1. Introduction
The sensitive and selective detection of small biomolecules in complex biological samples for clinical diagnosis has become of a great interest in the past few decades [
1]. As one kind of import dopamine (DA), is the most basic of the catecholamine neurotransmitters in the central nervous system. The determination of dopamine levels in the human body has a substantial level of interest due to its involvement in some psychiatric and neurological disorders such as Parkinson’s diseases and schizophrenia [
2]. The abnormal DA level indicates the state of human health and is used as a monitor of some diseases such as Parkinson’s schizophrenia diseases and HIV infection [
3]. Uric acid is the main end product in human urine nucleoside metabolism [
4]. UA concentration increase affects purine metabolism and results in many diseases such as gout, pneumonia, hyperuricemia, and Lesch-Nyhan syndrome [
5,
6]. Hence, the simultaneous and sensitive detection of Uric acid and dopamine is of crucial importance for human health monitoring [
7,
8,
9]. The determination of UA and DA has been achieved using various methods such as liquid chromatography (HLPC) [
9], fluorimetric method [
10], chemiluminescence method [
11] and electro-analytical technique.
The electrochemical methods have drawn much attention by offering major advantages including good stability, low cost, and simplicity compared to other analysis methods [
12]. Therefore, Simultaneous and sensitive analysis [
13,
14] of uric acid and dopamine [
15] is essential in the clinical diagnosis. DA and UA are both the primary products of purine metabolism. Therefore, they usually coexist in the extracellular fluid of the central nervous system and serum[
16]. At present, simultaneous and sensitive analysis of DA and UA is worthwhile for disease overseeing and is difficult because of the interference from potentially coexisting ascorbic acid (AA). The detection of DA and UA simultaneously in a mixture faces many challenges on traditional bare electrodes (metal and carbon). Given the oxidation reactions of DA and UA at the bare electrodes that occur at approximately the same potentials, also a fouling effect generally happens due to the use of bare electrodes [
17,
18]. Dealing with those difficulties demands the fabrication of modified electrodes based on novel functional electro-active materials such as metal nanoparticles (NP’s), conducting polymers, graphene, multiwall carbon nanotubes and so forth, which can sensitively and selectively observe DA and UA in their coexisting biological samples [
19,
20]. Lately, CNTs and MWCNTs have been used as electrode- modifying materials on the surface of electrodes. Modified electrodes with MWCNTs have been displayed to own enhanced sensitivity and selectivity toward the determination of many biomolecules such as phenol [
21].
Porphyrinoid system is a large family that encompasses phthalocyanines that enjoy an advantaged position [
22]. Phthalocyanines are macrocycle complexes, possess unique physicochemical characteristics, render these macrocycles valuable building blocks in materials science and have a two-dimensional 18-π electron aromatic system that is isoelectronic with that of porphyrins [
23]. Phthalocyanine derivatives that have a comparable structure to porphyrin, have been utilized in important functional materials in many fields. The effect of electron transfer abilities gives them much more useful proprieties. A metal phthalocyanine (metallophthalocyanine MPc) is a phthalocyanine containing one or two metal ions bound to a p-conjugated ligand and is well recognized for its excellent electrocatalytic activity for many reactions. M-PCs have been exploited in many fields such as optoelectronics, molecular electronics, photonics, etc... Metallophthalocyanines namely copper phthalocyanine (CuPc) and cobalt phthalocyanines (CoPc) have been widely used to fabricate electrochemical and biosensors and presented a significant catalytic activity for many compounds owing to their unique structural characteristics of aromatic heterocyclic molecules with high symmetry [
24]. Newly, many reports have shown that MPc-CNTs hybrids show improved electrochemical responses in comparison to the CNTs or MPc used alone [
25,
26].
In this work, a glassy carbon electrode (GCE) was coated with a novel metal phthalocyanine attached to an acrylate polymer and MWCNTs to detect DA and UA simultaneously. Before the validation of the efficacity of the developed sensor, the sensitive layer was characterized electrochemically. Several parameters were studied such as pH and scan rate influence. The fabricated sensor exhibits good potential in the determination of UA and DA.
2. Expremental
2.1. Reagents and Solutions
Multi-walled carbon nanotubes (purified to more than 95%) with an average diameter of 10 nm and an average length of 1.5µm have been purchased from DROP SENS. Dopamine, uric acid, ascorbic acid were obtained from Sigma-Aldrich and used as received. Copper monoamide-phthalocyanine functionalized acrylate polymer (Cu-Pc-mono amide–PnBA) was synthesized by the Electroanalytical Laboratory, Applied Organic Chemistry Department, National Research Centre, Dokki, Cairo, Egypt. Phosphate buffer 0.1 M (pH 7.2) was prepared by mixing Na2HPO4 and Na2H2PO4. Stock solutions of uric acid (10-2 mol L-1) and dopamine (10-2 mol L-1) were prepared daily by dissolving Uric acid and dopamine hydrochloride in distilled water. Prepared solutions were protected from light and stored at 4°C. Sample solutions were made by appropriate dilution to the desired concentration with distilled water. All experiments were realizsed at room temperature.
2.2. Apparatuses
All electrochemical measurements, differential pulse, cyclic voltammetry, and electrochemical impedance were performed using an electrochemical workstation VMP3 potentiostat, which was monitored by an integrated potentiostat/galvanostat system eDAQ (ER466E) with Echem software system and an SP-300 Bio-Logic Potentiostat/Galvanostat software in phosphate buffer solution (0.1M, pH 7,2), with a conventional three-electrode cell (5 mL) at room temperature. The working electrode was a modified glassy carbon electrode (3 mm diameter), while the counter and reference electrodes were platinum wire and a regular Ag/AgCl electrode (filled with 3 M KCl). The glassy carbon electrode was polished with 1.0, 0.3, and 0.05 µm alumina powders, then sonicated in acetone and distilled water and dried at room temperature before modification. All potentials are expressed in terms of Ag/AgCl. The SEM characterization of the samples was performed by a JEOL JSM-7600F, field emission scanning electron microscope using 5 kV electron acceleration voltage. A UV-Visible spectrophotometer Model S-2150 UV-Vis spectrophotometer was used to record optical absorption spectra (Cairo-Egypt). In a quartz cell, samples were loaded and measurements were taken over the wavelength range of 250–800 nm. All experiments were performed under the same conditions of room temperature (25 °C).
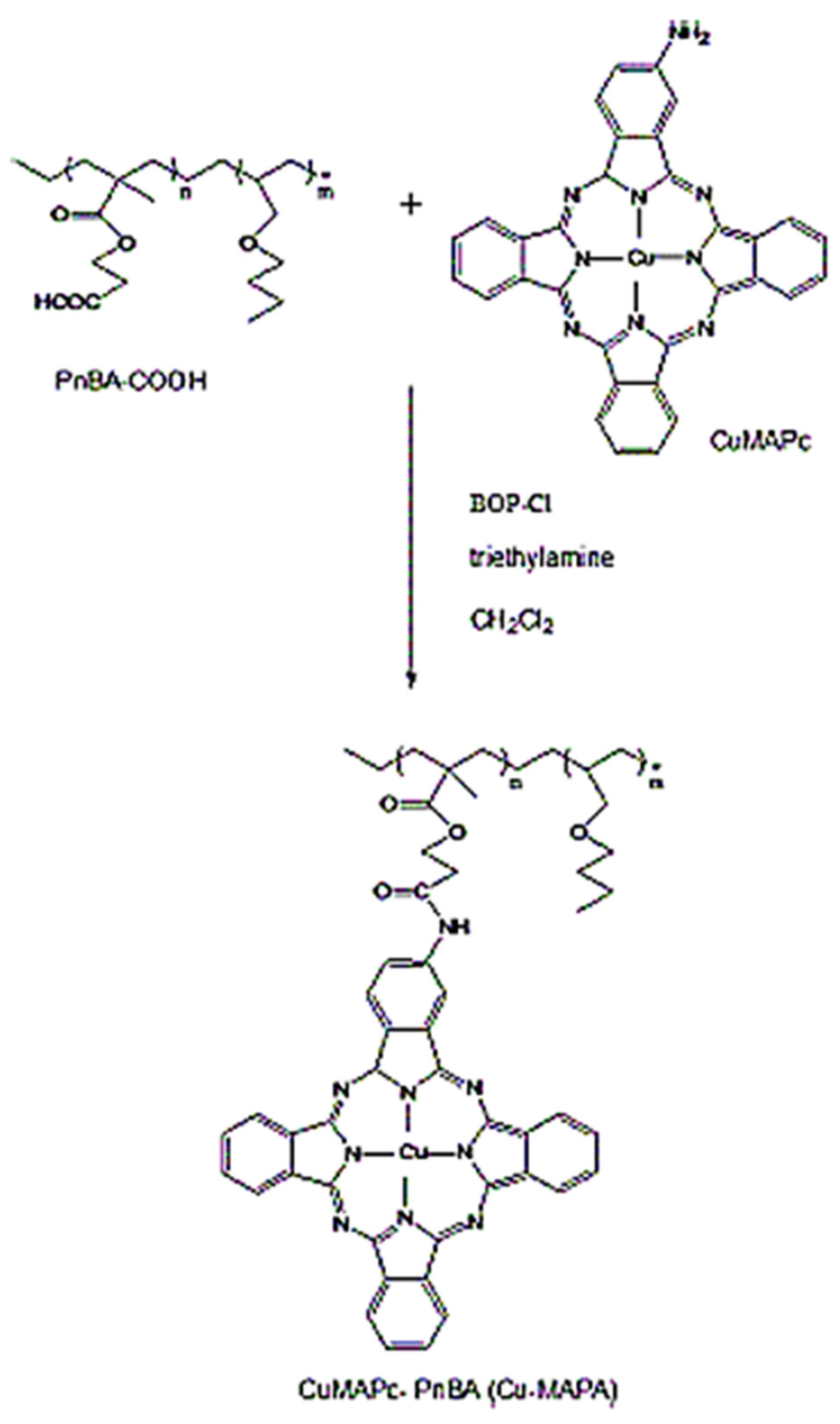
2.3. Synthesis of Cu-Pc-Mono Amide–PnBA
The novel copper phthalocyanine was synthesized with meticulous precision at the esteemed National Research Center of Cairo. The synthesis encompassed the production of two distinct entities: CuMAPc (ionophore I) and CuMAPc covalently tethered to PnBA–COOH polymer (ionophore II). The ensuing compounds were subjected to rigorous scrutiny employing fundamental analytical techniques, including basic analysis, UV-VIS, and IR spectroscopy, by a meticulously documented protocol [
27]. In a succinct procedural overview, the synthesis of copper phthalocyanine and copper mononitro-phthalocyanine transpired after the establishment of a reaction milieu conducive to the reflux of phthalic anhydride, 4-Nitrophthalic anhydride, copper acetate monohydrate, trace amounts of ammonium molybdate tetrahydrate as a catalyst, and urea, all within a nitrobenzene solution. The nitro-substituted copper complex underwent reduction to an amino derivative through the judicious application of sodium sulfide (Na
2S). Ionophore II, represented by CuMAPc covalently bound to PnBA–COOH polymer, was meticulously obtained through the amalgamation and stirring of PnBA–COOH with BOP-Cl, tri-ethylamine, and dry CH
2Cl
2 under a controlled nitrogen atmosphere. Separation of Copper phthalocyanine ionophore I from copper mononitro-phthalocyanine was facilely accomplished via liquid chromatography on a silica gel column. The covalent attachment of CuMAPc to PnBA–was executed by effecting amidation of the carboxylic group in the n-butyl acrylate polymer with CoMAPc in CH
2Cl
2, as visually depicted in
Figure 1. The process involved dissolving the acrylate polymer PnBA-COOH in dichloromethane, followed by the gradual addition of methanol, inducing the precipitation of CuPc-amide–PnBA in an orchestrated synthesis of notable scientific significance.
