You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
The Influence of High Dose Parenteral Vitamin C Supplementation on Incidence and Severity of Postoperative Pulmonary Complications after Cardiac Surgery with Extracorporeal Circulation: A Prospective, Randomized, Single-Blinded, Interventional Study
Altmetrics
Downloads
180
Views
63
Comments
0
This version is not peer-reviewed
Abstract
: Cardiac surgery (CS) with extracorporeal circulation (ECC), induces oxidative stress and systemic inflammatory response, which may seriously affect postoperative lung function. We aimed to test if high parenteral (200mg/kg/24h) perioperative (48h) doses of Vitamin C (VitC) may reduce the incidence and severity of postoperative pulmonary complications (PPC) in selected CS patients. Single centered, prospective, randomized, single-blinded, interventional study included 150 patients, assigned to control group A (n=75) and interventional group B (n=75). Group B intraoperatively received 1/4 of the planned daily Vit C dose (200mg/kg/24h), diluted in 10 ml of normal saline, divided into three parts, while Group A received an equal volume of normal saline at the same time frames. After 6 h from the first intraoperative dose, the following regimen was applied: Group B: 200 mg/kg/24h - 30 min i.v. infusion of VitC in 50 ml of normal saline, every 6h, for the next 48h, and Group A: 30 min i.v. infusion of an equal volume of normal saline every 6 hours, for the next 48h. Modified Kroenke’s score was used to determine the incidence and severity of PPC. The overall incidence of PPC was 36.7% and was significantly lower in Group B (13.3% v.s. 60.0%, p<0.001). The severity of PPC was also significantly lower in Group B [1(1) v.s. 3(2), p<0.001]. Besides, patients from Group B had significantly less damaged lungs and better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. High parenteral daily VitC doses (200mg/kg/24h) given to selected CS patients for 48h after CS are safe and effective in reducing the incidence and severity of PPC. Further multicenter RCT is needed to confirm these results.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Cardiac surgical (CS) operations with extracorporeal circulation (ECC), inevitably induce a complex, multi-etiological, oxidative stress (OS) and systemic inflammatory response (SIR), the intensity of which varies from mild, subclinical forms, to clinically manifest syndrome (SIRS), with or without multiorgan dysfunction (MODS). [1-5]
Within this continuum, the lungs are both the source and target organ for oxidative and inflammatory mediators. [3-6] Accordingly, postoperative pulmonary complications (PPC) are the most frequent complications of CS interventions with ECC and are responsible for significant morbidity, disability, mortality, and health care costs. [7-9] The incidence of PPC ranges between 3% and >50%, according to different criteria for their definition. [8,10-12] Commonly considered as a composite outcome measure, PPC are responsible for significant early (14%-30%) and late (one-year and five-year) mortality (45.9% and 71.4%, respectively). [7]
Thanks to its proven antioxidant and pleiotropic biochemical functions, and its ability to reduce the intracellular activation of NFκB, which determine the intensity and extent of systemic inflammation, vitamin C (VitC, ascorbic acid, ascorbate) is increasingly used in various conditions to reduce an excessive OS and SIR. [3,13-22] It was shown that non-CS patients need much more VitC (500mg-4gr/24h) than the recommended daily doses for healthy individuals (90mg/24h). [18,23,24] Cardiac surgical patients are even bigger VitC "consumers", [25] but, despite this, the majority of them (56%) enter CS with VitC deficiency. [18]
The effectiveness of VitC in CS was tested in a relatively small number of studies, [23-27], focusing mainly on postoperative atrial fibrillation, while the only one that examined the effect of VitC on PPC in low-risk CS patients, was limited to intraoperative parenteral administration of intermediate therapeutic doses (3x1g). [28]
Since 1970, onward, the scientific community has been divided upon the safety and efficacy of high-dose parenteral VitC supplementation. [29,30] Several studies conducted during the COVID-19 pandemic, NIH expert panel document on cancer treatment, and studies on critically ill patients, have reported no complications with high daily parenteral doses of up to 50g. [31-36]
We present the results of our study, designed primarily to investigate the influence of high parenteral (200mg/kg/24h) perioperative (48h) doses of VitC, on the incidence and severity of PPC in patients submitted to CS with ECC. The rationale for this therapeutic regimen relied on the fact that OS and SIR are most intense within the first 48 hours after CS, and so is the peak consumption of vitamin C. [5,25]
2. Materials and Methods
2.1. Design
This prospective, randomized, single-blinded, interventional study was conducted at the UC Clinical Centre of Serbia, Clinic for Cardiac Surgery, Belgrade, Serbia, starting on July 15, 2022., for the duration required for all respondents to complete the planned stages of the investigation. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical Faculty UC Belgrade, Serbia (protocol code 1322/VII-24 and date of approval 07.07.2022.). Informed consent was obtained from all subjects involved in the study. They also consented not to be informed of whether they were allocated to the control or the intervention group.
2.2. Participants
Inclusion criteria were: all patients aged ≥18 years undergoing an elective CS procedure with ECC, regardless of the type of planned operation. Exclusion criteria were: previous CS operation, emergency patients, clinically and/or radiographically active lung disease, the systolic pressure in the pulmonary artery >60 mmHg, allergy to ascorbic acid, gout, hemodialysis, significant oxaluria, uric nephrolithiasis, glucose-6-phosphate dehydrogenase enzyme deficiency, hemochromatosis, sickle cell anemia, sideropenic anemia, and thalassemia. The criteria for subsequent withdrawal from the study were: operations with an ECC time ≥6h, death during hospitalization caused by non-pulmonary reasons, and withdrawal of previously given consent to participate in the study.
2.3. Interventions
All patients were operated on and subsequently treated according to standard institutional anesthesiological, surgical, and intensive care protocols.
Patients from the intervention group (B) intraoperatively received 1/4 of the planned daily dose of Vit C (200mg/kg/24h), divided into three parts, diluted in 10 ml of normal saline, and administered via central venous catheter 10 minutes after induction of anesthesia, 10 minutes before removal of the aortic cross-clamp (reperfusion), and at the beginning of sternal closure. The control group (A) received an equal volume of normal saline at the indicated times. Postoperatively (6 hours after the first intraoperative dose), VitC was administered according to the following regimen:
- Intervention group (B): 200 mg/kg/24h - 48h - 30 min i.v. infusion of VitC in 50 ml of normal saline every 6 hours, under UV protection.
- Control group (A): 30 min i.v. infusion of an equal volume of normal saline every 6 hours, under UV protection.
Patients from group B continued to receive an enteral supplementation of VitC (2g/24h) until discharge and were advised to continue it for a week after.
For each patient included in the study, standard perioperative characteristics (i.e. demographic, anthropometric, clinical, and laboratory) were registered in a separate database. The American Society of Anesthesiologists (ASA) score was calculated to assess the preoperative physical status. [37] The degree of organ dysfunction after surgery was quantified by the Sequential Organ Failure Assessment (SOFA) score which was estimated 48h after CS. [38]
The incidence and severity of PPC were scored and agreed by two independent, blinded assistants, using the original operational definitions of Kroenke [39] and subsequent modifications. [40-43] Score values were determined daily, and as an individual PPK severity score, the worst registered value (i.e. the highest grade) within 7 days after surgery was used for the analyses. The severity of PPC was graded on an ordinal scale of 0 (no PPC) to 5 (death before discharge). The values from 1 to 4 depict the increasing severity of the PPC. (Supplement 1, Table S1). To determine the incidence of PPK, only scores ≥3 were considered.
2.4. Outcome
The primary end-point was to compare the incidence and severity of PPC in the control (A) and intervention (B) groups. Secondary end-points, aimed to compare the following parameters between the groups: pulmonary oxygenation and ventilation (Horowitz index: PaO2/FiO2, Alveolar–arterial gradient: A-aDO2, and time spent on mechanical ventilation), inflammatory markers (procalcitonin, C-reactive protein, leucocytes, neutrophils, lymphocytes, sedimentation rate, fibrinogen, albumin, D-dimer and ferritin), renal function (GFR < 60mL/min, creatinine, urea), non-pulmonary postoperative complications, postoperative organ dysfunction (SOFA score, ASA/SOFA ratio), intensive care unit – ICU parameters (re-admission, length of stay) and hospital parameters (length of stay, mortality).
2.5. Sample Size
The required number of subjects to be included in the study was calculated based on the primary objective - the assumption that high daily doses of parenterally administered VitC will reduce the severity of PPC after CS with ECC. To provide a study power of 0.90, 5% level of significance, and to detect a clinically significant difference of the mean value of the PPK score ≥0.3 (2.1, 95 % CI, 2.0-2.3 vs. 1.8, 95% CI, 1.7-2.0), as defined by previous studies, [28,40] the required sample size was 70 subjects in each group (i.e. a total of 140). To allow for 15% drop-out, we decided to include a minimum of 80 participants in each group (i.e. a total of 160).
2.6. Randomization and Masking
Randomization was done by allocating each consecutive respondent in a 1:1 manner to either control (A) or intervention (B) groups, the order of which was determined by manual random selection. Except for the principal investigator (M.K.K.) and two assistants (J.Č., R.K.), nobody else, involved in the patient treatment, clinical and laboratory data collection, and analysis had any information about the randomization and grouping. Two informed assistants prepared an unlabeled infusion solution (placebo or VitC), covered it with UV protective coating, and assigned it to the patient according to randomization and grouping. The principal investigator and two informed assistants were not engaged in the patient treatment, clinical and laboratory data collection, and analysis. Two independent, blinded specialists estimated and agreed on the ASA, SOFA, and PPC severity score grades.
2.7. Statistical Methods
Results are presented as count (%), means ± standard deviation, or median (interquartile range) depending on data type and distribution.
Groups are compared using parametric (t-test) and nonparametric (Chi-square, Mann-Whitney U test, Fisher’s Exact test) tests. All p-values less than 0.05 were considered significant.
To conduct all statistical analyses, we used SPSS 29.0 (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and R 3.4.2. (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
3. Results
After assessing for eligibility, 22 of 182 enrolled patients, who met the exclusion criteria were not included in randomization. A total of 160 patients were randomized and allocated to control group – A (n=80) and intervention group – B (n=80). A total of 10 patients were subsequently excluded from the analysis. In group A, one was for intraoperative death, three for ECC duration of ≥ 6 hours, and one for in-hospital non-pulmonary death. In group B, one was for intraoperative death, two for ECC duration of ≥ 6 hours, and two for in-hospital non-pulmonary death. As a result, a total of 150 patients (75 in each group) have entered the analysis of study data. (Figure 1).
The baseline perioperative characteristics of patients included in the study are shown in Table 1. There were no significant differences between the groups for the most of perioperative parameters, except for body mass index (BMI), diastolic arterial pressure (Tad), chronic renal failure (CRF), and duration of surgery, showing significantly higher values in group A.
Although the other characteristics are fairly evenly distributed between the groups, it is evident that all patients had advanced systemic disease (ASA scores 3 and 4). Both groups are elderly people with pronounced CVD risks (HTA 97.3% in group B and 100% in group A) and serious comorbidities. Interestingly, the proportion of patients with a history of COVID-19 infection was relatively small, keeping in mind that this study was conducted in a critical period (March 2020 to November 2022), when the most infected and deceased were recorded. [44] Yet, preoperative pulmonary status in both groups was quite good (PPC score grades 0 and 1).
