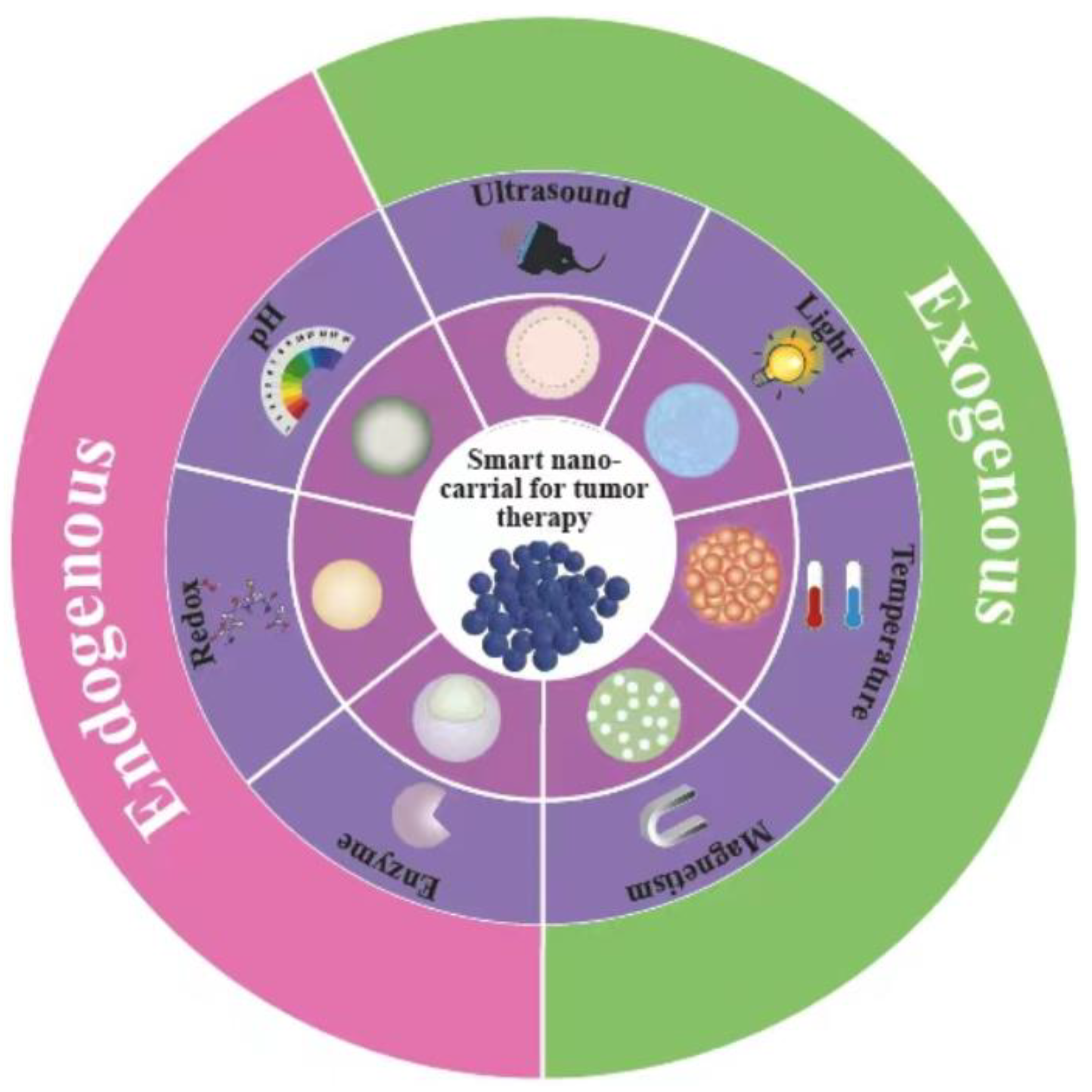
1. Introduction
Despite the remarkable advancements in recent years regarding diagnostic procedures and therapeutic techniques, cancer remains one of the most complex diseases affecting the human being health and a primary cause of mortality (around 10 million deaths annually) globally [1-3]. It is considered that around 27 million new cases of cancer would be detected by 2030. The clinical applicability of conventional cancer therapies, such as radiotherapy and chemotherapy, is limited due to their potential for harmful effects on normal cells and broad systemic toxicity [4-6]. In addition, surgery is a popular and successful cancer treatment; however, the broad metastatic lesions typically limit its use in the treatment of metastatic malignancies. The intrinsic limitations of these therapies have led to the progressive development and application of other, safer, and more effective therapeutic modalities. Nanomedicines appear with the tide of clinical requirements. The emphasis of the corresponding research is on the exploitation of nanocarrier-based delivery systems as a mean of overcoming these restrictions [
7,
8]. These systems have a variety of benefits, such as the capability to extend the circulation time, increase the aqueous solubility, and reduce the side reaction of therapeutic agents via delivering them to specific target positions. With the recent decades seeing a tremendous growth in materials science, nanotechnology, therapeutic methods, and even clinical failures, nanocarriers have emerged as a novel platform for more effective diagnostics, treatment and prevention of a variety of diseases. Furthermore, these nanocarriers can be preferentially accumulated in the leaky vasculature of tumors by passive and active targeting through enhanced permeation and retention (EPR) effects [9-11]. Against the backdrop, more than two dozen nanomedicine formulations have been ratified for applying in clinical settings, and more are undergoing clinical trials. Despite the promising progress, the translation of EPR into the clinic settings remains questionable. For example, most of the conventionally designed nanocarriers have the disadvantage of premature drug release during circulation, which can injure normal tissues and reduce their therapeutic efficacy. Thus, more delivery systems with higher efficiency are needed.
An alternative is the on-demand drug delivery process generally allows for customized release profiles with outstanding spatial, temporal and dosage control. On-demand drug delivery has become possible by adopting stimulus-responsive nanocarriers to recognize and dynamically respond to the specific triggers that mimic the responsiveness of living cells, tissues, or organs. Since stimulus-responsive drug delivery was introduced in the late 1970s [
12], researchers have conducted a large number of studies on the applications of stimuli-responsive materials in the field of drug delivery, especially in their design and application as nanocarriers. Specifically, the tumor microenvironment (TME) has utilized as an important target for cancer therapy and exhibits specific physiological properties. With quick tumor growth and vascular anisotropy, intra-tumor blood supply is often inadequate and chronically hypoxic, while cellular metabolism increases the accumulation of lactose and hydrogen ions, resulting in the formation of an acidic TME. In addition, higher redox potential, overexpression of enzymes, and increased metabolic activity are also characteristic of the TME. Together, these changes promote tumor angiogenesis, elevate tumor cell proliferation, migration capacity and immune escape ability, which in turn promote tumor development, and lead to therapeutic resistance and failure. Therefore, exploiting the unique properties of TME to design biomaterial platforms with TME responsiveness has been thought an effective strategy for cancer therapy.
Nanoscale stimuli-responsive carriers may be sensitive to specific triggers, such as internal (pH [13-15], enzyme [16-18], and redox reactions [
19,
20], etc.) and external (light [
21,
22], temperature [23-25], magnetic field [
26,
27], ionizing irradiation [28-30], etc.) triggers, as shown in
Figure 1. Upon specific stimuli, these smart nanocarriers will take the place of specific protonation, hydrolytic cleavage, or molecular or supramolecular conformational changes that resulting in the release of the contained drugs. With the unique characteristics of on-demand delivery, stimuli-responsive nanocarriers have greatly facilitated the utilization of nanotechnology in chemotherapy and medical translation. Although these promising tools have generated much interest in drug delivery, there is still a demand of comprehensive reviews that summarize updated achievements in novel stimuli-responsive nanocarriers for cancer therapy.
In this review, we emphasize the recent designs and approaches utilizing smart nanocarriers that can respond to physiological environments and external stimuli to address delivery challenges, such as delivery efficiency, targeting precision and gene silencing. Afterwards, the numerous types of stimuli-responsive nanocarriers employed are summarized and their roles and future potential in organ-restricted delivery are also discussed. In the end, the challenges and application prospects of the smart nanocarriers are elaborately described.
2. Approach to Smart Delivery Strategies
2.1. Internal Stimuli for Drug Delivery Systems
Specific biological factors within the TME or cancer cells, including enzymes, low pH, re dox potential, and high reactive oxygen content, could serve as specific triggers for intranuclear endosome/lysosome escape, controlled release of drug, activation of drug precursors, as well as tumor-specific diagnostics and therapeutics [31-33]. Intrinsic stimuli are those inherent in the TME or within the cancer cells. However, their low specificity and heterogeneous distribution in tumors may compromise the efficacy of nanocarriers that are sensitive to intrinsic stimuli. This section will focus on recent advances in drug delivery systems intrinsically responsive to stimuli substance (mainly pH, redox and enzymes) in cancers diagnosis and therapy.
2.1.1. pH-Responsive Nanocarriers
Tumor tissue tends to have lower pH (5.5-6.0) compared to the normal tissue (pH 7.2-7.4), which is typical of solid tumors [
13,
34]. The main reason for the difference in pH between tumor cells and normal cells is the unrestricted proliferation of tumor cells and their unique metabolic changes (Warburg effect), which allow the cells to take up more glucose [
35,
36]. However, the level of oxidative phosphorylation within cancer cells is insufficient. As the dominant energy supply mode of tumor cells, glycolysis produces a plentiful of lactic acid, giving rise to a large accumulation of lactic acid in tumor tissues. In addition, the incomplete vascular system of tumor tissues and the absence of lymphatic system accelerate the acidic microenvironment of tumors [
35,
37,
38]. One area of research that shows promise in responsive nanocarriers is exploiting the micro-acidity of tumor cells to control drug delivery and release. The release of drugs can be triggered by changes in internal pH levels. Nanoparticles have various gatekeeper molecules on their surface, which can be labeled using pH unstable groups like ester, hydrazine, and acetal bonds. These bonds are cleaved under acidic conditions. The transition from a neutral to an acidic environment leads to significant changes in the physical properties of substances' activity. For example, Zhang et al. designed and constructed a dihydralazine (HDZ)-pH-responsive delivery system based on a cyclic RGD (cRGD) peptide-modified dextran-hydrazone-doxorubicin (cRGD-Dex-DOX) precursor drug (
Figure 2A) [
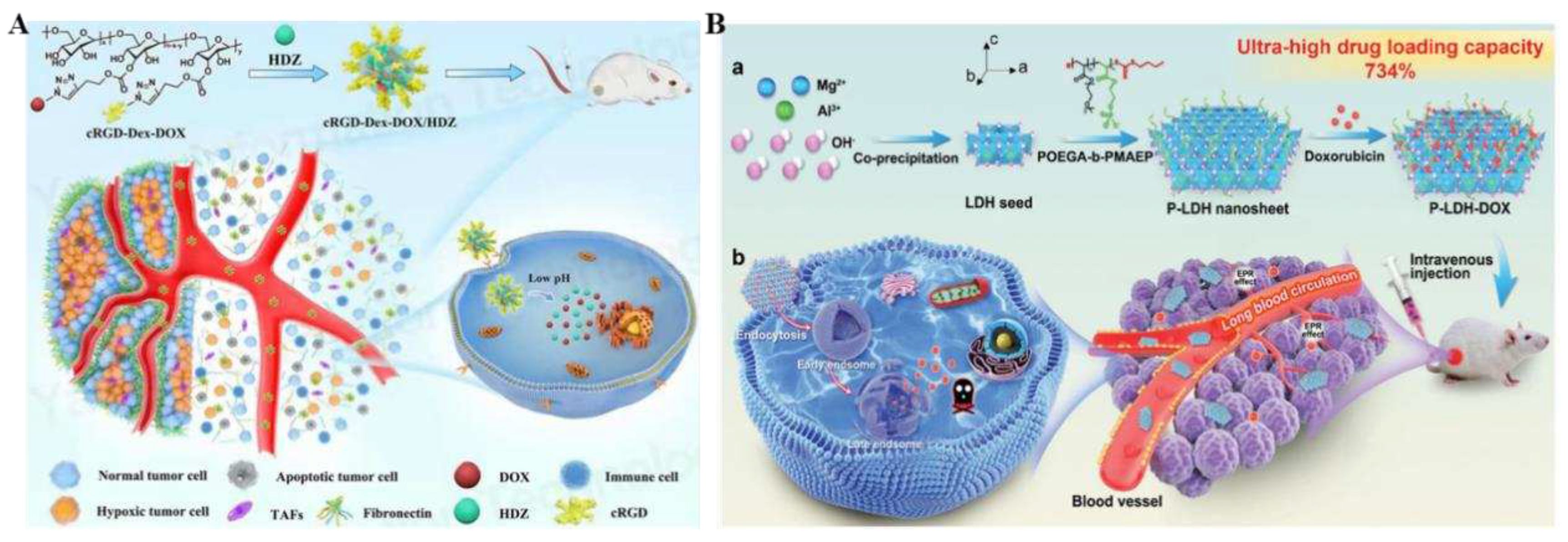
39]. This nanosystem not only improved active targeting ability through cRGD peptide introduction but also exhibited pH-responsive drug release characteristics for targeting tumor stromal microenvironments (TSMs). In addition, co-loading HDZ and doxorubicin injections into the nanosystem resulted in sustained normalization of tumor vasculature, enhancing penetration into deep tumor sites. After four rounds of injections, it significantly dilated tumor vasculature while down-regulation of hypoxia-inducible factor 1α (HIF-1α), α-smooth muscle actin (α-SMA), and fibronectin expression and so forth. These alterations within TSMs not only enhanced the NPs enrichment and infiltration but also facilitated activated T cells infiltration into the tumors, which indicated that implementing such a system could potentially offer an effective "multifunctional therapy" by enhancing the effectiveness of chemotherapy and boosting the immune response against tumors.