3. Characterization of CuMAPc and CuMAPc-PnBA
3.1. UV-Vis Spectra
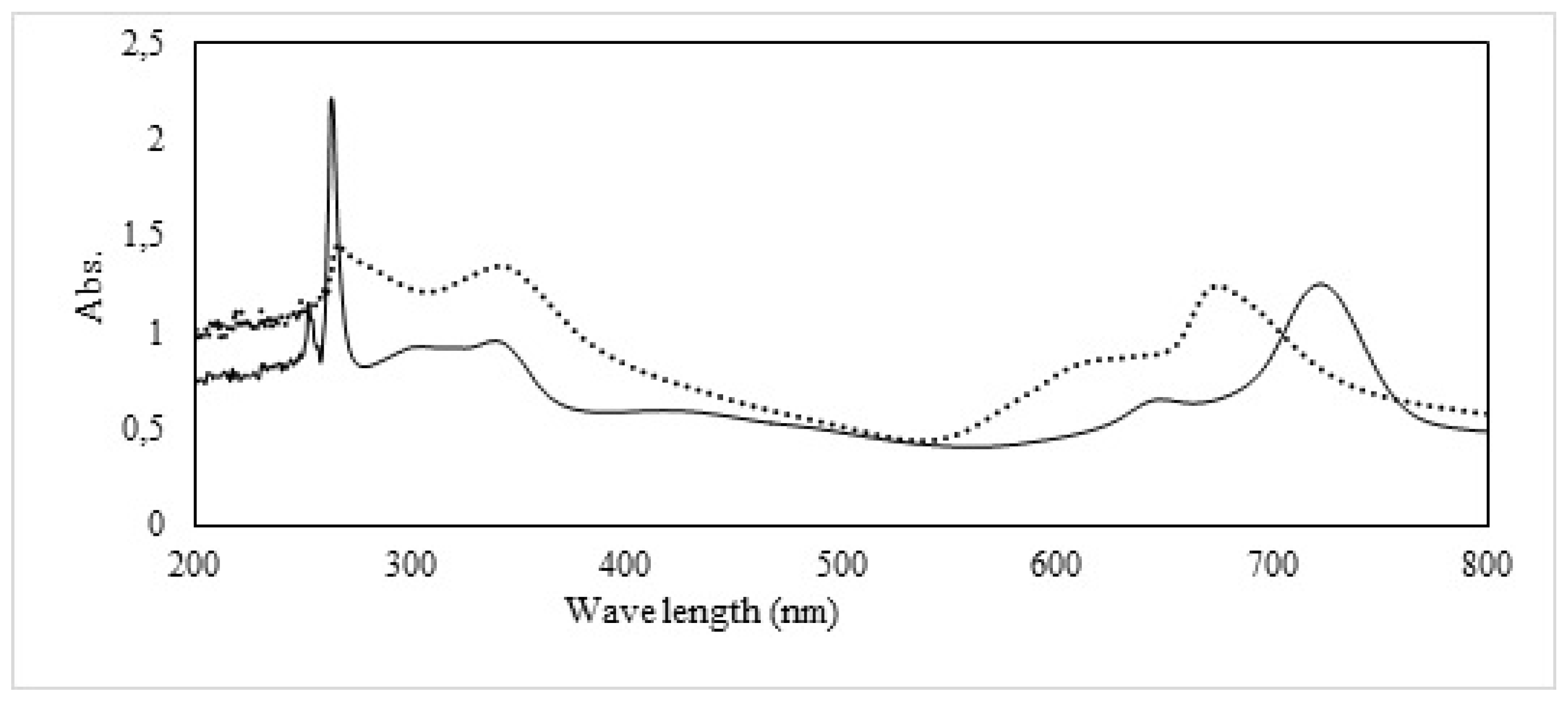
We systematically conducted UV-Vis absorption spectroscopy on both CuMAPc-PnBA and CuMAPc dispersed in dimethylformamide (DMF). The resulting UV-Vis absorption spectrum of CuMAPc is meticulously delineated in
Figure 2, revealing distinctive features, including a well-defined B-band at 264 nm, a minor absorption peak at 650 nm, and a pronounced peak at 726 nm. The CuMAPc-PnBA spectra exhibit the manifestation of two discernible peaks, one prominent at 617 nm and the other at 347 nm. Notably, an intricate interplay between the polymer matrix and the phthalocyanine moiety is elucidated through the observed spectral shifts. Specifically, the Q-band of CuMAPc-PnBA experiences a noteworthy blue shift of 47 nm compared to the absorption of CuMAPc. This observed shift signifies a systematic alteration in the electronic environment surrounding the phthalocyanine within the polymeric matrix. Additionally, the B-band of CuMAPc-PnBA is perceptibly broadened, indicating a modification in the electronic transitions and molecular interactions within the composite system. These spectroscopic findings provide crucial insights into the intimate association between the polymeric matrix and the embedded CuMAPc, offering a nuanced understanding of the spectral changes induced by their molecular interplay. The systematic analysis of these absorption spectra serves as a foundation for further elucidating the intricate photophysical properties and structural nuances inherent in the CuMAPc-PnBA composite system.
3.2. IR Spectra
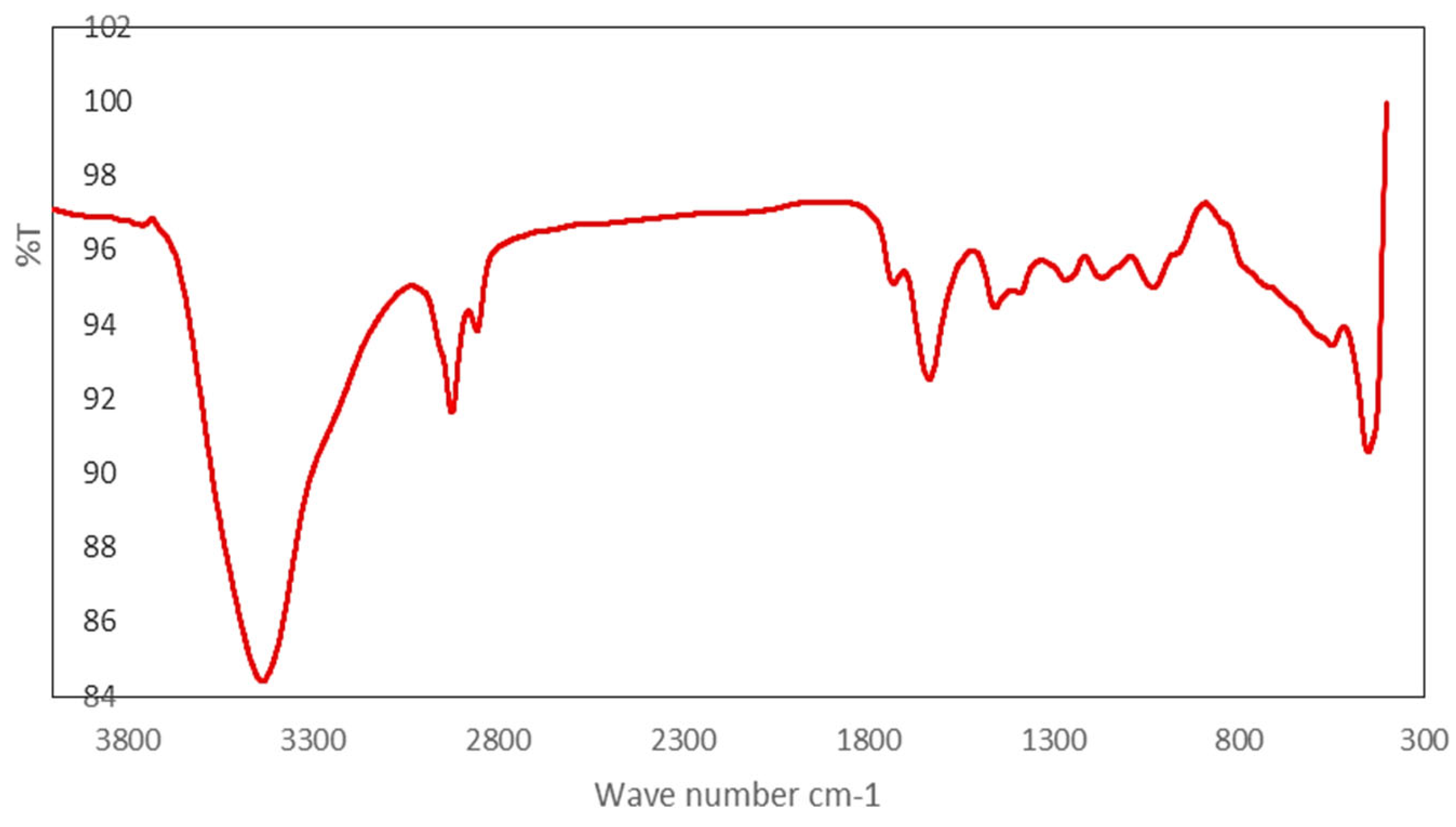
Figure 3 illustrates the infrared (IR) spectra of CuMAPc-Polymer, a pivotal examination undertaken to substantiate the successful linkage of the acrylate polymer with copper phthalocyanine. The primary objective of this spectroscopic characterization lies in assuring the veracity of the attachment between the acrylate polymer and CuMAPc. A salient feature in the IR spectrum manifests as a prominent absorption band at 1722 cm^-1, unequivocally indicative of the covalent linkage established between the acrylate polymer and CuMAPc. This specific absorption band corresponds to the characteristic vibrational mode associated with the amide bond formation (NH-C=O), providing compelling evidence for the creation of an amide bond between the acrylate polymer and CuMAPc. The discernment of this specific vibrational signature at 1722 cm
-1 serves as a conclusive confirmation of the successful formation of an amide bond, affirming the desired chemical conjugation between the acrylate polymer and CuMAPc. This thorough infrared spectroscopic analysis not only verifies the molecular connectivity but also elucidates the specific chemical nature of the formed bond, thereby enriching our understanding of the structural intricacies within the CuMAPc-Polymer composite.
Preceding to the sensor fabrication, the bare glassy carbon electrode (GCE) was polished on a polishing cloth to a mirror with alumina slurry (1µm), (0.3 µm), and (0.05 µm) respectively. Then sonicated in distilled water first followed by acetone and ethanol respectively for 5 min each and dried at room temperature. Copper monoamide phthalocyanine functionalized acrylate polymer solution was prepared by dissolving the phthalocyanine in THF tetrahydrofuran [
27]. Three types of functionalized electrodes were prepared: 1/3 CuMAPc-PnBA with 2/3 MWCNTs/GCE, 2/3 CuMAPc-PnBA with 1/3 MWCNTs/GCE, and 1/2 CuMAPc-PnBA with 1/2 MWCNTs/GCE. To prepare the Copper monoamide -phthalocyanine functionalized acrylate polymer/GCE, 5 µL of MWCNTs and CuMAPc-PnBA mixture was deposited on the surface of the glassy carbon electrode (GCE). The prepared electrodes were left to evaporate at room temperature for one night. The MWCNTs/CuMAPc-PnBA/GCE were immersed in PBS buffer before using it as the working electrode of the electrochemical measurements.
