Introduction
Hepatitis delta virus (HDV), belonging to the Herpeviridae family, is a small, defective RNA virus. Its existence is contingent upon concurrent infection with the hepatitis B virus (HBV), either through coinfection or superinfection 1,2. The ramifications of HDV infection can be severe, exacerbating chronic HBV infection and accelerating the progression to liver failure, cirrhosis, or hepatocellular carcinoma, especially in younger patients 1,3–5.
HDV is categorized into eight global genotypes, each with distinct geographical distributions, disease severities, and levels of accuracy in viral load quantification. Genotype 1 is notably widespread in Western countries 1–3. HDV's estimated prevalence is 0.8% among the general population and rises to 10-13% among those afflicted with HBV, indicating that around 48 to 60 million individuals are affected worldwide 5. Nevertheless, it is widely acknowledged that the true prevalence of HDV remains underestimated.
Historically, the primary treatment for HDV has been pegylated interferon alpha, administered for a minimum of 48 weeks. However, the overall virological response rate remains low 1,4. Recently, the European Medicines Agency (EMA) granted approval to an antiviral medication for HDV treatment—Bulevirtide (Hepcludex®). Its mode of action involves hindering the entry of HBV and HDV into hepatocytes by interacting with and deactivating the sodium/taurocholate cotransporting polypeptide (NTCP), a bile salt transporter functioning as a receptor. This drug is currently indicated for the treatment of chronic HDV infection in adults with positive HDV-RNA in plasma (or serum) and compensated liver disease. An HDV viral load reduction of 2 logs has been proposed as an endpoint to assess drug efficacy 6. Thus, precise HDV-RNA detection and quantification are pivotal for accurate diagnosis and treatment monitoring 7–9. However, currently, there are no FDA-approved assays available for HDV-RNA detection and quantification.
Considering the pivotal role of viral load monitoring in evaluating the efficacy of the newly approved HDV treatment, our study aims to evaluate and compare the performance attributes of three HDV RNA detection and quantification assays—two commercially available and one in developmental stages.
Materials and Methods
Study Population and Specimens
In our study, a total of 151 clinical samples were included from patients who tested positive for HBsAg. Out of these, 90 samples were derived from patients who were negative for both anti-HDV antibodies and HDV-RNA (45 from serum and 45 from plasma). Additionally, 61 samples of serum or plasma (33 from serum and 28 from plasma) were collected from patients who tested positive for both anti-HDV antibodies and HDV-RNA. Among the latter group, 36 samples originated from the Microbiology Department of the Clinical University Hospital San Cecilio in Granada. These samples represented patients from various countries including Spain (n=25), Senegal (n=5), Ukraine (n=2), Western Sahara (n=1), Romania (n=1), Ivory Coast (n=1), and Equatorial Guinea (n=1). The remaining 25 samples were obtained from an international patient collection (Cerba Research Biorepository, Gent, Belgium), with patients hailing from France (n=15), Cameroon (n=5), Romania (n=4), and Mauritania (n=1). Anti-HDV were tested using Liaison XL Murex Anti-HDV (Diasorin).
Molecular Assays for HDV Detection and Quantification
Our study involved the comparison of three different assays for the detection and quantification of Hepatitis D Virus (HDV). These assays included the Hepatitis Delta RT-PCR system kit from Vircell (Spain), the EurobioPlex HDV qRT-PCR assay from Eurobio Scientific (France), and the RoboGene HDV RNA Quantification kit 2.0 from Roboscreen Diagnostics (Germany). The latter two assays are labeled as Conformité Europeéne (CE) and in vitro diagnostics (IVD) tests, designed for HDV detection and quantification in routine clinical practice.
Before conducting reverse transcription and amplification with all assays, a nucleic acid extraction was performed on 300 μL of samples using the Maelstrom 4810 system (TANBead). The resulting nucleic acids were eluted in a volume of 60 μL. Real-time PCRs were carried out using a CFX-96 real-time thermocycler (Bio-Rad®, CA, USA). Each assay was conducted using the same RNA eluate for consistency following the PCR profile indicated in manufacturer´s instructions and maintaining the sample and master mix ratio recommended. Specifically, 5μl of RNA eluate was used for the Hepatitis Delta RT-PCR system kit and the RoboGene HDV RNA Quantification test, while 10μl of eluate was required for the EurobioPlex HDV assay.
