Submitted:
31 January 2024
Posted:
01 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Material and methods
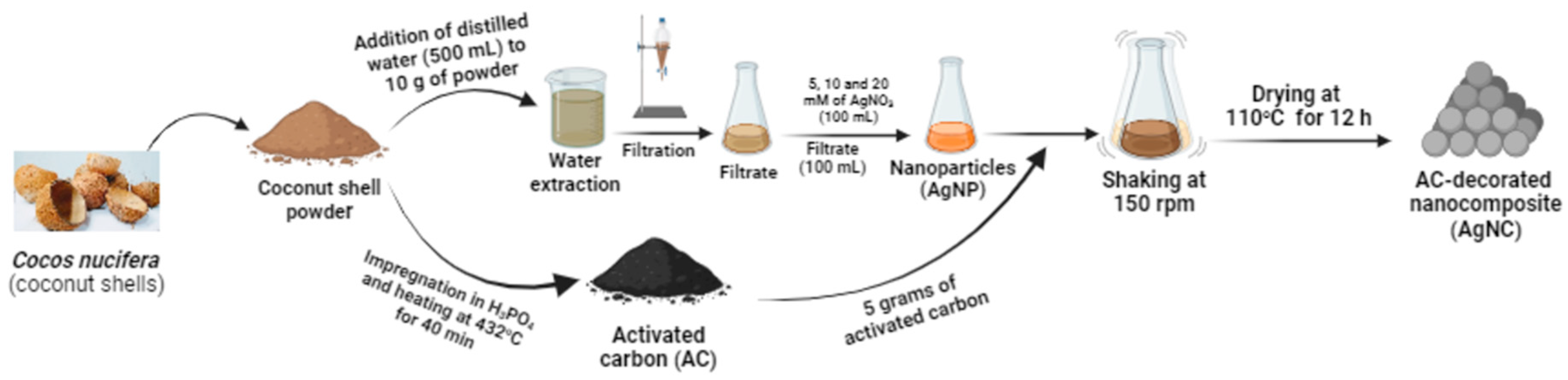
2.1. Material
2.2. Preparation of the activated carbon
2.3. Synthesis and characterisation of silver nanoparticles
2.4. Preparation of silver nanocomposite
2.5. Antimicrobial assay
2.5.1. Microbial species and culture media
2.5.2. Determination of minimum inhibitory concentrations
2.6. Time-kill kinetics test.
