|
360
|
99.2%
|
68
|
100%
|
|
|
|
| Type 2 diabetes mellitus |
0.565
|
0.452
|
-
|
| present |
84
|
19.5%
|
73
|
20.1%
|
11
|
16.2%
|
|
|
|
| absent |
347
|
80.5%
|
290
|
79.9%
|
57
|
83.8%
|
|
|
|
| Neurological conditions |
5.281
|
0.022*
|
2.195 (1.108
÷
4.347)
|
| present |
119
|
27.6%
|
108
|
29.8%
|
11
|
16.2%
|
|
|
|
| absent |
312
|
72.4%
|
255
|
70.2%
|
57
|
83.8%
|
|
|
|
| Renal / genital conditions |
0.160
|
0.689
|
-
|
| present |
112
|
26.0%
|
93
|
25.6%
|
19
|
27.9%
|
|
|
|
| absent |
319
|
74.0%
|
270
|
74.4%
|
49
|
72.1%
|
|
|
|
| Respiratory conditions |
0.213
|
0.644
|
-
|
| present |
44
|
10.2%
|
36
|
9.9%
|
8
|
11.8%
|
|
|
|
| absent |
387
|
89.8%
|
327
|
90.1%
|
60
|
88.2%
|
|
|
|
| Total |
431
|
100%
|
363
|
100%
|
68
|
100%
|
|
|
|
3.5. Multivariate logistic regression analyses of risk factors for infection and sepsis
Two6-step,logistic regression models were built to assess the combined risk of infection and, separately, of sepsis, exerted by the individual parameters previously identified as significantly associated with infection and, respectively, with sepsis.
The model generated to assess the risk of infection explained 42.5% of the variations recorded in the incidence of infected skin lesions and adequately classified 87.2% of cases with 97.5% sensitivity and 32.4% specificity (p<0.001). The Nagelkerke R2 coefficient was used to determine to what extent infection could be explained by independent variables of interest, and all the risk factors flagged as statistically significant are listed in Table 11.
Table 11.
Multivariate analysis of risk factors for infection.
Table 11.
Multivariate analysis of risk factors for infection.
| Risk factors |
|
|
|
95% CI |
| (statistically significant) |
Coef. B |
P-value |
Odds Ratio |
Lower |
Upper |
| Rural background |
1.066
|
0.001**
|
2.903
|
1.521
|
5.538
|
| Neurological conditions |
1.203
|
0.002**
|
3.329
|
1.527
|
7.260
|
| E. coli |
2.368
|
0.026*
|
10.679
|
1.335
|
85.416
|
| Venous ulcers |
2.205
|
<0.001**
|
9.073
|
3.833
|
21.480
|
| Cellulitis |
2.205
|
0.037*
|
9.071
|
1.137
|
72.347
|
| Microbial eczema |
1.130
|
0.002**
|
3.094
|
1.489
|
6.432
|
| Constant |
-0.789
|
0.009
|
0.454
|
|
|
The cumulated statistical effect of these risk factors was also analyzedto identify the probability of infection in the various combinations of factors (absent- 0 / present - 1). Probability values >0.5 were considered relevant, as illustrated with the following examples which interested readers may use to interpret other such results listed in Table 12. The risk of infection was highest (especially with E. coli) in rural residents with histories of neurological conditions, who were admitted with venous ulcers, cellulitis, and microbial eczema. The risk remained at the highest level even in the absence of one or two of these factors, if the rest were present.
Table 12.
Risk of infection in the combined presence of individual risk factors.
Table 12.
Risk of infection in the combined presence of individual risk factors.
