1. Introduction
During the winemaking process, yeast consumes the natural sugars in grapes (mainly glucose and fructose) and converts them into alcohol and carbon dioxide through the fermentation process. However, not all the sugar is fermented in some cases, and a certain amount remains in the finished wine [
1]. Reducing sugars are fermentable sugars that may be reduced by fermentation in a juice or wine. Residual sugars (“RS”) are all of the sugars that remain in the wine after fermentation is completed or terminated, including the non-fermentable sugars. The term “residual sugar” is used interchangeably with “reducing sugar” to mean the amount of glucose and fructose in the juice or wine [
2].
The level of residual sugars in a wine can vary widely, from dry wines with virtually no residual sugar to sweet wines with significant residual sugar content [
3].
Labeling of wine “sweetness” according to the residual content of the wine slightly differ between different counties. According to EU regulations: dry wines are those with sugar content up to 4 g·L
-1 (or 9 g·L
-1 if the total acidity expressed as tartaric acid is not more than 2 g·L
-1 lower than the residual sugar content); Medium-dry (i.e., semi-dry): sugar content of the wine is above 4 g·L
-1 but does not exceed 12 g·L
-1 (or 18 g·L
-1 if the total acidity expressed as tartaric acid is not more than 10 g·L
-1 lower than the residual sugar content). Mellow or semi-sweet wines contain at the most 45 g·L
-1, and Sweet wines contains at least 45 g·L
-1 RS [
4]. Control of the residual sugar levels in the wine is done through various techniques, including adjusting fermentation time, temperature, and yeast strains. The choice of when to stop fermentation or whether to leave some sugar unfermented is a deliberate decision that influences the wine’s style and sweetness level [
2,
3].
During wine fermentation, sugar measurements are taken regularly to monitor fermentation’s progress. Sugar measurements are typically taken by brix measurements and by specific gravity measurements. Both measurements are important in determining when to stop fermentation and when to rack the wine off the lees [
2].
It is easy to measure sugar contents in must using a refractometer or a hydrometer before alcoholic fermentation. However, as alcoholic fermentation progresses, alcohol interferes with sugar measurement, resulting in an inaccurate measurement of both brix and density. The data obtained from a refractometer is higher than the true brix of the sample, while that from a hydrometer is lower than the true value. Hydrometer brix decreases linearly with increasing percentage alcohol content, whereas refractometer Brix increases linearly [
5,
6].
Apart from the hydrometer and refractometer, there are other methods available for brix measurements including differential measurements, density measurements, osmotic potential measurements, mass flow measurements and various biosensors (e.g., optical, ultrasonic etc.). The demand over the years for a real-time, rapid, noninvasive, and continuous monitoring of sugar content analysis, yielded various novel techniques for sugar content determination [
7].
Precise measurement of sugar concentration, rather than brix measurement, is usually done by: “wet chemistry” techniques (i.e., reaction/titration), enzymatic assays, high-performance liquid chromatography (HPLC), spectroscopic based techniques and several alternative techniques (such as, remote-sensing technologies, colorimetric and digital image analysis) [
8,
9,
10]
Reaction/Titration includes two procedures commonly used: (i) Lane and Eynon and (ii) Rebelein methods (also known as Fehling test), both of which rely on reacting the sugars with alkaline cupric tartrate and then titrating to determine the excess copper ions. In the Lane and Eynon method, standard glucose solution is used as titrant, but in the Rebelein method, the concentration of Cu2+ is determined by reacting with excess iodine and then estimating the remaining iodine by titrating with standard thiosulfate solution. In both cases, red wines must be decolorized. It should be noted that both of these techniques measure all the reducing sugars including those like the pentose sugars that are not considered fermentable. These tests will therefore give higher results than tests that determine just the concentration of glucose and fructose. In the enzymatic assay a conversion of glucose and fructose by specific enzymes can be monitored directly by measuring the absorbance (340 nm) resulting from the generation of a by-product of the reaction (NADPH). The test is quite straightforward to conduct and requires only sample dilution. Kits for this assay are commercially available. The drawback of this method is its relatively high costs (reagents and a UV spectrophotometer).
HPLC, coupled with various detectors, is one of the common techniques for sugar determination. The most commonly applied detector is the refractive index (RI) detector [
11]. Other includes ultraviolet (UV) detector [
12], evaporating light scattering detector (ELSD) [
13], and (MS) detector [
14]. It offers several advantages, including potential for automation, high precision and it is relatively fast. However, this method is not environmentally friendly, time-consuming, and requires high-cost equipment and skilled personnel to operate and maintain the instrument [
8].
Alternative approaches based on spectroscopic techniques include near infrared (NIR), mid-infrared (MID) and Fourier-transform infrared spectroscopy (FTIR) [
15], have been proposed in order to simplify sugar quantitation [
16]. Some of these technologies are already in use in wine laboreteries with great results and ease of use (such as OenoFoss machine of Foss industries), but are highly expensive.
In general, all the methods for brix and sugar measurements are usually classified into two types: destructive or nondestructive. All the conventional methods such as hydrometry, refractometry, chromatography and few modern techniques (e.g., electronic tongue and SPR based sensors) are considered as destructive methods. Nondestructive methods includes vis/NIR Spectroscopy, Magnetic Resonance Methods, Spectroscopic Methods and Ultrasonic Methods. Commercially available in-line brix sensors can be effectively used to monitor the progress of the wine fermentation process. They are based on different principles including density, hydrostatic pressure, volume, osmotic potential, mass flow, absorbance, enzyme response and hydrostatic pressure [
17].
Despite the advantages of these above mentioned methods, most of them are less relevant for small wineries as they are either expensive or complicated to perform [
18,
19,
20,
21].
For this reason, simple and cost-effective approaches for quantification of reducing sugars were recently studied. Few studies have been published reporting the use of digital images to quantify sugar in food products [
22,
23]. Recent method combining colorimetric reaction with digital image analysis has been proved as an effective method for accurate and precise quantification in beverage samples by low-cost analytical procedures [
8]. In addition, novel methods were suggested for estimating the sugar concentration, based on brix measurements by various cost-effective sensors [
17], and by refractometer and hydrometer. Equations were empirically derived to calculate the accurate brix of must during alcoholic fermentation [
5,
24]. These methods are based on measurements made by either a hydrometer or refractometer (or both), but measure brix rather than residual sugars content [
7].
