1. Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease characterized by inflammation and tissue organ damage. Its etiology is complex and not fully understood, as contributing mechanisms include genetic, hormonal and environmental factors, the production of pathogenic antibodies, and the deposition of immune complexes [
1].
Advanced Glycation End-products (AGEs) are one type of the endogenous inducers (signals produced by stressed, damaged or otherwise malfunctioning tissues) postulated to have an important role in chronic inflammation [
2]. AGEs are a set of compounds whose formation is a complicated molecular process resulting from the non-enzymatic interaction of reducing sugars and associated metabolites with peptides, proteins, and amino acids, through the Maillard reaction [
3]. A nucleophilic addition reaction between a free amino group from a protein and a carbonyl group from a reducing sugar results in the formation of an unstable, freely reversible Schiff base, which is rearranged to a more stable intermediate, an Amadori product [
4]. Schiff bases and Amadori products are reversible reaction products that can react irreversibly with amino acid residues of peptides or proteins to form protein adducts or protein crosslinks [
5]. Or, alternatively, they can undergo further oxidation, dehydration, polymerization and oxidative breakdown reactions to give rise to numerous other AGEs [
6]. AGEs can also accumulate under hyperglycaemic and pro-oxidative conditions, including diabetes mellitus (DM) [
7], ageing [
8], cardiovascular disease [
9], chronic renal failure [
10], and neurological disorders [
11]. Although this reaction can occur in all proteins, its action is more common in those that present a slow metabolic turnover, such as collagen This is why these compounds have also been physiologically linked to aging processes, having been reported that AGEs levels correlate with age in the general population [
12].
The mechanisms of toxicity of AGEs are mainly related to two facts. On the one hand, glycation favors cross-links between the modified proteins, causing structural alterations and a resulting gradual deterioration in cell and tissue function [
13]. In addition, these unions decrease the solubility of proteins, making them more resistant to proteolysis and generating new immunological epitopes [
14]. On the other hand, AGEs are recognized by their own receptor (RAGE), a member of the immunoglobulin superfamily, which is expressed in multiple cells like neutrophils, macrophages, T lymphocytes and synovial fibroblasts [
15]. RAGE is divided into extracellular, transmembrane, and intracellular segments [
16]. The interaction of AGEs with RAGE can activate the downstream nuclear factor kappa-B (NF-ϰB) signaling pathway and promote the secretion of TNF-α, IL-1, IL-6, and other cytokines, contributing to inflammation [
17]. Furthermore, oxidative modification of proteins has been shown to elicit antibodies in a variety of diseases including SLE [
14].
sRAGE is a positively charged 48-kDa cleavage product from RAGE that keeps the ligand binding site but loses the other two domains [
18]. sRAGE binding to ligands terminates intracellular signal transduction due to the loss of the transmembrane and intracellular fragments and inhibits the proinflammatory processes mediated by RAGE and its ligands by acting as a decoy which competitively binds to RAGE ligands [
19]. However, sRAGE has also been linked to inflammation in SLE [
20].
So far, more than 20 AGEs have been described in tissues. Among them, the most studied are protein adducts, such as Nξ-(carboxymethyl)lysine (CML), Nεξ-(carboxyethyl)lysine (CEL) or pyrraline; and intra- and intermolecular linkages, including pentosidine, glucosepane, and imidazolium compounds [
21]. Due to their stability, the most measured AGEs are CML and pentosidine. The concentration of these, although present in tissues with up to 2 degrees of difference, has shown a high correlation with each other. Classical AGEs measurement methods include chromatographic techniques associated to mass spectrometry and immunochemical methods, such as immunosorbent assay enzyme linked (ELISA) [
22]. However, a part of the AGEs has the characteristic of being fluorescent (for example pentosidine) so it is possible to quantify them in a single measurement using an autofluorescence reader. This technique, developed by Meerwaldt et al. in 2004, allows, through a non-invasive method, the measurement of fluorescent AGEs stored in the skin. Measuring accumulated AGEs makes this assessment more appropriate to quantify the concentration of AGEs in an individual throughout their life than that of a single specific moment in relation to an acute process. It has been described that this autofluorescent measurement correlates with the concentration of AGEs, both fluorescent and not fluorescent, measured in skin biopsies [
23].