The primary end-point outcome measures of patients included in the study are shown in Table 2. The overall incidence of PPC was 36.7% and was significantly lower in Group B (13.3% v.s. 60.0%, p<0.001). The severity of PPC was also significantly lower in Group B [1(1) v.s. 3(2), p<0.001].
The secondary end-point outcome measures are shown in Table 3. The following parameters were significantly better in group B: Horowitz index (312.6±107.4 v.s. 268.9±112.6, p=0,008), C-reactive protein [95 (56.2) v.s. 167.4 (82.9), p<0.001], sedimentation rate [20 (6) v.s. 22 (18), p=0.023], acute renal failure (1.3%v.s. 10.7%, p=0,034), wound infection (6.7% v.s. 20%, p=0,016), GFR < 60mL/min (13.3% v.s. 32%, p=0,006), urea (6.3±1.9 v.s. 7.1±3, p=0,041), ICU re-admission (5.3% v.s. 20%, p=0,007), ICU stay [32 (24) v.s. 48 (24), p<0.001], and hospital mortality (1.3% v.s. 10.7%, p=0,034). Serum procalcitonin levels were significantly lower in group A [0.3 (0.6) v.s. 0.5 (0.8), p=0.032].
4. Discussion
The results of our study support parenteral administration of high daily doses of VitC (200 mg/kg/24h for 48h) to reduce the incidence and severity of PPC after CS with ECC. (see Table 2) Besides, patients from the VitC group had significantly less damaged lungs (i.e. better Horowitz index), better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. (see Table 3).
Trying to find the answers to the primary end-points of this study, we found a question: „Do we know what is meant by PPC in CS?“ In all the reports we preliminarily analyzed, we could not find the same or sufficiently similar definition of PPC which could allow robust comparisons. [8,11,12,45-49] Thus, comparing the guideline by two respectable task forces, [10,50] one can find seven listed complications in one, [50] and four in another definition [10] of PPC, as the composite outcome measure. But, is it just a lack of standardized PPC definition problem? Some believe that even more important are disparities in the clinical appraisal of PPC. [51,52] Accordingly, the incidences of PPC reported in the literature, widely range from 3% to >50%. [8,10-12] The overall incidence of PPC in our study (36.7%), fits within this range.
Yet, another seemingly simple question that appeared to us, was: “If it’s not severe enough, is it a complication at all?” Indeed, PPC after CS may vary from mild, subclinical forms (e.g. compensated abnormalities of respiratory mechanics) to severe respiratory failure with prolonged ventilator dependency. [53] Reviewing the literature, as for the definition of PCC, we could find very few reports defining standardized and objective criteria for quantification of the severity of PPC in CS. [10,39-42] We decided to use the modified [40-42] PPC severity score, originally defined by Kroenke et al. [39] for patients with severe chronic obstructive pulmonary disease. (Supplement 1, Table S1) It was recently compared with the Melbourne Group Scale, and both were deemed to be useful tools for grading PPC in CS patients. [54] To overcome Kroenke’s score inferiority in more severe cases, we found it appropriate to set the score value ≥3 as a cut-off in analysis of the PPC incidence.
The interventional part of our study relied on two sets of facts. The first is that CS operations with ECC, inevitably induce a complex, multi-etiological, phasic OS and SIR, the most intense within the first 48h after CS, which, in addition to the other factors, affect postoperative pulmonary function. [1-9] The second is the proven antioxidant and pleiotropic biochemical functions of the VitC, due to which it has been commonly used to reduce organ damage induced by excessive OS and SIR. [13-22]
About 63 million years ago, the primates and a few other species were „deceived“ by the abundance of plants containing VitC, and conserved the mutation and inactivation of the gene for the synthesis of L-gulono-γ-lactone oxidase (GULO) on chromosome 8p21, thus losing the ability to synthesize VitC from glucose. Accordingly, humans entirely rely on dietary intake and rational metabolic use of VitC to maintain homeostasis. [55] A recent study of VitC status and prevalence of deficiency suggests that dietary intake is globally insufficient. [56]
The first appreciation of the importance of VitC dietary intake came from the British sailors. Upon the observation of naval surgeon James Lind (1747) that oranges and lemons can prevent scurvy (lat. Scorbutus), Gilbert Blane (1795) managed to persuade the Admiralty to use citrus juice as a daily ration on board British naval vessels. [57] Two centuries and two Nobel Prizes later (Albert Szent-Györgyi and Walter Norman Haworth), this low-cost, essential substance was the subject of many controversial polemics, sometimes surpassing purely medical and scientific framework. [58] Seneca, a Roman stoic philosopher, once said, “Truth never gets old.” Thus, as a result of accumulated knowledge of its important pleiotropic functions, VitC therapy has continued to be tested in numerous contemporary studies, mainly in critically ill patients.
Regardless of preoperative status, CS rapidly consumes VitC and serious depletion may last for two weeks, deteriorating the defense against OS and SIR during cardiac operations. [14] To our knowledge, there is no study comparing the effectiveness of different therapeutic regimens (different doses and different duration of therapy) of parenterally administered VitC in CS patients. We aimed to test PPC in CS patients intervening with high daily parenteral doses of VitC (200mg/kg/24h) for the first 48 postoperative hours. Similar (i.e. 250mg/kg), single parenteral doses were tested in two studies on CS patients, one aimed to compare the postoperative dynamics of creatine kinase-MB and malondialdehyde, [59] and another to compare the dynamics of the cardiac index with a control group. [36] Both studies have reported the beneficial effect of VitC. [36,59] Study with the same dose of parenteral VitC as ours, given for 96h, in patients with severe sepsis, has proved its benefits in the dynamics of the inflammatory markers and SOFA score. [35] None of them have reported any adverse effects of high-dose parenteral VitC therapy. [35,36,59] We also didn’t have any side effects or complications with our VitC therapeutic regimen. To assess potential functional renal impairment induced by high doses of VitC, we included renal functional analysis in secondary outcome measures. Renal function was preoperatively significantly better in group B, and this remained the same up to 96h after CS. (Table 1 and Table 3, Supplement 1, Figure S3).
The incidence and the severity of PPC were significantly lower in group B (see Table 2). These results are in concordance with the only relatively comparable study. [28] Comparing the PPC by grade, in group A most of them were clustered in grades 2-4, while in group B they clustered in grades 1 and 2. (see Table 2, Supplement 1, Figure S1)
The most common types of PPC in our study were pleural effusion and pneumonia, all being significantly more frequent in group A. Similar incidences are reviewed by Tanner et al. [46] Grade 4 PPC (respiratory failure equivalent) was more frequent in group A (see Table 2). Wang et al. reported only one (2.7%) patient, from the control group, with PPC grade 4. Such discrepancy may be explained by comparing the baseline perioperative characteristics. Patients in our study were older, had advanced systemic disease, pronounced CVD risks, and serious comorbidities. Moreover, we had 26% of patients with a history of previous COVID-19 disease, while the study of Wang et al. was conducted before the first cases of COVID-19 were reported in Wuhan, China, in December 2019. [28,44]
Parameters indicating pulmonary function (i.e. oxygenation and ventilation) and selected inflammatory markers, were sampled at 48h after CS. The mean values of PaO2/FiO2 and A-aDO2 depict the presence of postoperative lung injury in both groups. A significantly better Horowitz index in group supports a potential lung protective effect of the VitC (see Table 3). In a study by Wang et al. there was no difference in these parameters between the groups. [28]
The median serum procalcitonin levels were not elevated as they are in septic patients but were significantly lower in group A. At 48h, there was no significant difference between the other inflammatory markers, except CRP and sedimentation rate, which were significantly lower in group B (see Table 2). The dynamics of those parameters were followed up for 96h. Interestingly, from 48h to 96h, statistically lower values were also recorded for leucocytes, neutrophils, and lymphocytes in group B, and fibrinogen in group A. (Supplement 1, Figure S2) These findings support the potential anti-inflammatory effect of VitC which spans more than 48h after CS.
Except for acute renal failure and wound infection, the other non-pulmonary complications didn’t show significant differences between the groups (see Table 3). Iizuka et al. found a positive correlation between low plasma levels of VitC and postoperative delirium in elderly CS patients, [60] but this was not the case in our study.
SOFA score, estimated 48h after CS didn’t show significant difference between the groups in our study. Median values of 4, indicate a mild degree of organ dysfunction in all patients at this time-frame. In other studies of high parenteral doses of VitC in critically ill patients, reviewed by Nabzdyk et al. a significant reduction in SOFA score grade was recorded in the intervention groups. [61] This difference may be interpreted in light of different underlying causes of organ dysfunction (i.e. severe sepsis and severe burns v.s. CS).
Patients in group B had significantly lower ICU re-admission rates and shorter ICU stays. Also, they had a significantly lower hospital mortality rate (see Table 3). These results are much better than those reported by Wang et al. [28], probably depicting the more physiological regimen (i.e. higher doses and longer duration) of parenteral VitC supplementation in our study.
This study has some limitations. First, as a single-centered and single-blinded study, it was difficult to avoid selection, observer, performance, and detection biases, affecting the validity and reliability of the study. To minimize these potential biases, the study was kept open only to three persons (the principal investigator and two informed assistants). The key study intervention (infusion of VitC solution) was masked, as explained earlier, to all but the principal investigator and two informed assistants. Similarly, the assessment of the key outcome parameter (PPC score) and other scoring systems (ASA, SOFA) was protected from the influence of informed researchers by engaging two independent, blinded assistants. Second, although the calculated sample size of 70 participants in each group provides this study with an acceptable level of statistical accuracy and validity, this number is still too small to draw any general conclusions, so the results of this study should be interpreted with caution. Third, highly inconsistent criteria for the definition of PPC make the results of this, like other similar studies, insufficiently suitable for comparisons and meta-analyses. That was why we decided to use the modified [40-42] PPC severity score, originally defined by Kroenke et al. [39], as an outcome measure for our primary end-point. Our decision is supported by the fact that this score was used in the only study published so far on the effect of VitC on PPC in CS. [28] Fourth, two score tools for practical use in the CS, one of them being Kroenke’s score, were compared and it was concluded that both can identify PPC effectively. [43] Yet, the validity of Kroenke’s score to identify severe PPC remains disputable. To overcome this, we set the score value ≥3 as a cut-off in the analysis of the PPC incidence. Thus, to “catch” the more severe cases, we probably lost some of the less severe PPC from the analysis of PCC incidence. Fifth, we could not measure plasma levels of the VitC, due to the complexity of the procedure and the excessive cost of the reagents. Because of the complex pharmacokinetics of VitC, [19] it would be the most reliable way to interpret the observed differences between the control (A) and the intervention group (B), in the context of the possible influence of the applied high parenteral VitC doses. Finally, we hope to see and participate in future, multicenter, prospective, randomized, controlled, double-blinded, interventional trials, which would (hopefully) confirm our results. Before that, it is necessary that a widely accepted, standardized, and comparable definition of PPC finally appears.
