Submitted:
29 January 2024
Posted:
02 February 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. What is available neuroscience integration for therapists?
1.2. Our goals
2. Three-Core-Network Model for Depression
3. Extensions to the Four-Network Model
4. Integration of Cognitive Behavioral Therapy Techniques into the Four-Network Model
5. Utilizing the Four-Network Model beyond Cognitive Behavioral Therapy
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cuijpers, P.; Karyotaki, E.; Weitz, E.; Andersson, G.; Hollon, S.D.; Van Straten, A. The Effects of Psychotherapies for Major Depression in Adults on Remission, Recovery and Improvement: A Meta-Analysis. J. Affect. Disord. 2014, 159, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Berking, M.; Andersson, G.; Quigley, L.; Kleiboer, A.; Dobson, K.S. A Meta-Analysis of Cognitive-Behavioural Therapy for Adult Depression, Alone and in Comparison with Other Treatments. Can. J. Psychiatry 2013, 58, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Papola, D.; Ostuzzi, G.; Tedeschi, F.; Gastaldon, C.; Purgato, M.; Del Giovane, C.; Pompoli, A.; Pauley, D.; Karyotaki, E.; Sijbrandij, M.; et al. Comparative Efficacy and Acceptability of Psychotherapies for Panic Disorder with or without Agoraphobia: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. Br. J. Psychiatry 2022, 221, 507–519. [Google Scholar] [CrossRef]
- Cuijpers, P.; Cristea, I.A.; Karyotaki, E.; Reijnders, M.; Huibers, M.J.H.H. How Effective Are Cognitive Behavior Therapies for Major Depression and Anxiety Disorders? A Meta-Analytic Update of the Evidence. World Psychiatry 2016, 15, 245–258. [Google Scholar] [CrossRef]
- Trauer, J.M.; Qian, M.Y.; Doyle, J.S.; Rajaratnam, S.M.W.; Cunnington, D. Cognitive Behavioral Therapy for Chronic Insomnia. Ann. Intern. Med. 2015, 163, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Hertenstein, E.; Trinca, E.; Wunderlin, M.; Schneider, C.L.; Züst, M.A.; Fehér, K.D.; Su, T.; Straten, A. v.; Berger, T.; Baglioni, C.; et al. Cognitive Behavioral Therapy for Insomnia in Patients with Mental Disorders and Comorbid Insomnia: A Systematic Review and Meta-Analysis. Sleep Med. Rev. 2022, 62, 101597. [Google Scholar] [CrossRef]
- Williams, A.C. de C.; Fisher, E.; Hearn, L.; Eccleston, C. Psychological Therapies for the Management of Chronic Pain (Excluding Headache) in Adults. Cochrane database Syst. Rev. 2020, 8, CD007407. [Google Scholar] [CrossRef]
- Leichsenring, F.; Steinert, C. Is Cognitive Behavioral Therapy the Gold Standard for Psychotherapy? The Need for Plurality in Treatment and Research. JAMA - J. Am. Med. Assoc. 2017, 318, 1323–1324. [Google Scholar] [CrossRef]
- Salzano, S.; Zappullo, I.; Baiano, C.; Conson, M. The Integrated Neuropsychological Therapy: A Psychotherapy Model Tying Neuropsychology and Cognitive Behavioral Therapy. J. Cogn. Psychother. 2023, JCP-2021-0020.R1. [CrossRef]