In this study, we suggest a novel model for predicting the residual sugar of the wine at a specific density point during fermentation, by measuring the initial brix of the must before fermentation. The model was developed by measurements of actual residual sugars as opposed to brix measurements used in other studies [
7]. Knowing the concentration of the residual sugars in wine is critical for a few wine styles such as semi-dry and fortified. The model can be used as a practical and rapid tool for estimating the exact point in the fermentation at which to terminate the fermentation in order to achieve the requested residual sugars in the wine, thus gaining precision and saving time and costs, as no special equipment is needed for the measurement but a simple hydrometer, usually present in any winery.
2. Materials and Methods
Fermentations were carried out both on natural and synthetic musts. The objective of using a synthetic medium was to standardize the experimental conditions and to obtain more reproducibility compared to the natural musts.
The synthetic medium used has been used as a wine model system in fermentation studies by several authors due to it good correspondence with grape juice [
25,
26,
27]. Composition of the medium was: 3 g·L
-1 tartaric acid, 2 g·L
-1 l(+)malic acid, 1 g·L
-1 KH
2PO
4, 0.5 g·L
-1 MgSO
4·7H
2O, 0.1 g·L
-1 NaCl, 0.1 g·L
-1 CaCl
2, 25 μg·L
-1 biotin, 0.25 g·L
-1 inositol and 100 μg·L
-1 of: H
3BO
3, ZnSO
4, MnCl
2, FeCl
2, CuSO
4, KI, thiamin, calcium pantothenate, pyridoxine, and nicotinic acid. The initial pH was adjusted to 3.3 with KOH. Glucose and Fructose (Sigma, Germany) were added in a 1:1 ratio in order to obtain five different initial sugar concentrations (3 replicates for each treatment). The brix values (°Bx) obtained were as follows: 18.1, 20.3, 22.4, 24.4 and 26.6. The Natural must contained Malbec grapes from Mevo-Horon, Israel (lat. 31.82, long. 35.02, alt. 200 m). A total yield of ca. 30 kg for each treatment; grapes were harvested at eight time points to create musts with varying degrees of initial brix (range of 17-26°Bx).
Fermentation conditions
Fermentations of synthetic musts were carried out in 2L wine bottles provided with a bubbling CO2 outlet and conducted in triplicate. In order to initiate alcoholic fermentation, 10g of the commercial Saccharomyces cerevisiae strain fx-33 (Laffort®, Bordeaux, France) was added. In addition, yeast nutrients were added according to the manufacturer’s instructions (Nutrivit Vinoferm, 3581 Beverlo, Belgium). The fermentation temperature was kept at 25°C.