Additionally, we evaluated the WHO international standard for HDV, PEI 7657/12, with a concentration of 575,000 IU/ml. Three serial dilutions of this standard were tested in triplicate, with concentrations of 5,750 IU/ml (3.76 log IU/mL), 575 IU/ml (2.76 log IU/mL), and 23 IU/ml (1.36 log IU/mL) respectively
The Vircell assay utilizes real-time PCR for the detection and quantification of HDV-RNA. It targets a specific region of the HDV genome (HDAg-L gene) and can be applied to both serum and plasma samples. The LoD of the assay is 23 IU/mL. The EurobioPlex HDV assay features primers and probes designed for the HDV antigen-coding region. Its sensitivity and specificity stand at 97.7% and 93.4% respectively, with a Limit of Detection (LOD) of 1x102 IU/mL 10,11. The RoboGene HDV RNA Quantification test employs primers and probes specific to a subsequence of the Hepatitis delta antigen. This kit boasts a 100% specificity, and its sensitivity and LOD vary depending on the real-time PCR instrument and purification kit utilized 12.
Statistical analysis
All the graphs, calculations, and statistical analyses were performed using GraphPad Prism software version 8.0 (GraphPad Software, San Diego, CA, USA). For qualitative variables, a concordance analysis was carried out, and the results were expressed as a percentage. To explore the correlation between quantitative results, which were transformed into Log IU/mL values, a linear regression analysis was conducted. This analysis aimed to assess the goodness-of-fit, and the correlation coefficient (r2) was calculated. Additionally, the Bland-Altman plot was generated to determine the Bias index. By plotting assay results against each other, a regression analysis was performed to compute the correlation coefficient.
HDV Genotyping
For the purpose of genotyping, a sequencing strategy based on near full-length amplicons with overlapping primers was employed
13. Subsequently, these amplicons underwent sequencing using Illumina's tagmentation-indexing strategy, and the resulting libraries were processed using a NextSeq 1000 system. The assembly process involved the utilization of CLC-Genomics-Workbench software, which employed reference sequences from various genotypes sourced from the Hepatitis Delta Virus Database (
https://hdvdb.bio.wzw.tum.de/hdvdb/).
Ethics Approval
The study adhered to the principles outlined in the Declaration of Helsinki and was both designed and conducted accordingly. It received approval from the local Ethics Committee of Hospital Universitario Clínico San Cecilio in Granada. Due to the deidentified nature of the testing conducted, individual patient consent was not deemed necessary for this study.
Results
Concordance between Tests
a) Qualitative Results
Among the 151 samples assessed, qualitative results indicated that 61 samples were positive using the Vircell assay, 61 with the EurobioPlex HDV kit, and 60 with the RoboGene HDV assay (one positive sample by Vircell & Eurobio could not be tested with Robogene). Using the RoboGene test as the reference, the overall concordance rates were 100% for Vircell and 100% for EurobioPlex. Sensitivity, specificity, positive predictive value, and negative predictive value, along with their 95% confidence intervals, were 100% (94.1 - 100%), 100% (96.0 - 100%), 100% (94.1 - 100%), 100% (96.0 - 100%) for the EurobioPlex assay, respectively. For the Vircell assay, the values were 100% (94.0 - 100%), 100% (96.0 – 100%), 100% (94.0 –100%), and 100% (96.0 - 100%), respectively. A detailed presentation of these findings can be seen in
Table 1.