- Yuan, S.; Wu, H.; Wu, Y.; Xu, H.; Yu, J.; Zhong, Y.; Zhang, N.; Li, J.; Xu, Q.; Wang, C. Neural Effects of Cognitive Behavioral Therapy in Psychiatric Disorders: A Systematic Review and Activation Likelihood Estimation Meta-Analysis. Front. Psychol. 2022, 13. [Google Scholar] [CrossRef]
- Månsson, K.N.T.; Lueken, U.; Frick, A. Enriching CBT by Neuroscience: Novel Avenues to Achieve Personalized Treatments. Int. J. Cogn. Ther. 2021, 14, 182–195. [Google Scholar] [CrossRef]
- Franklin, G.; Carson, A.J.; Welch, K. a. Cognitive Behavioural Therapy for Depression: Systematic Review of Imaging Studies. Acta Neuropsychiatr. 2015, 1–14. [Google Scholar] [CrossRef]
- Sankar, A.; Melin, A.; Lorenzetti, V.; Horton, P.; Costafreda, S.G.; Fu, C.H.Y. A Systematic Review and Meta-Analysis of the Neural Correlates of Psychological Therapies in Major Depression. Psychiatry Res. Neuroimaging 2018, 279, 31–39. [Google Scholar] [CrossRef]
- Picó-Pérez, M.; Fullana, M.A.; Albajes-Eizagirre, A.; Vega, D.; Marco-Pallarés, J.; Vilar, A.; Chamorro, J.; Felmingham, K.L.; Harrison, B.J.; Radua, J.; et al. Neural Predictors of Cognitive-Behavior Therapy Outcome in Anxiety-Related Disorders: A Meta-Analysis of Task-Based FMRI Studies. Psychol. Med. 2023, 53, 3387–3395. [Google Scholar] [CrossRef]
- Cohen, S.E.; Zantvoord, J.B.; Wezenberg, B.N.; Bockting, C.L.H.; van Wingen, G.A. Magnetic Resonance Imaging for Individual Prediction of Treatment Response in Major Depressive Disorder: A Systematic Review and Meta-Analysis. Transl. Psychiatry 2021, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Strege, M. V; Siegle, G.J.; Richey, J.A.; Krawczak, R.A.; Young, K. Cingulate Prediction of Response to Antidepressant and Cognitive Behavioral Therapies for Depression: Meta-Analysis and Empirical Application. Brain Imaging Behav. 2023, 17, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Kalin, N.H. Insights and Advances Into Treatments for Major Depression. Am. J. Psychiatry 2023, 180, 173–176. [Google Scholar] [CrossRef]
- Perrotta, D.; Perri, R.L. Mini-Review: When Neurostimulation Joins Cognitive-Behavioral Therapy. On the Need of Combining Evidence-Based Treatments for Addiction Disorders. Neurosci. Lett. 2022, 777, 136588. [Google Scholar] [CrossRef] [PubMed]
- Russell-Chapin, L.A. Integrating Neurocounseling into the Counseling Profession: An Introduction. J. Ment. Heal. Couns. 2016, 38, 93–102. [Google Scholar] [CrossRef]
- Russell-Chapin, L.A.; Pacheco, N.C.; DeFord, J.A. Practical Neurocounseling; Russell-Chapin, L., Pacheco, N., DeFord, J., Eds.; Routledge: New York, 2020; ISBN 9780367824402. [Google Scholar]
- Miller, R. Neuroeducation: Integrating Brain-Based Psychoeducation into Clinical Practice. J. Ment. Heal. Couns. 2016, 38, 103–115. [Google Scholar] [CrossRef]