2.7. Cytotoxicity assays
2.8. In vitro antioxidant activity
2.8.1. DPPH radical scavenging assay
2.8.2. ABTS radical scavenging assay
2.9. Statistical analysis
3. Results and discussion
3.1. Results
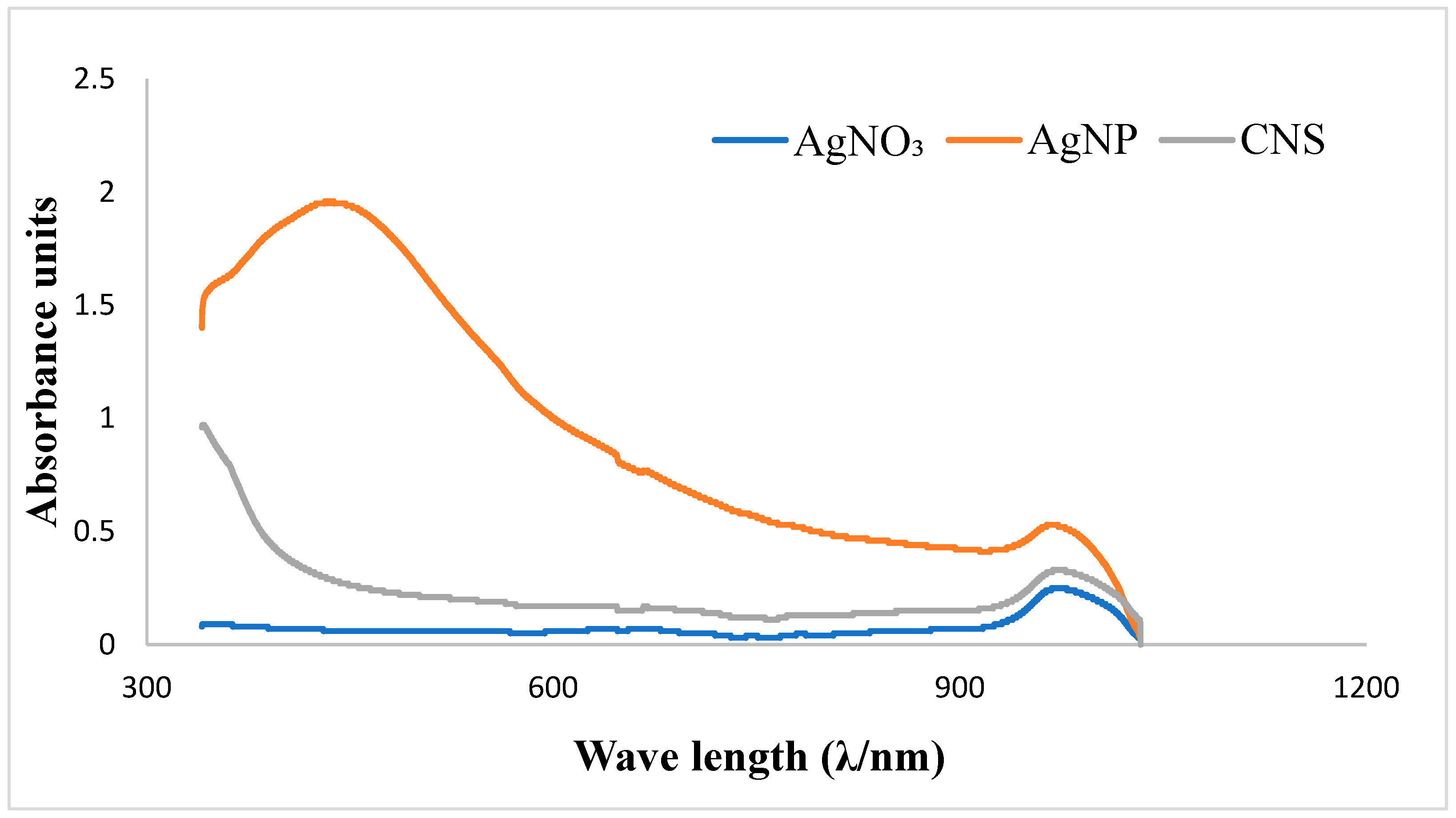
3.1.1. UV–visible spectral analysis
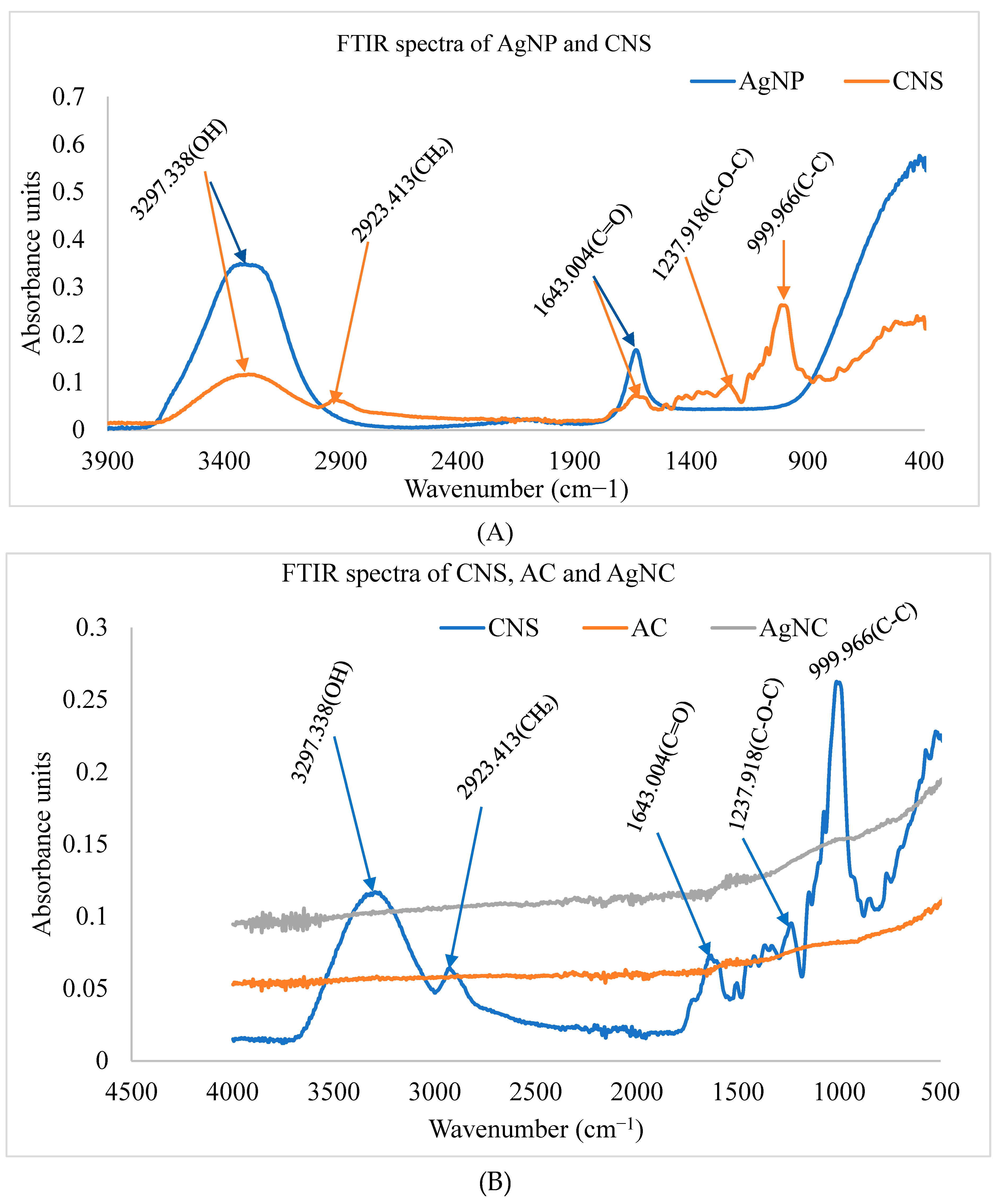
3.1.2. FTIR analysis of synthesized nanomaterials
3.1.3. Minimum inhibitory concentrations of the as-prepared nanomaterials
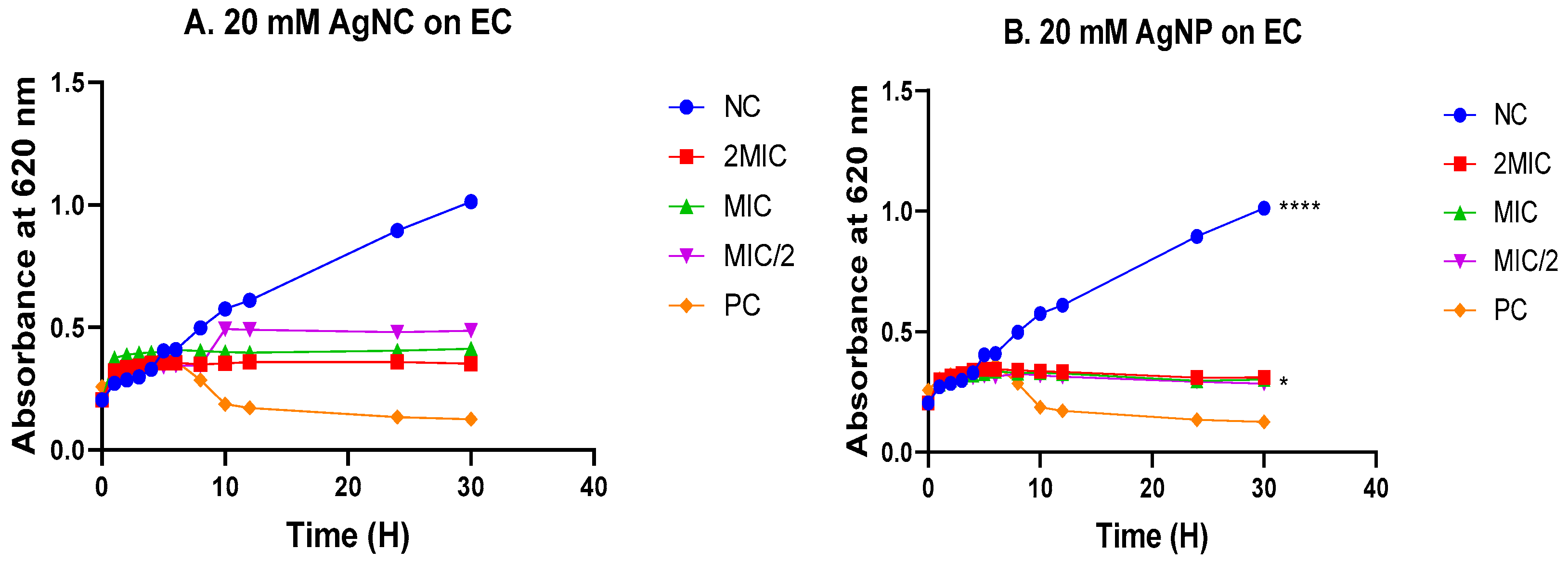