3.3. Scanning electron microscopy characterization
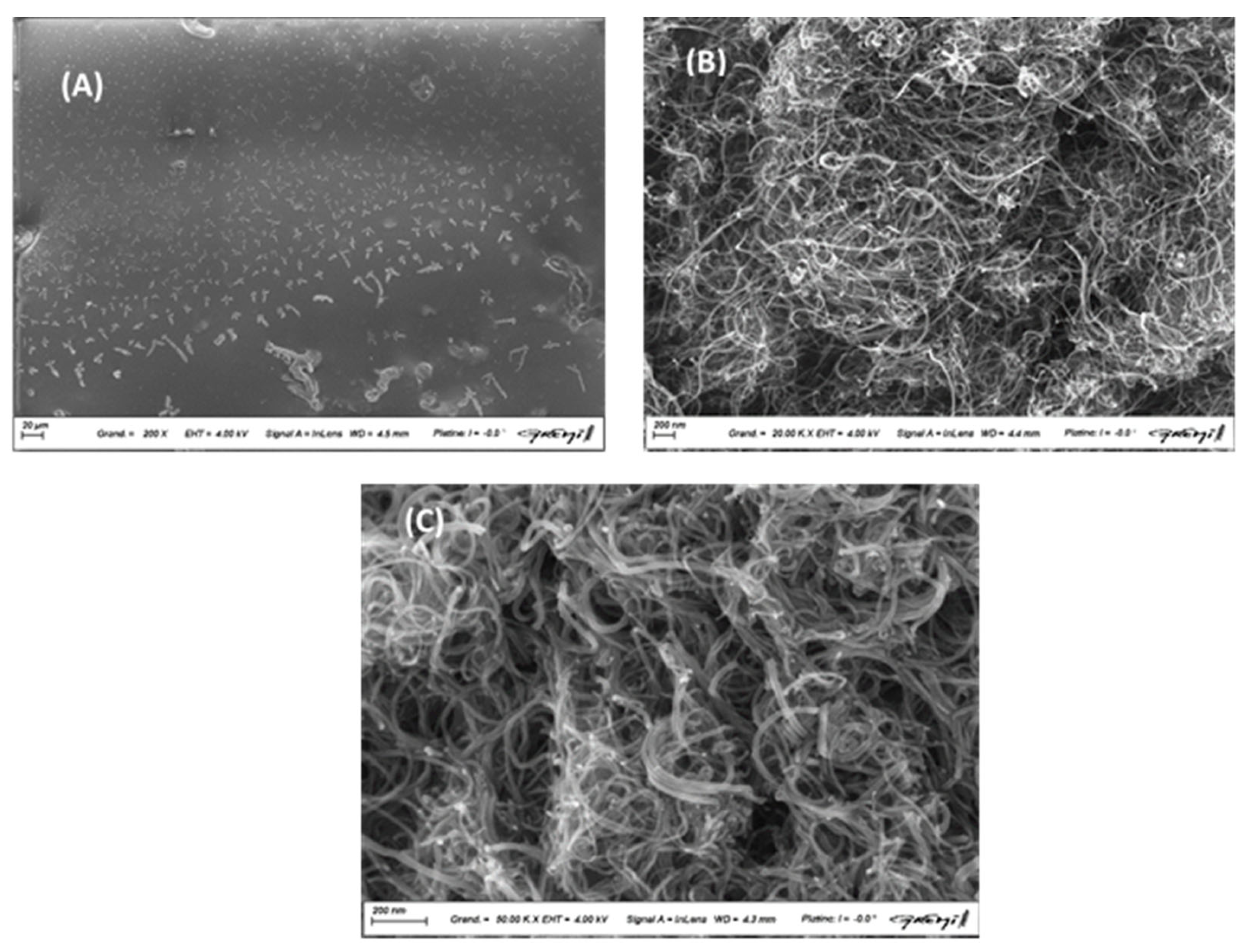
The morphology of the nano-composite layers was analyzed by scanning electron microscopy after deposition on silicon plates and drying for two days a room temperature. The CuMAPc-PnBA layer is a composite material composed of copper-phthalocyanine (CuMAPc) and p-nitrobenzoic acid (PnBA). The layer presents a rough surface due to the different characteristics of each material, which can result in a more porous structure
Figure 4A. This rough surface can increase the active area of the layer, allowing for more interaction with other materials or molecules. As seen in
Figure 4B, each pure MWCNTs is mainly a long and folded pipe with a diameter is in the 10 nm range. Observations showed a homogeneous distribution of MWCNTs in the CuMAPc-PnBA layer, forming elongated cylindrical aggregates horizontal to the surface of the thin film
Figure 4C. The presence of these aggregates provides a roughened surface, providing better grip and increased contact surface in applications where composite materials are used. These characteristics enhance the mechanical and electrical properties of the thin film CuMAPc-PnBA-MWCNTs.
4. Results and Discussion
4.1. Characterization of the MWCNTs-CuMAPc-PnBA
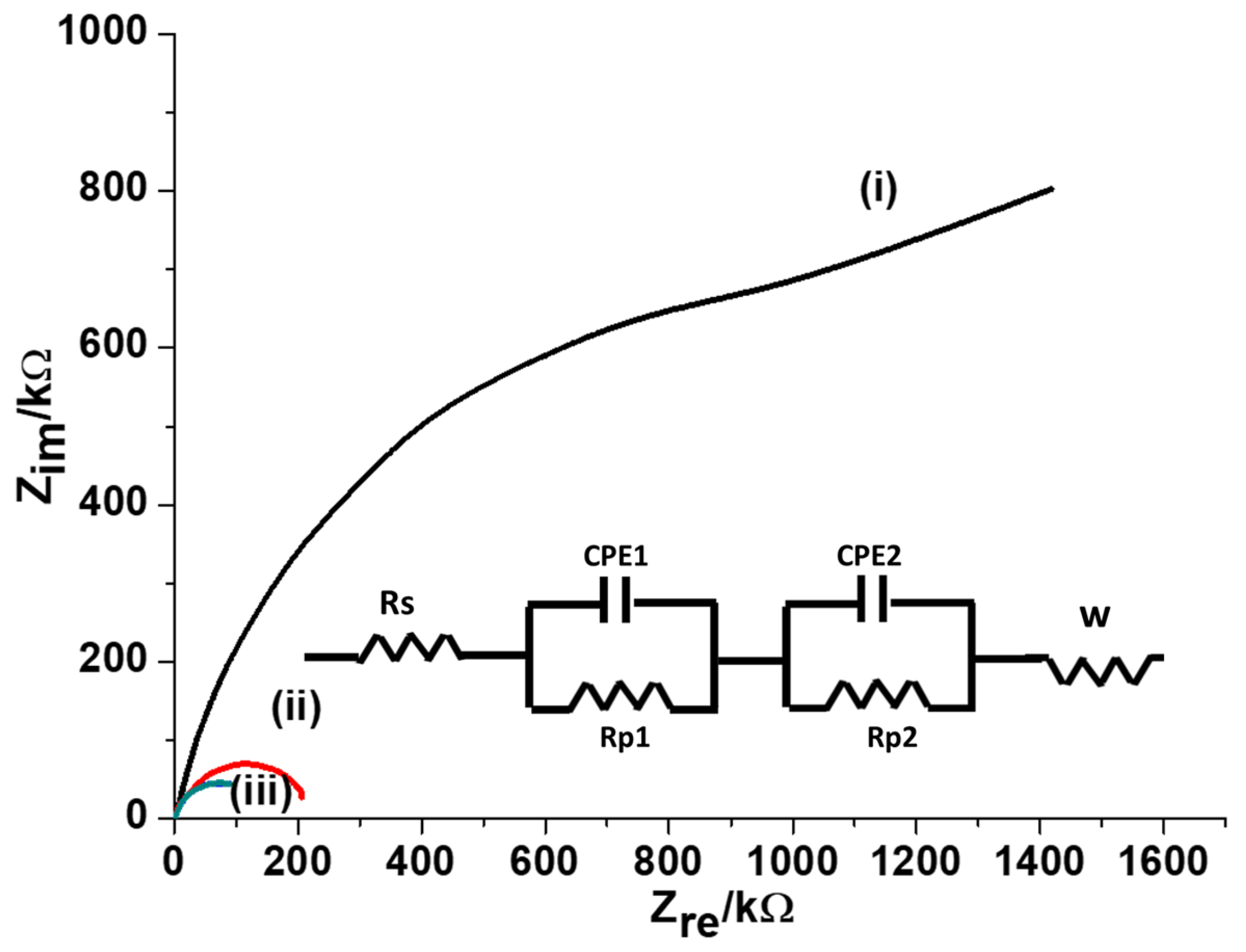
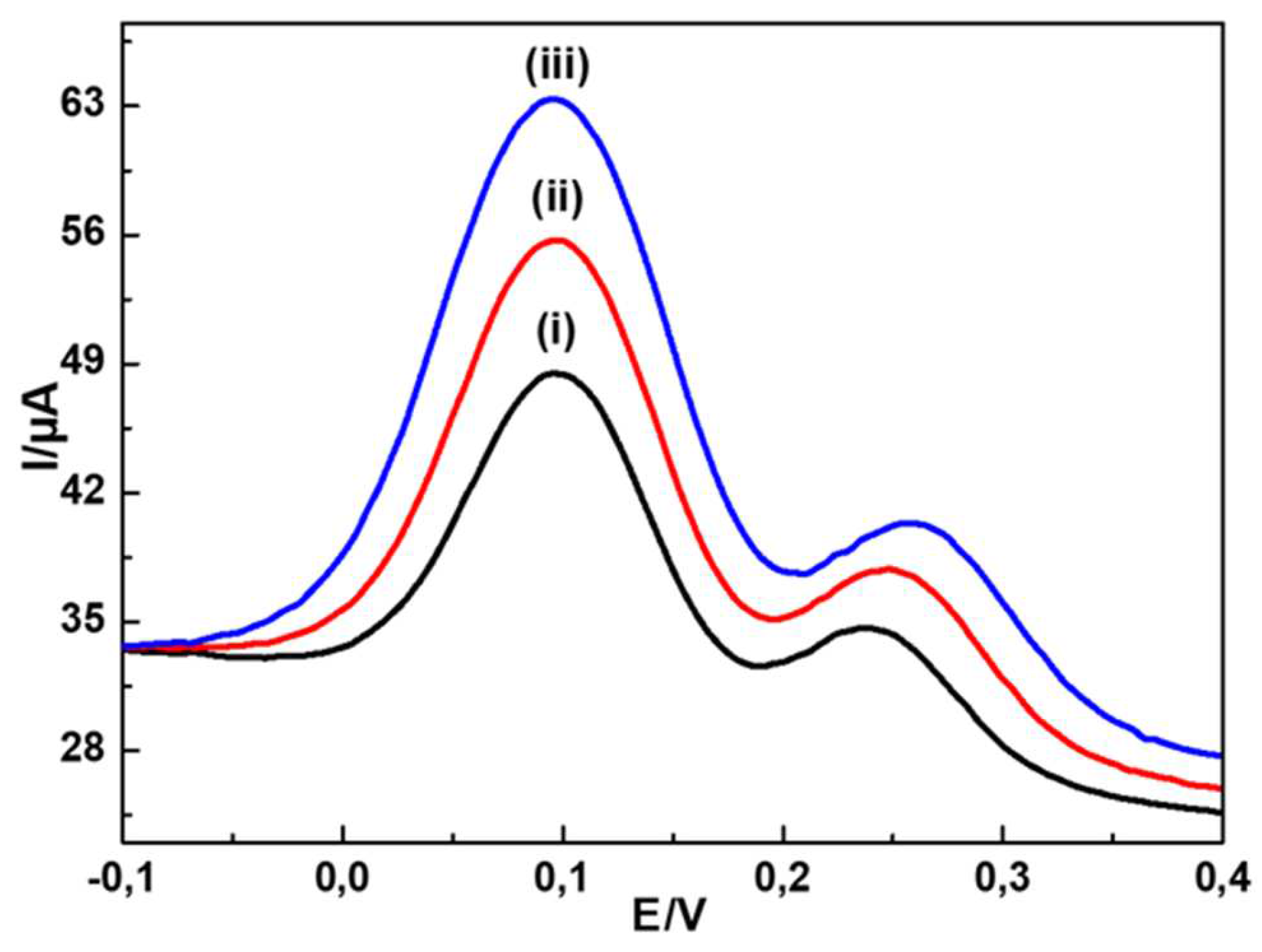
To investigate the conductivity of the modified GCE, EIS measurements were achieved in 5.0 mM [Fe(CN
6)]
3-/4- solution, the Nyquist plots were recorded using MWCNTs-CuMAPC-PnBA/GCE, CuMAPC-PnBA/GCE and a bare GCE
Figure 5. The obtained impedance curves were analyzed using the Randle circuit (equivalent circuit). R is the solution resistance, Rp
1 and Rp
2 are the polarization resistances, CPE
1 and CPE
2 are the constant phase elements and W is the Warburg element. According to Randle’s circuit, the transfer resistances of the bare GCE and CuMAPc-PnBA/GCE electrodes were much higher than MWCNTs-CuMAPC-PnBA/GCE. This proves that the MWCNTs-CuMAPc-PnBA layers enhance the conductivity of the developed sensor by making the rate of charge transfer process easier.