5. Conclusions
Our study has shown that high parenteral daily VitC doses (200mg/kg/24h) could be given to selected CS patients for 48h after CS without side effects or complications. By reducing the OS and SIR, along with many other pleiotropic functions, applied doses of VitC significantly reduce the incidence and severity of PPC in CS patients. Besides, patients receiving high parenteral daily VitC doses had significantly less damaged lungs and better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. Further multicenter RCT is needed to confirm these results.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: PPC severity score; Figure S1: PPC by grade; Figure S2: Dynamics of inflammatory markers; Figure S3: Dynamics of GFR < 60mL/min.
Author Contributions
Conceptualization, M.K.K., A.R., and D.M.; methodology, M.K.K., J.Č., and R.K.; software, I.S.; validation, M.K.K., A.R., and D.M.; formal analysis, I.S. and M.K.K.; investigation, M.K.K., D.L., M.G., D.T., J.Č., and R.K.; resources, M.K.K.; data curation, M.K.K., D.L., M.G., D.T., J.Č., and R.K; writing—original draft preparation, M.K.K.; writing—review and editing, M.K.K., A.R., M.K., I.P., and D.M.; visualization, M.K.K., M.K.; supervision, A.R., M.K. I.P., and D.M.; project administration, M.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical Faculty UC Belgrade (protocol code 1322/VII-24 and date of approval 07.07.2022.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J Cardiothorac Vasc Anesth 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am J Respir Crit Care Med 2015, 192, 958–964. [Google Scholar] [CrossRef]
- McGuinness, J.; Bouchier-Hayes, D.; Redmond, J.M. Understanding the inflammatory response to cardiac surgery. Surgeon 2008, 6, 162–171. [Google Scholar] [CrossRef]
- Semler, M.W.; Wheeler, A.P. Systemic inflammatory response syndrome after cardiac surgery: time for a change. Chest 2014, 145, 1181–1182. [Google Scholar] [CrossRef]
- Warltier, David C. ; Laffey, John G.; Boylan, John F.; Cheng, Davy C.H. The Systemic Inflammatory Response to Cardiac Surgery: Implications for the Anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- Joseph, D.; Puttaswamy, R.K.; Krovvidi, H. Non-respiratory functions of the lung. Continuing Education in Anaesthesia Critical Care & Pain 2013, 13, 98–102. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br J Anaesth 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Fischer, M.-O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Nigro Neto, C.; Kunstyr, J.; et al. Postoperative Pulmonary Complications After Cardiac Surgery: The VENICE International Cohort Study. Journal of Cardiothoracic and Vascular Anesthesia 2022, 36, 2344–2351. [Google Scholar] [CrossRef]
- Brown, P.P.; Kugelmass, A.D.; Cohen, D.J.; Reynolds, M.R.; Culler, S.D.; Dee, A.D.; Simon, A.W. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg 2008, 85, 1980–1986. [Google Scholar] [CrossRef]
- Abbott, T.E.F.; Fowler, A.J.; Pelosi, P.; Gama de Abreu, M.; Møller, A.M.; Canet, J.; Creagh-Brown, B.; Mythen, M.; Gin, T.; Lalu, M.M.; et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018, 120, 1066–1079. [Google Scholar] [CrossRef]
- Mali, S.; Haghaninejad, H. Pulmonary complications following cardiac surgery. Arch Med Sci Atheroscler Dis 2019, 4, e280–e285. [Google Scholar] [CrossRef] [PubMed]
- Naveed, A.; Azam, H.; Murtaza, H.G.; Ahmad, R.A.; Baig, M.A.R. Incidence and risk factors of Pulmonary Complications after Cardiopulmonary bypass. Pak J Med Sci 2017, 33, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Ballmer, P.E.; Reinhart, W.H.; Jordan, P.; Bühler, E.; Moser, U.K.; Gey, K.F. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations. J Thorac Cardiovasc Surg 1994, 108, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; O’Neill, L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Yamazaki, E. Vitamin C requirement in surgical patients. Current Opinion in Clinical Nutrition & Metabolic Care 2010, 13, 669–676. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Hess, A.S.; McCarthy, D.P.; DeCamp, M.M. Power for the Sickest: Vitamin C for Vasoplegia after Cardiac Surgery. J Cardiothorac Vasc Anesth 2020, 34, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis)interprete the clinical evidence. Redox Biol 2020, 34, 101532. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Borgs, C.; Fitzner, C.; Stoppe, C. Perioperative Vitamin C and E levels in Cardiac Surgery Patients and Their Clinical Significance. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Clasen, K.C.; Wendt, S.; Majoros Á, G.; Stoppe, C.; Adhikari, N.K.J.; Heyland, D.K.; Benstoem, C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mangoush, O.; Nakamura, K.; Al-Ruzzeh, S.; Athanasiou, T.; Chester, A.; Amrani, M. Effect of ascorbic acid on endothelium-dependent vasodilatation of human arterial conduits for coronary artery bypass grafting. Eur J Cardiothorac Surg 2003, 24, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Zhang, H.; Zhu, H.; Zhang, N.; Liu, J. Effect of Intravenous Injection of Vitamin C on Postoperative Pulmonary Complications in Patients Undergoing Cardiac Surgery: A Double-Blind, Randomized Trial. Drug Des Devel Ther 2020, 14, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Ascorbic acid and the common cold. Am J Clin Nutr 1971, 24, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Coppock, D.; Violet, P.C.; Vasquez, G.; Belden, K.; Foster, M.; Mullin, B.; Magee, D.; Mikell, I.; Shah, L.; Powers, V.; et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov 2020, 5, 100028. [Google Scholar] [CrossRef] [PubMed]
- PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Intravenous Vitamin C. Bethesda, MD: National Cancer Institute. Updated <06/17/2022>. Available at: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq. Accessed <12/10/2023>. [PMID: 26389504].
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Dingchao, H.; Zhiduan, Q.; Liye, H.; Xiaodong, F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg 1994, 42, 276–278. [Google Scholar] [CrossRef]
- Knuf, K.M.; Maani, C.V.; Cummings, A.K. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper Med (Lond) 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Lawrence, V.A.; Theroux, J.F.; Tuley, M.R. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med 1992, 152, 967–971. [Google Scholar] [CrossRef]
- Costa Leme, A.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; De Santis Santiago, R.R.; Osawa, E.A.; Pinheiro de Almeida, J.; Gerent, A.M.; Franco, R.A.; Zanetti Feltrim, M.I.; et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. Jama 2017, 317, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.; Helders, P.J.; Favié, N.J.; De Bie, R.A.; Brutel de la Riviere, A.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. Jama 2006, 296, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Huang, W.; Zhang, X.; Guo, Y.; Yu, P. Comparison of Tools for Postoperative Pulmonary Complications After Cardiac Surgery. J Cardiothorac Vasc Anesth 2023, 37, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, V.M.; Nikolić, N.; Radovanović Nenadić, U.; Öcal, A.; E, K.N.; Zečević, M. Preparedness and Preventive Behaviors for a Pandemic Disaster Caused by COVID-19 in Serbia. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Cheng, C.; Wei, X. Incidence of postoperative pulmonary complications in patients undergoing minimally invasive versus median sternotomy valve surgery: propensity score matching. Journal of Cardiothoracic Surgery 2021, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Hardman, J.; Sabaté, S.; Langeron, O.; Abreu, M.G.; Gallart, L.; Belda, J.; Markstaller, K.; Pelosi, P.; Mazo, V. PERISCOPE study: predicting post-operative pulmonary complications in Europe. Eur J Anaesthesiol 2011, 28, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 2004, 8, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015, 32, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, E.; Gologorsky, A.; Salerno, T.A. Lung-Centered Open Heart Surgery: A Call for a Paradigm Change. Front Cardiovasc Med 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Vaughan-Sarrazin, M.; Rosenthal, G.E.; Girotra, S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Yuqiang Wang; Yaxin Zhou; Zeruxin Luo; Wei Huang; Xiu Zhang; Yingqiang Guo; Yu, P. Comparison of Tools for Postoperative Pulmonary Complication Following Cardiac surgery. PREPRINT (Version 1) 2022, Research Square. [CrossRef]
- Hickey, S.; Roberts, H. Evolution and Deficiency. In Ascorbate: The Science of Vitamin C, Hickey, S., Roberts, H., Eds.; Lulu.com: 2004; pp. 66-72.
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.J. The discovery of vitamin C. Ann Nutr Metab 2012, 61, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Richards, E. Introduction. In Vitamin C and Cancer: Medicine or Politics?; Palgrave Macmillan UK: 1991; pp. 1-14.
- Li, C.C. [Changes of creatine phosphokinase and malondialdehyde in the serum and clinical use of large doses of vitamin C following open heart surgery]. Zhonghua Wai Ke Za Zhi 1990, 28, 16–17. [Google Scholar] [PubMed]
- Iizuka, Y.; Yoshinaga, K.; Takahashi, K.; Oki, S.; Chiba, Y.; Sanui, M.; Kimura, N.; Yamaguchi, A. Association between Plasma Ascorbic Acid Levels and Postoperative Delirium in Older Patients Undergoing Cardiovascular Surgery: A Prospective Observational Study. J Cardiovasc Dev Dis 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Nabzdyk, C.S.; Bittner, E.A. Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 2018, 7, 52–61. [Google Scholar] [CrossRef]
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart for each phase of the study.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart for each phase of the study.
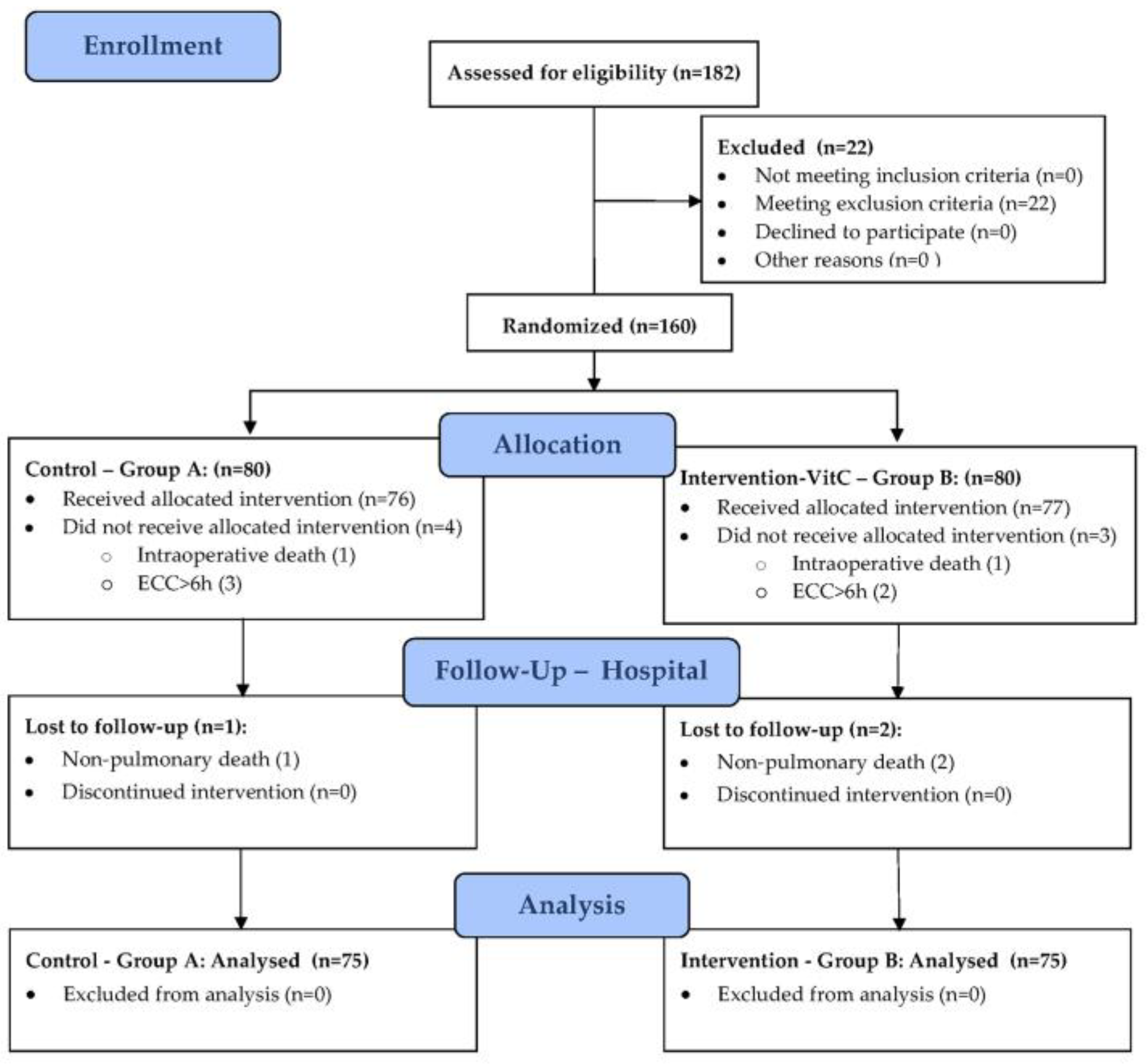
Table 1.