Rural
residence |
Neurological conditions |
E. coli |
Venous
ulcers |
Cellulitis |
Microbial
eczema |
Risk of
infection |
| 0 |
0 |
0 |
0 |
0 |
0 |
0.31 |
| 0 |
0 |
0 |
0 |
0 |
1 |
0.58 |
| 0 |
0 |
0 |
0 |
1 |
0 |
0.80 |
| 0 |
0 |
0 |
0 |
1 |
1 |
0.93 |
| 0 |
0 |
0 |
1 |
0 |
0 |
0.80 |
| 0 |
0 |
0 |
1 |
0 |
1 |
0.93 |
| 0 |
0 |
0 |
1 |
1 |
0 |
0.97 |
| 0 |
0 |
0 |
1 |
1 |
1 |
0.99 |
| 0 |
0 |
1 |
0 |
0 |
0 |
0.83 |
| 0 |
0 |
1 |
0 |
0 |
1 |
0.94 |
| 0 |
0 |
1 |
0 |
1 |
0 |
0.98 |
| 0 |
0 |
1 |
0 |
1 |
1 |
0.99 |
| 0 |
0 |
1 |
1 |
0 |
0 |
0.98 |
| 0 |
0 |
1 |
1 |
0 |
1 |
0.99 |
| 0 |
0 |
1 |
1 |
1 |
0 |
1.00 |
| 0 |
0 |
1 |
1 |
1 |
1 |
1.00 |
| 0 |
1 |
0 |
0 |
0 |
0 |
0.60 |
| 0 |
1 |
0 |
0 |
0 |
1 |
0.82 |
| 0 |
1 |
0 |
0 |
1 |
0 |
0.93 |
| 0 |
1 |
0 |
0 |
1 |
1 |
0.98 |
| 0 |
1 |
0 |
1 |
0 |
0 |
0.93 |
| 0 |
1 |
0 |
1 |
0 |
1 |
0.98 |
| 0 |
1 |
0 |
1 |
1 |
0 |
0.99 |
| 0 |
1 |
0 |
1 |
1 |
1 |
1.00 |
| 0 |
1 |
1 |
0 |
0 |
0 |
0.94 |
| 0 |
1 |
1 |
0 |
0 |
1 |
0.98 |
| 0 |
1 |
1 |
0 |
1 |
0 |
0.99 |
| 0 |
1 |
1 |
0 |
1 |
1 |
1.00 |
| 0 |
1 |
1 |
1 |
0 |
0 |
0.99 |
| 0 |
1 |
1 |
1 |
0 |
1 |
1.00 |
| 0 |
1 |
1 |
1 |
1 |
0 |
1.00 |
| 0 |
1 |
1 |
1 |
1 |
1 |
1.00 |
| 1 |
0 |
0 |
0 |
0 |
0 |
0.57 |
| 1 |
0 |
0 |
0 |
0 |
1 |
0.80 |
| 1 |
0 |
0 |
0 |
1 |
0 |
0.92 |
| 1 |
0 |
0 |
0 |
1 |
1 |
0.97 |
| 1 |
0 |
0 |
1 |
0 |
0 |
0.92 |
| 1 |
0 |
0 |
1 |
0 |
1 |
0.97 |
| 1 |
0 |
0 |
1 |
1 |
0 |
0.99 |
| 1 |
0 |
0 |
1 |
1 |
1 |
1.00 |
| 1 |
0 |
1 |
0 |
0 |
0 |
0.93 |
| 1 |
0 |
1 |
0 |
0 |
1 |
0.98 |
| 1 |
0 |
1 |
0 |
1 |
0 |
0.99 |
| 1 |
0 |
1 |
0 |
1 |
1 |
1.00 |
| 1 |
0 |
1 |
1 |
0 |
0 |
0.99 |
| 1 |
0 |
1 |
1 |
0 |
1 |
1.00 |
| 1 |
0 |
1 |
1 |
1 |
0 |
1.00 |
| 1 |
0 |
1 |
1 |
1 |
1 |
1.00 |
| 1 |
1 |
0 |
0 |
0 |
0 |
0.81 |
| 1 |
1 |
0 |
0 |
0 |
1 |
0.93 |
| 1 |
1 |
0 |
0 |
1 |
0 |
0.98 |
| 1 |
1 |
0 |
0 |
1 |
1 |
0.99 |
| 1 |
1 |
0 |
1 |
0 |
0 |
0.98 |
| 1 |
1 |
0 |
1 |
0 |
1 |
0.99 |
| 1 |
1 |
0 |
1 |
1 |
0 |
1.00 |
| 1 |
1 |
0 |
1 |
1 |
1 |
1.00 |
| 1 |
1 |
1 |
0 |
0 |
0 |
0.98 |
| 1 |
1 |
1 |
0 |
0 |
1 |
0.99 |
| 1 |
1 |
1 |
0 |
1 |
0 |
1.00 |
| 1 |
1 |
1 |
0 |
1 |
1 |
1.00 |
| 1 |
1 |
1 |
1 |
0 |
0 |
1.00 |
| 1 |
1 |
1 |
1 |
0 |
1 |
1.00 |
| 1 |
1 |
1 |
1 |
1 |
0 |
1.00 |
| 1 |
1 |
1 |
1 |
1 |
1 |
1.00 |
The model generated to assess the risk of sepsis explained 41.9% of the variations recorded in the incidence of sepsis (the Nagelkerke R2 coefficient), and correctly classified 84.8% of cases with 38.5% specificity and 94.8% sensitivity (p<0.001). The risk factors identified as statistically significant were different than for the risk of infection, except for the diagnosis of cellulitis, which featured in both models: mildly altered mental status, fever and chills, adenopathy, and established histories type 1 diabetes mellitus and anemia (see Table 13).