b) Quantitative Results
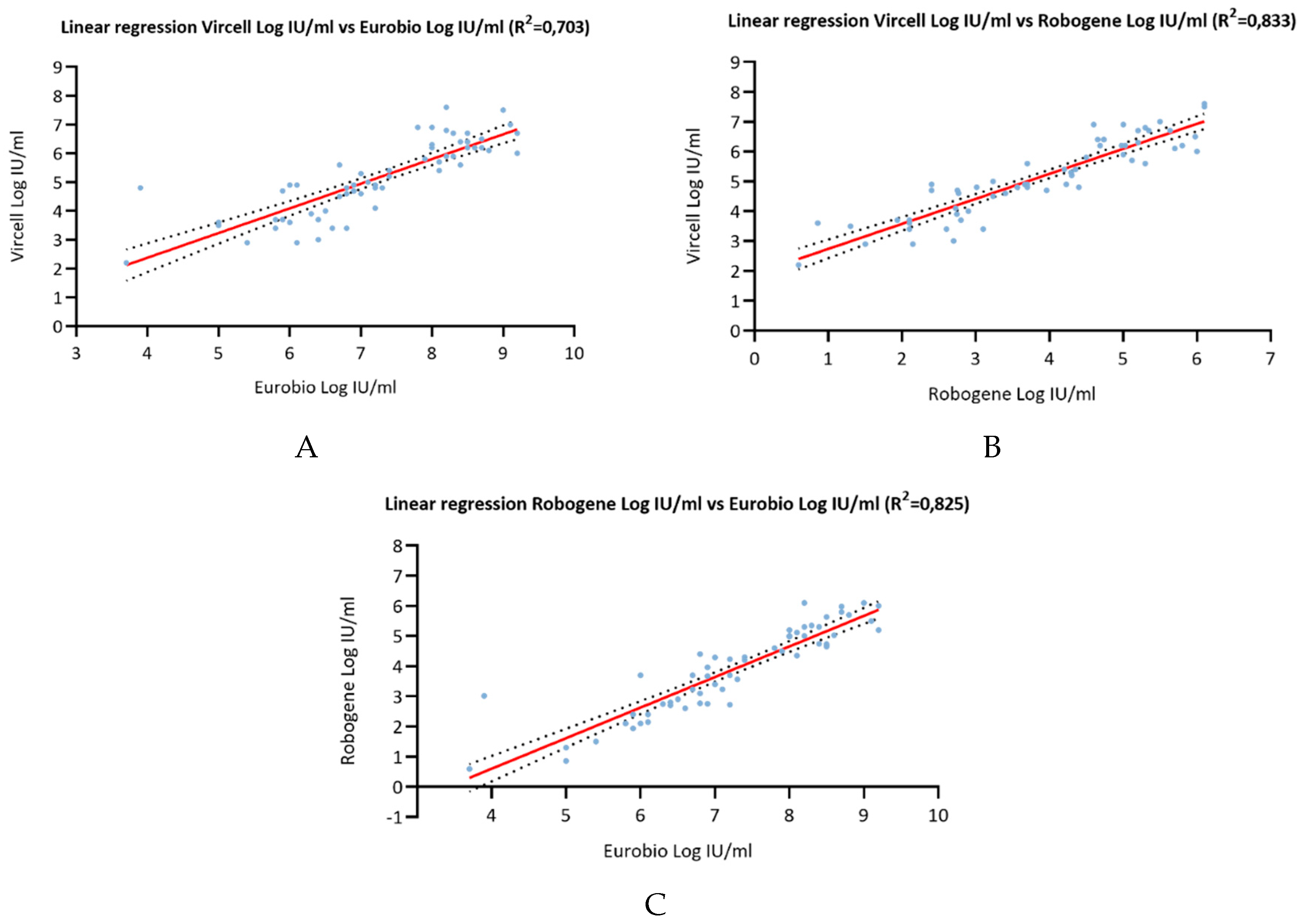
A goodness-of-fit analysis was employed to determine the correlation coefficients (r2) between the three tests (
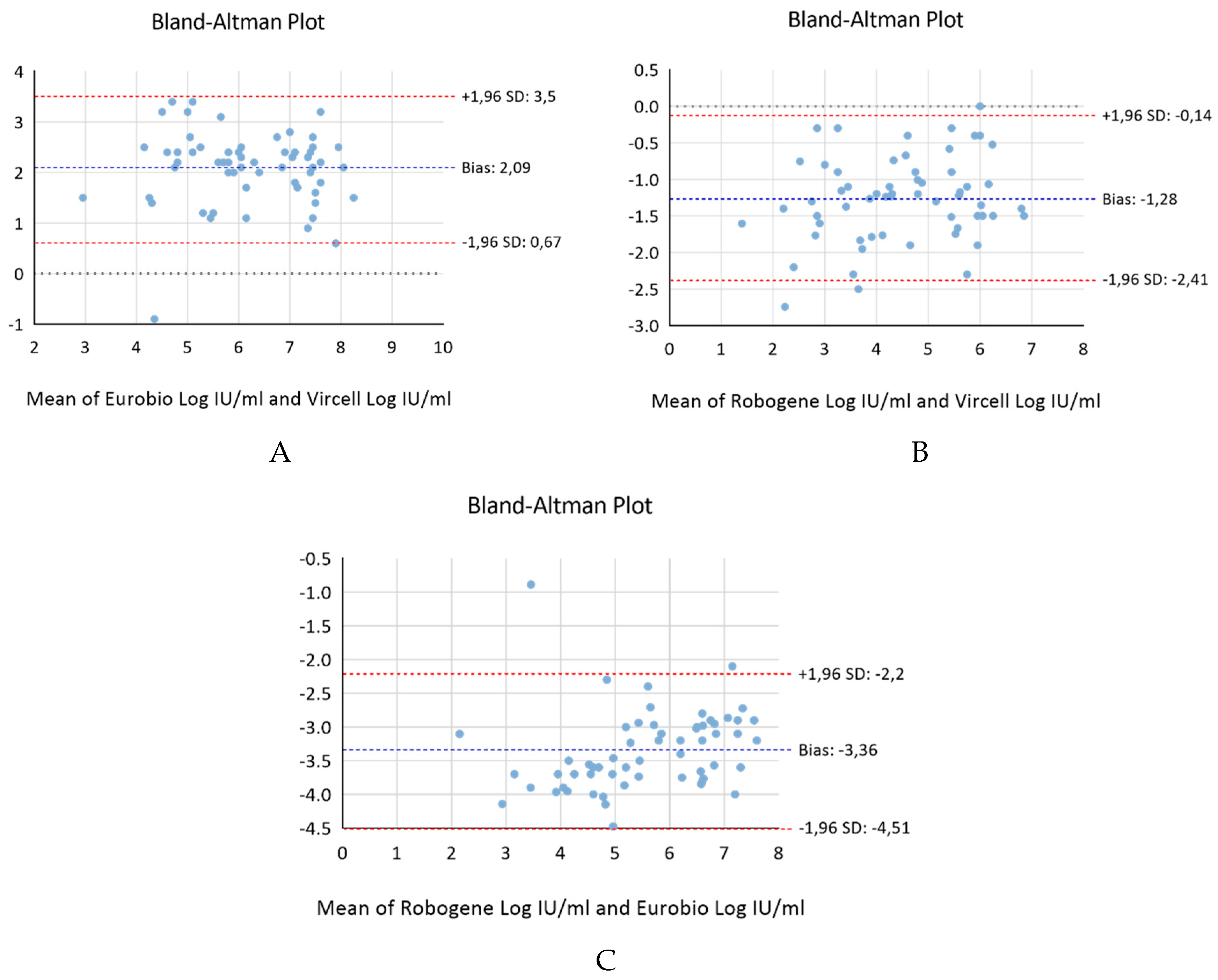
Figure 1). The calculated coefficients were as follows: r2= 0.703 for Vircell versus Eurobio, r2= 0.833 for Vircell versus RoboGene, and r2= 0.835 for Robogene versus Eurobio. The bias index, determined through a Bland-Altman analysis, produced results of 2.083 for Vircell versus Eurobio, -1.283 for Vircell versus RoboGene, and -3.36 for Robogene versus Eurobio (
Figure 2).