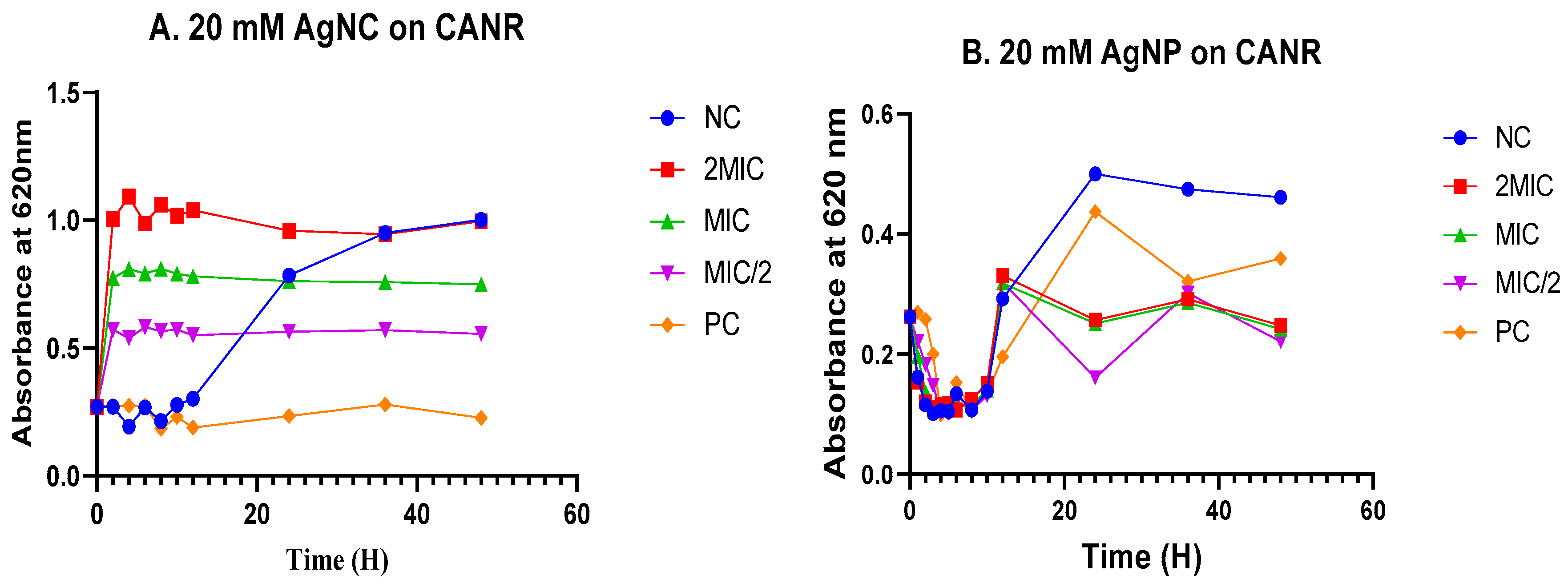
3.4. Time-kill kinetics
3.4.1. Time-kill kinetics in Escherichia coli
3.4.2. Time-kill kinetics in Candida albicans
3.5. Cytotoxicity assay
3.6. Antioxidant activity
3.6.1. The DPPH scavenging assay
| Nanomaterials/ascorbic acid | RSA50 (µg/mL) - | EC50 x 103 (µg/mol) - | ARP x 10-6 - (mol/µg) - |
|---|---|---|---|
| AgNC 5mM | >500 | na | na |
| AgNC 10mM | >500 | na | na |
| AgNC 20mM | >500 | na | na |
| AgNP 5mM | 382.5 ± 3.323ᵇ | 754.038 ± 6.552ᵇ | 198.529 ± .0115ᵇ |
| AgNP 10mM | >500 | na | na |
| AgNP 20mM | >500 | na | na |
| Ascorbic acid | 7.363 ± 0.312 a | 21.556 ± 0.615 a | 104.46 ± 2.92 a |
3.6.2. The ABTS scavenging test
4. Discussion
5. Limitations and perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Health Organization (WHO). Infectious Diseases. The Fact Sheets 2024. Available online: https://www.emro.who.int/health-topics/infectious-diseases/index.html (accessed on 6 January 2024).