Winemaking from the Malbec grapes was carried out according to the following procedure: grapes were harvested at different stages of grape ripping in order to produce a range of musts with different initial brix (8 treatments; brix range: 17.3-27). Grape clusters were destemmed, crushed, and placed in 25L tanks. In order to initiate alcoholic fermentation, 10g of the commercial Saccharomyces cerevisiae strain fx-10 (Lallemand Inc., Montreal, QC, Canada) was added following the manufacturer’s instructions. The cap punch-down operations were carried out twice a day during the seven days of fermentation at 25 °C. On day 7, the wine was separated from the pomace by pressing it using a hydraulic press and kept at the same temperature until its density dropped below 0.994 g·mL-1. Sulfur dioxide was added to the wine (as potassium metabisulfite) at a concentration of 60 mg·L-1.
Must and Wine Analysis
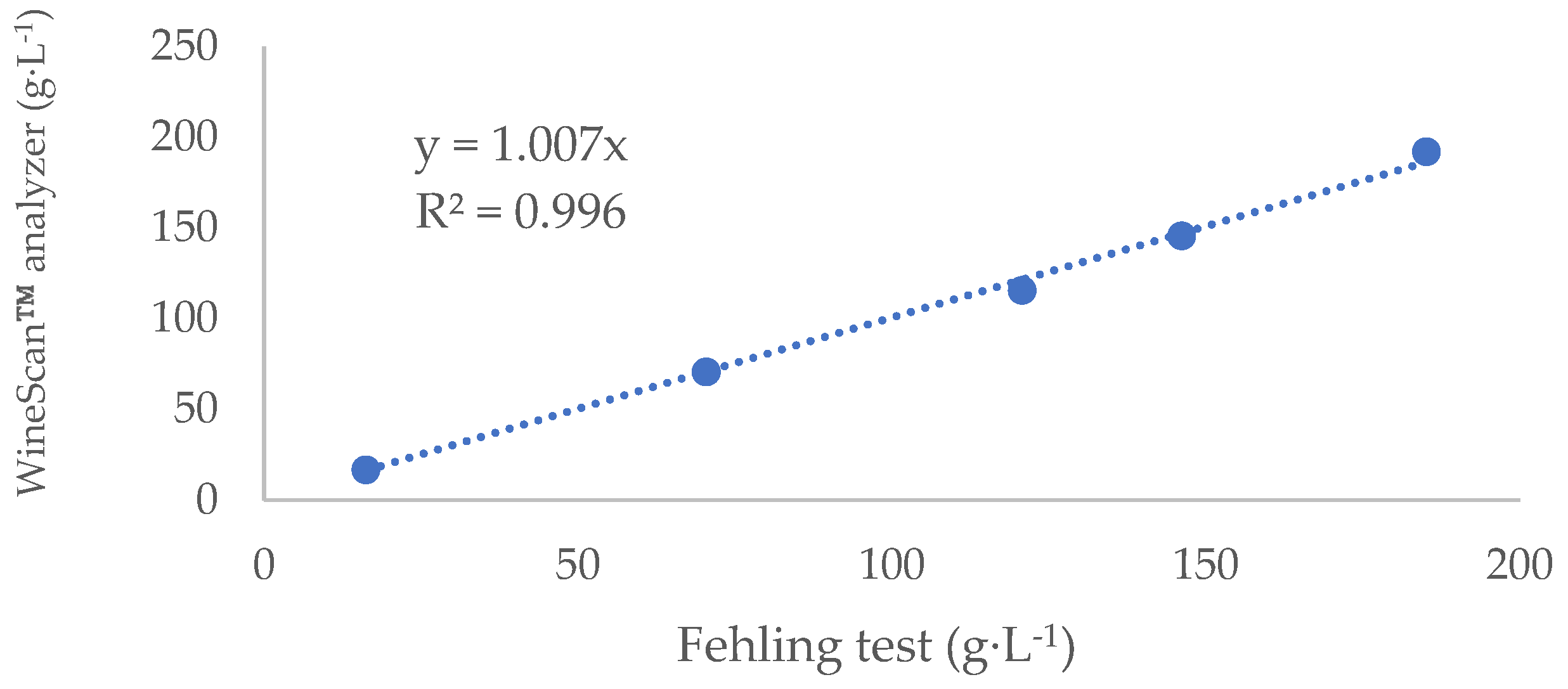
Samples were taken at indicated time points and analyzed for temperature, density and reducing sugars. Density was measured by a hydrometer (scale: 0 to 35 Brix, Triple scale hydrometer, France). Total soluble solids (brix determination) were measured by a digital refractometer (Pocket PAL-1, ATAGO, Tokyo, Japan). pH was measured by a Hanna HI 2211 pH meter (Hanna Instruments Inc, Woonsocket, RI, USA). Sugar concentrations were measured both by the Fehling test [
28] and by the WineScan™ analyzer (Foss, Hilleroed, Denmark). Fehling test (Also appear in the OIV compendium for analysis of residual sugars in wine/OIV-MA-AS311-01A) [
4] is a certified and accurate method and thus, we used the Fehling test results to validate measurements made by WineScan™ analyzer (
Figure 1). The WineScan™ analyzer makes use of Fourier-transformed infrared spectroscopy for these determinations [
29]. After the validation trials, we used the WineScan™ analyzer for the sugar measurements.
Statistical Analyses
Linear regressions were made and tested with Excel software; One-way ANOVA was conducted, followed by the Tukey post hoc test, for analyzing the differences among means of each wine characteristic (measurements were conducted in triplicates). Prism statistical software (GraphPad Software, Boston, USA) was used to determine the statistical significance of differences between the treatment means at α = 0.05.
3. Results
In order to achieve our goal, we conducted fermentation trials on both synthetic and natural grape musts (with different initial brix values). We measured residual sugars with concomitant brix/density measurements as follow-mentioned.
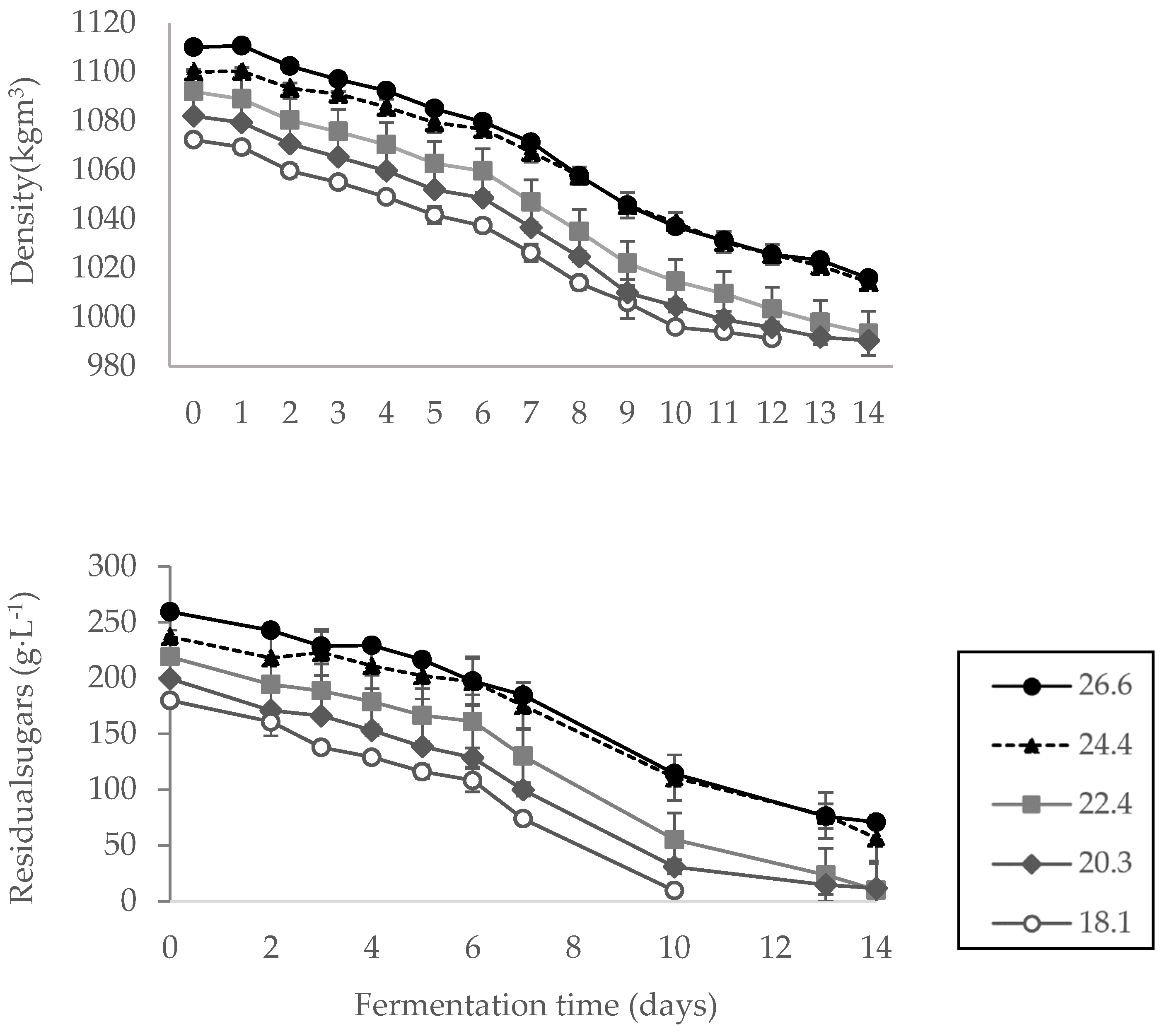
Measurement of actual residual sugars in synthetic musts
Fermentation trials of synthetic musts of different initial sugar concentrations were conducted at constant temperatures. Samples for density measurements by hydrometer, and the residual sugar analysis by Felling test were taken daily except at weekends. The results are summarized in
Figure 2.