- Nawaz, H.; Shah, I.; Ali, S. The Amygdala Connectivity with Depression and Suicide Ideation with Suicide Behavior: A Meta-Analysis of Structural MRI, Resting-State FMRI and Task FMRI. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2023, 124, 110736. [Google Scholar] [CrossRef]
- Bondi, E.; Maggioni, E.; Brambilla, P.; Delvecchio, G. A Systematic Review on the Potential Use of Machine Learning to Classify Major Depressive Disorder from Healthy Controls Using Resting State FMRI Measures. Neurosci. Biobehav. Rev. 2023, 144, 104972. [Google Scholar] [CrossRef]
- Kotoula, V.; Evans, J.W.; Punturieri, C.; Johnson, S.C.; Zarate, C.A. Functional MRI Markers for Treatment-Resistant Depression: Insights and Challenges. In Progress in brain research; Netherlands, 2023; 278, 117–148. [CrossRef]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-Analysis of Resting-State Functional Connectivity. JAMA psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef]
- Korgaonkar, M.S.; Goldstein-Piekarski, A.N.; Fornito, A.; Williams, L.M. Intrinsic Connectomes Are a Predictive Biomarker of Remission in Major Depressive Disorder. Mol. Psychiatry 2020, 25, 1537–1549. [Google Scholar] [CrossRef]
- Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. [Google Scholar] [CrossRef]
- Young, I.M.; Dadario, N.B.; Tanglay, O.; Chen, E.; Cook, B.; Taylor, H.M.; Crawford, L.; Yeung, J.T.; Nicholas, P.J.; Doyen, S.; et al. Connectivity Model of the Anatomic Substrates and Network Abnormalities in Major Depressive Disorder: A Coordinate Meta-Analysis of Resting-State Functional Connectivity. J. Affect. Disord. Reports 2023, 11, 100478. [Google Scholar] [CrossRef]
- Bertocci, M.A.; Afriyie-Agyemang, Y.; Rozovsky, R.; Iyengar, S.; Stiffler, R.; Aslam, H.A.; Bebko, G.; Phillips, M.L. Altered Patterns of Central Executive, Default Mode and Salience Network Activity and Connectivity Are Associated with Current and Future Depression Risk in Two Independent Young Adult Samples. Mol. Psychiatry 2023, 28, 1046–1056. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, Y.; Wu, X.; Huang, H.; Jia, Y.; Zhong, S.; Wu, X.; Zhao, L.; He, Y.; Huang, L.; et al. Shared and Specific Functional Connectivity Alterations in Unmedicated Bipolar and Major Depressive Disorders Based on the Triple-Network Model. Brain Imaging Behav. 2020, 14, 186–199. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhong, Y.; Wang, H.; Yan, B.; Zheng, K.; Wei, L.; Lu, H.; Li, B. Causal Interactions Between the Default Mode Network and Central Executive Network in Patients with Major Depression. Neuroscience 2021, 475, 93–102. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, S.; Zhao, P.; Cao, Q.; Lu, Q.; Yao, Z. Association Between Antidepressant Efficacy and Interactions of Three Core Depression-Related Brain Networks in Major Depressive Disorder. Front. Psychiatry 2022, 13. [Google Scholar] [CrossRef]