In vivo experiments conducted in 4T1 mice confirmed that this therapy has the most prominent anti-tumor effect with the fewest side effects than all other control regimens tested. The low drug-carrying capacity of nanocarrier carriers has been one of the major challenges encountered in smart nanomedicine systems and has largely limited the development of nanocarrier technology. Zhang and his co-workers constructed a 2D ultra-thin lamellar double hydroxide (LDH) nanosheet P-LDH with great drug loading capacity, outstanding colloidal stability, and extended blood circulation for cancer therapy (
Figure 2B) [
40]. P-LDH, synthesized by a biocompatible polymer-assisted bottom-up approach, has an ultra-thin two-dimensional lamellar structure with a large number of internal cargo-anchored sites available for drug loading, thus achieving a super drug loading capacity of 734% (doxorubicin/nanoparticle mass ratio). Under physiological pH conditions, P-LDH-delivered doxorubicin remained stable on the nanosheet carriers while exhibiting continued release in the TME and intracellular environment, suggesting on-demand drug release due to pH-responsive nanosheet biodegradation. In both
in vitro and
in vivo 4T1 models, based on an ultra-high-loading system, P-LDH exhibited improved efficacy in enhancing tumor cell uptake efficiency, prolonging drug circulation, improving therapeutic efficacy and reducing systemic toxicity compared to a multilayered high-loading system based on lactate dehydrogenase. The successful construction of this nanosystem also provides the possibility for the rapid development of nano-loading technology.
2.1.2. Redox-Responsive Nanocarriers
Reactive oxygen species termed ROS are known to be by-products of biological aerobic metabolism, containing oxygen ions, peroxides, and oxygen-containing free radicals, which play an essential role in various normal biological and pathological processes [
41,
42]. Scientific studies have shown that under normal physiological conditions, ROS are maintained at a very low equilibrium level in living organisms, but during the development of various diseases, including inflammation, cardiovascular diseases, sarcoidosis, and tumors, ROS level in local tissue mitochondria is much higher than that in normal ones [
43,
44]. Numerous studies have confirmed that the abnormal physiological behaviour of tumor tissues enable them to produce large amounts of ROS, the concentration of which is increased by more than ten times than that of normal tissues [
45]. Therefore, by utilizing the differences in ROS levels in and out of tumor tissues and cells, ROS-responsive smart nanomedicine carriers constructed with ROS-sensitive materials are a prerequisite for controlling the targeted release of drug molecules for drug delivery in tumor cells [
46,
47]. The ROS-sensitive chemical groups in the design skeletons of current ROS-responsive drug carriers mainly contain characteristic groups such as borate, thione, sulfide, selenium and ferrocene groups [
19,
48]. Recently, Dey et al. developed SPP@2ME, a cascade-amplified polymer precursor drug loaded with 2-methoxy-β-estradiol (2ME) [
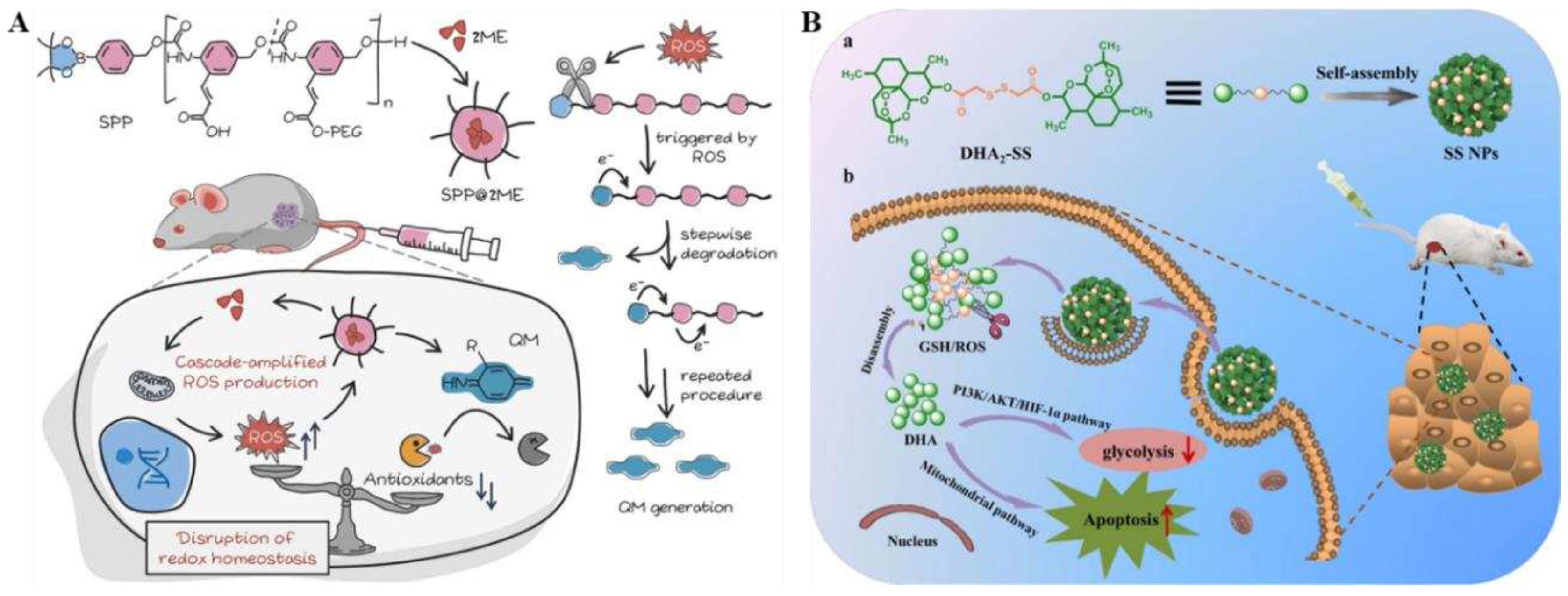
49]. In this study, selective activation of SPP@2ME by rich ROS in cancer cells triggers the progressive decomposition of spontaneous polymerization pre-drugs (SPPs) in tumor cells and the production of a large number of quinones and their analogs (QMs), which deplete glutathione (GSH). Meanwhile, SPP degradation leads to the rapid release of 2ME into cellular plasma, increasing ROS levels in cancer cells through a domino-like cascade amplification feedback mechanism (
Figure 3A). What's more, the synergistic effect of SPP and 2ME remarkably exacerbated the oxidative stress and destroyed redox equilibrium of cancer cells. The
in vivo and
ex vivo experiments confirmed the great potential of SPP@2ME in anti-tumor therapy and also provided important insights for innovative and precise nanomedicine.
There are significant redox potential differences between tumor tissues and normal physiological tissues as well as inside and outside tumor cells. The abnormal metabolism in tumor tissues leads to the generation of a more intense reducing environment [
50]. The concentration of GSH is about 2-20 μM in normal tissues, and the concentration of GSH in tumor tissues is usually two times that in normal tissues. The intracellular GSH concentration is about 0.5-10 mM, which is more than 100 times the extracellular concentration (2-20 μM) [
19,
51]. In addition to the reducing substance GSH, tumor cells contain small amounts of divalent iron ions (Fe
2+), cysteine (Cys), thioredoxin reductase and lysosomal thiol reductase [
52]. Therefore, this difference in the content of reduced substances inside and outside tumor cells (especially GSH) provides new possibilities for designing reduction-responsive nanodrug carriers to achieve reduction-responsive drug release inside the tumor cells. Reduction-sensitive chemical groups, such as disulfide bonds and diselenide bonds [
19,
53], are able to undergo rupture, thus causing changes in the structure of the drug carrier and achieving rapid drug release. For example, Li et al. reported a dihydroartemisinin (DHA) dimerized nanoprecursor drug (DHA2-SS) with disulfide bonds as connecting bonds [
51]. The nanoprecursor drug exhibited stability with high drug loads (up to 90.6 wt%). It demonstrated sensitivity to changes in redox levels within the TME, resulting in efficient release of DHA for chemotherapy (
Figure 3B). Notably,
in vitro and
in vivo therapeutic experiments revealed that these prepared SS NPs displayed strong endocytosis capabilities, intense cytotoxicity, and improved anti-tumor effects compared to free DHA. Simultaneously, RNA sequencing and bioinformatic analysis indicated that it could not only induced apoptosis through the intrinsic mitochondrial apoptotic pathway, but also inhibited glycolysis via the PI3K/AKT/HIF-1α signaling pathway, presenting a novel target for treating hepatocellular carcinoma. Consequently, this study provided valuable insights into designing responsive precursor drug nanoparticles while encouraging further research on traditional Chinese medicine and related natural active ingredients.
2.1.3. Enzyme-Responsive Nanocarriers
Enzymes, as macromolecular biocatalysts, act a crucial role in systemic metabolism and cellular regulation and are important parameters in the diagnosis of various diseases. Studies have shown that dysregulated expression of certain enzymes leads to increased enzyme concentrations at the tumor site, such as matrix metalloproteinases 2 (MMP2), glycosidase, cathepsin B, secretory phospholipase A2 (sPLA2), hyaluronidase (HAdase), and others (sPLA2), hyaluronidase (HAdase),
etc [
54,
55]. Therefore, based on the differences in enzyme expression between normal tissues and tumor sites, enzyme recognition and degradation nanosystems that can specifically recognize the differences in enzyme over-expression inside and outside the cell can be designed and constructed to achieve specific enzyme-responsive drug release at tumor sites [
55]. In addition, enzymes as triggers have many unique advantages, such as the mildness of the enzymatic reaction conditions and good enzyme selectivity of substrates. Therefore, the design of enzyme-responsive drug-release nanomedicines based on tumor-specific highly expressed enzymes can obtain highly specific controlled release functions and thus reduce side effects of drugs. A great number of studies have reported that matrix metalloproteinases (MMPs) are currently commonly used triggers for enzyme-responsive drug delivery in antitumor therapy [56-58]. For example, Cao et al. constructed the Nap-FFGPLGLARKRK, a surfactant-like peptide for cancer-targeted drug delivery [
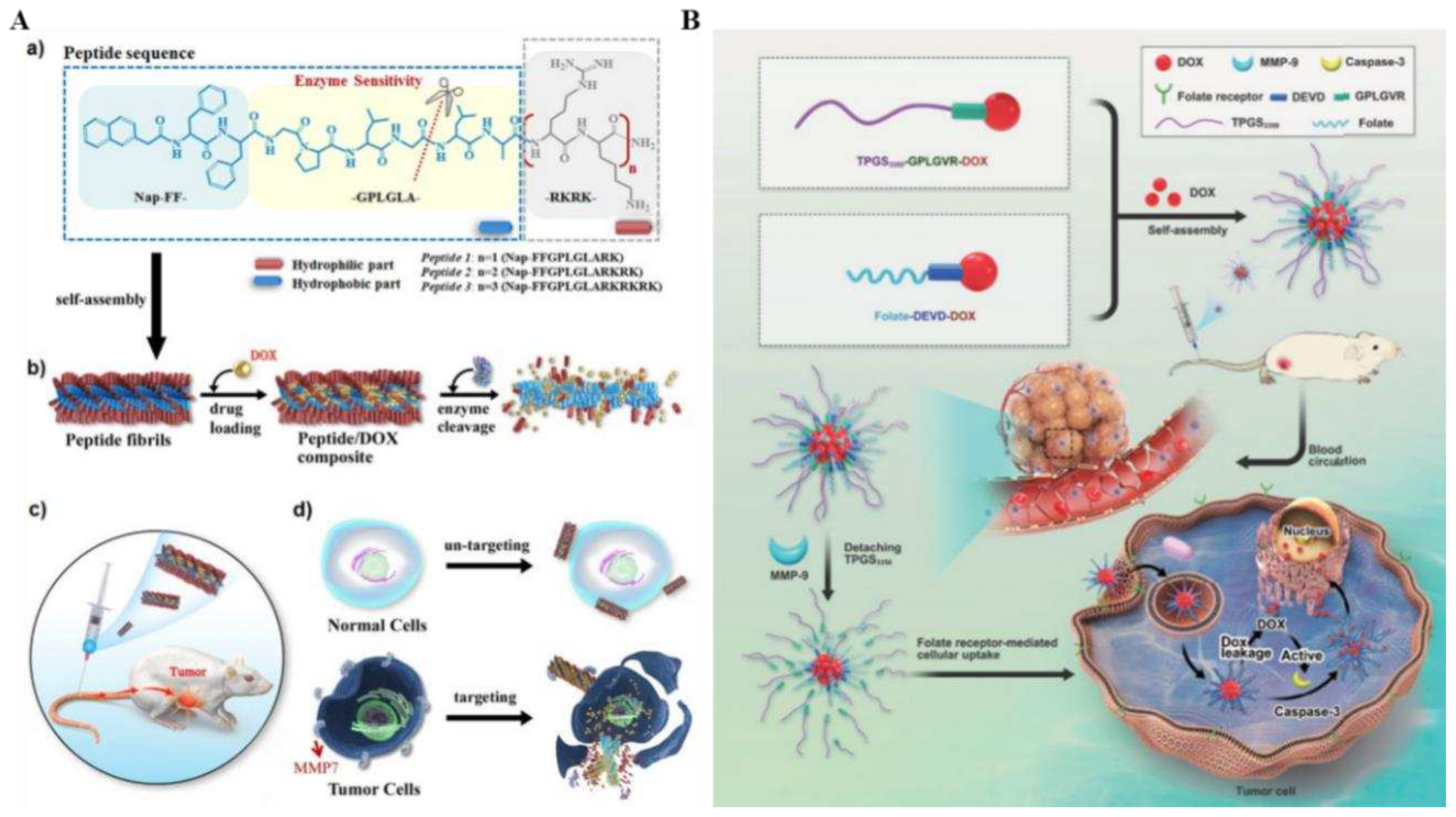
58]. This peptide consisted of three functional components: a Nap-FF-aromatic motif that facilitated it self-assembly, an enzyme-responsive cleavable -GPLGLA-segment, and a positively charged -RKRK-segment that balanced the molecule's amphiphilicity while promoted interactions with cell membranes, which self-assembled into elongated fibers with a hydrophobic core capable of encapsulating significant amounts of the anticancer drug DOX (
Figure 4A). Targeted drug delivery and selective anticancer therapy are achieved through tumor overexpression of matrix metalloproteinase-7 (MMP7) at the tumor site, which is degraded into finer fibers that precipitate out and slow the release of DOX.