In systemic autoimmune diseases, such as SLE, increased AGEs formation can be expected, as inflammation is one of the hallmarks of the disease. Chronic inflammation in SLE appears to be associated with an intensified glycation process and the formation of AGEs, having higher values of the latter compared to healthy controls (HC) been demonstrated in some studies [
24,
25,
26,
27,
28]. At the same time, through their pathogenic mechanisms, AGEs are also involved in the generation of inflammation and reactive oxygen species, creating a positive feedback that enhances inflammation and AGEs levels.
Regarding atherosclerosis, it has been observed that AGE-AGE covalent intermolecular unions in collagen I fibers induce an increase in molecular packing, causing an increase in vascular rigidity. In addition, the accumulation of AGEs in the vascular wall induces the adherence of blood cells to the endothelium, capturing immunoglobulins and apoproteins that favor the inflammatory process [
29]. Moreover, an AGE-modified form of LDL (low-density lipoproteins) has been found to circulate in human plasma, and AGE modifications have been identified as being present on both the apoprotein and the phospholipid components of LDL, converting them to glycated LDLs. It has been proposed that those AGE-modified peptides contribute to tissue injury by reattaching to susceptible target proteins both within and outside the vasculature, making them even more atherogenic [
30,
31]. In SLE, the presence of accelerated atherosclerosis that cannot be fully explained by traditional risk factors for cardiovascular disease is a well-recorded phenomenon [
32]. Some studies have suggested that increased levels of AGEs might contribute to the development of this accelerated atherosclerosis in SLE and, therefore, could be used as early markers for cardiovascular disease in this pathology [
27,
28].
Apart from age and atherosclerosis, some exogenous factors have also been reported to be positively correlated with AGEs levels like smoking status [
24,
33] or some foods [
34]. Treatment with drugs such as aminoguanidine, vitamins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin-II receptor blockers, statins, and metformin inhibit AGEs formation while Alagebrium breaks their crosslinks [
35]. Moreover, it seems that the level of circulating AGEs are genetically determined, as shown in a cohort study of healthy monozygotic and heterozygotic twins [
36].
Lately, there has been increased attention on the potential of RAGE and AGEs to target diseases, especially chronic inflammatory diseases such as SLE. Some studies have expounded on their usefulness as biomarkers of SLE diagnosis and prognosis, their relation with accelerated atherosclerosis, as well as their potential place as targets for new treatments. However, we find some controversial results in the literature, showing that more and better studies are needed to fully elucidate their role in SLE.
Having into account that the relation between skin AGEs and SLE have only been reported in one previous paper, the purpose of this work, that studies the levels of AGEs in a Spanish cohort suffering from SLE, tries to answer that unmet need encompassing several specific goals. First, to describe AGEs concentrations in SLE and compare them to age- and sex-matched HC. Secondly, to search for correlations between AGEs concentrations and SLE characteristics such as specific manifestations, indexes of activity or accrual damage, or patient reported outcomes (PROs). And finally, to explore AGEs relationship with cardiovascular disease and cardiovascular risk factors (CVRF). All of it with the ultimate aim of investigating AGEs role as potential SLE biomarkers in SLE, as well as their application in routine clinical practice as a tool for improving diagnosis, monitoring, and prognosis of the disease, or as surrogate markers for the assessment of cardiovascular risk in this population.
3. Results
3.1. Characteristics of patients and controls
The differences between the 189 HC and 62 cases are shown in Table 1: HC had a higher BMI and a higher incidence of dyslipidemia (both in total cholesterol and LDL values), obesity, hypertension and active smoking. Patients with SLE had higher AGEs values and creatinine concentrations. As all the HC were Caucasian, we performed a sensitivity analysis to assess the influence of ethnicity, testing only Caucasian patients against HC. We did not find any differences, so we kept all the ethnicities in the final analysis.