Table 13.
Multivariate analysis of risk factors for sepsis.
Table 13.
Multivariate analysis of risk factors for sepsis.
| Risk factors |
|
|
|
95% CI |
| (statistically significant) |
Coef. B |
P-value |
Odds Ratio |
Lower |
Upper |
| Glasgow score 13, 14 |
1.696
|
0.023*
|
5.451
|
1.268
|
23.432
|
| Fever, chills |
2.251
|
<0.001**
|
9.497
|
4.703
|
19.177
|
| Adenopathy |
1.384
|
0.003**
|
3.993
|
1.594
|
10.003
|
| Type 1 diabetes mellitus |
2.668
|
0.005**
|
14.404
|
2.205
|
94.101
|
| Anemia |
0.678
|
0.026*
|
1.971
|
1.086
|
3.578
|
| Cellulitis |
1.180
|
<0.001**
|
3.253
|
1.652
|
6.404
|
| Constant |
-3.703
|
<0.001
|
0.025
|
|
|
As for the probability of a patient to suffer from sepsis in the combined presence of significant individual risk factors identified above, the results are summarized in Table 14. The risk for sepsis was at the highest level in patients with several underlying conditions (type-1 diabetes mellitus, anemia), whose lesions were diagnosed as cellulitis, and who were admitted with fever, chills, adenopathy, and mildly altered mental status. The risk remained very high even in the absence of one, two, or even three of these factors. Absent fever and chills brought the risk of sepsis to irrelevant levels, as can be expected.
Table 14.
Risk of sepsis in the combined presence of individual risk factors.
Table 14.
Risk of sepsis in the combined presence of individual risk factors.
| Glasgow scores 13, 14 |
Fever,
chills |
Adenopathy |
T1DM |
Anemia |
Cellulitis |
Risk of
sepsis |
| 0 |
0 |
0 |
0 |
0 |
0 |
0.02 |
| 0 |
0 |
0 |
0 |
0 |
1 |
0.07 |
| 0 |
0 |
0 |
0 |
1 |
0 |
0.05 |
| 0 |
0 |
0 |
0 |
1 |
1 |
0.14 |
| 0 |
0 |
0 |
1 |
0 |
0 |
0.26 |
| 0 |
0 |
0 |
1 |
0 |
1 |
0.54 |
| 0 |
0 |
0 |
1 |
1 |
0 |
0.41 |
| 0 |
0 |
0 |
1 |
1 |
1 |
0.69 |
| 0 |
0 |
1 |
0 |
0 |
0 |
0.09 |
| 0 |
0 |
1 |
0 |
0 |
1 |
0.24 |
| 0 |
0 |
1 |
0 |
1 |
0 |
0.16 |
| 0 |
0 |
1 |
0 |
1 |
1 |
0.39 |
| 0 |
0 |
1 |
1 |
0 |
0 |
0.59 |
| 0 |
0 |
1 |
1 |
0 |
1 |
0.82 |
| 0 |
0 |
1 |
1 |
1 |
0 |
0.74 |
| 0 |
0 |
1 |
1 |
1 |
1 |
0.90 |
| 0 |
1 |
0 |
0 |
0 |
0 |
0.19 |
| 0 |
1 |
0 |
0 |
0 |
1 |
0.43 |
| 0 |
1 |
0 |
0 |
1 |
0 |
0.32 |
| 0 |
1 |
0 |
0 |
1 |
1 |
0.60 |
| 0 |
1 |
0 |
1 |
0 |
0 |
0.77 |
| 0 |
1 |
0 |
1 |
0 |
1 |
0.92 |
| 0 |
1 |
0 |
1 |
1 |
0 |
0.87 |
| 0 |
1 |
0 |
1 |
1 |
1 |
0.96 |
| 0 |
1 |
1 |
0 |
0 |
0 |
0.48 |
| 0 |
1 |
1 |
0 |
0 |
1 |
0.75 |
| 0 |
1 |
1 |
0 |
1 |
0 |
0.65 |
| 0 |
1 |
1 |
0 |
1 |
1 |
0.86 |
| 0 |
1 |
1 |
1 |
0 |
0 |
0.93 |
| 0 |
1 |
1 |
1 |
0 |
1 |
0.98 |
| 0 |
1 |
1 |
1 |
1 |
0 |
0.96 |
| 0 |
1 |
1 |
1 |
1 |
1 |
0.99 |
| 1 |
0 |
0 |
0 |
0 |
0 |
0.12 |
| 1 |
0 |
0 |
0 |
0 |
1 |
0.30 |
| 1 |
0 |
0 |
0 |
1 |
0 |
0.21 |
| 1 |
0 |
0 |
0 |
1 |
1 |
0.46 |
| 1 |
0 |
0 |
1 |
0 |
0 |
0.66 |
| 1 |
0 |
0 |
1 |
0 |
1 |
0.86 |
| 1 |
0 |
0 |
1 |
1 |
0 |
0.79 |
| 1 |
0 |
0 |
1 |
1 |
1 |
0.93 |
| 1 |
0 |
1 |
0 |
0 |
0 |
0.35 |
| 1 |
0 |
1 |
0 |
0 |
1 |
0.64 |
| 1 |
0 |
1 |
0 |
1 |
0 |
0.51 |
| 1 |
0 |
1 |
0 |
1 |
1 |
0.77 |
| 1 |
0 |
1 |
1 |
0 |
0 |
0.89 |
| 1 |
0 |
1 |
1 |
0 |
1 |
0.96 |
| 1 |
0 |
1 |
1 |
1 |
0 |
0.94 |
| 1 |
0 |
1 |
1 |
1 |
1 |
0.98 |
| 1 |
1 |
0 |
0 |
0 |
0 |
0.56 |
| 1 |
1 |
0 |
0 |