In vivo antitumor experiments in mice confirmed that this enzyme-responsive peptide drug carrier could effectively inhibited tumor growth and metastasis, while greatly reducing adverse reactions. This investigation demonstrated it’s desirable to utilize enzyme-sensitive peptide nanostructures in practical targeted drug delivery, which had significant prospects in biomedical cancer therapy. In addition, Wan et al. constructed dual enzyme-responsive micelles for the first time by physically loading enzyme-responsive substances into the micelles via π-π stacking, and obtained DOX micelles consisting of intra- and extracellular enzyme-responsive TPD&FPD&D micelles by dialysis preparation [
59]. Two of the enzyme-responsive substances were D-α-tocopherol polyethylene glycol 3350 succinate (TPGS3350)-Gly-Pro-Leu-Gly-Val-Arg (GPLGVR)-DOX (TPD) precursors specifically responding to the overexpression of MMP-9 in the TME, and FA-Asp-Glu-Val-Asp (DEVD)-DOX (FPD) precursors responding to the caspase-3, respectively (
Figure 4B). In addition, the micellar matrix material TPGS3350 was obtained by esterifying and synthesizing PEG3350 with vitamin E succinate to extend the circulation time of TPD&FPD&D micelles in the bloodstream
in vivo and to utilize the EPR effect for passive targeting enrichment in tumor tissues. In the presence of MMP-9 enzyme over-expressed in tumor tissues, the GPLGVR peptide in the micelles could be cleaved, thereby shedding the outer layer of TPGS3350 and exposing folate as itstargeting molecule. Subsequently, the micelles could enter the tumor cells through folate receptor-mediated endocytosis pathway. At the same time, free DOX was released is released from the micelles, inducing 4T1 apoptosis in tumor cells, activating the apoptotic protease caspase-3, which further cracked the peptide (DEVD) and increased the release of DOX, thus markedly enhancing the cytotoxicity of the intracellular drug and improving the antitumor effect. This study demonstrated that micelles, activated by a bi-enzymatic reaction, had the advantages of long cycle time, good stability, penetration, high drug loading, targeting, high biosafety, and rapid intracellular uptake, which provided an effective tumor inhibition and reduced systemic toxicity in 4T1 hormonal mice.
2.2. External Stimuli for Drug Delivery Systems
External stimuli can influence the state of nanocarriers in biosystems, mainly including light, ultrasound, magnetism and temperature. The enrichment of nanocarriers in a desired region is facilitated by external stimuli, such as magnetic fields, controlled release, intracellular drug delivery, and activation of imaging and therapeutics. Applying external stimulation for drug delivery are with multiple facilitations: (1) the timing, location, and intensity of external stimuli (e.g., magnetic field, laser irradiation) can be precisely controlled by artificial intelligence; (2) external stimuli can be attach or eliminated according to the therapeutic needs; (3) multifunctional, multimodal tumor diagnosis and treatment can be achieved through the superposition of multiple external stimuli; (4) it can be more easily implemented for multiple times or consecutively (e.g., for hours or days) drug delivery and therapeutic stimulation; and (5) injury to normal tissues and organs can be minimized by more perfect control of external stimulation. In addition, the external stimulus response system allows for interconversion between different stimuli, such as localized release of drugs under near-infrared irradiation with localized heat generation in tissues (thermal response). In this chapter, we will discuss the development of drug delivery systems utilizing external stimuli, including light, ultrasound, magnetic field, temperature changes, and their specific applications in tumor diagnosis and therapy.
2.2.1. Light-Responsive Nanocarriers
The tunability and real-time manipulation of the intensity and wavelength of light, good spatio-temporal precision, mini-invasiveness, higher confidence, and orthogonality to the intracellular light environment have led to the widespread use of photosensitized nanocarriers in biomedical fields [
60,
61]. Effective therapeutic methods of photostimulation include photothermal drugs and photosensitive drugs. The study of photosensitized drug release is, firstly, through light stimulation of microenvironmental changes, the carrier molecular structure changes to make the drug release; secondly, while irradiating the photosensitizer to produce therapeutic effects, the intermediates indirectly act on the chemical bond to make the drug release. The light source, which is the central element of photostimulation, can be ultraviolet-visible (UV-vis) or near-infrared (NIR) [
62,
63]. However, UV-vis light has the disadvantage of poor penetration ability, cannot penetrate deep into tissues and easily causing harm to the human body, while the maximum permeability of skin to light appears in the NIR window of 650-900 nm with a penetration depth of up to 2 cm. What’s more, the longer the wavelength, the greater the ability to penetrate into tissues in the NIR wavelength region [
64]. As a result, NIR light offers considerable advantages compared to UV and visible light in phototherapy and optical imaging of deep tissues [
65]. Therefore, NIR light is more suitable as a source of stimulating light for the construction of light-triggered drug delivery systems [
66].
In general, various strategies have been proposed in designing light-stimulated drug release systems for tumor therapy: (1) absorption of light dissociating the prodrug into its active form; (2) absorption of light by a photosensitive molecule, which triggers a physical change that releases the encapsulated drug [
67,
68]. The former commonly involves functionalizing the chemotherapeutic drug to obtain a prodrug with reduced cytotoxicity. Under light exposure at the target site, these precursor drugs undergo photolysis giving rise to the release of the chemotherapeutic drug and the realization of precision therapy. The latter usually employs light-responsive organic molecules such as azobenzene derivatives grafted onto the surface of functionalized nanoparticles. These photoresponsive moieties undergo isomerization (e.g., trans-cis-isomerization) in response to light, leading to conformational or molecular changes that result in the release of the encapsulated drug [
67,
69]. In addition to photo-responsive DDSs designed for chemotherapy, photosensitive nanomaterials have been applied for cancer diagnosis and treatment in other forms, such as photodynamic therapy (PDT), photothermal therapy (PTT), and photoacoustic (PA) imaging [70-72]. A successful construction of an intelligently designed and controlled synthesis of psar-modified gold nanovesicles (PSGVs) (
Figure 5A) was first reported by Lv et al [
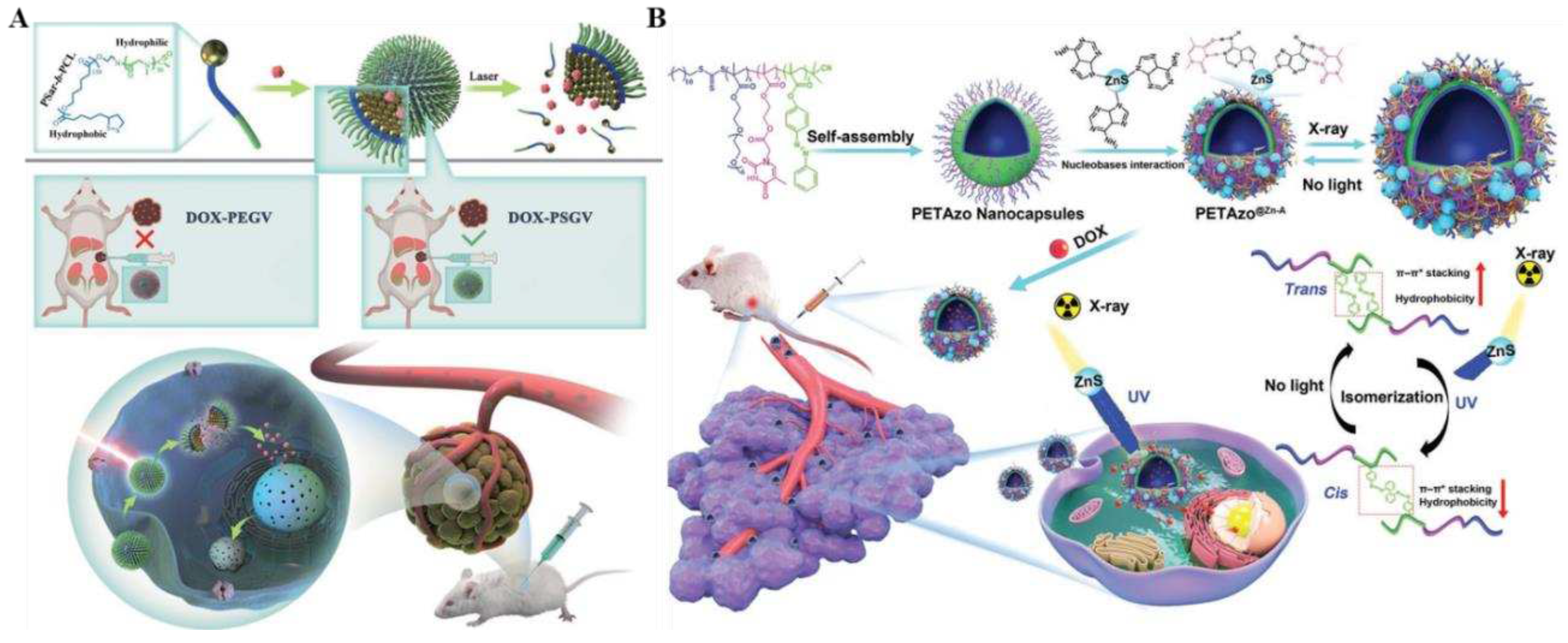
68]. By synthesizing a battery of lipoic acid-capped polysaccharide-b polycaprolactone block copolymers (PSar-b-PCL) and systematically investigating the self-assembly behavior of 26 nm gold nanoparticles mediated by block copolymer, the construction of NIR-light-responsive psar-modified gold nanovesicles (PSGVs) was enhanced to highly efficient photo-thermal conversion efficiency (up to 34.6%). Next, the anti-tumor drug DOX was efficiently loaded in the PSGV cavity, and the high loading capacity (up to 20.6%) and stability of DOX were realized. The encapsulated DOX was targeted for rapid release by 808 nm laser irradiation to achieve controlled drug delivery and tumor therapy. Impressively, DOX-loaded PSGV (DOX-PSGV) showed increased cellular uptake efficiency and higher tumor inhibition than DOX-loaded polyethylene glycol (PEG)-encapsulated gold nanovesicles (DOX-PEGV). Both in vitro and in vivo anti-tumor trials indicated that DOX-PSGV was equipped with favorable photothermal/chemotherapeutic synergistic therapeutic effects under 808 nm laser irradiation.
However, in practical clinical applications, the targeting properties and sustained drug release ability of nanostructures may be restricted by their irreversible and uncontrolled destruction. To address this challenge, Deng and his collaborators designed and prepared X-ray responsive biomimetic nanocapsules PETAzo@ZnS-A with reversible and tunable permeability by crosslinking poly(thymine) and photoisomerized poly(azobenzene) (PETAzo) with adenine-modified ZnS (ZnS-A) nanoparticles (NPs) (
Figure 5B). The ZnS-A nano particles efficiently converted X-ray into UV-visible light and induced isomerization of azobenzene groups, thus allowing the active load to diffuse controllably through the bilayer membrane for accurate drug delivery [
73]. In contrast to the conventional method of regulating the permeability of nanostructures by destroying the structure of nanocapsules, which was an irreversible process, PETAzo@ZnS-A remotely controlled bilayer permeability of nanocapsules allows for adjustable permeability while maintaining structural integrity, with long retention time, remotely controlled drug release, improved targeted aggregation, and potent anti-tumor effects. The outcomes of in vivo and ex vivo experiments proved that the X-ray-triggered nanocapsules can control the drug release to meet the complex sensitivities of various tumor cells and patients, enabling precision tumor therapy.
2.2.2. Ultrasound-Responsive Nanocarriers
Ultrasound, as a non-invasive method, belong to one of the most frequently used exogenous stimuli strategies in tumor therapy [
74,
75]. Furthermore, the inimitable benefits of ultrasound responsiveness are safer and deeper tissue penetration [
28,
76]. In drug delivery systems, the pressure waves of ultrasound are 20 kHz or higher, which is a crucial factor in local stimulation involving both site-specific and spatial release control, giving rise to a growing awareness of cancer therapy [77-79]. Wang et al. established an ultrasound-responsive nanoprodrug named CPT-t-R-PEG2000@BaTiO
3 (CRB), which encapsulated piezoelectric nanomaterials barium titanate nanoparticle (BaTiO
3) in amphiphilic prodrug molecules. The ROS-responsive thioketal bond (t) in this prodrug linked chemotherapy drug camptothecin (CPT) to NO-donor L-arginine (R) (
Figure 6A) [
80]. BaTiO
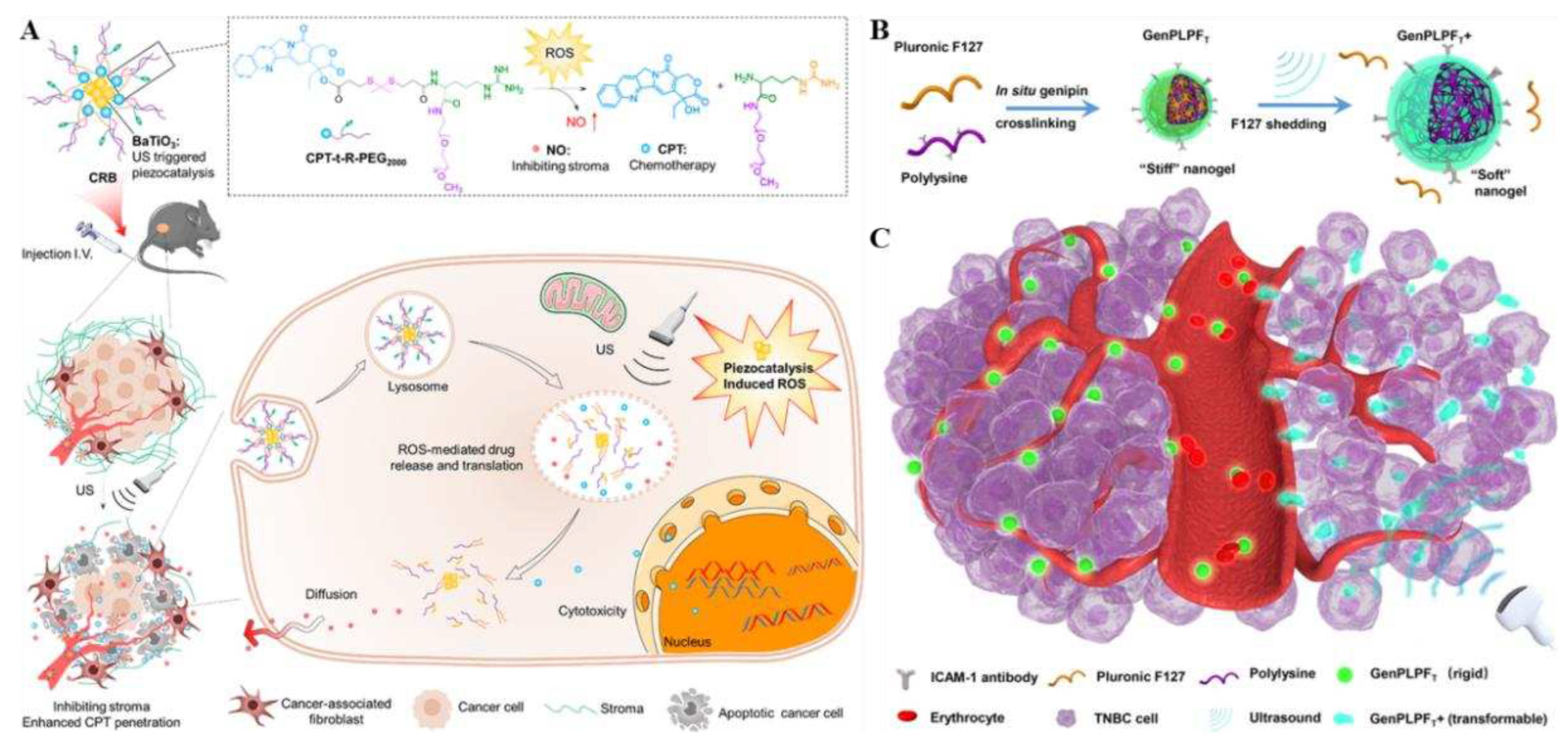
3, as a piezoelectric material, is triggered by ultrasound in the complicated hypoxic environment of tumors and water cleavage to continuously generate ROS through piezoelectric catalysis, which induces a cascade reaction process. Then due to breakage of thione bond, CPT is released and L-arginine (R) is oxidized to produce NO molecules, accomplishing timed and targeted delivery of NO and CPT at the tumor site. What's more, NO could modulate the matrix of the TME, promoting the deep tumor penetration of the nanoparticles and the release of CPT, further improving the chemotherapeutic effect on tumors. In vivo animal experiments confirmed that CRB significantly enhanced the anti-tumor effect by inhibiting resistance to chemotherapy in mice. This precursor drug delivery strategy was shown to have the great advantages of safer and better targeting, which might be a viable avenue for treatment of pancreatic cancer, inspiring further studies of drug deeper tumor infiltration.