- Menon, V. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, S.; Yin, S.; Ren, W.; He, R.; Li, J. The Fronto-Insular Cortex Causally Mediates the Default-Mode and Central-Executive Networks to Contribute to Individual Cognitive Performance in Healthy Elderly. Hum. Brain Mapp. 2018, 39, 4302–4311. [Google Scholar] [CrossRef]
- Mayberg, S. Limbic-Cortical Dysregulation: Depression. J. Neuropsychiatr. 1997, 9, 471–481. [Google Scholar] [CrossRef]
- Baker, J.T.; Dillon, D.G.; Patrick, L.M.; Roffman, J.L.; Brady, R.O.; Pizzagalli, D.A.; Öngür, D.; Holmes, A.J. Functional Connectomics of Affective and Psychotic Pathology. Proc. Natl. Acad. Sci. 2019, 116, 9050–9059. [Google Scholar] [CrossRef] [PubMed]
- Dahl, C.J.; Wilson-Mendenhall, C.D.; Davidson, R.J. The Plasticity of Well-Being: A Training-Based Framework for the Cultivation of Human Flourishing. Proc. Natl. Acad. Sci. 2020, 117, 32197–32206. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Xu, Y.; Zhou, W.; Chen, J.; Wei, N.; Lu, S.; Shang, D.; Wang, J.; Huang, M. Disrupted Intrinsic Functional Connectivity of the Cognitive Control Network Underlies Disease Severity and Executive Dysfunction in First-Episode, Treatment-Naive Adolescent Depression. J. Affect. Disord. 2020, 264, 455–463. [Google Scholar] [CrossRef]
- Sundermann, B.; Beverborg, M.O. lütke; Pfleiderer, B. Meta-Analysis of Resting-State FMRI in Depression: Generating Spatial Hypotheses for Potential Clinical Applications. PeerJ Prepr. 2014, 1–25. [CrossRef]
- Zhou, H.-X.; Chen, X.; Shen, Y.-Q.; Li, L.; Chen, N.-X.; Zhu, Z.-C.; Castellanos, F.X.; Yan, C.-G. Rumination and the Default Mode Network: Meta-Analysis of Brain Imaging Studies and Implications for Depression. Neuroimage 2020, 206, 116287. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A Critical Role for the Right Fronto-Insular Cortex in Switching between Central-Executive and Default-Mode Networks. Proc. Natl. Acad. Sci. 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Manoliu, A.; Meng, C.; Brandl, F.; Doll, A.; Tahmasian, M.; Scherr, M.; Schwerthöffer, D.; Zimmer, C.; Förstl, H.; Bäuml, J.; et al. Insular Dysfunction within the Salience Network Is Associated with Severity of Symptoms and Aberrant Inter-Network Connectivity in Major Depressive Disorder. Front. Hum. Neurosci. 2013, 7, 930. [Google Scholar] [CrossRef]
- Hugdahl, K.; Kazimierczak, K.; Beresniewicz, J.; Kompus, K.; Westerhausen, R.; Ersland, L.; Grüner, R.; Specht, K. Dynamic Up- and down-Regulation of the Default (DMN) and Extrinsic (EMN) Mode Networks during Alternating Task-on and Task-off Periods. PLoS One 2019, 14, e0218358. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 1–13. [Google Scholar] [CrossRef]
- Yokoyama, S.; Okamoto, Y.; Takagaki, K.; Okada, G.; Takamura, M.; Mori, A.; Shiota, S.; Ichikawa, N.; Jinnin, R.; Yamawaki, S. Effects of Behavioral Activation on Default Mode Network Connectivity in Subthreshold Depression: A Preliminary Resting-State FMRI Study. J. Affect. Disord. 2018, 227, 156–163. [Google Scholar] [CrossRef]