4.2. Optimization of Determination Conditions at the MWCNTs-CuMAPC-PnBA/GCE
4.2.1. Effect of pH
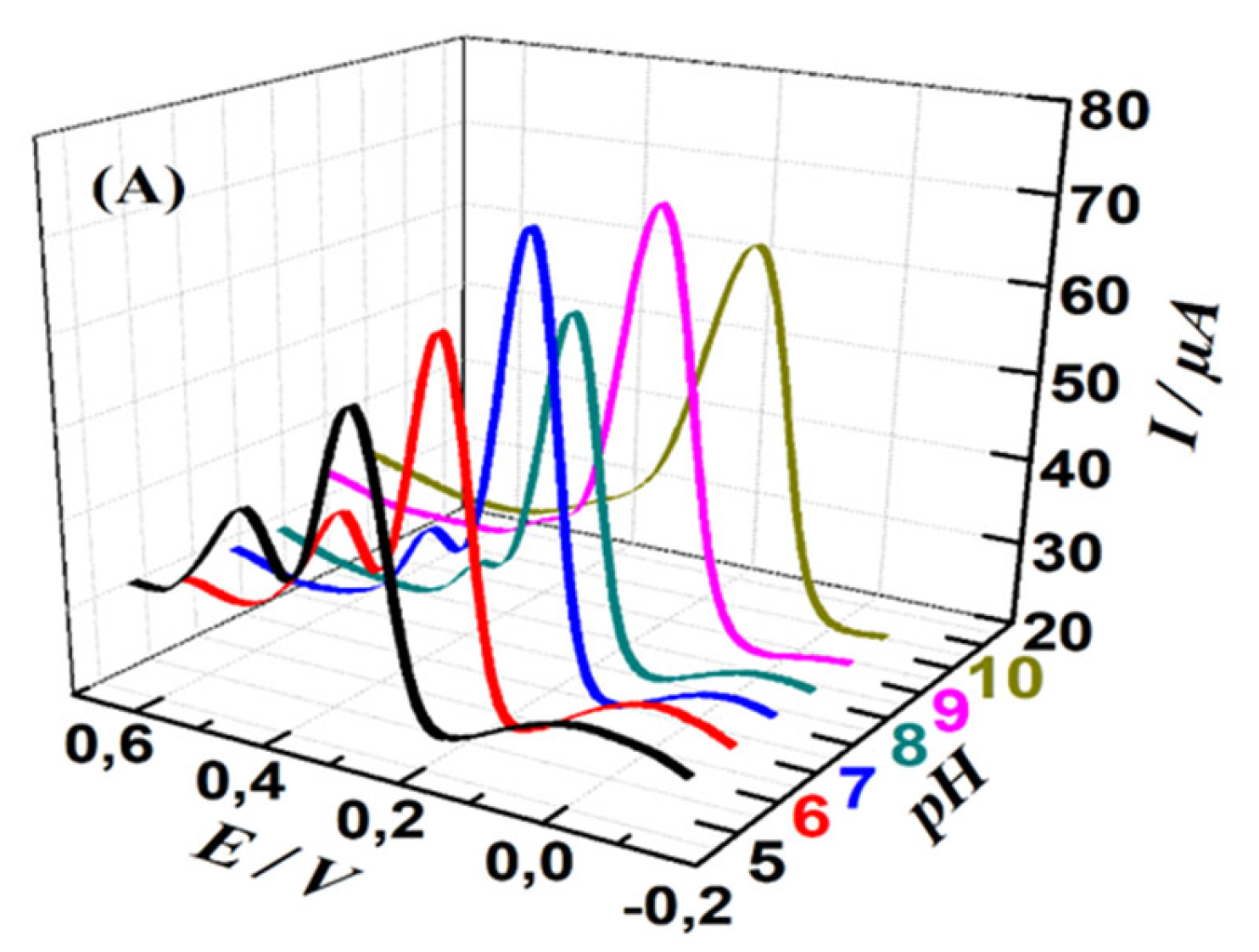
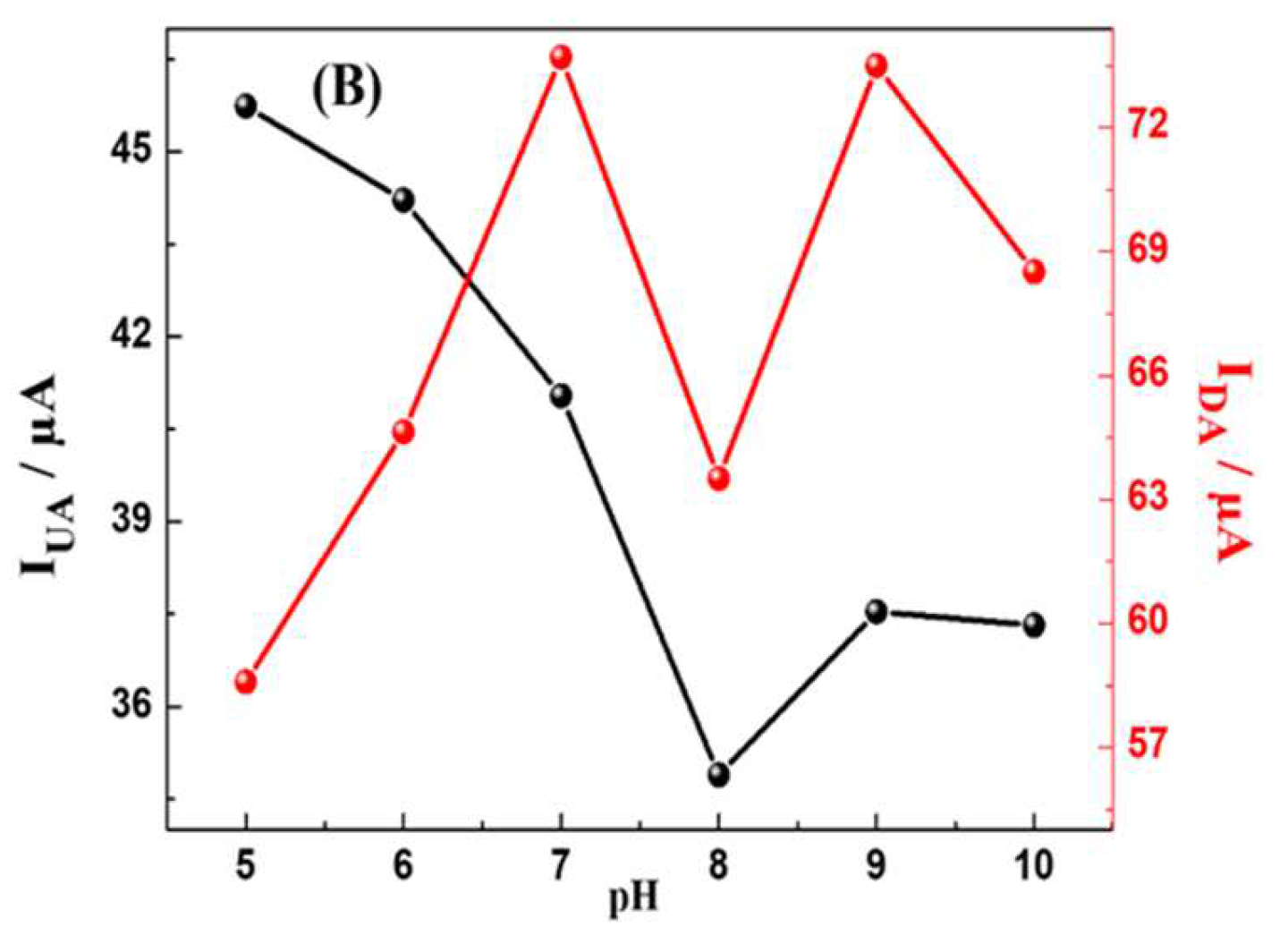
The effect of Buffer pH rate on the electrochemical responses of MWCNTs-CuMAPc-PnBA in the determination of UA and DA was investigated by DPV in the pH range of 5 to 10. As shown in
Figure 6, the maximum oxidation peak current of UA is at pH=5, then decreases within increases the pH to 8 and finally increasing again until pH =10. DA oxidation peak current reaches the maximum value at pH 7.0 and 9.0. Besides, we observe that the peak potentials of DA and UA gradually shifted negatively as the pH changed from 5 to 10, proving that the electro-oxidation of UA and DA at the MWCNTs-CuMAPc-PnBA/GCE is a proton-participated process. Thus, 0.1 M PBS solution with pH = 7.0 is chosen in the following measurements for the simultaneous and individual detection of UA and DA, and this is in consideration of physiological pH value (pH = 7.2) and to obtain relatively good sensitivity.
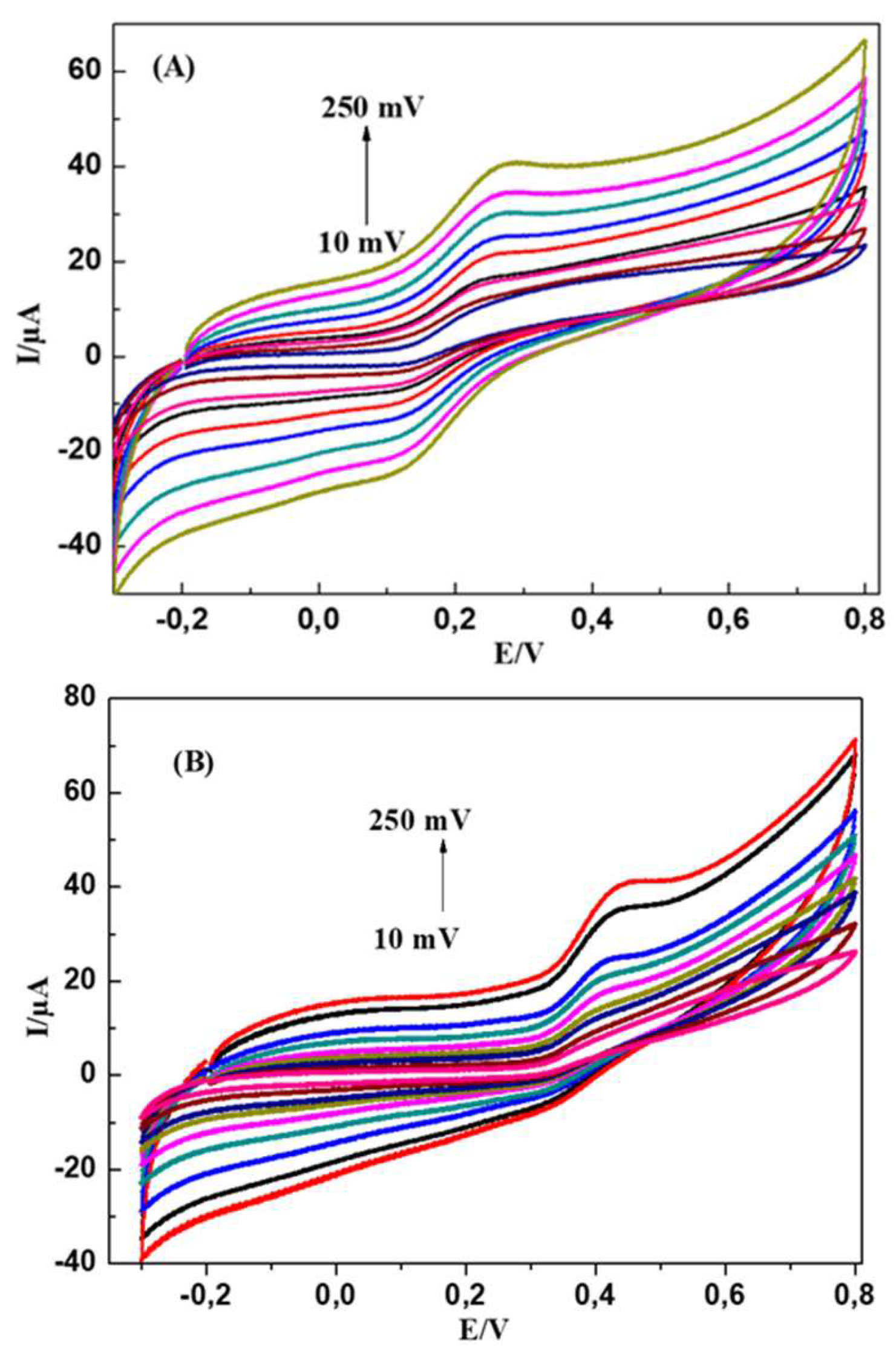
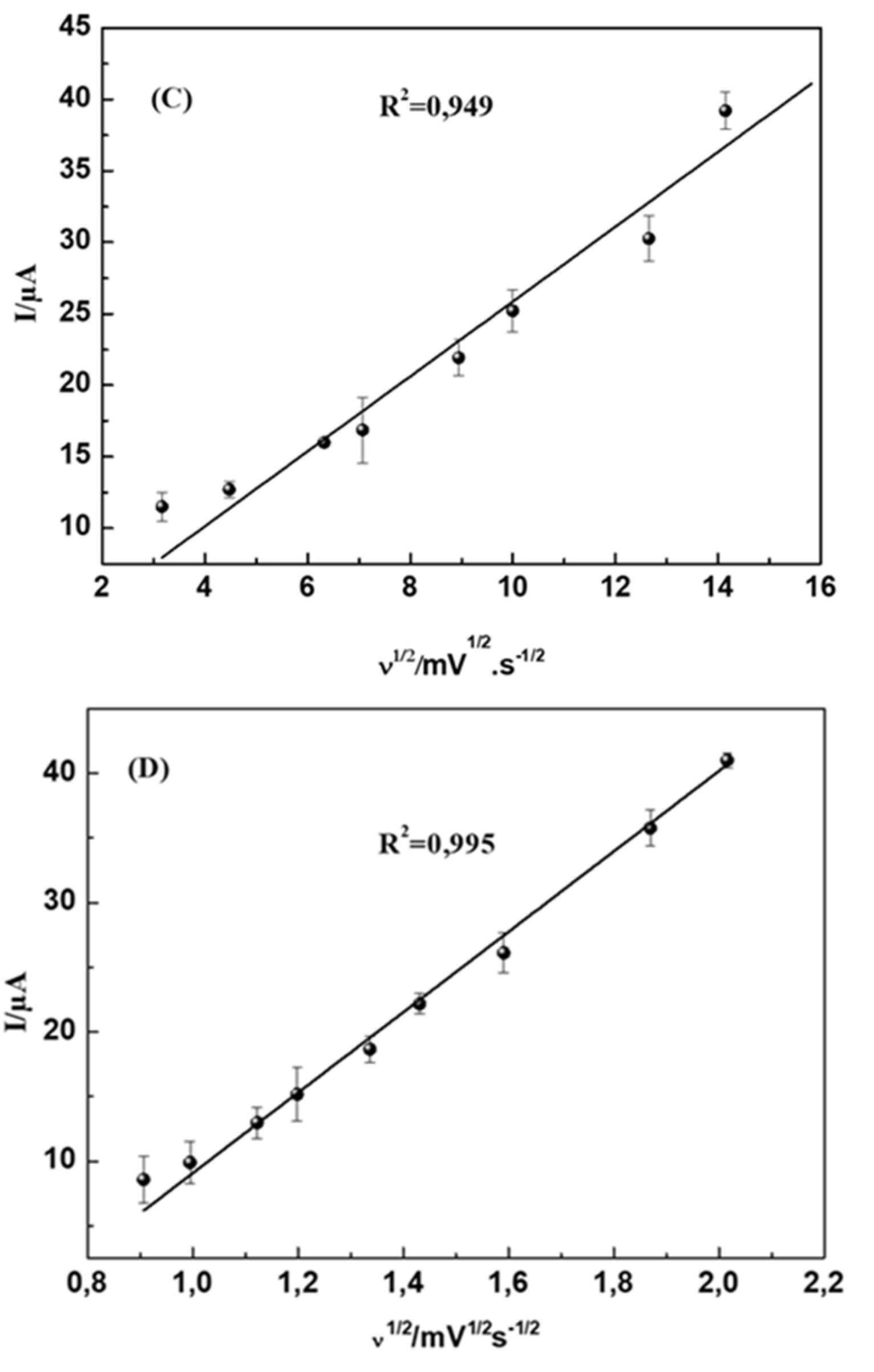
4.2.2. Influence of Potential Scan Rate
The effect of scan rate on the oxidation of DA and UA on the modified electrode was studied while the scan rate varies from 10 to 250 mV s
-1.