Sun and his group members developed a peptide nanogel that can undergo stiffness transformation by external ultrasound stimulation by using genipin crosslinked with polylysine, simultaneously, introducing a targeted drug (ICAM-1 antibody) and a chemotherapeutic drug (epirubicin) to obtain the gel prodrug GenPLPFT/EPI [
29]. By regulating the external ultrasound stimulation, its deformation ability and stiffness were achieved to reach deep penetration into the tumor and prolong blood circulation, further enabling it to effectively treat triple-negative breast cancer (TNBC) (
Figure 6B, C). In both
in vitro and
in vivo TNBC models, GenPLPFT/EPI was able to balance blood circulation and deep tumor infiltration to exert highly effective anti-cancer effects, which the investigators attributed likely to the active targeting and ultrasound-triggered deformability of GenPLPFT. In contrast, unsonicated GenPLPFT represented only a low level of tumor penetration. Overall, ultrasound-responsive peptide nanogels were an effective cancer drug treatment that could effectively balance the conflicting needs of deep tumor penetration and extended blood circulation.
2.2.3. Magnetic-Responsive Nanocarriers
In recent years, magnetically responsive nanosystems, owing to their unique and outstanding physical and chemical characteristics, have shown promising applications in many fields of biology and medicine, including tumor targeting and imaging. It is particularly worth mentioning for their roles in providing diversified delivery routes for drug delivery [
81]. Compared with endogenous stimulus-responsive nanosystems, magnetic-responsive nanosystems have the following advantages: with intrinsic magnetotactic properties, magnetic fields can be applied
in vivo by remote operation to increase the enrichment of drugs in tumors and target tumors; local high temperatures will be produced in an applied alternating magnetic field, which can kill tumor cells through targeted thermotherapy; through the action of an applied high-frequency magnetic field, different tissues of the organism radiate energy to the surroundings, which generates different resonance signals [82-84]. By applying high-frequency magnetic field, different tissues of the organism will radiate energy to the surrounding environment, thus generating different resonance signals and realizing magnetic resonance imaging (MRI), cellular imaging and tumor detection, as well as real-time dynamic tracking and evaluation of tumor diagnosis and treatment and therapeutic effect after drug administration [
85]. To date, a variety of magnetoresponsive nanocarriers have been studied and widely used, including magnetoresponsive nanoparticles, superparamagnetic iron oxide nanoparticles (SPIONs), magnetic nanogels and polymer micelles,
etc [86-89]. Recently, Guo et al. designed and prepared a magnetically responsive immunostimulatory nanoparticles (MINPs) based on SPIOs loaded with cytosine-phosphate-guanine oligodeoxynucleotides (CpG odn), which could be used not only as a contrast agent for photoacoustic/magnetic resonance (PA/MR) dual-modal imaging, but also a magnetically-targeted therapeutic agent for photothermal-triggered immunotherapy [
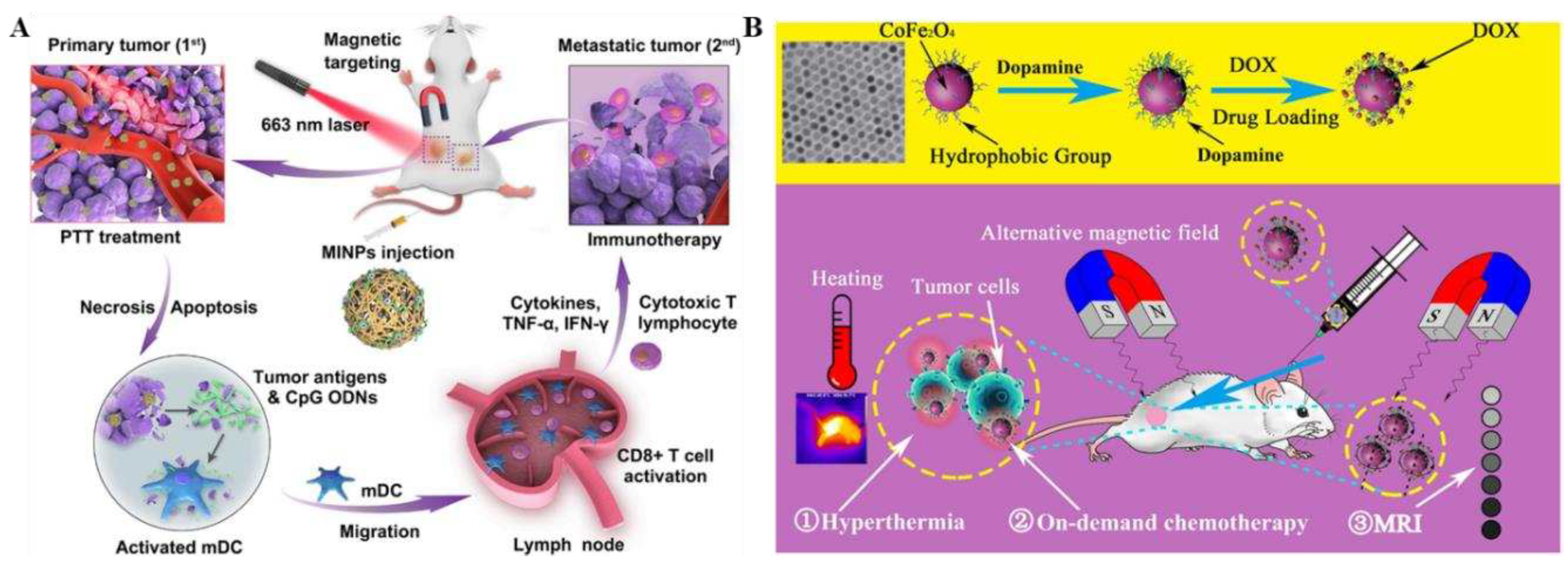
90]. Under the action of an external magnetic field, MINPs exhibited good magnetic targeting, which can lead to large number of photo-absorbents (SPIOs) and immuno-adjuvants (CpG ODNs) to accumulate within the tumor, achieving precise dual-modality imaging guidance. What's more, through near-infrared irradiation, MINPs have a good photothermal conversion effect, which can effectively photothermally destroy primary tumors, release tumor-associated antigens, and exhibit a function similar to that of an "autologous tumor vaccine", thus activating a force fulimmune response to fight against tumor and generating a synergistic photothermal/immunotherapy effect on both primaryand distant tumors (
Figure 7A). The successful construction of this strategy provided a platform to achieve a synergistic photothermal/immunotherapy effect and a new option for the precise individualized diagnosis and treatment of various tumors and metastases. Similarly, Jia et al. developed a versatilenanoplatform (CoFe
2O
4 nanoparticles@dopamine@DOX) for magnetically responsive on-demand thermotherapy and chemotherapy synergistic diagnosis of tumors based on magnetic CoFe
2O
4 MNPs [
91]. The nanoplatform had excellent magnetically triggered thermotherapy efficacy, magnetically responsive drug delivery capability, and strengthened MRI
T2-weighted signals. What's more,
in vivo experiments showed that CoFe
2O
4 nanoparticles@dopamine@DOX nanomedicine could achieve magnetically-responsive on-demand thermotherapy and DOX release, which significantly inhibited the growth of cancer cells and led to significant tumor regression with good safety (
Figure 7B). This multifunctional nanoplatform featured simple fabrication, green environmental protection, magnetically triggered high thermotherapy efficacy rate, magnetically responsive tumor on-demand therapy, and non-invasive imaging modalities, which offered great advantages over traditional single-drug therapeutic technologies, and provided an option for precise clinical treatment of tumors.
2.2.4. Temperature-Responsive Nanocarriers
Nowadays, temperature-sensitive drug delivery systems are much researched stimulus response strategies, which have been widely used in oncology therapy due to their advantages of flexible design, adjustable phase change temperature, multi-pathway stimulation (internal stimulation, including tumor, inflammation, and infection; and external stimulation, including light irradiation, triggered by magnetic or electric fields or external heating), and strong passive targeting ability [
24]. In addition, it has been reported that local thermal stimulation at 42.5-43.5°C in tumors benefits drugs to avoid cancer cells. On the other hand, thermal stimulation has a vasodilating effect and alters the permeability of tumor cell membranes, thus facilitating the delivery of antitumor drugs and local drug concentration, and enhancing the antitumor effect. Heat-sensitive materials are usually temperature-controlled by a temperature-sensitive material in a nanosystem, which triggers a change in the nanosystem upon warming, triggering drug release [
92,
93]. Typically, thermosensitive nanosystems remain stable at 37°C and can trigger rapid drug delivery after reaching 40-42°C, minimizing drug leakage due to blood circulation and metabolism at the tumor site [
31]. Polymeric micelles [94-96], hydrogel [
97,
98], liposomes [
92,
99,
100], and poly(N-isopropylacrylamide) [
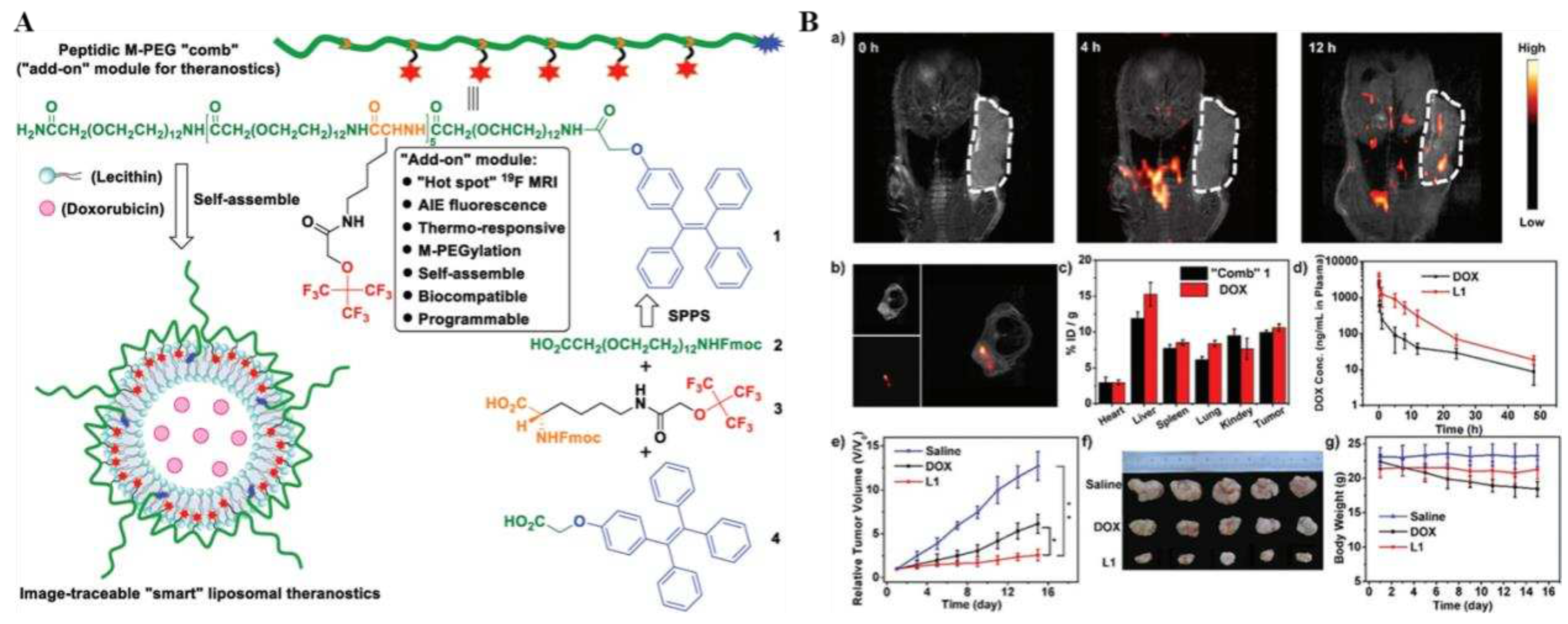
101] have all been used as carriers for thermoresponsive systems because they exhibit low critical dissolution temperatures. Temperature-sensitive liposomes are one of the most widely used thermoresponsive nanosystems, which can induce phase transitions in lipids and conformational changes in lipid bilayers under temperature changes (~40-50°C). A representative example was the recent construction of a multifunctional "smart" liposome diagnostic platform (liposomes L1) by Zhu et al., which consisted of the chemical structure of an M-PEGylated peptide programmed into the peptide M-PEG "comb" 1, which was equipped with an
19F MRI/fluorescence dual imaging and temperature-sensitive "add-on" modules, and were obtained by self-aggregation with the anticancer drug adriamycin (doxorubicin) and lecithin (
Figure 8A). Moreover, as a chemically accurate monodisperse peptide, it perfectly avoided the heterogeneity problem of polydisperse biomaterials. In addition, it could be precisely programmed during solid-phase peptide synthesis, enabling fine on-demand tuning of physicochemical and biological properties. And the
19F MRI/fluorescence dual imaging technology provides
ex vivo and
in vivo drug imaging and tumor imaging, which truly enabled visual dynamic imaging and efficacy tracking of therapy (
Figure 8B). What's more, this intelligent lipid diagnostic platform had the characteristics of long
in vivo half-life, good monodispersity, biodegradability, low toxicity and high tumor accumulation. The successful construction of this platform provided a new reference for the design of multifunctional "add-on" modular lipid nanodrug delivery systems for tumors, as well as the quick and facile development of various "smart" diagnostics.