3.2. Comparison of AGEs in SLE patients vs healthy controls
First of all, in order to evaluate possible confounding factors, we explored the associations between AGEs levels (stratified in tertiles) and data of all the participants of the study (both SLE patients and HC). The bivariate analysis showed a significant positive relationship between smoking and AGEs levels, while creatinine showed a trend in that same direction. On the contrary, the presence of dyslipidemia was associated with lower values of AGEs (Supplementary Table S2). Analyzing both groups separately, a significant positive association was found between tertiles of AGEs and both age and smoking, in the two groups. In HC, a significant negative association with dyslipidemia was also found (data not shown).
According to these results and the differences found between SLE patients and HC, interaction graphs were created to visually assess smoking, age, dyslipidemia, and creatinine as cofounding variables. We found differences in the slopes of age and dyslipidemia (Supplementary Figure S3) which were then evaluated in the fixed-effects analysis of covariance model Supplementary Table S3. Smoking was also added to the model due to extensive literature linking it to AGEs values. Furthermore, in the smoking interaction graph we observed that the slopes of non-smokers and former smokers behaved similarly, with only a slight increase in mean cumulative AGEs in non-smokers with SLE, but apparently insignificant, so we unified non-smokers and former smokers in the same group vs active smokers to increase statistical power (Supplementary Figure S3a).
Finally, according to all the data explored, the multivariate model was adjusted with age, smoking, dyslipidemia, creatinine, and the interaction terms. None of the interaction terms were statistically significant so they were finally removed from the model except for the interaction between dyslipidemia and group (LES or HC). This one, was not omitted because it allowed us to observe the effect (p=0.062) of dyslipidemia, granting a better estimation of the AGEs value (Table 2). This was verified by adjusting it without the interaction, where the main effect of dyslipidemia was lost. Dyslipidemia was also adjusted for age and smoking (since HC with dyslipidemia were younger and smoked less), and its effect remained unchanged, ruling out that it was confused by other variables (See Supplementary Table S3). The model reported a statistically significant difference between SLE and HC in AGEs values, showing that AGEs values in SLE patients were 0.721 (95% CI [0.566; 0.876]) units higher (p<0.001) than HC. See Table 2 for the analysis of covariance of fixed effects and Supplementary Figure S4 for the effects graphic.
3.3. Characteristics of SLE patients according to AGEs levels: bivariate analysis.
A total of 122 SLE patients were included. All the variables that showed statistically significant differences according to AGEs tertiles in the bivariate analysis are depicted in Table 3, adjusted by age (p-value M1) and by both age and smoking (p-value M2). The demographic characteristics and other SLE variables of interested are detailed in Supplementary Table S1.
3.4. Correlations between AGEs and SLE characteristics: multivariate analysis.
SLE characteristics that were significant in the exploratory analysis and that might be related to AGEs levels were tested in a model adjusted for previously selected confounding variables (see Methodology), avoiding spurious associations. After adjustment, several SLE characteristics showed associations with AGEs levels.