- Guha, A.; Yee, C.M.; Heller, W.; Miller, G.A. Alterations in the Default Mode-Salience Network Circuit Provide a Potential Mechanism Supporting Negativity Bias in Depression. Psychophysiology 2021, 58. [Google Scholar] [CrossRef] [PubMed]
- Krönke, K.-M.; Wolff, M.; Shi, Y.; Kräplin, A.; Smolka, M.N.; Bühringer, G.; Goschke, T. Functional Connectivity in a Triple-Network Saliency Model Is Associated with Real-Life Self-Control. Neuropsychologia 2020, 149, 107667. [Google Scholar] [CrossRef] [PubMed]
- Malejko, K.; Brown, R.C.; Plener, P.L.; Bonenberger, M.; Graf, H.; Abler, B. Differential Neural Processing of Unpleasant Sensory Stimulation in Patients with Major Depression. Eur. Arch. Psychiatry Clin. Neurosci. 2021, 271, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Macêdo, M.A.; Sato, J.R.; Bressan, R.A.; Pan, P.M. Adolescent Depression and Resting-State FMRI Brain Networks: A Scoping Review of Longitudinal Studies. Brazilian J. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The Role of the Salience Network in Cognitive and Affective Deficits. Front. Hum. Neurosci. 2023, 17. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.; Cha, J.; Choi, K.; Nemeroff, C.; Craighead, W.E.; Mayberg, H. Changes in Functional Connectivity in Remitters to CBT Versus Pharmacotherapy for Depression. Biol. Psychiatry 2022, 91, S51–S52. [Google Scholar] [CrossRef]
- Sun, J.; Ma, Y.; Guo, C.; Du, Z.; Chen, L.; Wang, Z.; Li, X.; Xu, K.; Luo, Y.; Hong, Y.; et al. Distinct Patterns of Functional Brain Network Integration between Treatment-Resistant Depression and Non Treatment-Resistant Depression: A Resting-State Functional Magnetic Resonance Imaging Study. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2023, 120, 110621. [Google Scholar] [CrossRef]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and Emotional Influences in Anterior Cingulate Cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Johnstone, T.; van Reekum, C.M.; Urry, H.L.; Kalin, N.H.; Davidson, R.J. Failure to Regulate: Counterproductive Recruitment of Top-Down Prefrontal-Subcortical Circuitry in Major Depression. J. Neurosci. 2007, 27, 8877–8884. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Wang, X.; Zhu, X.; Zhong, M.; Yi, J.; Rao, H.; Yao, S. First-Episode Medication-Naive Major Depressive Disorder Is Associated with Altered Resting Brain Function in the Affective Network. PLoS One 2014, 9, e85241. [Google Scholar] [CrossRef]