Assay Comparison using WHO International Standard
Table 2 presents results obtained for the three serial dilutions of the WHO international standard (IS) tested concurrently by the three kits. For an IS concentration of 5750 IU/mL (3.76 log IU/mL), the differences in log IU/mL quantification were -0.82 for Vircell, 1.26 for RoboGene, and 0.83 for Eurobio. At an IS concentration of 575 IU/mL (2.76 log IU/mL), the differences were -1.07 for Vircell, 1.57 for RoboGene, and 0.11 for Eurobio. Lastly, for an IS concentration of 23 IU/mL (1.36 log IU/mL), the differences were -0.98 for Vircell, 0.89 for RoboGene, and -1.06 for Eurobio. In summary, the Vircell kit overestimated the viral load by 0.98 log IU/mL, the Eurobio assay by 1.46 log IU/mL, and the RoboGene kit underestimated it by 0.98 log IU/mL.
Genotyping Analysis
Among the 59 samples subjected to full HDV genome sequencing, 51 were successfully classified as Genotype 1, 7 as Genotype 5, and 1 as Genotype 6. Samples classified as Genotype 1 came from Spain (n=20), France (n=4), Cameroon (n=4), Romania (n=2), Ukraine (n=2), Senegal (n=1), Mauritania (n=1), Equatorial Guinea (n=1) and Western Sahara (n=1). Genotype 5 samples originated from patients in France, Senegal, and Ivory Coast, while the Genotype 6 sample was from a Cameroonian patient. For eight samples the genotype could not be ascribed, 2 of them being excluded due to insufficient eluted volume, an additional two samples due to low RNA quantification, and 4 samples due to low quality sequencing. Correlation coefficients for Genotype 1 patients were r2= 0.686 for Vircell versus Eurobio, r2= 0.843 for Vircell versus RoboGene, and r2= 0.799 for Robogene versus Eurobio. Bias index values were 2.1 for Vircell versus Eurobio, -1.31 for Vircell versus RoboGene, and -3.4 for Robogene versus Eurobio. For Genotype 5 patients, correlation coefficients were r2= 0.947 for Vircell versus Eurobio, r2= 0.973 for Vircell versus RoboGene, and r2= 0.991 for Robogene versus Eurobio. Bias index values were 2.21 for Vircell versus Eurobio, -0.79 for Vircell versus RoboGene, and -3 for Robogene versus Eurobio.
Discussion
HDV exacerbates liver disorders, precipitating severe outcomes like liver failure, cirrhosis, and hepatocellular carcinoma 14. The limited success of conventional therapies accentuates the urgency for effective options, exemplified by Bulevirtide, heralding a novel era for these challenging patients 7. This accentuates the demand for accurate, standardized, and sensitive HDV-RNA assays 15. Our study meticulously scrutinized three HDV RNA detection assays (Vircell, EurobioPlex, and RoboGene), revealing considerable concordance rates that consistently discerned HDV-positive and HDV-negative samples. Nevertheless, quantitative assessment unveiled noteworthy discrepancies among the assays, reinforcing the imperative of monitoring HDV RNA levels with the same assay and laboratory for treatment monitoring to mitigate inter-laboratory and inter-assay variability.
In our investigation, both the EurobioPlex and Vircell assays achieved high sensitivity and specificity, when compared to the RoboGene assay as the benchmark. The robustness demonstrated by the three assays in identifying chronic HDV infection is remarkable. However, it is important to note that our study did not involve the examination of longitudinal samples from the same patient. Recent research has unveiled a decline in HDV viral load over time in a substantial subset of patients, especially those with cirrhosis 16, and is often accompanied by reduced aminotransferase levels, which emphasizes the significance of re-testing serum HDV RNA.
Quantitative analysis provided insights into the correlation and bias between the different assays. Correlation coefficients with values ranging from 0.703 to 0.835, revealed that all the three tests can be considered as highly correlated. Although Bland-Altman plots could reinforce these findings, with in theory acceptable bias indices across the assays, and indicating that while each assay may slightly differ in quantification, they generally yield comparable results, we believe, as others 15, that for clinical use the observed differences between tests do not warrant interchangeability to monitor the antiviral treatment of chronic HDV. The reduction of HDV replication stands as a pivotal objective in treating HDV infection. Hence, continuous monitoring of viral load throughout treatment utilizing rigorously standardized and validated real-time molecular assays is imperative 17. To circumvent inter-laboratory discrepancies and mitigate inter-assay variability 6,17, ensuring accurate and consistent measurements of HDV RNA viral load becomes paramount.