- Nii-Trebi, N.I. Emerging and neglected infectious diseases: Insights, advances, and challenges. Biomed. Res. Int. 2017, 2017, 5245021. [Google Scholar] [CrossRef]
- Kumwenda, P.; Adukwu, E.C.; Tabe, E.S.; Ujor, V.C.; Kamudumuli, P.S.; Ngwira, M.; Tsung, J.; Wu, S.; Chisale, M.R.O. Prevalence, distribution and antimicrobial susceptibility pattern of bacterial isolates from a tertiary Hospital in Malawi. BMC Infect. Dis. 2021, 21, 1–10. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-gutierrez, D. Fungal infections in humans: the silent crisis. Microb. Cell 2022, 7, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Pfavayi, L.T.; Denning, D.W.; Baker, S.; Sibanda, E.N.; Mutapi, F. Determining the burden of fungal infections in Zimbabwe. Sci.Rep. 2021, 1–13. [Google Scholar] [CrossRef]
- Mandengue, C.E.; Denning, D.W. The burden of serious fungal infections in Cameroon. J. Fungi. 2018, 4, 1–9. [Google Scholar] [CrossRef]
- Dall, C. Report highlights the deadly impact of bacterial infections. Antimicrobial Stewardship Public Health. CIDRAP. 2022. Available online: https://www.cidrap.umn.edu/antimicrobial-stewardship/report-highlights-deadly-impact-bacterial-infections (accessed on 21 January 2024).
- Muendlein, H. 2022. Bacterial infections caused most deaths in sub-Saharan Africa in 2019: Lancet. Available online: https://www.downtoearth.org.in/news/africa/bacterial-infections-caused-most-deaths-in-sub-saharan-africa-in-2019-lancet-86209#:~:text=Published%3A%20Monday%2028%20November%202022&text=Sub%2DSaharan%20Africa%20saw%20the,cause%20of%20death%20in%202019.
- Amvomo, N.L.D.; Sama, C.K.K.; Bouopda, R.; Minkeza, F.C.N.; Mbopda, L.P.; Tchoumi, C.L.Y.; Kenmoe, L.O.T.; Simeni, G.T.; Tolo, E.C.; Ngongang, R.; et al. Bacterial ecology and antibiotic susceptibility profile of isolated strains from surfaces and medical devices in some departments of the Jordan Medical Services, Cameroon: a descriptive cross-sectional study. PAMJ—One Health 2023, 11. [Google Scholar] [CrossRef]
- Giustarini, D.; Dalle-Donne, I.; Milzani, A.; Rossi, R. Oxidative stress induces a reversible flux of cysteine from tissues to blood in vivo in the rat. FEBS J. 2009, 276, 4946–4958. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The impact of oxidative stress in human pathology: Focus on gastrointestinal disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Novaes, R.D.; Teixeira, A.L.; de Miranda, A.S. Oxidative stress in microbial diseases: Pathogen, host, and therapeutics. Oxid. Med. Cell Longev. 2019, 2019, 8159562. [Google Scholar] [CrossRef]
- Gain, C.; Song, S.; Angtuaco, T.; Satta, S.; Kelesidis, T. The role of oxidative stress in the pathogenesis of infections with coronaviruses. Front. Microbiol. 2023, 13, 1111930. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Segal, E.; Elad, D. Special issue: Treatments for fungal infections. J. Fungi 2018, 4, 135. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Campitelli, M.; Zeineddine, N.; Samaha, G.; Maslak, S. Combination antifungal therapy: A review of current data. J. Clin. Med. Res. 2017, 9, 451–456. [Google Scholar] [CrossRef]
- Wahab, M.A.; Li, L.; Li, H.; Abdala, A. Silver nanoparticle-based nanocomposites for combating infectious pathogens: Recent advances and future prospects. Nanomaterials 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Nguimatsia, F.; Kenmogne, S.B.; Ngo Mback, M.N.L.; Kouamouo, J.; Nzenti Tchuitio, L.L.; Melogmo Dongmo, Y.K.; Jazet Dongmo, P.M. Antibacterial activities of the essential oil and hydroethanolic extract from Aeollanthus heliotropioides Oliv. Mediterr. J. Chem. 2021, 11, 95–103. [Google Scholar]