Figure 7 shows the effect of scan rate (v) on the electrochemical behaviour of 5 mM DA and 8 mM UA in 0.1M PBS (pH = 7.0) at MWCNTs-CuMAPC-PnBA/GCE. We observe that all the oxidation peak currents of UA and DA increases with a scan rate from 10mV s
-1 to 250 mV s
-1. Plots of the peak currents as a function of the square root of scan rate are shown in
Figure 7A, B and showed linear relationships indicating that the reactions of the two molecules DA and UA on MWCNTs-CuMAPC-PnBA/GCE are diffusion-controlled processes.
4.2.3. Optimization of the Ratio CuMAPc-PnBA / MWCNTs on the GCE
To investigate the ideal amount of MWCNTs, the ratio of MWCNTs to CuMAPc-PnBA was changed from V: 3V to 3V: V as presented in
Figure 8. The increase in the amount of MWCNTs involves the increase in the peak current. It reached the maximum when the ratio of MWCNTs to CuMAPc-PnBA was one. Thus, the optimal ratio of MWCNTs to CuMAPc-PnBA was V: V. This amount was used to carry out all the following experiments.
4.2.4. Electro-Catalytic Oxidation of UA and DA on the Modified Electrode
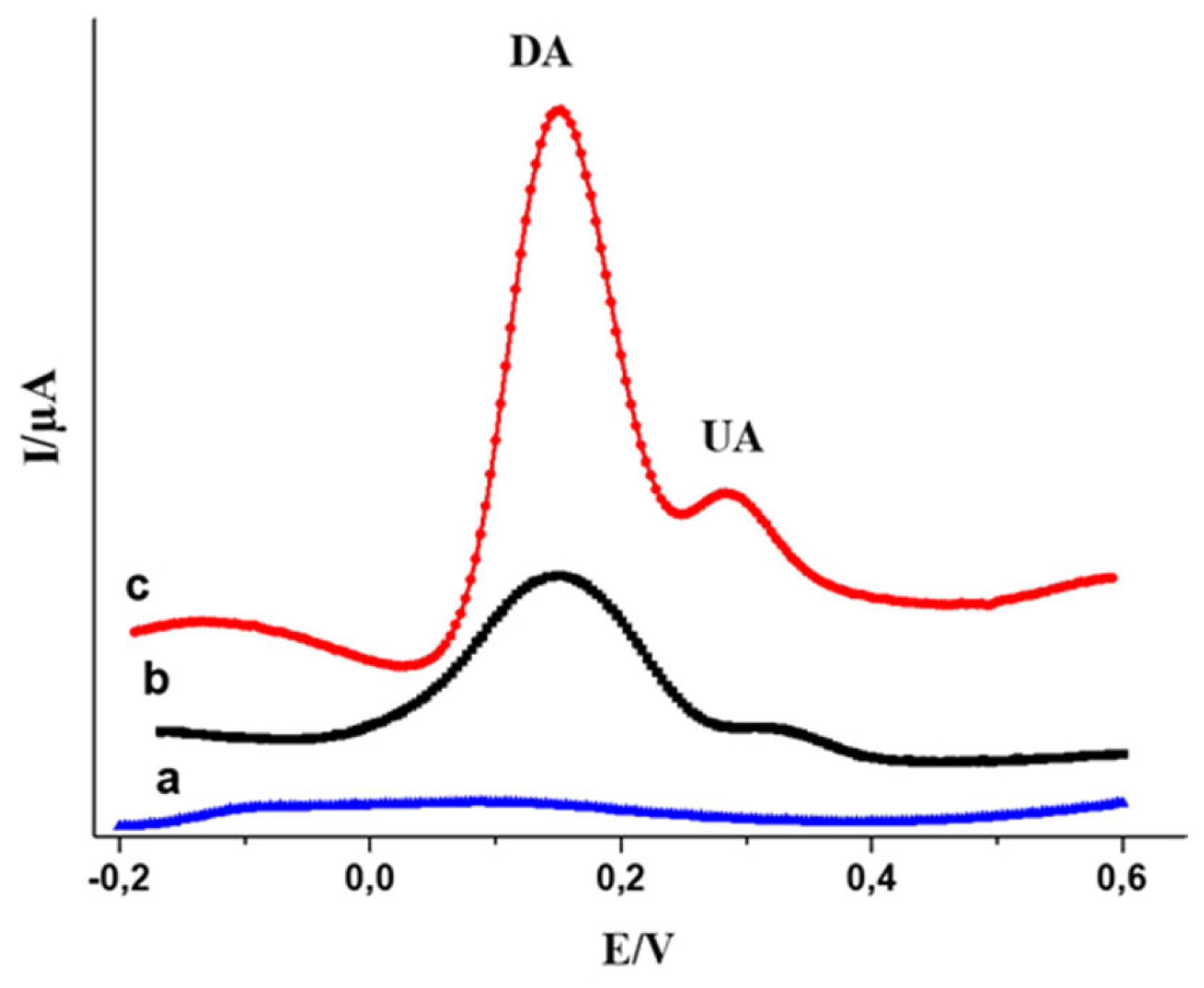
The electrocatalytic efficiency of the MWCNTs-CuMAPc-PnBA/GCE for the electro-oxidation of UA and DA was proved by recording the DPV and CV curves of the bare and modified electrodes in 0.1M PBS (pH 7.0) containing 250 µM of DA and 300 µM of UA. The simultaneous detection of DA and UA were investigated in
Figure 8. An outstanding increase in current compared with GCE and CuMAPc-PnBA/GCE is shown. In the meantime, DA and UA oxidation peak potentials are ≈ 0.1V and ≈ 0.27 respectively.
Figure 9.
DPV curves measured in 0.1 M PBS containing 250 μM DA plus 300 μM UA, (a) Bare GCE (b) CuMAPc-PnBA/GCE (c) MWCNT’s-CuMAPc-PnBA/GCE.
Figure 9.
DPV curves measured in 0.1 M PBS containing 250 μM DA plus 300 μM UA, (a) Bare GCE (b) CuMAPc-PnBA/GCE (c) MWCNT’s-CuMAPc-PnBA/GCE.