2.3. Dual and Multiple Stimuli Responsiveness
To further optimize the delivery of antitumor agents, there is an increasing interest in designing stimuli-responsive nanocarrier delivery systems. However, as tumor cells exhibit significant heterogeneity, most mono-responsive nanosystems possess limited performance of sluggish and inadequate drug release at target site, resulting in the reduction of therapeutic efficacy [
102,
103]. In comparison, multiple stimuli-responsive nanoparticles are more popular for dealing with the heterogeneity and complexity of the bio-microenvironment to achieve adequate site-specific delivery [
53,
104]. For example, in solid tumors, the abnormal metabolic patterns and levels of tumor tissues and tumor cells, which can co-exist with pH gradients and oxidative environments, are ideal combinations of intracellular stimulators for the construction of stimuli-responsive nanodrugs. Zhang et al. designed a self-assembled pH/ROS-responsive micellar drug delivery system (PPT/D(DMA)@DOX) loading adriamycin (DOX) [
105]. The system featured charge reversal and self-amplified drug release. Charge reversal occurs when the negatively charged surface micelles are exposed to acidic conditions (pH<6.8), generating good cell membrane penetration and enhancing tumor cell uptake. In addition, thioether molecules undergo oxidative decomposition and deliver drugs to tumor cells, exerting antitumor activity by increasing tumor ROS levels and inducing apoptosis. More importantly, the exposed α-tocopheryl succinate (TOS) fragments lead to increased intracellular ROS concentration, self-amplified breakdown of micelles, and accelerated release of DOX, resulting in potent tumor-killing capabilities (
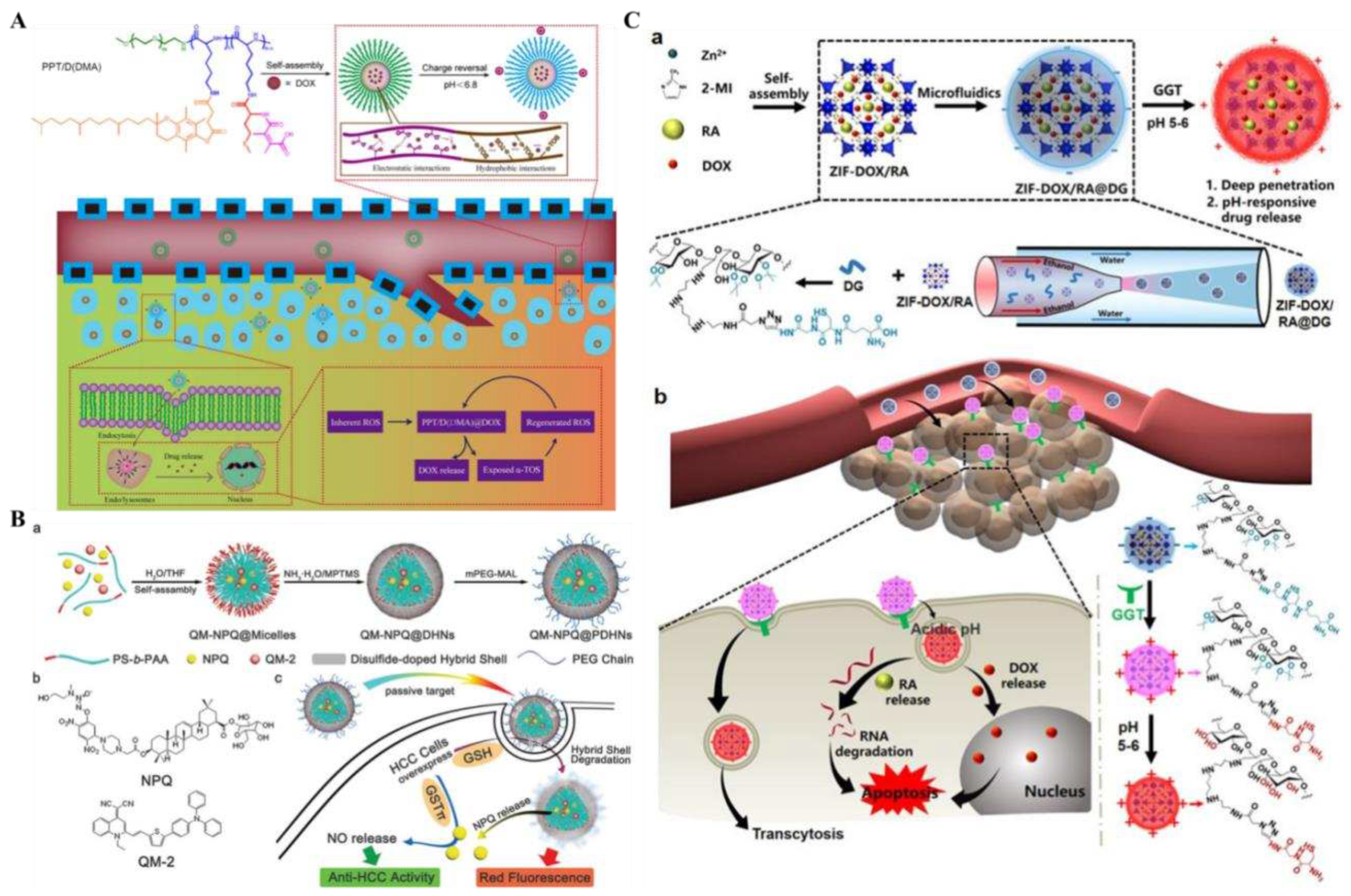
Figure 9A). In conclusion, this pH/ROS-responsive micellar drug delivery system has the unique advantages of desirable cellular uptake, triggered targeted release, and charge reversal system, showing significant prospects for improved treatment outcomes in tumor therapy.
Jia et al. reported an innovative intra-tumor dual redox/enzyme-responsive NO-releasing nanomedicine platform (QM-NPQ@PDHNs) by encapsulating NO-precursor drugs NPQ and AIEgen QM-2 in hybrid micelles doped with silicon disulfide [
106]. The nanoplatform exhibited efficient passive tumor-targeting ability, which allowed the encapsulated NPQ to be efficiently carried into the targeted tissue. The over-expression of GSH in the tumor would induce disulfide bond breaking and NPQ release. With high concentrations of GSTπ and GSH at the tumor site, NPQ was catalyzed to release large amounts of NO to kill tumor cells. Compared to free NPQ drugs, QM-NPQ@PDHN was more target-specific and had higher safety for normal tissues (
Figure 9B). This dual redox/enzyme-responsive nanomedicine provided a new way to increase the specificity of chemotherapeutic drugs, efficient anti-tumor therapy, and safe treatment.
Additionally, researchers have also dual expanded stimulus-responsive nanomaterials with pH and enzyme sensitivity for specific drug delivery. Considering that the barrier impermeability of solid tumors might limit the co-delivery of protein-based drugs and chemotherapeutic agents, Shen et al. established a novel pH/enzyme dual-activated ZIF-DOX/RA@DG nanosystem that carry ribonuclease a (RA) and DOX in the core of a zeolite-type imidazolium salt framework (ZIF-8) [
107]. External encapsulation with dextran coating (DG) using microfluidics was used to stabilize the polymer reactivity of the encapsulated drugs, the structure and biological activity of ZIF-8. In addition, the nanosystem exhibited dual reactivity owing to γ-glutamyl transpeptidase (GGT)-activated cationization and degradation arouse by the acidic microenvironment (
Figure 9C). Due to the surface charge of the DG layer from a slightly negative charge to a positive charge, the penetration ability of ZIF@DG NPs into solid tumors upon GGT activation was enhanced. Indeed, the pH-responsive DG coating inhibited circulating guest proteins and small-molecule drugs leaking prematurely from the ZIF-8 nanoparticles, thus achieving the targeted release of cargo molecules within the tumor. The results of
in vivo experiments confirmed the synergistic anticancer therapeutic effect of ZIF-DOX/RA@DG nanosystems with multiple drug-carrying capacity, tumor-selective permeability to deeper, and less easily penetrated lesion sites. This multidrug delivery system based on smart response design and microfluidic-assisted synthesis method offered prospects for clinical tumor therapy.
Apart from the synchronized internal dual response described above, there are external stimulus and internal stimulus synergistic, external and internal stimulus synergistic responses, and multi-response nanocarrier systems. For example, Chen et al. recently constructed a NIR light and pH dual-response multimodal synergistic diagnosis and treatment platform (PCN-DOX@PDA) [
108]. This platform could achieve tumor diagnosis and treatment through a triple synergistic strategy, which included: (1) using porous metal-organic framework (MOF) material PCN-600 as a carrier carrying antitumor agents and polydopamine (PDA), the ligand tetracarboxyphenylporphyrin (TCPP) in PCN600 as a photosensitizer generated monolinear oxygen (
1O
2) to kill tumor cells with an excitation of 633 nm, while PDA served as a photothermal agent to achieve photothermal treatment of tumors; (2) PCN-DOX@PDA achieved smart release of the anticancer drug DOX by responding to the weak acidic TME and thermal stimulation generated by near-infrared irradiation; (3) due to the presence of Fe
3+ in the center of the PCN, PCN-DOX@PDA could mediate tumor chemotherapy through magnetic resonance imaging and photothermal and photodynamic synergistic therapy to achieve diagnosis and treatment of tumors (
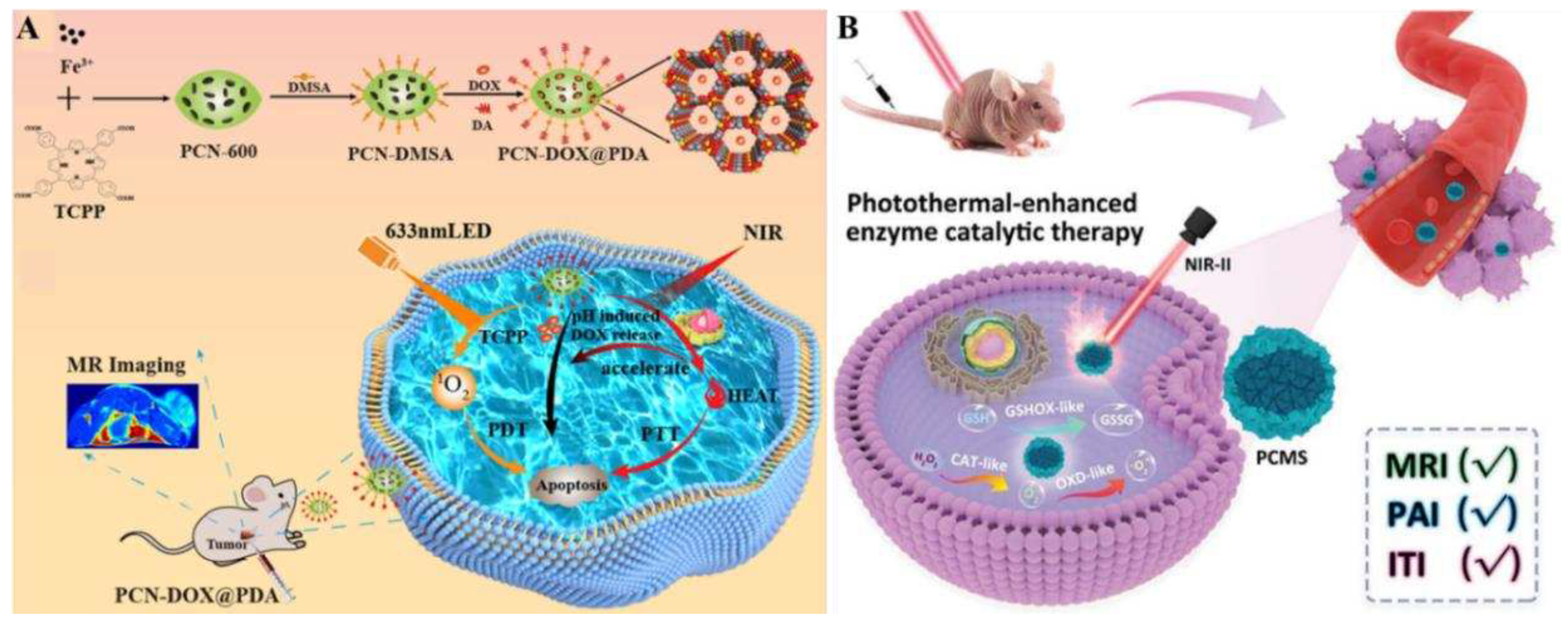
Figure 10A).
In vivo experimental results verified that PCN-DOX@PDA had good biocompatibility and anti-tumor ability, indicating that the dual-response multifunctional integrated diagnostic platform of NIR and pH had an unlimited potential in oncology precision therapy and diverse biomedical applications.