0 |
1 |
0.81 |
| 1 |
1 |
0 |
0 |
1 |
0 |
0.72 |
| 1 |
1 |
0 |
0 |
1 |
1 |
0.89 |
| 1 |
1 |
0 |
1 |
0 |
0 |
0.95 |
| 1 |
1 |
0 |
1 |
0 |
1 |
0.98 |
| 1 |
1 |
0 |
1 |
1 |
0 |
0.97 |
| 1 |
1 |
0 |
1 |
1 |
1 |
0.99 |
| 1 |
1 |
1 |
0 |
0 |
0 |
0.84 |
| 1 |
1 |
1 |
0 |
0 |
1 |
0.94 |
| 1 |
1 |
1 |
0 |
1 |
0 |
0.91 |
| 1 |
1 |
1 |
0 |
1 |
1 |
0.97 |
| 1 |
1 |
1 |
1 |
0 |
0 |
0.99 |
| 1 |
1 |
1 |
1 |
0 |
1 |
1.00 |
| 1 |
1 |
1 |
1 |
1 |
0 |
0.99 |
| 1 |
1 |
1 |
1 |
1 |
1 |
1.00 |
4. Discussion
4.1. Study results overview and general considerations
To prevent infection and further, life-threatening complications such as sepsis,dermatological conditions disruptive of the skin barrier should be promptly diagnosed and adequately treated. In this study, we analyzed the incidence of skin lesions associated with infections and sepsis in 509 adult patients fromNE Romania. The patients were admitted to the dermatology unit of the largest emergency hospital in the region over a three-year period (2020-2022), and those with concomitant infectious conditions or sepsis of other causes, including SARS-CoV-2, were not included. The patients presented with lesions featuring loss of tissue e.g., ulcerations, erosions, fissures, excoriations, as well as bullae and vesicles. The accompanying signs and symptoms ranged from pruritus, erythema, tumefaction to edema, pain, fever, chills, and adenopathy, telltale signs of inflammatory processes often caused by infection, but also mildly altered mental status. Etiological agents, mostly bacterial, were found to superinfect the patients’ skin lesions in 363 cases (71.3%), and 78 patients were in sepsis (15.3%).Lesions were uncontaminated in only 68 cases (13.3%), which include patients whose lesions may have been infected prior to, but not on admission, as a result of various (self-)treatments.
Currently available literature data on the incidence of acute skin infections and cutaneous-onset sepsis are scarce.An analysis of national US care data surveys from 1997 to 2005 revealed a 50% rise in visit rates from increasingly younger patients to diagnose and treat skin and soft tissue infections (SSTIs), especially in emergency settings, and mostly for cellulitis or abscesses [
13]. In addition, there were29% more hospital admissions in the US due to SSTIs in 2004 than in 2000, reaching close to 900,000 acute-care admissions, while pneumonia-related hospitalizations remained roughly the same [
14].
In Europe, researchers from Spainreviewed infection-related presentations in emergency departments and found that 1,250 patients had SSTIs (11%). Of them, 3.3% had signs and symptoms of septic syndrome [
15]. In a recent review focusing on the skin-sepsis relationship, sepsis or bacteremia cases were between 4.8% to 16% of SSTIs, with our result notably approaching the higher end of this range [
8].The7-year SENTRY Antimicrobial Surveillance Program implemented in Europe, North America and Latin America identified
Staphylococcus aureusas the main culprit behind the rising incidence of skin infections across all regions, along with
Pseudomonas aeruginosa, Escherichia coli, and
Enterococcus species [
16].