Baseline perioperative characteristics.
| Perioperative Parameters | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. Demographic and Anthropometric | |||
| Age (years) | 66.9±8.7 | 66.3±8.6 | 0.672 (t) |
| Male gender | 59 (78.7%) | 55 (73.3%) | 0.444 (chi) |
| BMI (kg/m2) | 28.4±3.9 | 26.7±3.3 | 0.005 (t) |
| 2. CVD Risk | |||
| HTA | 75 (100%) | 73 (97.3%) | 0.497 (fet) |
| DM | 36 (48%) | 36 (48%) | 1.000 (fet) |
| HLP | 72 (96%) | 68 (90.7%) | 0.190 (chi) |
| Smoking | 52 (69.3%) | 52 (69.3%) | 1.000 (chi) |
| 3. CV Status and Comorbidities | |||
| Recent MI | 41 (54.7%) | 34 (45.3%) | 0.253 (chi) |
| AP | 61 (81.3%) | 58 (77.3%) | 0.545 (chi) |
| TAs (mmHg) | 145.8±26.3 | 139.8±16.9 | 0.100 (t) |
| TAd (mmHg) | 81.9±13.1 | 77.3±11.6 | 0.022 (t) |
| EF-LV (%) | 46.5±9.1 | 48.4±8.0 | 0.187 (t) |
| HR (beats/min) | 70.4±9.3 | 69.5±9.7 | 0.565 (t) |
| Sinus | 68 (90.7%) | 66 (88%) | 0.597 (chi) |
| AF | 8 (10.7%) | 9 (12%) | 0.979 (chi) |
| CVD | 11 (14.7%) | 5 (6.7%) | 0.113 (chi) |
| CRF | 23 (30.7%) | 12 (16%) | 0.034 (chi) |
| COVID-19 | 22 (29.3%) | 17 (22.7%) | 0.352 (chi) |
| 4. Pulmonary status (PPC Score) | |||
| 0 | 20 (26.7%) | 25 (33.3%) | 0.373 (chi) |
| 1 | 55 (73.3%) | 50 (66.7%) | |
| 5. ASA Score | |||
| 3 | 65 (86.7%) | 66 (88.0%) | 0.806 (chi) |
| 4 | 10 (13.3%) | 9 (12.0%) | |
| 6. Surgery | |||
| CABG | 53 (70.7%) | 49 (65.3%) | 0.484 (chi) |
| Aortic valve | 9 (12%) | 13 (17.3%) | 0.356 (chi) |
| Mitral valve | 2 (2.7%) | 4 (5.3%) | 0.681 (fet) |
| Combined | 11 (14.7%) | 9 (12%) | 0.631 (chi) |
| Duration of surgery (min) | 245.7±40.2 | 219.9±45.0 | <0.001 (t) |
| ECC time (min) | 86.8±27.3 | 80.7±19.1 | 0.114 (t) |
| ACC time (min) | 55.6±20.7 | 56.3±15.4 | 0.799 (t) |
Legend: BMI – Body mass index; HTA – Arterial hypertension; DM – Diabetes Mellitus; HLP – Hy-perproteinemia; CV – Cardio-vascular; MI – Myocardial infarction; AP – Angina pectoris; Tas – Systolic arterial pressure; Tad – Diastolic arterial pressure; EF-LV – Left ventricular ejection fraction; CVD – Cerebro-vascular diseases; CRF – Chronic renal failure; COVID-19 - Coronavirus disease (SARS-CoV-2 virus); PPC – Postoperative pulmonary complications score; ASA - American Society of Anesthesiologists; CABG – Coronary artery bypass grafting; ECC – Extracorporeal circulation; ACC - Aortic cross-clamp. The results are presented as count (%) or mean±sd. * Statistical tests: (t) - Student’s t-test; (chi) - Chi-Square (Χ²) Test; (fet) - Fisher’s exact test.
Table 2.
Primary end-point outcome measures: PPC incidence, severity, and types.
| Primary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. PPC Incidence | |||
| PPC ≥ 3 (n,%) | 45 (60.0%) | 10 (13.3%) | <0.001 (chi) |
| 2. PPC Severity | |||
| PPC severity score | 3 (2) | 1 (1) | <0.001 (mw) |
| Grade 0 (n,%) | 5 (6.7%) | 14 (18.7%) | <0.001 (mw) |
| Grade 1 (n,%) | 3 (4.0%) | 31 (41.3%) | |
| Grade 2 (n,%) | 22 (29.3%) | 20 (26.7%) | |
| Grade 3 (n,%) | 23 (30.7%) | 7 (9.3%) | |
| Grade 4 (n,%) | 18 (24.0%) | 3 (4.0%) | |
| Grade 5 (n,%) | 4 (5.3%) | 0 | |
| 3. PPC Types | |||
| Pneumonia (n,%) | 32 (42.7%) | 13 (17.3%) | <0.001 (chi) |
| Pneumothorax (n,%) | 10 (13.3%) | 3 (4%) | 0.042 (chi) |
| Pleural effusion (n,%) | 51 (68%) | 38 (50.7%) | 0.031 (chi) |
| Re-intubation (n,%) | 16 (21.3%) | 2 (2.7%) | <0.001 (chi) |
Legend: PPC – Postoperative pulmonary complications. The results are presented as count (%), or median (IQR) * Statistical tests: (chi) - Chi-Square (Χ²) Test; (mw) - Mann–Whitney test.
Table 3.
Secondary end-point outcome measures.
| Secondary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* | ||
|---|---|---|---|---|---|
| 1. Pulmonary oxygenation and ventilation | |||||
| Horowitz index (PaO2/FiO2) 48h | 268.9±112.6 | 312.6±107.4 | 0.008 (t) | ||
| Alveolar–arterial gradient (A-aDO2) 48h | 17.3±5.4 | 17.9±4.7 | 0.432 (t) | ||
| Total MV time (h) | 5.2±1.6 | 5.4±1.2 | 0.493 (t) | ||
| 2. Inflammatory markers (48h) ** | |||||
| Procalcitonin | 0.3 (0.6) | 0.5 (0.8) | 0.032 (mw) | ||
| C-reactive protein | 167.4 (82.9) | 95 (56.2) | <0.001 (mw) | ||
| Leucocytes | 13.3±3.3 | 12.4±3.4 | 0.102 (t) | ||
| Neutrophils | 80.6±5.5 | 80.5±5.1 | 0.881 (t) | ||
| Lymphocytes | 12±4.6 | 12.6±4 | 0.418 (t) | ||
| Sedimentation rate | 22 (18) | 20 (6) | 0.023 (mw) | ||
| Fibrinogen | 5.1±1.4 | 5.2±1 | 0.775 (t) | ||
| Albumin | 31.8±3.5 | 31.4±3.2 | 0.479 (t) | ||
| D-dimer | 0.5 (0.5) | 0.6 (0.4) | 0.877 (mw) | ||
| Ferritin | 202 (277) | 200 (104) | 0.287 (mw) | ||
| 3. Postoperative complications (non-pulmonary) | |||||
| PONV | 20 (26.7%) | 30 (40.0%) | 0.083 (chi) | ||
| Delirium | 22 (29.3%) | 16 (21.3%) | 0.260 (chi) | ||
| Transfusion | 32 (42.7%) | 36 (48%) | 0.512 (chi) | ||
| Acute renal failure | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
| Wound infection | 15 (20%) | 5 (6.7%) | 0.016 (chi) | ||
| CPR | 4 (5.3%) | 0 (0%) | 0.120 (fet) | ||
| 4. Renal function(48h) ** | |||||
| GFR < 60mL/min | 24 (32%) | 10 (13.3%) | 0.006 (chi) | ||
| Creatinine | 99.6±44.6 | 87.8±27.5 | 0.054 (t) | ||
| Urea | 7.1±3 | 6.3±1.9 | 0.041(t) | ||
| 5. Postoperative organ dysfunction | |||||
| SOFA score | 4 (2) | 4 (1) | 0.132 (mw) | ||
| ASA/SOFA ratio | 1.0 (0.4) | 0.8 (0.25) | 0.190 (mw) | ||
| 6. ICU outcome measures | |||||
| ICU re-admission | 15 (20%) | 4 (5.3%) | 0.007 (chi) | ||
| ICU stay | 48 (24) | 32 (24) | <0.001 (mw) | ||
| 7. Hospital outcome measures | |||||
| Hospital stay | 8 (2) | 8 (2) | 0.092 (mw) | ||
| Hospital mortality | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
Legend: MV – Mechanical ventilation; PONV – Postoperative nausea and vomiting; CPR – Cardio-pulmonary resuscitation; GFR – Glomerular filtration rate; SOFA - Sequential Organ Failure Assessment, ASA - American Society of Anesthesiologists; ICU – Intensive care unit. The results are presented as count (%), mean±sd or median (IQR). * Statistical tests: (t) - Student’s t-test; (mw) - Mann–Whitney test; (chi) - Chi-Square (Χ²) Test; (fet) - Fisher’s exact test. ** Dynamics of inflammatory markers, measured at postoperative 0h, 24h, 48h. 72h and 96h time frame, available in Supplement 1, Figure S2, and for the GFR < 60mL/min in Supplement 1, Figure S3.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.zip (1.26MB )
Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Alerts
supplementary.zip (1.26MB )
This version is not peer-reviewed
Submitted:
30 January 2024
Posted:
31 January 2024
You are already at the latest version
Alerts
Abstract
: Cardiac surgery (CS) with extracorporeal circulation (ECC), induces oxidative stress and systemic inflammatory response, which may seriously affect postoperative lung function. We aimed to test if high parenteral (200mg/kg/24h) perioperative (48h) doses of Vitamin C (VitC) may reduce the incidence and severity of postoperative pulmonary complications (PPC) in selected CS patients. Single centered, prospective, randomized, single-blinded, interventional study included 150 patients, assigned to control group A (n=75) and interventional group B (n=75). Group B intraoperatively received 1/4 of the planned daily Vit C dose (200mg/kg/24h), diluted in 10 ml of normal saline, divided into three parts, while Group A received an equal volume of normal saline at the same time frames. After 6 h from the first intraoperative dose, the following regimen was applied: Group B: 200 mg/kg/24h - 30 min i.v. infusion of VitC in 50 ml of normal saline, every 6h, for the next 48h, and Group A: 30 min i.v. infusion of an equal volume of normal saline every 6 hours, for the next 48h. Modified Kroenke’s score was used to determine the incidence and severity of PPC. The overall incidence of PPC was 36.7% and was significantly lower in Group B (13.3% v.s. 60.0%, p<0.001). The severity of PPC was also significantly lower in Group B [1(1) v.s. 3(2), p<0.001]. Besides, patients from Group B had significantly less damaged lungs and better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. High parenteral daily VitC doses (200mg/kg/24h) given to selected CS patients for 48h after CS are safe and effective in reducing the incidence and severity of PPC. Further multicenter RCT is needed to confirm these results.