Nanocatalysts is one of nanomaterials with endogenous enzyme-like activity, which have been widely studied for tumor catalytic therapy recently. However, there is still a challenge for the construction of nanocatalysts with favorable enzyme catalytic activity and biocompatibility for tumor therapy. Recently, Zhu et al. constructed a TME/light-responsive, biodegradable, multimodal imaging-guided nanocatalyst, CuxMnySz (PCMS), for oncology diagnosis and treatment [
109]. PCMS possessed multi-enzymatic (CAT-like and OXD-like activities) properties, and was capable of catalyzing the endogenous hydrogen peroxide in the TME (H
2O
2) to oxygen (O
2) via a catalase-like activity cascade, and superoxide radicals through an oxidase-like activity cascade, resulting in excellent catalytic therapeutic effects. Meanwhile, PCMS depleted endogenous GSH in tumor cells through GSHOX-like activity, which effectively inhibited antioxidant defense and amplified the level of oxidative stress to ROS in tumor cells. In addition, under laser irradiation, PCMS exhibited excellent photothermal performance (up to ƞ = 56.7%) with an excitation at NIR-II 1064 nm, which significantly enhanced the therapeutic efficacy of tumor treatment. Notably, PCMS exhibited multimodal imaging (PAI, MRI, and ITI) performance
in vivo to effectively detect tumors and track nanocatalyst distribution and metabolism (
Figure 10B). This feature enabled PCMS to achieve optimal performance in the most efficient time window. In a hormonal mouse model, PCMS effectively inhibited tumor development, and significantly improved the therapeutic effect with few side effects. In summary, the present study provides an innovative strategy to efficiently generate ROS and consume ROS scavenging system through a cascade reaction, and rationally design a highly efficient nanocatalator for tumor catalytic therapy.
A large number of triple stimulus-responsive nanodrug delivery systems have been reported for tumor treatment in the TME, showing superior specificity, targeting, controllability and safety [
33], for example, pH/redox/NIR triple-responsive ZnO quantum dots-conjugated hollow mesoporous carbon nanoplatform [
110], light and dual-redox triple-responsive core-crosslinked micelles [
111], hypoxia/temperature/pH triple-responsive block copolymers [
112], and so forth. Recently, Wu et al. reported a pH/thermal/glutathione triple-responsive smart supramolecular nanomedicine (FUS/ICG@PEDD) which was constructed by utilizing particular and kinetic hydrogen bonding interactions [
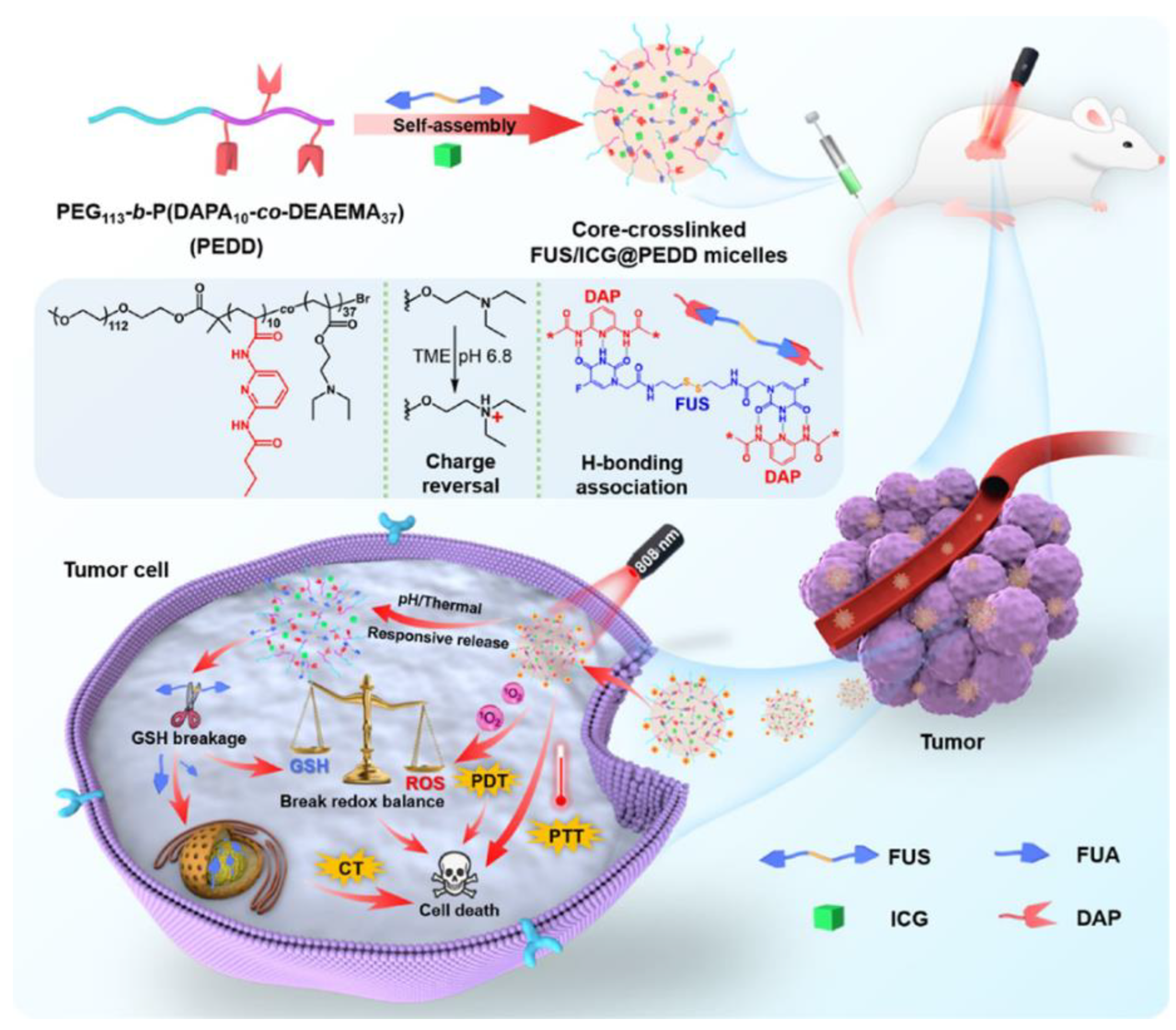
112]. The supramolecular polymeric micelles were co-loaded with α, ω-functionalized symmetric hydrogen bonding precursor drug 5-fluorouracil acetate -SS-5-fluorouracil acetate (FUS) and dual photothermal/photodynamic agent (indocyanine green, ICG). It also featured increased drug loading, cross-linking for robustness, pH-responsive charge reversal and intelligent drug delivery, and was perfectly synergistic with CT/PTT/PDT for tumor diagnosis and treatment (
Figure 11). For supramolecular micelle construction, the investigators designed a novel amphiphilic diblock copolymer PEG-b-P(DAPA-co-DEAEMA) (denoted as PEDD) as a drug delivery vehicle, in which hydrophilic PEG [poly(ethylene glycol)] and hydrophobic P(DAPA-co-DEAEMA) [poly(diaminopyridylacrylamide-co-2-(diethylamino)ethyl methacrylate] were equipped with dual functional groups: pH-responsive charge reversal DEAEMA motifs and H-bonded DAP motifs.
In vitro and
in vivo experiments revealed significant antitumor activity. Overall, the successful construction of this hydrogen-bonded nanomedicine provided new ideas and references to advancing intelligent nanomedicines and multimodal cancer therapy.
3. Conclusions and Perspectives
In summary, this paper reviews and assessments the strategies for the application of smart stimulus-responsive nanosystems in tumor therapy. Nowadays, stimuli-responsive nanomaterials have gained great attention in anti-tumor therapy investigation. Compared with traditional therapeutic methods, smart nanocarriers can change the carrier structure by endogenous or exogenous stimuli, which will release the encapsulated drug. It increased the biosafety and release rate of the drug, ensured it releasing at the desired point, and realized the targeted drug therapy. It also significantly improved the development of multi-drug resistance in tumors. Moreover, multifarious of stimuli-responsive nanocarriers have made significant progress in practical applications. Among them, stimulus-responsive nanocarriers such as pH, redox, enzyme, light, ultrasound, magnetism and temperature have been extensively researched in the fields of drug delivery and tumor therapy.
Generally, in intelligent stimulus-response nanosystems, different types of stimuli have different characteristics. For instance, pH stimulus-responsive nanocarriers have high sensitivity and a wide pH adjustment range. However, the complexity, heterogeneity, and uncertainty of tumorigenesis and development lead to different tumor pH values, which affect the mode of action and effect of pH stimulus-responsive nanocarriers. Of course, this difference in stimulus response factors is a common and generic problem with stimulus-responsive nanocarriers. Redox-stimulated responsive nanocarrier reactions are fastly being used for therapeutic applications such as antioxidants and tumors. Although they possess a well-defined chemical bonding reaction mechanism, the complexity of the material preparation process and its incomplete biodegradability in vivo are great challenges for such materials. Besides, enzyme-stimulated responsive polymer nanos with high selectivity and sensitivity are an excellent strategy for tumor therapy. Nevertheless, enzyme instability and the widely distributed nature of enzymes, the carrier drug may be released prematurely. Alternatively, temperature stimulus responsive nanocarriers with a wide temperature response range are not effective for single stimulus application and are suitable to be combined with other stimuli or therapies for optimal performance. Particularly, optical stimulus responsive nanocarriers have favorable selectivity and chargeability, but as mentioned earlier UV light has high energy but weak penetration, while NIR light is contrary, requiring prolonged light irradiation to achieve the targeted therapeutic effect. Consequently, light-responsive nanocarriers are suitable for the therapy of epidermal or superficial tumors, such as skin cancer.
Furthermore, despite the promising progress of smart nanomaterials in the field of tumor therapy, there are still many challenges away from practical applications.
(1) Biocompatibility and selectivity: this remains the most significant challenge. The majority of intelligent nanosystems continue to face challenges regarding their biocompatibility (toxicity associated with the materials used) as well as their limited selectivity, and thus novel/multiple approaches are imperative to adhere to the rigorous requirements in clinical environments.
(2) Mechanism clarity: most evaluations of the merits of materials have focused on their anti-tumor effects, but their anti-tumor biological mechanisms still need to be studied in greater depth, which will help to advance the understanding of tumor-anti-tumor drug-agent interactions, and to rationally develop novel and enhanced oncology agents.
(3) Pharmacogenetic and pharmacokinetic analyses: the therapeutic effects of smart nanomaterials in vivo, including selectivity and efficiency targets, biodistribution, biodegradation, and immune responses at the organ and system levels, still need to be studied in more depth.
(4) Animal experimental models: though in vitro bio-experiments could provide intelligent nanomaterials with rapid and prominent antitumor effect, and the in vitro results do not directly reflect the real situation.
(5) Clinical translational research: the establishment and advancement of various oncology smart nano-formulations are usually targeted to realize practical clinical applications, which involves the cooperation of experts from multi-disciplines.
Finally, the rapid development and explosive growth in the use of Artificial Intelligence (AI) is providing invaluable assistance in constructing predictive models of nanobio-interactions, hierarchical targeting and drug delivery efficiencies, modes of action, as well as nanomedicine safety and efficacy. Although it is still in its infancy, these tools and methods will revolutionize cancer nanomedicine in near future, at the same time, which also offering tremendous potential for improving cancer therapy.
Author Contributions
X.L.: initial draft writing, conceptualization, validation, visualization, and revision. F.H.: initial draft writing, conceptualization, validation, visualization, and revision. M.L.: conceptualization, final draft review, funding. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Hunan Province (No. 2021JJ41008), the Scientific Research Project of Health Commission of Hunan Province (No. B202313057213, No. W20243230), the Key Project of Changsha Science and Technology Plan (No. kh2201059), and the Youth Science Foundation of Xiangya Hospital (No. 2022Q16).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20-37. [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin. 2021, 71, 7-33.
- Guo, X.; Yang, N.; Ji, W.; Zhang, H.; Dong, X.; Zhou, Z.; Li, L.; Shen, H.M.; Yao, S.Q.; Huang, W. Mito-Bomb: targeting mitochondria for cancer therapy. Adv. Mater. 2021, 33, 2007778-2007817. [CrossRef]
- Chen, C.; Li, A.; Sun, P.; Xu, J.; Du, W.; Zhang, J.; Liu, Y.; Zhang, R.; Zhang, S.; Yang, Z. Efficiently restoring the tumoricidal immunity against resistant malignancies via an immune nanomodulator. J. Control Release 2020, 324, 574-585. [CrossRef]
- Vargason, A.M.; Anselmo, A.C.; Mitragotri, S. The evolution of commercial drug delivery technologies. Nat. Biomed. Eng. 2021, 5, 951-967. [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of clinical translation of cancer nanomedicines-lessons learned from successes and failures. Acc. Chem. Res. 2019, 52, 2445-2461. [CrossRef]
- Ma, Y.; Zhuang, Z.; Xing, L.; Li, J., Yang, Z.; Ji, S.; Hu, R.; Zhao, Z.; Huo, Y.; Tang, B.Z. The AIE-active dual-cationic molecular engineering: synergistic effect of dark toxicity and phototoxicity for anticancer therapy. Adv. Funct. Mater. 2021, 2106988-2106996. [CrossRef]
- Chen, S.; Liu, Y.; Liang, R.; Hong, G.; An, J.; Peng, X.; Zheng, W.H.; Song, F. Self-assembly of amphiphilic peptides to construct activatable nanophotosensitizers for theranostic photodynamic therapy. Chin. Chem. Lett. 2021, 32, 3903-3906. [CrossRef]
- Zuo, L.; Nie, W.; Yu, S.; Zhuang, W.; Wu, G.; Liu, H.; Huang, L.; Shi, D.; Sui, X.; Li, Y. Smart tumor-cell-derived microparticles provide on-demand photosensitizer synthesis and hypoxia relief for photodynamic therapy. Angew. Chem. Int. Ed. 2021, 60, 25365-25371.
- Manzari, M.T.; Shamay, Y.; Kiguchi, H.; Rosen, N.; Scaltriti, M.; Heller, D.A. Targeted drug delivery strategies for precision medicines. Nat. Rev. Mater. 2021, 6, 351-370. [CrossRef]
- Diep, Y.N.; Kim, T.J.; Cho, H.; Lee, L.P. Nanomedicine for advanced cancer immunotherapy. J. Control Release 2022, 351, 1017-1037. [CrossRef]
- Li, M.; Yu, B.; Wang, S.; Zhou, F.; Cui, J.; Su, J. Microenvironment-responsive nanocarriers for targeted bone disease therapy. Nano Today 2023, 50, 101838-101859. [CrossRef]
- Gontsarik, M.; Mansour, A.B.; Hong, L.; Guizar-Sicairos, M.; Salentinig, S. pH-responsive aminolipid nanocarriers for antimicrobial peptide delivery. J. Colloid Interface Sci. 2021, 603, 398-407.
- Rinaldi, F.; Forte, J.; Pontecorvi, G.; Hanieh, P.N.; Carè, A.; Bellenghi, M.; Tirelli, V.; Ammendolia, M.G.; Mattia, G.; Marianecci, C. pH-responsive oleic acid based nanocarriers: melanoma treatment strategies. Int. J. Pharm. 2022, 613, 121391-121403.