Age is generally known as a riskfactor in the progression to sepsis, aselderly patients are more likely to suffer from diminished immune response, malnutrition, multiple comorbidities, poorer body hygiene, skin injuries, etc. The combined effectsof these aspects of advanced age can complicate and accelerateinfection towards septic shock and even exitus. Numerous studies such as by Gabriel Wardi et al. concluded this and reported close correlations between the severity of associated pathologies and extent of organ dysfunction on one hand and unfavorable prognosis on the other [
17]. However, our results based on a significant sample of patients of all adult ages showed that age differences made far less of a difference thancomorbidities.
Information on sepsis epidemiology and patterns in patients under the age of 60 is limited, but not altogether absent. Carmen Bouza surveyed the national Spanish health data between 2006-2015, finding 28,351 cases of sepsis in patients aged 20-44 years, which amounted to 3.06‰ of all hospitalizations for this age group [
18]. Inour studyon patients aged 18 to 92, with a mean overall age of 64.22 (median 65), the patients with confirmed, cutaneous-onset sepsis were of all ages (18-88) and younger on average (mean 61.33, median 62) compared to patients without sepsis, at a weakly significant p=0.046. This result challenges existing notions related to age and sepsis, inviting further research into other potentiating factors such as comorbidities. It also underscores the importance of addressing skin lesions before they become contaminated.
Regarding background, rural residence has generally been proven to limit or delay access to health services. Primary and secondary outpatient or hospital care services are more readily available in towns and cities. Delayed diagnosis and management lead to further aggravation and complications that statistically show up as higher rates of infection, sepsis, and mortality among patients from rural areas [
19]. Our data focused exclusively on skin lesions makes this distinction very clear for all three studied situations: sepsis, uncomplicated infections, and uncontaminated lesions.
4.2. In-depth discussion of studied lesions
The onset of infectious dermatoses is often sudden, with soft tissue erythema, induration, local heat and pain, followed by more generalized symptoms such as fever, deterioration of vital functions, single or multiple organ dysfunction in the case of septic complications. Careful analysis of local and systemic manifestations allows a correct diagnosis to be made and appropriate treatment to be rapidly instituted. The acute skin infections diagnosed in our patients included trophic ulcers in advanced stages of chronic venous insufficiency, microbial eczema, cellulitis, erysipelas, bullous dermatoses (pemphigus, pemphigoid, erythema multiforme, Stevens-Johnson syndrome), erythroderma, and other dermatoses (ulcerated cutaneous neoplasms, atopic dermatitis in the exudative phase, tinea pedis intertriginosa).
Cellulitis typically involves a bacterial infection of the dermis and subcutaneous cellular tissue, most commonly the lower limbs (70-80% of cases) [
20].A recurrent medical emergency, it starts abruptly with soft tissue erythema, warmth, and local tenderness, and is most often caused by
Streptococcus pyogenes and/or
Staphylococcus aureus. In our study, more than 90% of the 93 patients with cellulitis had lower limb lesions, and just one patient was not infected. In approximately 40% of cases, multiple types of bacteria were evidenced, the other 60% being mono-bacterial infections. The main species found were
P. aeruginosa (38.7%) and
Staphylococcus (35.48%), while
K. pneumoniae, E. coli, β-hemolytic Streptococcus were present in 15-10% of cases. Some patients were also positive for
P. mirabilis, S. marcescens, C. freundi, P. stuartii, S. maltophilia, M. morganii, and
Enterobacter.
Erysipelas resembles cellulitis, with the difference that it usually affects the skin more superficially [
20]. The patient presents to the physician for sudden onset of an erythematous edematous plaque with unilateral inflammatory signs, chills, regional adenopathy, most often on the lower limbs (70-90% of cases). Less frequent upper limb localization (5-10%) has been associated with lymphoedema after breast neoplasia in women. Facial lesions are the rarest (5% of cases). The main pathogens involved in erysipelas are
Streptococcus pyogenes(58-67% of cases
), S. dysgalactiae sp. Echisimilis(14-25%), and
S. agalactiae(3-9%). Other bacteria found to accompany these streptococci are
Staphylococcus aureus (10-17%),
Pseudomonas aeruginosa, and enterobacteria (5-50%) [
21]. In our study, in addition to these species, cultured samples also revealed colonization with