It is clear from the figure that as the alcoholic fermentation progresses, there is a gradual drop in residual sugars, followed by a reduction in the wine’s density. In addition, as expected, it can be seen from the results that the time for completion of fermentation was longer for the treatment with higher initial brix than for the one with low initial brix (18 and 12 days, respectively). Fermentation of the must with 26.6 °Bx, was not fully completed during the 18 days of measurements, resulting in some residual sugar in the wine.
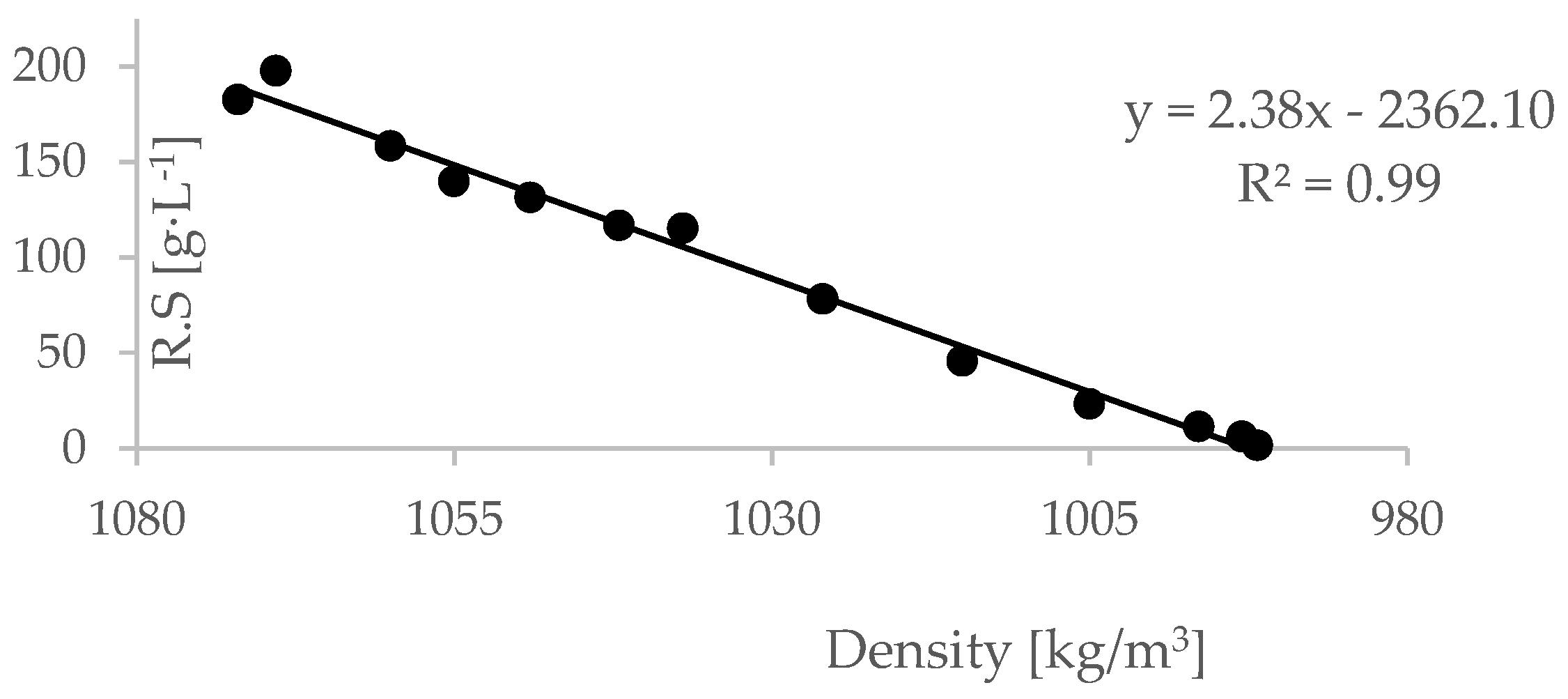
Linear regression between the residual sugar of the wine and its density was obtained for all treatments and repetitions (i.e., different values of must initial brix).
A sample of such regression is presented in
Figure 3 (the regression of the 26.6 Bx must).
Equations for the linear regression of the triplicates for all treatments (initial brix levels) are presented in
Table 1.