5. Individual Sensing of DA and UA on MWCNTs-CuMAPc-PnBA/GCE
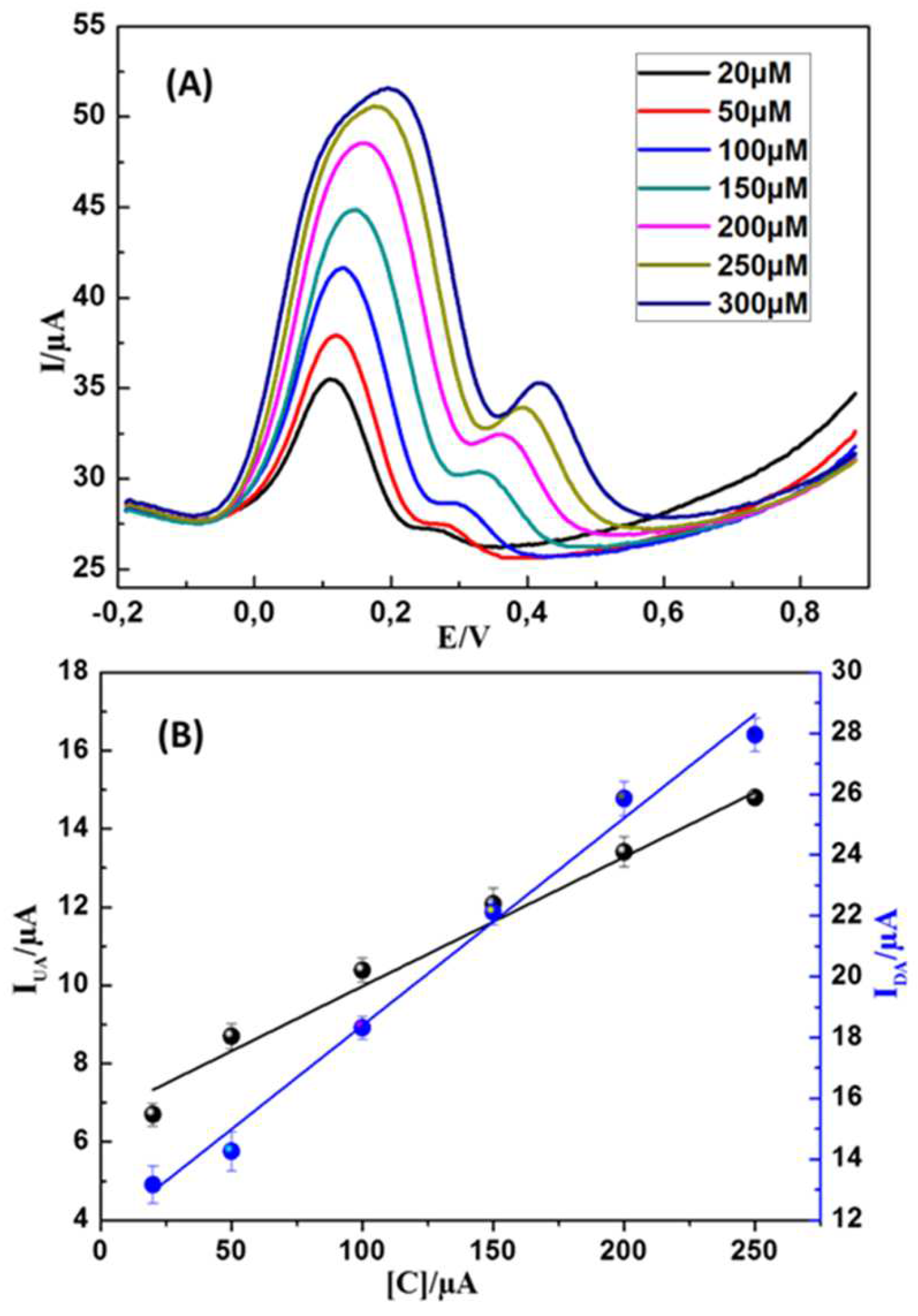
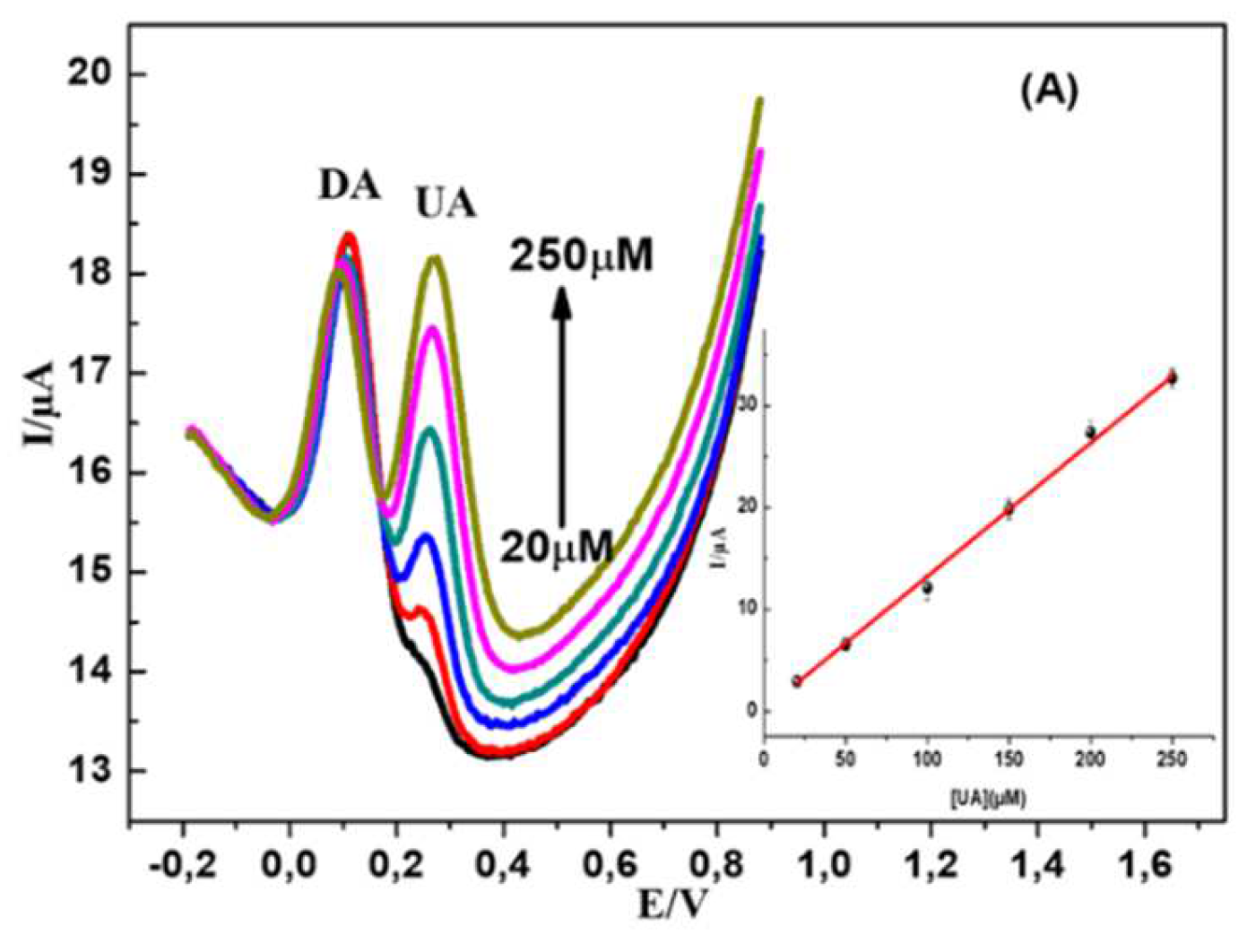
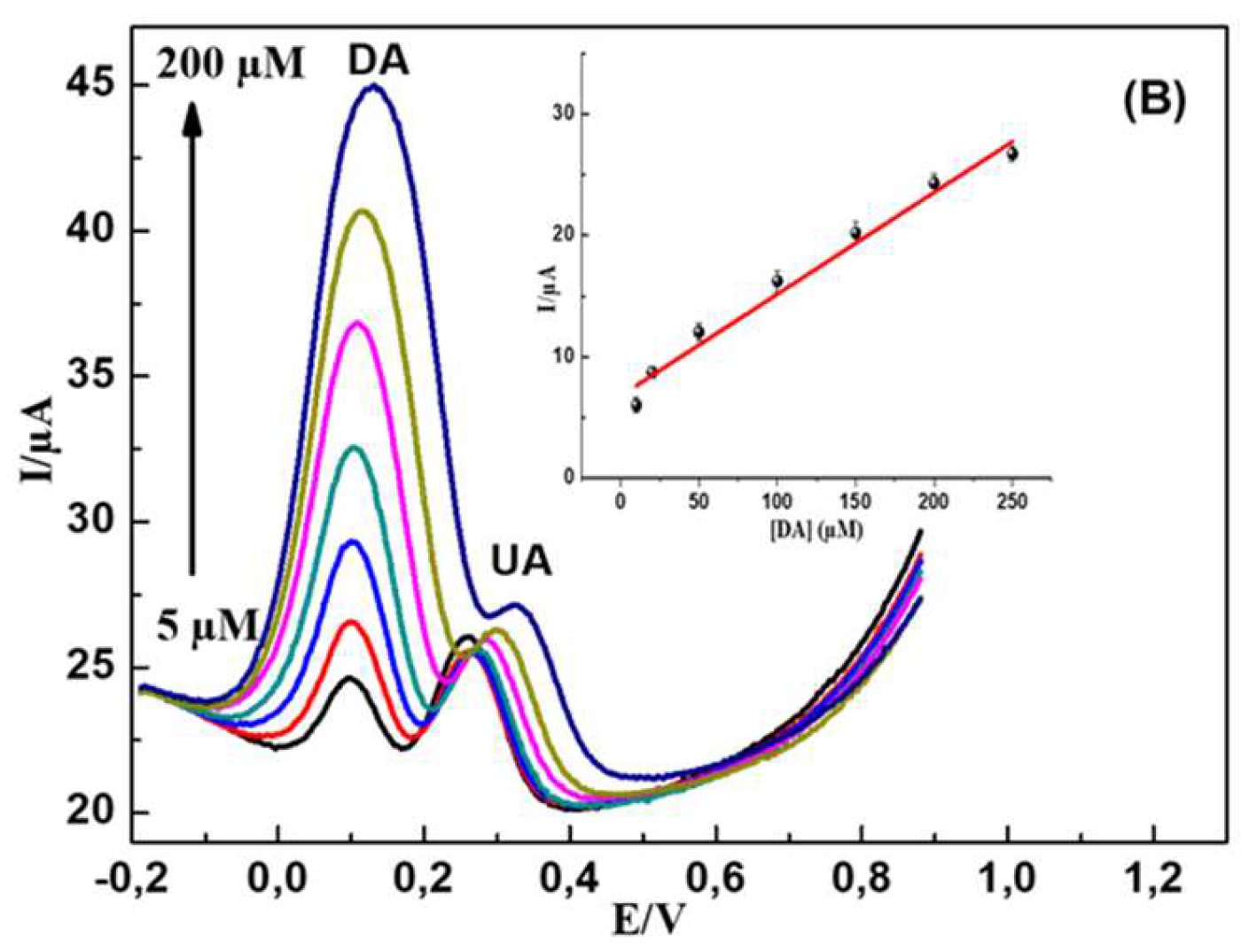
First, DA and UA were detected individually in PBS (0.1 M, pH 7.2) at MWCNTs-CuMAPC-PnBA/GCE with the DPV method. In the same buffer solution containing UA and the DA in their ternary mixture, one analyte concentration is changed while the other one is kept invariant. In
Figure 9 B, DA oxidation peak current increased regularly with a gradual increase in DA concentration from 5 to 200 µM, while the 200 µM concentration of UA is maintained constant. The incrimination of DA concentration hasn’t affected the oxidation current intensity of UA. In
Figure 10A, UA and DA coexisted while the DA was maintained constant, UA oxidation peak current increased regularly while the concentration of UA increased from 20 to 250 µM. These results confirm that the MWCNTs-CuMAPc-PnBA/GCE is are valuable for the individual sensing of UA and DA without any interference with each other. Furthermore, the previous results are comparable with those previously published in the literature. Rather, the developed sensor has been fabricated much easier than others (
Table 1).
6. Simultaneous Detection of DA and UA on MWCNTs-CuMAPc-PnBA/GCE
The practicality of the MWCNTs-CuMAPc-PnBA/GCE for the simultaneous detection of UA and DA was verified by the differential pulse voltammetric (DPV) technic. The curves were measured by MWCNTs-CuMAPc-PnBA/GCE immersed in PBS (0.1 M and pH 7.0) with the coexistence of UA and DA. The simultaneous sensing of DA and UA was achieved using the increment of DA and UA concentrations resulting in noticeable and regularly increased DPV curves. In
Figure 11 B we notice a well plotting linear relationship between concentration in the range of 20-300 µM and the corresponding oxidation current peak intensity for DA and UA, this linear relationship could be expressed by the regression equations:
I
UA (µA)=0.501+0.0032 C
UA (µM) (R
2=0.993) and I
DA (µA)=1.204+0.0063C
DA (µM) (R
2=0.988). The found detection limits are 9.813 µM and 0.176 µM for UA and DA respectively, based on the ratio of signal to noise (S/N = 3). Electrochemical sensors have many advantages including low cost, high sensitivity and simplicity, and have been utilized for UA and DA detection. The limit of detection (LOD) and the linear range of this work are comparable to those of previously reported sensors for UA and DA detection, Table1. Further, the MWCNTs-CuMAPc-PnBA composite was obtained by a very easy step technique and could be used for the fabrication of an electrochemical sensor with convenient performances. Differential pulse voltammetry as it is a sensitive technique and has been mostly undertaken for simultaneous electrochemical detection.
Figure 11.
(A) PV curves of DA and UA at the MWCNTs-CuMAPc-PnBA/GCE (B) Linear plotting realtionships of UA and DA concentrations versus the oxidation current peak intensities.
Figure 11.
(A) PV curves of DA and UA at the MWCNTs-CuMAPc-PnBA/GCE (B) Linear plotting realtionships of UA and DA concentrations versus the oxidation current peak intensities.