K. Pneumoniaeand
E. coli, more so in the group with confirmed sepsis (17.9% of cases) than in uncomplicated infections (8%
K. pneumoniae, 11.8%
E. coli).
Venous ulcers are a manifestation of chronic venous insufficiency (CVI), and they begin as small, round ulcerations that gradually expand to sometimes encompass the entire circumference of the calf.Edges are irregular, smooth or slightly elevated, and the surface is covered with cellular debris from microbial colonization. Venous ulcers are typically located in the distal 1/3 of the calf, the internal malleolar region (60-70% of cases), less often on the calf’s middlethird(18-20%), and most infrequently on the upper third (4-6%)or higher up the leg (3-4%) [
22]. Venous ulcers were the most common pathology diagnosed in our patients (>60% of sepsis and uncomplicated infection cases), associated with bacterialsuperinfection with
S. aureus,
P. aeruginosa,
K. pneomoniae,
E. coli, as well as with para-venous microbial eczema.
Bullous dermatoses including pemphigus, bullous pemphigoid, erythema multiforme, Stevens-Johnson syndrome, can compromisethe integrity of the epithelium and mucous membranes, thus undermining systemic homeostasis. Their etiopathogenic polymorphism is facilitated by numerous predisposing or favoring factors, and it correlates inversely with prognosis and quality of life [
23].In pemphigus, for instance, intraepidermal bullae are caused by the multifactorial activation of the immune system. The vesiculobullous lesions initially form in the mucous membranes and subsequently extend to the trunk. The buccal mucosa is damaged by flaccid vesicles that rupture easily and leave painful erosions, undermining the patient’s ability and willingness to eat, leading to weight loss and malnutrition. The cutaneous phase usually occurs after a latent period of several days to several months, and consists of a bullous, monomorphic rash that spreads rapidly, sometimes over the entire body [
24]. To achieve a good outcome, early bacteriological investigations, antibacterial therapy, electrolyte, and nutritional support are necessary [
25]. Bullous pemphigoid, anotherautoimmune condition from this category that can progress to sepsis, tends to be more common in patients aged 60-80 years. In the presence of staphylococcal or streptococcal infection (or of viral infection with herpes simplex or varicella-zoster), autoantibodies can be triggered against two hemidesmosomes (multiprotein complexes) that normally help basal epithelial cells adhere to the base membrane[
26]. Even if not the most frequent, we can see why such cases constitute dermatological emergencies. In our study, aggravation to severe forms with septic complications and substantial hydro-electrolyte imbalanceoccurred in 3.8% of the 29 patients with superinfected bullous dermatoses.
As the name suggests, erythroderma is clinically expressed as redness (erythema), and it is diagnosed as such when more than 90% of the body surface is affected. Redness is not always a sign of infection; it also occurs in other skin conditions, in neoplasia or as hypersensitivity to a certain drug the patient has taken. The incidence of erythroderma is about 1 in 100,000 adults, mostly men and typically aged 41-61, without racial predilection [27, 28]. In most cases, erythroderma is triggered by the exacerbation of a pre-existing dermatosis such as psoriasis (23% of cases), atopic dermatitis and contact dermatitis [
29]. The pathophysiological changes that justify treating a patient with erythroderma as a medical emergency are altered hemodynamic parameters, fever, hypoproteinemia/hypoalbuminemia, co-occurrence of edema, loss of fluids, electrolyte and/or acid-base imbalance, superinfection with risk of or confirmed sepsis [
30]. Erythroderma was rare in our study (only 11 patients – 4 in sepsis, 5 with uncomplicated infections, 2 uninfected), but ourclinical experience agrees with the medical literature.
Finally, a minority of patients also suffered from other dermatoses causing lesions disruptive of the skin barrier e.g., psoriasis, atopic dermatitis, dermatitis herpetiformis, vasculitis, and ulcerated skin neoplasms.
4.3. The role of comorbidities in acute skin infections and related sepsis
Comorbidities weaken the body’s defense mechanisms and can facilitate the spread of infection, eventually sepsis, by promoting the release of pro-inflammatory cytokines, altering coagulation processes, etc. In a previously cited study from Spain, 44% of patients hospitalized for soft tissue and skin infections had comorbidities, with diabetes and heart disease as significant risk factors for major adverse events [
15].The patients included in our study presented a range of underlying conditions representative of broader trends seen in cardiovascular, metabolic, neurological and other forms of chronic illness in our modern society. The multivariate analysis flagged type-1 diabetes mellitus and anemia as the most relevant comorbidities contributing to the risk for both infection and sepsis, particularly in patients with cellulitis.