- Karimifard, S.; Rezaei, N.; Jamshidifar, E.; Moradi Falah Langeroodi, S.; Abdihaji, M.; Mansouri, A.; Hosseini, M.; Ahmadkhani, N.; Rahmati, Z.; Heydari, M. pH-responsive chitosan-adorned niosome nanocarriers for co-delivery of drugs for breast cancer therapy. ACS Appl. Nano Mater. 2022, 5, 8811-8825.
- Harnoy, A.J.; Rosenbaum, I.; Tirosh, E.; Ebenstein, Y.; Shaharabani, R.; Beck, R.; Amir, R.J. Enzyme-responsive amphiphilic peg-dendron hybrids and their assembly into smart micellar nanocarriers. J. Am. Chem. Soc. 2014, 136, 7531-7534. [CrossRef]
- Saxena, S.; Jayakannan, M. π-conjugate fluorophore-tagged and enzyme-responsive l-amino acid polymer nanocarrier and their color-tunable intracellular fret probe in cancer cells. Biomacromolecules 2017, 18, 2594-2609. [CrossRef]
- Liu, Y.; Lin, A.; Liu, J.; Chen, X.; Zhu, X.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Enzyme-responsive mesoporous ruthenium for combined chemo-photothermal therapy of drug-resistant bacteria. ACS Appl. Mater. Interfaces 2019, 11, 26590-26606. [CrossRef]
- Lee, C. G.; Kwon, T. H. Controlling morphologies of redox-responsive polymeric nanocarriers for a smart drug delivery system. Chem. Eur. J. 2023, 29, 202300594-202300601. [CrossRef]
- Mahdieh, A.; Motasadizadeh, H.; Yeganeh, H.; Nyström, B.; Dinarvand, R. Redox-responsive waterborne polyurethane nanocarriers for targeted doxorubicin delivery. Int. J. Pharm. 2022, 628, 122275-122290. [CrossRef]
- Jia, S.; Tan, A.; Hawley, A.; Graham, B.; Boyd, B.J. Visible light-triggered cargo release from donor acceptor Stenhouse adduct (DASA)-doped lyotropic liquid crystalline nanoparticles. J. Colloid Interface Sci. 2019, 548, 151-159. [CrossRef]
- Roy, B.; Mengji, R.; Roy, S.; Pal, B.; Jana, A.; Singh, N.D.P. NIR-responsive lysosomotropic phototrigger: an “AIE + ESIPT” active naphthalene-based single-component photoresponsive nanocarrier with two-photon uncaging and real-time monitoring ability. ACS Appl. Mater. Interfaces 2022, 14, 4862-4870. [CrossRef]
- Yao, P.; Zou, A.; Tian, Z.; Meng, W.; Fang, X.; Wu, T.; Cheng, J. Construction and characterization of a temperature-responsive nanocarrier for imidacloprid based on mesoporous silica nanoparticles. Colloids Surf. B 2021, 198, 111464-111469. [CrossRef]
- Prawatborisut, M.; Jiang, S.; Oberländer, J.; Mailänder, V.; Crespy, D.; Landfester, K. Modulating protein corona and materials-cell interactions with temperature-responsive materials. Adv. Funct. Mater. 2021, 32, 2106353-2106368. [CrossRef]
- Prawatborisut, M.; Oberländer, J.; Jiang, S.; Graf, R.; Avlasevich, Y.; Morsbach, S.; Crespy, D.; Mailänder, V.; Landfester, K. Temperature-responsive nanoparticles enable specific binding of apolipoproteins from human plasma. Small 2021, 18, 2103138- 2103146. [CrossRef]
- Xue, D.; Meng, Q.B.; Song, X.M. Magnetic-responsive janus nanosheets with catalytic properties. ACS Appl. Mater. Interfaces 2019, 11, 10967-10974. [CrossRef]
- Li, Z.; Li, Y.; Chen, C.; Cheng, Y. Magnetic-responsive hydrogels: from strategic design to biomedical applications. J. Control Release 2021, 335, 541-556. [CrossRef]
- Mehta, S.; Bongcaron, V.; Nguyen, T. K.; Jirwanka, Y.; Maluenda, A.; Walsh, A. P. G.; Palasubramaniam, J.; Hulett, M. D.; Srivastava, R.; Bobik, A. An ultrasound-responsive theranostic cyclodextrin-loaded nanoparticle for multimodal imaging and therapy for atherosclerosis. Small 2022, 18, 2200967-2200981. [CrossRef]
- Sun, M.; Yue, T.; Wang, C.; Fan, Z.; Gazit, E.; Du, J. Ultrasound-responsive peptide nanogels to balance conflicting requirements for deep tumor penetration and prolonged blood circulation. ACS Nano 2022, 16, 9183-9194. [CrossRef]
- Paris, J.L.; Manzano, M.; Cabañas, M.V.; Vallet-Regí, M. Mesoporous silica nanoparticles engineered for ultrasound-induced uptake by cancer cells. Nanoscale 2018, 10, 6402-6408. [CrossRef]
- Simona Mura, J.N., Patrick Couvreur. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991-1003.
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557-4588. [CrossRef]
- Ashrafizadeh, M.; Delfi, M.; Zarrabi, A.; Bigham, A.; Sharifi, E.; Rabiee, N.; Paiva-Santos, A.C.; Kumar, A.P.; Tan, S.C.; Hushmandi, K. Stimuli-responsive liposomal nanoformulations in cancer therapy: Pre-clinical & clinical approaches. J. Control Release 2022, 351, 50-80. [CrossRef]
- Mohammadzadeh, V.; Zirak, M.R.; Hosseini khah, S.M.; Kamali, H.; Jaafari, M.R. pH-sensitive nanocarriers for curcumin delivery in cancer therapy. J. Drug Deliv. Sci. Technol. 2021, 66, 102879-102892.
- Park, H.; Saravanakumar, G.; Kim, J.; Lim, J.; Kim, W.J. Tumor microenvironment sensitive nanocarriers for bioimaging and therapeutics. Adv. Healthc. Mater. 2020, 10, 2000834-2000856. [CrossRef]
- Liu, T.; Du, Y.; Yan, Y.; Song, S.; Qi, J.; Xia, X.; Hu, X.; Chen, Q.; Liu, J.; Zeng, X.; et al. pH-responsive dual-functional hydrogel integrating localized delivery and anti-cancer activities for highly effective therapy in PDX of OSCC. Mater. Today 2023, 62, 71-97.
- Ding, C.; Chen, C.; Zeng, X.; Chen, H.; Zhao, Y. Emerging strategies in stimuli-responsive prodrug nanosystems for cancer therapy, ACS Nano 2022, 16, 13513-13553. [CrossRef]
- Liang, P.; Zhang, Y.; Schmidt, B.F.; Ballou, B.; Qian, W.; Dong, Z.; Wu, J.; Wang, L.; Bruchez, M. P.; Dong, X. Esterase-activated, pH-Responsive, and genetically targetable nano-prodrug for cancer cell photo-ablation. Small 2023, 19, 2207535-2207547.
- Zhang, L.; Huang, J.; Buratto, D.; Han, P.; Yang, Z.; Zhou, R. A pH-responsive nanoparticle delivery system containing dihydralazine and doxorubicin-based prodrug for enhancing antitumor efficacy. Aggregate 2023, 434-448. [CrossRef]
- Zhang, H.; Zhang, L.; Cao, Z.; Cheong, S.; Boyer, C.; Wang, Z.; Yun, S. L. J.; Amal, R.; Gu, Z. Two-dimensional ultra-thin nanosheets with extraordinarily high drug loading and long blood circulation for cancer therapy. Small 2022, 18, 2200299-2200318. [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Gems, D.; Kagan, V.E.; Kalyanaraman, B.; Larsson, N.G.; Milne, G.L.; Nyström, T.; Poulsen, H.E.; Radi, R.; Van Remmen, H.; Schumacker, P.T.; Thornalley, P.J.; Toyokuni, S.; Winterbourn, C.C.; Yin, H.; Halliwell, B. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651-662.
- Sies, H.; Jones, D. P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363-383. [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499-515. [CrossRef]
- Casas, A.I.; Nogales, C.; Mucke, H.A.M.; Petraina, A.; Cuadrado, A.; Rojo, A.I.; Ghezzi, P.; Jaquet, V.; Augsburger, F.; Dufrasne, F.; Soubhye, J.; Deshwal, S.; Di Sante, M.; Kaludercic, N.; Di Lisa, F.; Schmidt, H.H.H.W.; Touyz, R.M. On the clinical pharmacology of reactive oxygen species. Pharmacol. Rev. 2020, 72, 801-828. [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280-297. [CrossRef]
- Ma, W.; Sun, J.; Xu, J.; Luo, Z.; Diao, D.; Zhang, Z.; Oberly, P. J.; Minnigh, M.B.; Xie, W.; Poloyac, S.M. Huang, Y.; Li, S. Sensitizing triple negative breast cancer to tamoxifen chemotherapy via a redox-responsive vorinostat-containing polymeric prodrug nanocarrier. Theranostics 2020, 10, 2463-2478. [CrossRef]
- Sun, C.; Lu, J.; Wang, J.; Hao, P.; Li, C.; Qi, L.; Yang, L.; He, B.; Zhong, Z.; Hao, N. Redox-sensitive polymeric micelles with aggregation-induced emission for bioimaging and delivery of anticancer drugs. J. Nanobiotechnology 2021, 19, 14-29. [CrossRef]
- Huang, C.; Zhou, S.; Chen, C.; Wang, X.; Ding, R.; Xu, Y.; Cheng, Z.; Ye, Z.; Sun, L.; Wang, Z. j.; Hu, D.; Jia, X.; Zhang, G.; Gao, S. Biodegradable redox-responsive aiegen-based-covalent organic framework nanocarriers for long-term treatment of myocardial ischemia/reperfusion injury. Small 2022, 18, 2205062-2205075.
- Anup, D.; Jueun, J.; Been Y.; Yuce Li.; Jae H.P. Cascade-ampliffed self-immolative polymeric prodrug for cancer therapy by disrupting redox homeostasis. J. Control Release 2023, 358, 555-565.
- Zhang, W.; Liu, X.; Cao, S.; Zhang, Q.; Chen, X.; Luo, W.; Tan, J.; Xu, X.; Tian, J.; Saw, P.E.; Luo, B. Multifunctional redox-responsive nanoplatform with dual activation of macrophages and T cells for antitumor immunotherapy. ACS Nano 2023, 17, 14424-14441. [CrossRef]
- Y Li, Y.; Pei, Q.; Cui, B.; Zhang, H.; Han, L.; Li, W.; Zhu, W.; Feng, X.; Xie, Z. A redox-responsive dihydroartemisinin dimeric nanoprodrug for enhanced antitumor activity. J. Nanobiotechnology 2021, 19, 441-452.
- Li, H.; Chen, Y.; Chen, T.; Han, H.; Tong, H.; Jin, Q.; Ji, J. Methemoglobin as a redox-responsive nanocarrier to trigger the in situ anticancer ability of artemisinin. NPG Asia Mater. 2017, 9, 423-423. [CrossRef]
- Sun, B.; Luo, C.; Yu, H.; Zhang, X.; Chen, Q.; Yang, W.; Wang, M.; Kan, Q.; Zhang, H.; Wang, Y.; He, Z.; Sun, J. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018, 18, 3643-3650. [CrossRef]
- Dinakar, Y.H.; Karole, A.; Parvez, S.; Jain, V.; Mudavath, S.L. Organ-restricted delivery through stimuli-responsive nanocarriers for lung cancer therapy. Life Sci. 2022, 310, 121133-121143. [CrossRef]
- Kapalatiya, H.; Madav, Y.; Tambe, V.S.; Wairkar, S. Enzyme-responsive smart nanocarriers for targeted chemotherapy: an overview. Drug Deliv. Transl. Res. 2021, 12, 1293-1305. [CrossRef]
- Yao, Q.; Kou, L.; Tu, Y.; Zhu, L. MMP-responsive “smart” drug delivery and tumor targeting. Trends Pharmacol. Sci. 2018, 39, 766-781.