In order to ascertain whether the apparent bias detected during the analysis of clinical samples could be ascribed to the RNA extraction procedure or the specific real-time PCR platform employed (BIO-RAD CFX), we conducted a comparative assessment utilizing the WHO International Standard. Our investigations validated our initial findings, as all three tests consistently exhibited variations (either overestimating or underestimating) across all tested dilutions.
In addition, genotyping further enriched the study's findings. The considerable genetic diversity observed across distinct HDV genotypes and certain sub-genotypes has been demonstrated to contribute to the underestimation of viral load by numerous commercially accessible assays. This effect is particularly notable in instances involving African sub-genotype 1 and African genotypes 5-8 18. Regrettably, the prevalence of HDV genotypes within our study population was primarily confined to genotype 1. As anticipated, the correlation coefficients and bias indices for this genotype closely mirrored those of the broader global study. In the instances where we could assess a limited number of genotype 5 cases, the correlation was highly satisfactory; however, there remained an observable bias between the tests.
As already discussed, it is important to acknowledge the limitations of our study. The sample size and population diversity, while representative of certain regions, might not fully capture global HDV diversity. Additionally, the assays' performance might be influenced by various factors like operator experience, laboratory conditions, method of extraction of RNA and platform used for running the PCR.
In conclusion, this study demonstrates the importance of reliable HDV-RNA detection and quantification assays. The assays used for this study show promising performance in diagnosing HDV infections. Their accuracy, as shown by the concordance rates and quantitative analysis, makes them suitable tools for clinical practice. However, until there is harmonization across the different assays, quantitative HDV RNA monitoring in sequential serum samples should be performed in the same laboratory and with the same assay to avoid inter-laboratory and interassay variability. Efforts should be directed towards developing standardized HDV detection assays that consider genotypic diversity and global distribution. Collaborative studies involving larger and more diverse patient cohorts could further validate the findings and guide assay selection for specific genotypes and regions.
Author Contributions
Conceptualisation: Adolfo De Salazar, Federico García. Formal analysis: Adolfo De Salazar, Marta Illescas-López, Lucía Chaves-Blanco. Research: Adolfo de Salazar, Marta Illescas-López, Lucía Chaves-Blanco. Methodology: Adolfo De Salazar, Federico García, Marta Illescas-López, Lucía Chaves-Blanco. Validation: Adolfo De Salazar. Project Management: Adolfo De Salazar, Federico García. Data curation: Adolfo De Salazar, Marta Illescas-López. Resources: Adolfo De Salazar, Melisa Hernández-Febles, Raquel Carracedo, Eduardo Lagarejos, Ana Fuentes, Sara Pereira, Maria Cea, Alberto De La Iglesia, Carolina Freyre, Asunción Iborra, Valle Odero, Aurora García-Barrionuevo, Antonio Aguilera, María José Pena, Federico García. Supervision: Adolfo De Salazar, Federico García. Writing - original draft: Marta Illescas-López, Adolfo De Salazar. Writing - proofreading and editing: Adolfo De Salazar, Federico García.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Ethicts approval
This study was performed in line the participles of the Declaration of Helsinki. Approval was obtained from Hospital Universitario Clínico San Cecilio’s Ethics Committee.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Availability of data and material
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The results published are part of the thesis’s work, of the PhD candidate Marta Illescas López, in the Biomedicine Doctoral Program fo the University of Granada.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
References
- Hughes, S. A.; Wedemeyer, H.; Harrison, P. M. Hepatitis Delta Virus. Lancet 2011, 378, 73–85. [Google Scholar] [CrossRef]
- Negro, F.; Suk-Fong, A. Epidemiology, clinical manifestations and diagnosis of hepatitis D virus infection - UpToDate. 2023. Available online: https://www.uptodate.com/contents/epidemiology-clinical-manifestations-and-diagnosis-of-hepatitis-d-virus-infection?search=Pathogenesis, epidemiology, natural history, and clinical manifestations of hepatitis D virus infection&source=search_result&selectedTitle=2~54&usage_type=default&display_rank=2 (accessed on 13 August 2023).