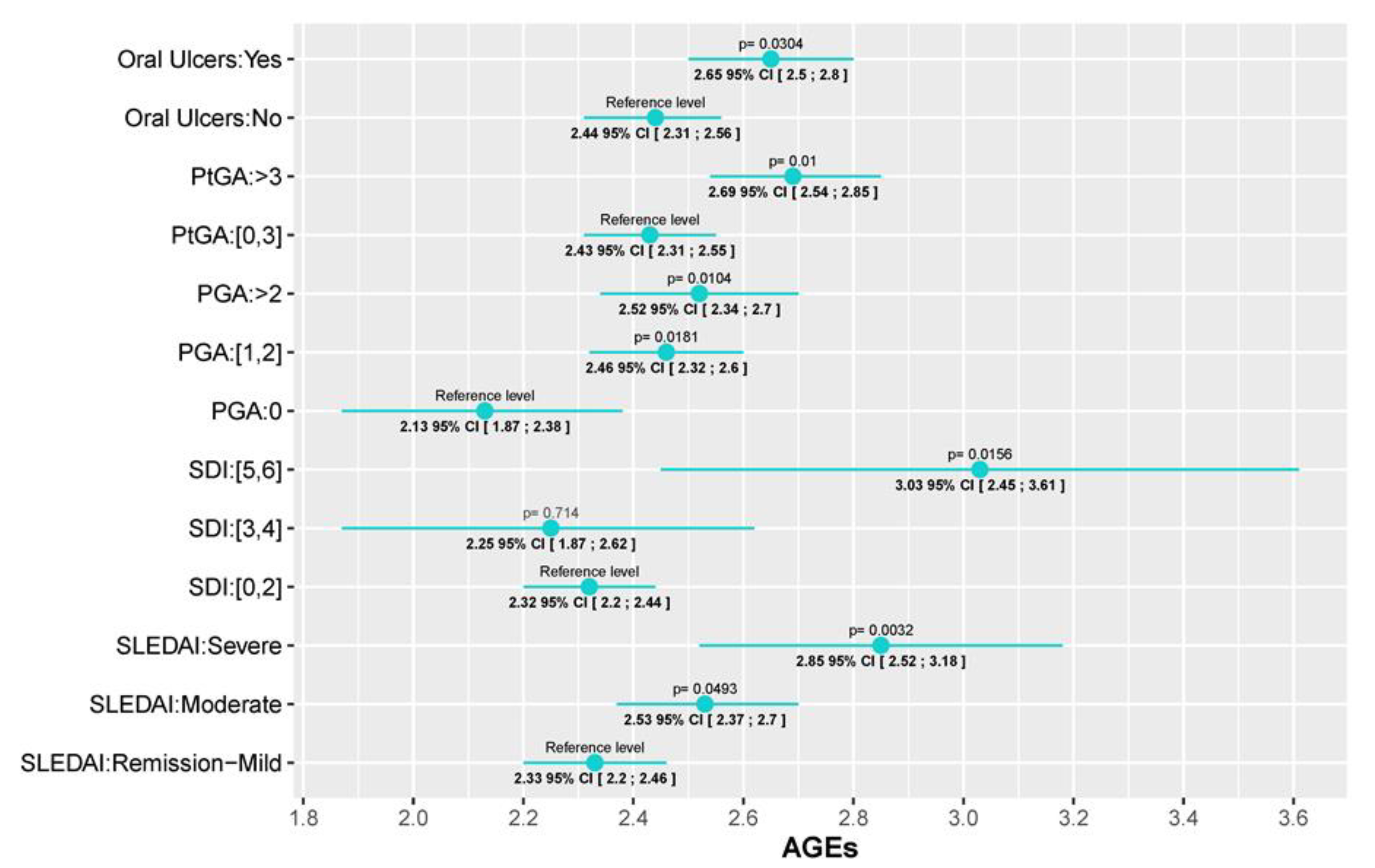
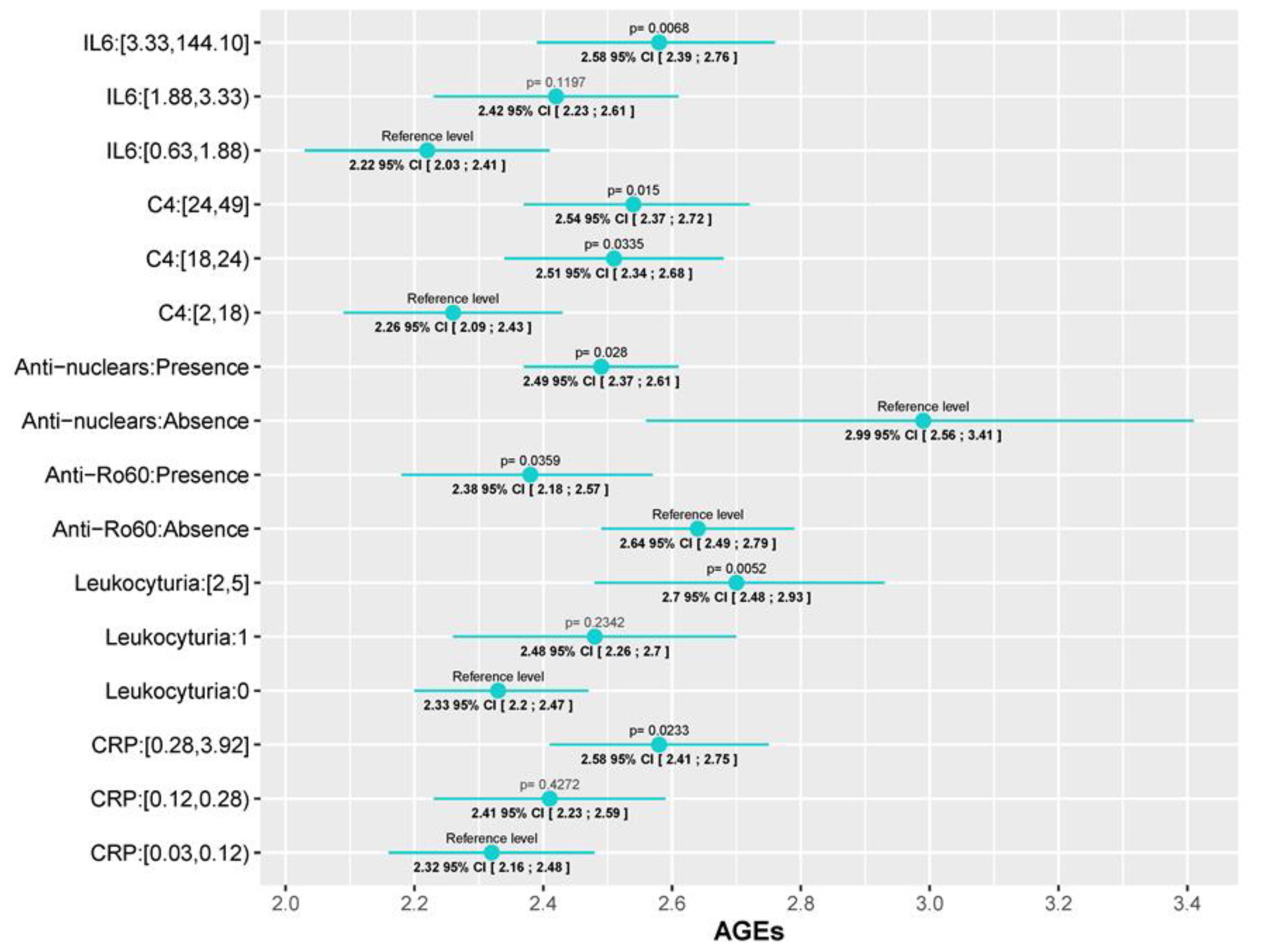
First of all, two of the most important SLE disease indexes, SLEDAI and SDI, were significantly associated with AGEs levels. While for the SLEDAI we found a progressive increase in AGEs values as the SLEDAI activity escalated (AGEs values in patients with moderate and severe activity were 0.2 (95% CI [0.0006; 0.4], p=0.0493) and 0.52 (95% CI [0.177; 0.86], p= 0.003) units higher than patients in remission/mild, respectively), we only found differences in SDI between those with low (0-2) and high scores (5, 6) (AGEs values 0.717 (95% CI [0.139;1.295], p=0.0156) units higher). This association with disease activity is also reflected in both the physician global assessment (PGA) and the patient global assessment (PtGA). In those cases, values higher than 1 (PGA) or 3 (PtGA) were associated with an AGEs level increase (PGA score of 1-2 and a PGA score higher than 2 had AGEs levels 0.033 (95% CI [0.058;0.61], p=0.018) and 0.39 (95% CI [0.094;0.694], p=0.01) units higher than patients with a PGA of 0, respectively; and patients with a PtGA score >3 had AGEs lelvels 0.26 (95% CI [0.063;0.46], p=0.01) units higher than patients with PtGA score ≤ 3. Regarding serum biomarkers, we observed an increment in AGEs levels as CRP and IL-6 increase, but significant differences were only detected between the 3
rd and 1
st tertile: 0.259 (95%CI [0.035;0.48], p=0,02) for CRP and 0.352 (95% CI [0.1;0.6], p=0.006) for IL-6. The same tendency was observed in the level of leukocyturia (0.369, 95%CI [0.112;0.626], p=0.005) and C4 complement, although in this last one, significant differences with the 2
nd tertile were also observed (0.25 (95%CI [0.02;0.48], p=0.0335) for the 2
nd tertile; and 0.28 (95%CI [0.056;0.514], p=0.015) for the 3