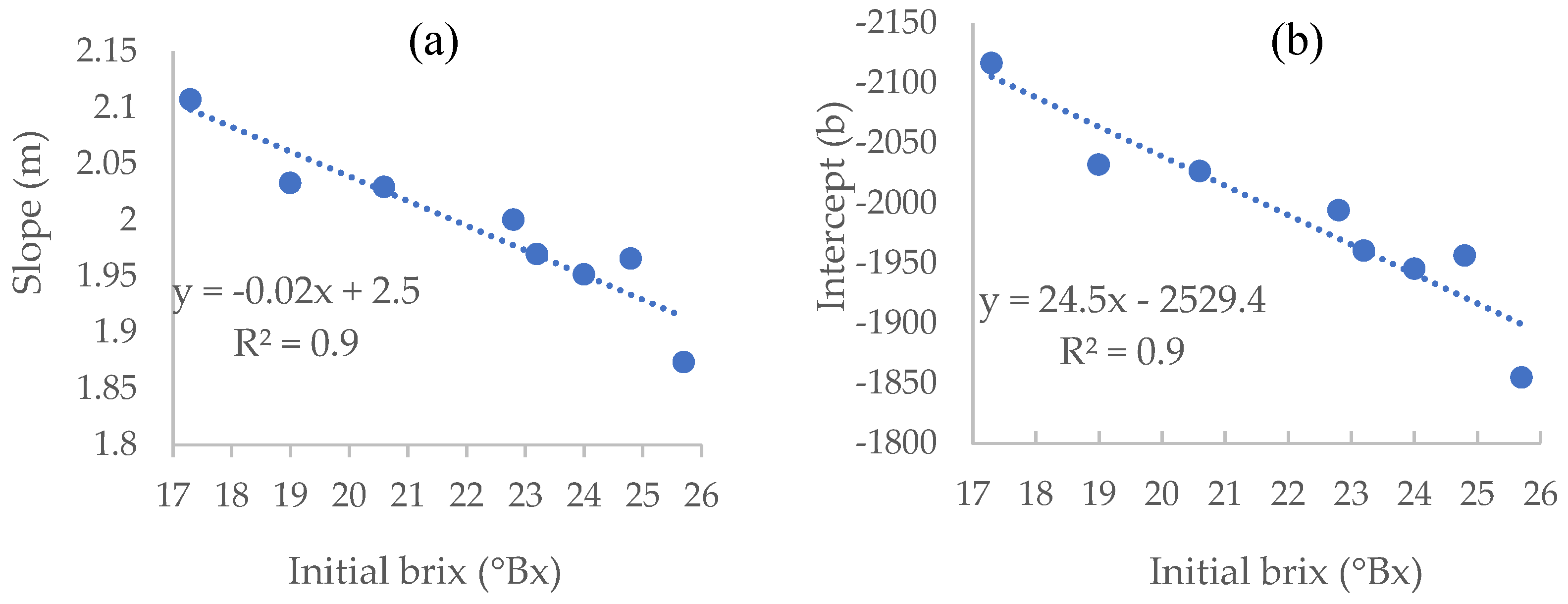
We used the equations to calculate the average slopes and intercepts to be used for each initial brix level.
Table 1 shows an apparent decrease in the regression slopes when initial brix levels are higher. This decrease is caused by the elevated accumulation of alcohol in the musts with higher initial brix, which lowers the measured density, as the density of ethanol, the primary alcohol in wines, is 0.78945 g/cm
3 at 20 °C [
24].
It is important to note that we used musts with brix values in the common range of fermented wines for our experiments. Thus, the regressions we present are practically all a winemaker needs in order to secure an accurate residual sugar in his final wine.
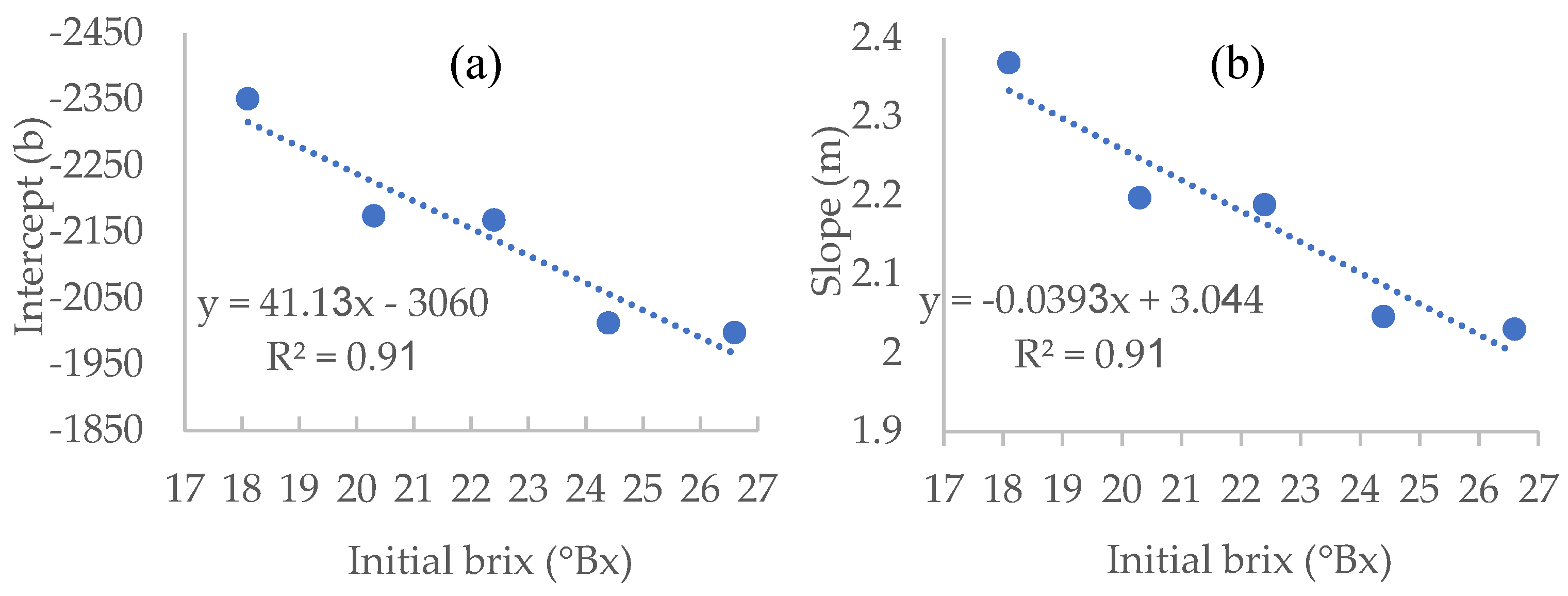
In order to predict the density needed for a specific residual sugar value for any given initial brix value within the range of 18.1-26.6 brix, other than that used for our study, we used the different linear equations presented in
Table 1 and plotted new curves, allowing the calculation of both intercept and slope for any given initial brix level (
Figure 4).
Using the equations derived from these graphs, which show a relatively high R
2 of 0.91 (
Figure 4), we can calculate the density values at which the fermentation will reach any desired residual sugar value, for any given initial brix level.
For example, achieving the basic equation for a brix value of 23.5 will be as follows:
The linear equation for calculating the slope (m) equals:
The linear equation for calculating the Intercept (b) equals:
Thus, the equation for calculating the density point for a specific desirable residual sugar level, for the 23.5 Bx must is as follows:
Using this equation, the winemaker can predict in what density he should stop the fermentation (x) to achieve the desired residual sugar concentration (y).