Keywords:
Subject: Medicine and Pharmacology - Cardiac and Cardiovascular Systems
1. Introduction
Cardiac surgical (CS) operations with extracorporeal circulation (ECC), inevitably induce a complex, multi-etiological, oxidative stress (OS) and systemic inflammatory response (SIR), the intensity of which varies from mild, subclinical forms, to clinically manifest syndrome (SIRS), with or without multiorgan dysfunction (MODS). [1-5]
Within this continuum, the lungs are both the source and target organ for oxidative and inflammatory mediators. [3-6] Accordingly, postoperative pulmonary complications (PPC) are the most frequent complications of CS interventions with ECC and are responsible for significant morbidity, disability, mortality, and health care costs. [7-9] The incidence of PPC ranges between 3% and >50%, according to different criteria for their definition. [8,10-12] Commonly considered as a composite outcome measure, PPC are responsible for significant early (14%-30%) and late (one-year and five-year) mortality (45.9% and 71.4%, respectively). [7]
Thanks to its proven antioxidant and pleiotropic biochemical functions, and its ability to reduce the intracellular activation of NFκB, which determine the intensity and extent of systemic inflammation, vitamin C (VitC, ascorbic acid, ascorbate) is increasingly used in various conditions to reduce an excessive OS and SIR. [3,13-22] It was shown that non-CS patients need much more VitC (500mg-4gr/24h) than the recommended daily doses for healthy individuals (90mg/24h). [18,23,24] Cardiac surgical patients are even bigger VitC "consumers", [25] but, despite this, the majority of them (56%) enter CS with VitC deficiency. [18]
The effectiveness of VitC in CS was tested in a relatively small number of studies, [23-27], focusing mainly on postoperative atrial fibrillation, while the only one that examined the effect of VitC on PPC in low-risk CS patients, was limited to intraoperative parenteral administration of intermediate therapeutic doses (3x1g). [28]
Since 1970, onward, the scientific community has been divided upon the safety and efficacy of high-dose parenteral VitC supplementation. [29,30] Several studies conducted during the COVID-19 pandemic, NIH expert panel document on cancer treatment, and studies on critically ill patients, have reported no complications with high daily parenteral doses of up to 50g. [31-36]
We present the results of our study, designed primarily to investigate the influence of high parenteral (200mg/kg/24h) perioperative (48h) doses of VitC, on the incidence and severity of PPC in patients submitted to CS with ECC. The rationale for this therapeutic regimen relied on the fact that OS and SIR are most intense within the first 48 hours after CS, and so is the peak consumption of vitamin C. [5,25]
2. Materials and Methods
2.1. Design
This prospective, randomized, single-blinded, interventional study was conducted at the UC Clinical Centre of Serbia, Clinic for Cardiac Surgery, Belgrade, Serbia, starting on July 15, 2022., for the duration required for all respondents to complete the planned stages of the investigation. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical Faculty UC Belgrade, Serbia (protocol code 1322/VII-24 and date of approval 07.07.2022.). Informed consent was obtained from all subjects involved in the study. They also consented not to be informed of whether they were allocated to the control or the intervention group.
2.2. Participants
Inclusion criteria were: all patients aged ≥18 years undergoing an elective CS procedure with ECC, regardless of the type of planned operation. Exclusion criteria were: previous CS operation, emergency patients, clinically and/or radiographically active lung disease, the systolic pressure in the pulmonary artery >60 mmHg, allergy to ascorbic acid, gout, hemodialysis, significant oxaluria, uric nephrolithiasis, glucose-6-phosphate dehydrogenase enzyme deficiency, hemochromatosis, sickle cell anemia, sideropenic anemia, and thalassemia. The criteria for subsequent withdrawal from the study were: operations with an ECC time ≥6h, death during hospitalization caused by non-pulmonary reasons, and withdrawal of previously given consent to participate in the study.
2.3. Interventions
All patients were operated on and subsequently treated according to standard institutional anesthesiological, surgical, and intensive care protocols.
Patients from the intervention group (B) intraoperatively received 1/4 of the planned daily dose of Vit C (200mg/kg/24h), divided into three parts, diluted in 10 ml of normal saline, and administered via central venous catheter 10 minutes after induction of anesthesia, 10 minutes before removal of the aortic cross-clamp (reperfusion), and at the beginning of sternal closure. The control group (A) received an equal volume of normal saline at the indicated times. Postoperatively (6 hours after the first intraoperative dose), VitC was administered according to the following regimen:
- Intervention group (B): 200 mg/kg/24h - 48h - 30 min i.v. infusion of VitC in 50 ml of normal saline every 6 hours, under UV protection.
- Control group (A): 30 min i.v. infusion of an equal volume of normal saline every 6 hours, under UV protection.
Patients from group B continued to receive an enteral supplementation of VitC (2g/24h) until discharge and were advised to continue it for a week after.
For each patient included in the study, standard perioperative characteristics (i.e. demographic, anthropometric, clinical, and laboratory) were registered in a separate database. The American Society of Anesthesiologists (ASA) score was calculated to assess the preoperative physical status. [37] The degree of organ dysfunction after surgery was quantified by the Sequential Organ Failure Assessment (SOFA) score which was estimated 48h after CS. [38]
The incidence and severity of PPC were scored and agreed by two independent, blinded assistants, using the original operational definitions of Kroenke [39] and subsequent modifications. [40-43] Score values were determined daily, and as an individual PPK severity score, the worst registered value (i.e. the highest grade) within 7 days after surgery was used for the analyses. The severity of PPC was graded on an ordinal scale of 0 (no PPC) to 5 (death before discharge). The values from 1 to 4 depict the increasing severity of the PPC. (Supplement 1, Table S1). To determine the incidence of PPK, only scores ≥3 were considered.
2.4. Outcome
The primary end-point was to compare the incidence and severity of PPC in the control (A) and intervention (B) groups. Secondary end-points, aimed to compare the following parameters between the groups: pulmonary oxygenation and ventilation (Horowitz index: PaO2/FiO2, Alveolar–arterial gradient: A-aDO2, and time spent on mechanical ventilation), inflammatory markers (procalcitonin, C-reactive protein, leucocytes, neutrophils, lymphocytes, sedimentation rate, fibrinogen, albumin, D-dimer and ferritin), renal function (GFR < 60mL/min, creatinine, urea), non-pulmonary postoperative complications, postoperative organ dysfunction (SOFA score, ASA/SOFA ratio), intensive care unit – ICU parameters (re-admission, length of stay) and hospital parameters (length of stay, mortality).
2.5. Sample Size
The required number of subjects to be included in the study was calculated based on the primary objective - the assumption that high daily doses of parenterally administered VitC will reduce the severity of PPC after CS with ECC. To provide a study power of 0.90, 5% level of significance, and to detect a clinically significant difference of the mean value of the PPK score ≥0.3 (2.1, 95 % CI, 2.0-2.3 vs. 1.8, 95% CI, 1.7-2.0), as defined by previous studies, [28,40] the required sample size was 70 subjects in each group (i.e. a total of 140). To allow for 15% drop-out, we decided to include a minimum of 80 participants in each group (i.e. a total of 160).
2.6. Randomization and Masking
Randomization was done by allocating each consecutive respondent in a 1:1 manner to either control (A) or intervention (B) groups, the order of which was determined by manual random selection. Except for the principal investigator (M.K.K.) and two assistants (J.Č., R.K.), nobody else, involved in the patient treatment, clinical and laboratory data collection, and analysis had any information about the randomization and grouping. Two informed assistants prepared an unlabeled infusion solution (placebo or VitC), covered it with UV protective coating, and assigned it to the patient according to randomization and grouping. The principal investigator and two informed assistants were not engaged in the patient treatment, clinical and laboratory data collection, and analysis. Two independent, blinded specialists estimated and agreed on the ASA, SOFA, and PPC severity score grades.
2.7. Statistical Methods
Results are presented as count (%), means ± standard deviation, or median (interquartile range) depending on data type and distribution.
Groups are compared using parametric (t-test) and nonparametric (Chi-square, Mann-Whitney U test, Fisher’s Exact test) tests. All p-values less than 0.05 were considered significant.
To conduct all statistical analyses, we used SPSS 29.0 (IBM Corp. Released 2023. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.) and R 3.4.2. (R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
3. Results
After assessing for eligibility, 22 of 182 enrolled patients, who met the exclusion criteria were not included in randomization. A total of 160 patients were randomized and allocated to control group – A (n=80) and intervention group – B (n=80). A total of 10 patients were subsequently excluded from the analysis. In group A, one was for intraoperative death, three for ECC duration of ≥ 6 hours, and one for in-hospital non-pulmonary death. In group B, one was for intraoperative death, two for ECC duration of ≥ 6 hours, and two for in-hospital non-pulmonary death. As a result, a total of 150 patients (75 in each group) have entered the analysis of study data. (Figure 1).
The baseline perioperative characteristics of patients included in the study are shown in Table 1. There were no significant differences between the groups for the most of perioperative parameters, except for body mass index (BMI), diastolic arterial pressure (Tad), chronic renal failure (CRF), and duration of surgery, showing significantly higher values in group A.
Although the other characteristics are fairly evenly distributed between the groups, it is evident that all patients had advanced systemic disease (ASA scores 3 and 4). Both groups are elderly people with pronounced CVD risks (HTA 97.3% in group B and 100% in group A) and serious comorbidities. Interestingly, the proportion of patients with a history of COVID-19 infection was relatively small, keeping in mind that this study was conducted in a critical period (March 2020 to November 2022), when the most infected and deceased were recorded. [44] Yet, preoperative pulmonary status in both groups was quite good (PPC score grades 0 and 1).
The primary end-point outcome measures of patients included in the study are shown in Table 2. The overall incidence of PPC was 36.7% and was significantly lower in Group B (13.3% v.s. 60.0%, p<0.001). The severity of PPC was also significantly lower in Group B [1(1) v.s. 3(2), p<0.001].