- Lima, E.B.; Sousa, C.N.; Meneses, L.N.; Ximenes, N.C.; Santos Júnior, M.A.; Vasconcelos, G.S.; Lima, N.B.; Patrocínio, M.C.; Macedo, D.; Vasconcelos, S.M. Cocos nucifera (L.) (Arecaceae): A phytochemical and pharmacological review. Braz. J. Med. Biol. Res. 2015, 48, 953–964. [Google Scholar] [CrossRef]
- Jadimurthy, R.; Jagadish, S.; Nayak, S.C.; Kumar, S.; Mohan, C.D.; Rangappa, K.S. Phytochemicals as invaluable sources of potent antimicrobial agents to combat antibiotic resistance. Life 2023, 13, 948. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; Silva, A.M.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Taha, A.; Ben Aissa, M.; Da’na, E. Green synthesis of an activated carbon-supported Ag and ZnO nanocomposite for photocatalytic degradation and its antibacterial activities. Molecules 2020, 25, 1586. [Google Scholar] [PubMed]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-based nanoparticles: antibacterial mechanisms and biomedical application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.-u.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: recent trends and future prospects. J. Nanobiotechnology 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Xulu, J.H.; Ndongwe, T.; Ezealisiji, K.M.; Tembu, V.J.; Mncwangi, N.P.; Witika, B.A.; Siwe-Noundou, X. The use of medicinal plant-derived metallic nanoparticles in theranostics. Pharmaceutics 2022, 14, 2437. [Google Scholar] [CrossRef]
- Ghazzy, A.; Naik, R.R.; Shakya, A.K. Metal-polymer nanocomposites: A promising approach to antibacterial materials. Polymers 2023, 15, 2167. [Google Scholar] [CrossRef]
- Pongener, C.; Kibami, D.; Rao, K.S.; Goswamee, R.L.; Sinha, D. Adsorption studies of fluoride by activated carbon prepared from Mucuna prurines plant. Biological Methods of Water Treatment. J. Water Chem. Technol. 2017, 39, 108–115. [Google Scholar] [CrossRef]
- Naphtali Odogu, A.; Daouda, K.; Paul, L.K.; Agbor Tabi, G.; Ngouateu Rene, L.; Nsami, N.J.; Mbadcam, K.K. Effect of doping activated carbon based Ricinodendron Heudelotti shells with AgNPs on the adsorption of indigo carmine and its antibacterial properties. Arab. J. Chem. 2020, 13, 5241–5253. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.S.; Kumar, A.; Vishnuprasad, C.N.; Patra, J.K. Photo-mediated optimized synthesis of silver nanoparticles using the extracts of outer shell fibre of Cocos nucifera L. fruit and detection of its antioxidant, cytotoxicity and antibacterial potential. Saudi J. Biol. Sci. 2021, 28, 980–987. [Google Scholar] [CrossRef]
- Clinical Laboratory Standard Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Ninth Edition M07 A9; Clinical Laboratory Standard Institute, 2012; 29(2). [Google Scholar]
- Bowling, T.; Mercer, L.; Don, R.; Jacobs, R.; Nare, B. Application of a resazurin-based high-throughput screening assay for the identification and progression of new treatments for human African trypanosomiasis. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 262–270. [Google Scholar] [CrossRef]
- Bassene, E. Initiation à la recherche sur les substances naturelles. Presse Universitaire de Dakar 2012. 147p.
- Khan, R.A.; Khan, M.R.; Sahreen, S.; Ahmed, M. Evaluation of phenolic contents and antioxidant activity of various solvent extracts of Sonchus asper (L.) Hill. Chem. Cent. J. 2012, 6, 12. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Sarkar, M.K.; Suriya, K.R.; Nithyanand, P.; Vadivel, V. Use of agricultural waste (coconut shell) for the synthesis of silver nanoparticles and evaluation of their antibacterial activity against selected human pathogens. Microb. Pathog. 2018, 124, 30–37. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Malik, M.A. Phytomediated photo-induced green synthesis of silver nanoparticles using Matricaria chamomilla L. and its catalytic activity against rhodamine B. Biomolecules 2020, 10, 1604. [Google Scholar] [CrossRef]