For example, in order to achieve a residual sugar value of 30 g·L
-1, the fermentation should be stopped at a density of 1001.6 kg/m
3, calculated as follows:
Application of the model to natural musts
The analysis method mentioned above was developed using synthetic musts. In order to investigate whether it could be applied to natural wines, Malbec grapes were harvested at different initial brix values and fermented (as detailed in the material and methods section). Density and residual sugar values during fermentation are summarized in
Table 2.
The results show that the natural must fermentations were faster than those of the synthetic musts, as expected and well-published [
30]. The basic reason for this is the well balanced nutritional status of the natural musts, which includes a wide variety of nitrogen and carbohydrate sources compared to the synthetic wine.
In addition, as with the synthetic fermentations, musts with higher brix values needed extended fermentation durations. Linear regression between the residual sugar of the wine and its density was obtained for all treatments, similar to the process we developed for the synthetic musts. The different equations are summarized in
Table 3.
As we have done with the synthetic wines, to predict the density needed for a specific residual sugar value for any given initial brix value, we used the different linear equations presented in
Table 3 and plotted new curves (
Figure 5).
Table 4 presents an example of the densities (and linear regression parameters) in which the fermentation should be terminated to achieve a residual sugar value of 30 g·L
-1, for different initial musts brix values.
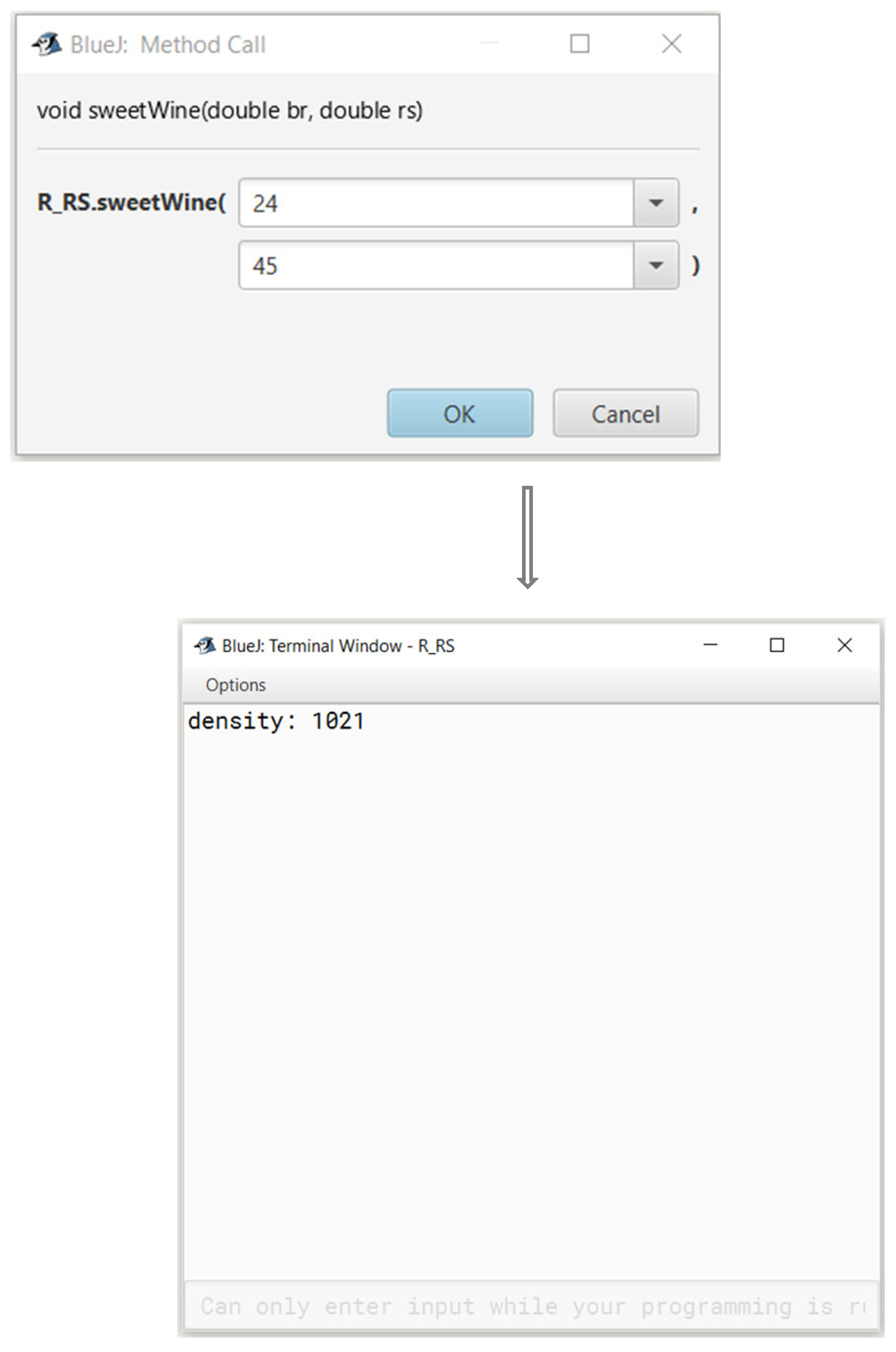
In order to achieve a practical tool that could be useful for the winemaker, we created an algorithm which uses the regressions we calculated for the natural musts for calculating the slope and intercept for any given initial brix level, and allows the user to ask for a, desirable residual sugar levels. The algorithm then creates the final equation and calculates the density point in which one should terminate the fermentation. A snapshot of this calculator is presented in
Figure 6; the code is available in the supplementary data.