Hypertension was the most common comorbidity across all studied groups, especially in the sepsis group (69.2% vs 58.8% of non-infected patients), but with non-significant differences. On the other hand, while 41% of patients with sepsis had established chronic heart failure, less than a quarter of patients with uncontaminated lesions did (23.5%). Importantly, sepsis is known to suppress myocardial function, favor diastolic dysfunction, decrease the cardiac index and output. According to literature data, patients with preserved ejection fraction are at a higher risk of mortality (2.4%) from sepsis than those without heart failure (0.4%),explained by insufficient cardiovascular reserves during systemic infection [
31]. In our study, the infectious risk of patients was 1.924-fold higher in the presence of NYHA class I, II heart failure.
During sepsis, the systemic release of pro-inflammatory cytokines, elevated levels of stress hormones, and changes in intravascular volume can compromise cardiovascular function, including electrical function, leading to the aggravation of pre-existing cardiac arrhythmiasor tonew-onset arrhythmias. One of the most common types among critically ill patients is atrial fibrillation [
32].Indeed, in our study, an established history of atrial fibrillation was significantly more frequent in the sepsis group (32.1%) compared to 27.8% of patients with uncomplicated infections and just 13.2% of non-infected patients. It appeared to increase infectious risk 2.527 times. Other published data show that sepsis is a risk factor for fibrillation, with new-onset atrial fibrillation being associated with a higher mortality rate than pre-existing fibrillation [
33].
In our study, microcytic hypochromic anemia was present in 37.2% of patients with acute skin infections and 55.1% of patients with sepsis, suggesting that patients with anemia are twice as likely to develop sepsis(odds ratio 2.075 at 95% confidence interval). Similar results were obtained by Czempik et al. in their prospective study of 90 patients with sepsis admitted to the ICU, where 42% had iron deficiency anemia. Iron is essential for host immunity and hemoglobin synthesis, which explains why patients with anemiaare more vulnerable to sepsis [
34]. In a study on 70 patients with sepsis and septic shock, as many as 82.6% hadvarious forms of anemia related to chronic illness and hemolysis in severe stages of sepsis, recurring phlebotomy and hemodilution. Hemolysis has been attributed to increased concentrations of hem, haptoglobin, hemopexin, and haemoxygenase-1 [
35]. Although the cases of anemia in our study were limited in type to disturbances of systemic iron homeostasis, our results align with international studies, thus providing a sound basis for future research on prognostic factors of cutaneous-onset sepsis, including markers of hemolysis.
Diabetesis a common underlying condition in patients who develop sepsis, and the prognosis of sepsis in these patients is controversial [
36,
37]. Severe infections may be facilitated by inadequate glycemic control, as insulin and oral antihyperglycemic drugs have been associated with lower incidence of sepsis. In a meta-analysis by Jiang et al., diabetes is said to not increase the risk of mortality for sepsis patients, although it can promote kidney failure [
38]. In both types 1 and 2, the pathological mechanisms contributing to higher risk of infection are complex and multifactorial. Glycemic imbalance can promote lesions via the irreversible glycation of protein chains, as well as by undermining the immune response and by generating oxygen reactive species. Neuropathy and vascular lesions are diabetes-related complications that can further increase infection risk by interfering with leukocyte migration towards affected tissues, while peripheral artery disease reduces blood flow, thus limiting the effectiveness of antibiotic treatment and allowbacteria to proliferate instead [
37]. We found that as many as a quarter of patients with cutaneous-onset sepsis and a fifth of those with uncomplicated infections had type-2 diabetes mellitus. In addition, despite the narrowly defined nature of our study, patients with type-1 diabetes were at a particularly high, 6.486-fold risk of sepsis.
4.4. Further comments on the predictive role of mildly altered mental status
Altered mental status is a major reason for concern in all medical specialties. It manifests as decreased cognitive function and level of awareness, confusion, behavioral changes, lack of alertness, and even coma. The Glasgow coma scores are often used to assess and monitor such types of neurological impairment [
39]. Sometimes, the nervous system may be the first to show signs of dysfunction, particularly in elderly and immunocompromised patients, leading to a variety of clinical syndromes including sepsis-associated encephalopathy (SAE), seizures, stroke, and neuromuscular disorders [
40].
Manifestations of sepsis-associated encephalopathy range from mild delirium to severe coma and are linked to increased long-term physical, mental, cognitive dysfunction and mortality rates [
41,
42]. Reported incidence ranges from 9% to 71% [43-45]. Although SAE is a reversible syndrome, mild to moderate residual neuropsychiatric symptoms, including depression, anxiety or cognitive impairment, may persist for up to one year in 40% of patients [46-47].