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control Release 2019, 308, 172-189. [CrossRef]
- Cao, M.; Lu, S.; Wang, N.; Xu, H.; Cox, H.; Li, R.; Waigh, T.; Han, Y.; Wang, Y.; Lu, J.R. Enzyme-triggered morphological transition of peptide nanostructures for tumor-targeted drug delivery and enhanced cancer therapy. ACS Appl. Mater. Interfaces 2019, 11, 16357-16366. [CrossRef]
- Wan, D.; Zhu, Q.; Zhang, J.; Chen, X.; Li, F.; Liu, Y.; Pan, J. Intracellular and extracellular enzymatic responsive micelle for intelligent therapy of cancer. Nano Res. 2022, 16, 2851-2858. [CrossRef]
- Singh, A.K.; Mengji, R.; Nair, A. V.; Shah, S.S.; Avijit, J.; Singh, N.D.P. Photoactivable AIEgen-based lipid-droplet-specific drug delivery model for live cell imaging and two-photon light-triggered anticancer drug delivery. ACS Appl. Bio Mater. 2023, 6, 4372-4382. [CrossRef]
- Zhang, Y.; Zhang, X.; Chen, W.; He, Y.; Liu, Y.; Ju, H. Self-assembled micelle responsive to quick NIR light irradiation for fast drug release and highly efficient cancer therapy. J. Control Release 2021, 336, 469-479. [CrossRef]
- Ray, S.; Banerjee, S.; Singh, A.K.; Ojha, M.; Mondal, A.; Singh, N.D.P. Visible light-responsive delivery of two anticancer drugs using single-component fluorescent organic nanoparticles. ACS Appl. Nano Mater. 2022, 5, 7512-7520. [CrossRef]
- Thang, D.C.; Wang, Z.; Lu, X.; Xing, B. Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective. Theranostics 2019, 9, 3308-3340. [CrossRef]
- Singh, A.K.; Banerjee, S.; Nair, A.V.; Ray, S.; Ojha, M.; Mondal, A.; Singh, N.D.P. Green light-activated single-component organic fluorescence-based nano-drug delivery system for dual uncaging of anticancer drugs. ACS Appl. Bio Mater. 2022, 5, 1202-1209. [CrossRef]
- Chen, Z.; Wei, X.; Zheng, Y.; Zhang, Z.; Gu, W.; Liao, W.; Zhang, H.; Wang, X.; Liu, J.; Li, H. Xu, W. Targeted co-delivery of curcumin and erlotinib by MoS2 nanosheets for the combination of synergetic chemotherapy and photothermal therapy of lung cancer. J. Nanobiotechnology 2023, 21, 333-345. [CrossRef]
- Chen, H.; Zhao, Y. Applications of light-responsive systems for cancer theranostics. ACS Appl. Mater. Interfaces 2018, 10, 21021-21034. [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote light-responsive nanocarriers for controlled drug delivery: advances and perspectives. Small 2019, 15, 1903060-1903093. [CrossRef]
- Lv, R.; Qian, Z.; Zhao, X.; Xiong, F.; Xu, Y.; Fan, W.; Yao, X.; Huang, W. Self-assembly of polysarcosine amphiphilic polymers-tethered gold nanoparticles for precise photo-controlled synergistic therapy. Nano Res. 2022, 16, 5685-5694. [CrossRef]
- Cheng, H.B.; Cui, Y.; Wang, R.; Kwon, N.; Yoon, J. The development of light-responsive, organic dye based, supramolecular nanosystems for enhanced anticancer therapy. Coord. Chem. Rev. 2019, 392, 237-254. [CrossRef]
- Liu, X.; Ren, Y.; Fan, D.; Huang, S.; Ma, Y.; Ding, J.; Luo, Z.; Chen, F.; Zeng, W. Shining light on multidrug-resistant bacterial infections: rational design of multifunctional organosilver-based AIEgen probes as light-activated theranostics for combating biofilms and liver abscesses. Adv. Funct. Mater. 2023, 33, 2304974-2304990. [CrossRef]
- Pearson, S.; Feng, J.; del Campo, A. Lighting the path: light delivery strategies to activate photoresponsive biomaterials in vivo. Adv. Funct. Mater. 2021, 31, 2105989- 2106115. [CrossRef]
- Liu, X.; Fan, D.; Ren, Y.; Huang, S.; Ding, J.; Liu, M.; Wegner, S. V.; Hou, J.; Rong, P.; Chen, F. Zeng, W. Photo-activable organosilver nanosystem facilitates synergistic cancer theranostics. ACS Appl. Mater. Interfaces 2023, 15, 711-722. [CrossRef]
- Deng, H.; Lin, L.; Wang, S.; Yu, G.; Zhou, Z.; Liu, Y.; Niu, G.; Song, J.; Chen, X. X-ray-controlled bilayer permeability of bionic nanocapsules stabilized by nucleobase pairing interactions for pulsatile drug delivery. Adv. Mater. 2019, 31, 1903443-1903450. [CrossRef]
- Lea-Banks, H.; O'Reilly, M. A.; Hynynen, K. Ultrasound-responsive droplets for therapy: a review. J. Control Release 2019, 293, 144-154. [CrossRef]
- Zhou, X.; Guo, L.; Shi, D.; Meng, D.; Sun, X.; Shang, M.; Liu, X.; Zhao, Y.; Li, J. Ultrasound-responsive highly biocompatible nanodroplets loaded with doxorubicin for tumor imaging and treatment in vivo. Drug Deliv. 2020, 27, 469-481. [CrossRef]
- Entzian, K.; Aigner, A. Drug delivery by ultrasound-responsive nanocarriers for cancer treatment. Pharmaceutics 2021, 13, 1135-1165. [CrossRef]
- Athanassiadis, A. G.; Ma, Z.; Moreno-Gomez, N.; Melde, K.; Choi, E.; Goyal, R.; Fischer, P. Ultrasound-responsive systems as components for smart materials. Chem. Rev. 2021, 122, 5165-5208. [CrossRef]
- Wei, P.; Cornel, E.J.; Du, J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 2021, 11, 1323-1339. [CrossRef]
- Huang, D.; Wang, J.; Song, C.; Zhao, Y. Ultrasound-responsive matters for biomedical applications. Innov. 2023, 4, 100421-100433. [CrossRef]
- Wang, Y.; Tang, Q.; Wu, R.; Sun, S.; Zhang, J.; Chen, J.; Gong, M.; Chen, C.; Liang, X. Ultrasound-triggered piezocatalysis for selectively controlled no gas and chemodrug release to enhance drug penetration in pancreatic cancer. ACS Nano 2023, 17, 3557-3573. [CrossRef]
- Zhao, C.; Song, X.; Jin, W.; Wu, F.; Zhang, Q.; Zhang, M.; Zhou, N.; Shen, J. Image-guided cancer therapy using aptamer-functionalized cross-linked magnetic-responsive Fe3O4@carbon nanoparticles. Anal. Chim. Acta 2019, 1056, 108-116. [CrossRef]
- Tung, W. L.; Hu, S. H.; Liu, D.M. Synthesis of nanocarriers with remote magnetic drug release control and enhanced drug delivery for intracellular targeting of cancer cells. Acta Biomater. 2011, 7, 2873-2882. [CrossRef]
- Guisasola, E.; Asín, L.; Beola, L.; de la Fuente, J. M.; Baeza, A.; Vallet-Regí, M. Beyond traditional hyperthermia: in vivo cancer treatment with magnetic-responsive mesoporous silica nanocarriers. ACS Appl. Mater. Interfaces. 2018, 10, 12518-12525. [CrossRef]
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of magnetic nanoplatforms for cancer theranostics. Biosensors 2022, 12, 38-58. [CrossRef]
- Ding, X.; Zhao, H.; Li, C.; Wang, Q.; Jiang, J. All-in-one theranostic nanoplatform with controlled drug release and activated MRI tracking functions for synergistic NIR-II hyperthermia-chemotherapy of tumors. Nano Res. 2019, 12, 2971-2981. [CrossRef]
- Yang, X.; Zhang, C.; Deng, D.; Gu, Y.; Wang, H.; Zhong, Q. Multiple stimuli-responsive mxene-based hydrogel as intelligent drug delivery carriers for deep chronic wound healing. Small 2021, 18, 2104368-2104377. [CrossRef]
- Ravichandran, M.; Oza, G.; Velumani, S.; Ramirez, J. T.; Garcia-Sierra, F.; Andrade, N. B.; Vera, A.; Leija, L.; Garza-Navarro, M. A. Plasmonic/magnetic multifunctional nanoplatform for cancer theranostics. Sci. Rep. 2016, 6, 34874- 34888. [CrossRef]
- lian, Y.; Wang, L.; Cao, J.; Liu, T.; Xu, Z.; Yang, B.; Huang, T.; Jiang, X.; Wu, N. Recent advances on the magnetic nanoparticle-based nanocomposites for magnetic induction hyperthermia of tumor: a short review. Adv. Compos. Hybrid Mater. 2021, 4, 925-937. [CrossRef]
- Rajan, A.; Sahu, N. K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanopart. Res. 2020, 22, 319-343. [CrossRef]
- Guo, Y.; Ran, Y.; Wang, Z.; Cheng, J.; Cao, Y.; Yang, C.; Liu, F.; Ran, H. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials 2019, 219, 119370-119387. [CrossRef]
- Jia, W.; Qi, Y.; Hu, Z.; Xiong, Z.; Luo, Z.; Xiang, Z.; Hu, J.; Lu, W. Facile fabrication of monodisperse CoFe2O4 nanocrystals@dopamine@DOX hybrids for magnetic-responsive on-demand cancer theranostic applications. Adv. Compos. Hybrid Mater. 2021, 4, 989-1001. [CrossRef]
- Zhu, J.; Zhang, H.; Chen, K.; Li, Y.; Yang, Z.; Chen, S.; Zheng, X.; Zhou, X.; Jiang, Z. X. Peptidic monodisperse PEG “Comb” as multifunctional “add-on” module for imaging-traceable and thermo-responsive theranostics. Adv. Healthc. Mater. 2019, 9, 1901331-1901340.
- Hemmatpour, H.; Haddadi-Asl, V.; Burgers, T.C.Q.; Yan, F.; Stuart, M.C.A.; Reker-Smit, C.; Vlijm, R.; Salvati, A.; Rudolf, P. Temperature-responsive and biocompatible nanocarriers based on clay nanotubes for controlled anti-cancer drug release. Nanoscale 2023, 15, 2402-2416. [CrossRef]
- Akimoto, J.; Nakayama, M.; Okano, T. Temperature-responsive polymeric micelles for optimizing drug targeting to solid tumors. J. Control Release 2014, 193, 2-8. [CrossRef]
- Long, H.; Tian, W.; Jiang, S.; Zhao, J.; Zhou, J.; He, Q.; Tang, Z.; Shen, W.; Wang, J. A dual drug delivery platform based on thermo-responsive polymeric micelle capped mesoporous silica nanoparticles for cancer therapy. Microporous Mesoporous Mater. 2022, 338, 111943-111954. [CrossRef]
- Gong, B.; Shen, Y.; Li, H.; Li, X.; Huan, X.; Zhou, J.; Chen, Y.; Wu, J.; Li, W. Thermo-responsive polymer encapsulated gold nanorods for single continuous wave laser-induced photodynamic/photothermal tumour therapy. J. Nanobiotechnology 2021, 19, 41-54. [CrossRef]
- Shang, H.; Yang, X.; Liu, H. Temperature-responsive hydrogel prepared from carboxymethyl cellulose-stabilized N-vinylcaprolactam with potential for fertilizer delivery. Carbohydr. Polym. 2023, 313, 120875-120885. [CrossRef]
- Boon-in, S.; Theerasilp, M.; Crespy, D. Temperature-responsive double-network cooling hydrogels. ACS Appl. Polym. Mater. 2023, 5, 2562-2574. [CrossRef]
- An, Y.; Yang, R.; Wang, X.; Han, Y.; Jia, G.; Hu, C.; Zhang, Z.; Liu, D.; Tang, Q. Facile assembly of thermosensitive liposomes for active targeting imaging and synergetic chemo-/magnetic hyperthermia therapy. Front. Bioeng. Biotechnol. 2021, 9, 691091- 691102.
- Li, J.; Gao, Y.; Liu, S.; Cai, J.; Zhang, Q.; Li, K.; Liu, Z.; Shi, M.; Wang, J.; Cui, H. Aptamer-functionalized quercetin thermosensitive liposomes for targeting drug delivery and antitumor therapy. Biomed. Mater. 2022, 17, 065003. [CrossRef]
- Xu, T.; Ma, Y.; Huang, J.; Lai, H.; Yuan, D.; Tang, X.; Yang, L. Self-organized thermo-responsive poly (lactic-co-glycolic acid)-graft-pullulan nanoparticles for synergistic thermo-chemotherapy of tumor. Carbohydr. Polym. 2020, 237, 116104- 116113. [CrossRef]
- Tong, T.; Guan, Y.; Gao, Y.; Xing, C.; Zhang, S.; Jiang, D.; Yang, X.; Kang, Y.; Pang, J. Smart nanocarriers as therapeutic platforms for bladder cancer. Nano Res. 2021, 15, 2157-2176. [CrossRef]
- Luo, S.; Lv, Z.; Yang, Q.; Chang, R.; Wu, J. Research progress on stimulus-responsive polymer nanocarriers for cancer treatment. Pharmaceutics 2023, 15, 1928- 1951. [CrossRef]
- Zhong, D.; Wu, H.; Wu, Y.; Li, Y.; Xu, X.; Yang, J.; Gu, Z. Rational design and facile fabrication of biocompatible triple responsive dendrimeric nanocages for targeted drug delivery. Nanoscale 2019, 11, 15091-15103. [CrossRef]
- Zhang, X.; Zhu, T.; Miao, Y.; Zhou, L.; Zhang, W. Dual-responsive doxorubicin-loaded nanomicelles for enhanced cancer therapy. J. Nanobiotechnology 2020, 18, 136-152. [CrossRef]
- Jia, X.; Zhang, Y.; Zou, Y.; Wang, Y.; Niu, D.; He, Q.; Huang, Z.; Zhu, W.; Tian, H.; Shi, J. Li, Y. Dual intratumoral redox/enzyme-responsive no-releasing nanomedicine for the specific, high-efficacy, and low-toxic cancer therapy. Adv. Mater. 2018, 30, 1704490-1704498.
- Shen, J.; Ma, M.; Shafiq, M.; Yu, H.; Lan, Z.; Chen, H. Microfluidics-assisted engineering of ph/enzyme dual-activatable zif@polymer nanosystem for co-delivery of proteins and chemotherapeutics with enhanced deep-tumor penetration. Angew. Chem. Int. Ed. 2022, 61, 202113703-202113713.
- Chen, Z.; Sun, Y.; Wang, J.; Zhou, X.; Kong, X.; Meng, J.; Zhang, X. Dual-responsive triple-synergistic Fe-MOF for tumor theranostics. ACS Nano 2023, 17, 9003-9013. [CrossRef]
- Zhu, Y.; Pan, Y.; Guo, Z.; Jin, D.; Wang, W.; Liu, M.; Zong, M.; Zheng, X.; Wu, Y.; Wang, L. Tian, C.; Cheng, J.; Liu, Y. Photothermal enhanced and tumor microenvironment responsive nanozyme for amplified cascade enzyme catalytic therapy. Adv. Healthc. Mater. 2022, 12, 2202198-2202209. [CrossRef]
- Feng, S.; Mao, Y.; Wang, X.; Zhou, M.; Lu, H.; Zhao, Q.; Wang, S. Triple stimuli-responsive ZnO quantum dots-conjugated hollow mesoporous carbon nanoplatform for NIR-induced dual model antitumor therapy. Colloid Interface Sci. 2020, 559, 51-64. [CrossRef]
- Ma, X.; Liu, J.; Lei, L.; Yang, H.; Lei, Z. Synthesis of light and dual-redox triple-stimuli-responsive core-crosslinked micelles as nanocarriers for controlled release. J. Appl. Polym. Sci. 2019, 136, 47946-47953.
- Ji, C.; Deng, Y.; Yuan, H.; Yuan, W.; Song, Y.; Li, Z. Hypoxia/temperature/pH triple stimuli-responsive block copolymers: synthesis, self-assembly, and controlled drug release. Macromol. Mater. Eng. 2021, 306, 2100073-2100085.
- Wu, Y.; Chen, S.; Zhu, J. Hydrogen-bond-driven core-crosslinked supramolecular micelles with pH/thermal/glutathione-responsive drug release toward enhanced cancer therapy. Chem. Mater. 2023, 35, 7655-767.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).