rd one). With reference to autoantibodies, a negative association was found between AGEs levels and both the presence of antinuclear antibodies (ANA) or anti-Ro60 antibodies in the blood test performed for the study, where AGEs values were 0.496 (95%CI [0.937;0.054], p=0.028) and 0.26 (95%CI [0.5;0.017], p=0.035) units lower, respectively. Finally, patients which had ever presented oral ulcers, a prevalent SLE manifestation, had AGEs values 0.216 (95% CI [0.02;0.41], p=0.03) units higher than patients who had never. All these data are depicted, according to the prediction of each model, in
Figure 1 and
Figure 2 which graphically represent the mean and its corresponding 95% CI of AGEs for each category of variables. P-values <0.05 indicate significant differences between the categories and the reference level of each variable. Also, the fixed-effects ANCOVA model between AGEs and each of the variables are provided in Supplementary Table S4.
4. Discussion
We observed statistically significant differences between AGEs values measured by skin autofluorescence in SLE patients vs HC. This difference has only been studied in two previous works [
27,
28] with small sample sizes (55 and 30 cases respectively, matched 1:1 with HC), having our study several stronger points. First, we have increased the sample size, especially the HC sample, by matching cases with HC in a 1:3 proportion instead of a 1:1 proportion, making the study more robust. Secondly, we selected HC that had at least one CVRF, so they would be more comparable to our patients who at least have one CVRF, being that the disease itself. This is based on the well-reported knowledge that AGEs are related to inflammation and cardiovascular risk on the one hand and, on the other, that patients with autoimmune diseases like rheumatoid arthritis, have an increased risk of cardiovascular disease that makes necessary to add a fixed multiplier of 1.5 to 2 to the established cardiovascular disease prediction general algorithms in order to adjust for the increased risk due to the disease [
27,
41],