4. Discussion
Developments in techniques for brix and sugar measurements in wine, among other fruits and products, were published in a review by Jaywant et al. [
17]. It was concluded that despite the various methods and instruments for sugar and Brix measurements in wine, there is still a need for prompt, low cost analysis, with minimal sample preparation. Vis/NIRS has been proposed as such alternative to traditional methods due to its rapidity, simplicity, cost effectiveness and potential for routine analysis (after proper calibration and validation). Residual sugars (g·L
-1) by such method was measured by Urbano-Cuadrado et al. with R
2 of 0.705 [
31] and total sugars (g·L
-1) were measured by Páscoa et al., with R
2 of 0.94 [
32]. Swe et al. developed a brix estimation model for both destructive and nondestructive measurements (R
2 of 0.72 and 0.85 respectively), using hyperspectral imaging combined with two different machine-learning approaches, ridge regression and the EBM [
10]. Recent study on combination of colorimetric reaction with digital image analysis proved to be a viable strategy for reducing sugar quantification [
8]. In our study, we aimed to achieve a model for estimating the residual sugars concentration rather than brix estimation. The residual sugars concentration (in units of g·L
-1) is critical for determination of the wine style and for vinification decisions (e.g., when to stop fermentation for achieving a specific sugar concentration), a well as for legislation of the wine in a proper category. Using our model enables the user to achieve an accurate measurement of sugar concentration with a simple measurement of initial brix followed by density measurements during fermentation (R
2 of 0.9), a very simple technique used regularly by wineries to monitor the fermentation status. The advantage of the model is by the prediction of the actual residual sugar concentration, rather than prediction of brix values, by simple and basic equipment that are common in every winery.
It is worth mentioning that our model is applicable for common brix values of grapes used for fermentation (i.e above 18 and lower than 27° Bx), mild fermentation conditions of temperature (circa 25°C), and low aeration conditions. Extreme condition (i.e very high or very low fermentation temperatures or extreme anaerobic/aerobic conditions) could influence the model by changing the ratio of alcohol yield versus initial residual sugar concentration. Temperature elevation influences the level of yeast biomass during fermentation [
33]. It has been reported that the higher the temperature- the lower the production of ethanol from the same initial level of sugars [
34]. However, the poor impact of the fermentation temperature on the final alcoholic strength has also been described by several studies [
35,
36]. According to this, even low fermentation temperatures have no effect on the final alcohol content as long as the temperature is high enough to allow the development of the yeast [
36].
Regarding the anaerobic/aerobic conditions, ethanol yields are lower under aerobic than under anaerobic conditions [
37]. Thus, further experiments should be held, elaborating our model to higher aeration conditions during fermentation. For example- the use of macro oxidation methods, which become increasingly applicable in recent years due to the development of available and feasible equipment [
38], might indeed change the sugar to alcohol ratio, and thus effect the predictability of residual sugar by the model we suggest
4. Conclusions
In this work, we have shown a straightforward approach for calculating the exact density point at which one can accurately terminate a fermentation to produce a wine with the requested residual sugar. The method is based on a measurement with a hydrometer- a basic tool used for monitoring the fermentation and can be used within the Brix range of most normal musts.
This novel approach is a rapid and practical tool that could be very useful for the winemaker for planning the wine’s residual sugar prior to fermentation, saving time, costs and effort. The practical calculator we coded can be implemented into a winery’s web site to be used freely during harvest time.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, code for
Figure 6.
Author Contributions
“Conceptualization, E.D.; methodology, E.D., R. Y., M.S. and E.S.; software, E.S..; formal analysis, E.S and V.B.H.; investigation, E.D., R.Y. and E.S.; resources, E.D.; data curation, E.S., V.B.A. and R.Y.; writing—original draft preparation, R.Y. E.S. and E.D.; writing—review and editing, E.D. and R.Y.; visualization, E.S., V.B.A. and R.Y.; supervision, E.D..; project administration, E.D., R.Y.; funding acquisition, E.D.. All authors have read and agreed to the published version of the manuscript.” Please turn to the CRediT taxonomy for the term explanation. Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Data Availability Statement
The data used for this work is presented in the manuscript or within the supplementary data section.
Acknowledgments
we wish to acknowledge the assistance of Daniel Schneiderman with the coding of the calculation tool.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maicas, S. Advances in Wine Fermentation. Fermentation 2021, 7. [Google Scholar] [CrossRef]
- Jacobson, J.L. Introduction to Wine Laboratory Practices and Procedures, 1st ed.; Springer US, 2006; ISBN 978-0-387-24377-1. [Google Scholar]
- Koone, R.; Harrington, R.J.; Gozzi, M.; McCarthy, M. The role of acidity, sweetness, tannin and consumer knowledge on wine and food match perceptions. J. Wine Res. 2014, 25, 158–174. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis Edition 2022 Volume 1; Paris, France, 2022; Vol. 1, ISBN 9782850380525. [Google Scholar]
- Son, H.S.; Hong, Y.S.; Park, W.M.; Yu, M.A.; Lee, C.H. A Novel Approach for Estimating Sugar and Alcohol Concentrations in Wines Using Refractometer and Hydrometer. 2009. 2009. [CrossRef]
- Rogerson, F.S.; Symington, C. A Method for the Estimation of Alcohol in Fortified Wines Using Hydrometer Baumé and Refractometer Brix. Am. J. Enol. Vitic. 2006. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural products—A review. Sci. Hortic. (Amsterdam). 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Teixeira, G.G.; Santos, P.M. Simple and cost-effective approaches for quantification of reducing sugar exploiting digital image analysis. J. Food Compos. Anal. 2022, 113, 104719. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Al Angari, Y.M.; Alotaibi, M.M.; Almulaiky, Y.Q. Novel and Facile Colorimetric Detection of Reducing Sugars in Foods via In Situ Formed Gelatin-Capped Silver Nanoparticles. Polymers (Basel). 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Swe, K.N.; Takai, S.; Noguchi, N. Novel approaches for a brix prediction model in Rondo wine grapes using a hyperspectral Camera: Comparison between destructive and Non-destructive sensing methods. Comput. Electron. Agric. 2023, 211, 108037. [Google Scholar] [CrossRef]
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid detection of apple juice concentrate adulteration with date concentrate, fructose and glucose syrup using HPLC-RID incorporated with chemometric tools. Food Chem. 2022, 370, 131015. [Google Scholar] [CrossRef]
- Jalaludin, I.; Kim, J. Comparison of ultraviolet and refractive index detections in the HPLC analysis of sugars. Food Chem. 2021, 365, 130514. [Google Scholar] [CrossRef]
- Lindqvist, D.N.; Pedersen, H.Æ.; Rasmussen, L.H. A novel technique for determination of the fructose, glucose and sucrose distribution in nectar from orchids by HPLC-ELSD. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1081–1082, 126–130. [Google Scholar] [CrossRef]
- Georgelis, N.; Fencil, K.; Richael, C.M. Validation of a rapid and sensitive HPLC/MS method for measuring sucrose, fructose and glucose in plant tissues. Food Chem. 2018, 262, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Croce, R.; Malegori, C.; Oliveri, P.; Medici, I.; Cavaglioni, A.; Rossi, C. Prediction of quality parameters in straw wine by means of FT-IR spectroscopy combined with multivariate data processing. Food Chem. 2020, 305, 125512. [Google Scholar] [CrossRef] [PubMed]
- Thanasi, V.; Catarino, S.; Ricardo-Da-Silva, J. Fourier transform infrared spectroscopy in monitoring the wine production. Cienc. e Tec. Vitivinic. 2022, 37, 79–99. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Singh, H.; Arif, K.M. Sensors and Instruments for Brix Measurement: A Review. Sensors 2022, 22, 1–20. [Google Scholar] [CrossRef]
- Amerine, M.A.; Ough, C.S. Methods for Analysis of Musts and Wines; Wiley: New York, 1980. [Google Scholar]
- Iland, P. Techniques for chemical analysis and quality monitoring during winemaking; Patrick Iland Wine Promotions. (2000).: Campbelltown, S. Aust.