7. Stability, Reproducibility, Long-Term Stability, and Interferences of the Sensing System
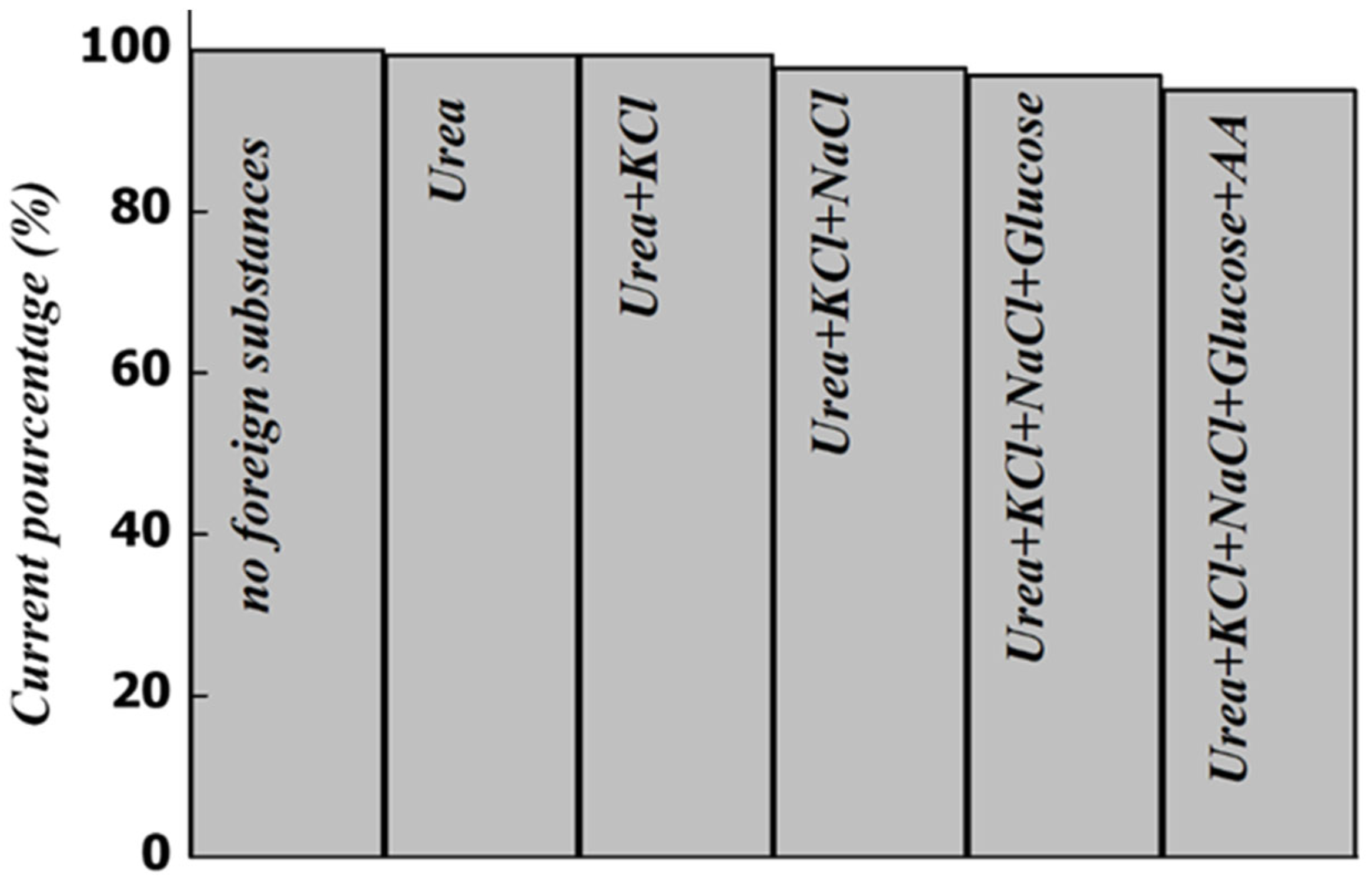
To validate the effectiveness of the developed sensor MWCNTs-CuMAPC-PnBA/GCE in the analysis of biological samples a few common co-existing compounds are considered by determining the responses of the modified electrode toward DA and UA in a mixture. The current response of UA and DA should change by less than ± 5.0 % to consider that the introduced foreign substances as non-interfering. The selected substances as interferences are KCl, NaCl, Glucose, Urea, and AA. These substances are chosen because they are the main interfering species for DA and UA in the real samples. Initially, the solution contains 200 µM DA and 400 µM UA then the interferences are added one by one while keeping the previous interferant at a concentration of 1 mM. In
Figure 12, the oxidation current peak intensities of UA and DA were measured and fixed as the standard value (100%), and the measures were repeated successively each time an interferant was added. These interferences had a very weak influence on the oxidation current peak intensities of DA and UA. Although, these results meant that the added substances have no significant interference on the sensing electrode which has a superior capability for highly selective determination of UA and DA.
To asses, the reproducibility of the MWCNTs-CuMAPC-PnBA/GCE, the DPV was the electrochemical measurement technique used for the evaluation with the same modified electrode in the mixture of (µM) UA and (µM) DA. Six repeated oxidation peak currents were measured on six prepared electrodes and the relative standard deviation values (RSD) were about 3.53 % (DA) and 4.75 % (UA). Operational stability of the sensor was studied by recording the analytical signal of the MWCNTs-CuMAPC-PnBA/GCE sensor in a mixture of DA and UA for 10 successive measurements. The relative standard deviation was 5.6% for DA and 7% for UA, which indicates good stability of the sensor’s response. The long term stability of the sensor was examined over a period of one month, and the current response of UA and DA was reduced by about 7.25 %. This result indicated that the MWCNTs-CuMAPC-PnBA/GCE sensor has long-term stability. Thus, the developed sensing system possessed high stability, repeatability, and reproducibility and this is proved by the low RSD values. Those results indicating the anti-interference ability and reproducibility of the MWCNTs-CuMAPC-PnBA/GCE validate its high feasibility as a sensing system for UA and DA.
Figure 12.
Current response of UA and DA considering the coexistence of foreign substances.
Figure 12.
Current response of UA and DA considering the coexistence of foreign substances.
8. Real Sample Analysis
Frequently, DA and UA coexist for the most biological fluid, then the suitability of the developed sensor for the real sample analysis was studied using human urine. The Urine sample was diluted with 0.1 M PBS (pH 7.0) and without any pretreatment process to avoid, any modifications to the simples matrix (kept authentic). The main idea is to compare the added amount of DA—UA and founded one [
34].
Table 2 summarizes all the analytical results and the detected results were calculated from the previous calibration plot. The proposed sensing method could be successfully used for the simultaneous detection of DA and UA in real samples because of the recovery rates of the samples that range between 92.16 % and 152.2 % and also because the human urine matrix doesn’t have any influence.
9. Conclusion
In this work, A MWCNTs-CuMAPc-PnBA/GCE sensing system was developed and used for the sensitive, individual, and simultaneous electrochemical detection of UA and DA. The prepared modified sensor exhibited excellent electrochemical signal responses toward the oxidation of UA and DA by showing a linear relation. Furthermore, the prepared sensor exhibited high analysis performances, reproducibility, repeatability, stability, and a strong anti-interference. In real human urine samples, the proposed electrochemical sensor exhibits high applicability for the simultaneous determination of UA and DA. The MWCNT-CuMAPc-PnBA/GCE is an ideal sensor for the determination of UA and DA referring to the recoveries ranging between 92.16 % and 152.2%.
Author Contributions
L. Barhoumi, O. Karker, S. Nasraoui, M. Ben Ali and O. Kanoun were responsible for conceptualizing and designing the methodology and overseeing the experimental work. L. Barhoumi, O. Karker and S. Nasraoui carried out the chemical elaboration, electrode fabrication, physical characterizations, and electrochemical measurements. The manuscript was collectively written by L. Barhoumi, O. Karker, and S. Nasraoui with contributions from all authors. M. Ben Ali, O. Kanoun contributed by proofreading and improvement of the manuscript. All authors have given approval for the final version of the manuscript.
Acknowledgements
The authors would like this manuscript to be a gift for the soul of the deceased among us, Professor Mohammed Nooredeen Abbas
References
- Kong, D.; Zhuang, Q.; Han, Y.; Xu, L.; Wang, Z.; Jiang, L.; Su, J.; Lu, C.H.; Chi, Y. Simultaneous voltammetry detection of dopamine and uric acid in human serum and urine with a poly(procaterol hydrochloride) modified glassy carbon electrode. Talanta 2018, 185, 203–212. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, G.; Tian, K.; Xu, C. A highly sensitive and stable electrochemical sensor for simultaneous detection towards ascorbic acid, dopamine, and uric acid based on the hierarchical nanoporous PtTi alloy. Biosensors and Bioelectronics 2016, 82, 119–126. [Google Scholar] [CrossRef]
- Benkert, O.; Müller-Siecheneder, F.; Wetzel, H. Dopamine agonists in schizophrenia: a review. European Neuropsychopharmacology 1995, 5, 43–53. [Google Scholar] [CrossRef]
- Sudha, V.; Krishnamoorthy, K.; Kumar, S.M.S. Thangamuthu, Copper oxide nanosheet modified electrodes for simultaneous determination of environmentally hazardous anions. Journal of Alloys and Compounds 2018. [Google Scholar] [CrossRef]
- de Oliveira, E.P.; Burini, R.C. High plasma uric acid concentration: causes and consequences. Diabetology & metabolic syndrome 2012, 4, 12. [Google Scholar]
- He, S.; Yu, Y.; Chen, Z.; Shi, Q.; Zhang, L. Synergistic Effect of Graphene and Multiwalled Carbon Nanotubes on a Glassy Carbon Electrode for Simultaneous Determination of Uric Acid and Dopamine in the Presence of Ascorbic Acid. Analytical Letters 2015, 48, 248–258. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, Q.; Zhou, W.; Zhao, J.; Zhang, B.; Sha, Y.; Pang, Z. Quantification of Dopamine in Brain Microdialysates with High-Performance Liquid Chromatography-Tandem Mass Spectrometry. Analytical Sciences 2016, 32, 419–424. [Google Scholar] [CrossRef]
- Casalini; Leonardi, F. ; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Organic Electronics 2013, 14, 156–163. [Google Scholar] [CrossRef]
- Contat-Rodrigo, L.; Pérez-Fuster, C.; Lidón-Roger, J.V.; Bonfiglio, A.; García-Breijo, E. Screen-printed Organic Electrochemical Transistors for the detection of ascorbic acid in food. Organic Electronics 2017, 45, 89–96. [Google Scholar] [CrossRef]
- Atack, C.V. The determination of dopamine by a modification of the dihydroxyindole fluorimetric assay. British Journal of Pharmacology 1973, 48, 699–714. [Google Scholar] [CrossRef]
- Xu, X.; Shi, H.; Ma, L.; Kang, W.; Li, S. Determination of trace amounts of dopamine by flow-injection analysis coupled with luminol–Ag(III) complex chemiluminescence detection. Luminescence 2011, 26, 93–100. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Ye, D.; Luo, J.; Su, B.; Zhang, S.; Kong, J. An electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan based on MWNTs bridged mesocellular graphene foam nanocomposite. Talanta 2014, 127, 255–261. [Google Scholar] [CrossRef]
- Fredj, Z.; Ali, M.B.; Abbas, M.N.; Dempsey, E. Determination of prostate cancer biomarker acid phosphatase at a copper phthalocyanine-modified screen printed gold transducer. Anal. Methods 2020, 12, 3883–3891. [Google Scholar] [CrossRef]
- Ahammad, A.J.S.; Odhikari, N.; Shah, S.S.; Hasan, M.; Islam, T.; Pal, P.R.; Qasem, M.A.A.; Aziz, A. Porous tal palm carbon nanosheets: preparation, characterization and application for the simultaneous determination of dopamine and uric acid. Nanoscale Adv. 2019, 1, 613–626. [Google Scholar] [CrossRef]
- Wei, X.; Guo, H.; Lu, Z.; Sun, L.; Pan, Z.; Liu, B.; Peng, L.; Yang, W. A novel electrochemical sensor based on DUT-67/ZnCo2O4-MWCNTs modified glassy carbon electrode for the simultaneous sensitive detection of dopamine and uric acid. J. Electroanal. Chem. 2023, 674, 131921. [Google Scholar] [CrossRef]
- EErgün; Kart, Ş.; Zeybek, D.K.; Zeybek, B. Simultaneous electrochemical determination of ascorbic acid and uric acid using poly(glyoxal-bis(2-hydroxyanil)) modified glassy carbon electrode. Sensors and Actuators B 2016, 224, 55–64. [Google Scholar] [CrossRef]
- Dursun, Z.; Gelmez, B. Simultaneous Determination of Ascorbic Acid, Dopamine and Uric Acid at Pt Nanoparticles Decorated Multiwall Carbon Nanotubes Modified GCE. Electroanalysis 2010, 22, 1106–1114. [Google Scholar] [CrossRef]