they selected a second control population with essential hypertension (EH), apart from the one conformed by HC. They found statistically significant differences in AGEs levels between SLE patients and HC but not between the SLE and the essential hypertension cohort, suggesting that finding differences when selecting HC with at least one CVRF could traduce a higher statistical power and a reduced probability of committing a type I error. Furthermore, they selected an SLE population with inactive disease, which might not reflect the reality of SLE patients in terms of disease characteristics in the way our patients might, which were included independently of their disease activity.
Additionally, we carefully examined all possible confounding factors to void drawing premature conclusions. Two controversial points were raised during the analyses. First, we observed only a positive trend shown by creatinine in the bivariate analysis of AGEs levels in the whole sample. We discussed if that trend could have a fictitious origin since patients with SLE had higher creatinine levels (although in normal range) and were mostly located in the third AGEs tertile, and also because the trend was not observed when we analyzed the two groups separately. However, we finally decided to include creatinine in the model because there is ample evidence of a higher accumulation of AGEs in patients with renal failure [
42] and lupus nephritis [
25], and a difference could exist between groups because renal disease was an exclusion criterion in the HC group. Secondly, we found a negative association between dyslipidemia and AGEs, which was observed both in the combined analysis of the whole sample and in the HC separately (suggesting that such association comes from the HC group). The only data in the literature that could explain this negative association comes from the reported effect of lipid-lowering drugs in reducing AGEs levels [
43]. Among HC, only 27 of the 85 with dyslipidemia (32%) were being treated with lipid-lowering agents, so we hypothesized that the rest could be controlling it with a lower-fat diet, which has also been associated with reduced AGEs levels [
34]. Hence, we ended up including dyslipidemia in the model.