- Compère, L.; Siegle, G.J.; Riley, E.; Lazzaro, S.; Strege, M.; Pacoe, E.; Canovali, G.; Barb, S.; Huppert, T.; Young, K. Enhanced Efficacy of CBT Following Augmentation with Amygdala RtfMRI Neurofeedback in Depression. J. Affect. Disord. 2023, 339, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gorka, S.M.; Young, C.B.; Klumpp, H.; Kennedy, A.E.; Francis, J.; Ajilore, O.; Langenecker, S.A.; Shankman, S.A.; Craske, M.G.; Stein, M.B.; et al. Emotion-Based Brain Mechanisms and Predictors for SSRI and CBT Treatment of Anxiety and Depression: A Randomized Trial. Neuropsychopharmacology 2019, 44, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Doerig, N.; Krieger, T.; Altenstein, D.; Schlumpf, Y.; Spinelli, S.; Späti, J.; Brakowski, J.; Quednow, B.B.; Seifritz, E.; Holtforth, M. grosse Amygdala Response to Self-Critical Stimuli and Symptom Improvement in Psychotherapy for Depression. Br. J. Psychiatry 2016, 208, 175–181. [Google Scholar] [CrossRef]
- Rive, M.M.; van Rooijen, G.; Veltman, D.J.; .Phillips, M.L.; Schene, A.H.; Ruhé, H.G. Neural Correlates of Dysfunctional Emotion Regulation in Major Depressive Disorder. A Systematic Review of Neuroimaging Studies. Neurosci. Biobehav. Rev. 2013, 37, 2529–2553. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Ferguson, M.A.; Grafman, J.; Cohen, A.L.; Fox, M.D. A Lesion-Derived Brain Network for Emotion Regulation. Biol. Psychiatry 2023, 94, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Zilverstand, A.; Parvaz, M.A.; Goldstein, R.Z. Neuroimaging Cognitive Reappraisal in Clinical Populations to Define Neural Targets for Enhancing Emotion Regulation. A Systematic Review. Neuroimage 2017, 151, 105–116. [Google Scholar] [CrossRef]
- Siegle, G.J.; Steinhauer, S.R.; Thase, M.E.; Stenger, V.A.; Carter, C.S. Can’t Shake That Feeling: Event-Related FMRI Assessment of Sustained Amygdala Activity in Response to Emotional Information in Depressed Individuals. Biol. Psychiatry 2002, 51, 693–707. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Etkin, A.; Furman, D.J.; Lemus, M.G.; Johnson, R.F.; Gotlib, I.H. Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data. Am. J. Psychiatry 2012, 169, 693–703. [Google Scholar] [CrossRef]
- Dunlop, B.W.; Cha, J.; Choi, K.S.; Nemeroff, C.B.; Craighead, W.E.; Mayberg, H.S. Functional Connectivity of Salience and Affective Networks among Remitted Depressed Patients Predicts Episode Recurrence. Neuropsychopharmacology 2023, 48, 1901–1909. [Google Scholar] [CrossRef]
- Amft, M.; Bzdok, D.; Laird, A.R.; Fox, P.T.; Schilbach, L.; Eickhoff, S.B. Definition and Characterization of an Extended Social-Affective Default Network. Brain Struct. Funct. 2015, 220, 1031–1049. [Google Scholar] [CrossRef]
- Göttlich, M.; Ye, Z.; Rodriguez-Fornells, A.; Münte, T.F.; Krämer, U.M. Viewing Socio-Affective Stimuli Increases Connectivity within an Extended Default Mode Network. Neuroimage 2017, 148, 8–19. [Google Scholar] [CrossRef]
- Manoliu, A.; Riedl, V.; Zherdin, A.; Mühlau, M.; Schwerthöffer, D.; Scherr, M.; Peters, H.; Zimmer, C.; Förstl, H.; Bäuml, J.; et al. Aberrant Dependence of Default Mode/Central Executive Network Interactions on Anterior Insular Salience Network Activity in Schizophrenia. Schizophr. Bull. 2014, 40, 428–437. [Google Scholar] [CrossRef]