- Hu, H.; Song, Y.; Feng, M.; Zhan, H. Carbon nanomaterials for simultaneous determination of dopamine and uric acid in the presence of ascorbic acid: from one-dimensional to the quasi one-dimensional. Electrochimica Acta 2016, 190, 40–48. [Google Scholar] [CrossRef]
- Ali, A.; Jamal, R.; Abdiryim, T.; Huang, X. Synthesis of monodispersed PEDOT/Au hollow nanospheres and its application for electrochemical determination of dopamine and uric acid Journal of Electroanalytical. Chemistry 2017, 787, 110–117. [Google Scholar] [CrossRef]
- CJia; Chen, L. ; Shao, Z.; Agarwal, U.P.; Hu, L.; Zhu, J.Y. Using a fully recyclable dicarboxylic acid for producing dispersible and thermally stable cellulose nanomaterials from different cellulosic sources. Cellulose 2017, 24, 2483–2498. [Google Scholar] [CrossRef]
- Kathirvelan, J.; Vijayaraghavan, R. Detection of methane using multi-walled carbon nanotubes. Bull Mater Sci 2015, 38, 909–913. [Google Scholar] [CrossRef]
- Mandoj, F.; Nardis, S.; Di Natale, C.; Paolesse, R. Porphyrinoid Thin Films for Chemical Sensing. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Anghelone, M.; Jembrih-Simbürger, D.; Schreiner, M. Identification of copper phthalocyanine blue polymorphs in unaged and aged paint systems by means of micro-Raman spectroscopy and Random Forest. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 149, 419–425. [Google Scholar] [CrossRef]
- Ivanov, A.N.; Younusov, R.R.; Evtugyn, G.A.; Arduini, F.; Moscone, D.; Palleschi, G. Acetylcholinesterase biosensor based on single-walled carbon nanotubes--Co phtalocyanine for organophosphorus pesticides detection. Talanta 2011, 85, 216–221. [Google Scholar] [CrossRef]
- Kurd, M.; Salimi, A.; Hallaj, R. Highly sensitive amperometric sensor for micromolar detection of trichloroacetic acid based on multiwalled carbon nanotubes and Fe(II)-phtalocyanine modified glassy carbon electrodeaterials. Science and Engineering: C 2013, 33, 1720–1726. [Google Scholar] [CrossRef]
- Abbas, M.N.; Radwan, A.L.A.; Nooredeen, N.M.; El-Ghaffar, M.A.A. Selective phosphate sensing using copper monoamino-phthalocyanine functionalized acrylate polymer-based solid-state electrode for FIA of environmental waters. J Solid State Electrochem 2016, 20, 1599–1612. [Google Scholar] [CrossRef]
- Barhoumi, L.; Baraket, A.; Nooredeen, N.M.; Ali, M.B.; Abbas, M.N.; Bausells, J.; Errachid, A. Silicon Nitride Capacitive Chemical Sensor for Phosphate Ion Detection Based on Copper Phthalocyanine—Acrylate-Polymer. Electroanalysis 2017, 29, 1586–1595. [Google Scholar] [CrossRef]
- Liu, X.; Peng, Y.; Qu, X.; Ai, S.; Han, R.; Zhu, X. Multi-walled carbon nanotube-chitosan/poly (amidoamine)/DNA nanocomposite modified gold electrode for determination of dopamine and uric acid under coexistence of ascorbic acid. Journal of Electroanalytical Chemistry 2011, 654, 72–78. [Google Scholar] [CrossRef]
- Meenakshi, S.; Devi, S.; Pandian, K.; Devendiran, R.; Selvaraj, M. Sunlight assisted synthesis of silver nanoparticles in zeolite matrix and study of its application on electrochemical detection of dopamine and uric acid in urine samples. Materials Science and Engineering: C 2016, 69, 85–94. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Zhong, J.; Yan, B.; Zhang, K.; Zhai, C.; Shiraishi, Y.; Du, Y.; Yang, P. Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite. Journal of Colloid and Interface Science 2017, 497, 172–180. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Shi, K.; Wang, Q.; Zhao, X.; Xiong, Z.; Zou, X.; Wang, Y. Graphene coated by polydopamine/multi-walled carbon nanotubes modified electrode for highly selective detection of dopamine and uric acid in the presence of ascorbic acid. Journal of Electroanalytical Chemistry 2016, 770, 56–61. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, W.-J.; Hou, B.-Q. Nano-Cu/PSA III modified glassy carbon electrode for simultaneous determination of ascorbic acid, dopamine and uric acid. Journal of Electroanalytical Chemistry 2013, 689, 135–141. [Google Scholar] [CrossRef]
- Lai, G.; Liu, Y.; Yu, A.; Han, D.; Zhang, H. Simultaneous Sensitive Determination of Dopamine and Uric Acid in the Presence of Excess Ascorbic Acid with a Magnetic Chitosan Microsphere/Thionine Modified Electrode. Analytical Letters 2013, 46, 1525–1536. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Gui, R.; Jin, H.; Xia, J.; Zhang, F. Simultaneous and selective measurement of dopamine and uric acid using glassy carbon electrodes modified with a complex of gold nanoparticles and multiwall carbon nanotubes. Sensors and Actuators B: Chemical 2018, 255, 2069–2077. [Google Scholar] [CrossRef]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric. Acid Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef]
Figure 1.
Synthesis of CuMAPc covalently attached to PnBA–COOH Polymer (ionophore II).
Figure 1.
Synthesis of CuMAPc covalently attached to PnBA–COOH Polymer (ionophore II).
Figure 2.
Representative UV-Vis spectra of (____) CuMAPc and (…….) CuMAPc-Polymer.
Figure 2.
Representative UV-Vis spectra of (____) CuMAPc and (…….) CuMAPc-Polymer.
Figure 3.
Representative IR spectra of CuMAPc-Polymer.
Figure 3.
Representative IR spectra of CuMAPc-Polymer.
Figure 4.
SEM images of (A) CuMAPc-PnBA matrix, (B) MWCNTs, and SEM image of (C) CuMAPc-PnBA-MWCNTs.
Figure 4.
SEM images of (A) CuMAPc-PnBA matrix, (B) MWCNTs, and SEM image of (C) CuMAPc-PnBA-MWCNTs.
Figure 5.
Nyquist plots of (i) bare GCE (ii) CuMAPc-PnBA/GCE and (iii) CuMAPc-PnBA-MWCNTs/GCE in a 5.0 mM [Fe(CN)6]3-/4-.
Figure 5.
Nyquist plots of (i) bare GCE (ii) CuMAPc-PnBA/GCE and (iii) CuMAPc-PnBA-MWCNTs/GCE in a 5.0 mM [Fe(CN)6]3-/4-.
Figure 6.
DPV curves of 150 µmol/L DA and 250 µM/L UA (A) at the MWCNTs-CuMAPc-PnBA/GCE in 0.1 mol/L PBS at different pH values with a scan rate of 100 mV.s-1 (5.0, 6.0, 7.0, 8.0, 9.0, 10), (B) pH influence on the Ipa for the oxidation of UA and DA at the MWCNTs-CuMAPc-PnBA/GCE.
Figure 6.
DPV curves of 150 µmol/L DA and 250 µM/L UA (A) at the MWCNTs-CuMAPc-PnBA/GCE in 0.1 mol/L PBS at different pH values with a scan rate of 100 mV.s-1 (5.0, 6.0, 7.0, 8.0, 9.0, 10), (B) pH influence on the Ipa for the oxidation of UA and DA at the MWCNTs-CuMAPc-PnBA/GCE.
Figure 7.
CV curves of 20 mmol/L DA (A) and 70 mmol/L UA (B) at the MWCNTs-CuMAPc-PnBA/GCE in 0.1mol/L PBS (pH=7.0) at different scan rates (10, 20, 40, 50, 80,100, 150,200, 250) The plots of Ipa versus the scan rate for DA (C) and UA (D).
Figure 7.
CV curves of 20 mmol/L DA (A) and 70 mmol/L UA (B) at the MWCNTs-CuMAPc-PnBA/GCE in 0.1mol/L PBS (pH=7.0) at different scan rates (10, 20, 40, 50, 80,100, 150,200, 250) The plots of Ipa versus the scan rate for DA (C) and UA (D).
Figure 8.
DPV curved mesured in 0.1 M PBS containg 250 µM DA plus 300 µM UA at different modified electrodes (i) MWCNTs ratio to CuMAPc-PnBA V: 3V (ii) MWCNTs ratio to CuMAPc-PnBA 2V:3V (iii) MWCNTs ratio to CuMAPc-PnBA V:V.
Figure 8.
DPV curved mesured in 0.1 M PBS containg 250 µM DA plus 300 µM UA at different modified electrodes (i) MWCNTs ratio to CuMAPc-PnBA V: 3V (ii) MWCNTs ratio to CuMAPc-PnBA 2V:3V (iii) MWCNTs ratio to CuMAPc-PnBA V:V.
Figure 10.
(A) DPV curves of PBS (0.1 M, pH 7.2) in the presence of 20–250 μM UA plus 0.15 mM DA (B) DPV curves of PBS in the presence of 5–200 μM DA plus 0.1 mM UA. CuMAPc-PnBA-MWCNTs/GCE was immersed in PBS and used for the working electrode for electrochemical measurements.
Figure 10.
(A) DPV curves of PBS (0.1 M, pH 7.2) in the presence of 20–250 μM UA plus 0.15 mM DA (B) DPV curves of PBS in the presence of 5–200 μM DA plus 0.1 mM UA. CuMAPc-PnBA-MWCNTs/GCE was immersed in PBS and used for the working electrode for electrochemical measurements.
Table 1.
Comparison of reported electrochemical sensors for the detection of DA and UA.
Table 1.
Comparison of reported electrochemical sensors for the detection of DA and UA.
| Sensors |
Limit of detection (µM)
|
Linear range (µM)
|
Ref |
| |
DA
|
UA
|
DA |
UA |
|
| ZnO/PANI/RGO/GCE |
0.017 |
0.122 |
0.1-90
90-1000 |
0.5-100
100-1000 |
[28] |
| DNA/PAMAM/MWNT-Chit/Au |
0.03 |
0.07 |
0.2-10
10-100 |
0.5-100 |
[29] |
| AgNP/Zeo-Y/GCE |
0.016 |
0.025 |
0.02-0.18 |
0.05-0.7 |
[30] |
| Cubic Pd/RGO/GCE |
0.18 |
1.6 |
0.45-421 |
4-469.5 |
[31] |
| Pdop@GR/MWCNTs |
1.0 |
15.0 |
7.0-297.0 |
20.0-320.0 |
[32] |
| Cunanoparticles–poly(sulfonazo III)/GCE |
0.01 |
0.1 |
0.02-65 |
0.25-107 |
[33] |
| MWNTs/MGF |
0.06 |
0.93 |
0.3-10 |
5-100 |
[13] |
| MCMS/Thionine/GCE |
0.50 |
2.30 |
2–30 |
9–100 |
[34] |
| Au@NAC-MWCNTs |
0.03 |
0.04 |
0.1-250 |
0.1-300 |
[35] |
| MWCNTs-CuMAPc-PnBA/GCE |
0.174 |
6.87 |
5-200 |
20-250 |
This work |
Table 2.
Determination of UA and DA in human urine by using MWCNTs-CuMAPc-PnBA/GCE.
Table 2.
Determination of UA and DA in human urine by using MWCNTs-CuMAPc-PnBA/GCE.
| Sample |
Spiked UA / µM |
Found UA / µM |
Recovery % |
Spiked
DA / µM |
Found
DA / µM |
Recovery % |
| Urine 1 |
0 |
11.19 |
__ |
0 |
0 |
__ |
| Urine 2 |
20 |
25.24 |
126.2 |
5 |
5.28 |
105.6 |
| Urine 3 |
100 |
105.46 |
105.46 |
10 |
12.37 |
123.7 |
| Urine 4 |
200 |
248.53 |
124.26 |
100 |
152.2 |
152.2 |
| Urine 5 |
300 |
331.41 |
110.47 |
200 |
184.32 |
92.16 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).