In the updated definition of sepsis-3, altered mental status in sepsis expressed as Glasgow coma scores is an important predictor of sepsis. Its relevance has also been acknowledged for the Sequential Organ Failure Assessment (SOFA) score and the Quick SOFA version (qSOFA). These assess the degree of respiratory, renal, cardiovascular, neurological, hepatic, and hematological impairment, and qSOFA is used in the emergency department for faster organ failure scoring, taking into account respiratory rate, hypotension, and the Glasgow coma score [48-49]. Higher mortality rates have been recorded in patients with GCSs <15, and some degree of neurological impairment has been noted in 25-33% of patients with sepsis [50-51].
In our study, mildly altered mental status (GCSsof 13 and 14) was associated with a substantially higher risk of sepsis(5.866-fold), and the multivariate analysis also identified it as a significant predictor alongside typical systemic manifestations of infectious processes (fever, chills, adenopathy).More severely impaired patients would not have been referred from the emergency department to the dermatology unit. Nonetheless, our results suggest that dermatologists can be valuable contributors in cases involving cutaneous-onset sepsis that may progress or has progressed to septic shock and other life-threatening complications.
4.5. Study limitations and further research opportunities
The results of the study should be considered in the context of certain limitations that raise interesting questions for further research. To begin with, we envisioned this as the first in a series of published studies, so our initial focus has been to assess the incidence of cutaneous-onset sepsis and infections in our region. The data hereby reported does not describetreatments and outcomes, which we could not collect in sufficient detail and with sufficient consistency from the type of electronic medical records we had access to for the purposes of this study. The reported results provide a helpful basis for a prospective study we have already initiated to assess specific biomarkers in patients with aggravated skin lesions, who present to the emergency department. We are alsoworking on reviewingdiagnostic and therapeutic approaches separately to put forth a pilot algorithm for more prompt and efficient management of cutaneous-onset sepsis.
Readers should also be aware that, in the group of patients with uninfected lesions on admission, the fact that lab samples were negative does not definitively rule out the possibility that these patients’ skin lesions had been infected previously. The opposite is suggested by the fact that these patients presented the clinical signs and symptoms typical of the studied conditions, which is why numbers for group C were not zero (e.g., the patient with clear signs of cellulitis but negative bacteriological exam). It is common for patients to attempt to treat skin conditions at home using traditional antibiotic remedies, over the counter or prescription medication, ointments, etc., not alwaysseeking or complying fully with professional advice from GPs or secondary care providers. This background information was not collected in this study, but it would be worth including it in future studies, whenever feasible.
At the other end of the spectrum, we remind readers that our study did not and, indeed, could not have enrolled patients presenting in emergency with both the primary skin lesions of interest and other, more pressing complications or concomitant viral infections, as protocols would dictate transfer for treatment in other units. The study period overlapped with the Covid-19 pandemic, so any patients carrying the SARS-CoV-2 virus were automatically directed to the hospital for infectious diseases.
5. Conclusions
Three years of admissions data at the largest emergency hospital in NE Romania have been retrospectively screened and analyzed to assess the incidence of acute skin infections and sepsis that can be attributed specifically to primary skin lesions with loss of tissue. Over five hundred cases were statistically processed to identify significant associations and risk factors for infection and cutaneous-onset sepsis among patients’ general characteristics, clinical manifestations, dermatological diagnoses, and established comorbidities.
Patients aged 18-92presented ulcerations, erosions, fissures, excoriations, bullae, vesicles, pruritus, tumefaction, edema, fever, chills, pain, adenopathy. They were diagnosed with venous ulcers, microbial eczema, cellulitis, superinfected bullous dermatoses, erysipelas, erythroderma, and other dermatoses. Of these, cellulitis was found to be the strongest predictor of both infection and sepsis. While S. aureus, P. aeruginosa, and E. coli were the most common bacterial species evidenced in the cultured samples harvested from lesions on admission, K. pneumoniae and S. β-hemolytic were flagged as significant risk factors for infection. Mildly altered mental status was also considered and found predictive of sepsis, especially in patients with type-1 diabetes mellitus and/or anemia. In fact, the patients’ comorbidities were more strongly associated with the risk of infection (chronic heart failure, pre-existing atrial fibrillation) and sepsis (type-1 diabetes mellitus, anemia) than age, sex, or background, even if infections were more common in men and in rural residents.
These results contribute meaningfully to currently scarce literature data on cutaneous-onset sepsis, including what primary skin lesions with loss of tissue can aggravate substantially to become medical emergencies, and what patients may be most at risk. Further research needs and opportunities are outlined, with some already underway, aiming to assess relevant biomarkers for cutaneous-onset sepsis that can be measured quickly and affordably, as well as a pilot algorithm for effective management.