- Delaveau, P.; Arruda Sanchez, T.; Steffen, R.; Deschet, K.; Jabourian, M.; Perlbarg, V.; Gasparetto, E.L.; Dubal, S.; Costa e Silva, J.; Fossati, P. Default Mode and Task-positive Networks Connectivity during the N-Back Task in Remitted Depressed Patients with or without Emotional Residual Symptoms. Hum. Brain Mapp. 2017, 38, 3491–3501. [Google Scholar] [CrossRef]
- Marwood, L.; Wise, T.; Perkins, A.M.; Cleare, A.J. Meta-Analyses of the Neural Mechanisms and Predictors of Response to Psychotherapy in Depression and Anxiety. Neurosci. Biobehav. Rev. 2018, 95, 61–72. [Google Scholar] [CrossRef]
- DeRubeis, R.J.; Siegle, G.J.; Hollon, S.D. Cognitive Therapy vs. Medication for Depression: Treatment Outcomes and Neural Mechanisms. Nat. Rev. Neurosci. 2008, 9, 788–796. [Google Scholar] [CrossRef]
- Alescio-Lautier, B.; Chambon, C.; Deshayes, C.; Anton, J.-L.; Escoffier, G.; Ferrer, M.-H.; Paban, V. Problem-Solving Training Modifies Cognitive Functioning and Related Functional Connectivity in Healthy Adults. Neuropsychol. Rehabil. 2023, 33, 103–138. [Google Scholar] [CrossRef] [PubMed]
- Baeken, C.; Wu, G.-R.; Rogiers, R.; Remue, J.; Lemmens, G.M.; Raedt, R. De Cognitive Behavioral Based Group Psychotherapy Focusing on Repetitive Negative Thinking: Decreased Uncontrollability of Rumination Is Related to Brain Perfusion Increases in the Left Dorsolateral Prefrontal Cortex. J. Psychiatr. Res. 2021, 136, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.H.; Watkins, E.R.; Peters, A.T.; Feldhaus, C.G.; Barba, A.; Carbray, J.; Langenecker, S.A. Targeting Ruminative Thinking in Adolescents at Risk for Depressive Relapse: Rumination-Focused Cognitive Behavior Therapy in a Pilot Randomized Controlled Trial with Resting State FMRI. PLoS One 2016, 11, e0163952. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Okamoto, Y.; Okada, G.; Takagaki, K.; Jinnin, R.; Takamura, M.; Kobayakawa, M.; Yamawaki, S. Behavioral Activation Can Normalize Neural Hypoactivation in Subthreshold Depression during a Monetary Incentive Delay Task. J. Affect. Disord. 2016, 189, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Shiota, S.; Okamoto, Y.; Okada, G.; Takagaki, K.; Takamura, M.; Mori, A.; Yokoyama, S.; Nishiyama, Y.; Jinnin, R.; Hashimoto, R.; et al. The Neural Correlates of the Metacognitive Function of Other Perspective: A Multiple Regression Analysis Study. Neuroreport 2017, 28. [Google Scholar] [CrossRef]
- Cernasov, P.; Walsh, E.C.; Kinard, J.L.; Kelley, L.; Phillips, R.; Pisoni, A.; Eisenlohr-Moul, T.A.; Arnold, M.; Lowery, S.C.; Ammirato, M.; et al. Multilevel Growth Curve Analyses of Behavioral Activation for Anhedonia (BATA) and Mindfulness-Based Cognitive Therapy Effects on Anhedonia and Resting-State Functional Connectivity: Interim Results of a Randomized Trial. J. Affect. Disord. 2021. [Google Scholar] [CrossRef]