- Rankine, B.C. Making good wine : a manual of winemaking practice for Australia and New Zealand; Sun Books: South Melbourne, 1989. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine analysis and production; Aspen Publishers: Maryland, 1995. [Google Scholar]
- Franco, M. de O.K.; Suarez, W.T.; dos Santos, V.B.; Resque, I.S. A novel digital image method for determination of reducing sugars in aged and non-aged cachaças employing a smartphone. Food Chem. 2021, 338, 127800. [Google Scholar] [CrossRef] [PubMed]
- Hernández-López, A.; Sanchez Felix, D.A.; Sierra, Z.Z.; Bravo, I.G.; Dinkova, T.D.; Avila-Alejandre, A.X. Quantification of reducing sugars based on the qualitative technique of Benedict. ACS Omega 2020, 5, 32403–32410. [Google Scholar] [CrossRef]
- Martens, M.; Hadrich, M.J.; Nestler, F.; Ouda, M.; Schaadt, A. Combination of Refractometry and Densimetry – A Promising Option for Fast Raw Methanol Analysis. Chemie-Ingenieur-Technik 2020, 92, 1474–1481. [Google Scholar] [CrossRef]
- D’Amato, D.; Corbo, M.R.; Del Nobile, M.A.; Sinigaglia, M. Effects of temperature, ammonium and glucose concentrations on yeast growth in a model wine system. Int. J. Food Sci. Technol. 2006, 41, 1152–1157. [Google Scholar] [CrossRef]
- Delfini, C.; Costa, A. Effects of the Grape Must Lees and Insoluble Materials on the Alcoholic Fermentation Rate and the Production of Acetic Acid, Pyruvic Acid, and Acetaldehyde. Am. J. Enol. Vitic. 1993, 44, 86 LP – 92. [Google Scholar] [CrossRef]
- Lema, C.L.; García-Jares, C.; Orriols, I.; Angulo, L.E.S. Contribution of Saccharomyces and Non-Saccharomyces Populations to the Production of Some Components of Albariño Wine Aroma. Am. J. Enol. Vitic. 1996. [Google Scholar] [CrossRef]
- Tiwari, A. Practical Biochemistry: A Student Companion; 2015; ISBN 3659757160. [Google Scholar]
- Berthels, N.J.; Cordero Otero, R.R.; Bauer, F.F.; Thevelein, J.M.; Pretorius, I.S. Discrepancy in glucose and fructose utilisation during fermentation by Saccharomyces cerevisiae wine yeast strains. FEMS Yeast Res. 2004, 4, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Viana, T.; Loureiro-Dias, M.C.; Prista, C. Efficient fermentation of an improved synthetic grape must by enological and laboratory strains of Saccharomyces cerevisiae. AMB Express 2014, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Urbano-Cuadrado, M.; Luque De Castro, M.D.; Pérez-Juan, P.M.; García-Olmo, J.; Gómez-Nieto, M.A. Near infrared reflectance spectroscopy and multivariate analysis in enology: Determination or screening of fifteen parameters in different types of wines. Anal. Chim. Acta 2004, 527, 81–88. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Porto, P.A.L.S.; Cerdeira, A.L.; Lopes, J.A. The application of near infrared spectroscopy to wine analysis: An innovative approach using lyophilization to remove water bands interference. Talanta 2020, 214, 120852. [Google Scholar] [CrossRef] [PubMed]
- Vamvakas, S.S.; Kapolos, J. Factors affecting yeast ethanol tolerance and fermentation efficiency. World J. Microbiol. Biotechnol. 2020, 36, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, F.J.; Jewett, M.C.; Nielsen, J.; Agosin, E. Growth temperature exerts differential physiological and transcriptional responses in laboratory and wine strains of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2008, 74, 6358–6368. [Google Scholar] [CrossRef]
- Rodrigues, A.J.; Raimbourg, T.; Gonzalez, R.; Morales, P. Environmental factors influencing the efficacy of different yeast strains for alcohol level reduction in wine by respiration. Lwt 2016, 65, 1038–1043. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Influence of temperature during pre-fermentative maceration and alcoholic fermentation on the phenolic composition of ‘cabernet sauvignon’ wines. Foods 2021, 10. [Google Scholar] [CrossRef]
- Tronchoni, J.; Gonzalez, R.; Guindal, A.M.; Calleja, E.; Morales, P. Exploring the suitability of Saccharomyces cerevisiae strains for winemaking under aerobic conditions. Food Microbiol. 2022, 101, 103893. [Google Scholar] [CrossRef]
- Rossi, S.; Bestulić, E.; Horvat, I.; Plavša, T.; Lukić, I.; Bubola, M.; Ganić, K.K.; Ćurko, N.; Jagatić Korenika, A.M.; Radeka, S. Comparison of different winemaking processes for improvement of phenolic composition, macro- and microelemental content, and taste sensory attributes of Teran (Vitis vinifera L.) red wines. Lwt 2022, 154. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).