- Rizzetto, M. Hepatitis D virus (HDV) infection and disease. Clin. Lab 1989, 19, 11–26. [Google Scholar] [CrossRef]
- Alvarado-Mora, M. V.; Locarnini, S.; Rizzetto, M.; Rebello Pinho, J. R. An Update on HDV: Virology, Pathogenesis and Treatment. Antivir. Ther. 2013, 18, 541–548. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, S.; Ou, X.; et al. Estimating the Global Prevalence, Disease Progression, and Clinical Outcome of Hepatitis Delta Virus Infection. J. Infect. Dis. 2020, 221, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Yurdaydin, C.; Abbas, Z.; Buti, M.; et al. Treating Chronic Hepatitis Delta: The Need for Surrogate Markers of Treatment Efficacy. J. Hepatol. 2019, 70, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Urban, S.; Neumann-Haefelin, C.; Lampertico, P. Hepatitis D Virus in 2021: Virology, Immunology and New Treatment Approaches for a Difficult-to-Treat Disease. Gut 2021, 70, 1782–1794. [Google Scholar] [CrossRef]
- European Medicines Agency. Ficha Técnica o Resumen de las Características del Producto: HEPCLUDEX. Available online: https://www.ema.europa.eu/en/documents/product-information/hepcludex-epar-product-information_es.pdf (accessed on 13 May 2023).
- Kang, C.; Syed, Y.Y. Bulevirtide: First Approval. 2020, 80, 1601–1605. [Google Scholar] [CrossRef]
- EurobioPlex HDV. Eurobio Scientific. Available online: https://eurobio-scientific.eu/fr/hepatite/5185-eurobioplex-hdv.html (accessed on 13 August 2023).
- Le Gal, F.; Dziri, S.; Gerber, A.; et al. Performance Characteristics of a New Consensus Commercial Kit for Hepatitis D Virus RNA Viral Load Quantification. J. Clin. Microbiol. 2017, 55, 431–441. [Google Scholar] [CrossRef]
- RoboGene HDV RNA Quantification Kit 2.0. Roboscreen Diagnostics. 2020. Available online: https://www.roboscreen.com/fileadmin/content/Manual_qHDV-TM2_e_rev_7_WEB.pdf (accessed on 13 August 2023).
- Çelik, I.; Karatayli, E.; Çevik, E.; et al. Complete Genome Sequences and Phylogenetic Analysis of Hepatitis Delta Viruses Isolated from Nine Turkish Patients. Arch. Virol. 2011, 156, 2215–2220. [Google Scholar] [CrossRef] [PubMed]
- Da, B. L.; Heller, T.; Koh, C. Hepatitis D Infection: From Initial Discovery to Current Investigational Therapies. Gastroenterol. Rep. 2019, 7, 231–245. [Google Scholar] [CrossRef]
- Association for the Study of the Liver, E.; Rossana Brunetto, M.; Ricco, G.; et al. EASL Clinical Practice Guidelines on Hepatitis Delta Virus. J Hepatol. 2023, 79(2), 433–460. [Google Scholar] [CrossRef]
- Palom, A.; Sopena, S.; Riveiro-Barciela, M.; et al. One-Quarter of Chronic Hepatitis D Patients Reach HDV-RNA Decline or Undetectability during the Natural Course of the Disease. Aliment. Pharmacol. Ther. 2021, 54, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, F.; Brichler, S.; Sahli, R.; Chevret, S.; Gordien, E. First International External Quality Assessment for Hepatitis Delta Virus RNA Quantification in Plasma. Hepatology 2016, 64, 1483–1494. [Google Scholar] [CrossRef]
- Brichler, S.; Le Gal, F.; Butt, A.; Chevret, S.; Gordien, E. Commercial Real-Time Reverse Transcriptase PCR Assays Can Underestimate or Fail to Quantify Hepatitis Delta Virus Viremia. Clin. Gastroenterol. Hepatol. 2013, 11, 734–740. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).