As for the interaction term between the main effect and dyslipidemia, although it was not found to be significant in the model, graphically the interaction seemed clear, especially in the group of SLE patients (Supplementary Figure S3). This could be due to a lack of statistical power, since in the group of SLE patients there were only 8 dyslipidemic cases, unlike the 85 dyslipidemic HC. Therefore, the statistical power to detect this difference was much lower in the patient group, generating a less precise CI to reject the alternative hypothesis and leading to a lack of significance.
Regarding the study of AGEs relationship with SLE characteristics, we have found associations between AGEs levels and some disease activity indexes: SLEDAI, PGA, PtGA, CRP, and IL6. As reflected in the Results section, the rise of AGEs levels with the increase of SLEDAI, which is the activity index most frequently used for SLE in clinical practice nowadays, showed a robust correlation. This association was also observed with other markers of activity commonly used to assess the disease state: PGA, PtGA and IL6. PGA is a part of the main indexes used currently to define remission or low disease activity in SLE. PtGA may be a more subjective parameter which can be influenced by external factors but that is clearly related to quality of life in SLE patients. IL6 is not used routinely in the follow-up of SLE patients but it is widely known its role in inflammation in general and in rheumatic diseases in particular.
In the case of PCR, a significant association was only found between the upper tertile (0.28-3.92 mg/dL) and the first (<0.12), suggesting that the highest levels of AGEs were found among the patients with higher CRP values, both normal and abnormal values (reference values in our laboratory <0.5 mg/dL). However, this correlation is only supported up to CRP values < 0.7 (R2=0.42, p<0.0001), as graphically reflected in Supplementary Figure S5. No correlation was found with higher PCR levels, which could be justified by a small number of patients with abnormal PCR levels. There was also a positive association with higher C4 levels, which draws attention because low C4 levels are the ones traditionally associated with high disease activity. However, although a decrease in complement levels is included in SLE classificatory criteria, there is wide controversy in the literature about the limited usefulness of the current techniques and types of complement measured in SLE and their ability to reflect disease activity [
44]. Other uncertainties about complement are whether low levels should be persistent or combined (both C3 and C4) to be significant [
45,
46]. In our study, C3 levels showed a statistically significant direct correlation with C4 values (p= <0.001) but not with AGEs levels. There was not an association between having normal C4 levels at the moment of the study and not having had hypocomplementemia ever: 43% of the patients with current normal C4 levels had history of hypocomplementemia and 57% did not, while 77% of the patients with history of low C4 had now normal levels). This could traduce either fluctuant titers or normalized levels of C4 in response to treatment/lower disease activity and a need for further studies to elucidate the relation between complement and AGEs.
We also found a relationship between AGEs and indexes of accrual damage, the SDI. There is only a previous work in the literature that analyzed this association [
28]. They found a correlation between AGEs and SDI in the univariate analysis that was lost after adjusting for age as well as in the multivariate analysis. In our case, the association persisted after adjusting for age and smoking status and any other possible confounding factor in the multivariate analysis. Taking into account this association, measuring AGEs levels could have a high impact in the prognosis of the disease helping to identify a subtype of patients with a more serious disease marked by higher accrual damage, which would be susceptible of a stricter follow-up and intensive treatment regimen, and subsequently allowing to improve these patients’ outcomes.
Specific manifestations (oral ulcers) or autoantibodies profile (less frequent anti-Ro60+ antibodies), could indicate a different clinical phenotype in SLE patients with less inflammation and thus, with lower AGEs levels. In clinical practice, it is very common to find overlaps of autoimmune diseases in the same patient, being especially frequent in SLE its overlap with Sjögren syndrome (SjS). It is known that both diseases have different inflammatory profiles [
47], which could explain why there could be differences in AGEs levels between patients anti-Ro60 positive and negative. AGEs concentrations have been scarcely studied in SjS and efforts have not been directed to skin AGEs but RAGE and sRAGE with conflicting results [
48,
49,
50], so more studies are needed to investigate AGEs levels in SjS and their differences both with SLE patients and with patients with a SLE-SjS overlap. Unfortunately, we could not validate this hypothesis in our study as the presence of SjS was recorded together with other autoimmune diseases as presence of overlapping syndrome in general, making not possible to study the association only in SjS. Furthermore, some patients had ongoing diagnostic SjS tests at the moment of our work. Similarly, oral ulcers are much more frequent in SLE than other autoimmune disease, potentially traducing a more typical SLE disease than in those without, which might justify differences in AGEs levels.