The secondary end-point outcome measures are shown in Table 3. The following parameters were significantly better in group B: Horowitz index (312.6±107.4 v.s. 268.9±112.6, p=0,008), C-reactive protein [95 (56.2) v.s. 167.4 (82.9), p<0.001], sedimentation rate [20 (6) v.s. 22 (18), p=0.023], acute renal failure (1.3%v.s. 10.7%, p=0,034), wound infection (6.7% v.s. 20%, p=0,016), GFR < 60mL/min (13.3% v.s. 32%, p=0,006), urea (6.3±1.9 v.s. 7.1±3, p=0,041), ICU re-admission (5.3% v.s. 20%, p=0,007), ICU stay [32 (24) v.s. 48 (24), p<0.001], and hospital mortality (1.3% v.s. 10.7%, p=0,034). Serum procalcitonin levels were significantly lower in group A [0.3 (0.6) v.s. 0.5 (0.8), p=0.032].
4. Discussion
The results of our study support parenteral administration of high daily doses of VitC (200 mg/kg/24h for 48h) to reduce the incidence and severity of PPC after CS with ECC. (see Table 2) Besides, patients from the VitC group had significantly less damaged lungs (i.e. better Horowitz index), better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. (see Table 3).
Trying to find the answers to the primary end-points of this study, we found a question: „Do we know what is meant by PPC in CS?“ In all the reports we preliminarily analyzed, we could not find the same or sufficiently similar definition of PPC which could allow robust comparisons. [8,11,12,45-49] Thus, comparing the guideline by two respectable task forces, [10,50] one can find seven listed complications in one, [50] and four in another definition [10] of PPC, as the composite outcome measure. But, is it just a lack of standardized PPC definition problem? Some believe that even more important are disparities in the clinical appraisal of PPC. [51,52] Accordingly, the incidences of PPC reported in the literature, widely range from 3% to >50%. [8,10-12] The overall incidence of PPC in our study (36.7%), fits within this range.
Yet, another seemingly simple question that appeared to us, was: “If it’s not severe enough, is it a complication at all?” Indeed, PPC after CS may vary from mild, subclinical forms (e.g. compensated abnormalities of respiratory mechanics) to severe respiratory failure with prolonged ventilator dependency. [53] Reviewing the literature, as for the definition of PCC, we could find very few reports defining standardized and objective criteria for quantification of the severity of PPC in CS. [10,39-42] We decided to use the modified [40-42] PPC severity score, originally defined by Kroenke et al. [39] for patients with severe chronic obstructive pulmonary disease. (Supplement 1, Table S1) It was recently compared with the Melbourne Group Scale, and both were deemed to be useful tools for grading PPC in CS patients. [54] To overcome Kroenke’s score inferiority in more severe cases, we found it appropriate to set the score value ≥3 as a cut-off in analysis of the PPC incidence.
The interventional part of our study relied on two sets of facts. The first is that CS operations with ECC, inevitably induce a complex, multi-etiological, phasic OS and SIR, the most intense within the first 48h after CS, which, in addition to the other factors, affect postoperative pulmonary function. [1-9] The second is the proven antioxidant and pleiotropic biochemical functions of the VitC, due to which it has been commonly used to reduce organ damage induced by excessive OS and SIR. [13-22]
About 63 million years ago, the primates and a few other species were „deceived“ by the abundance of plants containing VitC, and conserved the mutation and inactivation of the gene for the synthesis of L-gulono-γ-lactone oxidase (GULO) on chromosome 8p21, thus losing the ability to synthesize VitC from glucose. Accordingly, humans entirely rely on dietary intake and rational metabolic use of VitC to maintain homeostasis. [55] A recent study of VitC status and prevalence of deficiency suggests that dietary intake is globally insufficient. [56]
The first appreciation of the importance of VitC dietary intake came from the British sailors. Upon the observation of naval surgeon James Lind (1747) that oranges and lemons can prevent scurvy (lat. Scorbutus), Gilbert Blane (1795) managed to persuade the Admiralty to use citrus juice as a daily ration on board British naval vessels. [57] Two centuries and two Nobel Prizes later (Albert Szent-Györgyi and Walter Norman Haworth), this low-cost, essential substance was the subject of many controversial polemics, sometimes surpassing purely medical and scientific framework. [58] Seneca, a Roman stoic philosopher, once said, “Truth never gets old.” Thus, as a result of accumulated knowledge of its important pleiotropic functions, VitC therapy has continued to be tested in numerous contemporary studies, mainly in critically ill patients.
Regardless of preoperative status, CS rapidly consumes VitC and serious depletion may last for two weeks, deteriorating the defense against OS and SIR during cardiac operations. [14] To our knowledge, there is no study comparing the effectiveness of different therapeutic regimens (different doses and different duration of therapy) of parenterally administered VitC in CS patients. We aimed to test PPC in CS patients intervening with high daily parenteral doses of VitC (200mg/kg/24h) for the first 48 postoperative hours. Similar (i.e. 250mg/kg), single parenteral doses were tested in two studies on CS patients, one aimed to compare the postoperative dynamics of creatine kinase-MB and malondialdehyde, [59] and another to compare the dynamics of the cardiac index with a control group. [36] Both studies have reported the beneficial effect of VitC. [36,59] Study with the same dose of parenteral VitC as ours, given for 96h, in patients with severe sepsis, has proved its benefits in the dynamics of the inflammatory markers and SOFA score. [35] None of them have reported any adverse effects of high-dose parenteral VitC therapy. [35,36,59] We also didn’t have any side effects or complications with our VitC therapeutic regimen. To assess potential functional renal impairment induced by high doses of VitC, we included renal functional analysis in secondary outcome measures. Renal function was preoperatively significantly better in group B, and this remained the same up to 96h after CS. (Table 1 and Table 3, Supplement 1, Figure S3).
The incidence and the severity of PPC were significantly lower in group B (see Table 2). These results are in concordance with the only relatively comparable study. [28] Comparing the PPC by grade, in group A most of them were clustered in grades 2-4, while in group B they clustered in grades 1 and 2. (see Table 2, Supplement 1, Figure S1)
The most common types of PPC in our study were pleural effusion and pneumonia, all being significantly more frequent in group A. Similar incidences are reviewed by Tanner et al. [46] Grade 4 PPC (respiratory failure equivalent) was more frequent in group A (see Table 2). Wang et al. reported only one (2.7%) patient, from the control group, with PPC grade 4. Such discrepancy may be explained by comparing the baseline perioperative characteristics. Patients in our study were older, had advanced systemic disease, pronounced CVD risks, and serious comorbidities. Moreover, we had 26% of patients with a history of previous COVID-19 disease, while the study of Wang et al. was conducted before the first cases of COVID-19 were reported in Wuhan, China, in December 2019. [28,44]
Parameters indicating pulmonary function (i.e. oxygenation and ventilation) and selected inflammatory markers, were sampled at 48h after CS. The mean values of PaO2/FiO2 and A-aDO2 depict the presence of postoperative lung injury in both groups. A significantly better Horowitz index in group supports a potential lung protective effect of the VitC (see Table 3). In a study by Wang et al. there was no difference in these parameters between the groups. [28]
The median serum procalcitonin levels were not elevated as they are in septic patients but were significantly lower in group A. At 48h, there was no significant difference between the other inflammatory markers, except CRP and sedimentation rate, which were significantly lower in group B (see Table 2). The dynamics of those parameters were followed up for 96h. Interestingly, from 48h to 96h, statistically lower values were also recorded for leucocytes, neutrophils, and lymphocytes in group B, and fibrinogen in group A. (Supplement 1, Figure S2) These findings support the potential anti-inflammatory effect of VitC which spans more than 48h after CS.
Except for acute renal failure and wound infection, the other non-pulmonary complications didn’t show significant differences between the groups (see Table 3). Iizuka et al. found a positive correlation between low plasma levels of VitC and postoperative delirium in elderly CS patients, [60] but this was not the case in our study.
SOFA score, estimated 48h after CS didn’t show significant difference between the groups in our study. Median values of 4, indicate a mild degree of organ dysfunction in all patients at this time-frame. In other studies of high parenteral doses of VitC in critically ill patients, reviewed by Nabzdyk et al. a significant reduction in SOFA score grade was recorded in the intervention groups. [61] This difference may be interpreted in light of different underlying causes of organ dysfunction (i.e. severe sepsis and severe burns v.s. CS).
Patients in group B had significantly lower ICU re-admission rates and shorter ICU stays. Also, they had a significantly lower hospital mortality rate (see Table 3). These results are much better than those reported by Wang et al. [28], probably depicting the more physiological regimen (i.e. higher doses and longer duration) of parenteral VitC supplementation in our study.
This study has some limitations. First, as a single-centered and single-blinded study, it was difficult to avoid selection, observer, performance, and detection biases, affecting the validity and reliability of the study. To minimize these potential biases, the study was kept open only to three persons (the principal investigator and two informed assistants). The key study intervention (infusion of VitC solution) was masked, as explained earlier, to all but the principal investigator and two informed assistants. Similarly, the assessment of the key outcome parameter (PPC score) and other scoring systems (ASA, SOFA) was protected from the influence of informed researchers by engaging two independent, blinded assistants. Second, although the calculated sample size of 70 participants in each group provides this study with an acceptable level of statistical accuracy and validity, this number is still too small to draw any general conclusions, so the results of this study should be interpreted with caution. Third, highly inconsistent criteria for the definition of PPC make the results of this, like other similar studies, insufficiently suitable for comparisons and meta-analyses. That was why we decided to use the modified [40-42] PPC severity score, originally defined by Kroenke et al. [39], as an outcome measure for our primary end-point. Our decision is supported by the fact that this score was used in the only study published so far on the effect of VitC on PPC in CS. [28] Fourth, two score tools for practical use in the CS, one of them being Kroenke’s score, were compared and it was concluded that both can identify PPC effectively. [43] Yet, the validity of Kroenke’s score to identify severe PPC remains disputable. To overcome this, we set the score value ≥3 as a cut-off in the analysis of the PPC incidence. Thus, to “catch” the more severe cases, we probably lost some of the less severe PPC from the analysis of PCC incidence. Fifth, we could not measure plasma levels of the VitC, due to the complexity of the procedure and the excessive cost of the reagents. Because of the complex pharmacokinetics of VitC, [19] it would be the most reliable way to interpret the observed differences between the control (A) and the intervention group (B), in the context of the possible influence of the applied high parenteral VitC doses. Finally, we hope to see and participate in future, multicenter, prospective, randomized, controlled, double-blinded, interventional trials, which would (hopefully) confirm our results. Before that, it is necessary that a widely accepted, standardized, and comparable definition of PPC finally appears.
5. Conclusions
Our study has shown that high parenteral daily VitC doses (200mg/kg/24h) could be given to selected CS patients for 48h after CS without side effects or complications. By reducing the OS and SIR, along with many other pleiotropic functions, applied doses of VitC significantly reduce the incidence and severity of PPC in CS patients. Besides, patients receiving high parenteral daily VitC doses had significantly less damaged lungs and better postoperative renal function, shorter ICU stay, fewer ICU re-admissions, and lower hospital mortality. Further multicenter RCT is needed to confirm these results.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: PPC severity score; Figure S1: PPC by grade; Figure S2: Dynamics of inflammatory markers; Figure S3: Dynamics of GFR < 60mL/min.