- Cernasov, P.; Kinard, J.; Phillips, R.; Halverson, T.; Greene, R.; Arnold, M.; Lowery, S.; Luke, S.; Kelley, L.; McLamb, M.; et al. Mindfulness-Based Cognitive Therapy Attenuates Default-Mode Network Connectivity in Patients With Clinically Significant Anhedonia. Biol. Psychiatry 2020, 87, S197. [Google Scholar] [CrossRef]
- Bessette, K.L.; Jacobs, R.H.; Heleniak, C.; Peters, A.T.; Welsh, R.C.; Watkins, E.R.; Langenecker, S.A. Malleability of Rumination: An Exploratory Model of CBT-Based Plasticity and Long-Term Reduced Risk for Depressive Relapse among Youth from a Pilot Randomized Clinical Trial. PLoS One 2020, 15, e0233539. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, A.M.; Scholl, J.; Elmholdt, E.-M.; Fjorback, L.O.; Harmer, C.J.; Lazar, S.W.; O’Toole, M.S.; Smallwood, J.; Roepstorff, A.; Kuyken, W. Mindfulness Training Changes Brain Dynamics During Depressive Rumination: A Randomized Controlled Trial. Biol. Psychiatry 2023, 93, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bremer, B.; Wu, Q.; Mora Álvarez, M.G.; Hölzel, B.K.; Wilhelm, M.; Hell, E.; Tavacioglu, E.E.; Torske, A.; Koch, K. Mindfulness Meditation Increases Default Mode, Salience, and Central Executive Network Connectivity. Sci. Rep. 2022, 12, 13219. [Google Scholar] [CrossRef]
- Roiser, J.P.; Sahakian, B.J. Hot and Cold Cognition in Depression. CNS Spectr. 2013, 18, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Dunlop, B.W.; Cha, J.; Choi, K.S.; Rajendra, J.K.; Nemeroff, C.B.; Craighead, W.E.; Mayberg, H.S. Shared and Unique Changes in Brain Connectivity Among Depressed Patients After Remission With Pharmacotherapy Versus Psychotherapy. Am. J. Psychiatry 2023, 180, 218–229. [Google Scholar] [CrossRef]
- Trambaiolli, L.R.; Kohl, S.H.; Linden, D.E.J.; Mehler, D.M.A. Neurofeedback Training in Major Depressive Disorder: A Systematic Review of Clinical Efficacy, Study Quality and Reporting Practices. Neurosci. Biobehav. Rev. 2021, 125, 33–56. [Google Scholar] [CrossRef]
- Barbey, A.K.; Koenigs, M.; Grafman, J. Dorsolateral Prefrontal Contributions to Human Working Memory. Cortex 2013, 49, 1195–1205. [Google Scholar] [CrossRef]
- Backes, H.; Dietsche, B.; Nagels, A.; Stratmann, M.; Konrad, C.; Kircher, T.; Krug, A. Increased Neural Activity during Overt and Continuous Semantic Verbal Fluency in Major Depression: Mainly a Failure to Deactivate. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Hoy, K.E.; Daskalakis, Z.J.; Fitzgerald, P.B. Repetitive Transcranial Magnetic Stimulation for Treatment Resistant Depression: Re-Establishing Connections. Clin. Neurophysiol. 2016, 127, 3394–3405. [Google Scholar] [CrossRef] [PubMed]
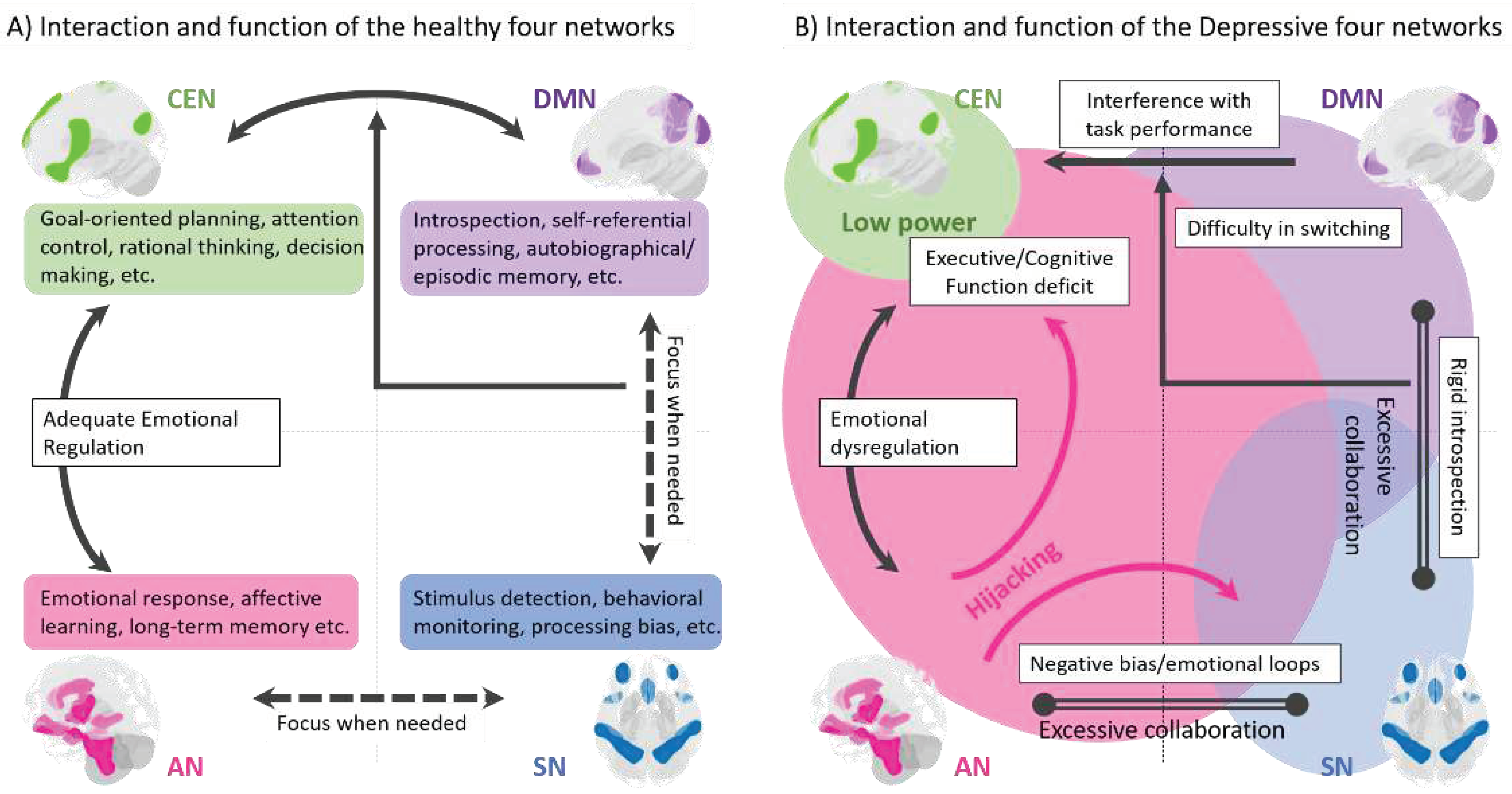
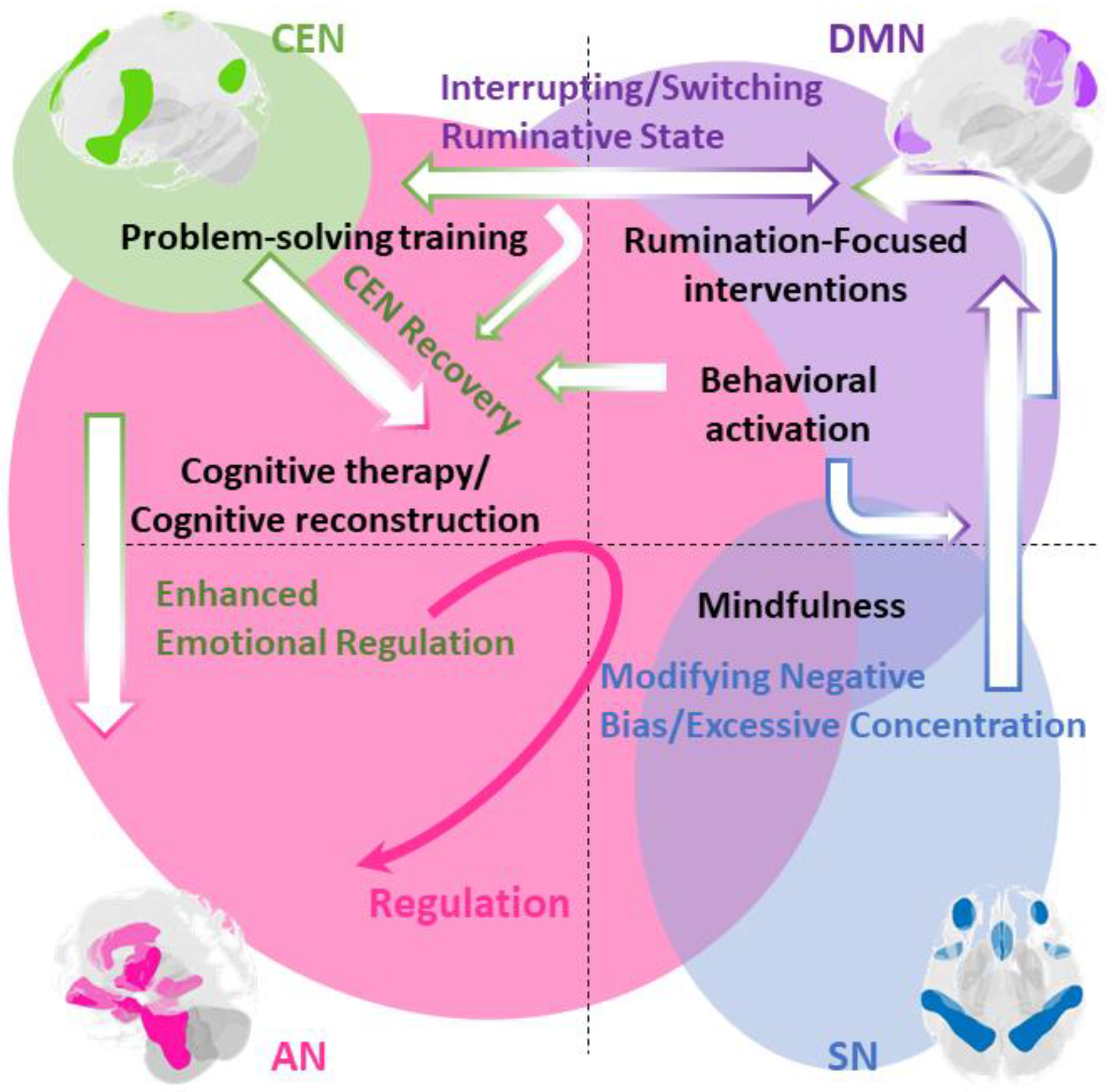
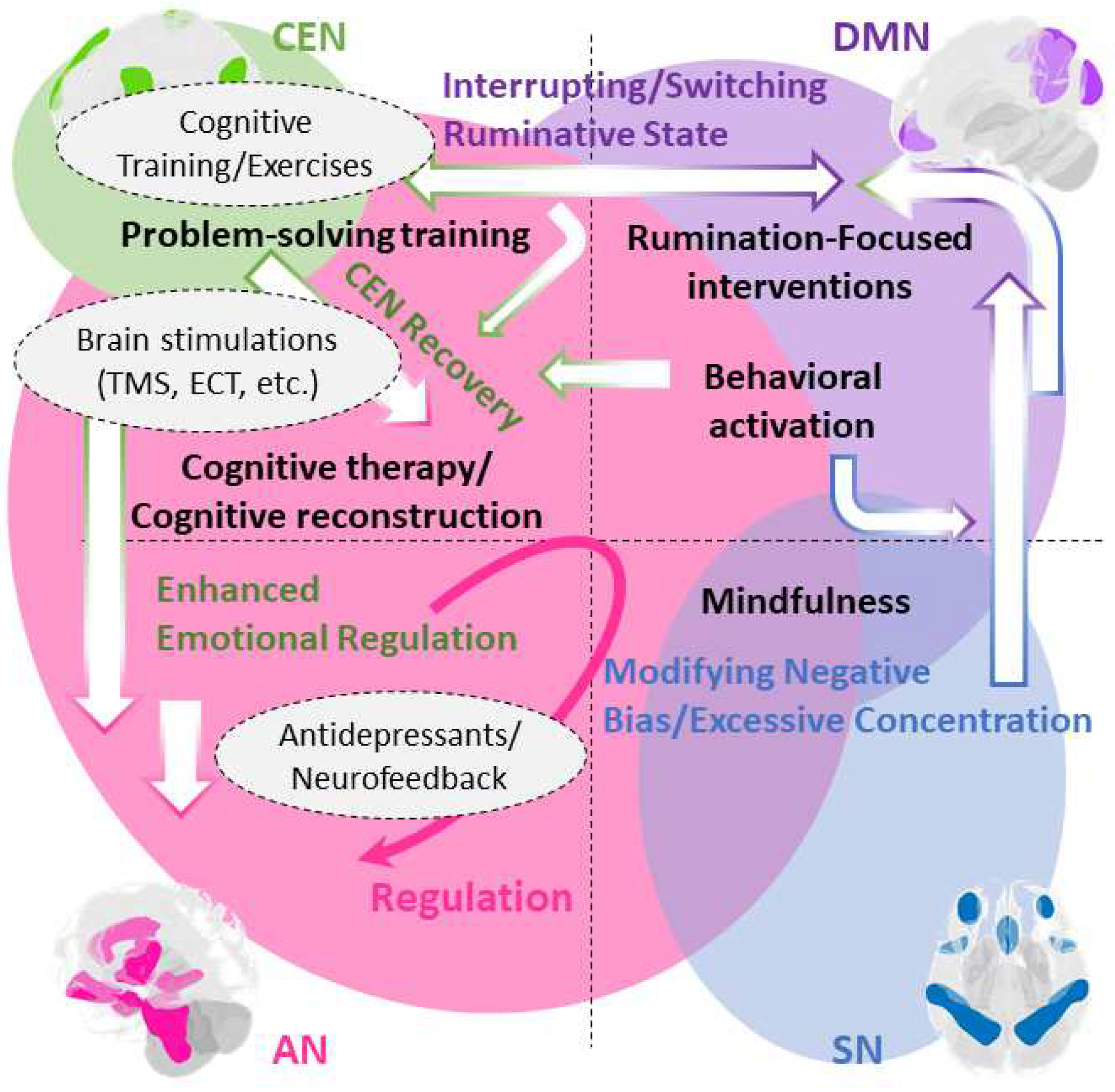
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