- Hano, C.; Abbasi, B.H. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules 2021, 12, 31. [Google Scholar] [CrossRef]
- Lee, I.; Park, J.Y.; Hong, K.; Son, J.H.; Kim, S.; Lee, J.L. The effect of localized surface Plasmon resonance on the emission colour change in organic light emitting diodes. Nanoscale 2016, 8, 6463–4647. [Google Scholar] [CrossRef]
- Rautela, A.; Rani, J.; Debnath, M. Green synthesis of silver nanoparticles from Tectona grandis seeds extract: characterization and mechanism of antimicrobial action on different microorganisms. J. Anal. Sci. Technol. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Paul, K.; Bag, B.G.; Samanta, K. Green coconut (Cocos nucifera Linn) shell extract mediated size controlled green synthesis of polyshaped gold nanoparticles and its application in catalysis. Appl. Nanosci. 2014, 4, 769–775. [Google Scholar] [CrossRef]
- Khalid Thebo, N.; Ahmed Simair, A.; Sughra Mangrio, G.; Ansari, K.; Ali Bhutto, A.; Lu, C.; Ali Sheikh, W. Antifungal potential and antioxidant efficacy in the shell extract of Cocos nucifera (L.) (Arecaceae) against pathogenic dermal mycosis. Medicines 2016, 3, 12. [Google Scholar] [CrossRef]
- Sulaeman, A.; Mathematics, F.; Sciences, N.; Bandung, I.T. Preliminary study of characterization of nanoparticles from coconut shell as filler agent in composites materials. MAYFEB J. Mater. Sci. 2016, 1, 1–9. [Google Scholar]
- Kumari, B.V.; Mani, R.; Asokan, B.R.; Balakrishnan, K.; Ramasamy, A.; Parthasarathi, R.; Kandasamy, C.; Govindaraj, R.; Vijayakumar, N.; Vijayakumar, S. Green synthesised silver nanoparticles using Anoectochilus elatus leaf extract: Characterisation and evaluation of antioxidant, anti-inflammatory, antidiabetic, and antimicrobial activities. J. Compos. Sci. 2023, 7, 453. [Google Scholar] [CrossRef]
- Franklin Loic, T.T.; Boniface, P.K.; Vincent, N.; Zuriatou, Y.T.; Yimgang, V.L.; Ndi, J.N.; Paul, K.L.; Fabrice, F.B. Biological synthesis and characterization of silver-doped nanocomposites: Antibacterial and mechanistic studies. Drugs Drug Candidates 2024, 3, 13–32. [Google Scholar] [CrossRef]
- Yahya, M.A.; Mansor, M.H.; Zolkarnaini, W.A.A.W.; Rusli, N.S.; Aminuddin, A.; Mohamad, K.; Sabhan, F.A.M.; Atik, A.A.A.; Ozair, L.N. A brief review on activated carbon derived from agriculture by-product. AIP Conf. Proc. 2018, 1972, 030023. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Silver Nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Burchacka, E.; Pstrowska, K.; Bryk, M.; Maciejowski, F.; Kułażyński, M.; Chojnacka, K. The properties of activated carbons functionalized with an antibacterial agent and a new SufA protease inhibitor. Materials 2023, 16, 1263. [Google Scholar] [CrossRef]
- Alvarez-Galvan, Y.; Minofar, B.; Futera, Z.; Francoeur, M.; Jean-Marius, C.; Brehm, N.; Yacou, C.; Jauregui-Haza, U.J.; Gaspard, S. Adsorption of hexavalent chromium using activated carbon produced from Sargassum ssp.: Comparison between lab experiments and molecular dynamics simulations . Molecules 2022, 27, 6040. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Mbaveng,, A.T.; Kuete, V. Antimicrobial Activities of African Medicinal Spices and Vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; 2017; pp. 207–237. [Google Scholar]
- Brice, R.P.; Boniface, P.K.; Eutrophe Le Doux, K.; Vincent, N.; Yanick Kevin, M.D.; Paul, K.L.; Fabrice, F.B. Extracts from Cardiospermum grandiflorum and Blighia welwitschii (Sapindaceae) reveal antibacterial activity against Shigella species. S. Afr. J. Bot. 2023, 164, 419–428. [Google Scholar] [CrossRef]
- Majoumouo, M.S.; Sibuyi, N.R.S.; Tincho, M.B.; Mbekou, M.; Boyom, F.F.; Meyer, M. Enhanced anti-bacterial activity of biogenic silver nanoparticles synthesized from Terminalia mantaly extracts. Int. J. Nanomed. 2019, 14, 9031–9046. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 1, 230–235. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Marrez, D.A.; Abdelmoeen, N.M.; Mahmoud, E.A.; Abdel-Shakur Ali, M.; Decsi, K.; Tóth, Z. Studying the antioxidant and the antimicrobial activities of leaf successive extracts compared to the green-chemically synthesized silver nanoparticles and the crude aqueous extract from Azadirachta indica. Processes 2023, 11, 1644. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress in Infection and Consequent Disease. Oxid. Med. Cell Longev. 2017, 3496043. [Google Scholar] [CrossRef] [PubMed]
| Bacterial/Fungal strains | Acronym | Reference number | Supplier |
|---|---|---|---|
|
Escherichia coli Klebsiella pneumoniae |
E. coli K. pneumoniae |
ATCC 25922 NR 41817 |
ATCC BEI Resources |
| Pseudomonas aeruginosa | P. aeruginosa | NR 48982 | BEI Resources |
| Methicillin-resistant Staphylococcus aureus | S. aureus | ATCC 33591 | BEI Resources |
|
Escherichia coli Klebsiella pneumoniae |
E. coli K. pneumoniae |
ATCC 25922 NR 41817 |
ATCC BEI Resources |
| Pseudomonas aeruginosa | P. aeruginosa | NR 48982 | BEI Resources |
| Methicillin-resistant Staphylococcus aureus | S. aureus | ATCC 33591 | BEI Resources |
|
Salmonella enteridis Shigella flexneri Shigella sonnei Staphylococcus aureus |
S. enteridis S. flexneri S. sonnei S. aureus |
Isolat NR 518 NR 519 ATCC 43300 |
CPC BEI resources BEI resources ATCC |
| Candida albicans | C. albicans | CA NR-29456 | BEI resources |
| Candida glabrata | C. glabrata | CG 100 | BEI resources |
| Candida krusei | C. krusei | CK ATCC-1415 | ATCC |
| Candida parapsilosis | C. parapsilosis | CP O31S | BEI resources |
| Candida albicans | C. albicans | CA ATCC-14516 | ATCC |
| Minimum inhibitory concentrations (µg/ml) | ||||||||
| Bacteria | EC | SONR | SA | SFNR | SAMR | SE | PANR | KPNR |
| AgNC 5mM | 62.5 | 125 | 62.5 | 250 | 250 | 250 | 125 | 62.5 |
| AgNC 10mM | 31.25 | 250 | 62.5 | 62.5 | 125 | 125 | 62.5 | 62.5 |
| AgNC 20mM | 31.25 | 62.5 | 31.25 | 31.25 | 62.5 | 31.25 | 31.25 | 62.5 |
| AgNP 5mM | 15.625 | 62.5 | 15.625 | 31.25 | 31.25 | 31.25 | 31.25 | 31.25 |
| AgNP 10mM | 15.625 | 62.5 | 15.625 | 31.25 | 15.625 | 125 | 15.625 | 31.25 |
| AgNP 20mM | 7.8125 | 15.625 | 15.625 | 31.25 | 31.25 | 62.5 | 31.25 | 31.25 |
| Ciprofloxacin | 0.078 | 0.078 | 0.039 | 0.078 | 0.078 | 0.156 | 0.078 | 0.039 |
| Minimum inhibitory concentrations (µg/ml) | |||||
| Fungi | CK | CP | CG | CANR | CA |
| AgNC 5 mM | 125 | 62.5 | 250 | 62.5 | 250 |
| AgNC 10 mM | 250 | 62.5 | 125 | 62.5 | 125 |
| AgNC 20 mM | 31.25 | 62.5 | 62.5 | 31.25 | 31.25 |
| AgNP 5 mM | 125 | 125 | 125 | 125 | 31.25 |
| AgNP 10 mM | 62.5 | 125 | 62.5 | 62.5 | 15.625 |
| AgNP 20 mM | 31.250 | 62.5 | 7.812 | 7.812 | 15.625 |
| Fluconazole | 0.153 | 0.153 | 0.3825 | 0.153 | 0.765 |
| Nanomaterials | CC50 (µg/ml) |
|---|---|
| AgNC 5 mM | >1000 |
| AgNC 10 mM | >1000 |
| AgNC 20 mM | >1000 |
| AgNP 5 mM | >1000 |
| AgNP 10 mM | >1000 |
| AgNP 20 mM | 60.52 ± 0.070711 |
| Podophyllotoxin | 0.4 ± 0.1 |
| Nanomaterials/ascorbic acid | RSA50 (µg/mL) | EC50 x 103 (µg/mol) | ARP x 10-5 (mol/µg) |
|---|---|---|---|
| AgNC 5mM | 53.855 ± 2.722a | 15.387 ± 0.778a | 6.619 ±1.703a |
| AgNC 10mM | 8.2695± 0.353a | 2.362 ± 0.101a | 41.704± 8.765a |
| AgNC 20mM | 6.586 ± 0.645a | 1.883 ± 0.186a | 55.055 ± 0.281a |
| AgNP 5mM | >500 | na | na |
| AgNP 10mM | >500 | na | na |
| AgNP 20mM | >500 | na | na |
| Ascorbic acid | 22.46 ± 2.729a | 6.417 ± 0.779a | 15.699 ± 0.19a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