Author Contributions
Conceptualization, M.K.K., A.R., and D.M.; methodology, M.K.K., J.Č., and R.K.; software, I.S.; validation, M.K.K., A.R., and D.M.; formal analysis, I.S. and M.K.K.; investigation, M.K.K., D.L., M.G., D.T., J.Č., and R.K.; resources, M.K.K.; data curation, M.K.K., D.L., M.G., D.T., J.Č., and R.K; writing—original draft preparation, M.K.K.; writing—review and editing, M.K.K., A.R., M.K., I.P., and D.M.; visualization, M.K.K., M.K.; supervision, A.R., M.K. I.P., and D.M.; project administration, M.K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical Faculty UC Belgrade (protocol code 1322/VII-24 and date of approval 07.07.2022.).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Squiccimarro, E.; Labriola, C.; Malvindi, P.G.; Margari, V.; Guida, P.; Visicchio, G.; Kounakis, G.; Favale, A.; Dambruoso, P.; Mastrototaro, G.; et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction After Cardiac Surgery. J Cardiothorac Vasc Anesth 2019, 33, 1682–1690. [Google Scholar] [CrossRef]
- Churpek, M.M.; Zadravecz, F.J.; Winslow, C.; Howell, M.D.; Edelson, D.P. Incidence and Prognostic Value of the Systemic Inflammatory Response Syndrome and Organ Dysfunctions in Ward Patients. Am J Respir Crit Care Med 2015, 192, 958–964. [Google Scholar] [CrossRef]
- McGuinness, J.; Bouchier-Hayes, D.; Redmond, J.M. Understanding the inflammatory response to cardiac surgery. Surgeon 2008, 6, 162–171. [Google Scholar] [CrossRef]
- Semler, M.W.; Wheeler, A.P. Systemic inflammatory response syndrome after cardiac surgery: time for a change. Chest 2014, 145, 1181–1182. [Google Scholar] [CrossRef]
- Warltier, David C. ; Laffey, John G.; Boylan, John F.; Cheng, Davy C.H. The Systemic Inflammatory Response to Cardiac Surgery: Implications for the Anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- Joseph, D.; Puttaswamy, R.K.; Krovvidi, H. Non-respiratory functions of the lung. Continuing Education in Anaesthesia Critical Care & Pain 2013, 13, 98–102. [Google Scholar] [CrossRef]
- Miskovic, A.; Lumb, A.B. Postoperative pulmonary complications. Br J Anaesth 2017, 118, 317–334. [Google Scholar] [CrossRef]
- Fischer, M.-O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Nigro Neto, C.; Kunstyr, J.; et al. Postoperative Pulmonary Complications After Cardiac Surgery: The VENICE International Cohort Study. Journal of Cardiothoracic and Vascular Anesthesia 2022, 36, 2344–2351. [Google Scholar] [CrossRef]
- Brown, P.P.; Kugelmass, A.D.; Cohen, D.J.; Reynolds, M.R.; Culler, S.D.; Dee, A.D.; Simon, A.W. The frequency and cost of complications associated with coronary artery bypass grafting surgery: results from the United States Medicare program. Ann Thorac Surg 2008, 85, 1980–1986. [Google Scholar] [CrossRef]
- Abbott, T.E.F.; Fowler, A.J.; Pelosi, P.; Gama de Abreu, M.; Møller, A.M.; Canet, J.; Creagh-Brown, B.; Mythen, M.; Gin, T.; Lalu, M.M.; et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018, 120, 1066–1079. [Google Scholar] [CrossRef]
- Mali, S.; Haghaninejad, H. Pulmonary complications following cardiac surgery. Arch Med Sci Atheroscler Dis 2019, 4, e280–e285. [Google Scholar] [CrossRef] [PubMed]
- Naveed, A.; Azam, H.; Murtaza, H.G.; Ahmad, R.A.; Baig, M.A.R. Incidence and risk factors of Pulmonary Complications after Cardiopulmonary bypass. Pak J Med Sci 2017, 33, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Oudemans-van Straaten, H.M.; Spoelstra-de Man, A.M.; de Waard, M.C. Vitamin C revisited. Crit Care 2014, 18, 460. [Google Scholar] [CrossRef] [PubMed]
- Ballmer, P.E.; Reinhart, W.H.; Jordan, P.; Bühler, E.; Moser, U.K.; Gey, K.F. Depletion of plasma vitamin C but not of vitamin E in response to cardiac operations. J Thorac Cardiovasc Surg 1994, 108, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; O’Neill, L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann Intern Med 2004, 140, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J Nutr 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.; Yamazaki, E. Vitamin C requirement in surgical patients. Current Opinion in Clinical Nutrition & Metabolic Care 2010, 13, 669–676. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Long, M.T.; Hess, A.S.; McCarthy, D.P.; DeCamp, M.M. Power for the Sickest: Vitamin C for Vasoplegia after Cardiac Surgery. J Cardiothorac Vasc Anesth 2020, 34, 1123. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J. On the effect of vitamin C intake on human health: How to (mis)interprete the clinical evidence. Redox Biol 2020, 34, 101532. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Borgs, C.; Fitzner, C.; Stoppe, C. Perioperative Vitamin C and E levels in Cardiac Surgery Patients and Their Clinical Significance. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Wendt, S.; Benstoem, C.; Neubauer, C.; Meybohm, P.; Langlois, P.; Adhikari, N.K.; Heyland, D.K.; Stoppe, C. Vitamin C to Improve Organ Dysfunction in Cardiac Surgery Patients-Review and Pragmatic Approach. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.; Clasen, K.C.; Wendt, S.; Majoros Á, G.; Stoppe, C.; Adhikari, N.K.J.; Heyland, D.K.; Benstoem, C. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Chalker, E. Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mangoush, O.; Nakamura, K.; Al-Ruzzeh, S.; Athanasiou, T.; Chester, A.; Amrani, M. Effect of ascorbic acid on endothelium-dependent vasodilatation of human arterial conduits for coronary artery bypass grafting. Eur J Cardiothorac Surg 2003, 24, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, M.; Zhang, H.; Zhu, H.; Zhang, N.; Liu, J. Effect of Intravenous Injection of Vitamin C on Postoperative Pulmonary Complications in Patients Undergoing Cardiac Surgery: A Double-Blind, Randomized Trial. Drug Des Devel Ther 2020, 14, 3263–3270. [Google Scholar] [CrossRef] [PubMed]
- Creagan, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N Engl J Med 1979, 301, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Ascorbic acid and the common cold. Am J Clin Nutr 1971, 24, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Coppock, D.; Violet, P.C.; Vasquez, G.; Belden, K.; Foster, M.; Mullin, B.; Magee, D.; Mikell, I.; Shah, L.; Powers, V.; et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life (Basel) 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov 2020, 5, 100028. [Google Scholar] [CrossRef] [PubMed]
- PDQ® Integrative, Alternative, and Complementary Therapies Editorial Board. PDQ Intravenous Vitamin C. Bethesda, MD: National Cancer Institute. Updated <06/17/2022>. Available at: https://www.cancer.gov/about-cancer/treatment/cam/hp/vitamin-c-pdq. Accessed <12/10/2023>. [PMID: 26389504].
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Dingchao, H.; Zhiduan, Q.; Liye, H.; Xiaodong, F. The protective effects of high-dose ascorbic acid on myocardium against reperfusion injury during and after cardiopulmonary bypass. Thorac Cardiovasc Surg 1994, 42, 276–278. [Google Scholar] [CrossRef]
- Knuf, K.M.; Maani, C.V.; Cummings, A.K. Clinical agreement in the American Society of Anesthesiologists physical status classification. Perioper Med (Lond) 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit Care 2019, 23, 374. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Lawrence, V.A.; Theroux, J.F.; Tuley, M.R. Operative risk in patients with severe obstructive pulmonary disease. Arch Intern Med 1992, 152, 967–971. [Google Scholar] [CrossRef]
- Costa Leme, A.; Hajjar, L.A.; Volpe, M.S.; Fukushima, J.T.; De Santis Santiago, R.R.; Osawa, E.A.; Pinheiro de Almeida, J.; Gerent, A.M.; Franco, R.A.; Zanetti Feltrim, M.I.; et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. Jama 2017, 317, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Futier, E.; Constantin, J.M.; Paugam-Burtz, C.; Pascal, J.; Eurin, M.; Neuschwander, A.; Marret, E.; Beaussier, M.; Gutton, C.; Lefrant, J.Y.; et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013, 369, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Hulzebos, E.H.; Helders, P.J.; Favié, N.J.; De Bie, R.A.; Brutel de la Riviere, A.; Van Meeteren, N.L. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. Jama 2006, 296, 1851–1857. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Z.; Huang, W.; Zhang, X.; Guo, Y.; Yu, P. Comparison of Tools for Postoperative Pulmonary Complications After Cardiac Surgery. J Cardiothorac Vasc Anesth 2023, 37, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, V.M.; Nikolić, N.; Radovanović Nenadić, U.; Öcal, A.; E, K.N.; Zečević, M. Preparedness and Preventive Behaviors for a Pandemic Disaster Caused by COVID-19 in Serbia. Int J Environ Res Public Health 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Cheng, C.; Wei, X. Incidence of postoperative pulmonary complications in patients undergoing minimally invasive versus median sternotomy valve surgery: propensity score matching. Journal of Cardiothoracic Surgery 2021, 16, 287. [Google Scholar] [CrossRef] [PubMed]
- Tanner, T.G.; Colvin, M.O. Pulmonary Complications of Cardiac Surgery. Lung 2020, 198, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Hardman, J.; Sabaté, S.; Langeron, O.; Abreu, M.G.; Gallart, L.; Belda, J.; Markstaller, K.; Pelosi, P.; Mazo, V. PERISCOPE study: predicting post-operative pulmonary complications in Europe. Eur J Anaesthesiol 2011, 28, 459–461. [Google Scholar] [CrossRef] [PubMed]
- Canet, J.; Gallart, L.; Gomar, C.; Paluzie, G.; Vallès, J.; Castillo, J.; Sabaté, S.; Mazo, V.; Briones, Z.; Sanchis, J. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010, 113, 1338–1350. [Google Scholar] [CrossRef]
- Weissman, C. Pulmonary complications after cardiac surgery. Semin Cardiothorac Vasc Anesth 2004, 8, 185–211. [Google Scholar] [CrossRef] [PubMed]
- Jammer, I.; Wickboldt, N.; Sander, M.; Smith, A.; Schultz, M.J.; Pelosi, P.; Leva, B.; Rhodes, A.; Hoeft, A.; Walder, B.; et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015, 32, 88–105. [Google Scholar] [CrossRef] [PubMed]
- Gologorsky, E.; Gologorsky, A.; Salerno, T.A. Lung-Centered Open Heart Surgery: A Call for a Paradigm Change. Front Cardiovasc Med 2016, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Khera, R.; Vaughan-Sarrazin, M.; Rosenthal, G.E.; Girotra, S. Racial disparities in outcomes after cardiac surgery: the role of hospital quality. Curr Cardiol Rep 2015, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef] [PubMed]
- Yuqiang Wang; Yaxin Zhou; Zeruxin Luo; Wei Huang; Xiu Zhang; Yingqiang Guo; Yu, P. Comparison of Tools for Postoperative Pulmonary Complication Following Cardiac surgery. PREPRINT (Version 1) 2022, Research Square. [CrossRef]
- Hickey, S.; Roberts, H. Evolution and Deficiency. In Ascorbate: The Science of Vitamin C, Hickey, S., Roberts, H., Eds.; Lulu.com: 2004; pp. 66-72.