Regarding the negative relation found between AGEs and ANA antibodies, all patients were ANA+ at SLE diagnosis but 10 of them (8.2%) converted during disease follow-up and were ANA- at the moment of the study. It has been reported that the reduction of ANA responses might reflect the natural history of the disease as well as the effects of therapy [
51]. Accordingly, these patients could have increased AGEs levels due to longer disease duration or more intense need for therapy due to more severe disease, and consequent more accrual damage and potentially higher AGEs levels. In our cohort, currently ANA- patients showed higher disease duration (15 vs 10 years) and higher SDI (same levels of p25 and p50 but differences in p75: 1.56 vs 0.68) although the differences were not statistically significant, probably due to lack of statistical power on account of the small sample size, also shown by the wide CI of this variable Supplementary Table S4. We didn’t observe differences in terms of taking immunosuppressants in the moment of the study between ANA+ and ANA- patients, but we didn’t retrieve data of the therapy history of patients, so we cannot rule out differences in the number of immunosuppressants or time taking therapy between both groups.
Despite the known relationship between AGEs and atherosclerosis, we did not find any correlation between AGEs levels and either CVRF or CVE. However, the p-value in the bivariate analysis was <0.1 and, considering that we have a small number of patients with CVE (N=9), it is likely that our results are limited by a lack of statistical power which prevents us from drawing conclusions about the role of AGEs in cardiovascular risk. Furthermore, we assessed cardiovascular disease only through traditional CVRF or CVE and did not perform additional tests like the intima-media thickness of the common carotid artery measured by ultrasound [
28] or the small artery elasticity measured by pulse-wave analysis using tonometric recordings of the radial artery [
27], both of which have been associated with AGEs levels in previous works. We also reassessed the correlation between AGEs and SDI excluding all variables related to cardiovascular disease (expressed as CVE in our study) as De Leeuw et al. do in their work [
28]. They found a correlation in the bivariate analysis between skin AGEs and SDI, also after correction for the damage caused by CV disease. This association was not seen after adjusting for age or in the multivariate analysis. In our cohort, this new analysis did not alter the statistical correlation between SDI and AGEs, indicating that the association is not attributable to AGEs being associated to CV damage.
Only one of the two previous works studying skin AGEs in SLE have analyzed their association with disease characteristics, finding an association with age, creatinine, disease duration, the intima-media thickness of the common carotid artery, and the SDI in the univariate analysis, and only with age and disease duration in the multivariate one [
28]. Our work has carried out a much more extensive analysis taking into account a great amount of demographic and clinical variables and performing a more complex statistical analysis considering all possible confounding factors, which provides a much deeper knowledge into these relationships and opens the door to the feasibility of using AGEs as a clinical tool for SLE management and prognosis.
Our study presents several limitations. Firstly, due to the retrospective nature of the study some data could not be retrieved like the cumulative GC dose that the patients had taken throughout the disease and we could only assess the impact of GC through the current dose at the moment of the study. Likewise, the design makes impossible to assess causality, which warrants future prospective studies. Secondly, and in order to clarify the effect on longstanding disease and therapy in AGEs levels, studies in newly diagnosed patients should be performed.
To our knowledge this is the second work to study and the first to find an association between SLE activity parameters and skin AGEs. We have found a correlation with, not one, but several SLE activity biomarkers and, also, with damage indexes. Furthermore, we have described, for the first time, skin AGEs associations with specific serological and clinical parameters that could define more precisely a specific type of patients in whom AGEs could have a particularly meaningful contribution. Therefore, our results are innovative and indicative of the promising role of AGEs and the AGEs skin reader as a tool to be implemented in daily clinical practice as a noninvasive, fast, real-time surrogate biomarker of SLE disease activity, damage and specific manifestations.