- Rowe, S.; Carr, A.C. Global Vitamin C Status and Prevalence of Deficiency: A Cause for Concern? Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.J. The discovery of vitamin C. Ann Nutr Metab 2012, 61, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Richards, E. Introduction. In Vitamin C and Cancer: Medicine or Politics?; Palgrave Macmillan UK: 1991; pp. 1-14.
- Li, C.C. [Changes of creatine phosphokinase and malondialdehyde in the serum and clinical use of large doses of vitamin C following open heart surgery]. Zhonghua Wai Ke Za Zhi 1990, 28, 16–17. [Google Scholar] [PubMed]
- Iizuka, Y.; Yoshinaga, K.; Takahashi, K.; Oki, S.; Chiba, Y.; Sanui, M.; Kimura, N.; Yamaguchi, A. Association between Plasma Ascorbic Acid Levels and Postoperative Delirium in Older Patients Undergoing Cardiovascular Surgery: A Prospective Observational Study. J Cardiovasc Dev Dis 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Nabzdyk, C.S.; Bittner, E.A. Vitamin C in the critically ill - indications and controversies. World J Crit Care Med 2018, 7, 52–61. [Google Scholar] [CrossRef]
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart for each phase of the study.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) flowchart for each phase of the study.

Table 1.
Baseline perioperative characteristics.
| Perioperative Parameters | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. Demographic and Anthropometric | |||
| Age (years) | 66.9±8.7 | 66.3±8.6 | 0.672 (t) |
| Male gender | 59 (78.7%) | 55 (73.3%) | 0.444 (chi) |
| BMI (kg/m2) | 28.4±3.9 | 26.7±3.3 | 0.005 (t) |
| 2. CVD Risk | |||
| HTA | 75 (100%) | 73 (97.3%) | 0.497 (fet) |
| DM | 36 (48%) | 36 (48%) | 1.000 (fet) |
| HLP | 72 (96%) | 68 (90.7%) | 0.190 (chi) |
| Smoking | 52 (69.3%) | 52 (69.3%) | 1.000 (chi) |
| 3. CV Status and Comorbidities | |||
| Recent MI | 41 (54.7%) | 34 (45.3%) | 0.253 (chi) |
| AP | 61 (81.3%) | 58 (77.3%) | 0.545 (chi) |
| TAs (mmHg) | 145.8±26.3 | 139.8±16.9 | 0.100 (t) |
| TAd (mmHg) | 81.9±13.1 | 77.3±11.6 | 0.022 (t) |
| EF-LV (%) | 46.5±9.1 | 48.4±8.0 | 0.187 (t) |
| HR (beats/min) | 70.4±9.3 | 69.5±9.7 | 0.565 (t) |
| Sinus | 68 (90.7%) | 66 (88%) | 0.597 (chi) |
| AF | 8 (10.7%) | 9 (12%) | 0.979 (chi) |
| CVD | 11 (14.7%) | 5 (6.7%) | 0.113 (chi) |
| CRF | 23 (30.7%) | 12 (16%) | 0.034 (chi) |
| COVID-19 | 22 (29.3%) | 17 (22.7%) | 0.352 (chi) |
| 4. Pulmonary status (PPC Score) | |||
| 0 | 20 (26.7%) | 25 (33.3%) | 0.373 (chi) |
| 1 | 55 (73.3%) | 50 (66.7%) | |
| 5. ASA Score | |||
| 3 | 65 (86.7%) | 66 (88.0%) | 0.806 (chi) |
| 4 | 10 (13.3%) | 9 (12.0%) | |
| 6. Surgery | |||
| CABG | 53 (70.7%) | 49 (65.3%) | 0.484 (chi) |
| Aortic valve | 9 (12%) | 13 (17.3%) | 0.356 (chi) |
| Mitral valve | 2 (2.7%) | 4 (5.3%) | 0.681 (fet) |
| Combined | 11 (14.7%) | 9 (12%) | 0.631 (chi) |
| Duration of surgery (min) | 245.7±40.2 | 219.9±45.0 | <0.001 (t) |
| ECC time (min) | 86.8±27.3 | 80.7±19.1 | 0.114 (t) |
| ACC time (min) | 55.6±20.7 | 56.3±15.4 | 0.799 (t) |
Legend: BMI – Body mass index; HTA – Arterial hypertension; DM – Diabetes Mellitus; HLP – Hy-perproteinemia; CV – Cardio-vascular; MI – Myocardial infarction; AP – Angina pectoris; Tas – Systolic arterial pressure; Tad – Diastolic arterial pressure; EF-LV – Left ventricular ejection fraction; CVD – Cerebro-vascular diseases; CRF – Chronic renal failure; COVID-19 - Coronavirus disease (SARS-CoV-2 virus); PPC – Postoperative pulmonary complications score; ASA - American Society of Anesthesiologists; CABG – Coronary artery bypass grafting; ECC – Extracorporeal circulation; ACC - Aortic cross-clamp. The results are presented as count (%) or mean±sd. * Statistical tests: (t) - Student’s t-test; (chi) - Chi-Square (Χ²) Test; (fet) - Fisher’s exact test.
Table 2.
Primary end-point outcome measures: PPC incidence, severity, and types.
| Primary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* |
|---|---|---|---|
| 1. PPC Incidence | |||
| PPC ≥ 3 (n,%) | 45 (60.0%) | 10 (13.3%) | <0.001 (chi) |
| 2. PPC Severity | |||
| PPC severity score | 3 (2) | 1 (1) | <0.001 (mw) |
| Grade 0 (n,%) | 5 (6.7%) | 14 (18.7%) | <0.001 (mw) |
| Grade 1 (n,%) | 3 (4.0%) | 31 (41.3%) | |
| Grade 2 (n,%) | 22 (29.3%) | 20 (26.7%) | |
| Grade 3 (n,%) | 23 (30.7%) | 7 (9.3%) | |
| Grade 4 (n,%) | 18 (24.0%) | 3 (4.0%) | |
| Grade 5 (n,%) | 4 (5.3%) | 0 | |
| 3. PPC Types | |||
| Pneumonia (n,%) | 32 (42.7%) | 13 (17.3%) | <0.001 (chi) |
| Pneumothorax (n,%) | 10 (13.3%) | 3 (4%) | 0.042 (chi) |
| Pleural effusion (n,%) | 51 (68%) | 38 (50.7%) | 0.031 (chi) |
| Re-intubation (n,%) | 16 (21.3%) | 2 (2.7%) | <0.001 (chi) |
Legend: PPC – Postoperative pulmonary complications. The results are presented as count (%), or median (IQR) * Statistical tests: (chi) - Chi-Square (Χ²) Test; (mw) - Mann–Whitney test.
Table 3.
Secondary end-point outcome measures.
| Secondary Outcome Measures | Group A (n=75) | Group B (n=75) | p-Value (test)* | ||
|---|---|---|---|---|---|
| 1. Pulmonary oxygenation and ventilation | |||||
| Horowitz index (PaO2/FiO2) 48h | 268.9±112.6 | 312.6±107.4 | 0.008 (t) | ||
| Alveolar–arterial gradient (A-aDO2) 48h | 17.3±5.4 | 17.9±4.7 | 0.432 (t) | ||
| Total MV time (h) | 5.2±1.6 | 5.4±1.2 | 0.493 (t) | ||
| 2. Inflammatory markers (48h) ** | |||||
| Procalcitonin | 0.3 (0.6) | 0.5 (0.8) | 0.032 (mw) | ||
| C-reactive protein | 167.4 (82.9) | 95 (56.2) | <0.001 (mw) | ||
| Leucocytes | 13.3±3.3 | 12.4±3.4 | 0.102 (t) | ||
| Neutrophils | 80.6±5.5 | 80.5±5.1 | 0.881 (t) | ||
| Lymphocytes | 12±4.6 | 12.6±4 | 0.418 (t) | ||
| Sedimentation rate | 22 (18) | 20 (6) | 0.023 (mw) | ||
| Fibrinogen | 5.1±1.4 | 5.2±1 | 0.775 (t) | ||
| Albumin | 31.8±3.5 | 31.4±3.2 | 0.479 (t) | ||
| D-dimer | 0.5 (0.5) | 0.6 (0.4) | 0.877 (mw) | ||
| Ferritin | 202 (277) | 200 (104) | 0.287 (mw) | ||
| 3. Postoperative complications (non-pulmonary) | |||||
| PONV | 20 (26.7%) | 30 (40.0%) | 0.083 (chi) | ||
| Delirium | 22 (29.3%) | 16 (21.3%) | 0.260 (chi) | ||
| Transfusion | 32 (42.7%) | 36 (48%) | 0.512 (chi) | ||
| Acute renal failure | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
| Wound infection | 15 (20%) | 5 (6.7%) | 0.016 (chi) | ||
| CPR | 4 (5.3%) | 0 (0%) | 0.120 (fet) | ||
| 4. Renal function(48h) ** | |||||
| GFR < 60mL/min | 24 (32%) | 10 (13.3%) | 0.006 (chi) | ||
| Creatinine | 99.6±44.6 | 87.8±27.5 | 0.054 (t) | ||
| Urea | 7.1±3 | 6.3±1.9 | 0.041(t) | ||
| 5. Postoperative organ dysfunction | |||||
| SOFA score | 4 (2) | 4 (1) | 0.132 (mw) | ||
| ASA/SOFA ratio | 1.0 (0.4) | 0.8 (0.25) | 0.190 (mw) | ||
| 6. ICU outcome measures | |||||
| ICU re-admission | 15 (20%) | 4 (5.3%) | 0.007 (chi) | ||
| ICU stay | 48 (24) | 32 (24) | <0.001 (mw) | ||
| 7. Hospital outcome measures | |||||
| Hospital stay | 8 (2) | 8 (2) | 0.092 (mw) | ||
| Hospital mortality | 8 (10.7%) | 1 (1.3%) | 0.034 (fet) | ||
Legend: MV – Mechanical ventilation; PONV – Postoperative nausea and vomiting; CPR – Cardio-pulmonary resuscitation; GFR – Glomerular filtration rate; SOFA - Sequential Organ Failure Assessment, ASA - American Society of Anesthesiologists; ICU – Intensive care unit. The results are presented as count (%), mean±sd or median (IQR). * Statistical tests: (t) - Student’s t-test; (mw) - Mann–Whitney test; (chi) - Chi-Square (Χ²) Test; (fet) - Fisher’s exact test. ** Dynamics of inflammatory markers, measured at postoperative 0h, 24h, 48h. 72h and 96h time frame, available in Supplement 1, Figure S2, and for the GFR < 60mL/min in Supplement 1, Figure S3.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Lung Protection Strategies during Cardiopulmonary Bypass Affect the Composition of Blood Electrolytes and Metabolites—A Randomized Controlled Trial
Katrine Buggeskov
et al.
JCM,
2018
Impact of Venoarterial Extracorporeal Membrane Oxygenation on Alkaline Phosphatase Metabolism after Cardiac Surgery
Thomas Poschner
et al.
Biomolecules,
2021
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated








